Abstract
Aim of the Study:
Controlled sequential elevation of the head and thorax (CSE) during active compression decompression (ACD) cardiopulmonary resuscitation (CPR) with an impedance threshold device (ITD) has been shown to increase cerebral perfusion pressure and cerebral blood flow in previous animal studies as compared to the traditional supine position. The potential for this novel bundled treatment strategy to improve survival with intact neurological function is unknown.
Methods:
Female farm pigs were sedated, intubated, and anesthetized. Central arterial and venous access were continuously monitored. Regional brain tissue perfusion (CerO2) was also measured transcutaneous. Ventricular fibrillation (VF) was induced and untreated for 10 minutes. Pigs were randomized to 1) Conventional CPR (C-CPR) flat or 2) ACD+ITD CSE CPR that included 2 min of ACD+ITD with the head and heart first elevated 10 and 8 cm, and then gradual elevation over 2 min to 22 and 9 cm, respectively. After 19 min of CPR, pigs were defibrillated and recovered. A veterinarian blinded to the intervention assessed cerebral performance category (CPC) at 24 hours. A neurologically intact outcome was defined as a CPC score of 1 or 2. Categorical outcomes were analyzed by Fisher’s exact test and continuous outcomes with an unpaired student’s t-test.
Results:
In 16 animals, return of spontaneous circulation rate was 8/8 (100%) with ACD+ITD CSE and 3/8 (25%) for C-CPR (p = 0.026). For the primary outcome of neurologically intact survival, 6/8 (75%) pigs had a CPC score 1 or 2 with ACD+ITD CSE versus 1/8 (12.5%) with C-CPR (p = 0.04). Coronary perfusion pressure (mmHg, mean ± SD) was higher with CSE at 18 minutes (41 ± 24 vs 10 ± 5, p = 0.004). rSO2 (%, mean ± SD) and ETCO2 (mmHg, mean ± SD) values were higher at 18 minutes with CSE (32 ± 9 vs 17 ± 2, p = 0.01, and 55 mmHg ± 10 vs 21 mmHg ± 4, p < 0.001), respectively.
Conclusions:
The novel bundled resuscitation approach of CSE with ACD+ITD CPR increased favorable neurological survival versus C-CPR in a swine model of cardiac arrest.
Keywords: Active compression-decompression CPR, Cardiac arrest, Cardiopulmonary Resuscitation, Cerebral perfusion, Head Up CPR, Head and thorax elevation, Controlled sequential elevation, Impedance Threshold Device, Mechanical CPR
Introduction
Neurologically intact survival after cardiac arrest remains abysmal, below 10%.1 A factor for this lack of progress is poor circulation during C-CPR. Animal studies have demonstrated that blood flow to the brain and heart during C-CPR is approximately 15–30% of normal values.2,3 However, there are recent advances that could overcome these limitations, including active compression-decompression (ACD) CPR with an impedance threshold device (ITD), while elevating the head and thorax in a controlled sequential manner, informally known as “Head Up” CPR.4,5
During the decompression phase of C-CPR, blood from the head and the rest of the body passively returns to the right heart while the coronary arteries are perfused. ACD+ITD CPR works by providing an upward force on the chest (ACD) while blocking the airway (ITD) during the decompression phase, generating negative intrathoracic pressure. The vacuum effect created in the chest enhances venous return to the right heart and promotes forward flow of blood. Previous animal and human studies have shown improved hemodynamics and rates of survival with the use of ACD+ITD CPR.4,6–10
Head Up CPR provides a similar benefit to elevating the head of a patient with a neurological emergency to decrease intracranial pressure (ICP). Provided enough mean aortic pressure (MAP) exists to drive blood uphill to the brain during cardiac arrest, gravity can be utilized to decrease ICP while maintaining MAP, thereby increasing cerebral perfusion pressure (CerPP). Head Up CPR provides a unique way to maintain cerebral perfusion and decrease cerebral venous congestion. Another hypothesized benefit of Head Up CPR is decreasing the concussive effect on the brain during the CPR compression phase, where the arterial and venous pressure waves generated can internally concuss the brain.11 Animal studies have consistently shown Head Up CPR used with a circulatory adjunct such as the ITD or ACD+ITD results in decreased ICP, increased CerPP, and improved cerebral blood flow.12–15 Clinically, a prospective observational study of patients who received Head Up CPR as part of an implementation of a new bundle-of-care showed improved pre-hospital ROSC rates as compared to historical numbers.5
Recently the combination of controlled sequential elevation of the head and thorax (CSE) during ACD+ITD CPR, where the head and thorax are slowly elevated over 2 minutes during ACD+ITD CPR to a target height, has been shown in pigs to improve hemodynamics over a prolonged CPR time approaching 20 minutes.16 CerPP values with this methodology have been shown to approach baseline values prior to cardiac arrest. By contrast, Head Up CPR performed with C-CPR results in CerPP values <10% of the normal baseline value after a similar period of time.13,17
The potential benefit of ACD+ITD CSE on neurological survival is unknown. The objective of the current study was to compare neurological function at 24 hours in a swine model of cardiac arrest, where animals were randomized to treatment with either C-CPR or ACD+ITD CSE CPR.
Materials and Methods:
Study Ethics:
This study was approved by the Institutional Animal Care Committee of the Hennepin Healthcare Research Institute (HHRI). Care of swine was compliant with the National Research Council’s 2011 Guidelines for the Care and Use of Laboratory Animals. A certified and licensed HHRI veterinarian assured study protocol was in compliance with these guidelines.
Study Preparation:
Study preparation and surgical techniques were performed as previously described.13,14,16 Female Yorkshire farm pigs weighing approximately 40 kg were acclimatized and fasted overnight prior to study. Pigs were sedated with intramuscular ketamine in the holding pen, transferred to the surgical suite and anesthetized with inhaled isoflurane, intubated with a 7.5 mm cuffed endotracheal tube (ETT), and mechanically ventilated with a tidal volume of 10 ml/kg (Narkomed, North American Dräger). The FiO2 and respiratory rate were adjusted to maintain O2 saturation above 92% and ETCO2 between 37 and 43 mmHg, which was constantly monitored (CO2SMO Plus® Machine, Novametrix Systems). Animals received an approximate 1000 ml normal saline bolus to achieve and maintain a right atrial (RA) pressure between 7 and 10 mmHg prior to induction of ventricular fibrillation (VF). All animals were treated with a bolus of 5,000 units of heparin followed by additional boluses hourly. Femoral aortic and venous access were obtained, and pressures were measured using micromanometer-tipped catheters (Mikro-Tip Transducer, Millar Instruments) at the level of the descending thoracic aorta. Catheter position was confirmed by fluoroscopy prior to the induction of VF. Temperature was monitored using an esophageal probe and maintained between 36.5°C and 38.5°C (TSD202F, BioPac Systems, Inc). Proximal airway pressure, a surrogate for intrathoracic pressure,18,19 was measured using a differential pressure transducer (TSD160C, BioPac Systems, Inc) located on the ETT. Regional cerebral tissue perfusion (rSO2) was measured using near-infrared spectroscopy (INVOS 5100C Cerebral/Somanetics Oximeter, Medtronic).
Data were continuously recorded throughout the study, including aortic pressure, RA pressure, electrocardiography, rSO2, and ETCO2 (BioPac; BioPac Systems Inc). Serial arterial blood gases (ABGs) were collected through the femoral artery catheter and analyzed using Gem Premier 3000 blood gas analyzer (Instrumentation Laboratory).
Following the preparatory phase, isoflurane was discontinued for 3 minutes. VF was then induced with electrical stimulation of the right ventricle using a pacing wire placed under fluoroscopy. After VF induction, animals were then randomized to ACD+ITD CSE CPR or C-CPR. was performed with an automatic piston device and suction cup on the chest (Caztek Engineering), at a rate of 100 compressions/minute, with a 50% duty cycle, depth of 22.5% antero-posterior chest diameter, and decompression force of 10 kg. C-CPR was performed with the same automatic piston device but with a washcloth underneath the suction cup to allow for full passive chest wall recoil. When used, the ITD (ResQPOD-16, ZOLL Medical) was placed at the end of the ETT. During CPR, manual positive pressure ventilation with a tidal volume of 10 mL/kg and oxygen was used as needed to maintain SpO2 ≥ 92%. If gasping occurred during CPR, a 100 mg bolus of intravenous succinylcholine (~2.5 mg/kg) was given.
If the animal was randomized to ACD+ITD CSE, the position of the body could be changed using an electric motor-powered customized elevation device (CED) for the animal. The CED can adjust for multiple torso and head elevation levels and is electro-mechanically coupled to the CPR machine to perform continuous chest compressions at a 90° to the sternum (Figure 1). Regardless of elevation of the upper thorax and head and neck, the lower thorax, abdomen and lower extremities rested flat without any elevation. During the preparation phase and the majority of VF, the animals were flat and supine. For animals randomized to ACD+ITD CSE, the CED was adjusted ~10 seconds prior to the start of CPR so that the thorax was elevated 8 cm and the occiput of the head was raised 10 cm. This 10 second period of time was intended to simulate placement of a patient in arrest on a head positioning device. ACD+ITD CPR was then performed at this low level of elevation for 2 minutes to prime the cardio-cerebral circuit prior initiation of higher elevation. In the highest CED position the thorax and occiput were elevated 9 and 22 cm, respectively.
Figure 1:
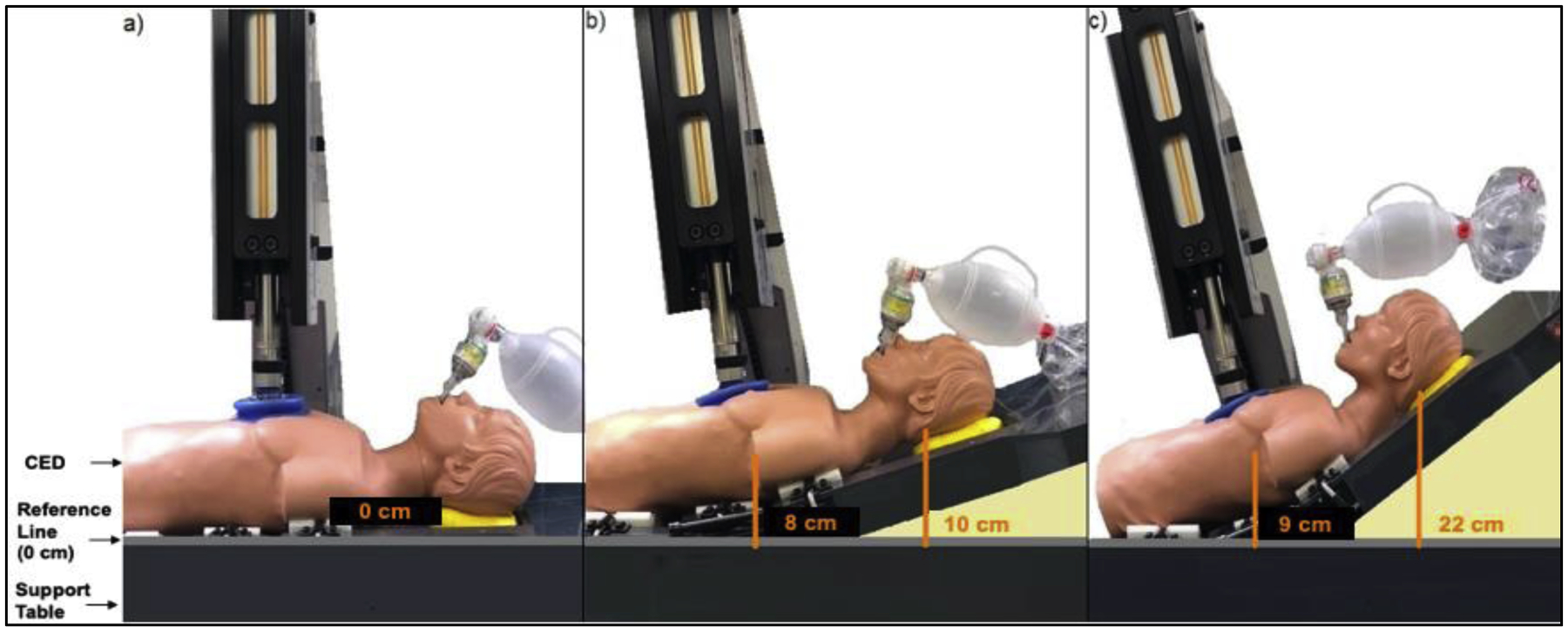
Diagram of the customized elevation device in the flat, lowest elevation and highest elevation
Experimental protocol
C-CPR Protocol
The experimental protocol is outlined in Figure 2. After 10 minutes of untreated VF, C-CPR was performed with a 30:2 compression: ventilation ratio for the first two minutes of the resuscitation, with manual positive pressure ventilation on room air. This was performed to simulate basic life support (BLS) guidelines.20 After 2 minutes of BLS C-CPR, CPR was performed continuously with a 10:1 compression: ventilation ratio. Animals were then given oxygen as needed to maintain SpO2 ≥ 92%. C-CPR was continued for a total of 19 minutes. ABGs were performed after 10 and 18 minutes of CPR. Intravenous amiodarone (50 mg) was given after 18 minutes of CPR. If the diastolic aortic pressure was ≤30 mmHg an intravenous bolus of epinephrine (0.50 mg) was administered. One minute later the animal was treated with up to three 200 J biphasic shocks (X-series Defibrillator, ZOLL Medical) to obtain return of spontaneous circulation (ROSC). If ROSC was unable to be obtained, up to 2 more cycles of medication and defibrillation attempts were performed.
Figure 2:
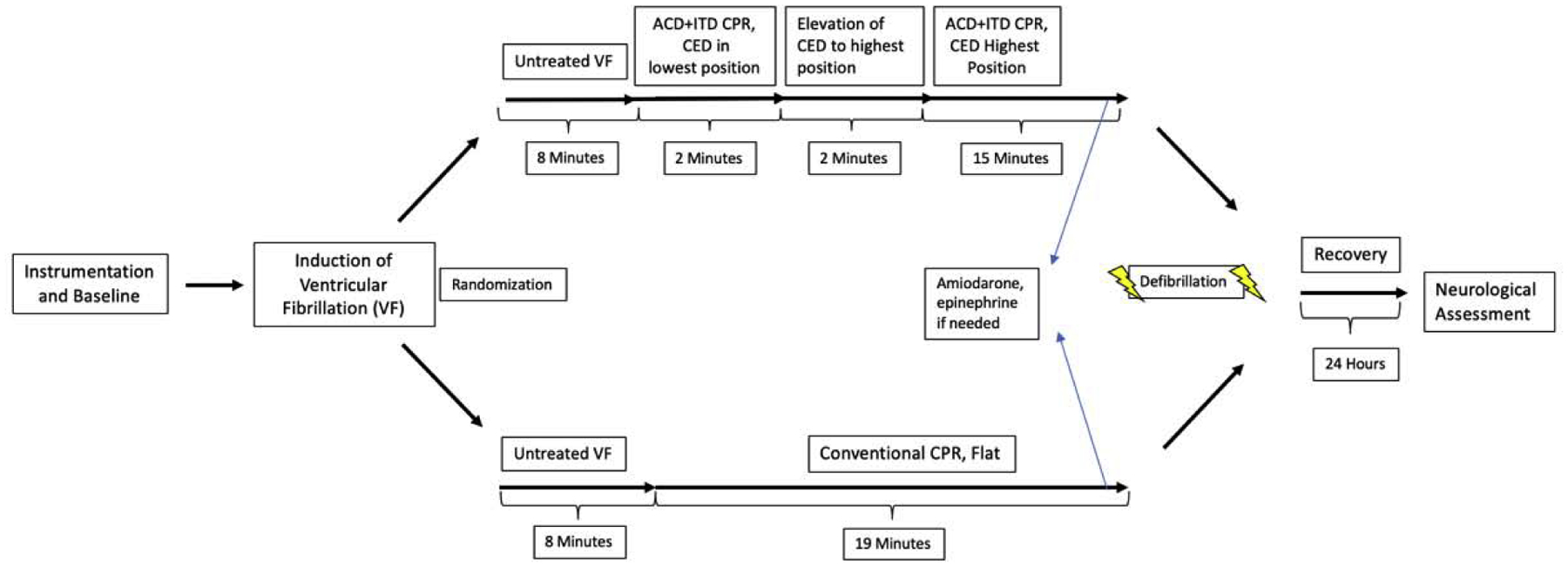
Representative graphic depicting the study protocol.
ACD+ITD CSE Protocol
After 10 minutes of untreated VF, ACD+ITD CPR was performed with a 30:2 compression: ventilation ratio for the first 2 minutes, with manual positive pressure ventilation on room air. After that, ACD+ITD CPR was performed continuously with a 10:1 compression: ventilation ratio. Animals were given oxygen as needed to maintain SpO2 ≥ 92%. Approximately ~10 seconds prior to CPR start, the CED was set to a low level of elevation as described above. After 2 minutes of CPR at minimal elevation using a 30:2 compression: ventilation ratio as described above, the head and thorax were elevated gradually over two minutes to a final elevation of 22 cm for the head and 9 cm for the heart. ACD+ITD CSE CPR was then continued for a total of 19 minutes of CPR and ABGs were collected as in the C-CPR protocol. After 19 minutes, medications and defibrillation attempts were performed as in the C-CPR protocol.
Post-ROSC protocol
Following ROSC all animals were maintained under anesthesia for 4 hours and then weaned off the anesthesia and ventilator. If the mean arterial pressure (MAP) decreased below 65 mmHg, epinephrine was administered as an infusion at 4 μg/mL to maintain MAP above 65 mmHg. Once stable, pigs were returned to the housing pen and observed. All animals were administered buprenorphine or transdermal fentanyl for pain control post-ROSC. Twenty-four hours after ROSC, a veterinarian of HHRI, but not a member of the study team and blinded to the randomization of the animal, performed a thorough neurological assessment. She assigned a Cerebral Performance Category (CPC) score modified for pigs,21 where a score of 1 = normal, 2 = slightly disabled, 3 = severely disabled but conscious, 4 = vegetative state, and 5 was assigned to animals that did not achieve ROSC or died prior to 24 hour assessment. A Neurological Deficit Score (NDS) for the animal was also calculated, which is a score that tests several reflexes and is scored between 0–260, with 0 being no deficit.22
Data analysis:
Hemodynamic data were analyzed during baseline and at each minute of CPR. Measurements of intrathoracic pressure, aortic pressure, RA pressure, and ETCO2 were made from five sequential compression and decompression cycles between breaths. rSO2 was measured each minute of CPR. These measurements were averaged to represent one value for each time point. The coronary perfusion pressure (CorPP) was calculated as the difference between the decompression phase aortic and RA pressures.
The primary endpoint of the study was 24-hour survival with good neurological function. Good neurological outcome was defined as a cerebral performance category (CPC) score of 1 or 2 based on neurological assessment. Assuming a α value of 0.05, 80% power, survival rate of 10% in the C-CPR group and 75% in the ACD+ITD group, we calculated a total sample size of 16 animals.
All data are presented as mean ± standard deviation. Fisher’s exact test was used to compare the animals that survived with a good neurological outcome and also for the endpoint of ROSC and animals that required epinephrine for ROSC. Kaplan-Meier survival curves were compared between ACD+ITD CSE and C-CPR curves with the log-rank test. Hemodynamic data and rSO2 values were compared at 18 minutes of CPR by using an unpaired two-tailed student’s t-test. Analyses were performed using STATA 15 (StataCorp).
Results:
A total of 16 pigs were randomized to C-CPR group (n = 8) or ACD+ITD CSE group (n = 8). Baseline hemodynamic, ABG, and rSO2 values are shown in Tables 1 and 2. There were no differences in baseline values between groups.
Table 1:
Hemodynamic and resuscitation parameter values recorded at baseline (prior to induction of ventricular fibrillation) and at multiple time points during ACD+ITD CSE CPR and C-CPR.
| N = 16 | Baseline | 5’ CPR | 10’ CPR | 15’ CPR | 18’ CPR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACD+ITD CSE | C-CPR | ACD+ITD CSE | C-CPR | ACD+ITD CSE | C-CPR | ACD+ITD CSE | C-CPR | ACD+ITD CSE | C-CPR | |
| Minimum Airway Pressure (mmHg) | 2 ± 0.5 | 2 ± 0.6 | −4 ± 2 | −1.0 ± 0.3 | −5 ± 1.5 | −1.0 ± 0.4 | −5 ± 2 | −1 ± 0.4 | −5 ± 1 | −1.0 ± 0.2 |
| Aortic Compression/Decompression (mmHg) | 86 ± 15/68 ± 13 | 93 ± 8/77 ± 7 | 75 ± 21/46 ± 21 | 46 ± 7/31 ± 5 | 76 ± 19/46 ± 20 | 41 ± 10/27 ± 6 | 75 ± 23/47 ± 23 | 33 ± 10/22 ± 6 | 75 ± 26/47 ± 25 | 31 ± 9/21 ± 6 |
| Right Atrial Compression/Decompression (mmHg) | 7 ± 2/8 ± 1 | 7 ± 2/8 ± 2 | 89 ± 26/7 ± 3 | 43 ±10/12 ± 2 | 83 ± 19/7 ± 4 | 37 ± 8/12 ± 2 | 81 ± 17/6 ± 4 | 46 ± 12/11 ± 2 | 80 ± 16/6 ± 4 | 30 ± 7/11 ± 2 |
| Coronary Perfusion Pressure (mmHg) | 60 ± 13 | 69 ± 6 | 39 ± 20 | 18.9± 4 | 40 ± 19 | 14 ± 5 | 46 ± 23 | 11 ± 5 | 41 ± 24 | 10 ± 5 |
| EtCO2 (mmHg) | 44 ± 2 | 44 ± 2 | 48 ± 6 | 26 ± 9 | 50 ± 7 | 24 ± 7 | 55 ± 7 | 20 ± 8 | 55 ± 9 | 19 ± 7.0 |
| Regional Brain Tissue Saturation (% Saturation) | 48 ± 9 | 50 ± 4 | 34 ± 6 | 24 ± 4. | 37 ± 6 | 23 ± 5 | 35 ± 7 | 17 ± 2 | 32 ± 9 | 17 ± 2 |
Table 2:
Arterial blood gas measurements taken at baseline, during CPR and after the return of spontaneous circulation (ROSC). Of note, only one C-CPR animal survived to ROSC 30 minutes after CPR.
| N = 16 | Baseline | CPR 9’ | CPR 18’ | ROSC 30’ | ROSC 60’ | ROSC 120’ | ROSC 240’ | |
|---|---|---|---|---|---|---|---|---|
| Arterial pH | ACD+ITD CSE | 7.46 ± 0.02 | 7.13 ± 0.05 | 7.10 ± 0.05 | 7.29 ± 0.03 | 7.33 ± 0.05 | 7.34 ± 0.03 | 7.36 ± 0.05 |
| C-CPR | 7.48 ± 0.02 | 7.31 ± 0.1 | 7.32 ± 0.13 | 7.22 ± 0.00 | 7.23 ± 0.00 | 7.32 ± 0.00 | 7.30 ± 0.00 | |
| Arterial pO2 | ACD+ITD CSE | 101± 12 | 116 ± 53 | 116 ± 48 | 138 ± 17 | 111 ± 18 | 105 ± 20 | 96 ± 15 |
| C-CPR | 98 ± 13.6 | 191 ± 80.9 | 246 ± 113 | 149 ± 0.0 | 137 ± 0.0 | 95 ± 0.0 | 77 ± 0.0 | |
| Arterial pCO2 | ACD+ITD CSE | 43 ± 3 | 67 ± 9 | 67 ± 10 | 42 ± 5 | 45 ± 4 | 41 ± 4 | 36 ± 5 |
| C-CPR | 44 ± 1 | 43 ± 11 | 34 ± 13 | 32.0 ± 0.0 | 44 ± 0.0 | 33 ± 0.0 | 41 ± 0.0 | |
| Arterial Base Excess | ACD+ITD CSE | 7.2 ± 1 | −6.8 ± 2 | −9.1 ± 2 | −6.4 ± 2 | −1.8 ± 4 | −3.5 ± 2 | −4.1 ± 4 |
| C-CPR | 9.4 ± 2 | −5.4 ± 2 | −9.5 ± 3 | −14.6 ± 0.0 | −9.2 ± 0.0 | −9.1 ± 0.0 | −6.2 ± 0.0 |
All pigs in the study intervention group (8/8) achieved ROSC versus 3/8 in the C-CPR group (p = 0.026). For the primary endpoint, a total of 6/8 pigs in the ACD+ITD CSE group survived to 24 hours with good neurological function compared with 1/8 in the C-CPR group (p = 0.04, Figure 3). For the neurological deficit score, the average score in the ACD+ITD CSE group was 15.8 ± 15 whereas the only pig that survived in the C-CPR group had a neurological deficit index score of 85. A total of 7 pigs died prior to 24 hours in the C-CPR group. Of these animals, 5 never had ROSC and 2 died within 1 hour after ROSC due to severe hypotension refractory to vasopressor therapy. Two of the ACD+ITD CSE animals did not survive to 24 hours, both of whom developed persistent seizures 8 hours after ROSC. Both of these animals had approximately 30 minutes of relative hypotension during the preparatory phase but were stable for >30 minutes prior to VF induction. No C-CPR animals were noted to have any difficulty in the preparatory phase. The Kaplan Meier survival curves were significantly different between groups (p = 0.0037, Figure 4).
Figure 3:
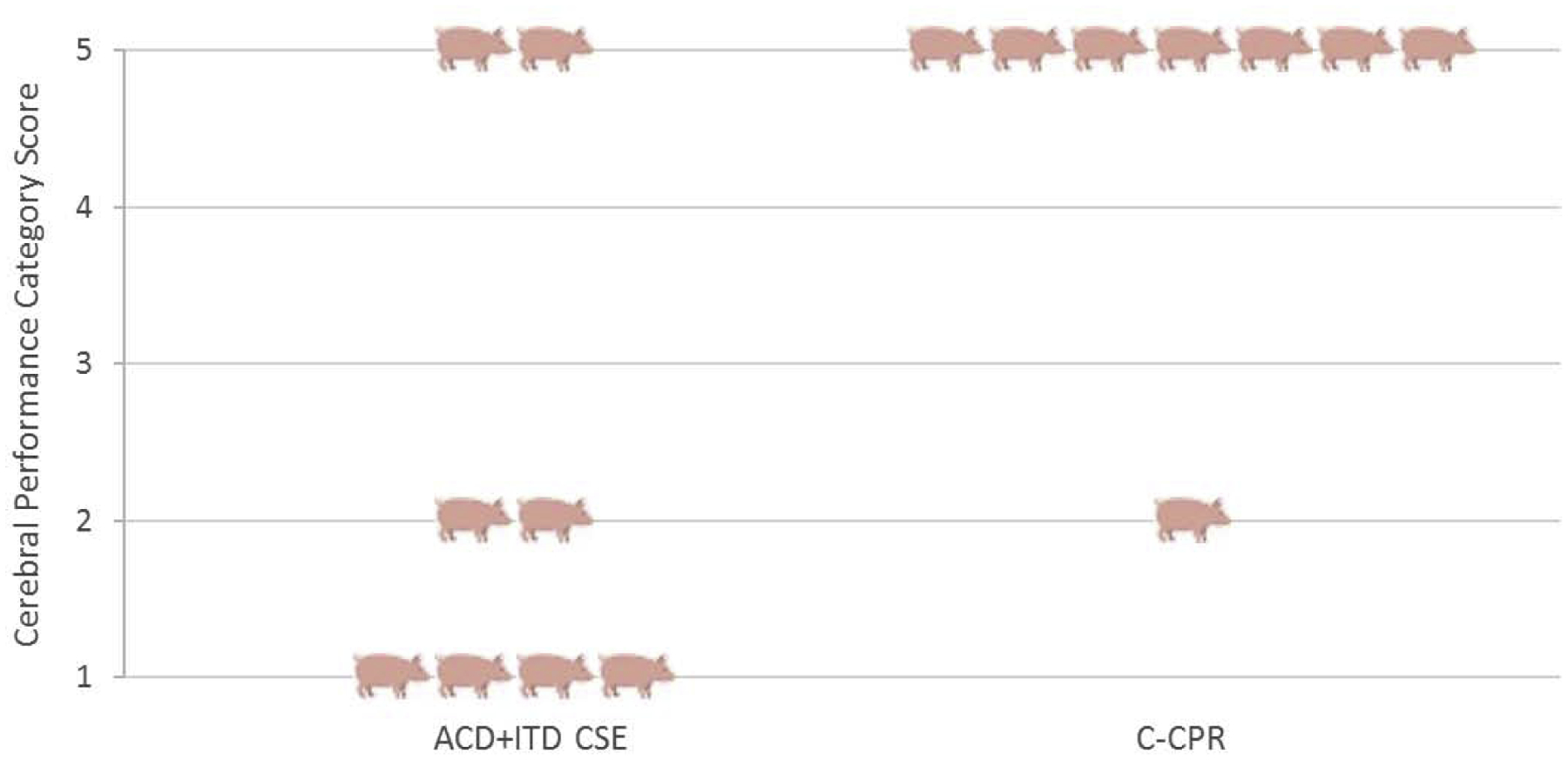
Representative histogram depicting the cerebral performance category (CPC) scores of each animal. CPC score is graded on a scale from 1 (No neurological impairment) to 5 (No ROSC/Dead before 24-hour assessment).
Figure 4:
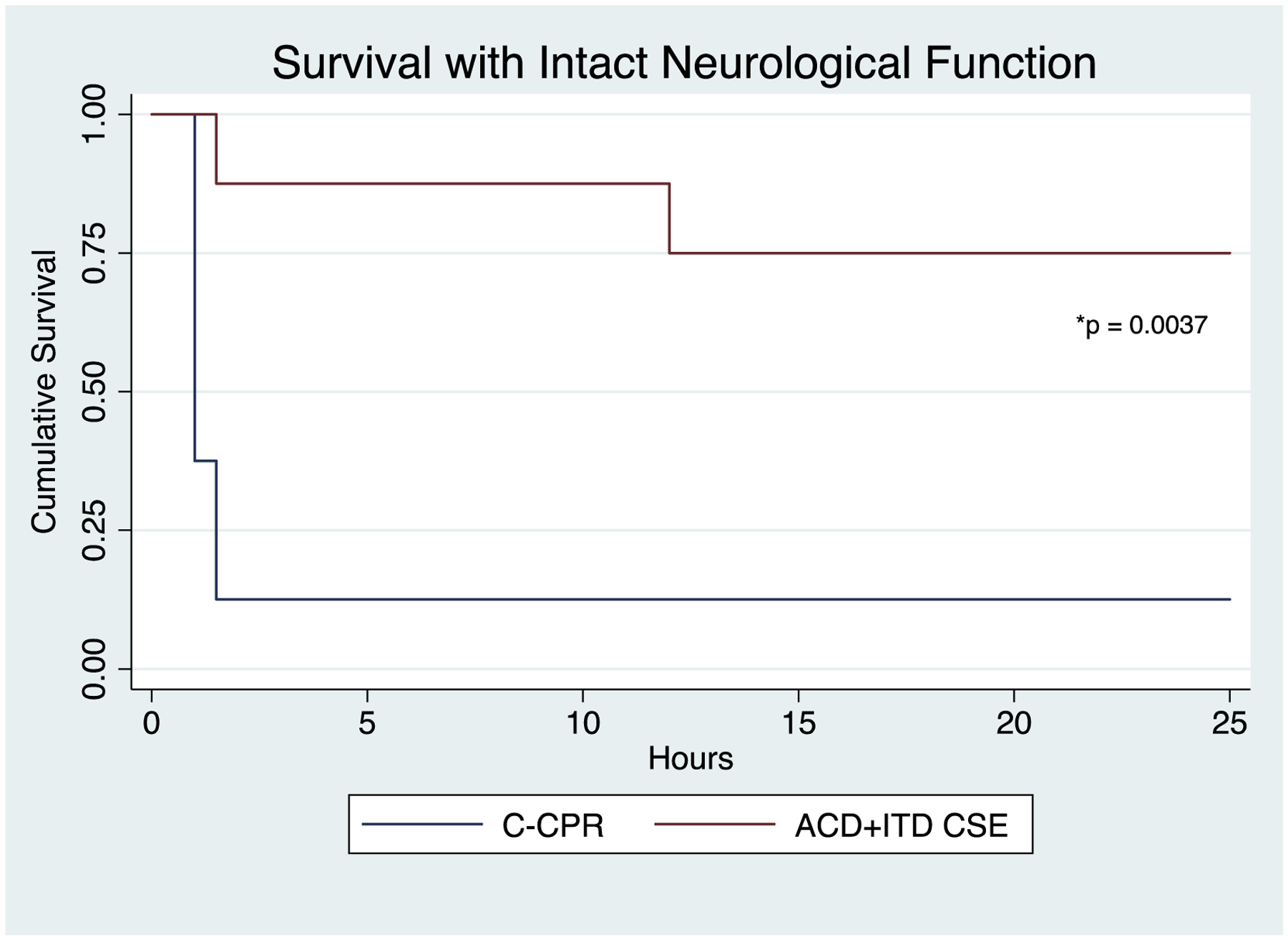
Kaplan Meier survival curves for two animal groups 1) Active compression-decompression (ACD) CPR with an impedance threshold device (ITD) and controlled sequential elevation (CSE) 2) Conventional (C) CPR in the flat position.
During CPR, there were a number of significant differences between the two study groups, as shown in Table 1 and Figure 5. CorPP at 18 minutes was 41 ± 24 for the CSE group versus 10 ± 5 for the C-CPR group (p = 0.004), prior to any epinephrine administration. CorPP decreased slowly over the entire 19-minute period of C-CPR whereas the opposite was observed in the ACD+ITD CSE group (Figure 5). A similar pattern was observed for ETCO2 and rSO2, (Figure 5). After 18 minutes of CPR and prior to any epinephrine administration, ETCO2 (55 mmHg ± 10 versus 21 mmHg ± 4, p < 0.001), rSO2 (32% ± 9 versus 17% ± 2, p = 0.01), and decompression phase aortic pressure (47 mmHg ± 24 versus 21 ± 5, p = 0.01) were higher in the ACD+ITD CSE group (Table 1). The MAP at 18 minutes was also higher in the ACD+ITD CSE group (61 mmHg ± 24 versus 26 mmHg ± 7, p = 0.003).
Figure 5:
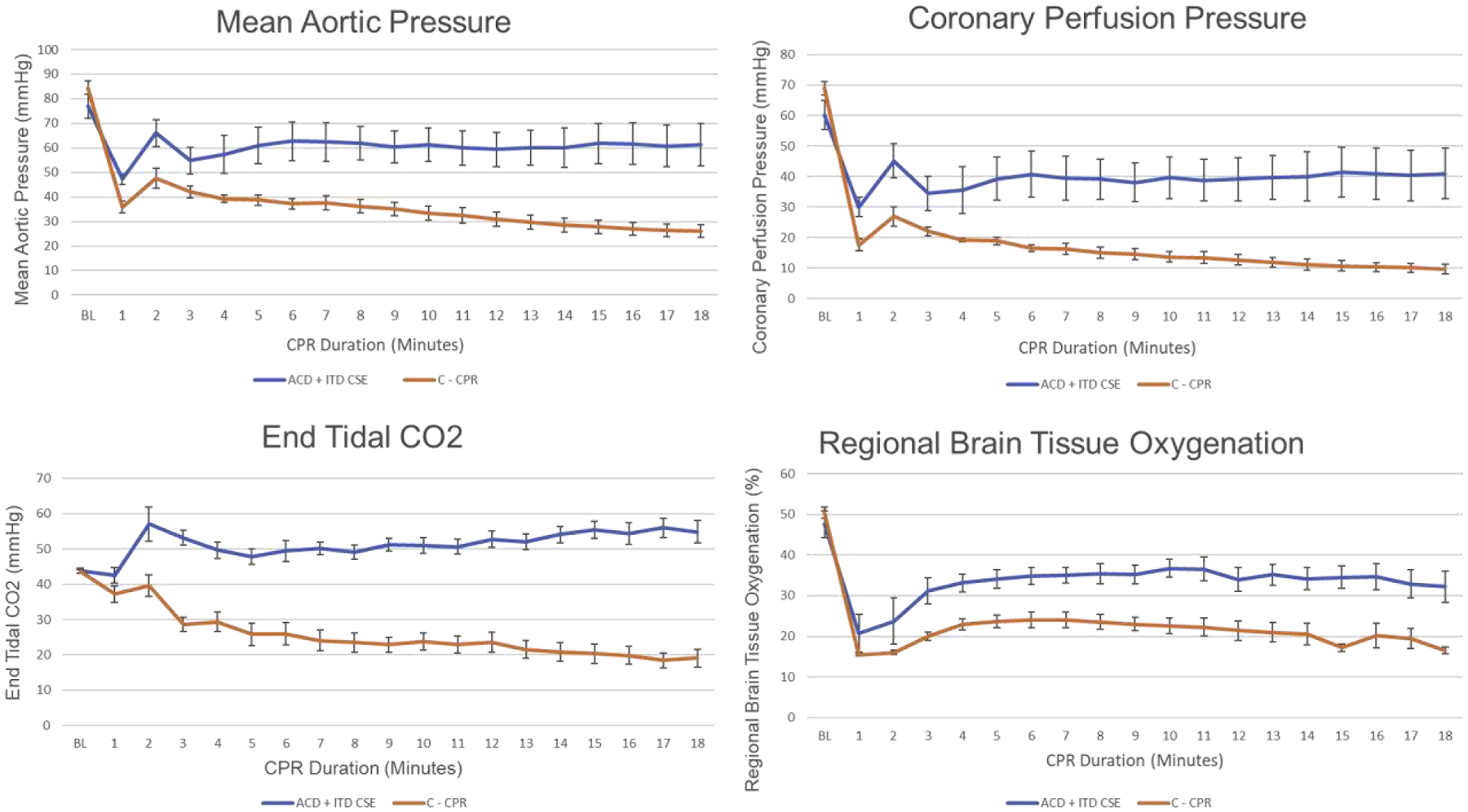
Hemodynamic and resuscitation parameter values recorded at baseline prior to induction of cardiac arrest, and during each minute of CPR, graphed over time.
A total of 8/8 C-CPR pigs and 2/8 pigs in the ACD+ITD CSE group required epinephrine after 18 minutes of CPR for a diastolic aortic blood pressure of <30 mmHg. In the C-CPR group, 1/8 pigs had a hemodynamic response to epinephrine, as indicated by an increase in systolic or diastolic arterial pressure of >5 mmHg within 60 seconds of drug administration. Both pigs in the intervention group responded with an increase in arterial pressure to epinephrine administration. Analysis of arterial blood gases obtained during CPR and after ROSC are shown in Table 2.
Discussion
Building upon past studies that have shown the hemodynamic and perfusion benefits of Head Up CPR, the current study determined if ACD+ITD CSE CPR would increase survival with intact neurological function versus C-CPR, the method of CPR that is most commonly used worldwide, and recommended by American Heart Association and International Liaison Committee on Resuscitation (ILCOR) Guidelines23,24. The results demonstrated that treatment with ACD+ITD CSE CPR resulted in significantly improved hemodynamics during resuscitation, and a significantly higher rate of ROSC and survival with favorable neurologic function versus C-CPR. This is the first demonstration of the potential neurological survival benefit associated with ACD+ITD CSE CPR.
This study also provides further insight into the importance of perfusion to the heart and brain during CPR in order to optimize post resuscitation brain function. Intracranial pressure was not measured directly out of concern that a pressure transducer in the brain had the potential to interfere with neurological recovery and cause pain to the animal upon awakening. However, rSO2 was significantly higher in ACD+ITD CSE animals. The intervention animals also had significantly higher ETCO2 values, diastolic aortic pressures, and CorPP, suggestive of improved circulation with ACD+ITD CSE. These findings are consistent with prior non-survival Head Up CPR studies showing increased blood flow to the brain,12,14 improved CorPP,12 and improved cerebral perfusion pressures12–14,25 as compared to the supine position.
Pigs were treated with a specific CSE technique carefully developed in previous studies using the same animal model. Initial Head Up CPR studies with ACD+ITD, and an initial 2 minute period of performing CPR with minimal elevation in order to “prime” the cardio-cerebral circuit, then a rapid elevation to the target elevation resulted in CerPP 40–80% of baseline pre-induction of VF values after nearly 20 minutes of CPR.13,14 This technique was refined with CSE over an additional 2 minute period after the 2 minute priming period, with resulting CerPP values >90% of baseline pre-induction of VF values after approximately 20 minutes of CPR.16
No safety concerns were identified with the use of ACD+ITD CSE. Both priming and ACD+ITD CPR method are both essential components of the therapies used in the intervention group. Previous Head Up CPR studies with C-CPR alone have shown very poor CerPP incompatible with life13 and cerebral metabolism,17 with CerPPs at 20 minutes of CPR < 10% of normal. C-CPR by itself is insufficient as it cannot provide enough forward blood flow “uphill” to the brain when the head and thorax are elevated.26 A recent study without any priming prior to rapid elevation of the whole body upwards also resulted in poor outcomes of the Head Up CPR animals.27
This study has limitations. Pigs were young, healthy and without known co-morbidities. Further, the model used VF, but many patients present with pulseless electrical activity or asystole. However, prior studies have demonstrated that interventions that have been shown to be of benefit in the porcine model of VF cardiac arrest translate well to treatment benefit in patients.9,10 The ROSC rate of the C-CPR animals was low, however these rates were consistent with previous animal studies.13 This C-CPR comparator group was specifically chosen as it is the current standard of care in the United States. The approach to resuscitation varies widely throughout the country in both the pre-hospital and hospital setting. Different comparator groups could be considered in further studies. The benefits of ACD+ITD CSE may have been underestimated as two of the pigs in the intervention arm experienced prolonged hypotensive episodes during the preparatory phase. These two animals did not survive to 24 hours. Finally, CSE was performed in combination with ACD+ITD, and the benefits observed cannot be attributed to any one element of this “triple therapy.” This was done intentionally, to compare a novel bundle of care that can be used clinically to the current standard.
Survival with intact neurological outcome after cardiac arrest is dependent on multiple factors. There is no one intervention that will uniformly lead to a good outcome. The chain of survival ranges from the rapid recognition of cardiac arrest and initiation of dispatch assisted CPR to more specialized therapies such as ECMO during cardiac arrest with subsequent cardiac catheterization. Optimization and refinement of all the elements involved in cardiac arrest care continues to be desperately needed. ACD+ITD CSE CPR has the potential to enhance the high-quality resuscitation component of the chain of survival, and can be applied rapidly and early to all cardiac arrest patients, regardless of age, comorbidities, or presenting rhythm.
Conclusion:
In a porcine model of cardiac arrest, there was improvement in overall survival and intact neurological survival with the use of a novel bundle of care including ACD+ITD and CSE of the head and thorax. There were no safety concerns with this device combination. These findings provide additional pre-clinical support for clinical assessment, which is already currently underway.
Supplementary Material
Acknowledgments
This study was funded by a NIH NHBLI SBIR grant, contract number 1R43HL139184-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Keith Lurie is an inventor of devices and methods to elevate the head and heart during CPR. He is the co-founder of Advanced CPR Solutions that makes resuscitation devices.
Contributor Information
Bayert Salverda, Hennepin Healthcare Research Institute, Minneapolis, MN, USA..
Carolina Rojas-Salvador, Department of Emergency Medicine, University of Minnesota, Minneapolis, MN, USA.
Michael Lick, Hennepin Healthcare Research Institute, Minneapolis, MN, USA..
Guillaume Debaty, University Grenoble Alps/CNRS/CHU de Grenoble Alpes/TIMC-IMAG UMR 5525, Grenoble, France.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Duggal C, Weil M, Gazmuri R, et al. Regional blood flow during closed chest cardiac resuscitation in rats. J Appl Physiol. 1973;74:147–152. [DOI] [PubMed] [Google Scholar]
- 3.Aufderheide TP, Lurie KG. Vital organ blood flow with the impedance threshold device. Crit Care Med. 2006;34(12 Suppl):S466–473. [DOI] [PubMed] [Google Scholar]
- 4.Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377(9762):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepe PE, Scheppke KA, Antevy PM, et al. Confirming the Clinical Safety and Feasibility of a Bundled Methodology to Improve Cardiopulmonary Resuscitation Involving a Head-Up/Torso-Up Chest Compression Technique. Crit Care Med 2019;47(3):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plaisance P, Lurie KG, Vicaut E, et al. Evaluation of an impedance threshold device in patients receiving active compression-decompression cardiopulmonary resuscitation for out of hospital cardiac arrest. Resuscitation. 2004;61(3):265–271. [DOI] [PubMed] [Google Scholar]
- 7.Wolcke BB, Mauer DK, Schoefmann MF, et al. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation. 2003;108(18):2201–2205. [DOI] [PubMed] [Google Scholar]
- 8.Plaisance P, Lurie KG, Payen D. Inspiratory impedance during active compression-decompression cardiopulmonary resuscitation: a randomized evaluation in patients in cardiac arrest. Circulation. 2000;101(9):989–994. [DOI] [PubMed] [Google Scholar]
- 9.Voelckel WG, Lurie KG, Sweeney M, et al. Effects of active compression-decompression cardiopulmonary resuscitation with the inspiratory threshold valve in a young porcine model of cardiac arrest. Pediatr Res. 2002;51(4):523–527. [DOI] [PubMed] [Google Scholar]
- 10.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91(6):1629–1632. [DOI] [PubMed] [Google Scholar]
- 11.Guerci AD, Shi AY, Levin H, Tsitlik J, Weisfeldt ML, Chandra N. Transmission of intrathoracic pressure to the intracranial space during cardiopulmonary resuscitation in dogs. Circ Res. 1985;56(1):20–30. [DOI] [PubMed] [Google Scholar]
- 12.Debaty G, Shin SD, Metzger A, et al. Tilting for perfusion: head-up position during cardiopulmonary resuscitation improves brain flow in a porcine model of cardiac arrest. Resuscitation. 2015;87:38–43. [DOI] [PubMed] [Google Scholar]
- 13.Ryu HH, Moore JC, Yannopoulos D, et al. The Effect of Head Up Cardiopulmonary Resuscitation on Cerebral and Systemic Hemodynamics. Resuscitation. 2016;102:29–34. [DOI] [PubMed] [Google Scholar]
- 14.Moore JC, Segal N, Lick MC, et al. Head and thorax elevation during active compression decompression cardiopulmonary resuscitation with an impedance threshold device improves cerebral perfusion in a swine model of prolonged cardiac arrest. Resuscitation. 2017. [DOI] [PubMed] [Google Scholar]
- 15.Kim T, Shin SD, Song KJ, et al. The effect of resuscitation position on cerebral and coronary perfusion pressure during mechanical cardiopulmonary resuscitation in porcine cardiac arrest model. Resuscitation. 2017;113:101–107. [DOI] [PubMed] [Google Scholar]
- 16.Rojas-Salvador C, Moore JC, Salverda B, Lick M, Debaty G, Lurie KG. Effect of Controlled Sequential Elevation Timing of the Head and Thorax during Cardiopulmonary Resuscitation on Cerebral Perfusion Pressures in a Porcine Model of Cardiac Arrest. Resuscitation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putzer G, Braun P, Martini J, et al. Effects of head-up vs. supine CPR on cerebral oxygenation and cerebral metabolism - a prospective, randomized porcine study. Resuscitation. 2018;128:51–55. [DOI] [PubMed] [Google Scholar]
- 18.Kwon Y, Debaty G, Puertas L, et al. Effect of regulating airway pressure on intrathoracic pressure and vital organ perfusion pressure during cardiopulmonary resuscitation: a non-randomized interventional cross-over study. Scand J Trauma Resusc Emerg Med. 2015;23(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shultz JJ, Coffeen P, Sweeney M, et al. Evaluation of standard and active compression-decompression CPR in an acute human model of ventricular fibrillation. Circulation. 1994;89(2):684–693. [DOI] [PubMed] [Google Scholar]
- 20.Kleinman ME, Brennan EE, Goldberger ZD, et al. Part 5: Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S414–435. [DOI] [PubMed] [Google Scholar]
- 21.Yannopoulos D, Matsuura T, Schultz J, Rudser K, Halperin HR, Lurie KG. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011;39(6):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sipos W, Holzer M, Bayegan K, et al. A novel highly observer-independent neurologic examination procedure for pigs in a model for cardiac arrest resuscitation. Vet Med Austria/Wien Tierarztl Monatsschr. 2008;95:28–38. [Google Scholar]
- 23.Perkins GD, Handley AJ, Koster RW, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation. 2015;95:81–99. [DOI] [PubMed] [Google Scholar]
- 24.Travers AH, Perkins GD, Berg RA, et al. Part 3: Adult Basic Life Support and Automated External Defibrillation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(16 Suppl 1):S51–83. [DOI] [PubMed] [Google Scholar]
- 25.Ageron FX, Debaty G. Survival is surfing on the guidelines wave. Resuscitation. 2016;98:e2–3. [DOI] [PubMed] [Google Scholar]
- 26.Moore JC, Segal N, Debaty G, Lurie KG. The “do’s and don’ts” of head up CPR: Lessons learned from the animal laboratory. Resuscitation. 2018;129:e6–e7. [DOI] [PubMed] [Google Scholar]
- 27.Park YJ, Hong KJ, Shin SD, et al. Worsened survival in the head-up tilt position cardiopulmonary resuscitation in a porcine cardiac arrest model. Clin Exp Emerg Med. 2019;6(3):250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


