Abstract
Objective
To assess whether delayed trigger during bolus-tracking for CT correlates with reduced heart function and suboptimal portovenous contrast phase.
Methods and Materials
Patients who underwent portovenous abdominal CT using bolus-tracking and echocardiography within 2 weeks were included and excluded if there was a non-standard contrast injection. The bolus trigger time (BTT) at 100 Hounsfield units in the abdominal aorta, patient age, congestive heart failure (CHF) history, and ejection fraction were recorded. Two radiologists scored the liver contrast phase (1–5, 5 being an optimal portovenous phase). When applicable, the BTT and contrast score of the most recent comparison examination with equivalent technical parameters were also recorded. Simple linear regression (univariate) was used to test for associations with trigger time.
Results
114 patients with a mean age of 61 ± 15 years fulfilled criteria. The mean trigger time was 18 ± 6 seconds (range: 6–38 seconds) and the mean ejection fraction was 52±12% (range: 19–69%).
A longer bolus trigger had a significant correlation with reduced ejection fraction (P=0.0018), lower hepatic contrast score (P<0.0001), history of CHF (P=0.0212) and older age (P =0.0223).
Contrast score differences between the study exam and available prior exams revealed score differences of 0 (n=73), 1 (n=15) and 2 (n=5); these were associated, respectively, with a mean bolus trigger time difference between exams of 2 seconds (range, 0–6 seconds), 6 seconds (range, 1–15 seconds), and 11 seconds (range, 5–13). The p-value comparing bolus trigger time and contrast score differences was less than 0.0001.
A lower ejection fraction also significantly correlated with suboptimal PV contrast phase (P<0.0001).
Conclusion
Delayed time to trigger during bolus-tracking for CT can indicate cardiac dysfunction and may not adequately adjust to provide an optimal portovenous contrast phase.
Keywords: Congestive heart failure, bolus-tracking, abdomen, CT, contrast
Introduction
Contrast-enhanced computed tomography (CT) provides an accurate and reliable evaluation of anatomy and pathology when the proper contrast phases are obtained. CT also provides an underappreciated facet of physiologic evaluation. While it is common to evaluate cardiac function during dedicated cardiac CT, the assessment of physiology is uncommon during non-cardiac CT and the knowledge of physiologic effects upon abdominal CT imaging quality have been under-evaluated.
Many physiologic variations encountered at CT are handled by the robust method of contrast bolus-tracking, which has been standard for many years. While the identification of contrast bolus arrival time by bolus-tracking largely adjusts for variations such as related to injection parameters and certain patient-related factors, multiple reports indicate the adjustment to be variably suboptimal [1–4].
Both controllable and uncontrollable factors are implicated in the resultant vascular and parenchymal opacification obtained during a contrast-enhanced examination. Controllable aspects of an examination include the type of contrast media used, injection technique and acquisition parameters. Uncontrollable factors of an examination include mainly patient-related components such as cardiac function, renal function, and hydration status [1,5]. A study by Johnson et al. evaluated 100 oncologic patients who each underwent four CT examinations of equivalent technique demonstrating significant intra-patient variability in abdominal contrast enhancement between examination time points [1]. They showed that the resultant trigger delay time during bolus tracking had the largest impact in variability implicating cardiac output as a suspected etiology, which had previously been suggested by Sakai et al. [6,1]. Bae et al. previously used a porcine model to show this correlation between a longer time to peak contrast enhancement and cardiac failure [5]. Shors et al. also demonstrated that a slower cardiopulmonary transit time predicted left heart failure [7]. This intuitive correlation was also shown through studies directly measuring cardiac output [8,9].
While it is known that reduced cardiac output results in slower contrast flow, the effect of cardiac dysfunction on portovenous contrast phase CT examinations and bolus-tracking in humans has not been previously reported. The purpose of our study is to assess whether the delayed time to Hounsfield unit trigger during bolus-tracking for CT correlates with reduced heart function on echocardiography and suboptimal portovenous contrast timing in the abdomen.
Materials and Methods
This retrospective study from March 2016 to December 2018 was approved by our institutional review board as Health Insurance Portability and Accountability Act compliant, and the need for informed consent was waived.
The electronic health record was searched for patients who underwent portovenous CT evaluation of the abdomen using bolus-tracking and who were also evaluated by echocardiography within 2 weeks of the CT examination. Patients were excluded if there was a non-standard contrast injection curve such as pressure peak above 300 p.s.i. or abnormal contrast flow rate/volume compared to our standard injection (Figure 1).
Figure 1.
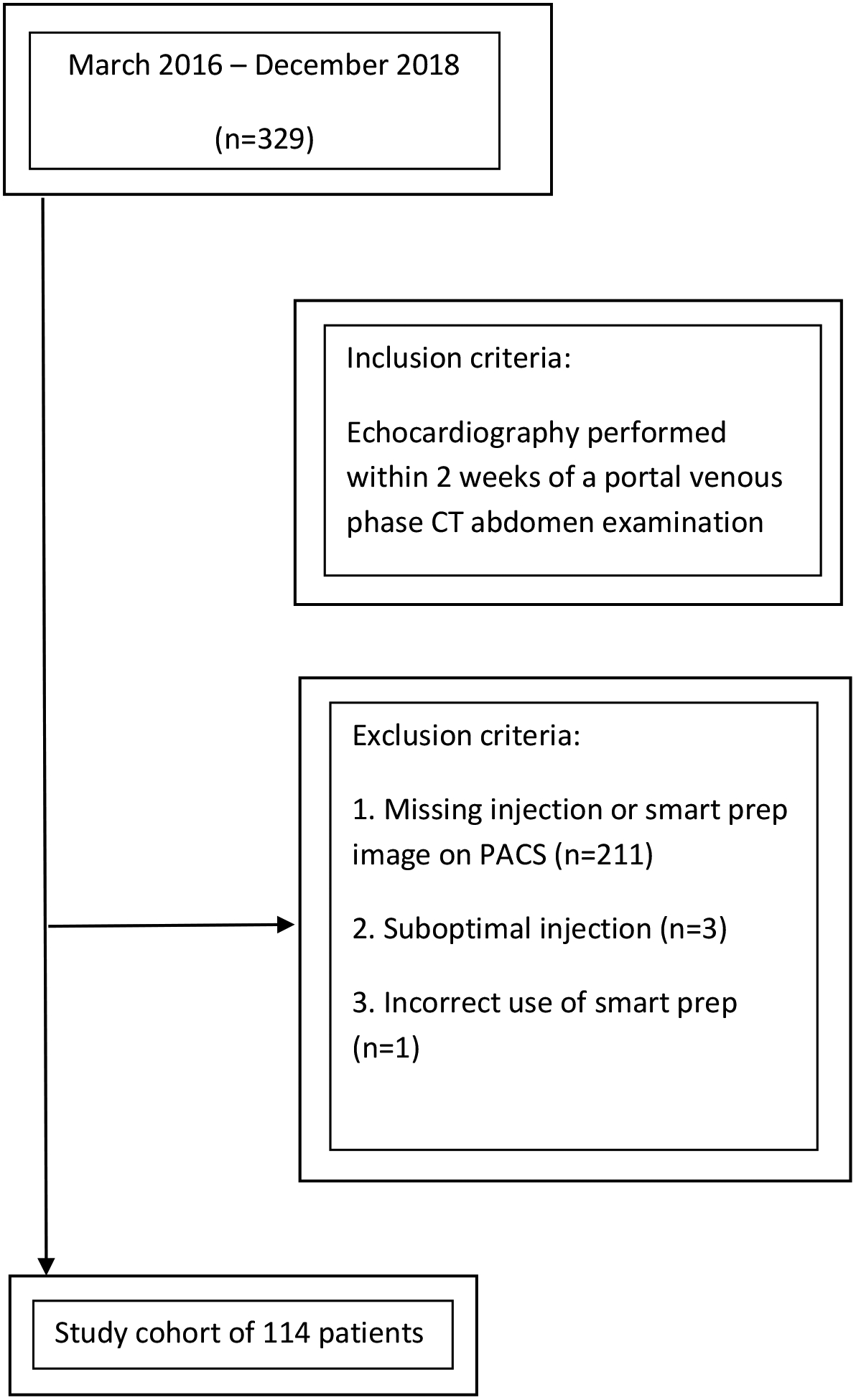
Flowchart of retrospective patient accrual.
Note—PACS-Picture archiving and communication system
Each patient’s age, sex, height, weight, body mass index and primary cancer diagnosis were recorded. Additionally, the ejection fraction from echocardiography and whether patients had a diagnosis of congestive heart failure (CHF) was recorded. Ejection fraction was calculated quantitatively using Biplane Method of Discs as recommended by the American Society of Echocardiography [10].
The study patients received a size-based intravenous contrast injection: 100 – 150 mL of iohexol 350 mgI/mL (Omnipaque 350, GE Healthcare) or 320 mgI/mL Iodixanol (Visipaque 320; General Electric) injected at 2–5 mL/sec, the lesser volume and rate given to smaller patients (digital field of view [DFOV], 40 cm or lower) and the higher given to larger patients (DFOV, greater than 40 cm). The injection parameters of our standard practice are set to yield equivalent injection durations and grams of Iodine per weight.
Bolus tracking was used with a 100 HU region of interest (ROI) trigger value in the abdominal aorta at the level of the celiac artery. Once the 100 HU value was reached, an additional diagnostic delay of 50 seconds was used to then scan in the portovenous phase: axial scan mode, single source, 64 – 128 × 0.6 mm, 40 mm beam collimation, 0.5 pitch, 0.4 s gantry rotation time, 120 kV tube potential, and 5mm section thickness. Tracking scans were set to begin 10 seconds after the start of injection, repeating every 2 seconds until the trigger value was reached; this bolus trigger time (BTT) was recorded.
The cardiac chambers were measured on CT using transverse dimension of the ventricles and right atria, and the anterior-posterior dimension for the left atria [11]. For each study, a 2-dimensional ROI was placed within each hepatic vein and the aorta to record mean HU; the aortic to hepatic vein HU ratio was calculated. The diameter of each ROI was chosen as approximately 75% of the width for each structure being measured.
Two radiologists (C.T.J. and R.K.) with 14 and 4 years of experience reading abdominal CT scans, respectively, reviewed each examination in consensus under standard clinical conditions with high-resolution monitors during the qualitative scoring of the portal venous phase using the following Likert scale: A score of 5 was given for an ideal portovenous phase consisting of avid portovenous enhancement and well-opacified hepatic veins. A score of 4 was given if there was mildly diminished hepatic vein enhancement, 3 for limited to no hepatic vein enhancement, 2 for limited to no hepatic vein enhancement with diminished portovenous enhancement, and 1 was not utilized, but would have been given if there was limited to no hepatic vein or portovenous enhancement (Figure 2).
Figure 2.
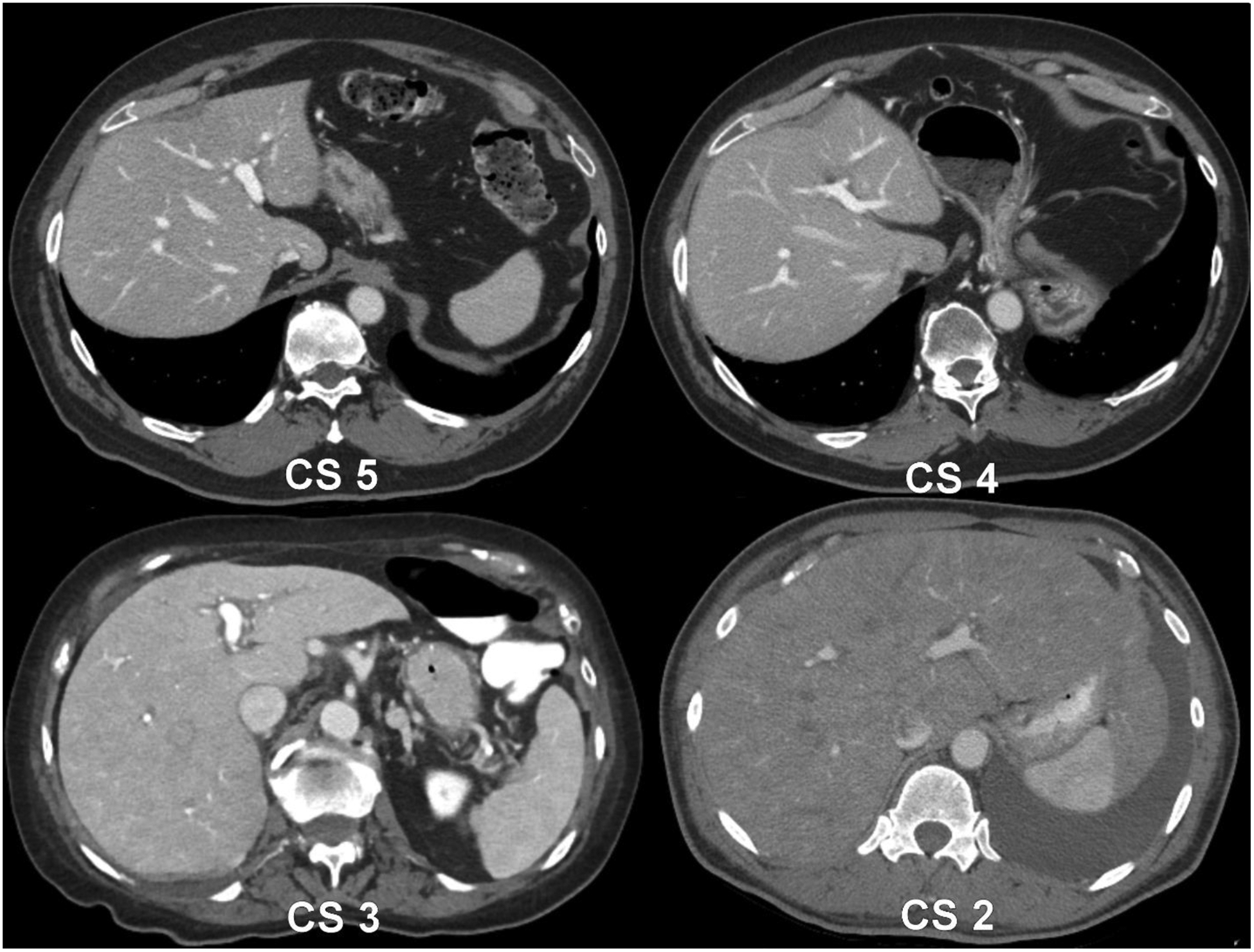
Example patient images from the study demonstrating the contrast scoring method. A contrast score of 5 represented an optimal portovenous phase. CS 4 was given when a mild reduction in enhancement was noted in the hepatic veins whereas CS 3 represented almost no hepatic vein contrast, but preserved avid enhancement remaining in the portovenous system. CS 2 was given when there was no hepatic vein enhancement and the portal vein enhancement was also diminished.
Note—CS-contrast score
When applicable, the BTT and contrast score of the most recent comparison examination with equivalent technical parameters as the study examination, including bolus-tracking settings, were also recorded.
Statistical Analysis
Sample size was chosen based upon power analysis to achieve 80% power using a two-sided Z test with a significance level of 0.05 and an expected ejection fraction (EF) distribution of 60% of patients with EF>55%, 25% with EF 40–55%, and 15% with EF<40%.
Patient demographics and CT imaging features were summarized using mean, standard deviation, minimum, median, and maximum. BTT or EF were associated with continuous predictors (e.g. age, weight, left ventricular size, etc.) using simple linear regression. BTT and EF values were compared between the levels of categorical predictors using two-sample t-test, analysis of variance (ANOVA), or Kruskal-Wallis test. Pairwise comparisons were conducted using Tukey-Kramer HSD or Steel-Dwass Method to control overall type I error rate. Simple logistic regression was used to correlate a lower contrast score with the predictors such as age, BTT, etc. Receiver operating characteristics (ROC) analysis was performed to determine an optimal threshold in predicting the presence of EF less than 50% on the basis of maximizing Youden index. Accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were reported for diagnostic performance. P-value was considered statistically significant at < 0.05. All statistical analyses were carried out using SAS (version 9.4, SAS Institute) and R (version 3.3.2, R Development Core Team).
Results
One hundred and fourteen patients (51 men: 63 women) fulfilled study criteria (Table 1). The primary oncologic diagnoses of each patient in the study group were breast cancer (N=27 patients), sarcoma (N=13), renal cell carcinoma (N=10), leukemia/lymphoma (N=8), melanoma (N=8), colorectal cancer (N=6), prostate cancer (N=6), urothelial cancer (N=5), endometrial cancer (N=5), lung cancer (N=4), germ cell tumor (N=3), cervical cancer (N=3), pancreatic adenocarcinoma (N=3), ovarian cancer (N=2), multiple myeloma (N=2), thyroid cancer (N=2) and pleural mesothelioma, peritoneal cancer, basal cell carcinoma, gastric cancer, fallopian tube cancer, neuroendocrine tumor and cholangiocarcinoma in 1 patient each.
Table 1.
Summary statistics for continuous variables.
| N | Mean | SD | Min | Median | Max | |
|---|---|---|---|---|---|---|
| Age (y) | 114 | 61 | 14.5 | 20 | 64 | 86 |
| Weight (kg) | 114 | 80.4 | 20.9 | 35.6 | 79.5 | 132.4 |
| Height (cm) | 114 | 169.0 | 11.63 | 139.5 | 168 | 193 |
| BMI (kg/m2) | 114 | 28.0 | 6.2 | 14.3 | 27.3 | 43.7 |
| Time between CT and U/S exams (days) | 114 | 0.39 | 5.87 | −14 | 0 | 14 |
| Transverse RV Size (cm) | 114 | 4.28 | 0.91 | 1.9 | 4.1 | 6.8 |
| Transverse LV Size (cm) | 114 | 4.63 | 0.93 | 2.3 | 4.6 | 7.2 |
| Transverse RA Size (cm) | 114 | 4.63 | 0.98 | 2.8 | 4.5 | 7.3 |
| Anterior-posterior LA Size (cm) | 114 | 3.89 | 0.93 | 1.8 | 3.8 | 6.2 |
| Bolus Trigger Time (s) | 114 | 18 | 6 | 6 | 18 | 38 |
| Contrast Volume (mL) | 114 | 133 | 14 | 99 | 125 | 150 |
| Contrast Flow Rate (mL/s) | 114 | 3.3 | 0.6 | 2.0 | 3 | 4.9 |
| Injected iodine per weight (gl/kg) | 114 | 1.7 | 0.4 | 1.1 | 1.7 | 3.1 |
| Aorta attenuation (HU) | 114 | 184 | 53 | 91 | 177 | 398 |
| Hepaticvein attenuation (HU) | 114 | 197 | 53 | 61 | 201 | 340 |
| Aortic : Hepatic Vein HU ratio | 114 | 0.98 | 0.39 | 0.58 | 0.89 | 3.95 |
| Ejection Fraction (%) | 114 | 51.6 | 11.6 | 19 | 55.5 | 69 |
| Comparison exam bolus trigger time (s) | 93 | 16.8 | 5.4 | 7 | 15 | 33 |
Note—HU-Hounsfield unit, SD-Standard Deviation, RV-right ventricle, LV-left ventricle, RA-right atrium, LA-left atrium, BMI-body mass index
The mean BTT was 18 seconds ± 6 (range, 6 – 38 seconds); a longer BTT correlated significantly with a reduced ejection fraction, history of CHF, lower hepatic contrast score, cardiac chamber enlargement and an increased aortic-to-hepatic vein ratio. Using our standardized technique, there was no significant BTT correlation with contrast volume, rate or type. Correlations of BTT, EF, and contrast score are shown with continuous variables in Tables 2 – 3 and with categorical variables in Table 4.
Table 2.
Correlation of bolus trigger time and ejection fraction with each continuous variable by simple linear regression.
| BolusTriggerTime (s) | Ejection Fraction (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% Cl Lower Limit | 95% Cl Upper Limit | P-value | Pearson correlation coefficient | Beta | 95% Cl Lower Limit | 95% Cl Upper Limit | P-value | Pearson correlation coefficient | |
| Age (y) | 0.091 | 0.013 | 0.170 | 0.0223 | 0.214 | −0.06 | −0.21 | 0.09 | 0.41 | −0.08 |
| Weight (kg) | 0.072 | 0.018 | 0.126 | 0.0098 | 0.241 | −0.06 | −0.16 | 0.04 | 0.26 | −0.11 |
| Height (cm) | 0.236 | 0.146 | 0.326 | <0.0001 | 0.440 | −0.25 | −0.43 | −0.07 | <0.01 | −0.25 |
| BMI (kg/m2) | 0.006 | −0.183 | 0.195 | 0.9504 | 0.006 | 0.06 | −0.29 | 0.41 | 0.72 | 0.03 |
| Time between CT and U/S exams (days) | 0.152 | −0.044 | 0.349 | 0.1268 | 0.144 | −0.04 | −0.41 | 0.33 | 0.83 | −0.02 |
| Transverse RV Size (cm) | 2.437 | 1.244 | 3.629 | 0.0001 | 0.357 | −1.26 | −3.62 | 1.10 | 0.29 | −0.10 |
| Transverse LV Size (cm) | 2.316 | 1.143 | 3.490 | 0.0002 | 0.347 | −7.57 | −9.41 | −5.73 | <0.0001 | −0.61 |
| Transverse RA Size (cm) | 2.857 | 1.800 | 3.913 | <0.0001 | 0.452 | −3.55 | −5.65 | −1.45 | <0.01 | −0.30 |
| Anterior-posterior LA Size (cm) | 3.239 | 2.139 | 4.339 | <0.0001 | 0.483 | −3.84 | −6.06 | −1.62 | <0.001 | −0.31 |
| Contrast Volume (mL) | −0.014 | −0.096 | 0.068 | 0.7380 | −0.032 | −0.24 | 0.06 | 0.24 | 0.24 | −0.11 |
| Contrast Flow Rate (mL/s) | −0.352 | −2.369 | 1.665 | 0.7303 | −0.033 | −5.45 | 2.02 | 0.37 | 0.37 | −0.09 |
| Injected iodine per weight (gl/kg) | −0.630 | −3.947 | 2.687 | 0.7076 | −0.036 | −8.63 | 3.67 | 0.43 | 0.43 | −0.08 |
| Aorta attenuation (HU) | −0.030 | −0.051 | −0.009 | 0.0065 | −0.254 | −0.04 | 0.04 | 0.98 | 0.98 | 0.00 |
| Hepatic vein attenuation (HU) | −0.061 | −0.080 | −0.042 | <0.0001 | −0.517 | −0.01 | 0.07 | 0.11 | 0.11 | 0.15 |
| Aortic : Hepatic Vein HU ratio | 3.966 | 1.076 | 6.857 | 0.0076 | 0.249 | −9.99 | 0.97 | 0.11 | 0.11 | −0.15 |
| Ejection Fraction (%) | −0.156 | −0.252 | −0.059 | 0.0018 | −0.290 | |||||
| Bolus Trigger Time (s) | −0.54 | −0.87 | −0.20 | <0.01 | −0.29 | |||||
Note—CI-Confidence interval, HU-Hounsfield unit, RV-right ventricle, LV-left ventricle, RA-right atrium, LA-left atrium, BMI-body mass index
Table 3.
Variable prediction of a contrast score of less than 5 by simple logistic regression.
| Odds Ratio | 95% Cl Lower Limit | 95% Cl Upper Limit | P-value | |
|---|---|---|---|---|
| Age (y) | 1.05 | 1.02 | 1.09 | 0.0036 |
| Weight (kg) | 1.02 | 1.00 | 1.04 | 0.0594 |
| Height (cm) | 1.05 | 1.01 | 1.09 | 0.0124 |
| BMI (kg/m2) | 1.02 | 0.96 | 1.09 | 0.5545 |
| Time between CT and U/S exams (days) | 1.01 | 0.95 | 1.08 | 0.7361 |
| Transverse RV Size (cm) | 2.24 | 1.41 | 3.73 | 0.0011 |
| Transverse LV Size (cm) | 3.10 | 1.84 | 5.64 | 0.0001 |
| Transverse RA Size (cm) | 3.01 | 1.86 | 5.26 | <0.0001 |
| Anterior-posterior LA Size (cm) | 3.10 | 1.90 | 5.42 | <0.0001 |
| Bolus Trigger Time (s) | 1.36 | 1.22 | 1.55 | <0.0001 |
| Contrast Volume (mL) | 0.98 | 0.96 | 1.01 | 0.2522 |
| Contrast Flow Rate (mL/s) | 0.56 | 0.27 | 1.14 | 0.1190 |
| Injected iodine per weight (gl/kg) | 0.45 | 0.13 | 1.44 | 0.1931 |
| Aorta attenuation (HU) | 0.99 | 0.98 | 1.00 | 0.0659 |
| Hepatic vein attenuation (HU) | 0.96 | 0.94 | 0.97 | <0.0001 |
| Aortic: Hepatic Vein HU ratio | 657.77 | 39.37 | 21342.27 | 0.0001 |
| Male/Female | 1.90 | 0.86 | 4.26 | 0.1167 |
Note—CI-Confidence interval, HU-Hounsfield unit, RV-right ventricle, LV-left ventricle, RA-right atrium, LA-left atrium, BMI-body mass index
Table 4.
Correlation of bolus trigger time and ejection fraction with categorical variables.
| Bolus Trigger Time (s) | Ejection Fraction (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Median | Max | P-value | Mean | SD | Min | Median | Max | P-value | |
| Female | 63 | 15.68 | 5.09 | 6 | 15 | 26 | <0.0001 | 54.18 | 9.92 | 25 | 57 | 69 | 0.0067 |
| Male | 51 | 20.94 | 6.32 | 8 | 20 | 38 | 48.33 | 12.69 | 19 | 50 | 69 | ||
| Diagnosis of CHF | |||||||||||||
| no | 44 | 16.34 | 6.13 | 6 | 16 | 36 | 0.02117 | ||||||
| yes | 70 | 19.10 | 6.09 | 7 | 18.5 | 38 | |||||||
| Administered IV Contrast | |||||||||||||
| lohexol 350 mg 1/mL | 88 | 17.90 | 6.58 | 6 | 17 | 38 | 0.6159 | ||||||
| lodixanol 320 mgl/mL | 26 | 18.50 | 4.93 | 10 | 18 | 28 | |||||||
| Contrast score | |||||||||||||
| 2 | 2 | 31.50 | 9.19 | 25 | 31.5 | 38 | <0.0001 | 22.00 | 4.24 | 19 | 22 | 25 | <0.0001 |
| 3 | 10 | 21.20 | 3.16 | 16 | 22 | 26 | 49.65 | 9.72 | 31 | 51.5 | 62 | ||
| 4 | 24 | 23.58 | 5.68 | 13 | 23.5 | 36 | 45.94 | 11.81 | 20 | 50 | 62.5 | ||
| All | 93 | 16.80 | 5.39 | 7 | 15 | 33 | |||||||
Note—CHF-congestive heart failure, SD-Standard Deviation
When assessing BTT difference between the current study and comparison exams available for 93 patients by contrast score difference between the studies, contrast score differences of 0 (n=72), 1 (n= 16) and 2 (n=5) were seen; these were associated, respectively, with a mean bolus trigger time difference of 2 seconds (range, 0 – 6 seconds), 6 seconds (range, 1 – 15 seconds), and 11 seconds (range, 5 – 13). The overall p-value correlating the differences in bolus trigger time and contrast score was less than 0.0001.
The best performance of BTT to dichotomize patients at an EF threshold of 50% occurred using a cutoff of 23 seconds, which is approximately a 30% delay from normal timing (Figure 3). For patients with a BTT of less than 23 seconds (N=90) and greater than 23 seconds (N=24), respectively, the mean aorta to hepatic vein HU ratio was 0.94 ± 0.36 and 1.13 ± 0.46, the mean ejection fraction was 53.5% ± 10.3 and 44.3% ± 13.4, the mean contrast score was 4.7 ± 0.6 and 4.0 ± 0.9; furthermore, the respective mean cardiac chamber measurements for the left atrium, right atrium, left ventricle, and right ventricle were 3.7 cm ± 0.8, 4.5 cm ± 0.9, 4.5 cm ± 0.9, 4.1 cm ± 0.8 and 4.5 cm ± 1.0, 5.2 cm ± 1.0, 5.2 cm ± 0.7, and 4.8 cm ± 1.0. The best performance of BTT to predict a suboptimal contrast score occurred using a cutoff of 21 seconds. (Figure 3).
Figure 3.
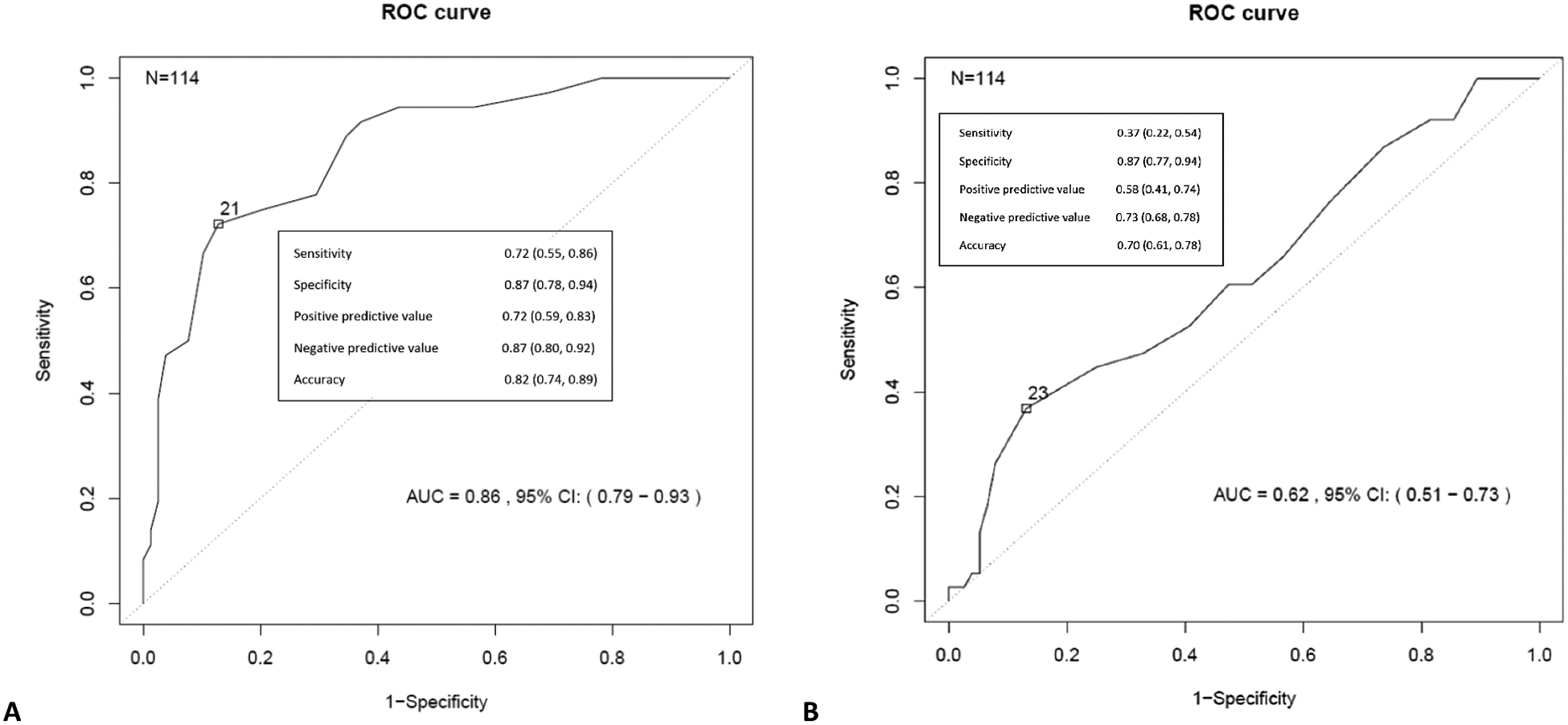
ROC curve analysis using bolus trigger time to predict a contrast score < 5 (A) and dichotomize EF at a threshold of 50% (B).
Note—EF-ejection fraction, ROC-receiver operating characteristic.
Discussion
The results of this study show that delayed time to HU triggering during bolus-tracking correlated with cardiac dysfunction measured by echocardiography (Table 2). With an overall mean BTT of 18 ± 6 seconds, there was 87% specificity for an EF<50% if the BTT was greater than or equal to 23 seconds, approximately a 30% delay from normal timing. Delayed BTT and cardiac dysfunction was also shown to correlate with a suboptimal early portovenous phase CT examination of the abdomen (e.g., decreased or no hepatic vein contrast). Internal validation identifying cardiac chamber enlargement and an increased aortic:hepatic vein HU ratio further supported significant correlations between BTT, EF, and contrast scores.
Study examinations were also compared to the most recent comparison examination that had equivalent technical parameters, when applicable (N=93). Seventy-three examinations had no difference in qualitative contrast score between time points, fifteen differed by a contrast score of 1 and five differed by a contrast score of 2 with each respective group mean BTT difference of 2 seconds, 6 seconds, and 11 seconds. This data further supports the correlation between a delayed BTT and suboptimal portovenous contrast phase, as well as an inference of potential cardiac dysfunction when a significant change in BTT is identified between examinations. Preliminarily, a BTT difference between examinations of 5 – 6 seconds, approximately a 30% change, appears to be a meaningful threshold by which to raise concern for cardiac dysfunction. Further investigation is needed to better quantify this finding.
Chaturvedi et al. suggested the use of a test bolus in patients with known cardiac dysfunction [12]; however, many patients either have no history or an unclear history of potential heart failure. At our institution, technologists review the health record and interview the patient for any history of cardiac dysfunction; while imperfect, this process identifies many patients appropriately, and they are given an additional delay to the standard portovenous timing. Other approaches such as using a slope-based bolus-tracking technique have been suggested [4]. Currently, a fixed threshold is typically used with a 100 HU trigger in the abdominal aorta whereas a proposed slope-based method would continue monitoring until a programmed decrease in HU curve slope occurs. While this is appropriate in the work by Bashir et al. regarding CT angiography and there would be benefit to more precisely identify peak ROI enhancement, there would still be a suboptimal correction for portovenous phase examinations [4]. For the same reason that the bolus arrival time is delayed, the bolus continuation from the abdominal aorta ROI until time of PV phase exam is delayed. Perhaps scanner software detection of a delayed BTT could be associated with a sliding-scale variance in PV phase delay time. Other potential solutions are prone to error such as manual triggering by an advanced technologist. ROIs have been used in the liver, but in the authors’ practice, this is also fraught with error (e.g., breathing motion, misplaced ROI) and confounded at times by lesions or fatty liver.
While a longer delay is needed in many patients with CHF to obtain an optimal PV phase exam, attempts to significantly lengthen the delay are limited by a concomitant increase in contrast dispersion that degrades the bolus geometry. For example, even if extrapolation from our study patient data in Figure 4 was made to suggest similar peak hepatic enhancement between the study obtained during CHF decompensation and baseline, the relative enhancement of the portal vein and hepatic vein compared to the hepatic parenchyma would be suboptimal if scanning near peak hepatic parenchymal enhancement (assuming the same injection parameters are used). To maintain the same volume of contrast, the injection duration could be lengthened to maintain a better balance of vascular to parenchymal enhancement in the setting of such a delayed portovenous phase. Note, while the magnitude of peak aortic enhancement increases in patients with reduced cardiac output, peak hepatic parenchymal enhancement remains relatively similar [5,13].
Figure 4.
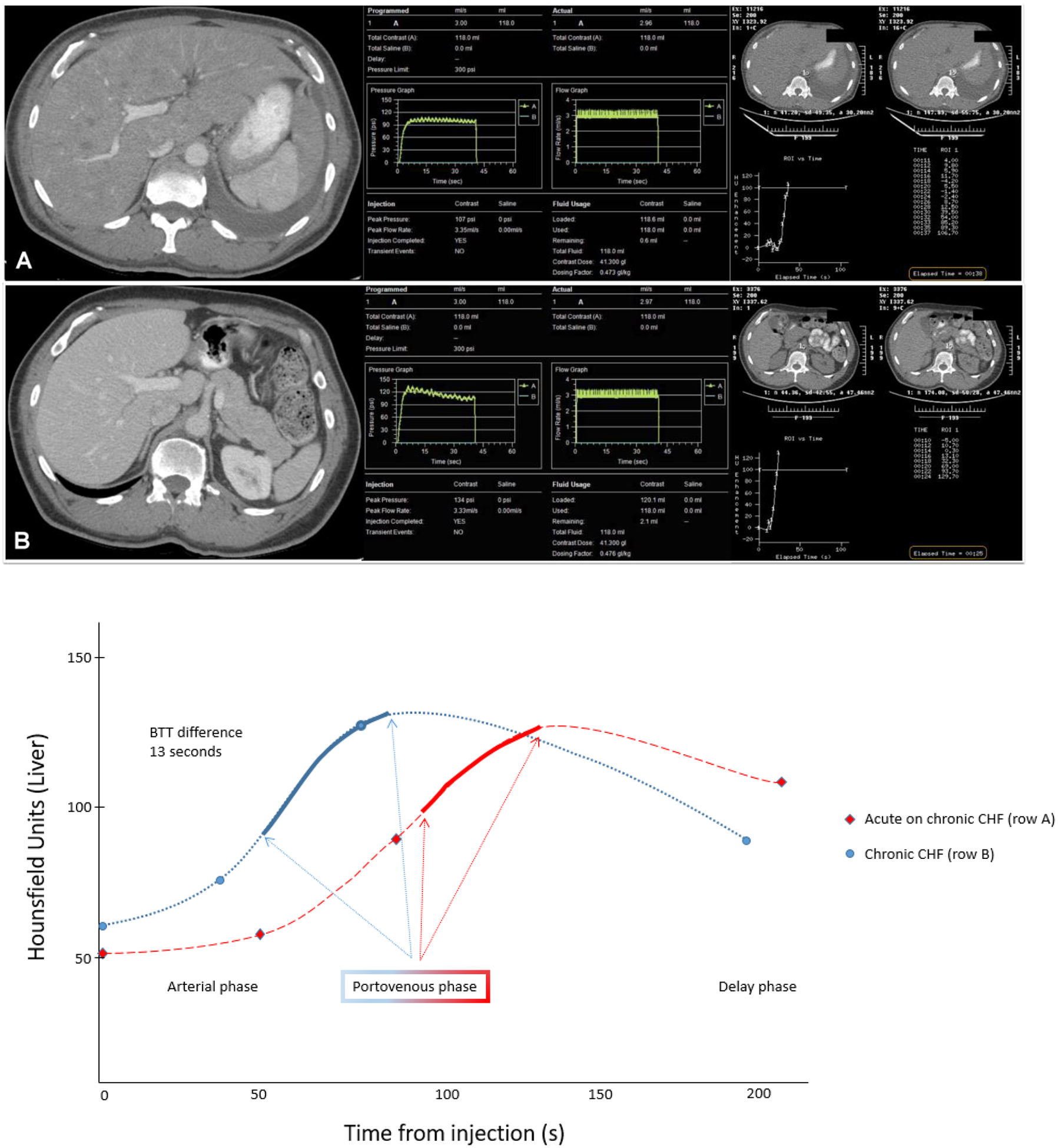
Study patient CT example (row A) demonstrates a contrast score of 2 in a patient with congestive heart failure (ejection fraction 19%) associated with a delayed trigger time during bolus tracking of 38 seconds. Prior to acute CHF decompensation (row B), 6 weeks earlier, the patient had a contrast score was 4 with a bolus tracking trigger time of 25 seconds. To demonstrate the rightward shift of hepatic enhancement curve that occurs due to CHF decompensation, the Hounsfield unit graphs of these two scans in this single patient are shown.
Note—BTT-bolus trigger time, CHF-congestive heart failure
While it may seem intuitive to simply increase the diagnostic delay to account for CHF patients that may have a delayed bolus or perhaps to use a fixed delay (i.e. without bolus-tracking), this brings about two main issues. First, the majority of patients, who do not have CHF, would obtain a later portovenous phase examination than is optimal, which can be limiting such as when assessing small-branch portovenous tumor involvement. Second, a discretely reproducible contrast phase is critical in oncologic imaging, most notable when assessing differences in tumor perfusion for therapeutic response. The use of a bolus tracking method helps to provide personalized imaging, at least partly accounting for the physiologic variations that occur between exams [14].
There are a few limitations to this study. First, any variations from the bolus-tracking settings we used such as kV, HU threshold and ROI placement may alter certain results of our study. Second, while we conducted a robust search of the electronic medical record to obtain CT examinations close to the time of echocardiography studies, a patient’s cardiac and physiologic status are inherently dynamic and could have changed during the period between the studies. Third, there are unknown variations between scans that were not accounted for such as hydration status and other conditions that may affect preload or afterload. Lastly, our results likely underestimate the correlation between the presence of CHF and a delayed BTT, as well as, suboptimal portovenous phase enhancement owing to patients with heart failure that may have had a preserved ejection fraction/diastolic CHF. Furthermore, our cardiac chamber measurements were carried out on non-cardiac-gated scans and thus they may underestimate the degree of cardiac enlargement in our study population.
In summary, an abnormally early portovenous contrast phase or delayed bolus trigger timing, in the absence of aberrant technical parameters, should raise suspicion for cardiac dysfunction. The standard bolus-tracking curve (from the scanner) provides useful clinical information and, along with the contrast injection curve (from the injector), is critical for quality assurance/improvement. A contrast bolus trigger time of 23 seconds or greater (using the settings in this study), approximately a 30% delay from normal trigger timing, or a BTT difference from prior exam of at least 6 seconds are preliminary thresholds by which to suggest possible reduced cardiac function; in such cases, consideration should then be made for echocardiography correlation. Tailored protocols or adjustments may prove useful for patients with suspected cardiac dysfunction to ensure that proper contrast phases are obtained in the abdomen to maintain accuracy.
Registry and Funding:
Supported by institutional CCSG (cancer center support grant) from the NIH/National Cancer Institute under award number P30CA016672.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: The authors have no relevant disclosures.
IRB Statement: This retrospective study was approved by our institutional review board as Health Insurance Portability and Accountability Act compliant, and the need for informed consent was waived.
References
- 1.Johnson DY, Farjat AE, Vernuccio F, Hurwitz LM, Nelson RC, Marin D (2020) Evaluation of Intraindividual Contrast Enhancement Variability for Determining the Maximum Achievable Consistency in CT. AJR Am J Roentgenol 214 (1):18–23. doi: 10.2214/AJR.19.21628 [DOI] [PubMed] [Google Scholar]
- 2.Kai N, Oda S, Utsunomiya D, Nakaura T, Funama Y, Kidoh M, Taguchi N, Iyama Y, Nagayama Y, Hirata K, Yuki H, Sakabe D, Hatemura M, Yamashita Y (2018) Dual-region-of-interest bolus-tracking technique for coronary computed tomographic angiography on a 320-row scanner: reduction in the interpatient variability of arterial contrast enhancement. Br J Radiol 91 (1081):20170541. doi: 10.1259/bjr.20170541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalra MK, Becker HC, Enterline DS, Lowry CR, Molvin LZ, Singh R, Rybicki FJ (2019) Contrast Administration in CT: A Patient-Centric Approach. J Am Coll Radiol 16 (3):295–301. doi: 10.1016/j.jacr.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 4.Bashir MR, Weber PW, Husarik DB, Howle LE, Nelson RC (2012) Improved aortic enhancement in CT angiography using slope-based triggering with table speed optimization: a pilot study. Int J Cardiovasc Imaging 28 (6):1533–1543. doi: 10.1007/s10554-011-9945-8 [DOI] [PubMed] [Google Scholar]
- 5.Bae KT, Heiken JP, Brink JA (1998) Aortic and hepatic contrast medium enhancement at CT. Part II. Effect of reduced cardiac output in a porcine model. Radiology 207 (3):657–662. doi: 10.1148/radiology.207.3.9609887 [DOI] [PubMed] [Google Scholar]
- 6.Sakai S, Yabuuchi H, Chishaki A, Okafuji T, Matsuo Y, Kamitani T, Setoguchi T, Honda H (2010) Effect of cardiac function on aortic peak time and peak enhancement during coronary CT angiography. Eur J Radiol 75 (2):173–177. doi: 10.1016/j.ejrad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 7.Shors SM, Cotts WG, Pavlovic-Surjancev B, Francois CJ, Gheorghiade M, Finn JP (2003) Heart failure: evaluation of cardiopulmonary transit times with time-resolved MR angiography. Radiology 229 (3):743–748. doi: 10.1148/radiol.2293021363 [DOI] [PubMed] [Google Scholar]
- 8.Mahnken AH, Henzler D, Klotz E, Hennemuth A, Wildberger JE, Gunther RW (2004) Determination of cardiac output with multislice spiral computed tomography: a validation study. Invest Radiol 39 (8):451–454. doi: 10.1097/01.rli.0000128655.58691.14 [DOI] [PubMed] [Google Scholar]
- 9.Garrett JS, Lanzer P, Jaschke W, Botvinick E, Sievers R, Higgins CB, Lipton MJ (1985) Measurement of cardiac output by cine computed tomography. Am J Cardiol 56 (10):657–661. doi: 10.1016/0002-9149(85)91030-6 [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 28 (1):1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 11.Hota P, Simpson S (2019) Going beyond Cardiomegaly: Evaluation of Cardiac Chamber Enlargement at Non–Electrocardiographically Gated Multidetector CT: Current Techniques, Limitations, and Clinical Implications. Radiology: Cardiothoracic Imaging 1 (1):e180024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi A, Oppenheimer D, Rajiah P, Kaproth-Joslin KA, Chaturvedi A (2017) Contrast opacification on thoracic CT angiography: challenges and solutions. Insights Imaging 8 (1):127–140. doi: 10.1007/s13244-016-0524-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae KT (2010) Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 256 (1):32–61. doi: 10.1148/radiol.10090908 [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Dercle L, Lichtenstein P, Wang D, Chen A, Zhu J, Piessevaux H, Zhao J, Schwartz LH, Lu L, Zhao B (2020) Automated Identification of Optimal Portal Venous Phase Timing with Convolutional Neural Networks. Acad Radiol 27 (2):e10–e18. doi: 10.1016/j.acra.2019.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]


