Abstract
Rationale:
Blood eosinophil (EOS) count and EOS cationic protein (ECP) associate with human cardiovascular diseases (CVD). Yet, whether EOS play a role in CVD remains untested. The current study detected EOS accumulation in human and murine abdominal aortic aneurysm (AAA) lesions, suggesting EOS participation in this aortic disease.
Objective:
To test whether and how EOS affect AAA growth.
Methods and Results:
Population-based randomized clinically controlled screening trials revealed higher blood EOS count in 579 male AAA patients than in 5,063 non-AAA control (0.236±0.182 vs 0.211±0.154, 109/L, P<0.001). Univariate (OR=1.381, P<0.001) and multivariate (OR=1.237, P=0.031) logistic regression analyses indicated that increased blood EOS count in AAA patients served as an independent risk factor of human AAA. Immunostaining and immunoblot analyses detected EOS accumulation and EOS cationic protein expression in human and murine AAA lesions. Results showed that EOS deficiency exacerbated AAA growth with increased lesion inflammatory cell contents, matrix-degrading protease activity, angiogenesis, cell proliferation and apoptosis, and smooth muscle cell (SMC) loss using angiotensin-II perfusion-induced AAA in Apoe–/– and EOS-deficient Apoe–/–ΔdblGATA mice. EOS deficiency increased lesion chemokine expression, muted lesion expression of IL4 and EOS-associated-ribonuclease-1 (mEar1, human ECP homolog), and slanted M1 macrophage polarization. In cultured macrophages and monocytes, EOS-derived IL4 and mEar1 polarized M2 macrophages, suppressed CD11b+Ly6Chi monocytes, and increased CD11b+Ly6Clo monocytes. mEar1 treatment or adoptive transfer of EOS from WT and Il13–/– mice, but not EOS from Il4–/– mice, blocked AAA growth in Apoe–/–ΔdblGATA mice. Immunofluorescent staining and immunoblot analyses demonstrated a role for EOS IL4 and mEar1 in blocking NF-κB activation in macrophages, SMCs, and endothelial cells.
Conclusions:
EOS play a protective role in AAA by releasing IL4 and cationic proteins such as mEar1 to regulate macrophage and monocyte polarization and to block NF-κB activation in aortic inflammatory and vascular cells.
Keywords: Eosinophil, abdominal aortic aneurysm, ECP, IL4, mEar1, NF-κB, vascular surgery, inflammation, Aneurysm, Vascular Disease
Graphical Abstract
INTRODUCTION
Studies from humans and experimental models suggest a role for asthma and allergic-immune responses in the development of abdominal aortic aneurysms (AAA). In the Danish National Registry of Patients study, patients with recently diagnosed asthma showed a two-fold higher risk of aortic rupture than those without asthma.1 In angiotensin-II (Ang-II) perfusion-induced AAA, ovalbumin-induced allergic airway hyperresponsiveness greatly increased AAA growth.2 A common phenotype of allergic lung diseases is the recruitment of airway inflammatory cells, including innate immune cells mast cells (MCs) and eosinophils (EOS).3–6 MCs develop from the bone marrow as progenitor cells and mature in the peripheral tissues.7,8 We previously reported a role for MCs in AAA by releasing their granular inflammatory molecules, IFN-γ and IL6, to promote angiogenesis and aortic smooth muscle cell (SMC) apoptosis. MC activation or inhibition directly affected AAA growth.9 EOS, innate immune cells that develop in the bone marrow under the control of transcription factor GATA-1 and cytokines IL5, IL3, and GM-CSF, accumulate in the peripheral tissues.10–13 Like MCs, EOS granules also richly contain inflammatory cytokines (e.g. IL4, IL5, IL13), chemokines, and growth factors. In addition, EOS contain a set of cationic proteins, including eosinophilic cationic protein (ECP), EOS-derived neurotoxin (EDN), major basic protein (MBP), and eosinophilic peroxidase (EPO).14–16
From a group of 3,742 patients undergoing coronary angiography, high blood EOS count associated positively with the major cardiovascular risk factors and cardiovascular disease (CVD) prevalence.17 In an England Biobank of 478,259 individuals, 1,377 died of CVD and 8,987 died of other causes throughout 7 years of follow-up. The CVD mortality group had a significantly higher blood EOS count than in those of total death (n=13,482) or alive (n=46,4797) groups.18 Serum ECP acted as a sensitive marker of EOS activation and associated with coronary atherosclerosis.19 Patients with high serum ECP had increased atherosclerosis burden.19 In patients with ST-segment elevation myocardial infarction (ST-STEMI) at the time of primary percutaneous coronary intervention (PCI), serum ECP predicted major adverse cardiac events (MACEs) at 24 months, independent of the baseline ejection fractions.20 These observations suggest a pathogenic role of EOS in CVD. Yet, in a retrospective study of 331 PCI patients for ST-STEMI, patients with MACEs had a significantly lower blood EOS-to-leukocyte ratio (ELR) calculated within 24 hrs after admission within 1 year of follow-up. Multivariate logistic regression test showed that ELR<0.1 acted as an independent predictor of MACEs (odds ratio [OR]=0.38, P<0.0001).21 In the England CALIBER program of 775,231 individuals and 55,004 with CVD and median 3.8 years follow-up, low EOS count associated inversely with peripheral arterial disease (hazard ratio [HR]=0.63).22 These clinical data suggest a beneficial role of EOS. Besides these conflicting clinical results, whether EOS play a role in AAA or other types of CVD remains untested.
The current study reports that blood EOS count remains higher in AAA patients than normal controls, and serves as an independent risk factor of human AAA. Using Ang-II perfusion-induced AAA in apolipoprotein E-deficient (Apoe–/–) mice, results show that EOS protect mice from AAA development by releasing interleukin-4 (IL4), IL13, mouse EOS-associated-ribonuclease-1 (mEar1), and possibly other untested molecules to slant M2 macrophage and Ly6Clo monocyte polarization, reduce extracellular matrix proteolysis and angiogenesis, increase SMC proliferation, and block aortic inflammatory and vascular cell nuclear factor (NF)-κB activation.
METHODS
The authors declare that all supporting data are available within the article and its Online Data Supplement. Major Resources Table, detailed descriptions of experimental materials and methods used in this study are provided in the Supplemental Materials.
RESULTS
Blood EOS count remains an independent risk factor of human AAA.
This study contains 5,642 men, including 579 AAA patients. Their mean age at analysis was 67.1±3.9 years. Online Table I shows the baseline characteristics, stratified by with and without AAA. From this population, t-test showed that among patients with current smoking status, diabetes, chronic obstructive pulmonary disease (COPD), previous acute myocardial infarction (AMI), uses of low dose aspirin, statins, beta blockers and corticosteroids, and peripheral arterial disease (PAD) (defined as ankle brachial index <0.9 or >1.4) at screening, patients with AAA all showed significantly higher blood EOS counts than those without AAA (Table 1). Pearson’s correlation test showed that systolic and diastolic blood pressures and lowest ankle brachial index associated negatively with blood EOS count, whereas body mass index (BMI) associated positively with blood EOS count (Table 1). Age did not associate with blood EOS count. In this population, men with AAA had significantly higher levels of blood EOS count than in those without AAA (0.236±0.182 vs. 0.211±0.154, 106/L, P<0.001). Univariate logistic regression analysis showed that an EOS count above median remained a significant risk factor of human AAA with a crude odds ratio (OR) of 1.38 (95% C.I.: 1.16-1.64, P<0.001) (Table 2). A multivariate logistic regression test also revealed this significance (OR=1.24 [95% C.I.: 1.02-1.50], P=0.031) after adjusting for current smoking, diabetes, previous AMI, COPD, use of statins, use of low dose aspirin, PAD at screening, BMI, and diastolic blood pressure. In this model, we did not adjust patients with asthma or allergy that is also a significant risk factor of human AAA1 because hospital-diagnosed cases of asthma were rare in this population. Yet, increase of blood EOS count is one of the pathologies of these patients.3,4 To consider the confounding from asthma, we adjusted the use of inhaling bronchodilators that contained glucocorticoids or β-agonists. Multivariate logistic regression test showed that blood EOS count remained a significant risk factor of human AAA with an unchanged OR (OR=1.24 [95% C.I.: 1.02–1.50], P=0.030) (Online Table II). Data regarding mean annual aneurysmal growth rate were available in 300 cases. Pearson’s correlation test showed no correlation between blood EOS count and annual aneurysmal growth rate (r=0.046, P=0.425), neither regarding the need for aneurysmal repair in a univariate logistic regression analysis (Hazard ratio [HR]=2.515 [95% C.I.: 0.361–17.526], P=0.361).
Table 1.
EOS count associations with baseline characteristics at screening for AAA.
| Blood EOS count (109/L) in patients |
|||
|---|---|---|---|
| Baseline characteristics | Without AAA Mean ± SD (n=5,648) | With AAA Mean ± SD (n=577) | P value* |
| Dichotomous clinical characteristics | |||
| First degree relative with AAA | 0.214 ±0.158 | 0.225 ± 0.150 | 0.266 |
| Current smoking | 0.211 ±0.158 | 0.230 ± 0.158 | 0.001 |
| Hypertension | 0.214 ±0.173 | 0.217± 0.144 | 0.539 |
| Diabetes | 0.212 ±0.156 | 0.243 ± 0.194 | <0.001 |
| Chronic obstructive pulmonary disease | 0.212 ±0.156 | 0.259 ± 0.208 | <0.001 |
| Previous stroke | 0.215 ±0.160 | 0.222 ± 0.175 | 0.379 |
| Previous acute myocardial infarction | 0.212 ±0.152 | 0.262 ± 0.258 | <0.001 |
| Previous peripheral arterial disease | 0.215 ±0.162 | 0.221 ± 0.129 | 0.669 |
| Use of low dose aspirin | 0.210 ± 0.147 | 0.235 ± 0.200 | <0.001 |
| Use of statin | 0.213 ± 0.158 | 0.234 ± 0.183 | 0.004 |
| Use of ACE inhibitor or AT2 blockage | 0.213 ± 0.156 | 0.220 ± 0.171 | 0.089 |
| Use of calcium-antagonist | 0.215 ± 0.165 | 0.218 ± 0.143 | 0.564 |
| Use of beta-blockage | 0.211 ± 0.150 | 0.239 ± 0.207 | <0.001 |
| Use of glucocorticoid | 0.212 ± 0.150 | 0.255 ± 0.267 | 0.002 |
| Ankle brachial index <0.9 or >1.4 at screening | 0.212 ± 0.153 | 0.234 ± 0.184 | 0.004 |
| AAA at screening | 0.211 ±0.154 | 0.236 ± 0.182 | <0.001 |
| Continuous clinical variables | Pearson’s correlation coefficient (r) | P value** | |
| Age (years) | 0.019 | 0.151 | |
| Body mass index (kg/m2) | 0.033 | 0.012 | |
| Systolic blood pressure (mmHg) | −0.068 | <0.001 | |
| Diastolic blood pressure (mmHg) | −0.065 | <0.001 | |
| Lowest ankle brachial index | −0.026 | 0.058 | |
Student t-test;
Pearson’s correlation analysis
Table 2.
Univariate and multivariate logistic regression analyses of AAA size as a dependent variable and EOS count as an independent variable.
| Univariate logistic regression analysis | B | SE | P value | OR | 95% C.I. |
|---|---|---|---|---|---|
| EOS count (109/L) > median | 0.323 | 0.089 | <0.001 | 1.381 | 1.161; 1.643 |
| Multivariate logistic regression analysis | B | SE | P value | OR | 95% C.I. |
| EOS count (109/L) > median | 0.213 | 0.098 | 0.031 | 1.237 | 1.020; 1.500 |
| Current smoking | 0.997 | 0.107 | <0.001 | 2.710 | 2.196; 3.344 |
| Diabetes | −0.118 | 0.150 | 0.432 | 0.889 | 0.662; 1.193 |
| Previous acute myocardial infarction | 0.219 | 0.173 | 0.206 | 1.245 | 0.887; 1.747 |
| COPD | 0.352 | 0.158 | 0.026 | 1.422 | 1.043; 1.939 |
| Use of low dose aspirin | 0.918 | 0.108 | <0.001 | 2.505 | 2.026; 3.096 |
| Use of statin | 1.736 | 0.113 | <0.001 | 5.674 | 4.546; 7.082 |
| ABI <0.9 or >1.4 at screening | 0.633 | 0.124 | <0.001 | 1.884 | 1.476; 2.405 |
| Body mass index (kg/m2) | −0.027 | 0.012 | 0.022 | 0.974 | 0.952; 0.996 |
| Diastolic blood pressure (mmHg) | 0.030 | 0.005 | <0.001 | 1.030 | 1.020; 1.040 |
Increased EOS accumulation in human and murine AAA lesions.
The Department of Surgery at the Brigham and Women’s Hospital provided human AAA lesions, and the Department of Surgery at the University of Florida provided normal human abdominal aortas. Immunoblot analysis revealed significantly increased expression of the 17-kDa ECP and 48-kDa Siglec-8 in AAA lesions, but negligible expression in normal abdominal aortas (Figure 1A). Immunostaining with anti-human Siglec-8 antibody yielded the same results. Human AAA lesions revealed Siglec-8+ EOS, which remained absent in normal aortas (Figure 1B). Not only EOS, but MCs also express Siglec-8 and these cells contribute to AAA development.9,23 Unlike EOS, human MCs express negligible CD11b.24 To prove that these Siglec-8-positive cells were EOS, we performed immunofluorescent double staining with both the Siglec-8 and CD11b antibodies. Results confirmed the presence of CD11b+Siglec-8+ EOS in human AAA lesions (Figure 1C). Mouse AAA lesions revealed the same observations. Immunoblot analysis with the anti-mouse Siglec-F antibody showed increased expression of this EOS-specific molecule in Ang-II perfusion-induced mouse AAA lesions from Apoe–/– mice (Figure 1D). Immunostaining with the Siglec-F antibody revealed that Siglec-F+ EOS accumulated in both the media and adventitia in mouse AAA lesions, but such cells remained absent in normal mouse abdominal aortas (Figure 1E). Different from human MCs, mouse MCs do not express Siglec-F,23 suggesting that these Siglec-F-positive cells in AAA lesions were EOS, but not MCs. Immunofluorescent double staining with CD11b and Siglec-F antibodies confirmed the accumulation of CD11b+Siglec-F+ EOS in mouse AAA lesions (Figure 1F). AAA lesions from EOS-deficient Apoe–/–ΔdblGATA mice confirmed the anti-Siglec-F antibody specificity (Figure 1G).
Figure 1.
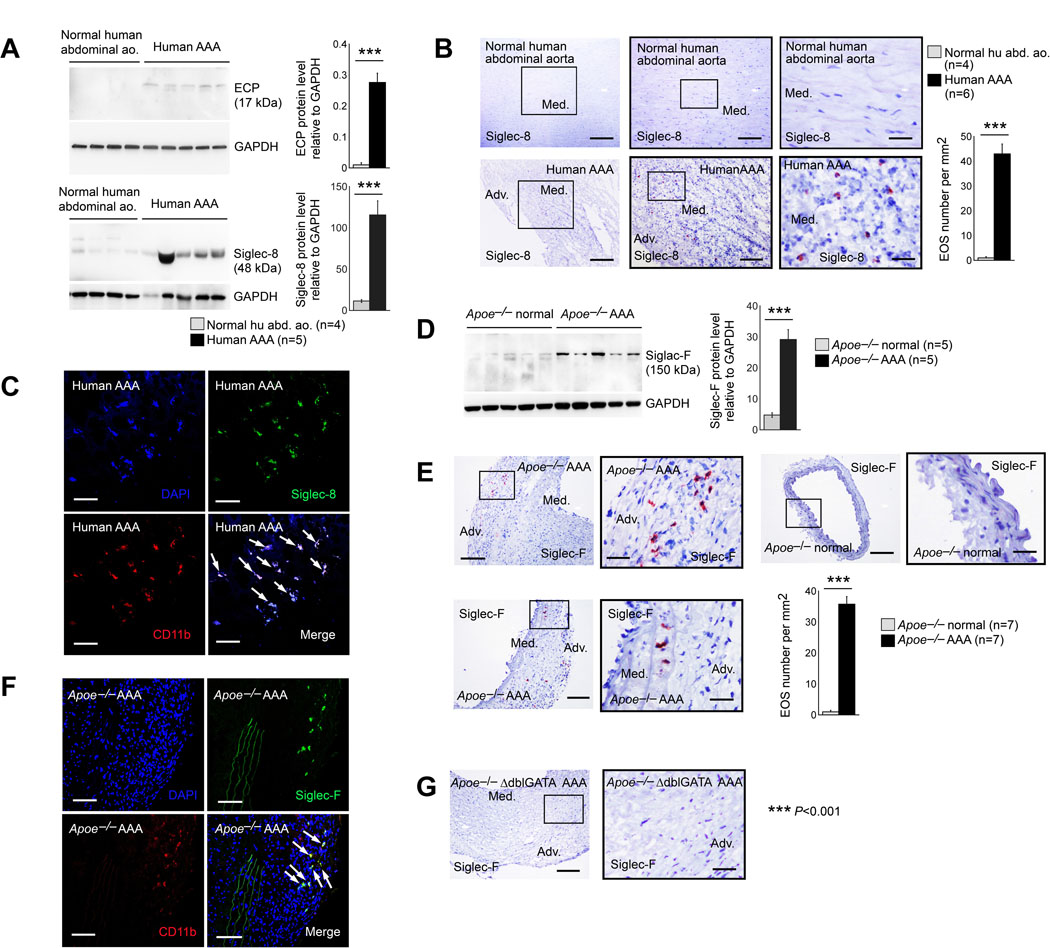
Increased EOS accumulation in human and mouse AAA lesions. A. Immunoblot analysis of human ECP and Siglec-8 in normal human abdominal aorta (n=4) and AAA lesions (n=5). B. Immunostaining detected Siglec-8+ EOS in human AAA lesions (n=6) but not in normal abdominal aortas (n=4). Scale: 400 μm, inset: 200 and 70 μm. C. Immunofluorescent double staining to confirm the accumulation of CD11b+Siglec-8+ EOS (arrows) in human AAA lesions (n=6). D. Immunoblot analysis of mouse Siglec-F in normal abdominal aorta and AAA lesions from Apoe–/– mice after 28 days of Ang-II perfusion-induced AAA (n=5 per group). E. Immunostaining localized Siglec-F+ EOS in Apoe–/– mouse AAA lesion (n=7) adventitia (Adv.) and media (Med.), but negligibly in normal mouse abdominal aorta (n=7). Scale: 200 μm, inset: 70 μm. F. Immunofluorescent double staining to confirm the accumulation of CD11b+Siglec-F+ EOS (arrows) in AAA lesions from Apoe–/– mice (n=4). G. Siglec-F immunostaining of AAA lesions from Apoe–/–ΔdblGATA mice to test antibody specificity (n=4). Scale: 200 μm, inset: 70 μm. Representative images in panels A, B, D, and E are shown to the left. Data are mean±SEM, ***P<0.001. Mann-Whitney U test.
EOS deficiency exacerbates AAA growth in mice.
EOS accumulation in human and murine AAA lesions suggests their participation in this aortic disease. Apoe–/– mice crossbred with the EOS-deficient ΔdblGATA mice, and the generation of the Apoe–/–ΔdblGATA mice and Apoe–/– littermates, tested a direct role of EOS in AAA. We produced Ang-II perfusion-induced AAA in both Apoe–/– and Apoe–/–ΔdblGATA mice. After 28 days, observations showed no significant differences in heart weight, body weight, heart weight-to-body weight ratio post-Ang-II infusion (Online Figure IA), nor systolic and diastolic blood pressures before and after Ang-II perfusion (Online Figure IB) between Apoe–/– and Apoe–/–ΔdblGATA mice. It happened to us many times that Ang-II perfusion induces aneurysms not only in the abdominal aortas, but also occasionally in thoracic aortas or even aortic arches. In this study, thoracic aortic aneurysm occurred in one mouse of each genotype. Apoe–/–ΔdblGATA mice showed much larger AAA size, bigger cross-section AAA lesion area as defined in Online Figure II, and higher AAA incidence than those of Apoe–/– mice, although the post-Ang-II perfusion survival rate did not differ between the groups (Figure 2A). Compared with those in Apoe–/– mice, Apoe–/–ΔdblGATA mice showed significantly increased AAA lesion Mac-3+ macrophage and CD4+ T-cell content, and CD31+ microvessel number, although the increase of lesion CD8+ T-cell in Apoe–/–ΔdblGATA mice did not reach statistic significance (Figure 2B–2D). AAA lesions from Apoe–/–ΔdblGATA mice also showed increased media elastica fragmentation, lesion cell proliferation (Ki67+ cell number), and media SMC loss (Figure 2E–2G). Immunofluorescent double staining demonstrated significantly reduced lesion Ki67+α-actin+ double-positive proliferating SMCs in AAA lesions from Apoe–/–ΔdblGATA mice (Figure 2H), but lesion caspase-3+α-actin+ double positive apoptotic SMCs did not differ between Apoe–/– and Apoe–/–ΔdblGATA mice (not shown), suggesting that reduced SMC proliferation, not SMC apoptosis, contributed to lesion SMC content reduction in Apoe–/–ΔdblGATA mice (Figure 2G). Indeed, Apoe–/–ΔdblGATA mice had significantly higher lesion total TUNEL-positive cell numbers than Apoe–/– mice (Figure 2I). To identify the cells that accounted for increased Ki67-positive cells in AAA lesions from Apoe–/–ΔdblGATA mice (Figure 2F), we performed immunofluorescent double staining of Ki67 with few common AAA lesion cell type markers Mac-2 (macrophages), CD31 (endothelial cells, ECs), and CD90 (fibroblasts). EOS deficiency greatly increased lesion proliferating macrophages, ECs, and fibroblasts (Online Figure IIIA-IIIC). Increases in lesion macrophage and T-cell accumulation in AAA lesions from Apoe–/–ΔdblGATA mice suggest increased inflammatory cell recruitment in these lesions. Real-time (RT)-PCR analysis demonstrated increased expression of chemokines E-selectin and ICAM-1 (intercellular adhesion molecule-1) in AAA lesions from Apoe–/–ΔdblGATA mice, although lesion VCAM-1 (vascular cell adhesion molecule-1) expression did not differ between Apoe–/– and Apoe–/–ΔdblGATA mice (Figure 2J). RT-PCR also revealed significant increases of M1 macrophage markers MCP-1 (monocyte chemoattractant protein-1), TNF-α (tumor necrosis factor-α), and iNOS (inducible nitric oxide synthase) (Figure 2K), and decrease of M2 marker Arg-1 (arginase-1) in AAA lesions from Apoe–/–ΔdblGATA mice, although IL10 expression did not differ from Apoe–/– mice (Figure 2L).
Figure 2.
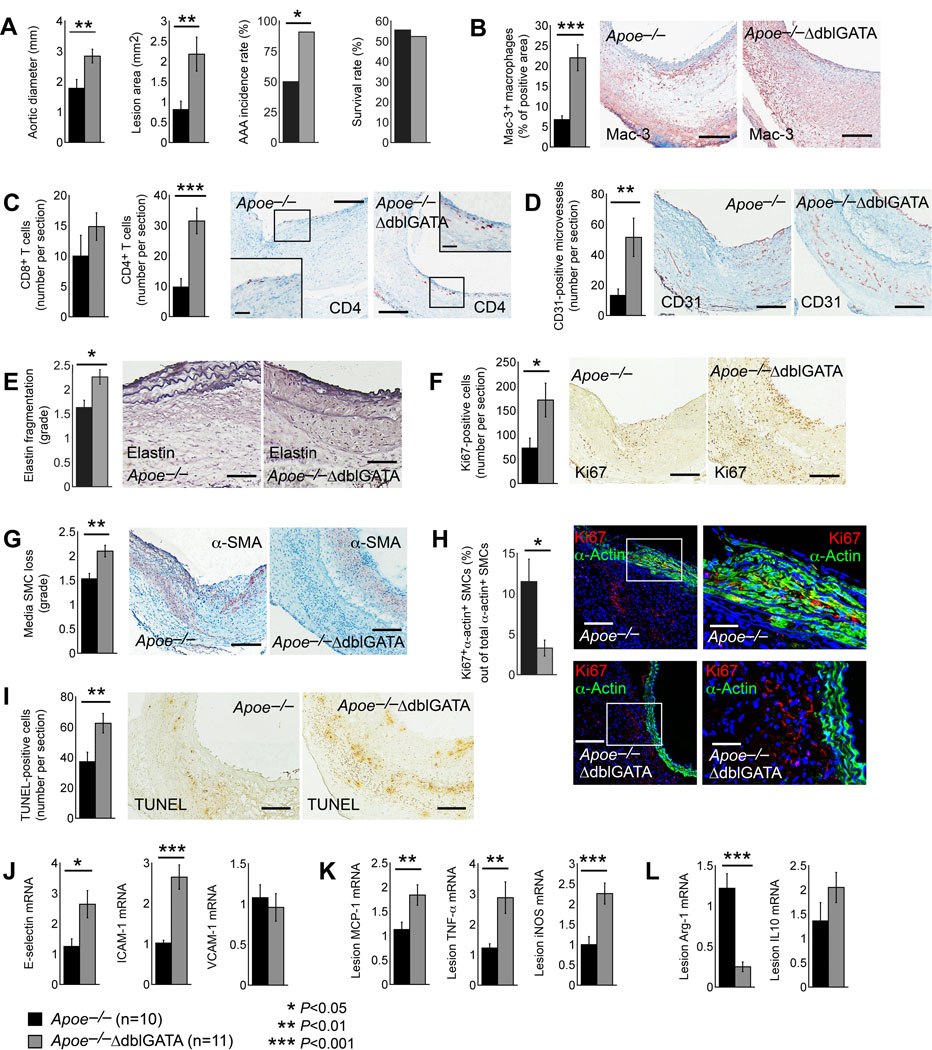
Ang-II perfusion-induced AAA in Apoe–/– and Apoe–/–ΔdblGATA mice. A. Abdominal aortic diameter, AAA lesion area, AAA incidence, and survival rate. B. Lesion Mac-3+ macrophage-positive area. Scale: 200 μm. C. Lesion CD8+ and CD4+ T-cell number. Scale: 200 μm, inset: 100 μm. D. CD31+ microvessel number. Scale: 200 μm. E. Elastin fragmentation grade. Scale: 100 μm. F. Ki67+ proliferating cell number. Scale: 200 μm. G. α-actin+ SMC loss in grade. Scale: 200 μm. H. Immunofluorescent double staining of α-actin and Ki67 to detect lesion SMC proliferation. Scale: 200 μm, inset: 70 μm. I. TUNEL staining detected lesion apoptotic cells. Scale: 200 μm. J-L. RT-PCR determined lesion mRNA levels of E-selectin, ICAM-1, and VCAM-1 (J), M1 macrophage markers MCP-1, TNF-α, and iNOS (K), and M2 macrophage markers Arg-1 and IL10 (L). Representative images for panels B-I are shown to the right. The mouse number and genotype of each cohort are indicated. Data are mean±SEM, *P<0.05, **P<0.01, ***P<0.001. Mann-Whitney U test.
EOS deficiency polarizes pro-inflammatory macrophage and monocyte differentiation.
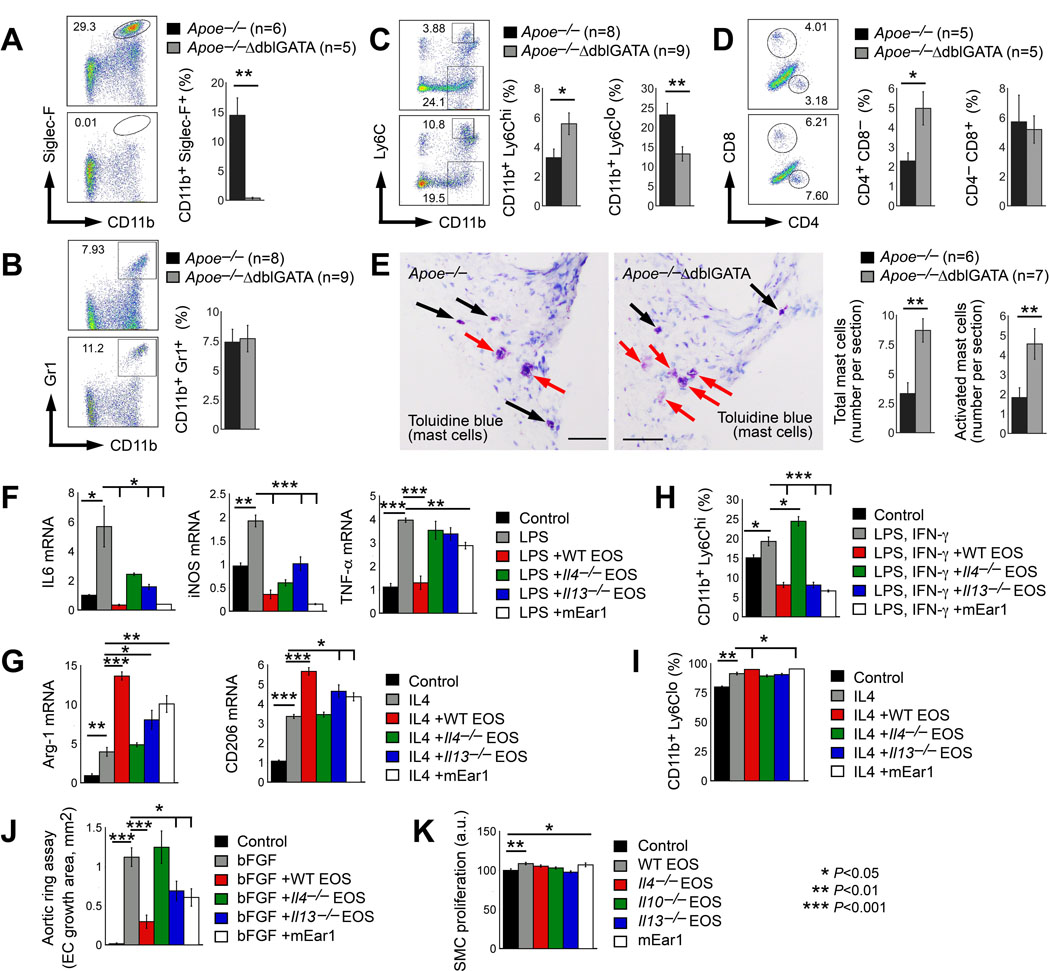
Increased lesion macrophages and T cells in AAA lesions from Apoe–/–ΔdblGATA mice (Figure 2B/2C) suggest that EOS deficiency affects both local (AAA lesion) and systemic inflammation. FACS analysis revealed increase of splenic CD11b+Gr1+ neutrophils and CD4+CD8− T cells in Apoe–/–ΔdblGATA mice, although the increase of neutrophils did not reach statistic significance and EOS deficiency did not affect splenic CD4–CD8+ T-cell content (Online Figure IVA/IVB). Apoe–/–ΔdblGATA mice had unexplained reduced levels of splenic CD11b+F4/80+ macrophages (Online Figure IVC), but had increased CD11b+Ly6Chi monocytes and reduced CD11b+Ly6Clo monocytes (Online Figure IVD). FACS analysis confirmed the loss of CD11b+Siglec-F+ EOS in AAA lesions from Apoe–/–ΔdblGATA mice (Figure 3A). Unlike in the spleens, EOS deficiency did not affect AAA lesion CD11b+Gr1+ neutrophil content (Figure 3B), but significantly increased lesion CD11b+Ly6Chi monocytes and CD4+CD8− T cells and reduced lesion CD11b+Ly6Clo monocytes, although CD4–CD8+ T-cell content did not change (Figure 3C/3D). Our earlier study demonstrated a pathogenic role for MCs in murine AAA.9 EOS deficiency in Apoe–/–ΔdblGATA mice may affect lesion MC contents as an indirect mechanism to enhance AAA growth. To test this hypothesis, we performed toluidine blue staining that allowed detection of AAA lesion degranulated (activated) and non-degranulated (inactive) MCs. Lesions from Apoe–/–ΔdblGATA mice contained many more total and activated MCs than the Apoe–/– mice (Figure 3E). Together with increased expression of M1 markers MCP1, TNF-α, and iNOS, but decreased expression of M2 marker Arg-1 in AAA lesions from Apoe–/–ΔdblGATA mice (Figure 2K/2L), we hypothesized a role for EOS in M2 macrophage and CD11b+Ly6Clo monocyte polarization. To test a role of EOS in macrophage polarization and to identify the molecules from EOS that were responsible for macrophage polarization, we prepared bone-marrow-derived macrophages (BMDMs) from wild-type (WT) mice and treated these BMDMs with LPS to induce M1 macrophage polarization or with IL4 to induce M2 macrophage polarization.25 To these LPS- and IL4-treated BMDMs, we added EOS lysate preparations from WT, Il4–/–, and Il13–/– mice and also mouse recombinant mEar1 to test whether EOS use these EOS molecules to control macrophage M1 or M2 polarizations. RT-PCR showed that LPS significantly increased BMDM expression of M1 markers IL6, iNOS, and TNF-α. WT EOS lysate fully suppressed such increases (Figure 3F). In contrast, suppression of M1 marker expression was much weaker when EOS lysates from Il4–/– and Il13–/– mice were used. Recombinant mEar1 acted similarly to WT EOS. mEar1 also blocked LPS-induced BMDM expression of IL6, iNOS, and TNF-α, although mEar1 activity in TNF-α expression suppression was much weaker than WT EOS (Figure 3F). IL4 induced BMDM expression of M2 markers Arg-1 and CD206. WT EOS increased IL4-induced expression of M2 markers Arg-1 and CD206 by 2~3 fold (Figure 3G). Such activity of EOS was muted when EOS lysate from Il4–/– mice was used. In contrast, EOS lysate from Il13–/– mice retained this activity, although much weaker than WT EOS. mEar1 also enhanced IL4-induced M2 marker expression (Figure 3G). These observations suggest that IL4, IL13, and mEar1 from EOS are potent suppressors of M1 macrophage polarization. EOS-derived IL4 and mEar1 are potent inducers of M2 macrophage polarization. LPS and IFN-γ together induce Ly6Chi monocyte differentiation, and IL4 induces Ly6Clo monocyte differentiation.26,27 EOS lysate preparations from WT and Il13–/– mice and recombinant mEar1significantly reduced Ly6Chi monocyte differentiation in mouse monocytes treated with LPS and IFN-γ. Similar activity did not occur with the use of EOS lysate prepared from Il4–/– mice (Figure 3H). Similarly, EOS lysate from WT mice and mEar1 also enhanced IL4-induced Ly6Clo monocyte differentiation, although EOS lysates from both Il4–/– and Il13–/– mice showed no effect (Figure 3I). These data suggest a role for EOS-derived IL4 and mEar1 in reducing Ly6Chi monocytes and increasing Ly6Clo monocytes, although our study does not exclude the possibility that changes of other immune cells due to EOS deficiency may indirectly contribute to M2 macrophage and Ly6Clo monocyte polarization in the spleens and AAA lesions from the Apoe–/–ΔdblGATA mice.
Figure 3.
EOS-derived IL4 and mEar1 activate macrophages, monocytes, ECs, and SMCs. FACS analysis of AAA lesion CD11b+Siglec-F+ EOS (A), CD11b+Gr-1+ neutrophils (B), CD11b+Ly-6chi and CD11b+Ly-6clow monocytes (C), CD4+CD8− and CD4–CD8+ T cells (D). E. Toluidine blue staining detected both inactive (black arrows) and active (red arrows) mast cells in AAA lesions. Scale: 200 μm. Representative images in A-E are shown to the left. F/G. RT-PCR determined the mRNA levels of M1 macrophage markers IL6, iNOS, and TNF-α in BMDMs treated with LPS and M2 macrophage markers Arg-1 and CD206 in BMDMs treated with IL4 with and without EOS lysate from WT, Il4–/– and Il13–/– mice, or mEar1. H/I. FACS detection of CD11b+Ly-6chi monocytes after bone-marrow-derived monocytes were treated with LPS and INF-γ, and CD11b+Ly-6clo monocytes after cells were treated with IL4, with and without EOS lysate from WT, Il4–/– and Il13–/– mice, or mEar1. J. Mouse aortic ring assay. bFGF was used as positive controls. K. Aortic SMC proliferation assay in the presence or absence of EOS lysates from WT, Il4–/– and Il13–/– mice, or mEar1. The numbers of mice are indicated in A-E. Results in F-K are mean±SEM of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001, Mann-Whitney U test (A-E) and one-way ANOVA test (F-K).
EOS activities in angiogenesis and SMC proliferation.
Increased microvessel numbers in AAA lesions from Apoe–/–ΔdblGATA mice (Figure 2D) suggests a role for EOS in inhibiting lesion angiogenesis. To test this hypothesis and to identify the essential EOS molecules responsible for this activity of EOS, mouse aortic ring assay was performed via embedding mouse aorta fragment from WT mice in matrigel, followed by covering with culture medium containing pro-angiogenic factor bFGF (basic fibroblast growth factor) with and without EOS lysates from WT, Il4–/–, and Il13–/– mice, or recombinant mEar1. EOS from WT and Il13–/– mice, as well as mEar1 significantly blunted bFGF-induced microvessel growth from the aortic ring. In contrast, EOS lysate from Il4–/– mice failed to block bFGF-induced microvessel growth (Figure 3J). These observations support a role for EOS-derived IL4 and mEar1 in inhibiting AAA lesion angiogenesis (Figure 2D). AAA lesions from Apoe–/–ΔdblGATA mice revealed reduced SMC content and SMC proliferation (Figure 2G/2H). EOS deficiency did not affect lesion SMC apoptosis (data not shown). These observations suggest a role for EOS in promoting SMC proliferation. Cultured mouse aortic SMCs with EOS lysates from different EOS or mEar1 tested this hypothesis. Lysate preparation from WT EOS and recombinant mEar1, but not lysates from Il4–/–, Il10–/–, and Il13–/– mice significantly increased aortic SMC proliferation (Figure 3K).
EOS-derived IL4 and mEar1 block AAA lesion nuclear factor (NF)-κB activation.
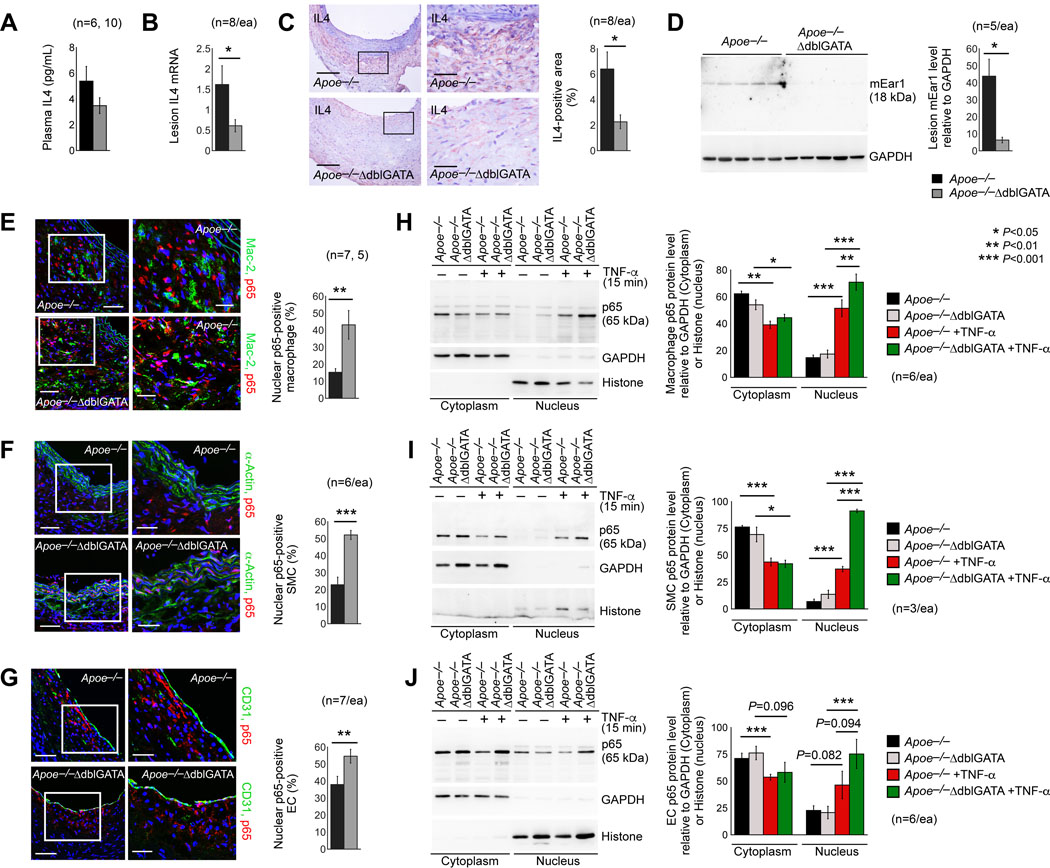
In hearts from mice with myocarditis, EOS accounted for 65% of total heart IL4 production.28–30 EOS may also produce a major fraction of IL4 in AAA lesions. ELISA showed comparable plasma IL4 levels between Apoe–/– and Apoe–/–ΔdblGATA mice after Ang-II perfusion-induced AAA (Figure 4A). Yet, both RT-PCR and immunostaining revealed IL4 deficiency in AAA lesions from Apoe–/–ΔdblGATA mice (Figure 4B/4C). Immunoblot analysis showed the absence of EOS molecule mEar1 in AAA lesions from the Apoe–/–ΔdblGATA (Figure 4D), confirming the EOS deficiency in these mice.
Figure 4.
AAA lesion EOS are essential source of IL4 and EOS block NF-κB activation in macrophages, SMCs, and ECs from mouse AAA lesions. A. ELISA analysis of plasma IL4 in Apoe–/– and Apoe–/–ΔdblGATA mice after Ang-II perfusion-induced AAA. RT-PCR (B), immunohistology (C), and immunoblot (D) detected lesion IL4 and mEar1 in these mice. Immunofluorescent double staining of p65 detected p65 accumulation in Mac-2+ macrophage (E), α-actin+ SMC (F), and CD31+ EC (G) nuclei in AAA lesions from Apoe–/– and Apoe–/–ΔdblGATA mice at 28 days after Ang-II perfusion. Scale: 200 μm, inset: 70 μm. The number and genotype of each cohort of mice are indicated. Immunoblot analysis of p65 in cytoplasm and nucleus of BMDM (H), SMC (I), and EC (J) from Apoe–/– and Apoe–/–ΔdblGATA mice after cells were treated with or without TNF-α for 15 min. The numbers of experiments are indicated. Data are mean±SEM. *P<0.05, **P<0.01, ***P<0.001, Mann-Whitney U test (A-G) and one-way ANOVA test (H-J).
NF-κB is a transcription factor that has been considered a prototypical pro-inflammatory signaling pathway, largely based on the activation of NF-κB by pro-inflammatory cytokines. It regulates both innate and adaptive immune responses.31,32 In the aortic wall, endothelial NF-κB activation up-regulates adhesion molecule expression,33 essential for inflammatory cell accumulation. NF-κB activation reduces SMC differentiation and proliferation following aortic injury34 and involves SMC apoptotic and inflammatory signaling.35 Macrophage NF-κB activation promotes M1 polarization.36 Using immunofluorescent double staining with both the NF-κB p65 antibody and cell type-specific antibodies, including Mac-2, α-actin, and CD31, we detected significantly increased NF-κB p65 nuclear localization in macrophages, SMCs, and ECs in AAA lesions from Apoe–/–ΔdblGATA mice, compared with that in Apoe–/– mice (Figure 4E–4G). These observations suggest a role for EOS in blocking NF-κB activation in these aortic inflammatory and vascular cells and may explain why AAA lesions from Apoe–/–ΔdblGATA mice showed increased macrophage and T-cell accumulation (Figure 2B/2C), reduced SMC proliferation (Figure 2H), increased expression of M1 macrophage markers (Figure 2K), and reduced expression of M2 macrophage marker (Figure 2L). Cultured BMDM, abdominal aortic SMCs, and ECs yielded the same conclusion. Cells from Apoe–/– and Apoe–/–ΔdblGATA mice were treated with TNF-α for 15 min, followed by preparation of cytoplasm and nucleus fractions. Immunoblot analysis showed that TNF-α reduced cytoplasm p65 levels, but increased nuclear p65 levels in these cells. Such increase was higher in cells from Apoe–/–ΔdblGATA mice than those from Apoe–/– mice (Figure 4H–4J), although this increase in ECs did not reach statistic significance (Figure 4J).
EOS are essential source of IL4 and mEar1 in AAA lesions (Figure 4B–4D) and these molecules may contribute to the activity of EOS in blocking p65 nuclear localization in AAA lesion macrophages, SMCs, and ECs (Figure 4E–4J). To test this hypothesis, we treated mouse BMDMs, aortic SMCs, and ECs with EOS lysate preparations from WT and Il4–/– mice, and mEar1. Immunofluorescent double staining showed that, in all three cell types, EOS from WT mice and mEar1 significantly blocked p65 nuclear translocation. In contrast, EOS lysate preparation from Il4–/– mice showed much weaker activities or no effect in p65 nuclear translocation (Figure 5A–5C). Immunoblot analysis revealed the same observations. EOS lysate preparation from WT mice and mEar1 blocked the expression of phosphor-p65 (p-p65) in BMDMs, aortic SMCs, and ECs over time (0, 15, or 30 min). In contrast, EOS lysate preparation from Il4–/– mice showed negligible effect on p-p65 expression in BMDMs, aortic SMCs, and ECs (Figure 5D–5F). These observations support a role of EOS-derived IL4 and mEar1 in controlling NF-κB activation in aortic wall inflammatory and vascular cells.
Figure 5.
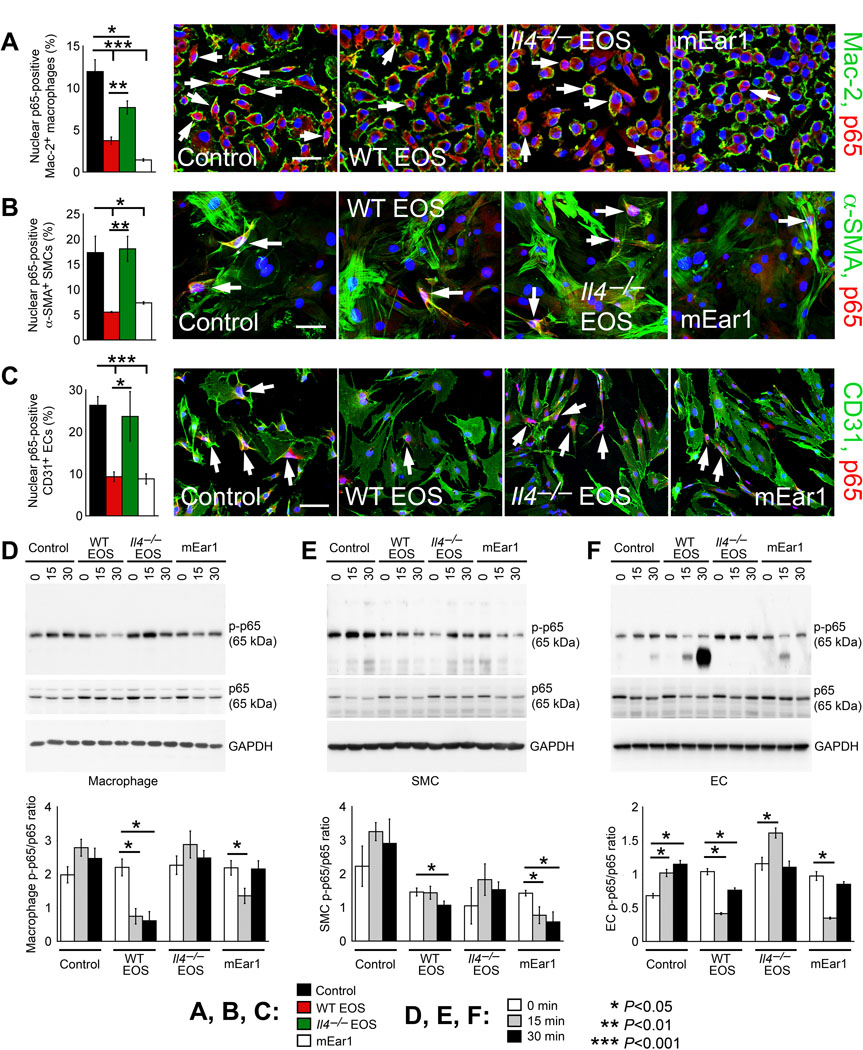
EOS-derived IL4 and mEar1 participate in NF-κB activation in cultured mouse BMDMs, SMCs, and ECs. A-C. Immunofluorescent double staining localized p65 accumulation in the nuclei of Mac-2-positive BMDMs (A), α-actin-positive aortic SMCs (B), and CD31-positive aortic ECs (C) treated with and without EOS lysates from WT and Il4–/– mice, or mEar1 for 15 min. Representative images are shown to the right panels. Scale: 70 μm. D-F. Immunoblot analyses of p-p65 and p65 and their ratios in BMDMs (D), aortic SMCs (E), and aortic ECs (F) treated with and without EOS lysates from WT or Il4–/– mice, or mEar1 at different time points as indicated. GAPDH immunoblots ensured equal protein loading. Representative blots are shown to the top of each panel. Data are mean±SEM from 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA test.
EOS-derived IL4 and mEar1 protect mice from AAA growth.
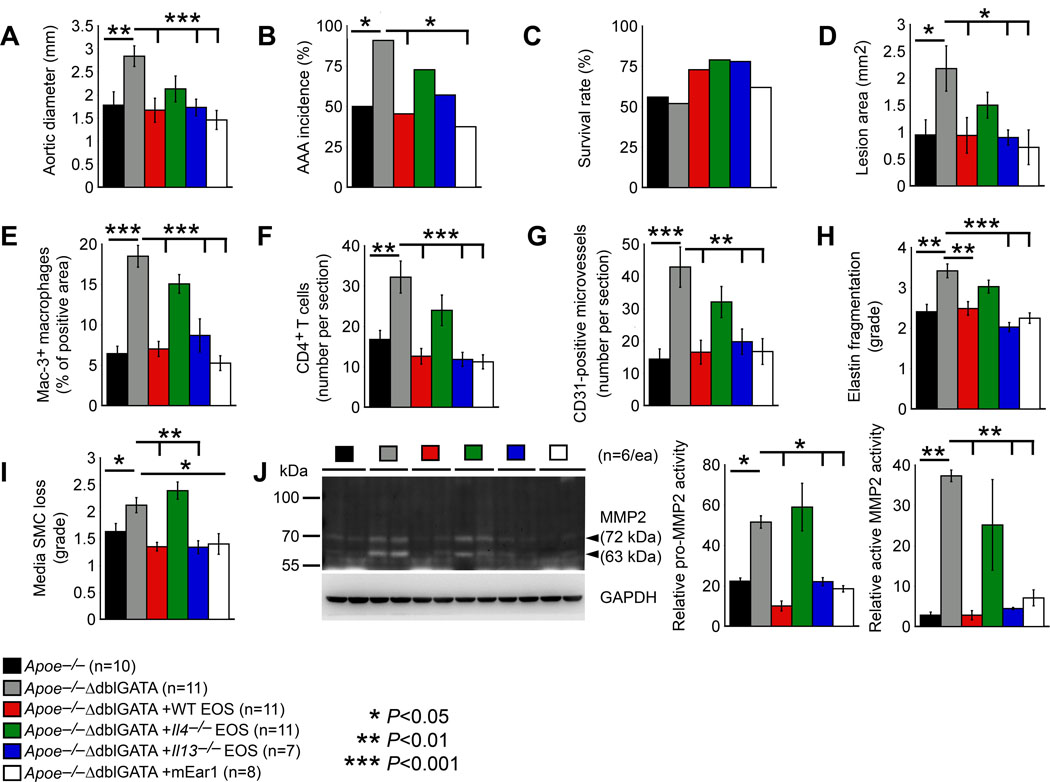
Immunofluorescent staining and immunoblot analysis of AAA lesions and cultured BMDMs, aortic SMCs, and ECs all indicated a role for EOS-derived IL4 and mEar1 in blocking NF-κB activation (Figures 4 and 5). EOS may use these molecules to reduce Ang-II perfusion-induced AAA. Increase of AAA growth and lesion complications in Apoe–/–ΔdblGATA mice (Figure 2) supports this hypothesis. To test a direct role of EOS in protecting mice from AAA and to identify essential molecules that facilitated EOS activity in reducing AAA growth, we performed adoptive transfer of EOS from WT, Il4–/–, and Il13–/– mice to Apoe–/–ΔdblGATA recipient mice. Due to the unavailability of mEar1-deficeint EOS, we treated Apoe–/–ΔdblGATA mice with mouse recombinant mEar1 by subcutaneous perfusion via an osmatic minipump to reduce mouse stress from daily administration. Among all tested groups of mice, both systolic and diastolic blood pressures increased after AAA production, but we did not detect significant differences in blood pressures among different treatments (Online Figure V). Yet, our results showed that adoptive transfer of EOS from WT mice and recombinant mEar1 but not EOS from Il4–/– mice blunted EOS deficiency-associated increases in aortic expansion, AAA incidence, and AAA cross section lesion area as defined in Online Figure II. WT EOS and mEar1 also increased the post-Ang-II perfusion-associated mouse survival rate, although such increases did not reach statistic significance (Figure 6A-6D). EOS from Il13–/– mice also reduced AAA growth and cross section lesion area in Apoe–/–ΔdblGATA recipient mice (Figure 6A/6D). Donor EOS from WT and Il13–/– mice and recombinant mEar1 reduced lesion Mac-3+ macrophage content, CD4+ T-cell number, CD31-positive microvessel number, aortic wall elastin fragmentation, and media SMC loss. These inhibitory activities disappeared if EOS from Il4–/– mice were used (Figure 6E-6I). Together, these observations demonstrated that EOS used at least IL4 and mEar1 to suppress AAA growth by blocking lesion inflammatory cell accumulation and lesion microvessel growth. EOS-derived IL13 may moderately affect AAA growth. In Figure 4 and 5, we proposed a role of EOS and EOS-derived IL4 and mEar1 in blocking aortic inflammatory and vascular cell NF-κB activation. We performed gelatin gel zymography to assess AAA lesion matrix metalloproteinase (MMP) activities that may serve as NF-κB activation downstream activity and explain aortic wall elastin fragmentation. Both MMP2 and pro-MMP2 activities increased in AAA lesions from Apoe–/–ΔdblGATA mice. Such increases were blocked when mice received donor EOS from WT and Il13–/– mice or treated with recombinant mEar1. In contrast, EOS from Il4–/– mice did not affect AAA lesion MMP2 or pro-MMP2 activities (Figure 6J).
Figure 6.
EOS-derived IL4 and mEar1 are essential to Ang-II perfusion-induced AAA. Apoe–/–ΔdblGATA mice received adoptive transfer of EOS from WT, Il4–/– and Il13–/– mice, or osmatic minipump delivery of mEar1 followed by Ang-II infusion-induced AAA for 28 days. A. Abdominal aortic diameter. B. AAA incidence rate. C. Survival rate after Ang-II perfusion. D. AAA lesion area. E. Lesion Mac-3+ macrophage-positive area. F. Lesion CD4+ T-cell number. G. Lesion CD31+ microvessel number. H. Lesion elastin fragmentation grade. I. Lesion media SMC loss grade. J. Gelatin gel zymography detected lesion MMP2 and pro-MMP2 activity. Representative blot is shown to the left. The number of experiments and the number, genotype, and treatment of each cohort of mice are indicated. Data are mean±SEM. *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA test.
With unexplained mechanisms, Apoe–/–ΔdblGATA mice showed reduced plasma cholesterol, LDL, and triglyceride levels and increased plasma HDL levels compared with those in Apoe–/– control mice after AAA production. These observations agreed with earlier studies that plasma HDL associated negatively with blood EOS count and plasma triglyceride associated positively with blood EOS count in patients with or without atopic asthma.37 Only EOS from WT and Il13–/– mice increased plasma triglyceride levels in Apoe–/–ΔdblGATA recipient mice. None of the treatments, donor EOS or mEar1, affected plasma cholesterol, LDL, or HDL in Apoe–/–ΔdblGATA recipient mice (Online Figure VIA-VID).
DISCUSSION
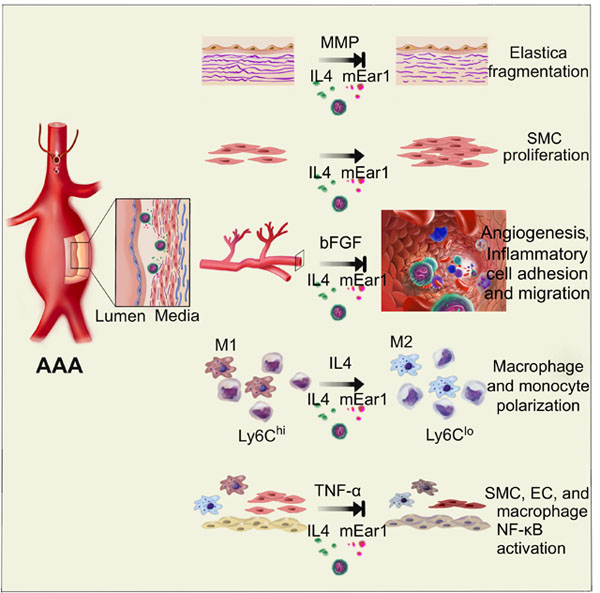
EOS and EOS cationic protein ECP are established risk factors of human CVD.17–20 EOS and ECP likely play a pathogenic role in human and murine AAA. Yet, this study demonstrates a beneficial role of EOS and human ECP homolog mEar1 in mouse AAA. In Ang-II-induced mouse AAA, EOS release IL4 and mEar1 to protect mice from AAA. Observations from in vitro mechanistic studies support the observations from AAA lesions. EOS release IL4 and mEar1 to polarize M2 macrophages and Ly6Clo monocytes, reduce angiogenesis, block aortic wall MMP2 activity, increase SMC proliferation, and blunt NF-κB activation in macrophages, SMCs, and ECs. All these activities support the conclusions that EOS deficiency in Apoe–/–ΔdblGATA mice increased AAA growth, lesion inflammatory cell content, angiogenesis, arterial wall elastica fragmentation, lesion cell apoptosis, SMC loss, and M1 macrophage marker expression. Therefore, as a compensatory mechanism, increased EOS contents in blood and in AAA lesions from humans and mice may play a protective role. A risk factor from population studies may not necessarily indicate a pathogenic role.
Yet, several questions linger from this study. Both adoptive transfer of live EOS from WT and Il4–/– mice to Apoe–/–ΔdblGATA mice with increased AAA growth, and in vitro cell culture experiments using EOS lysates from WT and Il4–/– mice all support a role for EOS-derived IL4 in protecting AAA growth. In mouse AAA lesions, EOS deficiency led to IL14 deficiency, suggesting that AAA lesion EOS are one of the major sources of lesion IL4. Th2 cells are also important sources of IL4.38,39 One question is why IL4 from Th2 cells cannot replace those from EOS in AAA lesions from Apoe–/–ΔdblGATA mice. Indeed, AAA is a Th2-dominant immune disease with increased lesion IL4 levels.40,41 Increased AAA in EOS-deficient Apoe–/–ΔdblGATA mice suggest a moderate role of AAA lesion EOS in type-2 immunity. Results from our study may support this hypothesis. We showed that Apoe–/–ΔdblGATA mice had comparable plasma IL4 levels to those from Apoe–/– mice. Therefore, EOS activity in AAA growth may affect not only lesion adaptive or innate immunity, but also other anti-inflammatory activities, such as promoting M2 macrophage and Ly6Clo polarizations and inhibiting matrix protease expression and degradation, angiogenesis, and inflammatory and vascular cell proliferation and NF-κB activation. IL4 deficiency in EOS possibly changed the overall anti-inflammatory activity of EOS. IL4 expression in EOS likely remains necessary to maintain the EOS anti-inflammatory activity, a hypothesis that merits detailed investigation.
Our data from mEar1-treated Apoe–/–ΔdblGATA mice in vivo and mEar1-treated macrophages, SMCs, and ECs in vitro all support a role for mEar1 in reducing AAA growth by blocking inflammatory and vascular cell activation. Evidence supports mEar1 as a homolog of human ECP based on its sequence identity.42 Yet, human ECP increases ICAM-1 expression in ECs, followed by increasing monocyte adhesion.43–45 These prior studies suggest a pathogenic role of human ECP in AAA. These conflicting observations from different studies with different species remain unexplained. Human ECP is highly positively charged because of its rich arginines in its primary amino acid sequence. Positively charged ECP binds to the negatively charged heparan sulfate proteoglycans on the cell surface and causes ECP cellular internalization and cell membrane disruption. Therefore, ECP is considered cytotoxic.46 The isoelectric point pI of human ECP is 11.4, much higher than mouse mEar1 (pI=9.2) that is close to human EDN (pI=8.9).47 It is possible that mouse mEar1 may be the homolog of human EDN, but not ECP. From the primary amino acid sequence alignment, human ECP and EDN share similar levels of homology to mEar1.42 Human EDN may act similarly to mouse mEar1 and may play a protective role in human AAA, a hypothesis that also merits further investigation.
EOS are key innate immune cells in the human and murine hyperresponsive airway by regulating macrophage phagocytosis, SMC contraction, and MC and basophil degranulation.48,49 Accumulating studies revealed additional roles of EOS in thymic T-cell development and recruitment,50,51 B-cell proliferation,52 Th2 polarization,53,54 myeloid dendritic cell chemoattraction and cytokine production,55,56 and tissue repairing.57,58 This study reveals a novel role of EOS on macrophage and monocyte polarization and macrophage and vascular cell NF-κB activation inhibition. This study also suggests that the human homolog of mouse mEar1, although not identified in this study, or recombinant mouse mEar1 have potential clinical significance to patients with AAA.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Blood eosinophil counts and eosinophil cationic proteins are associated with human cardiovascular diseases.
Like other granulocytes, eosinophils are rich in cytokines, chemokines, growth factors, and cationic proteins, but eosinophil function in cardiovascular diseases remains untested.
What New Information Does This Article Contribute?
Increased blood eosinophil count serves as an independent risk factor of human abdominal aortic aneurysms (AAA). Eosinophils accumulate in human and murine AAA lesions.
Eosinophil deficiency exacerbates Ang-II perfusion-induced AAA in mice.
Mouse eosinophil-derived IL4 and mEar1 promote M2 macrophage and Ly6Clo monocyte polarization, reduce angiogenesis, enhance smooth muscle cell proliferation, and blunt NF-κB activation in macrophages, smooth muscle and endothelial cells.
Exacerbated AAA formation in eosinophil-deficient mice can be blocked by adoptive transfer of in vitro-prepared eosinophils or administration of recombinant mEar1.
Whether eosinophils play a role in cardiovascular diseases is currently not clear. Here, we report elevated blood eosinophil count in patients with AAA, with high blood eosinophil count serving as an independent risk factor for this human aortic disease. In human and murine AAA lesions, we detect elevated eosinophil accumulation. Contrary to our expectation, these innate immune cells release IL4 and cationic proteins to protect mice from angiotensin-II perfusion-induced AAA by blocking angiogenesis and consequent lesion inflammatory cell accumulation, skewing M1 to M2 macrophage and Ly6Chi to Ly6Clo monocyte polarizations, reducing lesion macrophage, endothelial cell, and fibroblast proliferation but enhancing lesion smooth muscle cell proliferation; protecting aortic wall elastica from proteolytic fragmentation, and blunting lesion macrophage, smooth muscle cell, and endothelial cell NF-κB activation. These eosinophil-derived molecules may present a novel paradigm to slow human AAA growth.
ACKNOWLEDGMENTS
The authors thank Dr. Helene F. Rosenberg, previously of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD for helps with mouse eosinophil culture, Ms. Eugenia Shvartz for her technical assistance, and Ms. Chelsea Swallom for her editorial assistance.
SOURCES OF FUNDING
This study is supported by grants from the Finance Science and Technology Projects of Hainan Province (ZDYF2018102 to JG), the National Natural Science Foundation of China (81770487, 81460042 to JG; 81870328 to JZ), the University-College Joint Cultivation Fund of Zhengzhou University (2016_BSTDJJ-19 to JZ), the National Heart, Lung, and Blood Institute (HL60942, HL123568 to GPS; HL34636, HL80472 to PL), and the National Institute of Neurological Disorders and Stroke (AG063839 to GPS). Dr. Conglin Liu is supported by the American Heart Association Postdoctoral Fellowship # 17POST33670564).
Nonstandard Abbreviations and Acronyms:
- EOS
eosinophil
- AAA
abdominal aortic aneurysm
- OR
odds ratio
- HR
hazard ratio
- SMC
smooth muscle cell
- mEar1
mouse EOS-associated-ribonuclease-1
- Ang-II
angiotensin-II
- MC
mast cell
- ECP
eosinophilic cationic protein
- EDN
EOS-derived neurotoxin
- MBP
major basic protein
- EPO
eosinophilic peroxidase
- CVD
cardiovascular disease
- STEMI
ST-segment elevation myocardial infarction
- PCI
percutaneous coronary intervention
- MACE
major adverse cardiac event
- ELR
eosinophil-to-leukocyte ratio
- NF-κB
nuclear factor-κB
- COPD
chronic obstructive pulmonary disease
- AMI
acute myocardial infarction
- PAD
peripheral arterial disease
- BMI
body mass index
- ICAM-1
intercellular adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
- MCP-1
monocyte chemoattractant protein-1
- TNF-α
tumor necrosis factor-α
- iNOS
inducible nitric oxide synthase
- Arg-1
arginase-1
- BMDM
bone-marrow-derived macrophage
- WT
wild-type
- bFGF
basic fibroblast growth factor
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Liu CL, Wemmelund H, Wang Y, Liao M, Lindholt JS, Johnsen SP, Vestergaard H, Fernandes C, Sukhova GK, Cheng X, Zhang JY, Yang C, Huang X, Daugherty A, Levy BD, Libby P, Shi GP. Asthma associates with human abdominal aortic aneurysm and rupture. Arterioscler Thromb Vasc Biol. 2016;36:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu CL, Wang Y, Liao M, Wemmelund H, Ren J, Fernandes C, Zhou Y, Sukhova GK, Lindholt JS, Johnsen SP, Zhang JY, Cheng X, Huang X, Daugherty A, Levy BD, Libby P, Shi GP. Allergic lung inflammation aggravates angiotensin ii-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2016;36:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradding P. Asthma: Eosinophil disease, mast cell disease, or both? Allergy Asthma Clin Immunol. 2008;4:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone KD, Prussin C, Metcalfe DD. Ige, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. [DOI] [PubMed] [Google Scholar]
- 6.Nakagome K, Nagata M. Involvement and possible role of eosinophils in asthma exacerbation. Front Immunol. 2018;9:2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlin JS, Hallgren J. Mast cell progenitors: Origin, development and migration to tissues. Mol Immunol. 2015;63:9–17. [DOI] [PubMed] [Google Scholar]
- 8.Kambe N, Hiramatsu H, Shimonaka M, Fujino H, Nishikomori R, Heike T, Ito M, Kobayashi K, Ueyama Y, Matsuyoshi N, Miyachi Y, Nakahata T. Development of both human connective tissue-type and mucosal-type mast cells in mice from hematopoietic stem cells with identical distribution pattern to human body. Blood. 2004;103:860–867. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takamoto M, Sugane K. Synergism of il-3, il-5, and gm-csf on eosinophil differentiation and its application for an assay of murine il-5 as an eosinophil differentiation factor. Immunol Lett. 1995;45:43–46 [DOI] [PubMed] [Google Scholar]
- 12.John AE, Thomas MS, Berlin AA, Lukacs NW. Temporal production of ccl28 corresponds to eosinophil accumulation and airway hyperreactivity in allergic airway inflammation. Am J Pathol. 2005;166:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esnault S, Kelly EA. Essential mechanisms of differential activation of eosinophils by il-3 compared to gm-csf and il-5. Crit Rev Immunol. 2016;36:429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen BJ. Eosinophils: A review. Vet Res Commun. 1992;16:11–44. [DOI] [PubMed] [Google Scholar]
- 15.Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: Characteristics and roles in pathogenesis. Nat Rev Rheumatol. 2014;10:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bystrom J, Amin K, Bishop-Bailey D. Analysing the eosinophil cationic protein--a clue to the function of the eosinophil granulocyte. Respir Res. 2011;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verdoia M, Schaffer A, Cassetti E, Di Giovine G, Marino P, Suryapranata H, De Luca G. Absolute eosinophils count and the extent of coronary artery disease: A single centre cohort study. J Thromb Thrombolysis. 2015;39:459–466. [DOI] [PubMed] [Google Scholar]
- 18.Welsh C, Welsh P, Mark PB, Celis-Morales CA, Lewsey J, Gray SR, Lyall DM, Iliodromiti S, Gill JMR, Pell J, Jhund PS, Sattar N. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the uk biobank. Arterioscler Thromb Vasc Biol. 2018;38:1415–1423. [DOI] [PubMed] [Google Scholar]
- 19.Niccoli G, Ferrante G, Cosentino N, Conte M, Belloni F, Marino M, Baca M, Montone RA, Sabato V, Schiavino D, Patriarca G, Crea F. Eosinophil cationic protein: A new biomarker of coronary atherosclerosis. Atherosclerosis. 2010;211:606–611. [DOI] [PubMed] [Google Scholar]
- 20.Niccoli G, Calvieri C, Flego D, Scalone G, Imaeva A, Sabato V, Schiavino D, Liuzzo G, Crea F. Allergic inflammation is associated with coronary instability and a worse clinical outcome after acute myocardial infarction. Circ Cardiovasc Interv. 2015;8:e002554 [DOI] [PubMed] [Google Scholar]
- 21.Konishi T, Funayama N, Yamamoto T, Morita T, Hotta D, Nishihara H, Tanaka S. Prognostic value of eosinophil to leukocyte ratio in patients with st-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Atheroscler Thromb. 2017;24:827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: A caliber cohort study. Open Heart. 2016;3:e000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teodosio C, Mayado A, Sanchez-Munoz L, Morgado JM, Jara-Acevedo M, Alvarez-Twose I, Garcia-Montero AC, Matito A, Caldas C, Escribano L and Orfao A. The immunophenotype of mast cells and its utility in the diagnostic work-up of systemic mastocytosis. J Leukoc Biol. 2015;97:49–59. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Yu X, Chen H, Sjoberg S, Roux J, Zhang L, Ivoulsou AH, Bensaid F, Liu CL, Liu J, Tordjman J, Clement K, Lee CH, Hotamisligil GS, Libby P, Shi GP. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing m2 macrophages. Cell Metab. 2015;22:1045–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PY, Li Y, Kumagai Y, Xu Y, Weinstein JS, Kellner ES, Nacionales DC, Butfiloski EJ, van Rooijen N, Akira S, Sobel ES, Satoh M, Reeves WH. Type i interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol. 2009;175:2023–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chihara J, Yamamoto T, Kurachi D, Kakazu T, Higashimoto I and Nakajima S. Possible release of eosinophil granule proteins in response to signaling from intercellular adhesion molecule-1 and its ligands. Int Arch Allergy Immunol. 1995;108 Suppl 1:52–4. [DOI] [PubMed] [Google Scholar]
- 30.Bjerke T, Gaustadnes M, Nielsen S, Nielsen LP, Schiotz PO, Rudiger N, Reimert CM, Dahl R, Christensen I and Poulsen LK. Human blood eosinophils produce and secrete interleukin 4. Respir Med. 1996;90:271–7. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence T. The nuclear factor nf-kappab pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Zhang L, Joo D, Sun SC. Nf-kappab signaling in inflammation. Signal Transduct Target Ther. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito T, Hasegawa Y, Ishigaki Y, Yamada T, Gao J, Imai J, Uno K, Kaneko K, Ogihara T, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Importance of endothelial nf-kappab signalling in vascular remodelling and aortic aneurysm formation. Cardiovasc Res. 2013;97:106–114. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Yamashita M, Horimai C, Hayashi M. Smooth muscle-selective inhibition of nuclear factor-kappab attenuates smooth muscle phenotypic switching and neointima formation following vascular injury. J Am Heart Assoc. 2013;2:e000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrhof FB, Schmidt-Ullrich R, Dietz R, Scheidereit C. Regulation of vascular smooth muscle cell proliferation: Role of nf-kappab revisited. Circ Res. 2005;96:958–964. [DOI] [PubMed] [Google Scholar]
- 36.Liu CP, Zhang X, Tan QL, Xu WX, Zhou CY, Luo M, Li X, Huang RY, Zeng X. Nf-kappab pathways are involved in m1 polarization of raw 264.7 macrophage by polyporus polysaccharide in the tumor microenvironment. PLoS One. 2017;12:e0188317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barochia AV, Gordon EM, Kaler M, Cuento RA, Theard P, Figueroa DM, Yao X, Weir NA, Sampson ML, Stylianou M, Choy DF, Holweg CTJ, Remaley AT and Levine SJ. High density lipoproteins and type 2 inflammatory biomarkers are negatively correlated in atopic asthmatics. J Lipid Res. 2017;58:1713–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilmour J, Lavender P. Control of il-4 expression in t helper 1 and 2 cells. Immunology. 2008;124:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sofi MH, Qiao Y, Ansel KM, Kubo M, Chang CH. Induction and maintenance of il-4 expression are regulated differently by the 3’ enhancer in cd4 t cells. J Immunol. 2011;186:2792–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schonbeck U, Sukhova GK, Gerdes N, Libby P. T(h)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. [DOI] [PubMed] [Google Scholar]
- 42.Larson KA, Olson EV, Madden BJ, Gleich GJ, Lee NA, Lee JJ. Two highly homologous ribonuclease genes expressed in mouse eosinophils identify a larger subgroup of the mammalian ribonuclease superfamily. Proc Natl Acad Sci U S A. 1996;93:12370–12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venge P, Bystrom J, Carlson M, Hakansson L, Karawacjzyk M, Peterson C, Seveus L, Trulson A. Eosinophil cationic protein (ecp): Molecular and biological properties and the use of ecp as a marker of eosinophil activation in disease. Clin Exp Allergy. 1999;29:1172–1186. [DOI] [PubMed] [Google Scholar]
- 44.Chihara J, Yamamoto T, Kurachi D, Kakazu T, Higashimoto I, Nakajima S. Possible release of eosinophil granule proteins in response to signaling from intercellular adhesion molecule-1 and its ligands. Int Arch Allergy Immunol. 1995;108 Suppl 1:52–54. [DOI] [PubMed] [Google Scholar]
- 45.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: A caliber cohort study. Open Heart. 2016;3:e000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Oliveira PC, De Oliveira Lopes D, Do Vale Coelho IE, Pereira MC. Cytotoxic activities of eosinophil cationic protein and eosinophil-derived neurotoxin: In silico analysis. Cancer Genomics Proteomics. 2015;12:397–402. [PubMed] [Google Scholar]
- 47.Batten D, Dyer KD, Domachowske JB, Rosenberg HF. Molecular cloning of four novel murine ribonuclease genes: Unusual expansion within the ribonuclease a gene family. Nucleic Acids Res. 1997;25:4235–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefkowitz DL, Lincoln JA, Howard KR, Stuart R, Lefkowitz SS, Allen RC. Macrophage-mediated candidacidal activity is augmented by exposure to eosinophil peroxidase: A paradigm for eosinophil-macrophage interaction. Inflammation. 1997;21:159–172. [DOI] [PubMed] [Google Scholar]
- 49.Furuta GT, Nieuwenhuis EE, Karhausen J, Gleich G, Blumberg RS, Lee JJ, Ackerman SJ. Eosinophils alter colonic epithelial barrier function: Role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890–897 [DOI] [PubMed] [Google Scholar]
- 50.Throsby M, Herbelin A, Pleau JM, Dardenne M. Cd11c+ eosinophils in the murine thymus: Developmental regulation and recruitment upon mhc class i-restricted thymocyte deletion. J Immunol. 2000;165:1965–1975. [DOI] [PubMed] [Google Scholar]
- 51.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector t cells. J Exp Med. 2008;205:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong TW, Doyle AD, Lee JJ, Jelinek DF. Eosinophils regulate peripheral b cell numbers in both mice and humans. J Immunol. 2014;192:3548–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Ghahary A, Moqbel R. Cutting edge: Human eosinophils regulate t cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. [DOI] [PubMed] [Google Scholar]
- 54.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the tlr2-myd88 signal pathway in dendritic cells and enhances th2 immune responses. J Exp Med. 2008;205:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (edn), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. [DOI] [PubMed] [Google Scholar]
- 56.Yang D, Chen Q, Rosenberg HF, Rybak SM, Newton DL, Wang ZY, Fu Q, Tchernev VT, Wang M, Schweitzer B, Kingsmore SF, Patel DD, Oppenheim JJ, Howard OM. Human ribonuclease a superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol. 2004;173:6134–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogan SP. Functional role of eosinophils in gastrointestinal inflammation. Immunol Allergy Clin North Am. 2009;29:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, Chawla A. Eosinophils secrete il-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110:9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.