Abstract
Background
Survivors of childhood cancer exposed to cardiotoxic therapies are at significant cardiovascular risk. The utility of cardiac biomarkers to identify risk of future cardiomyopathy and mortality is unknown.
Methods
N-terminal pro-B-type natriuretic peptide (NT-proBNP) and cardiac troponin-T (cTNT) were assessed in 1213 adults ≥10 years from childhood cancer diagnosis, 786 exposed to anthracycline chemotherapy and/or chest-directed radiation therapy (RT). NT-proBNP above age- and sex-specific 97.5th percentiles were considered abnormal. Generalized linear models estimated cross-sectional associations between abnormal NT-proBNP and anthracycline or chest RT dose as risk ratios (RR) with 95% confidence intervals (CI). A Poisson distribution estimated rates and Cox-proportional hazards model estimated hazard ratios (HR) for future cardiac events and death.
Results
At a median age of 35.5 years (interquartile range, IQR 29.8–42.5), NT-proBNP and cTnT were abnormal in 22.5% and 0.4%, respectively. Exposure to chest RT and anthracycline chemotherapy were each associated with a dose-dependent increased risk for abnormal NT-proBNP (p-for-trend <0.0001). Among exposed survivors with no history of CTCAE graded cardiomyopathy and normal systolic function survivors with abnormal NT-proBNP had a higher rate/1000 person-years of cardiac mortality (2.93 vs. 0.96, p-value <0.0001) and future cardiomyopathy (32.10 vs. 15.98, p-value <0.0001) and an increased risk of future cardiomyopathy (HR 2.28, 95% CI 1.28–4.08) on multivariable assessment.
Conclusion
Abnormal NT-proBNP values were prevalent and, among survivors exposed to cardiotoxic therapy but without history of cardiomyopathy or current systolic dysfunction, identified those at increased risk for future cardiomyopathy. Further longitudinal studies are needed to confirm this novel finding.
Keywords: Biomarker, Cancer, Cardiotoxicity, Child, Survivors
Precis:
In this longitudinal cohort study that included 1213 adult survivors of childhood cancer, nearly 25% had an abnormal NT-proBNP value for age and sex at baseline. Among survivors exposed to cardiotoxic therapy who had a normal ejection fraction at baseline, those with abnormal NT-proBNP had more than twice the risk of future cardiomyopathy, a significant finding.
INTRODUCTION
Over 85% of children diagnosed with cancer today will become long-term survivors.1 However, by age 30, 8% will have at least one severe, disabling, life-threatening or fatal cardiovascular condition, a proportion that will increase to 35% by age 50.2 Known risk factors for cardiomyopathy among survivors of childhood cancer include anthracycline exposure and chest radiotherapy involving the heart.3,4 Traditional cardiovascular risk factors, including hypertension, dyslipidemia and diabetes further increase risk.5 Early detection provides opportunities for interventions to preserve systolic function and potentially limit heart failure associated morbidity and mortality, similar to findings observed in the general population.6,7 However, prior studies have been unsuccessful at identifying early markers of developing cardiac dysfunction in the childhood cancer survivor population.3,8
Natriuretic peptides including brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP), are released in response to myocardial wall stretch and remodeling and are widely utilized for the diagnosis and prognosis of symptomatic heart failure.6,7,9 Studies in the general population have identified that higher natriuretic peptide levels at baseline, even when within the normal range, are associated with increased risk of incident heart failure, a first major cardiovascular event, and death from any cause.10
Prior cross-sectional studies focused on the diagnostic yield of surveillance for cardiomyopathy in asymptomatic childhood cancer survivors suggested that natriuretic peptides were inadequate as the only method of surveillance due to low sensitivity and positive predictive value for detection of current cardiomyopathy.3,8,11,12 However, associations with future major cardiac events and death have not been systematically assessed in a large cohort of adult, long-term survivors of childhood cancer. Therefore, we aimed to assess cross-sectional associations between cardiac biomarkers and 1) cardiotoxic therapy exposure, 2) exercise intolerance and 3) their sensitivity and specificity as a screening marker of current cardiac dysfunction. We then aimed to assess longitudinal associations between abnormal NT-proBNP and new cardiac events or death, in the subset of survivors with cardiotoxic therapy exposure and no known cardiac dysfunction.
Methods
Study participants
Participants in the current study were recruited from the SJLIFE cohort.13,14 Eligibility included age ≥18 years at evaluation, ≥10 years from original cancer diagnosis, treated for childhood cancer at St. Jude Children’s Research Hospital (SJCRH) from 1962 through 2007, and had no history of congenital heart disease. Survivors were excluded if they had chronic kidney disease, defined as estimated glomerular filtration rate <60 ml/min/1.73 m2 or salt-wasting requiring electrolyte replacement, as renal dysfunction complicates the interpretation of NT-proBNP levels, at least in part due to decreased clearance of this peptide.15 Pregnant women were excluded.
Eligible survivors were stratified based on exposure to cardiotoxic therapy (any chest-directed radiation therapy (RT); any chest-directed RT and any anthracycline chemotherapy; anthracycline chemotherapy only, at dose categories of 1 to 199, 200 to 349 or ≥350 mg/m2; or neither anthracycline chemotherapy nor chest directed RT) and randomly assigned to order of recruitment. This study was approved by the institutional review board prior to recruitment and written, informed consent was obtained prior to enrollment.
Cardiac Biomarkers
Blood samples were obtained from fasting participants. Plasma NT-pro-BNP was measured with an electrochemiluminescence immunoassay (Elecsys 2008; Roche Diagnostics, Indianapolis, Indiana). The lower limit of detection was 5 pg/mL. Normal NT-pro-BNP values for age and sex were defined using previously established 97.5th quantile regression limit cut-points for healthy adults in the Framingham Heart Study Generation 3 cohort (Table S1).16 Cardiac troponin-T (cTnT) was measured with an electrochemiluminescence immunoassay (Elecsys 2008; Roche Diagnostics, Indianapolis, Indiana) with detectable levels at or above 0.01 ng/mL considered abnormal.
Demographic and exposure variables
Medical record abstraction ascertained cancer diagnostic and treatment information. Total anthracycline dose was calculated in milligrams per square meter as the doxorubicin equivalent dose.17 Radiotherapy records were centrally reviewed and the maximum target dose (maxTD) for chest directed RT was determined by summing the total prescribed dose from all overlapping treatment fields in the chest.18 Demographic, lifestyle and cardiac medication variables were obtained from SJLIFE questionnaires.
Cardiovascular risk factors, cardiac events (myocardial infarction, cardiomyopathy, stroke and other vascular events) and other chronic health conditions were identified either during clinical study evaluation or by participant self-report and validated by medical records. Events were graded using a modified Common Terminology Criteria for Adverse Events (CTCAE) v4.03 grading (Table S2).14 Among patients with cardiomyopathy diagnosed prior to the study visit, medications used to treat cardiomyopathy or heart failure were captured by participant self-report (Table S3).
Echocardiography
Baseline assessment included concurrent echocardiography and biomarker blood collection. Left ventricular volumes to determine ejection fractions were measured by three-dimensional (3D) echocardiography and diastolic function was assessed with two-dimensional measures and Doppler using a Vivid 7 machine (GE Medical Systems, Milwaukee, WI) according to the American Society of Echocardiography (ASE) guidelines with abnormal LVEF defined as <53%.19 Diastolic dysfunction was defined according to 2016 ASE guidelines by meeting any three of the following criteria: 1) E/e’ >14, 2) septal e’ velocity <7 cm/s or lateral e’ velocity <10 cm/s, 3) tricuspid regurgitation velocity >2.8 m/s, 4) left atrial volume index >34 ml/m2.20 From 2016 ASE guidelines, all participants with LVEF <53% were considered to have diastolic dysfunction. Global longitudinal peak systolic strain (GLS) was assessed using 2D echocardiography with speckle tracking and values >1.5 standard deviations above sex-, age- and vendor-specific means were defined as abnormal.21
Echocardiograms were centrally evaluated in a blinded manner by a core laboratory at Baylor College of Medicine. The core echocardiography lab included three cardiologist reviewers. We estimated interobserver and intraobserver variability for LVEF using a random selection of 20 echocardiograms, stratified by LVEF <53% versus ≥53%, which were originally read by the lead cardiologist and were then read by each of the three reviewers at a later date. Pairwise interobserver agreement was calculated for LVEF (normal vs. abnormal) as 95–100% for each of the three reviewer pairs. Intraobserver agreement for LVEF (normal vs. abnormal) was 95%.
Exercise testing
Exercise intolerance was defined as a relative peak oxygen uptake (VO2peak measured in milliliters per kilogram per minute) <85% of predicted during maximal cardiopulmonary exercise testing (CPET) on a treadmill using a modified Bruce protocol.13,22–24 A leg or arm ergometer ramping protocol was used for participants with amputation or balance impairments that prohibited treadmill testing. Safety and peak VO2 were monitored using a continuous 12-lead ECG and breath-by-breath gas analysis (Ultima CardiO2; MGC Diagnostics, St. Paul, MN). Lifestyle and performance measures known to impact exercise capacity were assessed and are described in detail in the Supplement.
Mortality and Major Cardiac Events
A National Death Index (NDI) search, supplemented by the SJCRH Cancer Registry continuous annual follow-up through December 31, 2018 was used to determine mortality. Cause of death was identified using the International Classification of Diseases, 9th and 10th Revisions from the NDI or review of death certificates, medical records, or next-of-kin interviews. Major non-fatal cardiac events subsequent to the baseline visit were identified by self-report during annual follow-up or clinical assessment during a subsequent SJLIFE visit and verified by clinician review of relevant medical records.
Statistical analyses
Descriptive statistics, with comparisons using t tests or χ2 statistics, characterized the study population. Median values were reported with interquartile ranges (IQR). Generalized linear models, adjusted for age at diagnosis, age at evaluation, sex and race/ethnicity, were first used to examine cross-sectional associations of abnormal NT-proBNP levels with treatment characteristics and then with chronic conditions. Estimates are reported as risk ratios (RR) with 95% confidence intervals (CI). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of abnormal NT-proBNP for identification of cross-sectional echocardiographic cardiac dysfunction (reduced LVEF, abnormal GLS or diastolic dysfunction) were calculated for survivors overall and those exposed to cardiotoxic therapy, excluding survivors previously diagnosed with CTCAE grade 3 or 4 cardiomyopathy. Generalized linear regression, using a binomial distribution and a logit link, was used to evaluate cross-sectional associations between abnormal NT-proBNP and exercise intolerance with estimates reported as odds ratios (OR) with 95% CI. For the longitudinal analysis of cardiac events and mortality, a Poisson distribution, using a log link with log of person-years as the offset, was used to estimate rates of death and cardiac events. Cox-proportional hazard regression estimated the association between abnormal NT-proBNP and cardiac event or mortality, adjusted for age at diagnosis, age at assessment, sex, race/ethnicity, and body mass index (BMI) reported as hazard ratios (HR) with 95% CI. SAS, version 9.4 (SAS Institute, Cary, NC) was used for analysis.
Results
Among 1,369 potentially eligible survivors, there were 109 with active or passive refusal, 44 excluded due to chronic kidney disease, and three for incomplete biomarker testing leaving a population of 1,213 evaluated for this analysis (88.6%; Figure S1). Survivors exposed to anthracycline chemotherapy and/or chest-directed RT (N=786), were older at diagnosis (median age 10.0 years, IQR 4.2–14.7; Table S4) than those not exposed (7.1 years, 3.1–12.9) but had a similar duration of follow-up from diagnosis (median 26.3 years, IQR 20.4–33.1, and 27.1 years, 18.7–35.1, respectively) at baseline evaluation. Exposed survivors were more likely to have been treated for lymphoma or bone tumors than unexposed survivors. The proportions of survivors with cardiovascular risk factors including diabetes mellitus, hypertension, dyslipidemia and obesity were similar (Table S4).
A total of 273 (22.5%, 95% CI 20.2–24.9%) survivors had an NT-proBNP value > age- and sex-specific 97.5th percentile values in the general population (Table S5; Figure S2 shows distributions by age and cardiac function). A higher proportion of survivors exposed to anthracyclines and/or chest RT had abnormal NT-proBNP values compared to unexposed survivors by sex (male 33.9% vs. 8.3%, p<0.0001; female 27.0% vs. 6.7%, p<0.0001; Figure 1, Table S5) and overall. Only five survivors (0.4%, 95% CI 0.0–0.8%) had an abnormal cTnT value, all of whom had been exposed to anthracycline chemotherapy or chest-directed RT.
Figure 1. Distribution of NT-proBNP level by anthracycline and chest radiation therapy exposure status and sex.
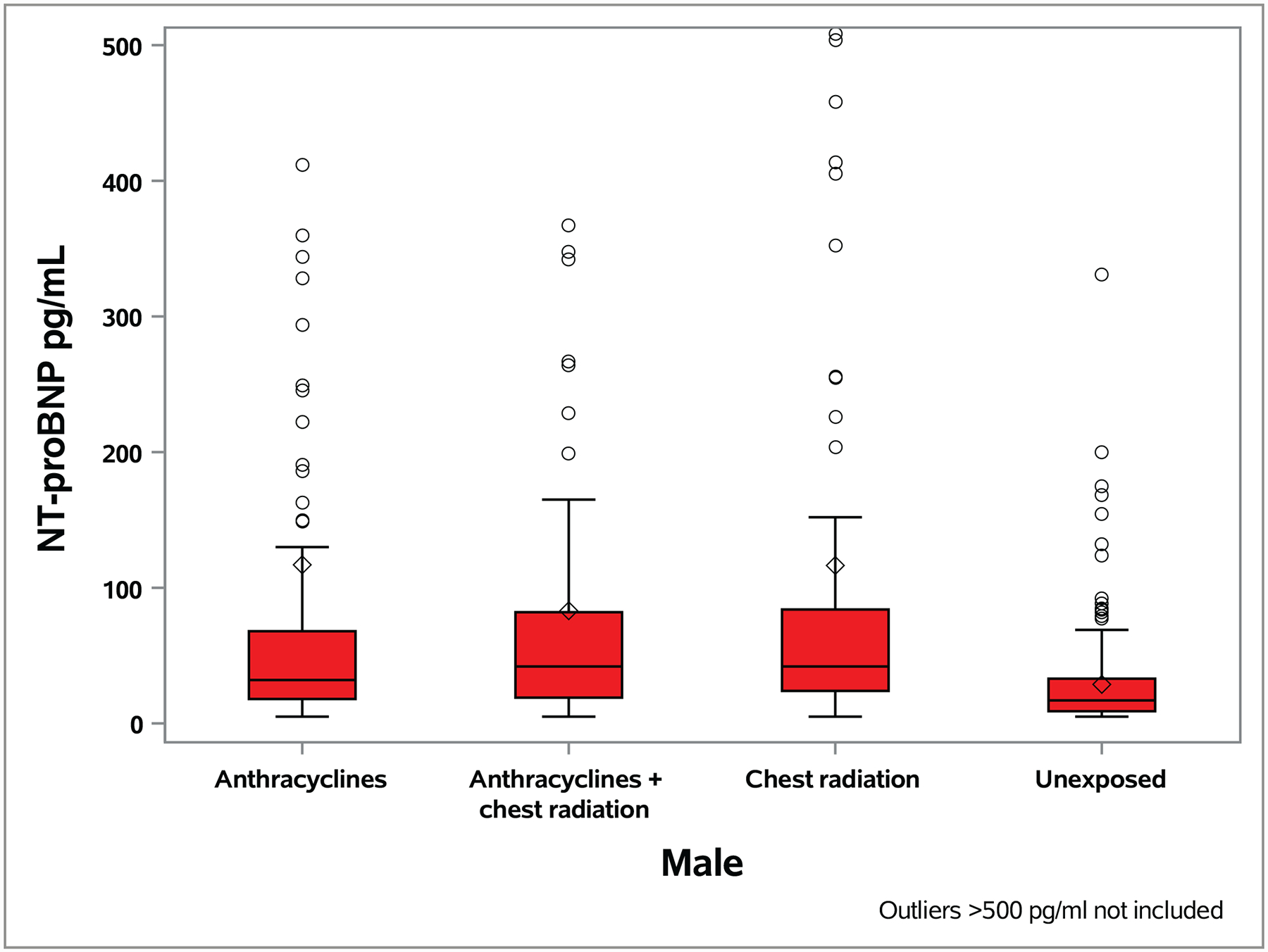
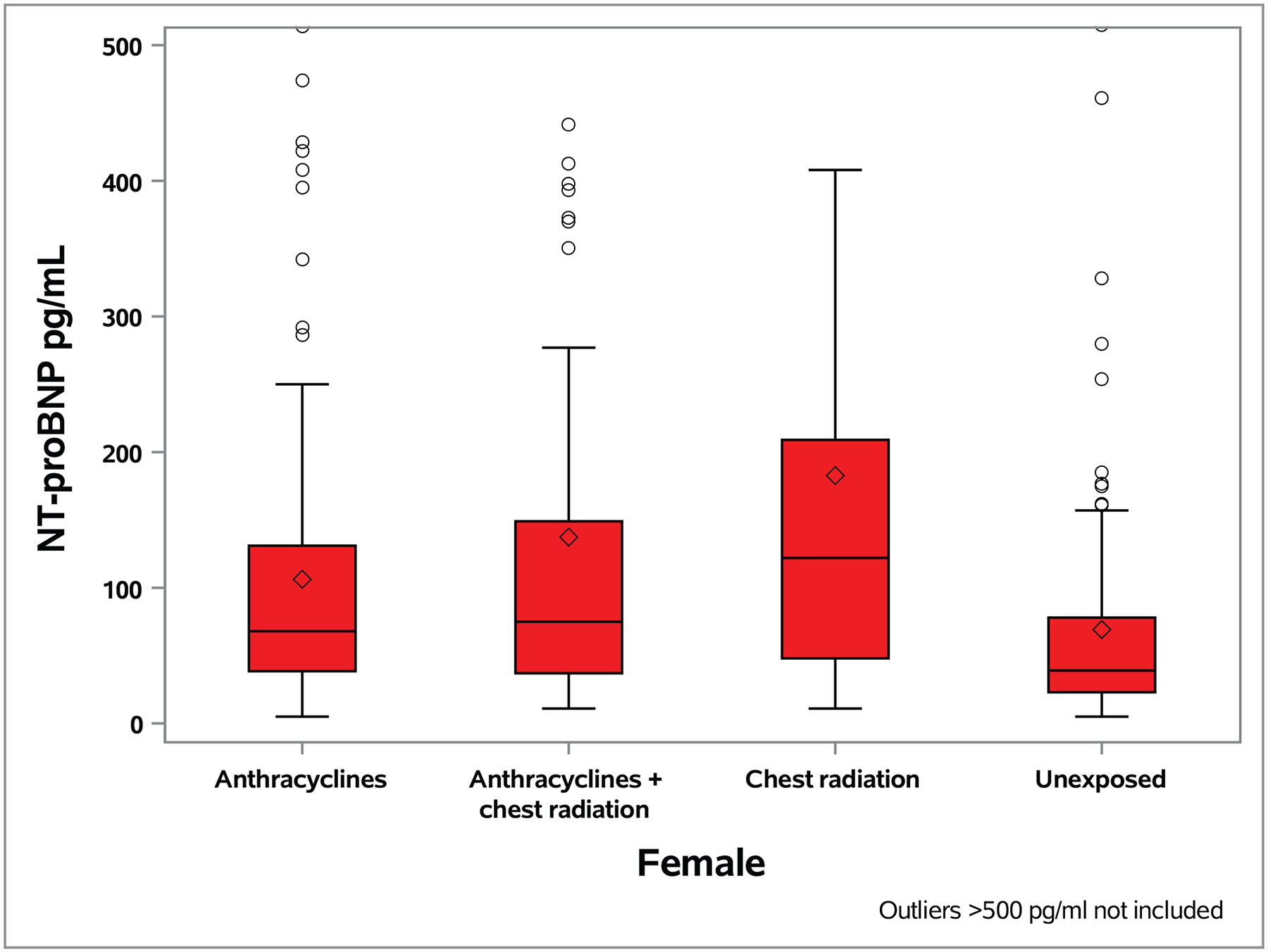
The median value is represented by the horizontal line within each box, the box is the interquartile range and the whiskers extend to 1.5 times the interquartile range. Circles represent outliers and the diamond represents the mean value
In a multivariable analysis, adjusted for demographic factors, we identified a dose-dependent increase in risk for abnormal NT-proBNP with exposure to chest RT (p-for-trend <0.0001) and anthracycline chemotherapy (p-for-trend <0.0001; Figure 2, Table S6). Survivors exposed to the highest dose level of anthracycline or chest RT, compared to no anthracycline or no chest RT, respectively, had at least a three-fold increase in risk for abnormal NT-proBNP (anthracycline dose >350 mg/m2, RR 2.99, 95% CI 2.27–3.95; chest RT ≥30 Gy, RR 3.66, 95% CI 2.89–4.64). Similarly, when a composite variable combining both agents and dose was generated, we identified that compared to those with no cardiotoxic agent exposure, survivors exposed to both agents at higher doses (>200 mg/m2 anthracycline and ≥20 Gy chest RT) had three-times the risk for abnormal NT-proBNP (RR 3.18, 95% CI 2.04–4.94; Table S7).
Figure 2. Multivariable association between treatment exposures and abnormal NT-proBNP as risk ratio (95% confidence interval).
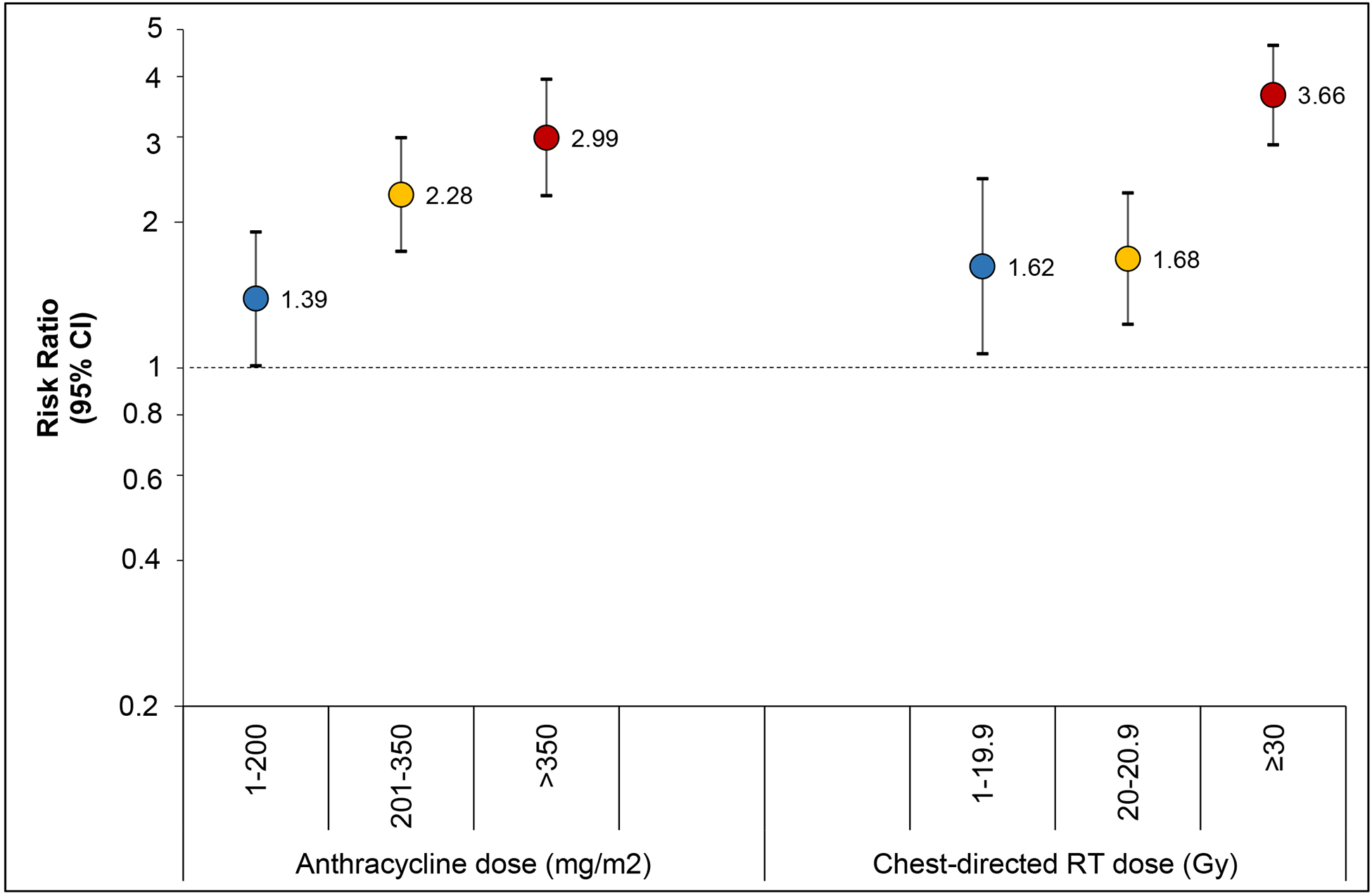
Analysis modeled the risk ratio of abnormal NT-proBNP adjusting for age at diagnosis, attained age, race, sex and treatment exposures in figure. Risk ratios for each treatment are compared to no exposure to that treatment, represented by the line of no difference at a risk ratio of 1.0.
Among survivors exposed to cardiotoxic therapy exercise intolerance was present in 64.4% (95% CI 62.6–66.3%) of those with normal NT-proBNP and 82.2% (95% CI 81.3–83.1%) of those with abnormal NT-proBNP (Table 1). Abnormal NT-proBNP was associated with an increased likelihood of exercise intolerance (OR 1.72, 95% CI 1.13–2.60), independent of age, sex, race/ethnicity, lifestyle habits and other organ system dysfunction.
Table 1.
Association of NT-proBNP with exercise intolerance adjusting for demographic, lifestyle factors and organ-based impairment among exposed survivors of childhood cancer
| N | % Exercise Intolerance (95% CI) | OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| NT-proBNP | |||||
| Normal | 545 | 64.4 (62.6–66.3) | 1.00 | ||
| Abnormal | 241 | 82.2 (81.3–83.1) | 1.72 | 1.13–2.60 | 0.01 |
| Race/Ethnicity | |||||
| White | 669 | 67.1 (66.3–68.0) | 1.00 | ||
| Non-white | 117 | 85.5 (84.4–86.6) | 2.34 | 1.49–3.65 | <0.001 |
| Sex | |||||
| Female | 367 | 65.4 (64.2–66.6) | 1.00 | ||
| Male | 419 | 73.7 (72.8–74.6) | 1.65 | 1.21–2.25 | 0.002 |
OR, odds ratio; CI, confidence interval
Analysis was limited to survivors exposed to chest-directed radiation or anthracycline chemotherapy. Odds were adjusted for age at assessment, race, sex, smoking (pack-years), diet quality, physical activity, and performance measures known to impact exercise capacity. (See Supplement for detailed description of all covariates)
Among the subset of survivors exposed to cardiotoxic therapy and previously undiagnosed with grade 3–4 cardiomyopathy, abnormal NT-proBNP at the time of the baseline evaluation had poor sensitivity (≤31% for all comparisons) and only moderate specificity for identifying survivors with new onset of abnormal LVEF <53%, GLS or diastolic dysfunction, 75%, 77%, and 76% respectively (Table S8).
Among exposed survivors with no previous history of grade 3–4 cardiomyopathy and normal LVEF (≥53%) at baseline (N=535), 24.7% (95% CI 21.0–28.4%, N=132) had an abnormal NT-proBNP value. With median follow-up of 5.4 (IQR 4.67–5.95) years, there were 74 major adverse cardiac events including 52 survivors with cardiomyopathy (Table S9) and 15 deaths attributable to second malignant neoplasms (n=4), cardiac conditions (n=4), liver failure (n=2), and other causes (n=5). The cardiac-related mortality rate was higher among those with abnormal vs. normal NT-proBNP values (Rate/1000 person-years 2.93 vs. 0.96; p-value <0.0001). Rates of any major adverse cardiac event and the development of cardiomyopathy were also higher among those with abnormal vs. normal NT-proBNP values (Any event: 35.76 vs. 24.56, Cardiomyopathy: 32.10 vs. 15.98, p-values<0.0001; Table 2). Compared to those with normal NT-proBNP, survivors with abnormal NT-proBNP exposed to cardiotoxic therapy with no history of cardiomyopathy and normal LVEF at baseline had an increased risk of any major adverse cardiac event (HR 1.75, 95% CI 1.04–2.94), driven by a two-fold increase in risk of future development of cardiomyopathy (HR 2.28, 95% CI 1.28–4.08) independent of age, sex, race/ethnicity, and baseline cardiovascular risk factors (BMI, hypertension, dyslipidemia, diabetes). Further, they had an increase in risk of cardiac mortality (HR 3.31, 95% CI 0.32–34.59); however, this finding did not reach statistical significance.
Table 2.
Crude incidence and multivariable associations of death and cardiac events by NT-proBNP status among exposed survivors with normal LVEF (≥53%) at baseline
| No of Events/Total No at Risk | NT-proBNP Status | Adjusted Hazard Ratio for Abnormal NT-proBNP | ||||
|---|---|---|---|---|---|---|
| Abnormal | Normal | |||||
| Rate/1000 person-years (95% CI) | p-value | HR | 95% CI | |||
| Cardiac Mortality | 4/535 | 2.93 (2.81–3.07) | 0.96 (0.92–1.00) | <0.0001 | 3.31 | 0.32–34.59 |
| 1st Major Adverse Cardiac Event | 70/535 | 35.76 (35.28–36.23) | 24.56 (24.34–24.78) | <0.0001 | 1.75 | 1.04–2.94 |
| Myocardial Infarction | 11/535 | 1.48 (1.38–1.57) | 4.86 (4.77–4.96) | <0.0001 | 0.41 | 0.05–3.40 |
| Cardiomyopathy | 52/535 | 32.10 (31.66–32.55) | 15.98 (15.81–16.16) | <0.0001 | 2.28 | 1.28–4.08 |
| Vascular Disease | 8/535 | 1.47 (1.39–1.57) | 3.38 (3.30–3.46) | <0.0001 | 0.59 | 0.07–5.19 |
| Stroke | 3/535 | 1.47 (1.39–1.57) | 0.96 (0.92–1.00 | <0.0001 | 1.38 | 0.11–17.88 |
HR, hazard ratio; CI, confidence interval
Major Adverse Cardiac Event included myocardial infarction, cardiomyopathy, stroke or other vascular disease (excluding stroke and myocardial infarction) as defined in CTCAE grading from Table S2.
Analyses were limited to survivors exposed to cardiotoxic therapy with no history of grade 3–4 cardiomyopathy and normal LVEF at baseline assessment. Cardiac events are CTCAE grade 2–4.
P-value is comparing the rate of cardiac event by NT-proBNP status.
HR was adjusted for demographic variables of age at diagnosis, age at evaluation, sex, race/ethnicity (non-Hispanic white vs other), BMI and presence/absence of baseline cardiovascular risk factors (hypertension, diabetes, dyslipidemia).
Discussion
We have demonstrated that an abnormal NT-proBNP identifies survivors at increased risk of future cardiomyopathy among a population exposed to cardiotoxic therapy but previously undiagnosed with cardiomyopathy and without echocardiographic evidence for systolic dysfunction at the time of biomarker evaluation. These novel findings should inform clinical care as an abnormal NT-proBNP, even in the setting of a normal LVEF, may identify individuals who should be considered for more frequent follow-up or prevention strategies.
To our knowledge, this is the first study to demonstrate that abnormalities in natriuretic peptide levels in survivors of childhood cancer exposed to cardiotoxic therapy are associated with current exercise intolerance and an increased risk of future cardiomyopathy. Among the general and elderly adult population, there is significant evidence for worse outcomes among individuals with elevated natriuretic peptide levels. A study of healthy adults in the Framingham Offspring cohort demonstrated that, after adjustment for age, sex, BMI, smoking status and traditional cardiovascular risk factors, a natriuretic peptide level above the 80th percentile for sex was associated with an increase in risk of death from all causes and first major cardiovascular event, with a 3- to 5-fold increase in risk of heart failure.10 Subsequent studies have confirmed that natriuretic peptide levels are an independent predictor of mortality in adults both with and without heart failure,25 and in specific populations including patients with hypertrophic cardiomyopathy,26 stable coronary artery disease,27 or aortic stenosis.28 Therefore, our findings are supported by those in other adult populations.
Among adult survivors of childhood cancer exposed to cardiotoxic therapy nearly one-third had an NT-proBNP level above the 97.5th percentile for age and sex, while abnormal levels were identified in only 7.5% of survivors not exposed. Furthermore, we identified a clear dose-dependent relationship between prior cardiotoxic therapy exposure and current NT-proBNP level, providing strong evidence that NT-proBNP is a marker of treatment-related toxicity. Despite this relationship, NT-proBNP had poor performance as a screening marker to identify survivors with current echocardiographic evidence of systolic or diastolic function. This confirms prior studies in childhood cancer survivors that determined NT-proBNP was unreliable as the only screening method for cardiac dysfunction.3,8,11,12
Only five survivors (0.4%) had abnormal cTNT levels. Although all five instances occurred in survivors with prior exposure to cardiotoxic therapy, only two had an LVEF <53% (both with abnormal NT-proBNP). This is expected, as elevated cardiac troponin levels are reflective of myocardial injury, and prior studies have demonstrated an association between elevated levels and short-term,29,30 but not late onset, anthracycline-related cardiotoxicity.8,12,31,32
Although our follow-up time for mortality and major cardiac events was relatively short, even at a median of five years we identified a significant increase in risk of cardiomyopathy among the over 500 survivors with exposure to cardiotoxic therapy who had no history of cardiomyopathy or echocardiographic systolic dysfunction at baseline. This suggests that there is a strong effect, however, longer follow-up time with longitudinal assessment of natriuretic peptide levels in conjunction with cardiac imaging will be necessary to more clearly define the utility and limits of natriuretic peptide levels for screening of survivors exposed to cardiotoxic therapy. Due to the low number of deaths, the elevated risk for cardiac mortality among survivors with abnormal NT-proBNP did not achieve statistical significance and we were unable to evaluate how cardiovascular risk factors (hypertension, diabetes, dyslipidemia) or baseline diet and exercise may modify observed associations. A limitation of our data is that it did not include assessment of clinical symptoms of heart failure. This information would have been useful to assess any possible association between NT-proBNP level and clinical heart failure in a non-acute setting among survivors as natriuretic peptide cut-points have been used to differentiate heart failure from other causes of dyspnea in the general adult population.9,33 Finally, although echocardiogram is the recommended screening tool for cardiomyopathy among survivors of childhood cancer,3,34 echocardiographic methods have high false-negative rates and, at best, moderate sensitivity to detect survivors with a reduced ejection fraction compared to cardiac magnetic resonance imaging (MRI).35 It is possible that a study investigating association between natriuretic peptide elevation and cardiac dysfunction using cardiac MRI may identify improved sensitivity and specificity for biomarker screening in this population.
In conclusion, abnormal NT-proBNP was highly prevalent among survivors previously exposed to cardiotoxic therapy and was associated with an increased risk for future cardiomyopathy among exposed survivors with no history of cardiomyopathy and normal LVEF at the time of baseline assessment. Further, abnormal NT-proBNP was associated with anthracycline chemotherapy and chest RT in a dose-dependent manner as well as exercise intolerance. Given that among exposed survivors with normal systolic function, those with abnormal NT-proBNP had increased rates of cardiac death and future major cardiac events, including cardiomyopathy, we believe these findings may inform current guidelines for long-term follow-up of survivors such that identification of abnormal NT-proBNP in the setting of normal LVEF may warrant more frequent follow-up. However, additional longitudinal studies including serial natriuretic peptide levels, cardiac imaging and long-term event data among survivors are needed to fully understand the utility of natriuretic peptides as a screening tool and prognostic marker for future cardiovascular events in this high-risk population of adult survivors of childhood cancer.
Supplementary Material
Funding:
Support to St. Jude Children’s Research Hospital provided by the National Cancer Institute (R01 CA157838, G. Armstrong, Principal Investigator; U01 CA195547, M. Hudson and L. Robison, Principal Investigators), the Cancer Center Support (CORE) grant (P30 CA21765, C. Roberts, Principal Investigator), and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
Additional Information: Data included in the manuscript was previously presented at the 2019 North American Symposium on Late Complications After Childhood Cancer, Atlanta, GA.
Author Disclosures:
Vijaya M. Joshi:
Stock and Other Ownership; Johnson & Johnson, Gilead Sciences
Daniel J. Lenihan:
Consulting or Advisory Role: Pfizer, Acorda, Lilly, Bristol Myers Squibb, Roche.
Research Funding Unrelated to Current Study: Myocardial Solutions, Inc
Melissa M. Hudson
Consulting or Advisory Role: Oncology Research Information Exchange Network
All other authors report no conflicts of interest to disclose.
References
- 1.Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute;2019. [Google Scholar]
- 2.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65(23):2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation. 2013;128(16):e240–e327. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. 2017;70(6):776–803. [DOI] [PubMed] [Google Scholar]
- 8.Leerink JM, Verkleij SJ, Feijen EAM, et al. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: a systematic review. Heart. 2019;105:210–216. [DOI] [PubMed] [Google Scholar]
- 9.Januzzi JL Jr., Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948–954. [DOI] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. [DOI] [PubMed] [Google Scholar]
- 11.Pourier MS, Kapusta L, van Gennip A, et al. Values of high sensitive troponin T in long-term survivors of childhood cancer treated with anthracyclines. Clin Chim Acta. 2015;441:29–32. [DOI] [PubMed] [Google Scholar]
- 12.Mavinkurve-Groothuis AM, Groot-Loonen J, Bellersen L, et al. Abnormal NT-pro-BNP levels in asymptomatic long-term survivors of childhood cancer treated with anthracyclines. Pediatr Blood Cancer. 2009;52(5):631–636. [DOI] [PubMed] [Google Scholar]
- 13.Ness KK, Plana JC, Joshi VM, et al. Exercise intolerance, mortality, and organ system impairment in adult survivors of childhood cancer. J Clin Oncol. 2020;38(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity-grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(5):666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srisawasdi P, Vanavanan S, Charoenpanichkit C, Kroll MH. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am J Clin Pathol. 2010;133(1):14–23. [DOI] [PubMed] [Google Scholar]
- 16.Fradley MG, Larson MG, Cheng S, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am J Cardiol. 2011;108(9):1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell RM, Smith SA, Weathers RE, Kry SF, Stovall M. Adaptations to a generalized radiation dose reconstruction methodology for use in epidemiologic studies: an update from the MD Anderson Late Effect Group. Radiat Res. 2019;192(2):169–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360. [DOI] [PubMed] [Google Scholar]
- 21.Takigiku K, Takeuchi M, Izumi C, et al. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J. 2012;76(11):2623–2632. [DOI] [PubMed] [Google Scholar]
- 22.Riebe D, Ehrman J, Liguori G, Magal M. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed. Philadelphia, PA: Wolters Kluwer; 2018. [Google Scholar]
- 23.Myers J, Kaminsky LA, Lima R, Christle JW, Ashley E, Arena R. A Reference Equation for Normal Standards for VO2 Max: Analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog Cardiovasc Dis. 2017;60(1):21–29. [DOI] [PubMed] [Google Scholar]
- 24.Kaminsky LA, Arena R, Myers J. Reference Standards for Cardiorespiratory Fitness Measured With Cardiopulmonary Exercise Testing: Data From the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc. 2015;90(11):1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.York MK, Gupta DK, Reynolds CF, et al. B-type natriuretic peptide levels and mortality in patients with and without heart failure. J Am Coll Cardiol. 2018;71(19):2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geske JB, McKie PM, Ommen SR, Sorajja P. B-type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61(24):2456–2460. [DOI] [PubMed] [Google Scholar]
- 27.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352(7):666–675. [DOI] [PubMed] [Google Scholar]
- 28.Clavel MA, Malouf J, Michelena HI, et al. B-type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol. 2014;63(19):2016–2025. [DOI] [PubMed] [Google Scholar]
- 29.Lipshultz SE, Miller TL, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30(10):1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351(2):145–153. [DOI] [PubMed] [Google Scholar]
- 31.Sherief LM, Kamal AG, Khalek EA, Kamal NM, Soliman AA, Esh AM. Biomarkers and early detection of late onset anthracycline-induced cardiotoxicity in children. Hematology. 2012;17(3):151–156. [DOI] [PubMed] [Google Scholar]
- 32.Soker M, Kervancioglu M. Plasma concentrations of NT-pro-BNP and cardiac troponin-I in relation to doxorubicin-induced cardiomyopathy and cardiac function in childhood malignancy. Saudi Med J. 2005;26(8):1197–1202. [PubMed] [Google Scholar]
- 33.Ewald B, Ewald D, Thakkinstian A, Attia J. Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008;38(2):101–113. [DOI] [PubMed] [Google Scholar]
- 34.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5.0 In. Monrovia, CA: Children’s Oncology Group; 2018. [Google Scholar]
- 35.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30(23):2876–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


