Abstract
Background
The effect of bisphosphonates on the resorption process of normal bone tissue has been clearly mentioned in the literature, while their effect on the grafting material is a new research area. Limited former study is not sufficient to determine the strength, reliability and dosage of bisphosphonates. In this study, our aim is to examine the effects of local and systemic use of bisphosphonates in bone graft applications on bone healing, histopathologically.
Methods
Therefore, 32 Sprague–Dawley rats are separated into four groups. In the first group, only an empty bone defect is made on tibia and the tissue is sutured primarily without any other application. In the second group, bone defect is filled with allograft material and closed without any other application. In the third group (LA), alendronate solution is locally added to the graft material before its application to the site of bone defect. In the fourth group, alendronate is applied systemically after the site of bone defect is grafted and primarily closed. After 6 weeks, all rats are killed and the obtained samples are examined histopathologically.
Results
Local and systemic application of alendronate increases new bone formation in a statistically significant degree. In LA group, newly formed bone was observed more mature and well developed. Alendronate application does not cause an increase in inflammation, fibrosis and necrosis. There is no increased necrosis with alendronate application.
Conclusion
Local and systemic application of alendronate in bone grafting increases bone formation without any other complication. But we believe that further research should be made on dosage, usage and possible side effects.
Keywords: Bisphosphonates, Alendronate, Bone graft, Bone healing
Introduction
Bone grafting operations are commonly used to repair hard tissue defects. Treatments of big defects are challenging problems for oral surgeons. To compensate bone deficiencies, a lot of grafts materials have been proposed in the literature [1]. Although a lot of graft material reported good results, autologous bone accepted the golden standard. But autologous grafts have limitations due to donor site and complications like donor site morbidity, pain, infections or hematoma [2, 3].
Bisphosphonates (BPs) have been used in treatment of osteoporosis for approximately four decades. Intravenous BP treatments have been used to treat and inhibit bone lesions of cancer and hyperkalemia especially for last 20 years [4]. Bisphosphonates inhibit the mineralization of the bone as well as the resorption by suppressing the osteoclast activity. The main effect at the tissue level is a decrease in bone turnover, secondarily the inhibition of bone resorption [5–7].
The literature had shown that a single dose of locally applied bisphosphonate could give an adequate distribution of bisphosphonate to the bone, because of the high affinity of bisphosphonates to bone mineral [8–10]. Also, reduced alveolar bone and periprosthetic bone resorption following mucoperiosteal surgery in rats after topical application of alendronate had been shown in the literature [11–13]. In addition, although the effect of the bisphosphonates on reducing bone resorption is well documented, their effect on bone formation is still uncertain.
Despite BPs’ wide usage in clinic, the effects on bone grafting procedures were not well defined. In the present study, our aim is to examine the effects of local and systemic use of bisphosphonates in bone graft applications on bone healing histopathologically. Our hypothesis behind this study is that anti-osteoclastic effects of alendronate will lead better graft integration.
Materials and Methods
This study was approved by the Local Ethics Committee of Animal Experiments of İstanbul University (2008/23). Thirty-two male Sprague–Dawley rats (mean weight 350 g ± 50 g) were used. The rats were housed, and all procedures were performed at the İstanbul University Experimental Medicine and Research Institute. The animals had unlimited access to water and soft chow.
Preparation of the Alendronate Solution
Alendronate sodium (Fosamax; MSD, Malmo, Sweden) was stored in a dark environment at − 20 °C. On the day of surgical procedure, alendronate solution was prepared at a concentration of 1 mg/mL of alendronate sodium in saline.
Surgical Procedure
All surgical procedure was performed under ketamine hydrochloride 35 mg/kg (Alfamine; Alfasan Int.) and xylazine hydrochloride 2.5 mg/kg (Alfazyne; Alfasan Int.) anesthesia. Procedure was performed on a heating pad used for maintenance of body temperature (36 °C to 37 °C). After shaving, a 2-cm skin incision was made to expose the tibia. A dental drill (3.5 mm, SU 100, BEGO, Germany) was used to create bone defect under saline irrigation. Rats are separated into four groups:
Control 1 Only an empty bone defect is made on tibia, and the tissue is sutured primarily without any other application.
Control 2 Bone defect is filled with allograft material (MinerOss®, USA) and closed without any other application.
Local Alendronate (LA) group: Graft material was soaked in the alendronate solution for 10 min and then rinsed three times in saline for 3 min each time; in the end, graft material was placed in the bone defect. The volume of saline was 2 ml, and volume of alendronate was 10 ml.
Systemic Alendronate (SA) group: Alendronate was applied (3 mg/kg) systemically 1 hour before the operation, and bone defect is filled with allograft material. The wounds were closed with absorbable sutures (Marlin, Germany).
After 6 weeks, all rats are killed and the obtained samples are examined histopathologically.
Histomorphometric Analysis
Tibia explants were fixed in 10% phosphate-buffered formalin for 1 day and then decalcified in 15% EDTA (pH 7.2 to 7.4) for 14 days. Then, specimens were embedded in paraffin. 2–4 µm sections were obtained from the median parts of the paraffin blocks. For standard histological evaluation, specimens were stained with hematoxylin–eosin (HE) and for evaluation of the osteoclasts tarte-resistant acid phosphatase (TRAP) and ED1, staining has been used. Histomorphometric evaluation was performed using planimetric analysis (AxioVision LE, Zeiss, Germany).
Statistical Analysis
Specimens were evaluated for new bone formation, infection, necrosis and fibrosis. Data were evaluated with GraphPad Prisma V3 statycal analyse programme. Chi-square test was used for quantitative data, and Mc Nemar’s test was used for repeated data. p < 0.05 was considered to indicate statistical significance.
Results
Inflammation scores for groups showed statistical significance. Control 1 group showed increased inflammation rates, and this is statistically significant compared to SA (p = 0.029) and Control 2 (p = 0.009) groups. Neither local nor systemic alendronate application did not cause increased inflammation (Table 1). Likewise, necrosis scores for Control 1 group were more statistically significant than other groups. There is no increased necrosis with alendronate application.
Table 1.
New bone formation in both LA and SA statistically significantly higher than control groups
| New bone p scores between groups | |
|---|---|
| LA/SA | 0.324 |
| LA/Control 2 | 0.001 |
| LA/Control 1 | 0.0002 |
| SA/Control 2 | 0.03 |
| SA/Control 1 | 0.004 |
| Control 2/Control 1 | 0.318 |
Statistically significantly increased fibrosis was observed in Control 1 group, and there are no statistically significant differences between other groups.
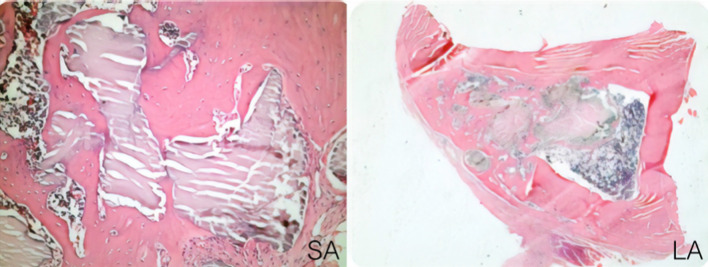
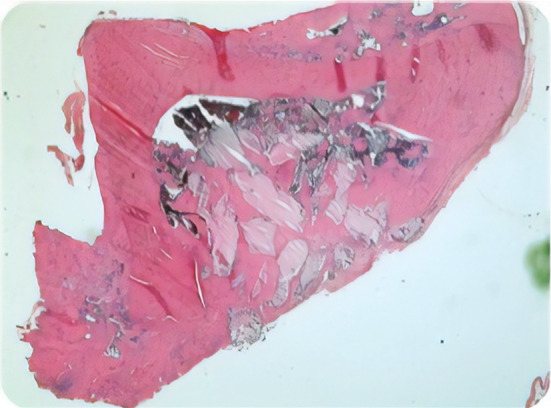
New bone formation in both LA and SA was more statistically significant than control groups. In Table 2, statistical scores can be seen between groups. In LA group, newly formed bone was observed more mature and well developed (Fig. 1). Bone bridges between graft and defect walls could be easily seen in both LA and SA groups (Fig. 2).
Table 2.
Control 1 group showed increased inflammation rates, and this is statistically significant compared to SA and Control 2 groups
| LA | SA | Control 2 | Control 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inflammation | |||||||||
| Positive | 10 | 62.5% | 13 | 81.2% | 14 | 87.5% | 6 | 37.5% | χ2: 10.9 |
| Negative | 6 | 37.5% | 3 | 18.8% | 2 | 12.5% | 10 | 62.5% | p = 0.012 |
Fig. 1.
In LA group, newly formed bone was observed more mature and well developed (H and E × 40)
Fig. 2.
Bone bridge between graft and defect can be easily seen in both SA and LA groups (H and E × 40)
Discussion
Our aim was to examine the effects of local and systemic use of bisphosphonates in bone graft applications on bone healing histopathologically. Previous studies focused on alendronate effects on rat extraction socket healing reported no necrosis but decreased bone formation and vascularity within the root socket and resorption of interdental alveolar bone after tooth extraction in rats [11, 14]. Chayary et al. [15] reported that alendronate increases bone volume and collagen accumulation in a rabbit dental extraction study. Tanoue et al. [16] evaluated short-term alendronate treatment effects in mice extraction sockets and reported that bone formation was not impeded by short-term ALN treatment. Rather, short-term ALN treatment enhanced bone formation [16]. In present study, we also observed increased new bone formation in both local and systemic alendronate usage. We think this difference was caused by anti-osteoclastic affects of alendronate.
Also, increased inflammation scores in bone healing with bisphosphonate usage were reported in the literature [17]. Nakamuro et al. reported that these increased inflammation scores have been seen with nitrogen-containing bisphosphonate compounds and researchers suggested that this was because of the suppressor effect of bisphosphonates on histamine synthesis. In the present study, we did not observe any increased inflammation score with bisphosphonates. But local alendronate applied group sho wed increased inflammation scores according to systemic alendronate group.
Aspenberg et al. and Altundal et al. [12, 13] reported that systemic alendronate application decreased otogen grafts resorption and increased new bone formation in experimental defects. Srisubut et al. [18] evaluated the local alendronate effects on bone graft healing in rats and reported statistically increased new bone formation. Lui et al. [19] reported that local administration of low-dose alendronate reduced peritunnel bone loss and increased bone tunnel mineralization, tunnel graft integrity, graft osteointegration and mechanical strength of the reconstructed complex at early healing period. Also, there is the literature about local and systemic bisphosphonate usage in dental implant operations [20, 21]. These studies also reported increased bone healing around implants both systemic and local bisphosphonate applications. In spite of these positive results, there are a lot of publications that reported bone necrosis due to bisphosphonate usage [22, 23]. That is why further research should be made on possible side effects.
Conclusion
Our study indicates that the local and systemic application of alendronate increases new bone formation in a statistically significant degree. Alendronate application does not cause an increase in inflammation, fibrosis and necrosis. Under these circumstances, we can say that local and systemic application of alendronate in bone grafting increases bone formation without any other complication. But we believe that further research should be made on dosage, usage and possible side effects.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants—a Cochrane systematic review. Eur J Oral Implantol. 2009;2:167–184. [PubMed] [Google Scholar]
- 2.Zouhary KJ. Bone graft harvesting from distant sites: concepts and techniques. Oral Maxillofac Surg Clin N Am. 2010;22:301–316. doi: 10.1016/j.coms.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Cordaro L, Amade DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implant Res. 2011;13:103–111. doi: 10.1034/j.1600-0501.2002.130113.x. [DOI] [PubMed] [Google Scholar]
- 4.Iwata K, Li J, Follet H, Phipps RJ, Burr DB. Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone. 2006;39:1053–1058. doi: 10.1016/j.bone.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Alp YE, Taskaldiran A, Onder ME, Karahan S, Kocyigit ID, Atil F, Tekin U. Effects of local low-dose alendronate injections into the distraction gap on new bone formation and distraction rate on distraction osteogenesis. J Craniofac Surg. 2017;28(8):2174–2178. doi: 10.1097/SCS.0000000000002615. [DOI] [PubMed] [Google Scholar]
- 6.Baiomy AA, Nassan MA, Abdellatif EM, Abdel Fattah A, El-Fekey AA, Abdel Aal AB. Experimental comparison of the effects of locally administered zoledronic acid and alendronate on the rate of mandibular distraction osteogenesis in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:35–42. doi: 10.1016/j.oooo.2012.09.086. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchimoto M, Azuma Y, Higuchi O, Sugimoto I, Hirata N, Kiyoki M, Yamamoto I. Alendronate modulates osteogenesis of human osteoblastic cells in vitro. Jpn J Pharmacol. 1994;66:25–33. doi: 10.1254/jjp.66.25. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe A, Iztkovich M, Earon Y, Alt I, Lilov R, Binderman I. Local delivery of an amino bisphosphonate prevents the resorptive phase of alveolar bone following mucoperiosteal flap surgery in rats. J Periodontol. 1997;68:884–889. doi: 10.1902/jop.1997.68.9.884. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe A, Binderman I, Breuer E, Pinto T, Golomb G. Disposition of alendronate following local delivery in a rat jaw. J Periodontol. 1999;70:893–895. doi: 10.1902/jop.1999.70.8.893. [DOI] [PubMed] [Google Scholar]
- 10.Astrand J, Aspenberg P. Topical, single dose bisphosphonate treatment reduced bone resorption in a rat model for prosthetic loosening. J Orthop Res. 2004;22:244–249. doi: 10.1016/j.orthres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Abtahi J, Agholme F, Sandberg O, Aspenberg P. Bisphosphonate-induced osteonecrosis of the jaw in a rat model arises first after the bone has become exposed. No primary necrosis in unexposed bone. J Oral Pathol Med. 2012;41(6):494–499. doi: 10.1111/j.1600-0714.2011.01125.x. [DOI] [PubMed] [Google Scholar]
- 12.Aspenberg P, Astrand J. Bone allografts pretreated with a bisphosphonate are not resorbed. Acta Orthop Scand. 2002;73(1):20–23. doi: 10.1080/000164702317281350. [DOI] [PubMed] [Google Scholar]
- 13.Altundal H, Sayrak H, Yurtsever E, Göker K. Inhibitory effect of alendronate on bone resorption of autogenous free bone grafts in rats. J Oral Maxillofac Surg. 2007;65(3):508–516. doi: 10.1016/j.joms.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010;16(7):674–685. doi: 10.1111/j.1601-0825.2010.01677.x. [DOI] [PubMed] [Google Scholar]
- 15.Chavarry NGM, Perrone D, Farias MLF, Dos Santos BC, Domingos AC, Schanaider A, Feres-Filho EJ. Alendronate improves bone density and type I collagen accumulation but increases the amount of pentosidine in the healing dental alveolus of ovariectomized rabbits. Bone. 2018;30(120):9–19. doi: 10.1016/j.bone.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Tanoue R, Koi K, Yamashita J. Effect of Alendronate on Bone Formation during Tooth Extraction Wound Healing. J Dent Res. 2015;94(9):1251–1258. doi: 10.1177/0022034515592867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura M, Umetsu R, Abe J, Matsui T, Ueda N, Kato Y, Sasaoka S, Tahara K, Takeuchi H, Kinosada Y. Analysis of the time-to-onset of osteonecrosis of jaw with bisphosphonate treatment using the data from a spontaneous reporting system of adverse drug events. J Pharm Health Care Sci. 2015;22(1):34. doi: 10.1186/s40780-015-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srisubut S, Teerakapong A, Vattraphodes T, Taweechaisupapong S. Effect of local delivery of alendronate on bone formation in bioactive glass grafting in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(4):e11–e16. doi: 10.1016/j.tripleo.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Lui PP, Lee YW, Mok TY, Cheuk YC. Local administration of alendronate reduced peri-tunnel bone loss and promoted graft-bone tunnel healing with minimal systemic effect on bone in contralateral knee. J Orthop Res. 2013;31(12):1897–1906. doi: 10.1002/jor.22442. [DOI] [PubMed] [Google Scholar]
- 20.Abtahi J, Tengvall P, Aspenberg P. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone. 2012;50(5):1148–1151. doi: 10.1016/j.bone.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Kellesarian SV, Abduljabbar T, Vohra F, Malignaggi VR, Malmstrom H, Romanos GE, Javed F. Role of local alendronate delivery on the osseointegration of implants: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2017;46(7):912–921. doi: 10.1016/j.ijom.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Dundar S, Yaman F, Gecor O, Cakmak O, Kirtay M, Yildirim TT, Karaman T, Benlidayi ME. Effects of local and systemic zoledronic acid application on titanium implant osseointegration: an experimental study conducted on two surface types. J Craniofac Surg. 2017;28(4):935–938. doi: 10.1097/SCS.0000000000003568. [DOI] [PubMed] [Google Scholar]
- 23.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]