We read with great interest the study by Coker et al.1 that provided supportive evidence for the role of oral microbiota in gastric cancer. A few studies also highlighted the possible link with esophageal cancer2-4. However, there is a lack of robust epidemiologic data on whether periodontal disease and tooth loss, indicators of oral microbial dysbiosis, are associated with these 2 cancers.
Here, we prospectively examined the association of periodontal disease and tooth loss with risk of esophageal and gastric adenocarcinoma in 98,459 women from the Nurses’ Health Study (1992-2014) and 49,685 men from the Health Professionals Follow-up Study (1988-2016). Dental measures, demographics, lifestyle, and diet were assessed using validated follow-up questionnaires. Self-reported cancer diagnosis was confirmed by review of medical records. We used Cox proportional hazards models to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs). We also examined the independent association of periodontal disease and tooth loss in a joint analysis.
Over 22-28 years of follow-up, we documented 199 cases of esophageal adenocarcinoma and 238 cases of gastric adenocarcinoma. Periodontal disease was associated with a 43% and 52% increased risk of esophageal adenocarcinoma (HR 1.43; 95% CI 1.05-1.96) and gastric adenocarcinoma (HR 1.52; 95% CI 1.13-2.04) (Table 1). Compared to individuals with no tooth loss, the risks of esophageal and gastric adenocarcinoma for those with ≥ 2 tooth loss were also modestly increased (HR 1.42; 95% CI 1.00-2.03; Ptrend 0.05 and HR 1.33; 95% CI 0.95-1.86; Ptrend 0.09, respectively). Among individuals with a history of periodontal disease, no tooth loss and ≥ 1 tooth loss were equally associated with 59% increased risk of esophageal adenocarcinoma (HR 1.59; 95% CI 1.04-2.41 and HR 1.59; 95% CI 1.04-2.44, respectively) compared to those with no periodontal disease and no tooth loss. Similarly, the same group of individuals had 50% and 68% greater risk of gastric adenocarcinoma (HR 1.50; 95% CI 1.01-2.23 and HR 1.68; 95% CI 1.13-2.50, respectively). (Figure 1).
Table 1.
Age-adjusted and multivariable associations of periodontal disease and tooth loss with esophageal and gastric adenocarcinoma
| Periodontal disease |
Number of tooth loss |
||||||
|---|---|---|---|---|---|---|---|
| No | Yes | P | 0 | 1 | ≥ 2 | Ptrendd | |
| Esophageal adenocarcinoma | |||||||
| Case | 134 | 65 | 123 | 25 | 51 | ||
| Person-year | 11033730 | 997259 | 10205784 | 1037319 | 787886 | ||
| Age-adjusted HR (95% CI)a | 1 (reference) | 1.72 (1.27-2.33) | 0.0005 | 1 (reference) | 1.26 (0.81-1.95) | 1.72 (1.22-2.42) | 0.002 |
| Multivariable-adjusted HR (95% CI)b | 1 (reference) | 1.64 (1.21-2.24) | 0.002 | 1 (reference) | 1.24 (0.80-1.94) | 1.64 (1.16-2.33) | 0.005 |
| Multivariable- + smoking-adjusted HR (95% CI)c | 1 (reference) | 1.43 (1.05-1.96) | 0.02 | 1 (reference) | 1.16 (0.74-1.81) | 1.42 (1.00-2.03) | 0.05 |
| Gastric adenocarcinoma | |||||||
| Case | 169 | 69 | 156 | 30 | 52 | ||
| Person-year | 11033734 | 997265 | 10205789 | 1037326 | 787884 | ||
| Age-adjusted HR (95% CI)a | 1 (reference) | 1.63 (1.22-2.18) | 0.001 | 1 (reference) | 1.14 (0.76-1.69) | 1.43 (1.03-1.99) | 0.03 |
| Multivariable-adjusted HR (95% CI)b | 1 (reference) | 1.60 (1.19-2.14) | 0.002 | 1 (reference) | 1.13 (0.76-1.69) | 1.40 (1.00-1.94) | 0.05 |
| Multivariable- + smoking-adjusted HR (95% CI)c | 1 (reference) | 1.52 (1.13-2.04) | 0.006 | 1 (reference) | 1.12 (0.75-1.67) | 1.33 (0.95-1.86) | 0.09 |
Abbreviation: BMI, body mass index; CI, confidence interval; HR, hazard ratio; HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent of task; NHS, Nurses’ Health Study.
Cox proportional hazards model stratified by age (continuous, month), cohort (NHS, HPFS), and study period (in 2-year intervals).
Further adjusted for race (white, nonwhite), history of diabetes mellitus (no, yes), BMI (<20, 20-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), physical activity (in quintiles, MET-hr/wk), fruits (in quintiles, serving/d), vegetables (in quintiles, serving/d), red/processed meat (in quintiles, serving/d), alcohol (women: never, <3.5, 3.5-6.9, ≥7.0 g/d; men: never, <7.0, 7.0-13.9, ≥14.0 g/d), regular aspirin use (no, yes), anti-acid medication (no, yes), menopausal hormone therapy (premenopausal, postmenopausal never user/men, postmenopausal past user, postmenopausal current user), and physical examination within the past 2 years (no, yes).
Further adjusted for smoking status (never smoker, past smoker, current smoker) and smoking intensity (continuous, pack-year)
P for trend was calculated by using the median number of tooth loss in each category as an ordinal variable.
Figure 1. Multivariable-adjusted hazard ratio of esophageal adenocarcinoma and gastric adenocarcinoma across history of periodontal disease and tooth loss.
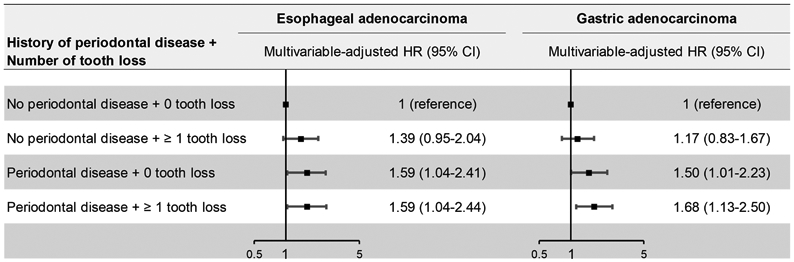
Cox proportional hazards models were stratified by age (continuous, month), cohort (NHS, HPFS), and study period (in 2-year intervals) and adjusted for the same covariates as the full model in Table 1. P for interaction between periodontal disease and tooth loss was calculated by including a cross-product interaction term between periodontal disease and tooth loss in the model and estimating the significance using the likelihood ratio test.
Prior findings on the relationship of periodontal disease and tooth loss with esophageal and gastric cancer have been inconsistent5. This is in part due to great variation in study design, exposure ascertainment, and confounding adjustment. Compared to prior studies, our analysis has the advantage of prospective design, long-term follow-up, examination of validated periodontal disease, a more specific marker for dysbiotic oral microbiome, in conjunction with tooth loss, as well as detailed covariate assessment that allowed us to control vigorously for confounding factors, especially smoking. Increasing number of studies have highlighted the strong scientific rationale for a link between oral microbiome and esophageal and gastric cancer. Tannerella forsythia and Porphyromonas gingivalis, as members of the “red complex” of periodontal pathogens6, have been associated with the presence or risk of esophageal cancer2-4. Coker et al.1 observed an over-representation of Tannerella forsythia, along with Peptostreptococcus stomatis and Streptococcus anginosus, among other oral microbes, in gastric cancer compared with other precancerous stages. From a mechanistic standpoint, Porphyromonas gingivalis may modulate the risk of esophageal cancer through anergy of activated T cells7, inhibition of apoptosis8, and dehydrogenation of ethanol to acetaldehyde, causing DNA damage, mutation, and excessive proliferation of epithelial cells9. Poor oral hygiene and periodontal disease could also promote the formation of endogenous nitrosamines known to cause gastric cancer through nitrate-reducing bacteria10.
Together, these data support the importance of oral microbiome in esophageal and gastric cancer. Further prospective studies that directly assess oral microbiome are warranted to identify specific oral bacteria responsible for this relationship. These additional findings may serve as readily accessible, non-invasive biomarkers and help identify individuals at high risk for these cancers.
ACKNOLWEDGEMENTS
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
FUNDING
This work was supported by the U.S. National Institutes of Health [UM1 CA186107, P01 CA87969, U01 CA167552; K07CA218377, R21AA027608 to Y.C.; R03 CA197879, R21 CA222940 to K.W.; P01 CA55075, R01 CA151993, R35 CA197735 to S.O.; R21 CA230873 to K.W. and S.O.; K24 DK098311, R01 CA137178, R01 CA202704 to A.T.C.; R00 CA215314 to M.S.]; the Siteman Investment Program [to Y.C.]; the American Institute for Cancer Research [to K.W.]; the American Cancer Society [MRSG-17-220-01 - NEC to M.S.]; the Project P Fund for Colorectal Cancer Research; the Bennett Family Foundation; and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. The funders had no role in design and conduct of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- FFQ
food frequency questionnaire
- HR
hazard ratio
- HPFS
Health Professionals Follow-up Study
- MET
metabolic equivalent of task
- NHS
Nurses’ Health Study
- P. gingivalis
Porphyromonas gingivalis
- T. forsythia
Tannerella forsythia
Footnotes
COMPETING INTERESTS
A.T.C. previously served as a consultant for Bayer Healthcare and Pfizer Inc. for work unrelated to the topic of this manuscript. All remaining authors have declared no conflicts of interest.
REFERENCES
- 1.Coker OO, Dai Z, Nie Y, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018;67:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017;77:6777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao S, Li S, Ma Z, et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agents Cancer 2016;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan X, Liu Y, Kong J, et al. Different frequencies of Porphyromonas gingivalis infection in cancers of the upper digestive tract. Cancer Letters 2017;404:1–7. [DOI] [PubMed] [Google Scholar]
- 5.Michaud DS, Fu Z, Shi J, et al. Periodontal disease, tooth loss, and cancer risk. Epidemiologic Reviews 2017;39:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol 1998;25:134–44. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz O, Jungas T, Verbeke P, et al. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infection and Immunity 2004;72:3743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salaspuro MP. Acetaldehyde, microbes, and cancer of the digestive tract. Critical Reviews in Clinical Laboratory Sciences 2003;40:183–208. [DOI] [PubMed] [Google Scholar]
- 10.Verna L N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacology & Therapeutics 1996;71:57–81. [DOI] [PubMed] [Google Scholar]


