Abstract
The three‐dimensional ultrastructure of the tendon is complex. Two main cell types are classically supported: elongated tenocytes and ovoid tenoblasts. The existence of resident stem/progenitor cells in human and equine tendons has been demonstrated, but their location and relationship to tenoblasts and tenocytes remain unclear. Hence, in this work, we carried out an ultrastructural study of the equine superficial digital flexor tendon. Although the fine structure of tendons has been previously studied using electron microscopy, the presence of telocytes, a specific type of interstitial cell, has not been described thus far. We show the presence of telocytes in the equine inter‐fascicular tendon matrix near blood vessels. These telocytes have characteristic telopodes, which are composed of alternating dilated portions (podoms) and thin segments (podomers). Additionally, we demonstrate the presence of the primary cilium in telocytes and its ability to release exosomes. The location of telocytes is similar to that of tendon stem cells. The telocyte–blood vessel proximity, the presence of primary immotile cilia and the release of exosomes could have special significance for tendon homeostasis.
Keywords: primary cilium, telocytes, tendon, tenocytes, transmission electron microscopy
This study describes the characteristics of telocytes, located near blood vessels, in the equine tendon. Telocytes may represent an early step in the tendon‐derived stem cell differentiation process towards mature tenocytes “in vivo” based on a previous hypothesis that highlights telocytes as potential mesenchymal progenitor cells. Of particular interest is the presence of the primary cilium as a signalling antenna involved in tenocyte differentiation.
1. INTRODUCTION
Tendons are anatomic structures interposed between muscles or muscles, and bones transmit the force created in the muscle to bone, making joint movement possible (Kannus, 2000; Rowson et al., 2016). Tendons are constantly subjected to large mechanical loads and, as a result, are prone to acute injuries (Khan & Maffuli, 1998). Tendinopathies represent major medical problems associated with physical activity and age‐related degeneration (Benjamin & Ralphs, 1997). The pathogenic mechanisms of tendinopathy are unclear. In fact, the restoration of the normal structure and function of injured tendons represents one of the most challenging areas in orthopaedic medicine (Zhang & Wang, 2010). Unfortunately, due to hypocellularity and hypovascularity, the natural healing ability of tendons is extremely low and inefficient (Benjamin & Ralphs, 1997).
Tendon research lags far behind research into other connective tissues, such as bone, muscle and cartilage. This is partly due to the lack of characterization of tendon cells (Chuen et al., 2004). Tendons consist of collagen (mostly type I collagen) and elastin embedded in a proteoglycan–water matrix. The three‐dimensional ultrastructure of tendon fibres and fibre bundles is complex. Within one collagen fibre, the fibrils are oriented not only longitudinally but also transversely and horizontally (Kannus, 2000). Emerging evidence shows that a fraction of the total amount of collagen is synthesized and removed on a daily basis without being incorporated into the permanent collagen as part of a circadian clock mechanism of protein homeostasis (Chang et al., 2020; Yeung & Kadler, 2019). This matrix is produced by tenoblasts and tenocytes that lie between the collagen fibres. Tenoblasts and tenocytes comprise approximately 90%–95% of the cellular elements of the tendon. The other 5%–10% includes chondrocytes localized at pressure and insertion sites, synovial cells of the tendon sheath and vascular cells, such as endothelial cells and smooth muscle cells of the arterioles (Jozsa & Kannus, 1997; Kannus, 2000).
Tenocytes are spindle‐ or stellate‐shaped cells with elongated nuclei and thin cytoplasm that sit on top of collagen fibres in tendons. Tenoblasts are relatively round cells with large ovoid nuclei. It has been suggested that tenoblasts are predominant in young tendons and that they transform into tenocytes during maturation and ageing (Chuen et al., 2004; Jozsa & Kannus, 1997). In 2007, (Bi et al. (2007) identified a cell population of resident tendon stem/progenitor cells (TSPCs). TSPCs exhibit classical adult mesenchymal stem cell characteristics, such as the presence of specific surface antigens, self‐renewal, clonogenicity and three‐lineage differentiation (adipogenic, osteogenic and chondrogenic) (Docheva et al., 2015). Zhang and Wang found that tendon stem cells in rabbits express the stem cell markers Oct‐4, SSEA‐4 and nucleostemin; these TSPCs possess smaller cell bodies and larger nuclei than ordinary tenocytes (Zhang & Wang, 2010). Despite the identification and isolation of stem cells from tendon tissues, the in vivo identity and roles of these cells in tendon homeostasis remain unclear (Tan et al., 2013).
Although the fine structure of tendons has been previously studied using electron microscopy (Jozsa et al., 1991), the presence of telocytes (TCs) has not been described thus far. Formerly called interstitial Cajal‐like cells, TCs are a peculiar interstitial cell type. Cajal discovered these interstitial cells in different types of viscera (intestine, pancreas and heart) and predicted their existence in other locations, considering them to be ‘primitive’ neurons in relation to the enteric nervous system (Ramón y Cajal, 1893). Popescu suggested naming these cells telocytes based on their long processes called telopodes (from Greek ‘telos’ = distance) (Popescu & Faussone‐Pellegrini, 2010). These cells have a small fusiform body that is mainly occupied by the nucleus, which is surrounded by scant cytoplasm. The perinuclear cytoplasm is rich in mitochondria and contains a small Golgi apparatus as well as rough and smooth endoplasmic reticulum and cytoskeletal elements (thin and intermediate filaments). The cell periphery is comprised of the typical plasmalemma, with no (or thin and discontinuous) basal lamina and some caveolae. As their main characteristic, telocytes have two or three thin and long processes known as telopodes that show a dichotomic branching pattern. Telopodes are composed of alternating dilated portions named podoms (250–300 nm), which contain mitochondria and endoplasmic reticulum, and thin segments called podomers (~80 nm) (Aleksandrovych et al., 2017; Cretoiu & Popescu, 2014). Telocytes are different from fibroblasts, mesenchymal stem cells and endothelial cells (Cretoiu & Cretoiu, 2016; Popescu et al., 2005).
Interestingly, telocytes have been found in a large variety of tissues and organs in vertebrates, such as the epicardium (Popescu et al., 2010), myocardium (Kostin, 2010; Suciu et al., 2010; Zhou et al., 2010), endocardium (Gherghiceanu et al., 2010), eye (Luesma et al., 2013), duodenal lamina propria (Cantarero Carmona et al., 2011a), pleura (Hinescu et al., 2011) and prostate (Corradi et al., 2013), in relation to blood vessels (Cantarero et al., 2011b; Li et al., 2014). A more detailed list of locations can be found in Aleksandrovych et al. (2017).
Transmission electron microscopy (TEM) seems to be the optimal method to identify TCs, since it has been widely demonstrated that immunohistochemistry alone is not sufficient to characterize TCs (Cantarero et al., 2016).
We collected healthy superficial digital flexor tendon samples for ultrastructural examination. In this paper, we show the presence of telocytes located on the equine endotendon or inter‐fascicular tendon matrix near blood vessels by TEM. Moreover, we demonstrated the presence of a primary cilium in telocytes. Primary cilia project from the surface of most vertebrate cell types, where they detect and transmit extracellular cues to regulate diverse cellular processes during development and tissue homeostasis (Anvarian et al., 2019).
Several functions have been proposed for TCs. Here, it is shown that telocyte–blood vessel proximity and the presence of the primary cilium could have special significance for tendon homeostasis.
2. MATERIALS AND METHODS
The project was approved by the Ethical Committee for Animal Experiments at the University of Zaragoza (project license PI36/07; date of approval February 15, 2008). The care and use of animals were performed in accordance with the Spanish Policy RD53/2013, which meets the guidelines of European Union Directive 2010/63 on the protection of animals used for scientific purposes.
2.1. Sample collection and preparation
Superficial digital flexor tendon samples were obtained from two cross‐breed geldings (aged 8 years) among the 12 horses (H1–H12) used in a previous study (Romero et al., 2017), which were determined to be healthy and free of tendon injury, as shown by their antecedents, clinical assessment and ultrasonographic exam. These horses were used in a previous investigation in which a comparison of the use of autologous bone marrow, and adipose tissue‐derived mesenchymal stem cells and platelet‐rich plasma for treating surgically induced lesions in the equine superficial digital flexor tendon was performed. At week 45, the horses were sedated with 0.04 mg/kg IV romifidine (Sedivet, Boehringer‐Ingelheim) and 0.02 mg/kg IV butorphanol (Torbugesic, Pfizer) and euthanized with 200 mg/kg sodium pentobarbital (Euthasol, Esteve) via IV. Samples from the zones used as off‐lesion controls in one tendon of each horse were collected. Therefore, the equine superficial digital flexor tendon samples used in this study were injury‐free and used as controls.
2.2. Transmission electron microscopy
For the TEM studies, the sample was fixed in 2.5% glutaraldehyde in phosphate buffer overnight at room temperature, washed in 0.1 M phosphate buffer for 5 minutes, post‐fixed with 2% osmium, rinsed, dehydrated in graded acetone (30%, 50%, 70% with 2% uranyl acetate, 90% and 100%), cleared in propylene oxide and embedded in araldite (Durcupan, Fluka AG, Buchs SG, 82). Semithin sections (1 μm) were cut with a glass knife, stained lightly with 1% toluidine blue and examined by light microscopy (Olympus BX51 microscope, Olympus Imaging Corporation). Later, ultrathin (0.05 μm) sections were cut with a diamond knife, collected on Formvar‐coated single‐slot grids, counterstained with 1% uranyl acetate and Reynold's lead citrate for 10 minutes and examined under an FEI Tecnai G2 SpiritTEM. The images were captured with using a charge‐coupled device (CCD) imaging system (Advanced Microscopy Techniques).
3. RESULTS
The elongated tenocytes were immersed in a wide area of extracellular matrix (ECM) rich in collagen fibrils and were separated at regular intervals of 10–15 microns. They showed polygonal and stellate shapes (Figure 1a). The tenocytes had a prominent nucleus and long slender cytoplasmic processes that extended across large distances to make contact with other tenocytes, allowing intercellular communication, although several contacts were difficult to visualize in the same plane (Figure 1b).
FIGURE 1.
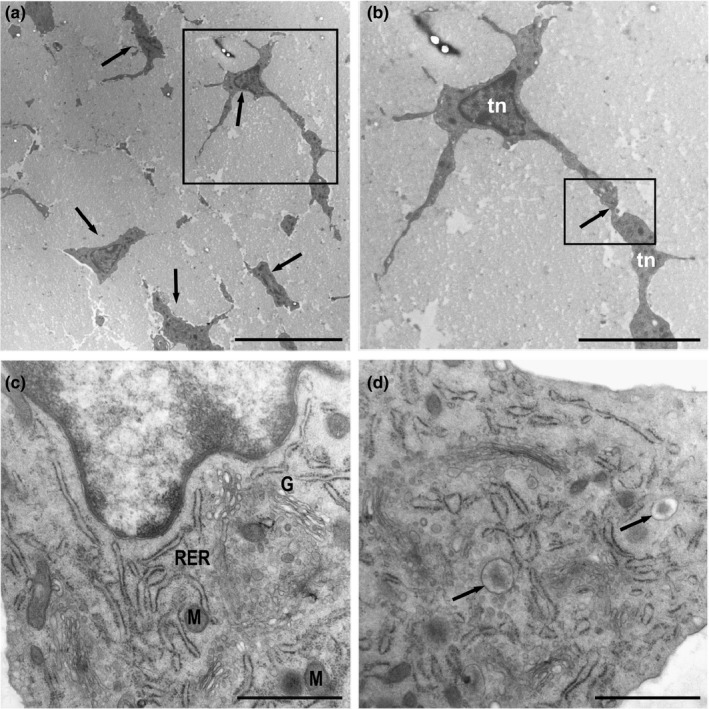
Ultrathin sections of the equine digital flexor tendon. (a) Tendon areas showing polygonal and stellate tenocytes (arrows) immersed in a wide area of extracellular matrix. (b) Detail of A‐Tenocytes (Tn) with long slender cytoplasmic processes that contact neighbouring tenocytes (inset). (c) Tenocytes with rough endoplasmic reticulum (RER), a well‐developed Golgi apparatus (G) and few mitochondria (M). The nucleus contains a narrow layer of marginal chromatin. (d) A large number of vesicles released from the Golgi apparatus showing an electron‐dense central core containing matrix proteins were observed (arrows). Scale bars: (a) 10 µm; (b) 5 µm; (c) 1 µm; (d) 1 µm
These prolongations surrounded the bundles of collagen fibrils, which were organized in a parallel arrangement. The disposition and quantity of cellular organelles were in accordance with the abundant synthesis of extracellular matrix proteins by these cells, so they had a highly developed granular endoplasmic reticulum, abundant dictyosomes in the Golgi apparatus and a small number of mitochondria, which are necessary to synthesize the rich surrounding extracellular matrix (Figure 1c). A large number of vesicles with different contents were released from the dictyosomes of the Golgi apparatus. Some of these vesicles seemed to contain extracellular matrix proteins that condensed into an electron‐dense central core (Figure 1d).
Tenoblasts were also observed. They presented different ultrastructural features from those of mature tenocytes. These cells were wider than tenocytes (10 microns) with a voluminous nucleus and prominent nucleolus (Figure 2a). The cytoplasmic prolongations were wider and shorter and established contact with other tenoblasts (Figure 2b). The tenoblast membranes made punctate contacts with electron‐dense reinforcements as well as numerous pinocytic vesicles (Figure 2c) and coated pits, which were indicative of endocytic processes mediated by clathrin (Figure 2d). The rough endoplasmic reticulum (RER) was, in appearance, less well developed than that in tenocytes, while more mitochondria were observed. Multivesicular bodies full of exosomes also appeared in tenoblasts (Figure 2d).
FIGURE 2.
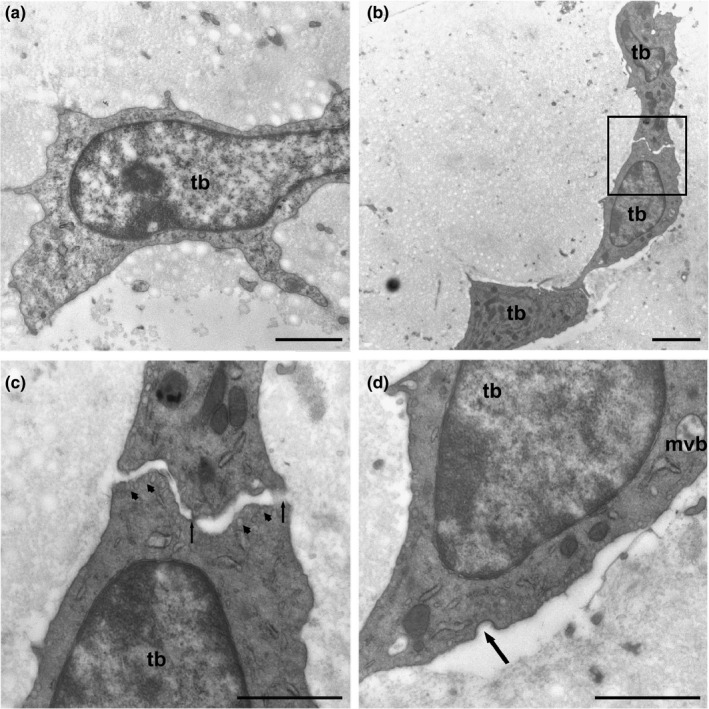
(a) Tenoblast (tb) showing a voluminous nucleus and prominent nucleolus. (b) The cytoplasmic prolongations were wider and shorter than those in tenocytes and established contacts with neighbouring tenoblasts (inset). (c) Detail of B‐Tenoblast membranes made punctate contacts with electron‐dense reinforcements (arrows). Numerous pinocytic vesicles appear below the plasma membrane (arrowheads). (d) Tenoblasts (tb) have small saccules of rough endoplasmic reticulum and multivesicular bodies (mvb) containing exosomes. Numerous pinocytic vesicles and coated pits are indicative of endocytic processes mediated by clathrin (arrow). Scale bars: (a) 1 µm; (b) 2 µm; (c) 1 µm; (d) 1 µm
Fusiform cells were found around tendon capillaries (Figure 3a). These cells showed voluminous nuclei and a sparse perinuclear cytoplasm (Figure 3b); however, the cells were extended with very long prolongations. These prolongations, which are named telopodes, comprised alternating dilated portions termed podoms, which contained mitochondria and small RER saccules, and small filiform diameter segments named podomers. The prolongations allowed contact with neighbouring telocytes through gap‐like junctions (Figure 3c) and peg‐and‐socket junctions, which involve a finger‐shaped evagination that extends from a cell to penetrate an invagination in the other cell (Figure 3d). Fine podomers were sometimes observed in relation to small vesicles (exosomes) (Figure 4).
FIGURE 3.
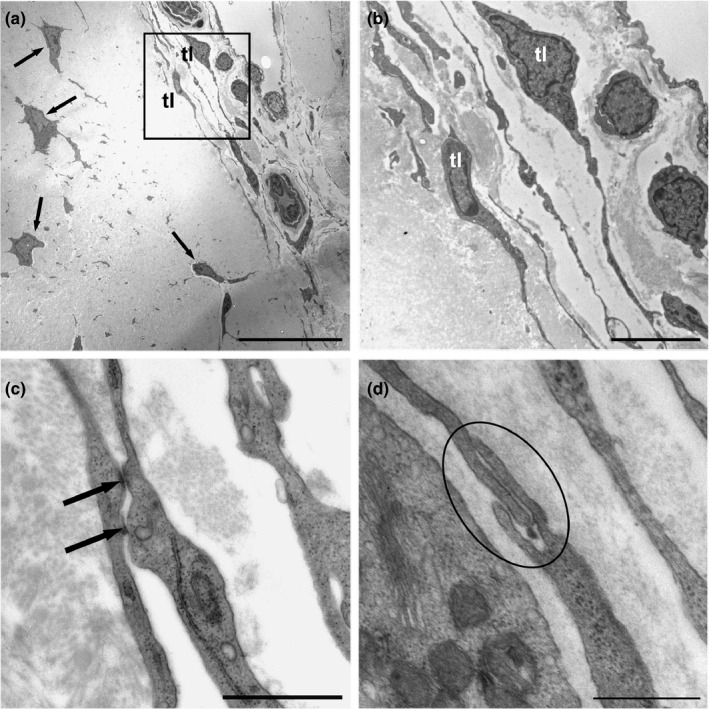
(a) Tenocytes (arrows) embedded in the extracellular matrix. Various telocytes (tl) can be observed around the tendon capillaries (inset). (b) Telocytes (tl) showed voluminous nuclei and scant perinuclear cytoplasm (c) and extended via very long extensions to make contact with neighbouring cells through gap‐like junctions (arrows), and (d) peg‐and‐socket contacts (encircled). Scale bars: (a) 20 µm; (b) 5 µm; (c) 1 µm; (d) 0.5 µm
FIGURE 4.
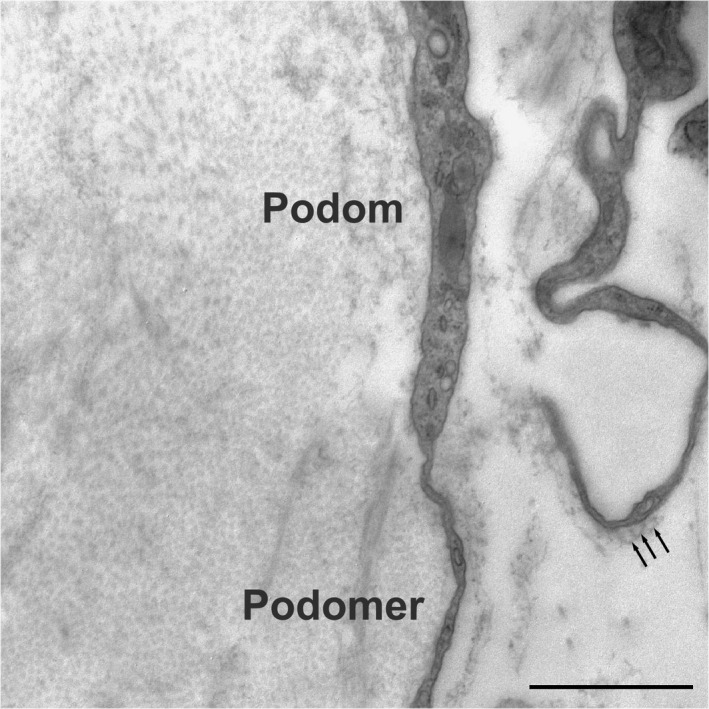
The prolongations of the telocytes, named telopodes, comprised dilated portions named podoms containing mitochondria, small saccules of RER, and filiform segments named podomers. The podomers were observed in relation to small vesicles (exosomes) (arrows). Scale bar: 1 µm
Telocytes were found to contain a primary cilium (this type of cilium is characterized by a 9 + 0 pattern and lacks motility‐related components such as inner and outer dynein arms and a radial spoke). The parental centriole of the diplosomal pair from which the cilium originates was clearly observed (Figure 5a). The primary cilium was projected outside of telocytes and directly protruded into the extracellular space. The axoneme was a direct extension of a typical basal body. The cilium remained partially intracellular within a membrane invagination in the ciliary pocket. Both the ciliary pocket and the axonema were surrounded by a membrane that was continuous with the plasma membrane of the cell. The transition zone between the basal body and the cilium contained a moderately opaque structure known as the terminal plate, which defines the boundary between the plasma membrane and the ciliary membrane. The distal end of the basal body made contact with the plasma membrane through transitional fibres (alar sheets). The parental and daughter centrioles displayed peripheral satellites. The daughter centriole was located near the basal body of the primary cilium. In proximity, we observed well‐developed Golgi dictyosomes surrounded by multiple secretory vesicles, suggesting the occurrence of active ciliary transport that was probably related to intraflagellar transport. Exosomes were also common close to the telocyte membrane (Figure 5b).
FIGURE 5.
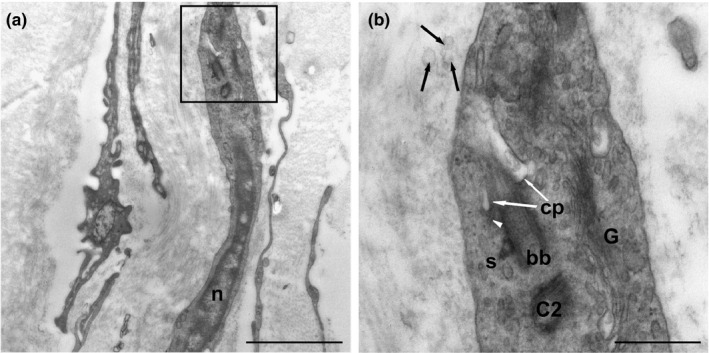
(a) Location of the primary cilium of a telocyte (inset). n: nucleus. (b) Detail of A‐Primary cilium emerging from a basal body (bb). The proximal region of the cilium is located within an invagination of the plasma membrane (ciliary pocket) (white arrows) (cp). The distal end of the basal body (bb) contacts the plasma membrane through transitional fibres (arrowheads). Extracellular vesicles can be observed in close relationship with the primary cilium (black arrows). C2, daughter centriole; G, Golgi apparatus; S, satellite. Scale bars: (a) 2 µm; (b) 0.5 µm
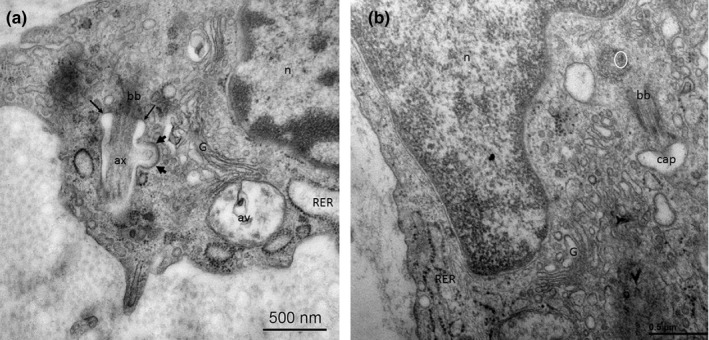
Primary cilia can also appear in tenocytes and tenoblasts. In Figure 6a, a tenocyte primary cilium is observed. Microtubules show a longitudinal disposition thorough the axoneme, immerse in the ciliary pocket. This structure presents an endocytic dominium with clathrin‐coated invaginations (identified by an electron‐dense reinforce in the inner membrane). (Figure 6a). In Figure 6b, primary ciliogenesis in a tenoblast is shown. The mother centriole initiates its elongation covered by the ciliary vesicle, which will originate (once it binds the cell membrane) the ciliary pocket. Primary cilia assembly and disassembly suppose a very dynamic process, which may be transiently present in cellular differentiation. Thus, quantitative analyses of primary cilia result difficult.
FIGURE 6.
Pseudoglandular, canalicular, and saccular phase of lung development (a‐c). Exemplary micro‐CT images of coronary cross sections at ED15 (a), ED20 (b), and N0 (c). Additional histological sections (H&E) of corresponding pulmonary tissue (×10 magnification; d,e,f). Images were adjusted for best visualization. In the pseudoglandular stage, lung parenchyma had a glandular structure and terminal buds grew out into the surrounding mesenchyme (a,d). In the later stages, branching and penetration of the epithelial tubules into the surrounding tissue were observed. During the canalicular stage the airways expanded (b,e). In the saccular stage, transition from branching of the airways to alveolarization could be observed (c,f). Scalebar 1 mm
4. DISCUSSION
Tendon is a key element within the locomotor system that is often subjected to significant stress and tendinopathies. Although cell heterogeneity in tendons has been recognized for many years, the identities of various cell types have not been studied in detail (Chuen et al., 2004). An exhaustive study of tendon structure and the accurate identification and characterization of all its elements are of the utmost importance to understand tissue homeostasis and promote tendinopathy prevention and treatment.
Tenoblasts are sometimes regarded as activated forms of tenocytes, especially in the case of the intrinsic healing of tendon injuries (Davidson et al., 1997). Interconversion between tenoblasts and tenocytes might occur, and their ratio in tendons may govern the tissue responses to various stimuli (Chuen et al., 2004). Tenoblasts and tenocytes may represent different statuses of differentiation and play distinct roles in the tendon in adults (Rolf et al., 2001).
The proliferation index and apoptosis index of tenoblasts are higher than those of tenocytes, which are presumably terminally differentiated; this supports the view that tenoblasts are precursor cells for tenocyte differentiation (Chuen et al., 2004).
With respect to their origins, tenoblasts may be remnants of embryonic development (Jozsa & Kannus, 1997) or derived from connective tissue progenitor cells (Chuen et al., 2004; Muschler & Midura, 2002). Bi et al. identified a cell population of resident tendon stem/progenitor cells (TSPCs) (Bi et al., 2007; Lui & Chan, 2011). The perivascular space of tendon tissue seems to be the source of local stem/progenitor cells (Docheva et al., 2015).
Our main contribution in this work has been the identification of telocytes in the tendon for the first time. Telocytes are located precisely near the blood vessels of the tendon interfascicular matrix. The special relationship between TCs and blood vessels has been documented in different organs by several authors, including us (Cantarero et al., 2011b; Gherghiceanu & Popescu, 2012; Popescu & Faussone‐Pellegrini, 2010). Moreover, we suggest that blood vessels and nerve fibres can create a favourable microenvironment in the differentiation process of mesenchymal precursor cells. This is what our team has described previously as the ‘mesenchymal cell niche’, which is composed of telocytes and blood vessels (Cantarero et al., 2011b).
It has been shown that telocytes have powerful potential effects for tissue repair and regeneration (in heart, lung, skeletal muscle, skin, meninges and choroid plexus, eye, liver, uterus and the urinary system) (Aleksandrovych et al., 2017; Bei et al., 2015). Moreover, different studies have suggested that telocytes could represent an early stage in the differentiation process of mesenchymal cells (Anvarian et al., 2019; Cantarero et al., 2011b; Maxia et al., 2018; Suciu et al., 2010). We propose that telocytes may be a subpopulation of mesenchymal progenitor cells and a source of tenoblasts. Furthermore, telocytes (as well as interstitial cells of Cajal) express stem cell markers, including c‐kit, CD34, CD44 (Blondheim et al., 2006) and nestin (Carmona et al., 2011; Lendahl et al., 1990), which would support this hypothesis.
Different authors have described the ultrastructural characteristics of telocytes in different locations, and common ultrastructural features have been described in all these cases (Aleksandrovych et al., 2017). Our results are consistent with previous findings, although we describe the presence of a primary cilium in tendon telocytes for the first time. In previous works, we showed that the primary cilium in telocytes and interstitial cells of Cajal is in a different location (Cantarero et al., 2011b; Junquera Escribano et al., 2011; Junquera et al., 2007).
Primary cilia have subsequently been observed in a wide variety of connective tissue cells, including chondrocytes, fibroblasts, osteocytes, osteoblasts and tenocytes (Donnelly et al., 2010; Hellio Le Graverand et al., 2001; Jensen et al., 2004; Luesma et al., 2016; Poole, 1997; Xiao et al., 2006). Primary cilia have been hypothesized to play a role in establishing the orientation of cells and their secreted ECM molecules (Donnelly et al., 2010; Poole, 1997). Thus, the orientation of the primary cilium may be related to the establishment of anisotropic ECM organization in skeletal tissues.
Primary cilia function as a hub for various signalling pathways essential for development and tissue homeostasis, including mechanotransduction and TGFβ (transforming growth factor β), Wnt and Hedgehog signalling (Nauli et al., 2003; Rohatgi et al., 2007; Rowson et al., 2016; Schweitzer et al., 2010; Wann et al., 2012).
Several authors have reported the presence of a single cilium as an ultrastructural characteristic of precursor cells (Seeley & Nachury, 2010) that is necessary for chemically induced differentiation of human mesenchymal stem cells (Tummala et al., 2010), which is another piece of evidence that would support the fact that telocytes represent an early step in the differentiation of tendon‐derived stem cells or that the telocyte primary cilium may be particularly important in signalling within the vascular niche, where tendon‐derived stem cells reside.
On the other hand, the presence of a large number of vesicles appears to be a conserved feature of TCs regardless of their location (Yang et al., 2015). Telocytes secrete growth factors, chemoattractants and cytokines/chemokines by means of vesicles, indicating that telocytes may regulate stem cell growth and differentiation (Cretoiu et al., 2009; Hutchings et al., 2009; Pasternak et al., 2016; Pieri et al., 2008). Our group described in 2011 the presence of exosomes in the telocytes of the duodenum (Cantarero Carmona et al., 2011a; Junquera Escribano et al., 2011). Therefore, we have been able to verify the release of exosomes by tendon telocytes. We believe that telocytes might contribute to the regulation of the microvascular niche and participate in heterocellular communication mediated by gap junctions and exosomes.
It is important to know the cell composition and the interconnections between different cell types in a tissue. Therefore, improving the understanding of these aspects can provide insights into the maintenance and repair of tendon tissues. Our findings that telocytes may function as a new cellular entity located in the tendon interfascicular matrix and the presence of a primary cilium in telocytes could be relevant to tenogenesis.
5. CONCLUSION
This study has revealed the characteristics of telocytes in the equine tendon. We believe that telocytes may represent an early step in the tendon‐derived stem cell differentiation process towards mature tenocytes ‘in vivo’ based on a previous hypothesis that highlights telocytes as potential mesenchymal progenitor cells. Of particular interest is the presence of the primary cilium as a signalling antenna involved in tenocyte differentiation.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
ACKNOWLEDGMENTS
The authors declare the following contributions to the conduct of the study: tendon sample collection (RC), data analysis (SAI and CI), optical microscopy interpretation and ultrastructural study (JC, LMJ and CI), drafting of the manuscript (LMJ and JC), critical revision of the manuscript for important intellectual content (all authors) and final approval of the manuscript (all authors). LMJ (mjluesma@unizar.es) takes responsibility for the integrity of the work as a whole. We thank Mario Soriano, CIPF ME technician (Valencia), for generating the serial ultrafine sections, and the authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación‐SAI, Universidad de Zaragoza.
Luesma MJ, Cantarero I, Sánchez‐Cano AI, Rodellar C, Junquera C. Ultrastructural evidence for telocytes in equine tendon. J. Anat.2021;238:527–535. 10.1111/joa.13335
REFERENCES
- Aleksandrovych, V. , Pasternak, A. , Basta, P. , Sajewicz, M. , Walocha, J.A. & Gil, K. (2017) Telocytes: facts, speculations and myths (Review article). Folia Medica Cracoviensia, 57(1), 5–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28608858 [Accessed: 12 December 2019] [PubMed] [Google Scholar]
- Anvarian, Z. , Mykytyn, K. , Mukhopadhyay, S. , Pedersen, L.B. & Christensen, S.T. (2019) Cellular signalling by primary cilia in development, organ function and disease. Nature Reviews Nephrology, 15(4), 199–219. 10.1038/s41581-019-0116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei, Y. , Wang, F. , Yang, C. & Xiao, J. (2015) Telocytes in regenerative medicine. Journal of Cellular and Molecular Medicine, 19(7), 1441–1454. 10.1111/jcmm.12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, M. & Ralphs, J.R. (1997) Tendons and ligaments — an overview. Histology and Histopathology, 12(4), 1135–1144. [PubMed] [Google Scholar]
- Bi, Y. , Ehirchiou, D. , Kilts, T.M. , Inkson, C.A. , Embree, M.C. , Sonoyama, W. et al. (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Medicine, 13(10), 1219–1227. 10.1038/nm1630 [DOI] [PubMed] [Google Scholar]
- Blondheim, N.R. , Levy, Y.S. , Ben‐Zur, T. , Burshtein, A. , Cherlow, T. , Kan, I. et al. (2006) Human +sition. Stem Cells and Development, 15(2), 141–164. 10.1089/scd.2006.15.141 [DOI] [PubMed] [Google Scholar]
- Cantarero Carmona, I. , Luesma Bartolomé, M.J. & Junquera Escribano, C. (2011a) Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy. Journal of Cellular and Molecular Medicine, 15(1), 26–30. 10.1111/j.1582-4934.2010.01207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero, I. , Luesma, M.J. & Junquera, C. (2011b) The primary cilium of telocytes in the vasculature: electron microscope imaging. Journal of Cellular and Molecular Medicine, 15(12), 2594–2600. 10.1111/j.1582-4934.2011.01312.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero, I. , Jose Luesma, M. , Miguel Alvarez‐Dotu, J. , Muñoz, E. & Junquera, C. (2016) Transmission electron microscopy as key technique for the characterization of telocytes. Current Stem Cell Research & Therapy, 11(5), 410–414. 10.2174/1574888x10666150306155435 [DOI] [PubMed] [Google Scholar]
- Carmona, I.C. , Luesma Bartolomé, M.J. , Lavoie‐Gagnon, C. & Junquera Escribano, C. (2011) Distribution of nestin protein: immunohistochemical study in enteric plexus of rat duodenum. Microscopy Research and Technique, 74(2), 148–152. 10.1002/jemt.20884 [DOI] [PubMed] [Google Scholar]
- Chang, J. , Garva, R. , Pickard, A. , Yeung, C.‐Y.‐C. , Mallikarjun, V. , Swift, J. et al. (2020) Circadian control of the secretory pathway maintains collagen homeostasis. Nature Cell Biology, 22(1), 74–86. 10.1038/s41556-019-0441-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuen, F.S. , Chuk, C.Y. , Ping, W.Y. , Nar, W.W. , Kim, H.L. & Ming, C.K. (2004) Immunohistochemical characterization of cells in adult human patellar tendons. Journal of Histochemistry and Cytochemistry, 52(9), 1151–1157. 10.1369/jhc.3A6232.2004 [DOI] [PubMed] [Google Scholar]
- Corradi, L.S. , Jesus, M.M. , Fochi, R.A. , Vilamaior, P.S.L. , Justulin, L.A. , Góes, R.M. et al. (2013) Structural and ultrastructural evidence for telocytes in prostate stroma. Journal of Cellular and Molecular Medicine, 17(3), 398–406. 10.1111/jcmm.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretoiu, D. & Cretoiu, S.M. (2016) Telocytes in the reproductive organs: current understanding and future challenges. Seminars in Cell & Developmental Biology, 55, 40–49. 10.1016/j.semcdb.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Cretoiu, S.M. , Cretoiu, D. , Suciu, L. & Popescu, L.M. (2009) Interstitial Cajal‐like cells of human Fallopian tube express estrogen and progesterone receptors. Journal of Molecular Histology, 40(5–6), 387–394. 10.1007/s10735-009-9252-z [DOI] [PubMed] [Google Scholar]
- Cretoiu, S.M. & Popescu, L.M. (2014) Telocytes revisited. Biomolecular Concepts, 5(5), 353–369. 10.1515/bmc-2014-0029 [DOI] [PubMed] [Google Scholar]
- Davidson, C.J. , Ganion, L.R. , Gehlsen, G.M. , Verhoestra, B. , Roepke, J.E. & Sevier, T.l. (1997) Rat tendon morphologic and functional changes resulting from soft tissue mobilization. Medicine and Science in Sports and Exercise, 29(3), 313–319. 10.1097/00005768-199703000-00005 [DOI] [PubMed] [Google Scholar]
- Docheva, D. , Müller, S.A. , Majewski, M. & Evans, C.H. (2015) Biologics for tendon repair. Advanced Drug Delivery Reviews, 84, 222–239. 10.1016/j.addr.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly, E. , Ascenzi, M.‐G. & Farnum, C. (2010) Primary cilia are highly oriented with respect to collagen direction and long axis of extensor tendon. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 28(1), 77–82. 10.1002/jor.20946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu, M. , Manole, C.G. & Popescu, L.M. (2010) Telocytes in endocardium: electron microscope evidence. Journal of Cellular and Molecular Medicine, 14(9), 2330–2334. 10.1111/j.1582-4934.2010.01133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu, M. & Popescu, L.M. (2012) Cardiac telocytes ‐ their junctions and functional implications. Cell and Tissue Research, 348(2), 265–279. 10.1007/s00441-012-1333-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellio Le Graverand, M.P. , Ou, Y. , Schield‐yee, T. , Barclay, L. , Hart, D. , Natsume, T. et al. (2001) The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. Journal of Anatomy, 198(Pt5), 525–535. 10.1046/j.1469-7580.2000.19850525.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinescu, M.E. , Gherghiceanu, M. , Suciu, L. & Popescu, L.M. (2011) Telocytes in pleura: two‐ and three‐dimensional imaging by transmission electron microscopy. Cell and Tissue Research, 343(2), 389–397. 10.1007/s00441-010-1095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings, G. , Williams, O. , Cretoiu, D. & Ciontea, S.M. (2009) Myometrial interstitial cells and the coordination of myometrial contractility. Journal of cellular and molecular medicine, 13(10), 4268–4282. 10.1111/j.1582-4934.2009.00894.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, C.G. , Poole, C.A. , McGlashan, S.R. , Marko, M. , Issa, Z.I. , Vujcich, K.V. et al. (2004) Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biology International, 28(2), 101–110. 47010.1016/j.cellbi.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Jozsa, L.G. & Kannus, P. (1997) Structure and metabolism of normal tendons In: Jozsa, L.G. and Kannus, P. (Eds.) Human tendons: anatomy, physiology, and pathology. Human Kinetics, pp. 46–95. [Google Scholar]
- Junquera, C. , Martínez‐Ciriano, C. , Castiella, T. , Serrano, P. , Azanza, M.J. & Ramón y Cajal Junquera, S. (2007) Immunohistochemical and ultrastructural characteristics of interstitial cells of Cajal in the rabbit duodenum. Presence of a single cilium. Journal of Cellular and Molecular Medicine, 11(4), 776–787. 10.1111/j.1582-4934.2007.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junquera Escribano, C. , Cantarero Carmona, I. , Luesma Bartolomé, M.J. , Soriano Navarro, M. , Martínez Ciriano, C. , Castiella, M.T. et al. (1991) Three‐dimensional ultrastructure of human tendons. Acta Anatomica, 142(4), 306–312. 10.1159/000147207 [DOI] [PubMed] [Google Scholar]
- Junquera Escribano, C. , Cantarero Carmona, I. , Luesma Bartolomé, M.J. , Soriano Navarro, M. , Martínez Ciriano, C. , Castiella Muruzabal, T. et al. (2011) The primary cilium: a relevant characteristic in interstitial cells of rat duodenum enteric plexus. Histology and Histopathology, 26(4), 461–470. 10.14670/HH-26.461 [DOI] [PubMed] [Google Scholar]
- Kannus, P. (2000) Structure of the tendon connective tissue. Scandinavian Journal of Medicine and Science in Sports, 10(6), 312–320. 10.1034/j.1600-0838.2000.010006312.x [DOI] [PubMed] [Google Scholar]
- Khan, K.M. & Maffuli, N. (1998) Tendinopathy: an Achilles’ heel for athletes and clinicians. Clinical Journal of Sport Medicine, 8(3), 151–154. [PubMed] [Google Scholar]
- Kostin, S. (2010) Myocardial telocytes: a specific new cellular entity. Journal of cellular and molecular medicine, 14(7), 1917–1921. 10.1111/j.1582-4934.2010.01111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl, U. , Zimmerman, L.B. & McKay, R.D.G. (1990) CNS stem cells express a new class of intermediate filament protein. Cell, 60(4), 585–595. 10.1016/0092-8674(90)90662-X [DOI] [PubMed] [Google Scholar]
- Li, H. , Zhang, H. , Yang, L. , Lu, S. & Ge, J. (2014) Telocytes in mice bone marrow: electron microscope evidence. Journal of Cellular and Molecular Medicine, 18(6), 975–978. 10.1111/jcmm.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luesma, M.J. , Cantarero, I. , Ranera, B. , Remacha, A.R. , Castiella, T. , Romero, A. et al. (2016) Primary cilia in chondrogenic differentiation of equine bone marrow mesenchymal stem cells: ultrastructural study. Journal of Equine Veterinary Science, 47, 47–54. 10.1016/J.JEVS.2016.08.002 [DOI] [Google Scholar]
- Luesma, M.J. , Gherghiceanu, M. & Popescu, L.M. (2013) Telocytes and stem cells in limbus and uvea of mouse eye. Journal of Cellular and Molecular Medicine, 17(8), 1016–1024. 10.1111/jcmm.12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui, P.P.Y. & Chan, K.M. (2011) Tendon‐derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Reviews and Reports, 7(4), 883–897. 10.1007/s12015-011-9276-0 [DOI] [PubMed] [Google Scholar]
- Maxia, C. , Murtas, D. , Isola, M. , Tamma, R. , Zucca, I. , Piras, F. et al. (2018) Immunophenotypic characterization of telocyte‐like cells in pterygium. Molecular Vision, 24, 853–866. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30713424 [Accessed: 12th December 2019]. [PMC free article] [PubMed] [Google Scholar]
- Muschler, G.F. & Midura, R.J. (2002) Connective tissue progenitors: practical concepts for clinical applications. Clinical Orthopaedics and Related Research, 1(395), 66–80. 10.1097/00003086-200202000-00008 [DOI] [PubMed] [Google Scholar]
- Nauli, S.M. , Alenghat, F.J. , Luo, Y. , Williams, E. , Vassilev, P. , Li, X. et al. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics, 33(2), 129–137. 10.1038/ng1076 [DOI] [PubMed] [Google Scholar]
- Pasternak, A. , Szura, M. , Gil, K. & Matyja, A. (2016) Interstitial cells of Cajal ‐ systematic review. Folia Morphologica, 75(3), 281–286. 10.5603/FM.a2016.0002 [DOI] [PubMed] [Google Scholar]
- Pieri, L. , Vannucchi, M.G. & Faussone‐Pellegrini, M.S. (2008) Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. Journal of Cellular and Molecular Medicine, 12(5B), 1944–1955. 10.1111/j.1582-4934.2008.00461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, C.A. (1997) Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biology international, 21(8), 483–494. 10.1006/cbir.1997.0177 [DOI] [PubMed] [Google Scholar]
- Popescu, L.M. & Faussone‐Pellegrini, M.‐S. (2010) TELOCYTES ‐ a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal‐Like Cells (ICLC) to TELOCYTES. Journal of Cellular and Molecular Medicine, 14(4), 729–740. 10.1111/j.1582-4934.2010.01059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu, L.M. , Hinescu, M.E. , Ionescu, N. , Ciontea, S.M. , Cretoiu, D. & Ardeleanu, C. (2005) Interstitial cells of Cajal in pancreas. Journal of Cellular and Molecular Medicine, 9(1), 169–190. 10.1111/j.1582-4934.2005.tb00347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu, L.M. , Manole, C.G. , Gherghiceanu, M. , Ardelean, A. , Nicolescu, M.I. , Hinescu, M.E. et al. (2010) Telocytes in human epicardium. Journal of Cellular and Molecular Medicine, 14(8), 2085–2093. 10.1111/j.1582-4934.2010.01129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal, S . (1893) Los ganglios y plexos nerviosos del intestino de los mamíferos y pequeñas adiciones a nuestros trabajos sobre la médula y gran simpático general. Madrid. [Google Scholar]
- Rohatgi, R. , Milenkovic, L. & Scott, M.P. (2007) Patched1 regulates hedgehog signaling at the primary cilium. Science (New York, NY), 317(5836), 372–376. 10.1126/science.1139740 [DOI] [PubMed] [Google Scholar]
- Rolf, C.G. , Fu, B.S. , Pau, A. , Wang, W. & Chan, B. (2001) Increased cell proliferation and associated expression of PDGFRbeta causing hypercellularity in patellar tendinosis. Rheumatology (Oxford), 40(3), 256–261. 10.1093/rheumatology/40.3.256 [DOI] [PubMed] [Google Scholar]
- Romero, A. , Barrachina, L. , Ranera, B. , Remacha, A.R. , Moreno, B. , de Blas, I. et al. (2017) Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. The Veterinary Journal, 224, 76–84. 10.1016/j.tvjl.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Rowson, D. , Knight, M.M. & Screen, H.R.C. (2016) Zonal variation in primary cilia elongation correlates with localized biomechanical degradation in stress deprived tendon. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 34(12), 2146–2153. 10.1002/jor.23229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer, R. , Zelzer, E. & Volk, T. (2010) Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development, 137, 2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, E.S. & Nachury, M.V. (2010) The perennial organelle: assembly and disassembly of the primary cilium. Journal of Cell Science, 123(Pt 4), 511–518. 10.1242/jcs.061093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suciu, L. , Nicolescu, M.I. & Popescu, L.M. (2010) Cardiac telocytes: serial dynamic images in cell culture. Journal of Cellular and Molecular Medicine, 14(11), 2687–2692. 10.1111/j.1582-4934.2010.01185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suciu, L. , Popescu, L.M. , Gherghiceanu, M. , Regalia, T. , Nicolescu, M.I. , Hinescu, M.E. et al. (2010) Telocytes in human term placenta: morphology and phenotype. Cells, Tissues, Organs, 192(5), 325–339. 10.1159/000319467 [DOI] [PubMed] [Google Scholar]
- Tan, Q. , Lui, P.P. & Lee, Y.W. (2013) In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells and Development, 22(23), 3128–3140. 10.1089/scd.2013.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala, P. , Arnsdorf, E.J. & Jacobs, C.R. (2010) The Role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cellular and Molecular Bioengineering, 3(3), 207–212. 10.1007/s12195-010-0127-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wann, A.K. , Zuo, N. , Haycraft, C.J. , Jensen, C.G. , Poole, C.A. , McGlashan, S.R. et al. (2012) Primary cilia mediate mechanotransduction through control of ATP‐induced Ca2+ signaling in compressed chondrocytes. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 26(4), 1663–1671. 10.1096/fj.11-193649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Z. , Zhang, S. , Mahlios, J. , Zhou, G. , Magenheimer, B.S. , Guo, D. et al. (2006) Cilia‐like structures and polycystin‐1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. The Journal of Biological Chemistry, 281(41), 30884–30895. 10.1074/jbc.M604772200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Chi, C. , Liu, Z. , Yang, G. , Shen, Z.‐J. & Yang, X.‐J. (2015) Ultrastructure damage of oviduct telocytes in rat model of acute salpingitis. Journal of Cellular and Molecular Medicine, 19(7), 1720–1728. 10.1111/jcmm.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, C. & Kadler, K. (2019) Importance of the circadian clock in tendon development. Current Topics in Developmental Biology, 133, 309–342. 10.1016/bs.ctdb.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Zhang, J. & Wang, J.H. (2010) Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskeletal Disorders, 11, 10 10.1186/1471-2474-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Zhang, Y. , Wen, X. , Cao, J. , Li, D. , Lin, Q. et al. (2010) Telocytes accompanying cardiomyocyte in primary culture: two‐ and three‐dimensional culture environment. Journal of Cellular and Molecular Medicine, 14(11), 2641–2645. 10.1111/j.1582-4934.2010.01186.x [DOI] [PMC free article] [PubMed] [Google Scholar]


