Abstract
Purpose
To evaluate transdermal diclofenac in terms of analgesic efficacy, safety, compliance and cost-effectiveness and to compare it with oral tablets and intramuscular (IM) injections following surgical removal of impacted mandibular third molars.
Subjects and Methods
A prospective, single-centre, multi-arm parallel, randomized study on subjects undergoing extraction of impacted mandibular third molars was conducted between January 2016 and December 2017. The study included 90 participants, 30 in each group. Participants received the standard once daily (OD) dosages of diclofenac in each group for three post-operative days and were advised to consume paracetamol 500 mg as rescue analgesics if the pain was not alleviated. Outcome measures such as demographics, duration of surgery, post-operative pain, the number of rescue analgesics taken, adverse drug reactions experienced and overall global assessment for three post-operative days were recorded by the participants on a questionnaire.
Results
Transdermal and oral forms achieved similar analgesia on all 3 days. Injectable diclofenac had significantly better pain control on the second and third post-operative days compared to tablets and on the third day compared to transdermal diclofenac. A higher number of rescue analgesics was consumed in oral group on day 1. Gastritis and vomiting were seen in 36.66% and 10% cases, respectively, in oral group. 100% of those in IM group had pain on injection. 6.6% complained of dry skin due to patch, while 3.33% had rash and pruritus. Transdermal group had better overall global assessment by patients with 16.67%, 46.67% and 20% participants reporting excellent, very good and good pain control, respectively. The cost in INR was maximum for the transdermal group.
Conclusion
Transdermal diclofenac is an excellent alternative to oral and parenteral routes of drug administration in oral surgical procedures with adequate analgesic efficacy, good compliance and fewer side effects.
Keywords: Transdermal, Oral, Injectable diclofenac, Oral surgery
Introduction
Striving to achieve adequate post-operative analgesia that works conjointly with patient compliance has been an unremitting task for surgeons. Diclofenac is a non-steroidal anti-inflammatory agent (NSAID) frequently prescribed for post-operative pain relief and available in various formulations like injections, tablets, suppositories, gel preparations, transdermal patch and suspension.
Of these, the transdermal delivery system has gained momentum over the last decade. Initially introduced to circumvent the gastric side effects of the oral and injectable forms, it has emerged as a patient-friendly alternative to the latter. It can overcome the pharmacokinetic barriers of oral and parenteral routes by bypassing the hepatic first-pass metabolism, maintaining a steady-state plasma concentration over a prolonged time and minimizing inter- and intrapatient variability [1]. Its reduced dosage frequency compared to the oral route, non-invasiveness, ease of administration and termination also results in better patient compliance [2]. Besides, patients are delivered from the unnecessary pain and phobia associated with injectable forms.
Although there have been several studies on the effect of transdermal diclofenac usage in fields like acute muscle spasm [3], sports injuries [4], laparoscopic surgeries [5], its impact on pain control in oral surgical procedures is less known, making surgeons depend on the more familiar oral and parenteral routes. Hence, the purpose of this study was to highlight the effectiveness and compliance of diclofenac in a transdermal mode in minor oral surgeries, with impacted mandibular third molar extraction as a standard model.
This study aimed to evaluate transdermal diclofenac in terms of analgesic efficacy, safety, compliance and cost-effectiveness and to compare the same with that of oral tablets and intramuscular injections following surgical removal of impacted mandibular third molars.
It was hypothesized that diclofenac transdermal patch would be as effective as an oral tablet or intramuscular injection in reducing pain following removal of impacted third molars.
Subjects and Methods
This was a prospective, single-centre, multi-arm parallel, randomized study of subjects undergoing extraction of impacted mandibular third molars conducted between January 2016 and December 2017, and approved by the Institutional Ethics Committee.
Adult participants between 18 and 43 years of age, with ASA physical status I & II, who had impacted mandibular third molar diagnosed with a clinical and radiological examination, were included in this study after obtaining informed consent. Participants with blood coagulation disorders, peptic ulcer or gastritis, skin disorders, known allergy to NSAIDs or polyvinylpyrrolidone (PVP) or ethyl chloride (EC), history of analgesic or alcohol consumption in the 24 h preceding the surgery were excluded from the study. Vulnerable subjects like pregnant and lactating women, mentally challenged or immunocompromised patients were also excluded from this study.
Sample Size Determination and Randomization
The sample size was calculated using standard alpha and beta errors as 0.05 and 0.2, respectively. Based on this, the sample was adjusted to 90 participants with an equal allocation ratio of 1:1:1 in the three groups—oral (Diclo tab), parenteral (Diclo inj) and transdermal (Diclo patch). Each participant was assigned to one of the groups following simple randomization procedures. The allocation sequence was generated using the programme MATLAB. Each treatment group was attributed a number, i.e. 1, 2 and 3 for oral, parenteral and transdermal, respectively, and a vector of size 90 was created containing only these 3 numbers. A random permutation of these 3 numbers with each number appearing 30 times was generated from this vector using MATLAB. This sequence was concealed from the researcher and participants in sequentially numbered, opaque and sealed envelopes by an investigator with no clinical involvement in the trial.
Intervention
All surgical procedures were performed by a single surgeon using identical protocols. Following 0.2% chlorhexidine mouth rinse, participants were placed in supine position and vitals were recorded. Inferior alveolar and buccal nerve anaesthesia were achieved using 2% lignocaine hydrochloride with 1:200,000 adrenaline. Envelope flaps were raised exposing the buccal cortical plate, distal spur of bone and occlusal surface of third molar teeth. Bone removal and tooth sectioning were done with a straight fissure bur and extracted using straight or Cryer elevator from the sockets. After a complete inspection of sockets and toileting with normal saline, interrupted silk sutures were placed apposing the buccal and lingual mucosa. Saline-soaked gauze packs were placed over the operated area, and the participants were asked to bite the gauze for 30–45 min to achieve haemostasis. The operating time ranged from 15 to 30 min.
Participants were advised to take the standard once daily (OD) dosages of diclofenac in each group for three post-operative days. The oral group took diclofenac 100 mg SR tablet (Voveran SR 75) just before the procedure and once daily thereafter. Diclofenac injection 75 mg (Dixer aqua) was administered intramuscularly to the parenteral group just after completion of the procedure and then once daily. The transdermal group was advised to place a 50-cm [2] patch containing 100 mg of diclofenac diethylamine (Dicloplast) on the arm, 5 h before the procedure, which was changed daily for the next 3 days by the patient, each application on a new hairless skin site such as back, shoulder or upper arm. All patients received paracetamol tablets 500 mg (Aiopar 500) as rescue analgesics with the advice to consume a maximum of 4 tablets per day in case the pain was not alleviated. On discharge, they were given a questionnaire that had to be filled at home for post-operative pain assessment, the number of rescue analgesics taken, adverse drug reactions experienced and overall global assessment for three post-operative days. All participants were recalled after 3 days, and the findings were recorded by the observer.
Outcome Measures
The patient demographics such as age, sex, type of impaction along with the duration of surgery were recorded.
The pain scores were marked by the patients over three consecutive post-operative days using a 100-mm Visual Analogue Scale (VAS) [6]. This was repeated at an interval of 2 h, for 12 h after surgery or till the time at which the patient complained of pain and consumed rescue analgesics. Then, the average VAS score for the day was calculated. The necessity for rescue analgesics and the number of paracetamol 500 mg tablets consumed were recorded.
Various gastrointestinal adverse effects such as abdominal pain, constipation, diarrhoea, dyspepsia, flatulence, melaena, vomiting, nausea; skin reactions like dry skin, pruritus, rash, paraesthesia, vesicle or bulla formation and other effects including pain on injection, dizziness, oedema, headache, halitosis, taste perversion, asthma attack were noted if present.
The patient satisfaction was assessed using the Overall Global Assessment Scale at the end of the third day in the questionnaire by the participant during the study period. The cost of the drugs in INR was recorded for the three groups.
Statistical Analysis
Quantitative variables like age, duration of surgery, pain scores were expressed as mean ± standard deviation and compared using one-way analysis of variance test. Rescue analgesic consumption among the three groups was compared using Kruskal–Wallis test. Categorical variables like gender distribution and overall global assessment by patients were described as frequencies and proportions and compared using Chi-square test. The level of statistical significance was set at 0.05. All statistical analyses were performed using Statistical Package of Social Science, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Table 1 shows the baseline characteristics of the subjects, where no significant differences were found. The analgesic efficacy of the three modes of diclofenac in terms of the VAS score is represented in Tables 2 and 3. The need for rescue analgesics and the number of rescue analgesic consumption are represented in Tables 4 and 5. Figure 1 shows the adverse effects encountered in the three study groups during the study period. The Overall Global Assessment Scale rating given by the participants at the end of the 3 days is shown in Fig. 2. The overall cost incurred in 3 days is shown in Fig. 3.
Table 1.
Baseline characteristics of the participants
| Variables | Group tab | Group inj | Group patch |
|---|---|---|---|
| Gender | |||
| Male | 15 (50%) | 14 (46.7%) | 16 (53.3%) |
| Female | 15 (50%) | 16 (53.3%) | 14 (46.7%) |
| Age (years) | |||
| Mean | 27.50 ± 7.06 | 32.03 ± 9.67 | 25.93 ± 6.37 |
| Range | 17–35 | 18–43 | 17–35 |
| DOS (in min) | 25.93 ± 3.73 | 25.16 ± 5.33 | 23.33 ± 5.14 |
DOS duration of surgery
Table 2.
VAS pain score during follow-up period
| Follow-up period | Group tab | Group inj | Group patch | p value |
|---|---|---|---|---|
| Day 1 | 49.66 ± 19.02 | 41.33 ± 16.76 | 44.33 ± 13.33 | 0.148 |
| Day 2 | 30.66 ± 16.17 | 19.00 ± 15.61 | 28.00 ± 12.70 | 0.008 |
| Day 3 | 15.00 ± 12.52 | 6.33 ± 9.99 | 13.33 ± 10.61 | 0.008 |
VAS Visual Analogue Scale
Table 3.
Intergroup comparison of pain
| Days | Parameters | Group tab | Group inj | Group patch | |||
|---|---|---|---|---|---|---|---|
| Inj diclofenac | Patch diclofenac | Tab diclofenac | Patch diclofenac | Tab diclofenac | Inj diclofenac | ||
|
Day 1 VAS |
Mean difference | 8.33 | 5.33 | − 8.33 | − 3.00 | − 5.33 | 3.00 |
| p value | 0.16 | 0.64 | 0.16 | 1.00 | 0.64 | 1.00 | |
|
Day 2 VAS |
Mean difference | 11.66 | 2.66 | − 11.66 | − 9.00 | − 2.66 | 9.00 |
| p value | 0.01 | 1.00 | 0.01 | 0.06 | 1.00 | 0.06 | |
|
Day 3 VAS |
Mean difference | 8.66 | 1.66 | − 8.66 | − 7.00 | − 1.66 | 7.00 |
| p value | 0.01 | 1.00 | 0.01 | 0.05 | 1.00 | 0.05 | |
Table 4.
Necessity for rescue analgesics
| Rescue analgesic usage | ||||
|---|---|---|---|---|
| Particulars | Diclo tab | Diclo inj | Diclo patch | p value |
| Day 1 n (%) | 25 (83.33) | 21 (70.00) | 23 (76.66) | 0.261 |
| Day 2 n (%) | 19 (63.33) | 8 (26.66) | 19 (63.33) | |
| Day 3 n (%) | 15 (50.00) | 4 (13.33) | 9 (30.00) | |
Table 5.
Rescue analgesics (paracetamol 500 mg) consumed in 3 days
| Particulars | Diclo tab | Diclo inj | Diclo patch | p value |
|---|---|---|---|---|
| Day 1 median (IQR) RA | 2.0 (1.0–2.0) | 1.0 (0.0–1.0) | 1.0 (0.75–2.0) | 0.007 |
| Day 2 median (IQR) RA | 1.0 (0.0–1.0) | 0.0 (0.0–1.0) | 1.0 (0.0–1.0) | 0.003 |
| Day 3 median (IQR) RA | 0.5 (0.0–1.0) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.008 |
Fig. 1.
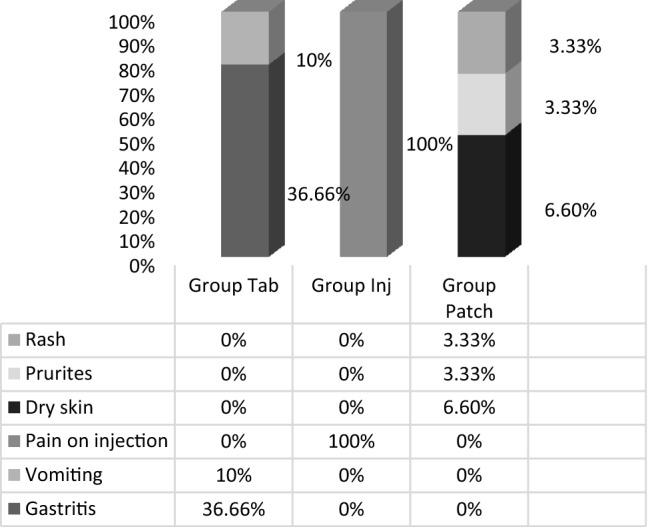
Adverse effects exhibited to diclofenac by the three study groups
Fig. 2.
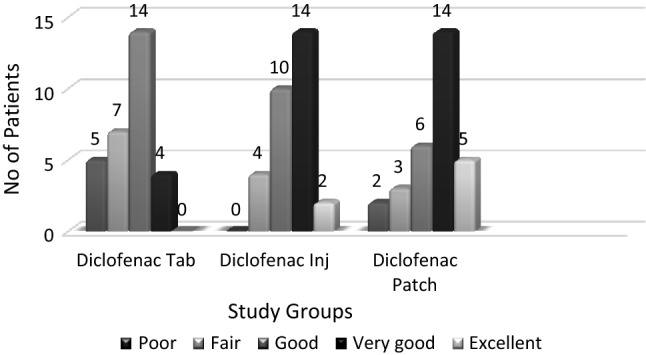
Overall Global Assessment Scale for three study groups
Fig. 3.
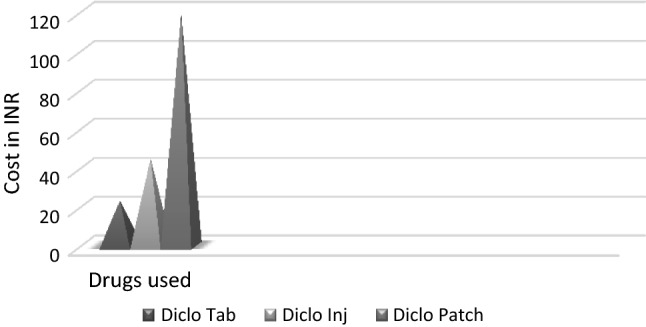
Cost of the three modes of diclofenac used in the study for 3 days
Discussion
Intraoperative analgesia having been studied enough in the literature, the efficient management of post-operative analgesia needs attention, which was the intent of this study. We assessed and compared the analgesic efficacy of diclofenac in three different modes of administration, namely transdermal, oral and parenteral following extraction of impacted mandibular third molar. With third molar surgery as a chosen model, we have been able to perform the study with minimal bias. Trans-alveolar extraction of impacted mandibular third molar serves as an ideal model for any research pertinent to analgesia, because it causes pain due to both incisional and inflammatory injuries, thus eliciting both central and peripheral responses. It results in acute, consistent, moderate to severe pain, appearing about 1 h after wear out of local anaesthesia and continuing for the next 24–48 h, thus making the usage of analgesics mandatory. Besides, the patients are usually healthy and homogeneous, and the surgery is elective and performed on an outpatient basis [7].
We hypothesized that diclofenac transdermal patch would be as effective as oral tablet or injections in reducing pain during post-operative period following removal of impacted mandibular third molar.
NSAIDs act by the inhibition of cyclooxygenases 1 and 2 which are the key enzymes in prostaglandin synthesis. Diclofenac, a non-selective cyclooxygenase inhibitor, is a common orally and parenterally used analgesic. The oral route carries the risk of high first-pass metabolism and reduced systemic absorption; about 50% of the drug fails to reach the systemic circulation [1]. Dose-dependent adverse effects like gastric irritation and renal toxicity remain a major concern. To overcome this, topical administration of drug was introduced as an alternative, offering the advantage of local and enhanced drug delivery with reduced systemic adverse effects, which have been mainly attributed to the lower plasma drug levels in topical compared to oral and parenteral ones. Parenteral drug delivery by intramuscular injection is yet another commonly used route of administration, which can gain easy access to systemic circulation. But its rapid drug absorption is accompanied by a swift decline of drug levels in circulation [8].
The transdermal system is an innovative mode of drug delivery replacing traditional forms of administration. Its prime advantage is controlled drug delivery with maintenance of uniform plasma concentrations, avoiding hepatic first-pass metabolism. The drug passively diffuses into systemic circulation due to existence of a concentration gradient, with a higher concentration in the patch and zero in the skin and subcutaneous vessels. Diffusion of drug depends upon factors such as molecular weight, lipophilicity, potency and the presence of a rate-controlling barrier membrane. Since optimal dose is delivered at a constant rate and diffusion no longer occurs once a steady state is achieved between the patch and plasma, the transdermal route emerges as a safer mode of drug administration. Moreover, patch may be removed any time, terminating further absorption, unlike oral and injectable forms which once given as a bolus cannot be retrieved again. It differs from topical methods in that while both modes use the skin as a port of entry, the topical agent has a local action in the area of application, while the transdermal has systemic action in remote areas similar to oral and parenteral routes.
Transdermal patches are available as reservoir or matrix types [9]. In reservoir-type patches, the drug along with the excipient is present in a reservoir layer and is separated from the adhesive layer by a rate-controlling barrier membrane. In the matrix-type patches, the drug is present within the adhesive layer itself. Diclofenac diethylamine can be formulated into the transdermal matrix-type patches to sustain its release characteristics. Arora and Mukherjee tested various polymeric compositions of polyvinylpyrrolidone (PVP) and ethyl cellulose (EC), and the ratio of 1:2 was found to have the maximum capability for controlled and sustained drug release as well as physical stability [10]. However, it has a delayed onset of action with various studies reporting a range from 4 to 12 h [11].
When the analgesic efficacy of oral, intramuscular and transdermal diclofenac was compared, the post-operative VAS scores were similar in all three groups on day 1 (p = 0.148). On second day, there was highly significant difference in pain between oral and intramuscular ones (p = 0.01), but no significant difference among intramuscular–transdermal (p = 0.06) or transdermal–oral groups (p = 1.00). On day 3, intramuscular diclofenac had better results compared to both oral (p = 0.01) and transdermal groups (p = 0.05), while the latter two had similar scores (p = 1.00). This suggests a higher decrease in pain scores and thus better pain relief in intramuscular group than in the other two, while similar pain relief in oral and transdermal groups. This difference may be because only 50% of orally dosed diclofenac reaches systemic circulation [1] and the transdermal route sustainably releases the drug till equilibrium is achieved between patch and the system, making the effective dose in circulation at a given point of time, less than that of injectable form. Krishnan et al. [12] compared oral and transdermal diclofenac in 40 healthy subjects with carious third molars and concluded that both routes afforded similar pain relief. In contrast, Bachalli et al. [13] showed significant pain relief in oral group when compared with transdermal group at 12-h interval following extraction of mesioangular impacted mandibular third molar but no significant difference in analgesic efficacy over the next 2 post-operative days between the two routes of administration. This contradiction may be explained by the timing of drug administration in the present study, where Diclo patch was administered 5 h before the procedure, taking into consideration its slower onset of action. Selvi et al. [14] demonstrated no significant difference in analgesic efficacy or duration of action between transdermal and injectable forms in bilateral mesioangular impacted mandibular third molars contrary to this study where a difference between these groups existed on the third day. Nevertheless, the therapeutic efficacy of these two was found to be similar in the first two post-operative days when the pain is maximum.
The percentage of people requiring rescue analgesics was similar in all groups (p = 0.261), but, a higher quantity was consumed in the Diclo tab group on first post-operative day when compared with the other groups. In a study by Bhaskar et al. [15] to compare oral and transdermal diclofenac, where one out of 20 participants in transdermal group required rescue analgesic, whereas none in the oral group required any, albeit this difference was not significant. Preemptive analgesic administration in the present study may have made the difference. We could not find any other study comparing rescue analgesic consumption between injection and patch groups.
In this study, adverse effects of Diclo tab like gastritis were seen in 11 participants, which were diagnosed by clinical history and endoscopic studies. Rash and pruritus were seen in 3.33% of participants in Diclo patch group. Similar findings are seen in a study conducted by Bhaskar et al. [15] for multiple premolar extractions in orthodontic patients, where 2 out of 20 participants developed gastric irritation. However, multiple studies over the years have found no significant difference in adverse reactions among the varied routes of diclofenac administration. Krishna et al. [16] have demonstrated similar rates of complications between transdermal and injection groups, in lower limb orthopaedic surgeries.
In this study, Diclo patch had an overall global assessment of excellent pain control in 16.67%, very good and good pain control in 46.67% and 20% of participants, respectively, suggesting patient satisfaction and compliance. Similar findings were reported by Hsieh et al. [17].
The overall cost of the drug in INR was the highest for transdermal group and lowest for tablet group. Special manufacturing needs and limited usage of patch may be a factor in its higher cost. We can expect that with widespread use it would be reduced. Nevertheless, cost is a small price to pay in the light of the other advantages it has to offer.
Perepa et al. [11] evaluated the analgesic efficacy of patch and intramuscular injection of diclofenac in immediate post-operative period of bi-jaw surgery for correction of dentofacial deformities in 60 patients and concluded that a single dose of 100 mg transdermal diclofenac was more efficient than the diclofenac injection with a significantly higher mean duration of analgesia and better adverse effect profile. Similarly, Hsieh et al. [17] demonstrated in their study of 153 subjects with myofascial pain syndrome of upper trapezius that diclofenac patch had significantly better VAS score, range of motion with no adverse effects when compared with placebo.
This study is singular in comparing three different routes of diclofenac administration using a standardized model and evaluated not only analgesic efficacy but overall global assessment indicating the patient compliance levels, which forms the basis for efficient management for any post-operative pain. This study accepts the null hypothesis that the diclofenac patch group was as efficacious in pain control as oral and parenteral routes. The present study has its demerits though. Blinding has not been done as is desirable in an analgesic assay [7]. VAS was the only pain scale used to evaluate the analgesic efficacy of the drug. Qualitative scales assessing pain relief have not been considered. Timing of rescue analgesic consumption has not been recorded which could have given better knowledge about the duration of action of the drugs.
Conclusion
Transdermal diclofenac was found to be a promising analgesic modality to combat mild to moderate post-operative pain in impacted third molar extractions. With its proven analgesic efficacy, good patient compliance and side-effect profile, transdermal diclofenac can serve as an excellent alternative to oral and parenteral routes of drug administration. Its usage can, therefore, be extended to post-surgical and post-traumatic pain. Further clinical trials on transdermal diclofenac with larger sample size are needed to establish its scope in other oral surgical procedures.
Compliance with Ethical Standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dipti Samal, Email: diptisamal87@gmail.com.
Niranjan Mishra, Email: drniranjanmishra@gmail.com.
Brundabati Meher, Email: brundabatimeher@gmail.com.
Indu Bhusan Kar, Email: indubkar@gmail.com.
Rosalin Kar, Email: kar.rosalin@gmail.com.
R. H. Saipooja, Email: saisunith.omfs@gmail.com
References
- 1.Willis JV, Kendall MJ, Finn RM, Thornhill DP, Welling PG. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur J Pharmacol. 1979;16:405–410. doi: 10.1007/BF00568201. [DOI] [PubMed] [Google Scholar]
- 2.Keith AD. Polymer matrix consideration for transdermal devices. Drug Dev Ind Pharm. 1983;9:605–621. doi: 10.3109/03639048309044695. [DOI] [Google Scholar]
- 3.Predel HG, Koll R, Pabst H, Dieter R, Gallacchi G, Giannetti B, Bulitta M, Heidecker JL, Mueller EA. Diclofenac patch for topical treatment of acute impact injuries: a randomised, double-blind, placebo-controlled, multicentre study. Br J Sports Med. 2004;38:318–323. doi: 10.1136/bjsm.2003.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galer BS, Rowbotham M, Perander J, Devers A, Friedman E. Topical diclofenac patch relieves minor sports injury pain: results of a multicenter controlled clinical trial. J Pain Symptom Manage. 2000;19:287–294. doi: 10.1016/S0885-3924(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 5.Alessandri F, Lijoi D, Mistrangelo E, Nicoletti A, Crosa M, Ragni N. Topical diclofenac patch for postoperative wound pain in laparoscopic gynecologic surgery: a randomized study. J Minim Invasive Gynecol. 2006;13:195–200. doi: 10.1016/j.jmig.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain. Arthritis Care Res. 2011;63:240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 7.Cooper SA. Models for clinical assessment of oral analgesics. Am J Med Novemb. 1983;14:24–29. doi: 10.1016/0002-9343(83)90229-2. [DOI] [PubMed] [Google Scholar]
- 8.Vaile JH, Davis P. Topical NSAIDs for musculoskeletal conditions. A Rev Lit Drugs. 1998;56:783–799. doi: 10.2165/00003495-199856050-00004. [DOI] [PubMed] [Google Scholar]
- 9.Scheindlin S. Transdermal drug delivery: past, present, future. Mol Interv. 2004;4:308–312. doi: 10.1124/mi.4.6.1. [DOI] [PubMed] [Google Scholar]
- 10.Arora P, Mukherjee B. Design, development, physicochemical, and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J Pharm Sci. 2002;91:2076–2089. doi: 10.1002/jps.10200. [DOI] [PubMed] [Google Scholar]
- 11.Perepa A, Sinha R, Uppada UK, Kumar AVSSS. Diclofenac transdermal patch: a potential ingress to maxillofacial surgery. J Maxillofac Oral Surg. 2016 doi: 10.1007/s12663-016-0941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan S, Sharma P, Sharma R, Kumar S, Verma M, Chaudhary Z. Transdermal diclofenac patches for control of post-extraction pain. Pilot randomized controlled double-blind study. Oral Maxillofac Surg. 2013 doi: 10.1007/s10006-013-0422-5. [DOI] [PubMed] [Google Scholar]
- 13.Bachalli PS, Nandakumar H, Srinath N. A comparative study of diclofenac transdermal patch against oral diclofenac for pain control following removal of mandibular impacted third molars. J Maxillofac Oral Surg. 2009;8:167–172. doi: 10.1007/s12663-009-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvi UPG, Kamatchi D, Babu C, Keerthana S, Jeeva SVL. Comparison of transdermal diclofenac patch with intramuscular diclofenac injection as an analgesic modality following surgical extraction of impacted mandibular third molars: a cross over efficacy trail. Int J Sci Study. 2016;4:117–123. [Google Scholar]
- 15.Bhaskar H, Pranav Kapoor R. Comparison of transdermal diclofenac patch with oral diclofenac as an analgesic modality following multiple premolar extractions in orthodontic patients: a cross over efficacy trial. Contemp Clin Dent. 2010;1:158–163. doi: 10.4103/0976-237X.72783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishna R, Nataraj MS. Efficacy of a single dose of a transdermal diclofenac patch as pre-emptive postoperative analgesia: a comparison with intramuscular diclofenac. South Afr J Anaesth Analg. 2012;18:194–197. doi: 10.1080/22201173.2012.10872852. [DOI] [Google Scholar]
- 17.Hsieh LF, Hong CZ, Chern SH, Chen CC. Efficacy and side effects of diclofenac patch in treatment of patients with myofascial pain syndrome of the upper trapezius. J Pain Symptom Manage. 2010;39:116–125. doi: 10.1016/j.jpainsymman.2009.05.016. [DOI] [PubMed] [Google Scholar]


