ABSTRACT
The anatomy of the hypothalamus includes many nuclei and a complex network of neurocircuits. In this context, some hypothalamic nuclei reside closer to the blood‐brain barrier, allowing communication with the peripheral organs through some molecules, such as leptin. Leptin is considered the main adipokine for energy homeostasis control. Furthermore, leptin signalling in the hypothalamus can communicate with insulin signalling through the activation of phosphoinositide 3‐kinase (PI3k). Previous data suggest that isoforms of PI3k are necessary to mediate insulin action in the hypothalamus. However, obese animals show impairment in the central signalling of these hormones. Thus, in the current study, we evaluated the role of acute exercise in the leptin and insulin pathways in the hypothalamus, as well as in food intake control in obese mice. Although acute physical exercise was not able to modulate leptin signalling, this protocol suppressed the increase in the suppressor of cytokine signalling 3 (SOCS3) protein levels. In addition, acute exercise increased the content of PI3k‐p110α protein in the hypothalamus. The exercised animals showed a strong tendency to reduction in cumulative food intake. For the first time, our results indicate physical exercise can increase PI3k‐p110α protein content in the hypothalamus of obese mice and regulate food intake.
Keywords: hypothalamus, leptin, obesity, physical exercise, PI3k
Acute physical exercise increases the PI3k‐p110α protein content in the hypothalamus of obese mice and reduces food intake.
1. INTRODUCTION
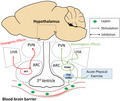
Obesity is characterized by an imbalance in energy homeostasis and is considered a public health problem worldwide (Araujo et al., 2016; Gluckman et al., 2011). During the development of obesity, it was observed that one of the first tissues impaired when exposed to a diet rich in saturated fat is the hypothalamus (Thaler et al., 2012). Anatomically located below the thalamus, the hypothalamus is close to the blood‐brain barrier, which is responsible for transporting nutrients and hormones into the brain. Leptin, an essential controller of energy metabolism (Haddad‐Tóvolli et al., 2017), is one of the main hormones needed in the brain. Thus, understanding the role of leptin in the hypothalamus is essential to clarify the genesis and outcomes associated with obesity.
Leptin is produced in white adipose tissue and plays a critical role in the control of food intake and energy expenditure (Andermann & Lowell, 2017). In the hypothalamic arcuate nucleus (ARC), there are predominantly two neural populations capable of regulating food intake. One neuron population can express the agouti‐related peptide (AgRP), which is characterized by an appetite‐stimulating signal, while the other neuron population can express proopiomelanocortin (POMC), a vital appetite suppressant which is sensitive to leptin stimulation (Rossi & Stuber, 2018). Hypothalamic leptin signalling starts at the leptin receptor (LepR) with subsequent activation of the protein Janus kinase 2 (JAK2). When activated, JAK2 can activate the signal transducer and the transcription activator 3 (STAT3), promoting the transcription of the neuropeptide proopiomelanocortin (POMC) and reducing food intake. In addition, the STAT3 in the nucleus transcribes the suppressor of cytokine‐3 signalling (SOCS3) gene, which is inhibited via leptin by negative feedback in JAK2 (Reed et al., 2010; Seoane‐Collazo et al., 2020). Finally, leptin signalling is linked to the insulin pathway through PI3k, which can control food intake (Razolli et al., 2019).
Previous evidence showed PI3k activation by leptin signalling, and the isoforms of PI3k are present in the hypothalamus (Al‐Qassab et al., 2009; Carvalheira et al., 2005; Tups et al., 2010). In addition, the PI3k isoforms are necessary to mediate insulin activation and reduce food intake (Tups et al., 2010). Moreover, it has been observed that aerobic physical exercise can increase PI3k‐p110α, and this subunit is essential for mediating exercise‐induced cardioprotection (McMullen et al., 2007; Weeks et al., 2012). The hypothalamic increase in PI3 K‐p110α was related to reduced food intake in lean exercised mice (Gaspar et al., 2019). Despite it, no evidence has been described as the effects of exercise in PI3k‐p110α hypothalamic in obese conditions.
Even considering the beneficial properties of leptin, its use for obesity treatment showed moderate results as obese patients are usually leptin resistant (Izquierdo et al., 2019; Seoane‐Collazo et al., 2020). Diets containing high amounts of saturated fatty acids promote hypothalamic inflammation and, consequently, hypothalamic leptin resistance (De Souza et al., 2005; Ozcan et al., 2009). On the other hand, treatment using the association of leptin with other hormones, leptin receptor agonists and amino acids has shown promising effects on energy homeostasis (Izquierdo et al., 2019).
In this scenario, physical exercise is considered a nonpharmacological strategy for obesity prevention and treatment. Although physical exercise has been shown to attenuate hypothalamic leptin resistance (Laing et al., 2016; Ropelle et al., 2010), its molecular mechanisms are not entirely understood. Therefore, the present study verified the acute effects of physical exercise associated with intraperitoneal leptin stimulation in the hypothalamus of obese mice, as well as its impact on food intake. Based on previous studies (Gaspar et al., 2019; Ropelle et al., 2010), we hypothesized that acute physical exercise improves leptin sensitivity and increases PI3k‐p110α after leptin stimulation.
2. EXPERIMENTAL PROCEDURES
2.1. Experimental animals
All the experimental protocols were approved by the Ethics Committee on the use of Animals (CEUA) of the Institute of Biological Sciences, UNICAMP ‐ Campinas‐SP, protocol nº. 3946‐1. Four‐week‐old male Swiss mice from the Multidisciplinary Center for the Biological Investigation Laboratory of Animal Science (CEMIB ‐ UNICAMP) were divided into three groups: Control Group (CTL) = animals that were fed with a commercial diet (NUVILAB®) (n = 5), High‐fat Diet Sedentary (HFD) = animals that were fed with a high‐fat diet and were not submitted to the physical exercise protocol (n = 10); High‐fat Diet Exercised (HFD‐EXE) = animals that were fed with a high‐fat diet and were subjected to an acute exercise protocol (n = 10) (Figure 1a).
FIGURE 1.
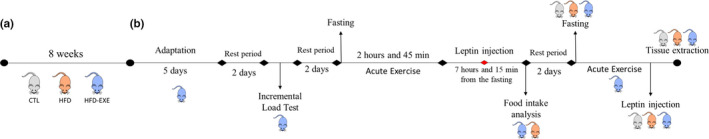
Experimental design. (a) The Swiss mice were divided into three groups: control (CTL), fed a chow diet; HFD ‐ fed a high‐fat diet for 8 weeks and HFD‐EXE ‐ fed a high‐fat diet for 8 weeks and were subjected to an acute exercise protocol. (b) The HFD‐EXE animals underwent a treadmill adaptation period and then performed an incremental load test. Two days after the incremental test, the animals were submitted to the acute physical exercise protocol, which consisted of three 45 min exercise sessions with 15 min intervals between each session. After that, the mice were stimulated by leptin, and the food intake was analysed. Two days after the mice were exercised and the tissue was collected after leptin stimulus
2.2. Diet composition
The HFD composition was adapted from a previous study performed by our research group (Cintra et al., 2012) (Control Diet: 42.75% Corn Starch, 20% Casein, 13.2% Sucrose, 10% Dextrinated Starch, 4% Soybean Oil, 5% Cellulose, 3.5% Mineral Mix, 1% Vitamin Mix, 0.3% L‐Cysteine, 0.25% Choline; High‐Fat Diet: 11.55% Corn Starch, 20% Casein, 13.2% Sucrose, 10% Dextrinated Starch, 4% Soybean Oil, 31.2% Lard, 5% Cellulose, 3.5% Mineral Mix, 1% Vitamin Mix, 0.3% L‐Cysteine, 0.25% Choline).
2.3. Acute physical exercise protocol
After the treadmill adaptation (5 days, 10 min/day at a speed of 3 m/min), mice were submitted to the incremental load test. The animals ran with 0% inclination, and increments of 3 m/min every 3 min were applied until exhaustion. The exhaustion velocity (EV) was used to prescribe the intensity of the acute exercise protocol as previously standardized (Gaspar et al., 2018). The intensity used was 60% of the EV. Thus, the mice ran for a total f 2 h and 45 min, distributed into three bouts of 45 min with a 15‐min interval between them (Gaspar et al., 2018) (Figure 1b).
2.4. Blood collection and lactate analysis
During the incremental load test, blood samples (12.5 µl) were collected before and 0, 3, 5, 7 and 9 min after the test using heparinized capillary tubes. Subsequently, the blood samples were transferred to microcentrifuge tubes containing trichloroacetic acid at 4% (200 µl). The samples were centrifuged for 3 min (3000 rpm) at room temperature and 50 µl of plasma was placed in a microplate. Next, the glycine/EDTA, hydrazine hydrate 24%, lactate dehydrogenase and beta‐nicotinamide adenine dinucleotide were added. After 60 min of incubation at room temperature, the absorbance was determined at 340 nm using a spectrophotometer (BioTek®).
2.5. Fasting blood glucose and glucose tolerance test (GTT)
After 8 hours of fasting, a distal cut was made in the tail of the animals for basal blood glucose dosage by glucometer (Roche Accu‐Chek®). The HFD‐EXE group started the fasting concomitant to the physical exercise protocol. After that, an intraperitoneal (IP) injection of glucose solution (50%) in a dose of 2 g/kg body weight was applied. Blood samples were collected at 30, 60, and 120 min. The area under the curve (AUC) was calculated for each experimental group.
2.6. Leptin administration
After 7 h and 15 min of fasting, the animals received an intraperitoneal injection of leptin (BioVision® #4367) (2 mg/kg). The, 45 min after leptin stimulation, the food intake was analysed and the animals were euthanized (Gaspar et al., 2018) (Figure 1b).
2.7. Evaluation of food intake
The food intake of the HFD and HFD‐EXE groups was measured at 5 (7 p.m.), 12 (7 a.m.), and 24 hr (7 p.m.) after the exercise protocol and leptin injection. The weight measurement was taken using a Gehaka® analytical scale (BK3000).
2.8. Tissue extraction
After completion of the physical exercise protocol and leptin intraperitoneal stimulation (previously described), the animals were euthanized with ketamine hydrochloride (100 mg/kg) and xylazine (5 mg/kg). The hypothalamus was dissected according to the following anatomical limits: anterior, optic chiasm, posterior, mammillary bodies, lateral, optic tracts, and superior, the apex of the hypothalamic third ventricle. The brain was removed, and the hypothalamus collected (Gaspar et al., 2019; Ropelle et al., 2010). The adipose tissue was collected and weighed on a Gehaka® analytical scale (BK3000). The hypothalamus was homogenized in extraction buffer, and the homogenate was used for determination of the total protein content according to a previous study from our research group (Muñoz et al., 2017).
2.9. Western Blotting (WB)
After determination of the total protein content, equal amounts of protein (70 μg/μl) were subjected to SDS‐PAGE polyacrylamide gel electrophoresis. Next, the membranes were incubated overnight at 4°C with the following primary antibodies (dilution 1:1000 ul): Cell Signaling Technology: p‐STAT3 Tyr705 [rabbit, 9145], STAT3 [rabbit, 12640], SOCS3 [rabbit, 2923s] and β‐Actin [rabbit, 4967s]. Santa Cruz Biotechnology: PI3‐kinase p110α [goat, sc‐1331]. Next, the membranes were incubated for 1 h with the specific secondary antibodies (dilution 1:2000 ul; Anti‐rabbit IgG, HRP‐linked Antibody [Cell Signaling Technology #7074]; donkey anti‐goat IgG‐FITC sc‐2024 [Santa Cruz Biotechnology]), and the bands were visualized with enhanced chemiluminescence (ECL) and quantified by densitometry. Ponceau staining was applied to check membrane transfer (Nakandakari et al., 2019).
2.10. RNA extraction and RT‐qPCR
Hypothalamic total RNA was isolated using the TRIzol reagent (Invitrogen). A total of 2‐μg of RNA was used as a template for the synthesis of cDNA using the SuperScript® III First‐Strand Synthesis System (Invitrogen). The cDNA was subjected to a quantitative real‐time polymerase chain reaction (RT‐qPCR) using the Taqman amplification protocol on the 7500 Fast Real‐Time PCR System (Applied Biosystems). The data were evaluated in the StepOne Software program by calculating ΔΔCt. POMC (Mm00435874_m1), NPY (Mm03048253_m1) and GAPDH (Mm99999915_g1) primers were used (Nakandakari et al., 2019).
2.11. Statistical analysis
This was a pre‐clinical study. All results are expressed as median and interquartile range (IQR). The Western blotting results were presented and quantified by densitometry using the Un‐Scan‐It Gel 6.1® program (Silk Scientific) and normalized by respective endogen control, which was analysed Kruskal–Wallis nonparametric test. For the GTT was used Friedman test. For food intake and RT‐qPCR experiments, the Wilcoxon rank sum test was used. Statistical significance was set at p < 0.05. The program “GraphPad Prism 8.01 (GraphPad Software)” was used.
3. RESULTS
3.1. Incremental load test performance
The individual performance in the incremental load test was used to set the exhaustion velocity (EV) as well as the intensity corresponding to 60% of EV (Figure 2a). We reported the exhaustion velocity (EV) as well as the 60% EV. The lactate concentration increased immediately after the exercise to 5.01 mM and decreased over time (3 min = 3.59; 5 min = 3.38; 7 min = 3.19; 9 min = 2.92), while the mean basal lactate was 1.24 mM (Figure 2b).
FIGURE 2.
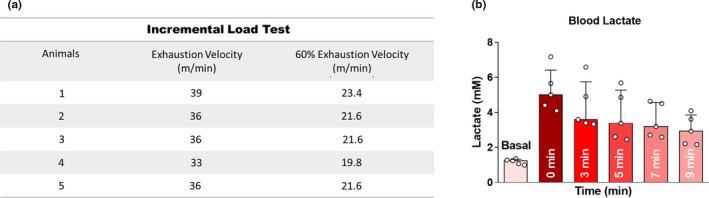
Exercise performance (n = 5). (a) Exhaustion velocity and intensity corresponding to 60% of EV for each mice. (b) Lactate before (basal) and after the incremental load test (0, 3, 5, 7 and 9 min). The bar charts represent the median and IQR of each experimental group
3.2. Acute physical exercise reduced fasting glucose but did not change body weight
After establishing 60% of EV, we applied the acute exercise protocol and verified if the body weight changed. As expected, no differences were observed compared to the initial body weight (Figure 3a). The HFD group presented increased final body weight compared to the CTL group, which was mirrored in the fat pad content of the epididymal adipose tissue (Figure 3b,c). No differences were observed in the body weight and epididymal adipose mass between the HFD and HFD‐EXE groups (Figure 3c). In addition, the HFD group showed an increase in fasting glucose compared to the CTL (Figure 3d). Besides, HFD‐fed presented a glucose intolerance compared with CTL mice (Figure 3e,f). However, no difference was found in the glucose tolerance and AUC between HFD and HFD‐EXE groups (Figure 3e,f).
FIGURE 3.
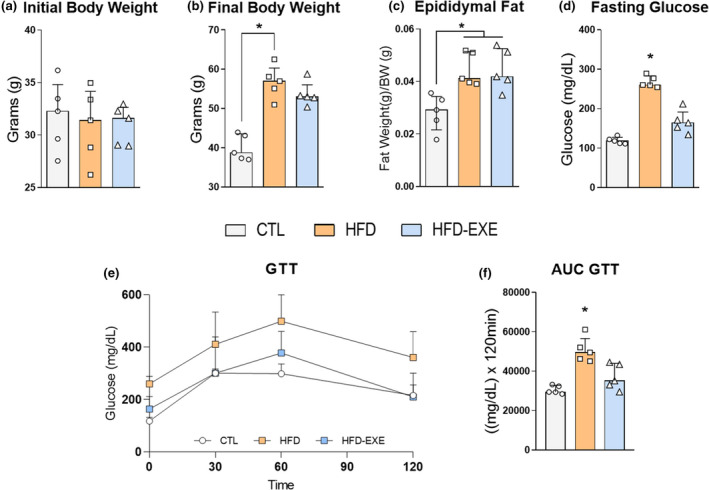
Physiological parameters (CTL n = 5, HFD n = 5, HFD‐EXE n = 5). (a) Initial body weight. (b) Final body weight. (c) Epididymal fat mass. (d) Fasting glucose. (e) Glucose tolerance test. (f) AUC GTT. The bar charts represent the median and IQR of each experimental group. *p < 0.05 HFD vs. CTL, #p < 0.05 HFD‐EXE vs. HFD
3.3. Exercise increased PI3k‐p110α content in the hypothalamus and decreased the food intake
In the molecular analysis, no difference was observed for the p‐STAT3 (Figure 4a). The HFD group presented increased SOCS3 protein content compared to the CTL group (Figure 4b). However, the HFD‐EXE mice were preserved from an increase in SOCS3 levels, although without statistical significance (Figure 4b). On the other hand, when we analysed the cross‐talk with the insulin pathway, we found that the physical exercise group exhibited higher PI3k‐p110α protein content than the HFD group (Figure 4c). No difference was observed between the CTL and HFD groups (Figure 4c). We also measure the Akt phosphorylation and no difference was found among the groups (Figure 4d). After that, food intake analysis was performed between the HFD and HFD‐EXE groups after leptin stimulation. Thus, we identified that the exercised mice presented strong tendency (p = 0.06) to reduced cumulative food intake during the 24‐h time point (Figure 4e,f). No changes were found in POMC and NPY mRNA levels (Figure 4g).
FIGURE 4.
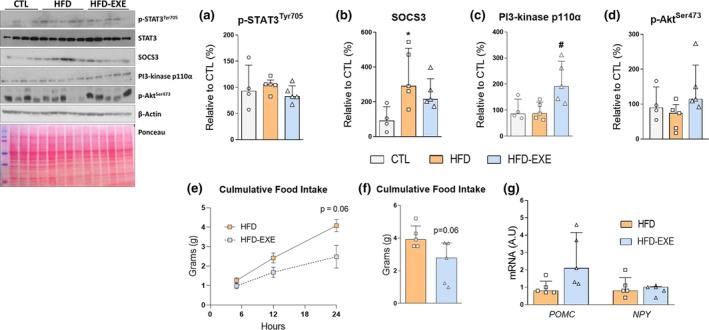
Molecular analysis (CTL n = 4, HFD n = 5, HFD‐EXE n = 5) and energy intake assessment (HFD n = 5, HFD‐EXE n = 5) after leptin stimulation. (a) p‐STAT3 Tyr705/STAT3 (b) SOCS3/β‐Actin, (c) PI3‐kinase p110α/β‐Actin and (d) p‐Aktser473/β‐Actin. (e) Cumulative food intake at 5, 12 and 24 h. (f) Total cumulative food intake for 24 h. (g) mRNA levels of hypothalamic POMC and NPY. The bar charts represent the median and IQR of each experimental group. *p < 0.05 HFD vs. CTL, #p < 0.05 HFD‐EXE vs. HFD
4. DISCUSSION
The hypothalamus is an essential tissue for food intake control. As it is anatomically close to the blood‐brain barrier, the hypothalamus receives peripheral stimuli, making it an area sensitive to obesity damage (Haddad‐Tóvolli et al., 2017). Leptin stimulates the POMC neuron in the arcuate nucleus (Timper & Brüning, 2017). Once activated, POMC neurons activate secondary neurons in the paraventricular nucleus (PVN) and inhibit the neurons in the lateral hypothalamic area (LHA) (Cone, 2005). This mechanism results in anorexigenic effects (food intake reduction and increased energy expenditure) (Timper & Brüning, 2017). In addition, leptin can inhibit the orexigenic pathway (Andermann & Lowell, 2017). Therefore, obesity is associated with leptin resistance and, consequently, hyperphagia. The high fat‐fed induced the expression of inflammatory pathways in the hypothalamus through the toll‐like receptor 4 (TLR4) (De Souza et al., 2005). Thus, the inflammatory molecules promote leptin resistance (Dragano, Haddad‐Tovolli & Velloso, 2017). On the other hand, physical exercise has been shown an important anti‐inflammatory hypothalamic effect and then increases leptin sensitivity (Marinho et al., 2018; Ropelle et al., 2010). Herein, we found that physical exercise can increase hypothalamic PI3k‐p110α and decrease food intake (Figure 5).
FIGURE 5.
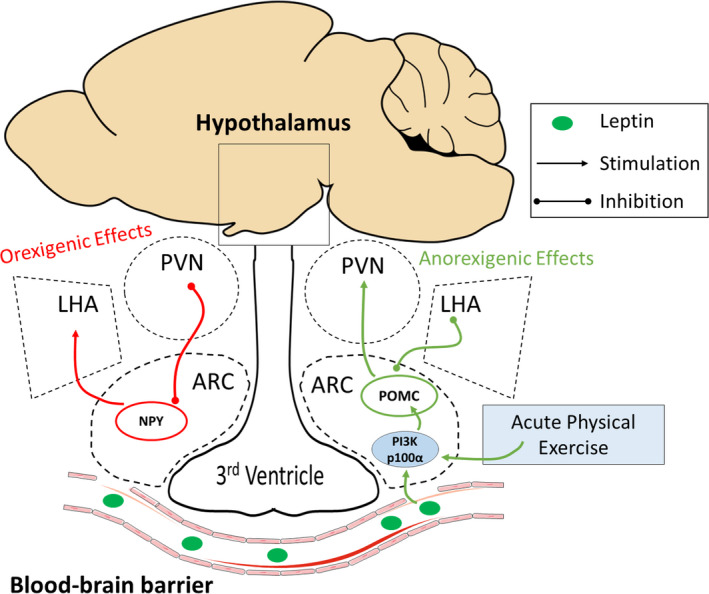
Schematic figure. POMC neurons in the hypothalamic arcuate nucleus activate secondary neurons in the paraventricular nucleus (PVN) and inhibit the neurons in the lateral hypothalamic area (LHA), resulting in a reduction in food intake and increasing energy expenditure. In contrast, in the resistance leptin context, NPY increases food intake and reduces energy expenditure. In this study, exercise increased the PI3k subunit and POMC levels and decreased food intake
Among the effects of physical exercise on the obesity condition, it is known that exercise reduces food intake (Laing et al., 2016; Rodrigues et al., 2015). Recently, our research group showed the prevention effects of exercise (Marinho et al., 2018). Thus, a high‐fat diet associated with physical training in mice prevented inflammation and leptin hypothalamic resistance as well as reducing neuron apoptosis compared with sedentary obese mice (Marinho et al., 2018). Moreover, acute exercise can reduce food intake in obese mice and improve leptin sensitivity (Marinho et al., 2018; Ropelle et al., 2010). On the other hand, when the treatment effects were analysed in obese mice, physical exercise and intraperitoneal leptin stimulation did not demonstrate hypothalamic effects (Borg, Andrews & Watt, 2014). Additionally, Carvalho et al. (2018) verified the hypothalamic effects of exercise without body weight change and reported no differences in the leptin and insulin signalling (Carvalho et al., 2018). Furthermore, in the present study, no difference was observed in STAT3 phosphorylation. Therefore, the chronic effects of exercise on the hypothalamus might be more effective for prevention during obesity development rather than as a treatment.
Our current data show that the HFD group had higher SOCS3 levels than the control mice, while the exercised mice were preserved from the SOCS3 increase. SOCS3 is an important negative feedback of the leptin signalling pathway, inhibiting LepR and JAK2 activation (Seoane‐Collazo et al., 2020). In this context, previous studies observed higher levels of SOCS3 in the hypothalamus of obese mice (Kang, Kim & Shin, 2013; Marinho et al., 2018). However, changes in lifestyle reduce the hypothalamic SOCS3 in obese conditions. Obese rats submitted to exercise training led to a decrease in hypothalamic SOCS3 (Kang, Kim & Shin, 2013).
Although we did not find a difference in the leptin pathway, the exercised mice presented higher PI3k‐p110a. Interestingly, a previous investigation of our research group verified an increase in hypothalamic PI3k‐p110a, and a reduction in food intake in lean mice after acute exercise (Gaspar et al., 2019). PI3k signalling is crucial to mediate hypothalamic leptin and insulin action. In addition, it was verified that the pharmacological inhibition of PI3k decreases acute leptin‐induced neuronal activity (Williams et al., 2011). Tups et al. (2010) suggested that PI3k isoforms are necessary to mediate insulin action in the hypothalamus (Tups et al., 2010).
Other mechanisms are associated with PI3k and food intake. Our group showed that physical exercise increased the association between PI3k‐p110α and the adaptor protein containing the pleckstrin homology domain, phosphotyrosine‐binding domain and leucine zipper motif 1 (APPL1), a crucial protein for adiponectin signalling (Gaspar et al., 2019). Acute exercise in lean mice increased PI3k‐p110α and APPL1 in the hypothalamic arcuate nucleus (Gaspar et al., 2019). Moreover, obese mice presented a reduction in hypothalamic APPL1 protein levels after leptin stimulation. However, exercise reverted this damage and reduced food intake (Gaspar et al., 2018). Moreover, a positive correlation was observed between APPL1 and PI3k mRNA levels in human samples (Gaspar et al., 2019).
In obese humans, using magnetic resonance images, Thaler et al. (2012) identified cerebral gliosis in the mediobasal hypothalamus (Thaler et al., 2012). In addition, using functional magnetic resonance imaging (fMRI), obese individuals showed different hypothalamic functional activity compared with lean individuals after glucose consumption (Van De Sande‐Lee et al., 2011). Obese children were verified with hypothalamic gliosis, which was associated with alterations in the glucose‐stimulated hypothalamic functional response (Sewaybricker et al., 2019). After bariatric surgery, changes in hypothalamus activity after glucose intake were observed in fMRI analysis, bringing these responses closer to the lean group (Van De Sande‐Lee et al., 2011). Furthermore, obese children who performed high‐intensity exercise (75%VO2max) showed lower energy intake during lunch and dinner than the sedentary and low‐intensity exercise group, as well as lower 24‐h total energy intake. However, more studies are necessary to understand the role of physical exercise on hypothalamic alterations and food intake in humans (Thivel et al., 2012).
Altogether, our study shows for the first time that acute physical exercise increases the PI3k‐p110α protein content in the hypothalamus of obese mice and reduces food intake. Thus, these results might aid understanding of how exercise can control food intake and how exercise could be used in the improvement of obesity and hypothalamic disorders.
AUTHOR CONTRIBUTIONS
R.C.G. was responsible for the experimental design and all data. R.C.G. and S.C.B.R.N. were responsible for tissue extraction and western blotting experiments. R.C.G. and V.R.M. were responsible for the mRNA and lactate analysis. R.C.G. and V.R.M., R.C.G. and R.F.L.V. were responsible for food intake data. R.C.G. and J.R.P. were responsible for manuscript writing. A.S.R.S., D.E.C., E.R.R. and L.P.M. were responsible for the support technique and the manuscript review. J.R.P., L.P.M., E.R.R. provided the laboratory support. All authors approve this submission and are following the journal guidelines.
ACKNOWLEDGEMENTS
The authors thank Obesity and Comorbidities Research Center – OCRC, the supported by National Council for Scientific and Technological Development (CNPq; process number 442542/2014‐3 and 306535/2017‐3), Coordination for the Improvement of Higher Education Personnel (CAPES; finance code 001) and São Paulo Research Foundation (FAPESP; process numbers 2017/20542‐3, 2018/07568‐6, and 2018/20872‐6).
Gaspar RC, Nakandakari SCBR, Muñoz VR et al. Acute physical exercise increases PI3K‐p110α protein content in the hypothalamus of obese mice. J. Anat. 2021;238:743–750. 10.1111/joa.13342
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Al‐Qassab, H. , Smith, M.A. , Irvine, E.E. , Guillermet‐Guibert, J. , Claret, M. , Choudhury, A.I. et al. (2009) Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell metabolism, 10(5), 343–354. 10.1016/j.cmet.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann, M.L. & Lowell, B.B. (2017) Toward a wiring diagram understanding of appetite control. Neuron, 95(4), 757–778. 10.1016/j.neuron.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo, E.P. , Moraes, J.C. , Cintra, D.E. & Velloso, L.A. (2016) Mechanisms in endocrinology: Hypothalamic inflammation and nutrition. European Journal of Endocrinology, 175(3), R97–R105. 10.1530/EJE-15-1207 [DOI] [PubMed] [Google Scholar]
- Borg, M.L. , Andrews, Z.B. & Watt, M.J. (2014) Exercise training does not enhance hypothalamic responsiveness to leptin or ghrelin in male mice. Journal of Neuroendocrinology, 26(2), 68–79. 10.1111/jne.12130 [DOI] [PubMed] [Google Scholar]
- Carvalheira, J.B.C. , Torsoni, M.A. , Ueno, M. , Amaral, M.E. , Araújo, E.P. Velloso, L.A. et al. (2005) Cross‐talk between the insulin and leptin signaling systems in rat hypothalamus. Obesity Research, 13(1), 48–57. 10.1038/oby.2005.7 [DOI] [PubMed] [Google Scholar]
- Cintra, D.E. , Ropelle, E.R. , Moraes, J.C. , Pauli, J.R. , Morari, J. , de Souza, C.T. et al. (2012) Unsaturated fatty acids revert diet‐induced hypothalamic inflammation in obesity. PLoS One, 7(1), 10.1371/journal.pone.0030571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone, R.D. (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci, 8(5), 571–578. 10.1038/nn1455 [DOI] [PubMed] [Google Scholar]
- de Carvalho, F.P. , Moretto, T.L. , Benfato, I.D. , Barthichoto, M. , Ferreira, S.M. , Costa‐Júnior, J.M. et al. (2018) Central and peripheral effects of physical exercise without weight reduction in obese and lean mice. Bioscience Reports, 38(2), 10.1042/BSR20171033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza, C.T. , Araujo, E.P. , Bordin, S. , Ashimine, R. , Zollner, R.L. , Boschero, A.C. et al. (2005) Consumption of a fat‐rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology, 146(October), 4192–4199. 10.1210/en.2004-1520 [DOI] [PubMed] [Google Scholar]
- Dragano, N.R.V. , Haddad‐Tovolli, R. & Velloso, L.A. (2017) Leptin, neuroinflammation and obesity. Frontiers of Hormone Research, 48, 84–96. 10.1159/000452908 [DOI] [PubMed] [Google Scholar]
- Gaspar, R.C. , Muñoz, V.R. , Formigari, G.P. , Kuga, G.K. , Nakandakari, S.C.B.R. , Botezelli, J.D. et al. (2018) Acute physical exercise increases the adaptor protein APPL1 in the hypothalamus of obese mice. Cytokine, 110(April), 87–93. 10.1016/j.cyto.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Gaspar, R.C. , Muñoz, V.R. , Kuga, G.K. , Nakandakari, S.C.B.R. , Crisol, B.M. ., Lenhare, L. et al. (2019) Acute physical exercise increases APPL1/PI3K signaling in the hypothalamus of lean mice. European Journal of Neuroscience, 50(7). 10.1111/ejn.14490 [DOI] [PubMed] [Google Scholar]
- Gluckman, P.D. , Hanson, M. , Zimmet, P. & Forrester, T. (2011) Losing the war against obesity: the need for a developmental perspective. Science Translational Medicine, 3, 93cm19 10.1126/scitranslmed.3002554 [DOI] [PubMed] [Google Scholar]
- Haddad‐Tóvolli, R. , Dragano, N.R.V. , Ramalho, A.F.S. & Velloso, L.A. (2017) Development and function of the blood‐brain barrier in the context of metabolic control. Frontiers in Neuroscience, 11(APR), 1–12. 10.3389/fnins.2017.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo, A.G. , Crujeiras, A.B. , Casanueva, F.F. & Carreira, M.C. (2019) Leptin, obesity, and leptin resistance: where are we 25 years later? Nutrients, 11(11). 10.3390/nu11112704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S. , Kim, K.B. & Shin, K.O. (2013) Exercise training improve leptin sensitivity in peripheral tissue of obese rats. Biochemical and Biophysical Research Communications, 435(3), 454–459. 10.1016/j.bbrc.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Laing, B. , Do, K. , Matsubara, T. , Wert, D.W. , Avery, M.J. , Langdon, E.M. et al. (2016) Voluntary exercise improves hypothalamic and metabolic function in obese mice. The Journal of Endocrinology, 229(2), 109–122. 10.1530/JOE-15-0510 [DOI] [PubMed] [Google Scholar]
- Marinho, R. , Munõz, V.R. , Pauli, L.S.S. , Ropelle, E.C.C. , de Moura, L.P. , Moraes, J.C. et al. (2018) Endurance training prevents inflammation and apoptosis in hypothalamic neurons of obese mice. Journal of Cellular Physiology, 234(1), 880–890. 10.1002/jcp.26909 [DOI] [PubMed] [Google Scholar]
- McMullen, J.R. , Amirahmadi, F. , Woodcock, E.A. , Schinke‐Braun, M. , Bouwman, R.D. , Hewitt, K.A. et al. (2007) Protective effects of exercise and phosphoinositide 3‐kinase(p110α) signaling in dilated and hypertrophic cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America, 104(2): 612–617. doi: 10.1073/pnas.0606663104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, V.R. , Gaspar, R.C. , Crisol, B.M. , Formigari, G.P. , Sant’Ana, M.R. , Botezelli, J.D. et al. (2017) Physical exercise reduces pyruvate carboxylase (PCB) and contributes to hyperglycemia reduction in obese mice. The Journal of Physiological Sciences, 68(4), 493–501. 10.1007/s12576-017-0559-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakandakari, S.C.B.R. , Muñoz, V.R. , Kuga, G.K. , Gaspar, R.C. , Sant'Ana, M.R. , Pavan, I.C.B. et al. (2019) Short‐term high‐fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain, Behavior, and Immunity, 79, 284–293. 10.1016/j.bbi.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Ozcan, L. , Ergin, A.S. , Lu, A. , Chung, J. , Sarkar, S. , Nie, D. et al. (2009) Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metabolism, 9(1), 35–51. 10.1016/j.cmet.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Razolli, D.S. , Moura‐Assis, A. , Bombassaro, B. & Velloso, L.A. (2019) Hypothalamic neuronal cellular and subcellular abnormalities in experimental obesity. International Journal of Obesity, 43(12), 2361–2369. 10.1038/s41366-019-0451-8 [DOI] [PubMed] [Google Scholar]
- Reed, A.S. , Unger, E.K. , Olofsson, L.E. , Piper, M.l. , Myers, M.G. & Xu, A.W. (2010) Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long‐term energy homeostasis. Diabetes, 59(4), 894–906. 10.2337/db09-1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, B.d.A. , Pauli, L.S.S. , De Souza, C.T. , Da silva, A.S.R. , Cintra, D.E.C. , Marinho, R. et al. (2015) Acute exercise decreases tribbles homolog 3 protein levels in the hypothalamus of obese rats. Medicine & Science in Sports & Exercise, 47(8), 1613–1623. 10.1249/MSS.0000000000000585 [DOI] [PubMed] [Google Scholar]
- Ropelle, E.R. , Flores, M.B. , Cintra, D.E. , Rocha, G.Z. , Pauli, J.R. , Morari, J. et al. (2010) IL‐6 and IL‐10 anti‐inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLoS Biology, 8(8), 31–32. 10.1371/journal.pbio.1000465 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rossi, M.A. & Stuber, G.D. (2018) Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metabolism, 27(1), 42–56. 10.1016/j.cmet.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane‐Collazo, P. , Martínez‐Sánchez, N. , Milbank, E. & Contreras, C. (2020) Incendiary leptin. Nutrients, 12(2), 472 10.3390/nu12020472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewaybricker, L.E. , Schur, E.A. , Melhorn, S.J. , Campos, B.M. , Askren, M.K. , Nogueira, G.A.S. et al. (2019) Initial evidence for hypothalamic gliosis in children with obesity by quantitative T2 MRI and implications for blood oxygen‐level dependent response to glucose ingestion. Pediatric Obesity, 10.1111/ijpo.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler, J.P. , Yi, C.‐X. , Schur, E.A. , Guyenet, S.J. , Hwang, B.H. , Dietrich, M.O. et al. (2012) Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation, 122, 153–162. 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivel, D. , Isacco, L. , Montaurier, C. , Boirie, Y. , Duché, P. & Morio, B. (2012) The 24‐h energy intake of obese adolescents is spontaneously reduced after intensive exercise: A randomized controlled trial in calorimetric chambers. PLoS One, 10.1371/journal.pone.0029840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timper, K. & Brüning, J.C. (2017) Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech, 10(6), 679–689. 10.1242/dmm.026609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tups, A. , Anderson, G.M. , Rizwan, R.A. , Chaussade, C. & Shepherd, P.R. (2010) Both p110 a and p110 b isoforms of phosphatidylinositol 3‐OH‐kinase are required for insulin signalling in the hypothalamus neuroendocrinology. Journal of Neuroendocrinology, 22(8), 534–542. 10.1111/j.1365-2826.2010.01975.x [DOI] [PubMed] [Google Scholar]
- Van De Sande‐Lee, S. , Pereira, F.R.S. , Cintra, D.E. , Fernandes, P.T. , Cardoso, A.R. , Garlipp, C.R. et al. (2011) Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes, 60(6), 1699–1704. 10.2337/db10-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks, K.L. , Gao, X. , Du, X.‐J. , Boey, E.J.H. , Matsumoto, A. , Bernardo, B.C. et al. (2012) Phosphoinositide 3‐kinase p110α is a master regulator of exercise‐induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circulation Heart Failure, 5(4), 523–534. 10.1161/CIRCHEARTFAILURE.112.966622 [DOI] [PubMed] [Google Scholar]
- Williams, K.W. , Sohn, J.‐W. , Donato, J. , Lee, C.E. , Zhao, J.J. , Elmquist, J.K. et al. (2011) The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. Journal of Neuroscience, 31(37), 13147–13156. 10.1523/JNEUROSCI.2602-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


