Abstract
The purpose of this study was to examine sex cognition and behavioral strategy correlates for chlamydia, gonorrhea, and HIV testing among a national sample of young adults ages 18–20. Young adults (18–20 years) were recruited nationally (N=1,144). The sample was restricted (n=817) based on inclusion/exclusion criteria for analysis. The outcome variables were gonorrhea, chlamydia, and HIV testing, respectively, in the last 12 months. Covariates included demographic variables, alcohol use, perceived vulnerability, protective behavioral strategies, and sexual behavior in the last 3 months. Adjusted logistic regression models were estimated in SAS 9.4. Approximately 24% of respondents were tested for chlamydia and gonorrhea, and 21% were tested for HIV in the past year. Women were more likely than men to be tested for chlamydia (OR=1.67, 95%CI 1.13, 2.46) and gonorrhea (OR=1.55, 95%CI 1.05, 2.28). Persons who were worried about an STI after a sexual encounter and who engaged in casual sex were more than two times as likely to be tested for all three STIs. Similarly, persons who used more non-condom related protective behavioral strategies were more likely to be tested. Future studies may consider these correlates as potential intervention points for promoting STI testing among young adults.
Keywords: sexual behavior, sexually transmitted infections, testing, alcohol, young adults
Introduction
Young adults in the United States are a high-risk population for sexually transmitted infections (STIs), and thus, screening for STIs are recommended for young adult sub-populations. In an effort to understand how to promote STI testing among this population, recognizing the cognitions and protective behaviors related to alcohol and alcohol-related sexual behavior may identify targets for future intervention.
Sexually Transmitted Infections and Young Adults
Rates of STIs are on the rise in the United States, especially chlamydia (Chlamydia trachomatis), gonorrhea (Neisseria gonorrhoeae), and Human Immunodeficiency Virus (HIV) infections (Centers for Disease Control and Prevention 2018). Specific populations, including young adult populations, are disproportionately impacted by these STIs. Young adults age 15–24 are approximately 25% of the sexually active population in the US, but account for over half of the 19 million STIs diagnosed per year (Satterwhite et al. 2013). There are significant societal economic and health costs associated with STIs (Owusu-Edusei et al. 2013). As such, efforts to prevent STIs among young adult populations and the corresponding negative health outcomes and costs are needed.
Although primary prevention strategies, such as condom use and reduction in the number of sexual partners exist to reduce STI transmission, secondary prevention via STI and HIV screening is a cost-effective approach among young adult populations. As these STIs can often be asymptomatic, routine screening may reduce the transmission and the associated complications among young adults (Miller and Shafer 2008). Screening young adult women for chlamydia and gonorrhea is cost-effective and, in some populations, results in cost-savings compared to the treatment for the resulting reproductive sequelae (Gottlieb et al. 2010). Because of this, screening for chlamydia and gonorrhea are recommended annually for sexually active women under age 25, while screening for these infections are recommended at least once year for sexually active men who have sex with men (MSM) (Lefevre 2014; Workowski and Bolan 2015). HIV screening among young adults has also been shown to be cost-effective (Paltiel et al. 2005; Walensky et al. 2007). Because HIV screening is cost-effective, the CDC recommends than all men and women age 13–64 be tested for HIV at least once in their lifetime as part of their regular medical care (Centers for Disease Control and Prevention 2006) or if they are seeking evaluation or treatment for other STIs (Workowski and Bolan 2015). HIV screening is recommended at least once in young adult populations, with those at higher risk for HIV infection (MSM, risky sexual behaviors) screened at least annually (Workowski and Bolan 2015; DiNenno et al. 2017).
Despite these recommendations, screening rates for chlamydia, gonorrhea, and HIV are low among young adults. Studies indicate just 40% of young adult women are screened for chlamydia (Berman and Satterwhite 2011), and these rates are even lower for men (National Committee for Quality Assurance 2016; Hoover et al. 2014). According to the National Survey of Family Growth, 39% of women and 54% of men age 15–44 reported never receiving an HIV test; never having received an HIV test was more common among young adults ages 15–24 years (64% of women, 74% of men) compared to those 25 or older (Febo-Vazquez et al. 2018).
Cognitions and Protective Behaviors for Sexually Transmitted Infections
The relationship between STIs and sexual behaviors (e.g., hooking up, condom use resistance, etc.) is well-established (Davis et al. 2014; Sheeran and Taylor 1999; Wegner et al. 2018). Although “hooking up” is interpreted differently among both researchers and young adults, there is a consensus that hooking up involves sexual activity outside a committed romantic relationship (Claxton and van Dulmen 2013; Fielder et al. 2014; Lewis et al. 2013). The lack of commitment in hook-up relationships typically result in shorter time gaps between partners or concurrent partnerships, which increases STI risk (Fielder et al. 2014; Kraut-Becher and Aral 2003). In spite of increased knowledge that condom use is effective in reducing STIs, young adults continue to report inconsistent condom use (Copen 2017). The incongruence may be explained cognitively by a lack of perceived STI vulnerability (Gerrard et al. 1996; James et al. 2004).
Perceived vulnerability, also known as perceived susceptibility, is an individual’s belief about the likelihood of a health threat’s occurrence (Gerrard et al. 1996). Perceived STI vulnerability is not only associated with risky sexual behavior and STI history but also past STI testing behavior and current STI testing intentions (de Visser and O’Neill 2013; Gerrard et al. 1996; Martin-Smith et al. 2018; Wolfers et al. 2010). Additionally, women with low perceived STI vulnerability report more frequent and varied condom use resistance tactics like risk-level reassurance (e.g., reassuring their partner they are ‘clean’ from STIs) and seduction, thus increasing risk of STIs (Wegner et al. 2018). Research demonstrates that condom protective behavioral strategies (PBS) (e.g., having a mental plan to use a condom, discussing condom use with partner, etc.) increased use of condoms at most recent vaginal sexual experience (Lewis et al. 2009). However, the relationship on condom PBS and STI testing behavior remains unknown.
Moreover, alcohol use has been consistently linked to sexual behavior and sexual assault (Howells and Orcutt 2014; Krebs et al. 2009; Rehm et al. 2012; Testa and Hoffman 2012). Young adults who engage in heavy drinking are at an increased risk for engaging in high-risk sexual practices that result in negative health outcomes, such as the contraction of STIs and unwanted pregnancies (Lewis et al. 2012; Patrick and Maggs 2009). The association between alcohol consumption and sexual risk taking is attributed to the acute pharmacological effects of alcohol, specifically alcohol myopia, which limits a person’s cognitive functions to attend to situational cues (Steele and Josephs 1990; Abbey 2002). Alcohol has been shown to impair both cognitive functioning and motor skills and also reduce inhibitions, which in turn increases individual’s willingness to engage in risky sexual behavior (Rehm et al. 2012; Townshend et al. 2014).
Despite higher risk of STIs, young adult heavy episodic drinkers are less likely to engage in protective behaviors such as consistent condom usage to avoid risk (George and Stoner 2000; Hingson et al. 2005; Cooper 2002). Moreover, alcohol-related sexual cognitions impact future engagement in both adverse and sexual health promoting behaviors (Lewis et al. 2012; Lewis et al. 2014). Alcohol-related sexual expectancies and perceived vulnerability of alcohol-related consequences may impact young adults’ likelihood to get tested for STIs.
Given the young adults in the 18- to 20-year-old age group are at high-risk for STIs, examining correlates of STI testing can identify potential intervention points to promote recommended screening among this population. The purpose of this study is to assess alcohol and sex cognition and behavioral strategy correlates for chlamydia, gonorrhea, and HIV testing. We hypothesize that STI testing will be higher among women than men, condom-related protective behavioral strategies will be correlated with increased STI testing, and perceived vulnerability will increase the odds of STI testing.
Materials and Methods
Participants and Procedures
Participants for the present study included 1,144 18- to 20-year-old young adults who were participating in a larger study evaluating an intervention for alcohol-related sexual behavior. Data for the present analyses come from the baseline assessment of the longitudinal intervention study. Demographics for the baseline sample include mean age of 19.17 years old (SD = .79) and a gender representation of 54.5% female. Ethnic and racial representation of the baseline sample was as follows: 15.1% Hispanic/Latino, 70.5% White, 3.9% Other/More than one race, 7.9% African American, 9.7% Asian, 1.2% American Indian/Alaska Native, and 0.4% Native Hawaiian/Pacific Islander. Current educational background representation consisted of 15.7% not enrolled in any form of college, 72.7% attending a 4-year university, 8.0% attending a community college, 0.7% attending a technical/vocational college, 0.5% attending a graduate/professional school, and 1.7% in high school.
Participants for this study were recruited nationally through various methods and asked to complete a brief, five-minute web-based screening survey to determine if they met inclusion criteria for the longitudinal study. Recruitment methods included online recruiting (e.g., Facebook, Craigslist, Amazon Mechanical Turk), in-print advertisements, flyers, participant referrals, and in-person recruiting. The most commonly endorsed recruitment sources were Craigslist (47.88%), Instagram (11.76%), Participant referral (8.99%), Facebook (7.93%), while each of the other sources of recruitment (e.g., Researchmatch.org, Twitter, radio, flyer) were endorsed by less than 5% of the sample. All advertisements and recruitment efforts included a URL to a study website that included a brief information statement describing the study and access to a short three-minute online eligibility survey. Initial eligibility criteria included the following: reside in the US; age 18–20; provide a birthdate consistent with their age; provide a phone number, first and last name, birth sex, gender, sexual desire, and valid email address; correctly answer check questions (i.e., select 2 for what is 4 minus 2, select the color blue from a list of colors); not be in a monogamous relationship, or be in a monogamous relationship for less than three months and be open to having a sexual relationship with someone other than a monogamous partner; have had sex in the past three months; and have had an alcoholic drink at least twice a month on average over the past 3 months. Potential participants (N=17,899) who completed the eligibility survey were then moved to the next part of the screening process (N= 2,690; 15.02%).
Participants who met minimum eligibility criteria after completing the screening survey were placed into a database for study staff to review. Study staff screened participants in the database dependent on the demographic needs of the study and, if appropriate, moved them to a telephone contact list. Those in the contact list were then called by study staff to verify eligibility and provide more details about study procedures. Participants who were unable to verify eligibility information during the phone call were marked as ineligible. Participants were also able to decline from providing additional information via phone or decline from the study if they no longer wished to participate after providing the additional information.
Additionally, to ensure a diverse sample, participants were stratified by birth sex, education level, race, and ethnicity prior to the verification call. Participants who were in excess of a quota of one of the stratification groups (e.g., women, in college, or white), based on demographic information, were not invited to the baseline survey as assessed throughout the recruitment in the study. Out of the 1,480 participants who met all inclusion criteria, 1,144 (77.3%) were invited to complete the baseline survey and longitudinal study participation.
Participants who verified their information with study staff and wished to continue were sent the invitation to complete the baseline survey. Upon receiving the invitation to the survey, participants were presented with a full information statement. Those who agreed to participate and indicated their consent were immediately routed to the online baseline assessment. Of the 1,144 participants invited to the baseline survey, 1,065 (93.1%) completed the survey. Participants who completed the survey received a $25 gift certificate. A Federal Certificate of Confidentiality was obtained to help ensure privacy of research participants. All study procedures were approved by the University’s Institutional Review Board, and no adverse events were reported.
The sample from the baseline survey were further subset to meet the criteria for this study analysis. The sample was restricted to persons who had not had a past chlamydia, gonorrhea, or HIV diagnosis since the timing of the past diagnosis could not be confirmed (n=1,092), were not in a serious relationship or married (n=1,046), did not have outliers for sexual experiences variables (n=1,025), had sex in the last three months (n=1,003), reported yes or no for STI testing in the last three months (n=978), and responded to all other covariates for this analysis (n=817).
Measures
Measures for this analysis included STI testing, demographics, alcohol use, alcohol-related sexual cognitions, and sexual behaviors.
STI testing.
The outcome variables for this analysis were reported STI testing in the last three months for gonorrhea, chlamydia, and HIV, respectively. All were dichotomous responses (yes/no).
Demographic variables.
Demographic variables included biological sex (male, female) and relationship status (single, not dating, dating, not serious).
Typical number of drinks per week.
The Daily Drinking Questionnaire (DDQ; Collins, Parks, & Marlatt, 1985) was used to assess number of typical drinks per week. Participants were asked to “Consider a typical week in the past 3 months. How much alcohol, on average (measured in number of drinks), do you drink on each day of a typical week?” Weekly drinking was computed by summing the standard number of drinks for each day of the week.
Perceived vulnerability for STIs.
Participants responded to perceived vulnerability for experiencing a health problem, such as an STI, after drinking and non-drinking sexual scenarios. These scenarios included (1) a casual partner while not drinking, (2) a partner he/she would not normally have sex with (i.e., other partner) while not drinking, (3) a casual partner while drinking 5/4 or more drinks, and (4) a partner he/she would not normally have sex with (i.e., other partner) while drinking 5/4 or more drinks. Response options were a 5-point Likert scale from very unlikely to very likely. Due to the distribution of the response options, the responses were aggregated to unlikely (very unlikely and unlikely), neither likely nor unlikely, and likely (likely, very likely).
Alcohol-related sexual consequences.
A range of alcohol-related sexual consequences from less severe to more severe were assessed using the 41-item Alcohol-related Sexual Consequences Scale (Lewis et al. 2019). Sexual behavior included digital, oral, vaginal, and anal sex. Participants indicated which of the problems they had experienced as a result of drinking alcohol in the past month by responding yes (1) or no (0). The 41 items were summed to create a total score (α ranged from .89 to .92 across the three assessments). Items covered a variety of sexual consequences or behaviors resulting from alcohol such as regretted sexual activity, unprotected sexual activity, and casual sex. Examples include “vaginal sex without a condom,” “oral sex later regretted,” and “vaginal sex with someone just met.”
Protective behavioral strategies.
Two scales were used to measure safer-sex protective behavioral strategies, responding to the stem, “Please indicate how much you engaged in the following sex-related behaviors during the past 3 months.” Condom-related protective behavioral strategies comprised 6 items (Cronbach’s alpha=0.87) and non-condom protective behavioral strategies comprised 8 items (Cronbach’s alpha=0.80). Example items for condom-related PBS included “buy condoms,” “told a partner I wanted to use a condom,” and “have a mental plan to use a condom.” Example items for non-condom PBS included “talked about partner’s history of safe sex behaviors prior to sex,” “had a mental plan to NOT have sex with someone you just met (i.e., one-night stand),” and “made sure you went home with a trusted friend.”
Sexual behavior last three months.
Participants reported their sexual behavior in the last three months. This included the number of partners for the following types of sex: casual sex, casual sex after consuming alcohol, any sex after consuming alcohol, and number of times used a condom during sex. These variables were recoded as yes or no for each item (0=no, and 1 or more=yes).
Data Analysis
The distributions of the variables were examined using univariate descriptive statistics. For each outcome variable, bivariate frequencies were estimated for each covariate. Separate independent variables selected for this analysis were based on the theoretical relevance to protective behavioral skills, perceived vulnerability, and alcohol-related sexual consequences. Additionally, alcohol use, relationship status, and gender were screened as potential confounders for bivariate associations with the outcome variable. In SAS 9.4, logistic regression models were estimated for each outcome variable modeling the odds of STI testing in the last three months. Adjusted odds ratios (OR) and 95% confidence intervals (CI) were reported.
Results
Sample Overview
Among the 817 18- to 20-year-old participants in the study, over half were female (54.3%), and most were single and not dating (74.2%) (Table 1). On average, participants reported typically consuming 13 drinks per week. With regard to perceived vulnerability, most reported they were unlikely to contract an STI, regardless of type of partner, when not drinking; however, the distribution was more evenly split across likelihood for scenarios while drinking. Over three-quarters of participants reported they have not worried about an STI as an alcohol-related sexual consequence. The average number of condom-related protective behavioral strategies was 3.13 (out of 6 items), and average number of non-condom-related protective behavioral strategies was 2.82 (out of 8 items). Most participants reported using a condom, having casual sex, having sex with alcohol, and having casual sex with alcohol in the last 3 months.
Table 1.
Descriptive Characteristics among 18–20 Year Old Young Adults (n=817)
| % Tested Chlamydia | % Tested Gonorrhea | % Tested HIV | Total | |
|---|---|---|---|---|
| 210 (24.1) | 210 (24.1) | 185 (21.2) | ||
| Demographics | ||||
| Sex | ** | ** | ||
| Male | 66 (31.4) | 69 (32.9) | 81 (43.8) | 398 (45.7) |
| Female | 144 (68.6) | 141 (67.1) | 104 (56.2) | 473 (54.3) |
| Relationship Status | ** | ** | ** | |
| Single, Not Dating | 138 (65.7) | 141 (67.1) | 123 (66.5) | 646 (74.2) |
| Dating, Not Serious | 72 (34.3) | 69 (32.9) | 62 (33.5) | 225 (25.8) |
| Alcohol Use | ** | ** | ** | |
| # Drinks per Week | 12.49 (9.54) | 12.54 (9.59) | 13.07 (10.44) | 13.28 (10.45) |
| Perceived Vulnerability for STI | ||||
| Casual Partner While Not Drinking | ||||
| Unlikely | 107 (51.0) | 109 (51.9) | 102 (55.1) | 495 (56.8) |
| Neither | 54 (25.7) | 52 (24.8) | 44 (23.8) | 217 (24.9) |
| Likely | 49 (23.3) | 49 (23.3) | 39 (21.1) | 159 (18.3) |
| Other Partner While Not Drinking | ||||
| Unlikely | 94 (44.8) | 95 (45.2) | 85 (46.0) | 405 (46.5) |
| Neither | 48 (22.9) | 46 (21.9) | 40 (21.6) | 216 (24.8) |
| Likely | 68 (32.4) | 69 (32.9) | 60 (32.4) | 250 (28.7) |
| Casual Partner While Drinking | ** | * | ||
| Unlikely | 57 (27.1) | 60 (28.6) | 54 (29.2) | 273 (31.3) |
| Neither | 54 (25.7) | 52 (24.8) | 49 (26.5) | 266 (30.5) |
| Likely | 99 (47.1) | 98 (46.7) | 82 (44.3) | 332 (38.1) |
| Other Partner While Drinking | * | * | ||
| Unlikely | 41 (19.5) | 44 (21.0) | 40 (21.6) | 220 (25.3) |
| Neither | 56 (26.7) | 56 (25.2) | 47 (25.4) | 246 (28.2) |
| Likely | 113 (53.8) | 113 (53.8) | 98 (53.0) | 405 (46.5) |
| Alcohol-Related Sexual Consequence | ||||
| Had sex and worried about STI | ** | ** | ** | |
| Yes | 50 (23.8) | 48 (22.9) | 38 (20.5) | 115 (13.2) |
| No | 160 (76.2) | 162 (77.1) | 147 (79.5) | 756 (86.8) |
| Protective Behavioral Strategies | ** | ** | ** | |
| Condom Related | 3.28 (1.20) | 3.29 (1.21) | 3.39 (1.19) | 3.13 (1.15) |
| Non-Condom Related | 3.12 (0.95) | 3.11 (0.95) | 3.08 (0.92) | 2.82 (0.94) |
| Sex in the Last 3 Months | ||||
| Condom Use | ||||
| Yes | 152 (72.4) | 152 (72.4) | 134 (72.4) | 599 (68.8) |
| No | 58 (27.6) | 58 (27.6) | 51 (27.6) | 272 (31.2) |
| Casual Sex | * | |||
| Yes | 192 (91.4) | 192 (91.4) | 172 (93.0) | 763 (87.6) |
| No | 18 (8.6) | 18 (8.6) | 13 (7.0) | 108 (12.4) |
| Any Sex with Alcohol | ||||
| Yes | 165 (78.6) | 165 (78.6) | 139 (75.1) | 669 (76.8) |
| No | 45 (21.4) | 45 (21.4) | 46 (24.9) | 202 (23.2) |
| Casual Sex with Alcohol | ||||
| Yes | 150 (71.4) | 151 (71.9) | 131 (70.8) | 596 (68.4) |
| No | 60 (28.6) | 59 (28.1) | 54 (29.2) | 275 (31.6) |
Indicates statistically significant bivariate test, p<0.05
Indicates statistically significant bivariate test, p<0.01
For STI testing, 24% reported chlamydia testing, 24% reported gonorrhea testing, and 21% reported HIV testing in the last 3 months. Table 1 describes the bivariate differences for persons tested and not tested for chlamydia, gonorrhea, and HIV, respectively, for each predictor variable.
Chlamydia Testing
Women were more likely to be tested for chlamydia in the last three months (OR=1.67, 95%CI 1.13, 2.46) compared to men (Table 2). Relationship status, alcohol use, and perceived vulnerability for an STI in drinking and non-drinking scenarios were not significantly associated with chlamydia testing. Participants who reporting being worried about an STI after a sexual encounter were more than two times the odds of being tested for chlamydia (OR=2.77, 95%CI 1.77, 4.35) compared to persons who were not worried. Similarly, persons who reported having casual sex in the last three months were also more likely to be tested for chlamydia (OR=2.58, 95%CI 1.18, 5.66). Other types of sexual encounters (e.g., sex with alcohol or condom use) were not significantly associated with chlamydia testing. While condom-related protective behavioral strategies were not significantly associated with chlamydia testing, reported use of non-condom related strategies increased the odds of chlamydia testing (OR=1.45, 95%CI 1.18, 1.79).
Table 2.
Adjusted Models Correlates of STI Testing among 18–20 Year Old Young Adults (n=817)
| Chlamydia | Gonorrhea | HIV | |
|---|---|---|---|
| Demographics | |||
| Sex | |||
| Male | Referent | Referent | Referent |
| Female | 1.67 (1.13, 2.46) | 1.55 (1.05, 2.28) | 0.85 (0.58, 1.26) |
| Relationship Status | |||
| Single, Not Dating | Referent | Referent | Referent |
| Dating, Not Serious | 1.38 (0.96, 1.99) | 1.26 (0.87, 1.81) | 1.41 (0.97, 2.05) |
| Alcohol Use | |||
| # Drinks per Week | 0.99 (0.97, 1.01) | 0.99 (0.97, 1.01) | 1.00 (0.98, 1.01) |
| Perceived Vulnerability | |||
| Casual Partner While Not Drinking | |||
| Unlikely | Referent | Referent | Referent |
| Neither | 1.53 (0.92, 2.54) | 1.44 (0.87, 2.39) | 1.12 (0.67, 1.87) |
| Likely | 1.32 (0.73, 2.37) | 1.30 (0.73, 2.32) | 1.06 (0.58, 1.93) |
| Other Partner While Not Drinking | |||
| Unlikely | Referent | Referent | Referent |
| Neither | 0.63 (0.36, 1.08) | 0.65 (0.37, 1.12) | 0.73 (0.42, 1.28) |
| Likely | 0.69 (0.39, 1.20) | 0.75 (0.43, 1.30) | 0.86 (0.50, 1.51) |
| Casual Partner While Drinking | |||
| Unlikely | Referent | Referent | Referent |
| Neither | 0.55 (0.29, 1.02) | 0.55 (0.29, 1.02) | 0.73 (0.39, 1.36) |
| Likely | 0.91 (0.47, 1.75) | 0.86 (0.45, 1.63) | 0.86 (0.45, 1.64) |
| Other Partner While Drinking | |||
| Unlikely | Referent | Referent | Referent |
| Neither | 1.85 (0.94, 3.63) | 1.57 (0.81, 3.07) | 1.38 (0.70, 2.73) |
| Likely | 1.51 (0.74, 3.06) | 1.44 (0.72, 2.88) | 1.50 (0.74, 3.01) |
| Alcohol-Related Sexual Consequence | |||
| Worried about STI (Yes vs. No) | 2.77 (1.77, 4.35) | 2.53 (1.61, 3.97) | 2.07 (1.30, 3.31) |
| Protective Behavioral Strategies | |||
| Condom Related | 1.05 (0.88, 1.26) | 1.07 (0.89, 1.28) | 1.13 (0.94, 1.36) |
| Non-Condom Related | 1.45 (1.18, 1.79) | 1.44 (1.17, 1.78) | 1.43 (1.16, 1.78) |
| Sex in the Last 3 Months | |||
| Condom Use (Yes vs. No) | 1.10 (0.73, 1.66) | 1.08 (0.72, 1.63) | 0.98 (0.65, 1.49) |
| Casual Sex (Yes vs. No) | 2.58 (1.18, 5.66) | 2.33 (1.07, 5.06) | 2.39 (1.06, 5.36) |
| Any Sex with Alcohol (Yes vs. No) | 1.32 (0.66, 2.66) | 1.21 (0.61, 2.41) | 0.88 (0.44, 1.77) |
| Casual Sex with Alcohol (Yes vs. No) | 0.66 (0.32, 1.38) | 0.77 (0.37, 1.60) | 0.93 (0.45, 1.96) |
All models adjusted for gender, relationship status, alcohol use, perceived vulnerability, alcohol-related sexual consequence, protective behavioral strategies, and sex in the last three months.
Bold values indicate statistical significance, p<0.05
Gonorrhea Testing
Women were more likely to be tested for gonorrhea in the last three months (OR=1.55, 95%CI 1.05, 2.28) compared to men. Similar to chlamydia testing, relationship status, alcohol use, and perceived vulnerability for an STI in drinking and non-drinking scenarios were not significantly associated with gonorrhea testing. Those participants who reporting being worried about an STI after sex were more than two times the odds of being tested for gonorrhea (OR=2.53, 95%CI 1.61, 3.97) compared to persons who were not worried. Similarly, persons who reported having casual sex in the last three months were also more likely to be tested for gonorrhea (OR=2.33, 95%CI 1.07, 5.06). Persons who reported use of non-condom related strategies had increased the odds of gonorrhea testing (OR=1.45, 95%CI 1.18, 1.79), while condom-related strategies were not significantly associated with gonorrhea testing.
HIV Testing
Gender, relationship status, alcohol use, and perceived vulnerability were not significantly associated with HIV testing. Additionally, condom-related protective behavioral strategies and condom use during sex in the last three months were not significantly associated with HIV testing. Sex with alcohol use was also not significantly associated. Participants who reported worrying about an STI after a sexual encounter were more likely to report HIV testing (OR=2.07, 95%CI 1.30, 3.31). With each additional non-condom-related protective behavioral strategy reported, the odds of HIV testing increased (OR=1.43, 95%CI 1.16, 1.78). Finally, persons who had casual sex in the last three months were more likely to be tested for HIV (OR=2.39, 95%CI 1.06, 5.36).
Non-Condom Related Protective Behavioral Strategies
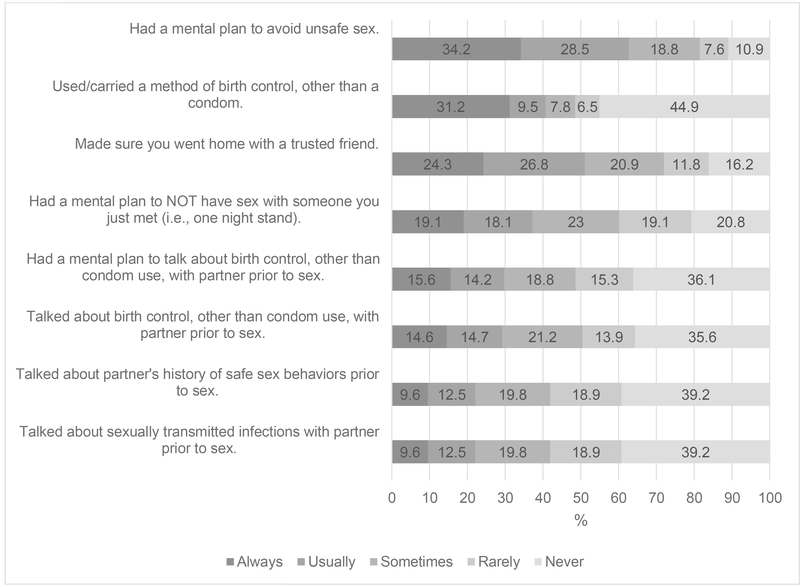
Due to significant association of non-condom related protective behavioral strategies across STI testing types, the item responses to the scale were assessed (Figure 1). The most common strategies always used by participants was had a mental plan to avoid unsafe sex (34%) and used/carried a method of birth control, other than a condom (31%). In contrast, the strategies most frequently cited as never used was used/carried a method of birth control, other than a condom (45%), talked about partner’s history of safe sex behaviors prior to sex (39%), and talked about STIs with partner prior to sex (39%). Note, that used/carried a method of birth control, other than a condom was significantly different (p<0.001) for women and men. All non-condom related protective behavioral strategies were significantly, positively, correlated with one another. A strong correlation was observed for talked about birth control other than condom use with partner prior to sex and had a mental plan to talk about birth control, other than condom use, with partner prior to sex (r=0.76). Similarly, a strong correlation was observed for talked about partner’s history of safe sex behaviors prior to sex and talked about sexually transmitted infections with partner prior to sex (r=0.78).
Fig 1.
Proportion of Responses to Non-Condom Related Protective Behavioral Strategies (N=871)
Discussion
This study examined the alcohol and sex cognition correlates of chlamydia, gonorrhea, and HIV testing among 18- to 20-year-old young adults. Approximately a quarter of young adults reported testing for chlamydia and gonorrhea in the last three months, and one out of five reported testing for HIV in the past three months. The main correlates identified for STI testing were related to being female, alcohol-related sexual consequences, non-condom protective behavioral strategies, and having a casual sexual partner.
Since condoms are one of the primary prevention strategies for STI prevention, we hypothesized that increased use of condom PBS would be associated with higher STI testing behavior. Essentially, persons engaged in one type of prevention would be more likely to be engage in other prevention behaviors. However, in this study, condom-related PBS were not associated with STI testing; instead, non-condom related PBS were significantly associated. The two most common non-condom PBS were having a mental plan to avoid unsafe sex and using a method of birth control other than a condom; yet, other birth control methods cannot prevent STIs. As a result, young adults may be engaging in STI testing as a non-condom related PBS for STI prevention in the absence of using condom-related PBS. These same persons may also be engaging in other reproductive prevention behaviors, such as the use of contraception for the prevention of pregnancy. Future research may consider STI testing a type of sex-related protective behavioral strategy for the prevention of STIs and how it may relate to other sexual and reproductive health behaviors, particularly among a high-risk age group of young adults. Additional studies are needed to test the validity and reliability of adding STI testing as a protective behavioral skill.
Furthermore, we hypothesized that perceived vulnerability for STIs would be associated with increased STI testing. While cognitions related to hypothetical scenarios for perceived vulnerability were not significantly associated, immediate perceived vulnerability with an alcohol-related sexual consequence was significantly associated with STI testing. The finding that perceiving oneself to be at risk for an STI is more strongly associated with STI testing over one’s reaction to a hypothetical scenario is not surprising given the differences in level of specificity; perceived risk from one’s experiences should predict STI testing over perceived risk from hypothetical scenarios. Moreover, these findings suggest that interventions promoting STI testing might be timed for when individuals directly perceive themselves to be vulnerable to contracting a STI after a given event. For instance, a STI testing message might be triggered after a risk event as part of a text message intervention aiming to reduce sexual risk taking. Text message interventions are emerging with the aim to reduce alcohol and sexual risk taking; however, most sexual risk taking interventions focus on increasing condom use rather than increasing STI testing (i.e., (Mastroleo et al. 2019; Monti et al. 2016). Alternative digital engagement through social media or apps may be beneficial options for providing real-time feedback to this age group in risk taking scenarios. Future studies should focus on assessing technology and intervention preferences for program design related to sexual health and alcohol use among young adults.
In regards to alcohol use, findings from the current study indicate that typical drinking behavior is not associated with STI testing, which suggests that engaging in a high-risk health behavior associated with sexual decision-making does not relate to taking a protective action, such as STI testing. As such, drinking behavior may be too distal from STI testing and may not be the optimal target in an intervention if the aim is to increase STI testing. However, findings did suggest that engaging in casual sex does associate with STI testing, which parallels previous studies finding that number of sex partners is associated with STI screening (Griner et al. 2020; Moore, 2013). Given these findings and high rates of hooking up behaviors in this population, future research examining the STI testing decision-making process following casual sex behavior may identify salient patterns and partner characteristics that influence STI testing. One example of this may be knowledge of a partner’s sexual history. In this study, 39% of participants reported that they did not talk to their partners about previous sexual behaviors or about STIs. Because having less knowledge of a partner’s sexual history may prompt individuals to test for STIs, future qualitative research focused on non-condom related PBS in this population may identify specific behavioral strategies to be targeted in intervention development. Moreover, since U.S. Preventive Screening Task Force recommends more frequent STI screening among those with new or multiple sexual partners, more readily available methods of screening, such as self-sampling methods, may be an option for young adults. Young adult women report the availability of self-screening methods meets their needs of screening on their own time, rather than scheduling provider appointments (Griner et al. 2020).
Additionally, we hypothesized that gender would be associated with increased STI testing, particularly among women compared to men. These findings indicate women had higher odds than men for receipt of chlamydia and gonorrhea testing in the past three months, consistent with previous literature indicating that women are more likely to seek STI screening and other sexual and reproductive healthcare than are men (Bersamin et al. 2017; Moore 2013). However, there was no significant association between gender and receipt of HIV testing in this study. Previous research has noted that among those with high risk sexual behaviors, women had higher rates of chlamydia and gonorrhea screening, but conversely, men were significantly more likely to receive HIV testing than women (Tao and Irwin 2008). One potential factor influencing the higher rates of chlamydia and gonorrhea testing in women may be the recommendations from professional organizations to annually screen for these STIs in sexually active women under age 25 and more often in those at high risk, such as those with new partners, those more than one partner in the past 12 months, or those with sex partners with concurrent partners (American Academy of Pediatrics Committee on Adolescence and Society for Adolescent Health and Medicine 2014; United States Preventive Services Task Force 2019a; Workowski and Bolan 2015). However, similar recommendations to screen for chlamydia and gonorrhea do not exist for men in this age group, and therefore may not be proactively recommended by healthcare providers. Differences in STI testing by gender may also exist due to differential patterns in reproductive healthcare recommended for women, including contraceptive counseling and cervical cancer screening. The equivalent rates of HIV testing among men and women in this analysis may be influenced by the recommendations for testing, as HIV testing is recommended at least once for those age 15 to 65 years old and more often among those with high risk sexual practices, regardless of gender (United States Preventive Services Task Force 2019b).
Even with the recommendations for testing, less than a quarter of participants in this study received chlamydia and gonorrhea testing and just 21% received HIV testing. These low rates of testing in the young adult age group have been associated with concerns about confidentiality and privacy barriers to seeking screening (Bersamin et al. 2017; Doll et al. 2018; Fielder et al. 2013; Peralta et al. 2007). These concerns suggest a need to explore alternative methods to traditional, in-clinic, provider-recommended testing for chlamydia, gonorrhea, and HIV that are acceptable to this age group. These approaches may include oral and rapid HIV testing (Estem et al. 2016; Peralta et al. 2007) and self-collected sampling methods for chlamydia and gonorrhea testing (Eaton et al. 2016; Gaydos 2018; Paudyal et al. 2015; Wiesenfeld et al. 2000). Additional studies are needed to examine the acceptability of these methods, specifically among men, and how to promote use among this high-risk age group.
These findings are not withstanding limitations. First, STI testing was self-reported by participants. Given the similarities in the proportions of young adults reporting the three types of STI testing, participants may conflate the type of STIs that are being tested at a visit. A study conducted among an urban sample of patients at an STI clinic found most patients incorrectly identified the STIs they were tested for (Goodman and Black 2018). Thus, misclassification of the outcomes may have occurred. Secondly, the cognitions and reports of STI testing were collected at the same time point by participants, which limited any causal inferences from these findings.
STIs are common among young adults, and STI testing is a key prevention strategy for this public health issue. Among a national, young adult sample, we found that key cognitions related to STI testing were immediate perceived vulnerability and non-condom protective behavioral strategies. Future studies may consider these correlates as potential intervention points for promoting STI testing among young adults.
Acknowledgments
Funding: Data collection and manuscript preparation were supported by National Institute on Alcohol Abuse and Alcoholism Grants R01AA021379 awarded to Melissa A. Lewis.
Footnotes
Conflict of Interest: The authors have no conflicts to disclose.
Compliance with Ethical Standards:
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the university’s institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References Cited
- Abbey A (2002). Alcohol-related sexual assault: a common problem among college students. J Stud Alcohol Suppl(14), 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Adolescence and Society for Adolescent Health and Medicine (2014). Screening for nonviral sexually transmitted infections in adolescents and young adults. Pediatrics, 034, e302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, & Satterwhite CL (2011). A paradox: Overscreening of older women for chlamydia while too few younger women are being tested. Sexually transmitted diseases, 38(2), 130–132. [DOI] [PubMed] [Google Scholar]
- Bersamin M, Fisher DA, Marcell AV, & Finan LJ (2017). Reproductive health services: Barriers to use among college students. Journal of community health, 42, 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2006). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep, 55(14), 1–17. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2018). Sexually Transmitted Disease Surveillance, 2017. U.S. Department of Health and Human Services. [Google Scholar]
- Claxton SE, & van Dulmen MH (2013). Casual sexual relationships and experiences in emerging adulthood. Emerging Adulthood, 1(2), 138–150. [Google Scholar]
- Cooper ML (2002). Alcohol use and risky sexual behavior among college students and youth: evaluating the evidence. J Stud Alcohol Suppl(14), 101–117. [DOI] [PubMed] [Google Scholar]
- Copen CE (2017). Condom Use During Sexual Intercourse Among Women and Men Aged 15–44 in the United States: 2011–2015 National Survey of Family Growth. Natl Health Stat Report(105), 1–18. [PubMed] [Google Scholar]
- Davis KC, Stappenbeck CA, Norris J, George WH, Jacques-Tiura AJ, Schraufnagel TJ, et al. (2014). Young men’s condom use resistance tactics: A latent profile analysis. The Journal of Sex Research, 51(4), 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser RO, & O’Neill N (2013). Identifying and understanding barriers to sexually transmissible infection testing among young people. Sexual Health, 10(6), 553–558. [DOI] [PubMed] [Google Scholar]
- DiNenno EA, Prejean J, Irwin K, Delaney KP, Bowles K, Martin T, et al. (2017). Recommendations for HIV Screening of Gay, Bisexual, and Other Men Who Have Sex with Men - United States, 2017. MMWR Morb Mortal Wkly Rep, 66(31), 830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll M, Fortenberry D, Roseland D, McAuliff K, Wilson CM, & Boyer CB (2018). Linking HIV-Negative Youth to Prevention Services in 12 U.S. Cities: Barriers and Facilitators to Implementing the HIV Prevention Continuum. Journal of Adolescent Health, 62(4), 424–433. [DOI] [PubMed] [Google Scholar]
- Eaton S, Biggerstaff D, Pink J, Petrou S, Osipenko L, Gibbs J, et al. (2016). Factors affecting young people’s preferences for emerging technologies for chlamydia testing and treatment: a discrete choice experiment in England. The Lancet, 388(2), S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estem KS, Catania J, & Klausner JD (2016). HIV Self-Testing: a Review of Current Implementation and Fidelity. Current HIV/AIDS Reports, 13(2), 107–115. [DOI] [PubMed] [Google Scholar]
- Febo-Vazquez I, Casey CE, & Daugherty J (2018). Main Reasons for Never Testing for HIV Among Women and Men Aged 15–44 in the United States, 2011–2015. National Health Statistics Reports(107). [PubMed] [Google Scholar]
- Fielder RL, Carey KB, & Carey MP (2013). Acceptability of sexually transmitted infection testing using self-collected vaginal swabs among college women. Journal of American College Health, 61, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielder RL, Walsh JL, Carey KB, & Carey MP (2014). Sexual hookups and adverse health outcomes: a longitudinal study of first-year college women. The Journal of Sex Research, 51(2), 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydos CA (2018). Let’s Take A “Selfie”: Self-Collected Sample for Sexually Transmitted Infections. Sexually transmitted diseases, 45, 278–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George WH, & Stoner SA (2000). Understanding acute alcohol effects on sexual behavior. Annu Rev Sex Res, 11, 92–124. [PubMed] [Google Scholar]
- Gerrard M, Gibbons FX, & Bushman BJ (1996). Relation between perceived vulnerability to HIV and precautionary sexual behavior. Psychological Bulletin, 119(3), 390. [DOI] [PubMed] [Google Scholar]
- Goodman K, & Black CM (2018). Patient knowledge of STI testing in an urban clinic. Journal of the American Academy of PAs, 31(4), 36–41. [DOI] [PubMed] [Google Scholar]
- Gottlieb SL, Berman SM, & Low N (2010). Screening and treatment to prevent sequelae in women with Chlamydia trachomatis genital infection: How much do we know?. The Journal of infectious diseases, 201(S2), 156–167. [DOI] [PubMed] [Google Scholar]
- Griner SB, Beckstead JW, Vamos CA, Puccio JA, Perrin K, & Daley EM (2020). Sexually Transmitted Infection Screening among College Women by Race/Ethnicity and Number of Male Sex Partners: National Survey of Family Growth, 2013–2015. International Journal of Sexual Health, 1–10. [Google Scholar]
- Hingson R, Heeren T, Winter M, & Wechsler H (2005). Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: changes from 1998 to 2001. Annu Rev Public Health, 26, 259–279. [DOI] [PubMed] [Google Scholar]
- Hoover KW, Leichliter JS, Torrone EA, Loosier PS, Gift TL, & Tao G (2014). Chlamydia screening among females aged 15–21 years--multiple data sources, United States, 1999–2010. MMWR Suppl, 63(2), 80–88. [PubMed] [Google Scholar]
- Howells NL, & Orcutt HK (2014). Diary study of sexual risk taking, alcohol use, and strategies for reducing negative affect in female college students. J Stud Alcohol Drugs, 75(3), 399–403. [DOI] [PubMed] [Google Scholar]
- James S, Reddy S, Taylor M, & Jinabhai CC (2004). Young people, HIV/AIDS/STIs and sexuality in South Africa: the gap between awareness and behaviour. Acta Paediatrica, 93(2), 264–269. [PubMed] [Google Scholar]
- Kraut-Becher JR, & Aral SO (2003). Gap length: an important factor in sexually transmitted disease transmission. Sexually transmitted diseases, 30(3), 221–225. [DOI] [PubMed] [Google Scholar]
- Krebs CP, Lindquist CH, Warner TD, Fisher BS, & Martin SL (2009). College women’s experiences with physically forced, alcohol- or other drug-enabled, and drug-facilitated sexual assault before and since entering college. J Am Coll Health, 57(6), 639–647. [DOI] [PubMed] [Google Scholar]
- Lefevre ML (2014). Screening for Chlamydia and Gonorrhea: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine, 161(12), 902–910. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Atkins DC, Blayney JA, Dent DV, & Kaysen DL (2013). What Is Hooking Up? Examining Definitions of Hooking Up in Relation to Behavior and Normative Perceptions. The Journal of Sex Research, 50(8), 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MA, Granato H, Blayney JA, Lostutter TW, & Kilmer JR (2012). Predictors of hooking up sexual behaviors and emotional reactions among U.S. college students. Arch Sex Behav, 41(5), 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MA, Litt DM, Cronce JM, Blayney JA, & Gilmore AK (2014). Underestimating protection and overestimating risk: examining descriptive normative perceptions and their association with drinking and sexual behaviors. J Sex Res, 51(1), 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MA, Logan DE, & Neighbors C (2009). Examining the Role of Gender in the Relationship Between Use of Condom-Related Protective Behavioral Strategies when Drinking and Alcohol-Related Sexual Behavior. Sex Roles, 61(9), 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MA, Rhew IC, Fairlie AM, Swanson A, Anderson J, & Kaysen D (2019). Evaluating Personalized Feedback Intervention Framing with a Randomized Controlled Trial to Reduce Young Adult Alcohol-Related Sexual Risk Taking. Prev Sci, 20(3), 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Smith HA, Okpo EA, & Bull ER (2018). Exploring psychosocial predictors of STI testing in University students. [journal article]. BMC Public Health, 18(1), 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroleo NR, Celio MA, Barnett NP, Colby SM, Kahler CW, Operario D, et al. (2019). Feasibility and Acceptability of a Motivational Intervention Combined with Text Messaging for Alcohol and Sex Risk Reduction with Emergency Department Patients: A Pilot Trial. Addict Res Theory, 27(2), 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, & Shafer MB (2008). Chlamydia trachomatis In L. S. Neinstein, C. M. Gordon, D. K. Katzman, D. S. Rosen, & E. R. Woods (Eds.), Adolescent Health Care: A Practical Guide (Fifth Edit ed, pp. 805–818). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Monti PM, Mastroleo NR, Barnett NP, Colby SM, Kahler CW, & Operario D (2016). Brief motivational intervention to reduce alcohol and HIV/sexual risk behavior in emergency department patients: A randomized controlled trial. J Consult Clin Psychol, 84(7), 580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E (2013). Human Immunodeficiency Virus and chlamydia/gonorrhea testing among heterosexual college students: Who is getting tested and why do some not? Journal of American College Health, 61, 196–202. [DOI] [PubMed] [Google Scholar]
- National Committee for Quality Assurance (2016). Chlamydia Screening in Women: The HEDIS Measure. Washington, DC. [Google Scholar]
- Owusu-Edusei K, Chesson HW, & Gift TL (2013). The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sexually transmitted diseases, 40, 197–201. [DOI] [PubMed] [Google Scholar]
- Paltiel AD, Weinstein MC, Kimmel AD, Seage GR, Losina E, Zhang H, et al. (2005). Expanded Screening for HIV in the United States — An Analysis of Cost-Effectiveness. New England Journal of Medicine, 352, 586–595. [DOI] [PubMed] [Google Scholar]
- Patrick ME, & Maggs JL (2009). Does drinking lead to sex? Daily alcohol-sex behaviors and expectancies among college students. Psychol Addict Behav, 23(3), 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudyal P, Llewellyn C, Lau J, Mahmud M, & Smith H (2015). Obtaining Self-Samples to Diagnose Curable Sexually Transmitted Infections: A Systematic Review of Patients’ Experiences. PLoS One, 10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta L, Griffin Deeds J, Hipszer S, & Ghalib K (2007). Barriers and Facilitators to Adolescent HIV Testing. AIDS patient care and STDs, 21(6). [DOI] [PubMed] [Google Scholar]
- Rehm J, Shield KD, Joharchi N, & Shuper PA (2012). Alcohol consumption and the intention to engage in unprotected sex: systematic review and meta-analysis of experimental studies. Addiction, 107(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, & Ocfemia MC (2013). Sexually transmitted infections among US women and men. Sexually transmitted diseases, 40, 187–193. [DOI] [PubMed] [Google Scholar]
- Sheeran P, & Taylor S (1999). Predicting Intentions to Use Condoms: A Meta-Analysis and Comparison of the Theories of Reasoned Action and Planned Behavior 1. Journal of Applied Social Psychology, 29(8), 1624–1675. [Google Scholar]
- Steele CM, & Josephs RA (1990). Alcohol myopia. Its prized and dangerous effects. Am Psychol, 45(8), 921–933. [DOI] [PubMed] [Google Scholar]
- Tao G, & Irwin KL (2008). Receipt of HIV and STD testing services during routine general medical or gynecological examinations: variations by patient sexual risk behaviors. Sexually transmitted diseases, 35(2), 167–171. [DOI] [PubMed] [Google Scholar]
- Testa M, & Hoffman JH (2012). Naturally occurring changes in women’s drinking from high school to college and implications for sexual victimization. J Stud Alcohol Drugs, 73(1), 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend JM, Kambouropoulos N, Griffin A, Hunt FJ, & Milani RM (2014). Binge drinking, reflection impulsivity, and unplanned sexual behavior: impaired decision-making in young social drinkers. Alcohol Clin Exp Res, 38(4), 1143–1150. [DOI] [PubMed] [Google Scholar]
- United States Preventive Services Task Force (2019a). Final Update Summary: Chlamydia and Gonorrhea Screening. [Google Scholar]
- United States Preventive Services Task Force (2019b). Screening for HIV Infection US Preventive Services Task Force Recommendation Statement. Journal of the American Medical Association. [Google Scholar]
- Walensky RP, Freedberg KA, Weinstein MC, & Paltiel AD (2007). Cost-Effectiveness of HIV Testing and Treatment in the United States. Clinical Infectious Diseases, 45(S4), S248–S254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner R, Lewis MA, Davis KC, Neilson EC, & Norris J (2018). Tactics young women use to resist condom use when a partner wants to use a condom. The Journal of Sex Research, 55(7), 817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld HC, Lowry DL, Heine RP, Krohn MA, Bittner H, Kellinger K, et al. (2000). Self-collection of vaginal swabs for the detection of Chlamydia, Gonorrhea, and Trichomoniasis: Opportunity to encourage sexually transmitted disease testing among adolescents. Sexually transmitted diseases, 28(6), 321–325. [DOI] [PubMed] [Google Scholar]
- Wolfers ME, Kok G, Mackenbach JP, & de Zwart O (2010). Correlates of STI testing among vocational school students in the Netherlands. [journal article]. BMC Public Health, 10(1), 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workowski KA, & Bolan GA (2015). Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep, 64(3). [PubMed] [Google Scholar]