Abstract
Rapid phosphoester hydrolysis of endogenous purine and pyrimidine nucleotides has challenged the characterization of the role of P2 receptors in physiology and pathology. Nucleotide phosphoester stabilization has been pursued on a number of medicinal chemistry fronts. We investigated the in vitro and in vivo stability and pharmacokinetics of prototypical nucleotide P2Y1 receptor (P2Y1R) agonists and antagonists. These included the riboside nucleotide agonist 2-methylthio-ADP and antagonist MRS2179, as well as agonist MRS2365 and antagonist MRS2500 containing constrained (N)-methanocarba rings, which were previously reported to form nucleotides that are more slowly hydrolyzed at the α-phosphoester compared with the ribosides. In vitro incubations in mouse and human plasma and blood demonstrated the rapid hydrolysis of these compounds to nucleoside metabolites. This metabolism was inhibited by EDTA to chelate divalent cations required by ectonucleotidases for nucleotide hydrolysis. This rapid hydrolysis was confirmed in vivo in mouse pharmacokinetic studies that demonstrate that MRS2365 is a prodrug of the nucleoside metabolite AST-004 (MRS4322). Furthermore, we demonstrate that the nucleoside metabolites of MRS2365 and 2-methylthio-ADP are adenosine receptor (AR) agonists, notably at A3 and A1ARs. In vivo efficacy of MRS2365 in murine models of traumatic brain injury and stroke can be attributed to AR activation by its nucleoside metabolite AST-004, rather than P2Y1R activation. This research suggests the importance of reevaluation of previous in vitro and in vivo research of P2YRs and P2XRs as there is a potential that the pharmacology attributed to nucleotide agonists is due to AR activation by active nucleoside metabolites.
Keywords: P2Y1 receptor, Prodrug, Adenosine, Ectonucleotidase, A3 receptor, A1 receptor
Introduction
Early research on the diverse pharmacology of adenine-containing compounds, starting with the classic research of Drury and Szent-Györgyi, led to the classification of ATP as a neurotransmitter with extracellular effects [1–3]. Observations of the diverse effects of extracellular ATP in a variety of systems ultimately led to the proposal of two families of G protein-coupled purinergic receptors [4]. The adenosine receptors (ADORA receptor gene family or P1 receptors) are comprised of four receptor subtypes (A1, A2A, A2B, and A3) for which adenosine is the primary endogenous ligand [5, 6]. The P2 receptor (P2R) family is comprised of two subfamilies of receptors: the ATP-gated ion channel P2X receptor (P2XR) family for which ATP is the primary endogenous ligand and the G protein coupled P2Y receptor (P2YR) family for which purine and pyrimidine nucleotides are the primary endogenous ligands [7, 8].
A major challenge in delineating the functional roles of the P2Rs in vitro and in vivo is the rapid phosphate ester hydrolysis of both endogenous nucleotides and pharmacological tool compounds[9]. Ectonucleotidases are ubiquitous enzymes located on cell membrane surfaces and in circulating blood that rapidly dephosphorylate adenine and uridine nucleotides to adenosine and uridine, respectively [10]. These enzymes play a critical function in regulating the effects of nucleotides released from cells [11]. Ex vivo studies with perfused organs such as the lung or heart have reported ATP half-lives of 0.2 s or less [12, 13]. Consensus opinion is that the in vivo half-lives of endogenous mononucleotides are in the range of seconds or less.
Specific agonists and antagonists that are resistant to ectonucleotidase-mediated dephosphorylation are required to fully investigate and characterize the role of P2 receptors in physiology and disease. One approach is to incorporate methylene phosphonates which are non-hydrolyzable bonds, but often the P2YR affinity of such phosphonates is reduced [14]. Nevertheless, incorporation of methylene, dihalomethylene, boronophosphate, or phosphorothioate substitutions within the phosphodiester bonds of adenosine or uracil nucleotides has been reported to impart significant in vitro stability to dephosphorylation [14–17]. Another modification of P2Y1R agonists and antagonists that was found to reduce but not eliminate the hydrolysis was the replacement of the phosphorylated riboside of purine nucleotide analogs with a phosphorylated ring-constrained (N)-methanocarba moiety containing bicyclo[3.1.0]hexane. This general modification increased the affinity and selectivity of P2Y1R agonists relative to other P2 receptors and was also noted to increase in vitro stability to dephosphorylation by nucleotidases. The hydrolysis by CD73 of (N)-methanocarba-AMP in a pure single enzymatic system occurred at < 1% of the rate for AMP [18], and it was deemed “somewhat stable” to dephosphorylation. An (N)-methanocarba 3′,5′-bisphosphate P2Y1R antagonist MRS2500 retained in vivo antithrombotic potency considerably longer (~ 1 h) than a related 2′-deoxyribonucleotide antagonist MRS2179 [19], suggesting its relative stability to hydrolysis.
Despite the lack of definitive in vivo demonstration of resistance to ectonucleotidases for these chemical modifications, a number of research groups used both ribose-containing and (N)-methanocarba nucleotide analogs to evaluate the in vitro and in vivo role of P2Y and P2XRs in normal and pathophysiology [19–29]. Most of this research has not included any reported attempts to quantitate actual systemic or target tissue pharmacokinetics for these receptor agonists and antagonists. The lack of definitive stability data for these compounds is a potentially major limitation for the mechanistic interpretation of these studies.
Our research included a specific focus on the in vitro and in vivo metabolism and pharmacokinetics of the prototypical high-affinity and specific (N)-methanocarba P2Y1R agonist MRS2365. We also evaluated the in vitro plasma and blood stability of 2-MeS-ADP, the high-affinity 3′,5′-bisphosphate (N)-methanocarba P2Y1R antagonist MRS2500, and the 3′,5′-bisphosphate riboside antagonist MRS2179 that have also been utilized extensively in previous in vitro and in vivo studies [19, 24, 27, 30–33]. Our data reveal that these compounds are still highly susceptible to rapid metabolism in vitro and in vivo to dephosphorylated nucleoside analogs. The metabolism is so rapid in vivo that the P2Y1R agonist MRS2365 acts as a prodrug of its more stable nucleoside metabolite AST-004 (MRS4322, (1R,2R,3S,4R,5S)-4-(6-amino-2-(methylthio)-9H-purin-9-yl)-1-(hydroxymethyl)bicyclo[3.1.0]hexane-2,3-diol) in our studies. We show that AST-004 has affinity for adenosine receptors, most notably the A1 and A3 receptors. We further suggest that the efficacy of the P2Y1R agonist MRS2365 in in vivo murine models of TBI and stroke is actually due to interactions of its metabolite AST-004 with adenosine receptors. Considering that the vast majority of research performed to date with P2Y1R agonists and antagonists has not included evaluation of in vitro/in vivo stability and pharmacokinetics, our data suggest that previous reports of the pharmacology of P2Y and P2X receptors could be due to activation of adenosine receptors. We conclude by advocating for additional pharmacokinetic studies in any future in vivo studies when investigating phosphorylated receptor agonists and antagonists regardless of chemical modification.
Materials and methods
Chemicals
MRS2365, MRS2500, MRS2179, 2-methylthio-ADP (2-MeS-ADP), and 2-methylthioadenosine were obtained from Tocris Bioscience (Bristol, UK), and 2-methylthio-AMP (2-MeS-AMP) was from Sigma-Aldrich (St. Louis, MO). AST-004 (MRS4322) was synthesized at the National Institute of Diabetes, Digestive and Kidney Diseases (Bethesda, MD), as published [18]. MRS1523 was obtained from Sigma-Aldrich (St. Louis, MO). Analytical grade tolbutamide was obtained from commercial supplies at Seventh Wave Laboratories (Maryland Heights, MO). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Radioligands were obtained from PerkinElmer (Boston, MA).
Animals
Female C576BL/6 J mice weighing approximately 0.02 kg were used for this study, supplied by the University of Texas Health at San Antonio (UTHSA). All studies were conducted under approved UTHSA IACUC protocols.
In vitro stability and metabolism studies
Media and astrocyte culture stability determination
Mouse and human astrocyte media and cell cultures were prepared as previously described [34]. Astrocytes were cultured in 96-well plates at a density of 1 × 104 cells per well, and stability studies were conducted 24 h following plating. MRS2365 stability was determined in phosphate-buffered saline (pH 7.4), astrocyte cell culture media, and in mouse and human astrocyte cell cultures. MRS2365 was solubilized in cold phosphate-buffered saline at a concentration of 100 μM. Solutions and cell cultures were pre-warmed to 37 °C in a humidified, 5% CO2 incubator. Stability incubations were initiated with the addition of 6 μl of 100 μM MRS2365 (1 μM final concentration). Stability incubations utilized incubation timepoints of 1, 15, 30, 60, 120, and 240 min. At each timepoint, 50 μl aliquots of incubations were acquired and immediately quenched with 150 μl ice-cold methanol containing the bioanalytical internal standard tolbutamide. Quenched samples were immediately centrifuged, and supernatants were placed in microtainer tubes and stored at − 80 °C until analysis.
Plasma stability determination
MRS2365, enalapril, and procaine were dissolved in phosphate-buffered saline, pH 7.4. Enalapril and procaine were utilized as plasma and blood stability standards with known in vitro stability half-lives. Plasma samples (prepared from blood with either EDTA or lithium heparin as anticoagulants) were pre-warmed for 60 min in a humidified, 5% CO2 incubator maintained at 37 °C. Stability incubations were initiated with the addition of MRS2365 (1 μM final concentration). Initial assessments of stability in EDTA-generated plasma utilized incubation timepoints of 0, 10, 30, 60, 120, and 240 min. Subsequent studies comparing EDTA- and heparin-generated plasma utilized incubation timepoints of 0, 1, 2.5, 5, 7.5, 10, and 30 min. Additional plasma stability incubations comparing EDTA- and heparin-generated plasma were performed using timepoints of 0, 5, 10, 20, 30, 45, 60, and 90 s. At each timepoint, stability was determined by quantitation of concentrations of parent compound assessed by LC-MS/MS (see below). For metabolite scouting analyses, MRS2365 was incubated in heparinized human plasma at a concentration of 100 μM for 10 or 30 min. In all studies, 50 μl aliquots of incubations were acquired at each timepoint and immediately quenched with 150 μl ice-cold methanol containing the bioanalytical internal standard tolbutamide and centrifuged at 4 °C. Supernatants were placed in microtainer tubes, frozen on dry ice, and stored at − 80 °C until analysis.
Blood stability determination
MRS2365, MRS2179, MRS2500, 2-MeS-ADP, enalapril, and procaine were dissolved in phosphate-buffered saline, pH 7.4. Blood samples (EDTA- or lithium heparin-treated) were pre-warmed for 60 min in a humidified, 5% CO2 incubator maintained at 37 °C. Stability incubations were initiated with the addition of individual compounds (1 μM final concentration). Fifty microliter blood sample aliquots were obtained at 0, 1, 2.5, 7.5, 10, and 30 min and placed in microtainer tubes. Fifty microliters of ice-cold distilled water were added to the blood aliquots to lyse blood cells, immediately followed by addition of 150 μl ice-cold methanol containing the bioanalytical internal standard tolbutamide, vortexing, and centrifugation at 4 °C. Supernatants were placed in microtainer tubes, frozen on dry ice, and stored at − 80 °C until analysis. At each timepoint, stability was determined by quantitation of concentrations of parent compound assessed by LC-MS/MS (see below). In the case of 2-MeS-ADP, stability was determined by the rate of formation of 2-methylthioadenosine assessed by LC-MS/MS. For metabolite scouting analyses, MRS2365, MRS2179, and MRS2500 were incubated in heparinized human or mouse whole blood at a concentration of 100 μM for 10 or 30 min; plasma was immediately prepared by centrifugation at 4 °C and stored at − 80 °C until structural elucidation analyses (see below).
In vivo pharmacokinetic studies
Drug administration
MRS2365 was dissolved in phosphate-buffered saline and then diluted in phosphate-buffered saline to prepare dosing solutions. The final dosing solution concentration of MRS2365 was 100 μM or 1 μM. A 100 μl volume of dosing solution was administered intraperitoneally or intravenously to each mouse per 20-g body weight; MRS2365 was administered at 0.5 and 5.0 μmol/kg or 0.27 and 2.7 mg/kg. Three mice were administered MRS2365 for each sampling timepoint. Pharmacokinetic studies utilized sampling times of 1 or 5, 15, 30, 60, 120 and 180 min following intraperitoneal administration.
Pharmacokinetic studies were also performed for the MRS2365 dephosphorylated nucleoside metabolite, AST-004. AST-004 was solubilized in DMSO and then diluted in saline to prepare dosing solution. Final dosing solution concentration of AST-004 was 100 μM. A 100 μl volume of dosing solution was administered intraperitoneally to each mouse per 20-g body weight; AST-004 was administered intraperitoneally at 0.15 mg/kg. Three mice were administered AST-004 for each sampling timepoint. Plasma and brain samples were obtained at 0, 0.083, 0.25, 0.5, 1, 2, and 8-h post-dose.
Pharmacokinetic studies were also performed to assess the pharmacokinetics of the nucleoside metabolite AST-004 following intraperitoneal administration of MRS2365. Dosing and sampling conditions were identical to those described above for MRS2365; however, the concentrations of the metabolite AST-004 were determined in plasma and the brain. Plasma and brain samples were obtained at 0, 0.083, 0.25, 0.5, 1, 2, and 8-h post-dose.
Tissue sampling
At each timepoint, mice (3/timepoint) were euthanized in a carbon monoxide chamber. Whole blood was obtained by cardiac puncture into Microtainer tubes containing heparin and immediately centrifuged for preparation of plasma, plasma was stored at − 80 °C. At each timepoint, whole brain samples were obtained by decapitation, rinsed in ice-cold phosphate-buffered saline, and weighed. Brain samples were then immediately flash-frozen in liquid nitrogen and stored at − 80 °C.
Bioanalytical and pharmacokinetic analyses
Concentrations of MRS2365, AST-004, MRS2500, MRS2179, and 2-methylthioadenosine in in vitro and/or in vivo samples were determined by single reaction monitoring LC-MS/MS in negative or positive (2-methylthioadenosine) ion mode. The systems consisted of Shimadzu (Columbia, MD) Prominence or Waters (Milford, MA) Acquity HPLC units and AB Sciex API4000 or API5500 mass spectrometers. Parent compounds were monitored for Q1/Q3 masses with tolbutamide as an internal standard. For each analyte, bioanalytical methods were identical for all matrices; standard curve statistics (e.g., Fit, Intercept, Slope, Correlation Coefficient) were determined for each matrix but were not significantly different. For each tissue matrix, standard curves were created, and lower (LLOQ) and upper (ULOQ) limits of quantitation were determined. Generally, bioanalytical LLOQs for these studies were ≤ 2.4 ng/mL or less, and ULOQs were up to 5000 ng/mL. Pharmacokinetic parameters were calculated by non-compartmental analysis using Phoenix WinNonlin (Certara, Princeton, NJ).
In vitro and in vivo structural elucidation of MRS2365, 2-MeS-ADP, MRS2179, and MRS2500 metabolites
Following in vitro incubation of MRS2365 in mouse and human plasma and blood, or mouse in vivo intravenous administration, targeted metabolite scouting for parent MRS2365 and putative metabolites in plasma, blood and brain samples were conducted using LC-MS/MS in negative and positive ion mode. Negative ion mode was utilized to monitor nucleotide metabolite formation, and positive ion mode was utilized for nucleoside metabolite formation. Analyses were conducted with a Shimadzu Prominence HPLC unit and an AB Sciex (Redwood City, CA) API4000 mass spectrometer. Samples were analyzed for the product ions for parent MRS2365, its putative monophosphate metabolite MRS2347, and its putative dephosphorylated nucleoside metabolite AST-004.
Following 2-MeS-ADP, MRS2179, and MRS2500 mouse whole blood in vitro incubations, targeted metabolite scouting for their monophosphate (MRS2179 and MRS2500) and dephosphorylated nucleoside metabolites (2-MeS-ADP, MRS2179, and MRS2500) were conducted using Shimadzu Prominence or Waters Acquity HPLC units and AB Sciex API4000 or API5500 mass spectrometers in negative and positive ion mode. Samples were analyzed for the product ions for parent compounds, putative monophosphate metabolites, and putative dephosphorylated nucleoside metabolites.
In vitro receptor affinity and functional studies
A1, A2A, A2B, or A3 adenosine receptor affinity
For AST-004, membrane preparations of recombinant CHO or HEK293 cells stably expressing adenosine receptor subtypes A1, A2A, A2B, or A3 were conducted as previously described [35] or purchased at PerkinElmer. Rat cerebral cortical and rat striatal membranes were obtained as previously described [36]. Radioligand binding assays at human, rat, and mouse for A1, A2A, A2B, and A3 receptors were performed utilizing specific radioligands for each receptor [37]. [3H] CCPA was used as an A1 agonist radioligand, [3H]CGS21680 as an A2A agonist radioligand and [3H]PSB-603 as an A2B antagonist radioligand, since no selective agonist radioligand is currently available for A2B receptors. [3H]5′-N-Ethylcarboxamidoadenosine (NECA) was employed as an A3 agonist radioligand. The non-selective agonist [3H]NECA could be used because CHO cells do not natively express adenosine receptors. Concentration-dependent displacement of the radioligands by AST-004 was determined. Non-specific binding was determined using the compounds (final concentration): 2-chloroadenosine (10 μM), NECA (50 μM), and R-PIA (100 μM), for A1, A2A, and A3ARs, respectively. Previously published NECA affinities for the adenosine receptors were used as a reference to compare to AST-004 [37]. Assays measuring inhibition of forskolin-induced cAMP accumulation in CHO cells recombinantly expressing human, rat, and mouse A1 and A3 receptors were performed [37], using the non-selective agonist NECA as a control.
For 2-methylthioadenosine, binding affinities were determined as previously described [38]. [3H]N6-R-phenylisopropyladenosine ([3H]R-PIA) was used as an A1 agonist radioligand, [3H]2-[p-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine ([3H]CGS21680) as an A2A agonist radioligand, and [3H]NECA was used as a non-selective A2B agonist radioligand. [125I]N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide ([125I]I-AB-MECA) was employed as an A3 agonist radioligand.
Receptor and enzyme target selectivity
AST-004 was profiled against a collection of 160 receptor and enzyme targets using a commercially available target profiling panel (Bioprint, Eurofins Cerep, Le Bois I’Evêque B.P. 30001, 86 600 Celle I’Evescault, France).
Results
In vitro stability of P2Y1R agonists and antagonists
The stabilities of commonly used phosphorylated (N)-methanocarba P2Y1R agonist and antagonist (MRS2365 and MRS2500, respectively) and phosphorylated riboside P2Y1R agonist and antagonist (2-MeS-ADP and MRS2179, respectively) were assessed in vitro.
MRS2365 stability was assessed in in vitro incubations conducted at 37 °C and analyzed by LC-MS/MS. Following incubation in phosphate-buffered saline (pH 7.4), no hydrolysis of MRS2365 is observed (Table 1). However, in cell-free astrocyte culture media, or in mouse and human astrocyte cell culture incubations, MRS2365 was found to have in vitro half-lives of 42, 25, and 26 min, respectively. By 2 h, no MRS2365 was detectable in either media or cell culture incubations.
Table 1.
In vitro stability of nucleotides MRS2365, MRS2179, and MRS2500 in phosphate-buffered saline, astrocyte culture media or mouse, and human astrocyte cell culture
| Media | ||||||||
|---|---|---|---|---|---|---|---|---|
| PBS, pH 7.4 | Astrocyte culture media | Mouse astrocyte cell culture | Human astrocyte cell culture | |||||
| Compound | T1/2 (min) | % remaining (240 min) | T1/2 (min) | % remaining (240 min) | T1/2 (min) | % remaining (240 min) | T1/2 (min) | % remaining (240 min) |
| MRS2365 | > 240 | 100% | 42 | 2.5% | 25 | < 1% | 26 | < 1% |
| MRS2179 | > 60 | 100% | - | - | - | - | - | - |
| MRS2500 | > 60 | 100% | - | - | - | - | - | - |
Results are the mean values of 3 replicates per incubation conducted at 37 °C
-Not tested
The stability of MRS2365 was also assessed in in vitro incubations at 37 °C in mouse and human plasma and whole blood prepared with either EDTA or heparin as anticoagulants (Table 2). EDTA is a commonly used anticoagulant, but it is also an inhibitor of ectonucleotidases responsible for dephosphorylation of nucleotides [39]. MRS2365 was completely stable over 240 min in EDTA-prepared mouse plasma and had a half-life of 47 min in EDTA-prepared mouse blood. In EDTA-prepared human plasma and blood, MRS2365 was stable over 240 min in both matrices. However, our initial stability studies in heparinized mouse and human plasma and whole blood did not detect quantifiable concentrations of MRS2365 at any incubation timepoint from 1 to 60 min. Additional studies were conducted to evaluate stability at timepoints from 5 to 90 s in both EDTA- and heparin-prepared mouse and human. In these shorter incubation studies, 80–100% MRS2365 remained after 20 s in EDTA-prepared plasma, but only 5–10% remained in heparin-prepared plasma.
Table 2.
In vitro stability of nucleotides MRS2365, MRS2179, MRS2500, and 2-MeS-ADP in mouse and human plasma and blood treated with the anticoagulants EDTA or heparin
| Half-life (T1/2, min) in treated media | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mouse plasma | Mouse whole blood | Human plasma | Human whole blood | |||||
| Compound | EDTA | Heparin | EDTA | Heparin | EDTA | Heparin | EDTA | Heparin |
| MRS2365 | > 240 | < 0.1 | 47 | 2.5 | > 240 | < 0.1 | > 240 | < 0.1 |
| MRS2179 | – | – | > 180 | 67 | - | - | - | - |
| MRS2500 | - | - | > 180 | < 1 | - | - | - | - |
| 2-MeS-ADP | - | - | > 60 | 11.7 | - | - | - | - |
| Enalapril | - | 59 | 59–65 | 64–75 | - | - | - | - |
| Procaine | - | - | - | - | - | 0.4 | - | 1.1 |
Results are the mean values of 3 replicates per incubation conducted at 37 °C
-Not tested
The stability of the phosphorylated ribose P2Y1R agonist 2-MeS-ADP was then assessed in mouse blood using either EDTA or heparin as anticoagulants. In mouse blood prepared with EDTA, 2-MeS-ADP was completely stable over a 2-h incubation. But again, in mouse blood prepared with heparin, the half-life of 2-MeS-ADP was approximately 11.7 min and associated with the formation of the dephosphorylated nucleoside metabolite 2-methylthioadenosine. By 1 h, 2-MeS-ADP was completely metabolized to 2-methylthioadenosine.
Since numerous in vitro and in vivo research studies have utilized the phosphorylated P2Y1R antagonists MRS2179 and MRS2500, their in vitro stability was also measured. In phosphate-buffered saline, neither compound exhibited any detectable hydrolysis. In addition, both compounds were stable over 60-min incubation periods in mouse whole blood utilizing EDTA as the anticoagulant. However, in mouse blood utilizing heparin as the anticoagulant, MRS2179 had a half-life of 67 min, and MRS2500 was undetectable at all timepoints.
In all incubations, the in vitro half-lives of the stability standards, enalapril and procaine were within the range reported throughout the literature.
Identification of P2Y agonist and antagonist metabolites in vitro
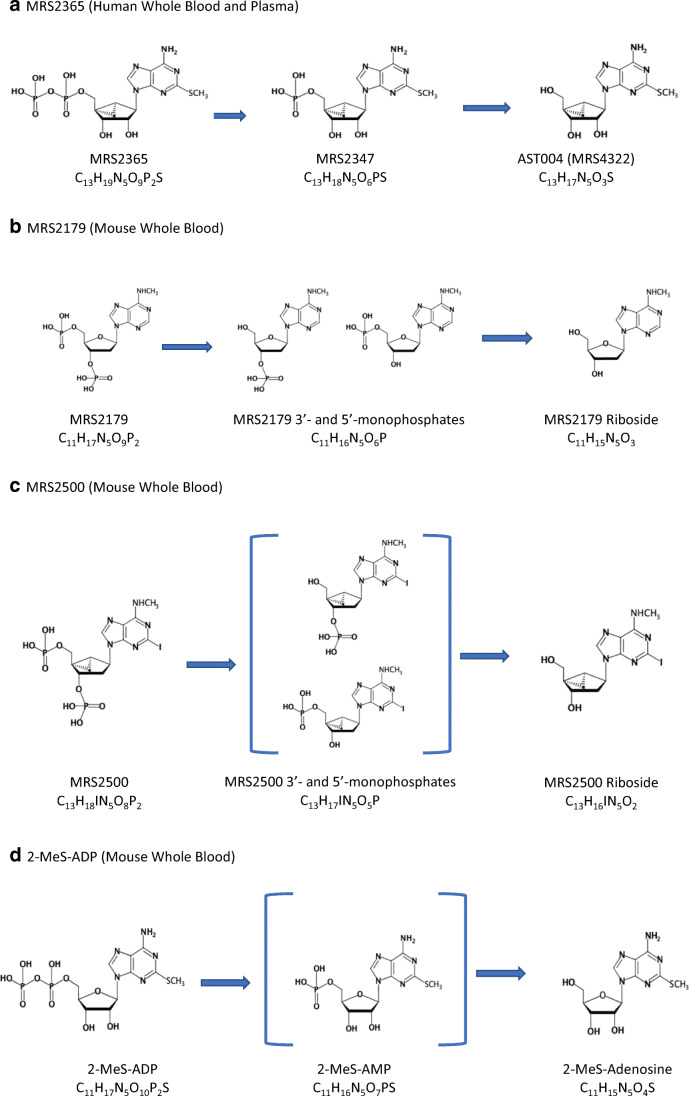
Metabolite scouting was conducted using LC-MS/MS monitoring product ions of putative in vitro metabolites of MRS2365, 2-MeS-ADP, MRS2179, and MRS2500. Negative ion mode was utilized to monitor for nucleotide metabolites and positive ion mode utilized for monitoring nucleoside metabolites. The identified in vitro metabolic pathways of MRS2365, 2-MeS-ADP, MRS2179, and MRS2500 that result from phosphoester hydrolysis by ectonucleotidases are illustrated (Fig. 1). No other metabolites were detected.
Fig. 1.
Metabolism by phosphoester enzymatic hydrolysis of nucleotides MRS2365 (a), MRS2179 (b), MRS2500 (c), and 2-MeS-ADP (d) in heparinized mouse and/or human plasma and whole blood. a MRS2365 (human whole blood and plasma), b MRS2179 (mouse whole blood), c MRS2500 (mouse whole blood), and d 2-MeS-ADP (mouse whole blood)
To identify potential metabolites of MRS2365 in vitro, the compound was incubated in heparinized human whole blood or plasma for 10 min at 37 °C at a concentration of 100 μM. Product ions for the nucleoside metabolite AST-004 and its corresponding 5′-monophosphate MRS2347 were observed in incubation extractions. These data confirmed that the in vitro instability of MRS2365 in blood and plasma was due to rapid sequential dephosphorylation to the nucleoside AST-004. No other metabolites were observed in whole blood or plasma incubations.
Following 60-min incubations of MRS2179 in heparinized mouse whole blood, product ions consistent with two monophosphate metabolites and one dephosphorylated nucleoside metabolite were identified. For MRS2500, only the dephosphorylated nucleoside metabolite was identified, suggesting that for this compound, dephosphorylation rapidly progressed through hydrolysis of either the 3′ and 5′ phosphate moieties on the (N)-methanocarba ring to form monophosphate metabolites that were not directly detected by LC-MS/MS. For 2-MeS-ADP, the nucleoside metabolite 2-methylthioadenosine was identified, suggesting rapid dephosphorylation via 2-MeS-AMP that could not be detected by LC-MS/MS.
In all cases, the predominant route of metabolism, as expected, was dephosphorylation to the resulting dephosphorylated nucleoside metabolites. Metabolism of the agonists MRS2365 and 2-MeS-ADP resulted in metabolites that are analogs of adenosine. Interestingly, the metabolism of the (N)-methanocarba and phosphorylated riboside P2Y1R antagonists MRS2500 and MRS2179 resulted in 2′-deoxy nucleoside metabolites, analogs of 2′-deoxyadenosine.
In vivo pharmacokinetics of MRS2365
The plasma and brain pharmacokinetics of the P2Y1R agonist MRS2365 were evaluated in mice following intravenous or intraperitoneal administration, utilizing a sensitive LC-MS/MS bioanalytical method (1–5 ng/mL lower limits of quantitation) and multiple protocols utilizing different dose levels (0.27 and 2.7 mg/kg) as well as blood sampling as early as 1-min post-dose. In all of these studies, detectable concentrations of MRS2365 were never observed in either plasma or the brain following intraperitoneal or intravenous administration, suggestive of very high plasma clearance consistent with the rapid metabolism observed for endogenous nucleotides (Table 3).
Table 3.
In vivo plasma and brain concentrations and pharmacokinetics of MRS2365 and its metabolite AST-004 in mice following intraperitoneal or intravenous administration of either MRS2365 or AST-004
| Compound administered | Compound monitored | Route of administration | Dose (mg/kg; μmol/kg) | Tissue | Concentration (ng/mL or ng/g) at time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.02 | 0.08 | 0.25 | 0.5 | 1 | 1.5 | 2 | 2.5 | |||||
| MRS2365 | MRS2365 | i.p. | 0.27; 0.5 | Plasma | - | - | < LLOQ | < LLOQ | < LLOQ | < LLOQ | - | < LLOQ | |
| i.v. | 0.27; 0.5 | Plasma | - | < LLOQ | < LLOQ | < LLOQ | < LLOQ | < LLOQ | - | < LLOQ | |||
| i.v. | 2.7; 5.0 | Plasma | - | < LLOQ | < LLOQ | - | - | - | - | - | |||
| Brain | - | - | < LLOQ | - | - | - | - | - | |||||
| MRS2365 | AST-004 | i.p. | 0.27; 0.5 | Plasma | - | - | 57.1 | 45.7 | 26.1 | 3.3 | - | 0.8 | - |
| Brain | - | - | 5.8 | 3.8 | 2.9 | < LLOQ | - | < LLOQ | - | ||||
| AST-004 | AST-004 | i.p. | 0.15; 0.5 | Plasma | - | - | 49.5 | 41.8 | 21.1 | 5.8 | - | 0.8 | - |
| Brain | - | - | 2.4 | 3.1 | 2.4 | < LLOQ | - | < LLOQ | - | ||||
| AST-004 | AST-004 | i.v. | 0.25; 0.8 | Plasma | - | - | 79.4 | 38.0 | 9.2 | 6.0 | 2.2 | 0.8 | 1.04 |
Results are the means of 3 mice per timepoint
LLOQ for MRS2365 was 5 ng/mL plasma or 5 ng/g brain tissue
LLOQ for AST-004 was 0.1 ng/mL plasma or 2.4 ng/g brain tissue
-Not sampled
Identification of MRS2365 metabolites in vivo
Based on initial pharmacokinetic studies which failed to detect circulating concentrations of MRS2365, the identification of circulating metabolites was pursued. Given the extremely high clearance of endogenous nucleotides due to dephosphorylation, it was hypothesized that the likely major route of MRS2365 in vivo metabolism was dephosphorylation, similar to that observed in in vitro studies.
Following intraperitoneal administration of MRS2365 to mice, neither MRS2365 nor its monophosphate metabolite MRS2347 was detected in plasma or brain samples even as early as 1-min post-dose. However, the dephosphorylated nucleoside metabolite AST-004 was detected in both plasma and brain samples (Table 3). These data indicated that MRS2365 was rapidly dephosphorylated in mice to the (N)-methanocarba nucleoside AST-004.
In vivo pharmacokinetics of the MRS2365 nucleoside metabolite AST-004
Identification of AST-004 as the only drug-related material in mice following administration of MRS2365 led to measuring the pharmacokinetics of AST-004. Following intravenous administration to mice, AST-004 was found to have a high plasma clearance, a volume of distribution indicating dispersal into tissues and a plasma half-life of 0.5 h (Table 4). AST-004 is primarily renally eliminated unmetabolized, and the high clearance observed in mice is likely due to the substantially higher body weight-adjusted glomerular filtration rate in that species. Subsequent pharmacokinetic studies in rats, dogs, neonatal pigs, and cynomolgus monkeys are beyond the scope of this report, but in these species, the plasma clearance has been demonstrated to be moderate and the predicted human half-life by i.v. administration to be in the range of 19–24 h (data not shown). AST-004 brain/plasma concentration ratios in mice based on either Cmax or AUC values ranged from 4.0 to 9.6% indicating distribution of AST-004 into brain tissue following administration of either MRS2365 or AST-004; ratios in other species range from 10 to 30% (data not shown).
Table 4.
In vivo plasma and brain pharmacokinetic parameters of AST-004 in mice following intraperitoneal or intravenous administration of either MRS2365 or AST-004
| Compound administered | Compound monitored | Route of administration | Dose (mg/kg; μmol/kg) | Tissue | Pharmacokinetic parameters | ||||
|---|---|---|---|---|---|---|---|---|---|
| C0 or Cmax (ng/mL or ng/g) | CLp (mL/min/kg) | Vdss (L/kg) | AUC (ng-h/mL) |
T1/2 (h) | |||||
| MRS2365 | AST-004 | I.P. | 0.27; 0.5 | Plasma | 64.5 | - | - | 29.3b | 0.3 |
| Brain | 6.2 | - | - | 1.7c | - | ||||
| AST-004 | AST-004 | I.P. | 0.15; 0.5 | Plasma | 49.5 | - | - | 30.1b | 0.3 |
| Brain | 3.2 | - | - | 1.2c | - | ||||
| AST-004 | AST-004 | I.V.a | 0.25; 0.8 | Plasma | 115 | 132 | 2.2 | 31.6b | 0.5 |
Results are the means of 3 mice per timepoint
aLLOQ for AST-004 plasma was 0.1 ng/mL
bAUC (0-Inf)
cAUC (0–0.5 h)
-Not determined
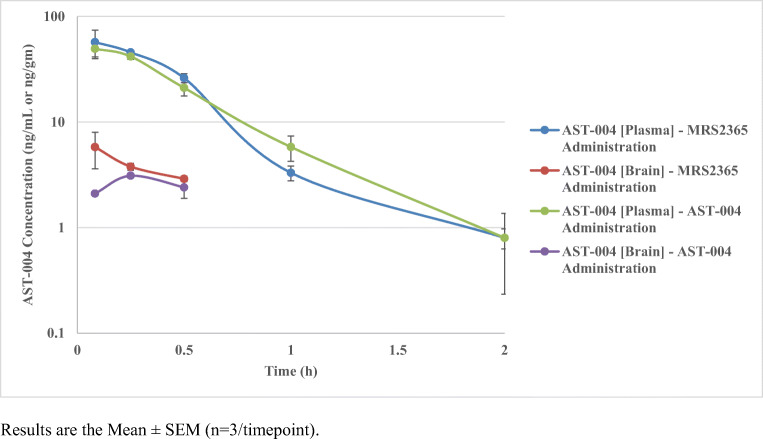
Importantly, when equimolar doses of MRS2365 and AST-004 were intraperitoneally administered to mice and AST-004 plasma and brain concentrations were assessed by LC-MS/MS, the overall plasma concentration profiles and pharmacokinetics of AST-004 were not significantly different (Fig. 2, Table 4). This strongly suggests that MRS2365 is rapidly and quantitatively metabolized to AST-004 in mice. The clearance of MRS2365 is so rapid that this P2Y1R agonist can be considered an in vivo prodrug of the more stable nucleoside metabolite AST-004.
Fig. 2.
Plasma and brain concentration-time profiles of AST-004 following intraperitoneal doses of either MRS2365 or AST-004 at equimolar doses. Results are the mean ± SEM (n = 3/timepoint)
Receptor affinity and cAMP accumulation analyses of the nucleoside metabolite AST-004
The receptor affinity of structurally similar analogs of the MRS2365 major metabolite, AST-004, has previously been reported. For example, the 6-chloro analog MRS1873 has affinity for the adenosine receptor family and appears to be a partial agonist [40, 41]. Likewise, the nucleoside metabolite of 2-MeS-ADP, 2-methylthioadenosine, also interacts with adenosine receptors, primarily the adenosine A3 receptor and to a lesser extent the adenosine A1 receptor, with affinities for human A1, A2A, A2B, and A3 receptors of 1.9, 11.6, > 30, and 0.7 μM, respectively (Table 5).
Table 5.
Affinities of AST-004, 2-methylthioadenosine, or NECA at adenosine receptors determined in radioligand binding studies
| Human A3AR | Rat A3AR |
Mouse A3AR |
Human A1AR | Rat A1AR |
Mouse A1AR |
|
|---|---|---|---|---|---|---|
| Agonist | Ki ± SEM (nM) | |||||
| AST-004 | 1490 ± 410 | 10,800 ± 1200 | 4940 ± 974 | 1590 ± 853 | 1880 ± 507 | 3690 ± 877 |
| 2-Methylthio-adenosineb | 720 ± 280 | - | - | 1900 ± 770 | - | - |
| NECA | 6.18 (KD) a | 48.6 (KD)a | 15.1 (KD) a | 14a | 5.1a | 2.49a |
| Human A2AAR |
Rat A2AAR |
Mouse A2AAR |
Human A2BAR |
Rat A2BAR |
Mouse A2BAR |
|
|---|---|---|---|---|---|---|
| Agonist | Ki ± SEM (nM) | |||||
| AST-004 | 16,000 ± 6170 | 10,200 ± 1990 | 9040 ± 4290 | > 10,000 | > 10,000 | 10,000 |
| 2-Methylthio-adenosineb | 11,600 ± 1000 | - | - | > 30,000 | - | - |
| NECA | 20a | 9.7a | 43.4a | 1890a | 1110a | 656a |
aData from Alnouri et al. (2015)
bMethods from Gao et al. (2003)
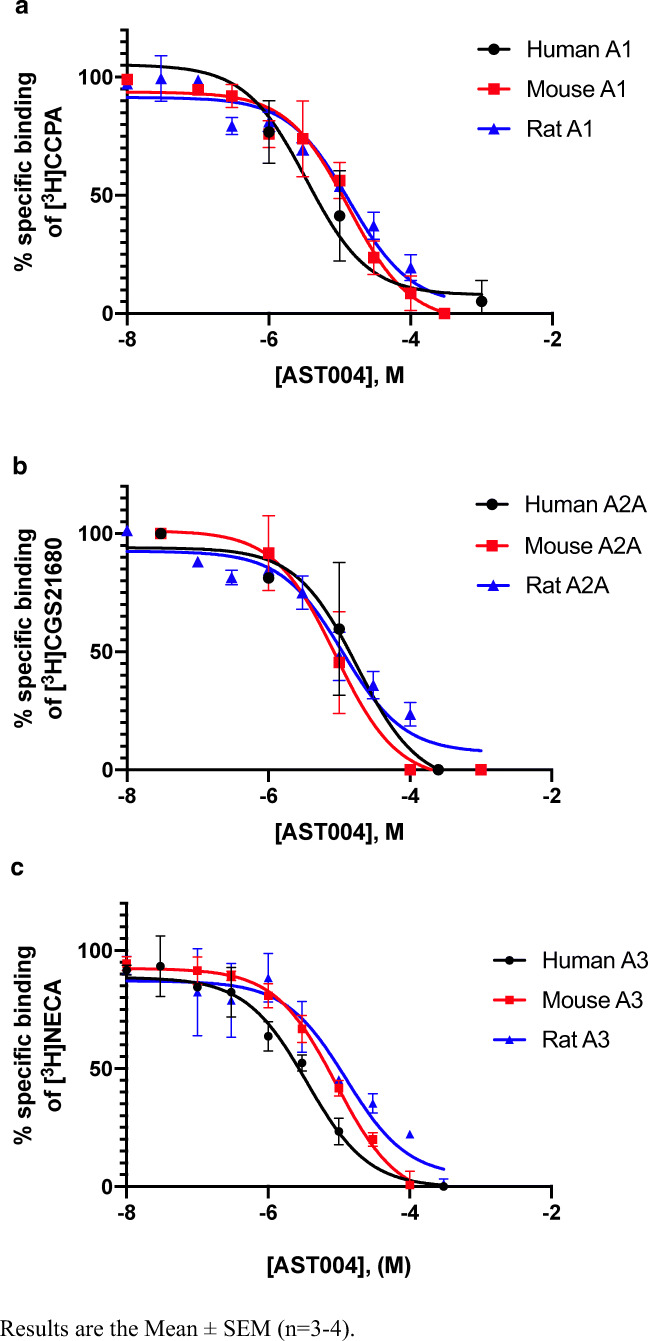
Our binding displacement studies indicated that the affinity of AST-004 in rodent species is primarily to the A3 receptor, and to a less extent the A1 receptor, although affinity for the human A3 and A1 receptors appears to be similar (Fig. 3, Table 5). A broad commercial human target selectivity panel (Cerep Bioprint) was also utilized to identify potential additional receptor or enzyme targets for AST-004 and none of significance have been identified other than the adenosine A3 and A1 receptors (data not shown). Affinity for the adenosine A2A receptor was significantly lower, and affinity for the A2B receptor could not be measured.
Fig. 3.
Competition binding experiments of AST-004 versus the adenosine radioligands at human, mouse, and rat A1 (a), A2A (b), and A3 (c) receptors expressed in CHO cells. Results are the mean ± SEM (n = 3–4)
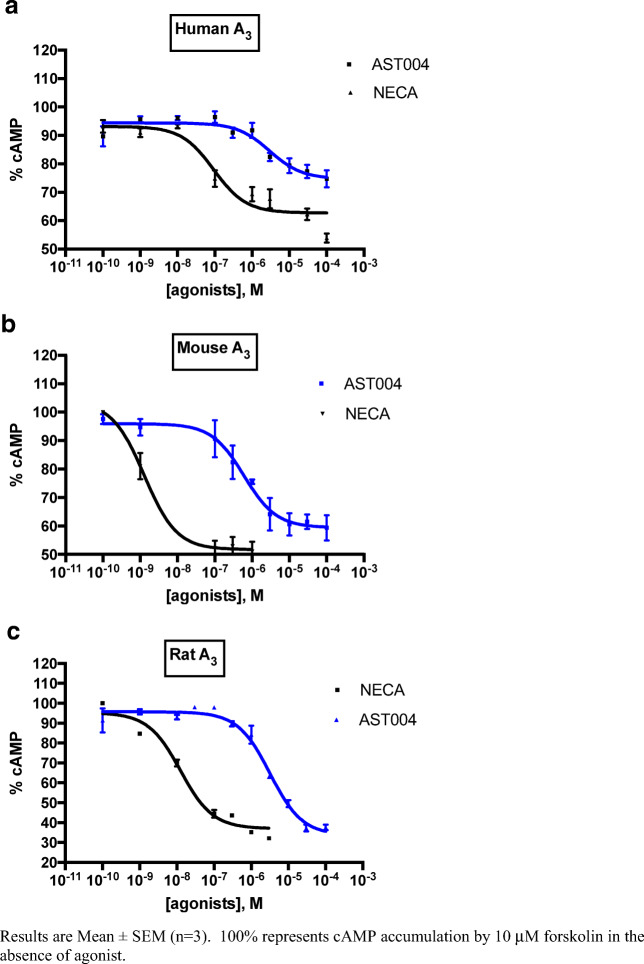
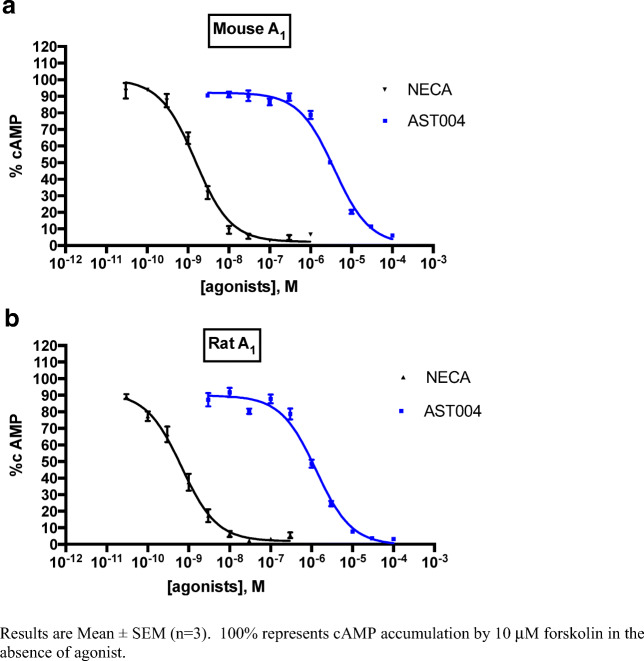
Additionally, cAMP accumulation experiments were conducted in CHO cells recombinantly expressing mouse, rat, or human adenosine A1 or A3 receptors, with the non-selective agonist NECA used as a control agonist (Figs. 4 and 5, Table 6). In these studies, EC50 values ranged from 0.76 μM in mouse A3-expressing CHO cells to 3.6 μM in human A3-expressing cells. In these engineered cell lines, AST-004 appeared to be a full or nearly full agonist compared with the full agonist NECA. The EC50 of NECA and AST-004 were not determined for the human A1R due to lack of a functional CHO cell line expressing this receptor.
Fig. 4.
Inhibition of forskolin-mediated camp accumulation by AST-004 and NECA at human (a), mouse (b), and rat (c) A3 receptors expressed in CHO cells. Results are mean ± SEM (n = 3). 100% represents cAMP accumulation by 10 μM forskolin in the absence of agonist
Fig. 5.
Inhibition of forskolin-mediated cAMP accumulation by AST-004 and NECA at mouse (a) and rat (b) A1 receptors expressed in CHO Cells. Results are mean ± SEM (n = 3). 100% represents cAMP accumulation by 10 μM forskolin in the absence of agonist
Table 6.
Potencies of AST-004 and reference agonist NECA at A3 and A1 adenosine receptors stably expressed in CHO cells determined in camp accumulation assays
| Human A3AR |
Rat A3AR |
Mouse A3AR |
|
|---|---|---|---|
| Agonist | EC50 ± SEM (nM) (efficacy at 100 μM concentration, in % relative to maximal effect of NECAa) | ||
| AST-004 | 3630 ± 370 (70 ± 12) | 3260 ± 983 (98 ± 1) | 759 ± 170 (72 ± 18) |
| NECA | 41.8 ± 6.3 | 16.3 ± 1.13 | 6.85 ± 0.88 |
|
Human A1AR |
Rat A1AR |
Mouse A1AR |
|
| Agonist | EC50 ± SEM (nM) (efficacy at 100 μM concentration, in % relative to maximal effect of NECAa) | ||
| AST-004 | n.d. |
1330 ± 259 (100 ± 1) |
3780 ± 480 (102 ± 2) |
| NECA | n.d. | 0.781 ± 0.343 | 1.50 ± 0.28 |
a10 μM NECA
Together, these data highlight the likelihood of nucleoside metabolites of P2Y1R agonists to interact with adenosine receptors.
Relative efficacies of MRS2365 and its metabolite AST-004 in in vivo murine model of traumatic brain injury (TBI)
Previous data demonstrated that intraperitoneal administration of MRS2365 significantly reduced cerebral edema and reactive gliosis in an in vivo model of traumatic brain injury, attributed to P2Y1R agonism [29]. Based on the observations of rapid in vivo metabolism of MRS2365, the efficacy of its adenosine A3 agonist metabolite AST-004 was compared in a controlled cortical impact (CCI) model of TBI in mice.
An equimolar intraperitoneal bolus dose of AST-004, compared with MRS2365 (0.22 mg/kg), or vehicle, was administered to mice following a CCI. Cell death, blood brain barrier permeability and reactive gliosis (astrocytes and microglia), were measured 3–7 days post-injury. Administration of AST-004 resulted in a statistically significant reduction in seizures, gliosis, and learning impairments (Bozdemir et al., 2020; manuscript in preparation). Indistinguishable neuroprotective efficacy of MRS2365 and AST-004 was also observed in a murine model of stroke (data not shown, manuscript in preparation). Together, these data indicate that the observed efficacy of MRS2365 in mouse models of TBI and stroke are mediated by adenosine receptors, not by P2Y1R.
Discussion
The susceptibility of purine and pyrimidine nucleotide P2Y or P2X agonists and antagonists to rapid dephosphorylation by ectonucleotidases has long been recognized [10, 11]. Multiple ectonucleotidases and phosphatases, which are present at different levels in different cells and organs, are responsible for this metabolism [42, 43]. ATP and ADP and their analogs, similarly to the P2Y1R agonists examined here, are first hydrolyzed by E-NTPDase (CD39, along with its NTPDase family) to produce AMP, followed by CD73 to cleave the 5′-phosphate of AMP [39]. Many in vitro and in vivo studies of P2YRs and P2XRs have noted the potential of applied adenine nucleotide agonists to form adenosine, which can activate adenosine receptors and complicate the data interpretation. In some cases, either ectonucleotidase inhibitors or adenosine receptor antagonists are added to minimize this effect [44, 45]. However, these compounds can produce their own confounding effects through other interactions or by blocking endogenous adenosine [46]. Furthermore, enzymes other than CD73 can hydrolyze AMP to adenosine, such as alkaline phosphatase in human airways [43]. It is worth noting that the activity of the ectonucleotidases that metabolize the P2Y1R agonists and antagonists in our study is also subject to temperature and pH dependence [42, 47]. The pH of maximal activity of human CD73 was found to be 7.2–7.5, and the corresponding optimal pH for E-NTPDase was reported to be 8.5. Therefore, many factors may affect the complication of phosphate hydrolysis in situ associated with P2Y1R tool compounds, leading to activation of adenosine receptors or other activities.
Extensive medicinal chemistry efforts have been conducted to stabilize the nucleotide phosphoester groups by structural modification to prevent hydrolysis [14–19, 48, 49]. However, very few analogs have been evaluated in both in vitro and in vivo pharmacokinetic or metabolic studies in preclinical species or in humans. Those few examples have reported in vivo half-lives or durations of activity on the order of 2 min [48, 49], consistent with an extremely high plasma clearance. Our data are a systematic assessment of in vitro and in vivo metabolism and pharmacokinetics of multiple P2Y agonists and antagonists, including examples that have previously been reported to have increased in vitro stability to ectonucleotidase-mediated dephosphorylation relative to endogenous nucleotides [18, 19]. In addition, we have presented the pharmacological implications of the metabolism of P2 receptor ligands to adenosine receptor-active metabolites.
The current data reveal a rapid in vitro and in vivo metabolism of the high-affinity and specific P2Y1R agonist MRS2365 to a dephosphorylated nucleoside AST-004 with affinity for the adenosine A3 receptor and A1 receptors. These findings also indicate that while the ring constraint imparted by the (N)-methanocarbo moiety in these P2Y1R agonists may result in greater in vitro metabolic stability of the α-phosphoester relative to AMP, as previously demonstrated, these structural modifications are still prone to rapid dephosphorylation in vitro and in vivo. Moreover, this finding of rapid metabolism is not unique to MRS2365, as significant and rapid in vitro dephosphorylation was also observed with the (N)-methanocarba P2Y1R antagonist MRS2500 as well as the phosphorylated riboside P2Y1R agonists and antagonists 2-MeS-ADP and MRS2179. The major metabolite of 2-MeS-ADP, 2-methylthioadenosine, has also been characterized as an adenosine receptor ligand.
In in vitro mouse and human serum and blood experiments, the rapid metabolism of these compounds was significantly inhibited in samples prepared with 1 mM EDTA as the anticoagulant. Since ectonucleotidases require divalent cation cofactors for their activity, the inhibition of metabolism in EDTA-treated samples is likely due to cation chelation and loss of ectonucleotidase activity in these matrices [39]. When heparin was utilized as the plasma and blood anticoagulant, rapid dephosphorylation was observed. Although ectonucleotidase activity was not quantified in this study, the effects of EDTA on the metabolism of these compounds are consistent with ectonucleotidase inhibition as previously reported [39].
Initial studies have found no evidence of rephosphorylation of the nucleoside metabolite AST-004 back to the monophosphoester nucleotide MRS2347 or the diphosphoester nucleotide MRS2365. Together, these data, along with the preliminary findings in mouse models of TBI and stroke (manuscripts in preparation), suggest that AST-004 efficacy is due to interaction with cell surface A3AR and not due to extracellular or intracellular rephosphorylation back to a P2Y1R agonist. A role for adenosine to reduce TBI damage, possibly through either A1 or A3 receptors, has been proposed [50].
Additional research in this report describes the in vitro metabolism of the P2Y1R antagonists MRS2179 and MRS2500 to their dephosphorylated nucleoside metabolites. Although receptor affinity studies were not conducted for the metabolites of these antagonists, structurally it is conceivable that these 2′-deoxynucleosides would also interact weakly with the adenosine receptors and might have other biological activities. The 2′-deoxynucleoside metabolites are also analogs of 2′-deoxyadenosine, a compound that has been studied extensively in the fields of immune function and oncology [51]. For example, the anticancer drug cladribine, which is the 2′-deoxy analog of potent, non-selective adenosine receptor agonist 2-chloroadenosine, displays affinity at human A1 and A2A (Ki = 1.57 and 15.1 μM, respectively), but not A2B and A3 (Ki > 100 μM) receptors [52]. Thus, the adenosine receptor affinity of this riboside is reduced by > 100-fold by the removal of the 2′-hydroxyl group. Furthermore, the 2-iodo modification of adenosine analogs further decreases adenosine receptor affinity [53]; thus, it is unlikely that a metabolite of MRS2500 would substantially activate adenosine receptors. The product of phosphate removal from the 5′ position of MRS2500 (Fig. 1c) is reported to have a Ki of 1.56 μM at the human P2Y1R, i.e., ~ 2000-fold weaker than MRS2500 [54, 55]. Thus, based on our findings, it would seem very important to evaluate the in vivo pharmacokinetics and metabolism of these antagonists as a key component of any in vivo efficacy study. Paradoxically, a single report has been published on the in vivo pharmacokinetics of MRS2500 following intravenous administration to mice, reporting a low plasma clearance value (determined using mouse serum) [56], which is consistent with antithrombotic efficacy of MRS2500 in the mouse observed by Hechler et al. [19]. Another report refers instead to the compound’s short half-life in monkeys [24]. Further research is required to explain the discrepancy between the apparent rapid metabolism reported here and the previously reported mouse in vivo data.
Many studies using as P2Y1R pharmacological probes, riboside nucleotide agonist 2-methylthio-ADP and antagonist MRS2179, or the (N)-methanocarba agonist MRS2365 and antagonist MRS2500 have been validated using other evidence for direct effects on this receptor. For example, the use of both agonist and antagonist (at moderate concentrations) in control experiments or following the selective loss of P2Y1R due to desensitization [57, 58], or the use of P2Y1R-knockout mice [59], increases confidence in the use of these compounds. Also, it is worth noting that stable antagonists of P2Y1R are available, such as allosteric antagonist BPTU, a urea derivative (although much weaker than MRS2500), which was used to block P2Y1R neuromuscular responses to MRS2365 in intestinal muscle strips [57]. Therefore, we are not recommending discontinuation of the use of these tool compounds in research. Nevertheless, our results suggest caution in interpretation of results with these ligands, especially for in vivo application. The unusual characteristics of P2 receptor pharmacology, compared with many other GPCRs and ligand gated ion channels for which there are chemically and enzymatically stable tool compounds, require taking into consideration phosphate ester hydrolysis and its consequences.
In addition to (N)-methanocarba modifications of nucleotides, other structural modifications have been pursued to impart hydrolytic stability to the phosphoester groups of P2Y or P2X agonists and antagonists. These modifications include incorporation of β,γ-methylene, α-BH3. β,γ-dihalomethylene, or phosphorothionate groups into the phosphoester backbone of these nucleotides. These compounds appear to have greater in vitro stability than ATP or ADP in in vitro human serum incubations and/or various preparations of ectonucleotidases. However, the vast majority of these compounds has not been evaluated in in vivo pharmacokinetic studies to date. Interestingly, one β,γ-dihalomethylene ATP analog has been assessed in vivo in rat, dog, and human, with a reported in vivo human half-life of only 2 min, indicative of extremely high in vivo clearance [48, 49]. Surprisingly, this same compound was reported to be completely stable to dephosphorylation in human serum.
In previous publications on boronophosphate P2Y1R agonists, the reference in vitro half-life of ATP in plasma has been reported to be unusually long, up to 4 h [15]. This is in contrast to many studies that have reported in vitro ATP half-lives ranging from seconds to min and ex vivo perfused tissue and in vivo studies that have reported ATP half-lives < 1 s [12, 13]. These discrepancies in the reported stability of the natural endogenous nucleotides make interpretation of relative stabilities of various structural modifications very difficult to interpret. A common methodological approach to both the conduct and the analysis of nucleotide stability studies is warranted. For example, fresh heparinized whole blood could be a fairly consistent in vitro matrix with which to conduct nucleotide stability studies and compare results across chemical series and research groups, utilizing LC-MS/MS to ensure specific monitoring of substrate concentrations and metabolite formations. Obviously, it would be important to avoid the addition of EDTA to in vitro nucleotide stability assays for the reasons mentioned above regarding chelation of divalent cations required for ectonucleotidase activity.
At this time, however, there is not a consistent approach to scaling between in vitro methods to assess nucleotide stability and the actual in vivo pharmacokinetics of these compounds, further emphasizing the need for in vivo pharmacokinetic confirmation of the disposition of these nucleotide analogs. Unlike other drug-metabolizing enzyme families such as the cytochrome P450 family that are predominantly expressed in the liver, ectonucleotidases are an ubiquitous family of multiple enzymes that are not only expressed on the surface of every cell but circulated in the blood as well [10, 11]. In the case of cytochrome P450-mediated metabolic clearance, techniques have been developed and summarized to predict in vivo intrinsic clearance of compounds from in vitro microsomal or hepatocyte stability data [60]. Although these techniques have not been applied to the unique challenges of the broad tissue expression of ectonucleotidases, they should be able to be adapted, modified, and applied to at least allow an initial whole blood in vitro-in vivo scaling of blood intrinsic clearance of nucleotide analogs. Further research will be pursued in this area.
Our observations present a number of challenges and potential dangers for the broader field of purinergic pharmacology, not just the field of P2Y1R pharmacology. First, the vast majority of published research in both the P2Y and P2X receptor fields makes no effort to confirm in vitro, in vivo circulating, or in vivo target tissue concentrations of nucleotide P2 receptor ligands. The research groups conducting these studies administered various P2Y or P2X receptor agonists and antagonists and assume that the observed effects were due to the unmetabolized administered ligand. Second, although great progress has been made in the synthesis of non-nucleotide P2 receptor antagonists [61], current research in the P2 receptor field still relies heavily on phosphorylated receptor agonists and, in many cases, both purine-containing and pyrimidine-containing agonists and antagonists. Given the susceptibility of phosphorylated nucleotides to hydrolysis, it is critically important that previous research is reevaluated to ensure that effects attributed to P2Y or P2X receptor ligands are indeed due to the interactions with P2Y or P2X receptors and not due to nucleoside metabolites that may actually be activating adenosine receptors or other potential targets.
Acknowledgments
We thank Jeanne Rumsey, Christin Vielmuth and Angelika Fischer for skillful technical assistance.
Abbreviations
- AST-004
(1R,2R,3S,4R,5S)-4-(6-amino-2-(methylthio)-9H-purin-9-yl)-1-(hydroxymethyl)bicyclo[3.1.0]hexane-2,3-diol
- CCPA
2-Chloro-N6-cyclopentyladenosine
- CGS21680
2-(4-(2-carboxyethyl)phenethylamino)-5′-N-ethylcarboxamidoadenosine
- CHO
Chinese hamster ovary
- 2-MeS-ADP
2-methylthio-adenosine 5′-diphosphate
- HEK
Human embryonic kidney
- I-AB-MECA
[125I]N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide
- LLOQ
Lower limit of quantitation
- NECA
Adenosine-5′-N-ethyluronamide
- R-PIA
[3H]N6-R-phenylisopropyladenosine
- PSB-603
8-[4-[4-(4-chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine
- ULOQ
Upper limit of quantitation.
Funding
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R41NS093756, the NIDDK Intramural Research Program (ZIADK31117) and the Deutsche Forschungsgemeinschaft (DFG).
Compliance with ethical standards
Competing interest
The authors declare they have no competing interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Deutsche Forschungsgemeinschaft.
Ethics approval
All studies were conducted under approved University of Texas Health at San Antonio (UTHSA) and Inotiv, Inc. IACUC protocols.
Footnotes
Significance statement
Extensive chemical efforts have attempted to stabilize nucleotide phosphoester groups to prevent hydrolysis and allow investigation of the in vitro and in vivo effects of P2Y and P2X receptor ligands. Our research demonstrates that prototypical P2Y1 receptor agonists containing an (N)-methanocarba ring system to impede α-phosphoester hydrolysis are rapidly metabolized to nucleoside metabolites with affinity for adenosine receptors, and antagonists are similarly unstable. This research suggests that some P2YR pharmacology observed to date may actually be due in part to adenosine receptor activation.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galindo A, Krnjevic K, Schwartz S. Micro-iontophoretic studies on neurones in the cuneate nucleus. J Physiol. 1967;192(2):359–377. doi: 10.1113/jphysiol.1967.sp008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock GA (1978) A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L (eds) Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach. Raven Press, pp 107–118
- 5.van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 6.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98(3):1591–1625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 8.Boeynaems JM, Communi D, Gonzalez NS, Robaye B. Overview of the P2 receptors. Semin Thromb Hemost. 2005;31(2):139–149. doi: 10.1055/s-2005-869519. [DOI] [PubMed] [Google Scholar]
- 9.Zimmet J, Jarlebark L, Hammarberg T, van Galen PJ, Jacobson KA, Heilbronn E. Synthesis and biological activity of novel 2-Thio derivatives of Atp. Nucleosides Nucleotides. 1993;12(1):1–20. doi: 10.1080/07328319308016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev Res. 2001;52:44–56. doi: 10.1002/ddr.1097. [DOI] [Google Scholar]
- 11.Zimmermann H (2006) Ectonucleotidases in the nervous system. Novartis found Symp 276:113-128; discussion 128-130, 233-117, 275-181 [PubMed]
- 12.Binet L, Burstein M. Lung and vascular action of adenosin triphosphate. Presse Med. 1950;58(68):1201–1203. [PubMed] [Google Scholar]
- 13.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Tayeb A, Qi A, Muller CE. Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem. 2006;49(24):7076–7087. doi: 10.1021/jm060848j. [DOI] [PubMed] [Google Scholar]
- 15.Eliahu SE, Camden J, Lecka J, Weisman GA, Sevigny J, Gelinas S, Fischer B. Identification of hydrolytically stable and selective P2Y(1) receptor agonists. Eur J Med Chem. 2009;44(4):1525–1536. doi: 10.1016/j.ejmech.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azran S, Forster D, Danino O, Nadel Y, Reiser G, Fischer B. Highly efficient biocompatible neuroprotectants with dual activity as antioxidants and P2Y receptor agonists. J Med Chem. 2013;56(12):4938–4952. doi: 10.1021/jm400197m. [DOI] [PubMed] [Google Scholar]
- 17.Azran S, Danino O, Forster D, Kenigsberg S, Reiser G, Dixit M, Singh V, Major DT, Fischer B. Identification of highly promising antioxidants/neuroprotectants based on nucleoside 5'-phosphorothioate scaffold. Synthesis, activity, and mechanisms of action. J Med Chem. 2015;58(21):8427–8443. doi: 10.1021/acs.jmedchem.5b00575. [DOI] [PubMed] [Google Scholar]
- 18.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a northern methanocarba conformation: enhanced stability and potency as P2Y(1) receptor agonists. J Med Chem. 2002;45(10):2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316(2):556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen JB, Cronin C, Sonin D, Joshi BV, Gongora Nieto M, Harrison D, Jacobson KA, Liang BT. P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy: implications for the treatment of heart failure. Am J Physiol Heart Circ Physiol. 2007;292(2):H1077–H1084. doi: 10.1152/ajpheart.00515.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuboyama K, Harada H, Tozaki-Saitoh H, Tsuda M, Ushijima K, Inoue K. Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31(9):1930–1941. doi: 10.1038/jcbfm.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenker IC, Sobrinho CR, Takakura AC, Mulkey DK, Moreira TS. P2Y1 receptors expressed by C1 neurons determine peripheral chemoreceptor modulation of breathing, sympathetic activity, and blood pressure. Hypertension. 2013;62(2):263–273. doi: 10.1161/HYPERTENSIONAHA.113.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch H, Bespalov A, Drescher K, Franke H, Krugel U. Impaired cognition after stimulation of P2Y1 receptors in the rat medial prefrontal cortex. Neuropsychopharmacology. 2015;40(2):305–314. doi: 10.1038/npp.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong PC, Watson C, Crain EJ. The P2Y1 receptor antagonist MRS2500 prevents carotid artery thrombosis in cynomolgus monkeys. J Thromb Thrombolysis. 2016;41(3):514–521. doi: 10.1007/s11239-015-1302-7. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Cheng Y, Zhang R, Liu D, Luo YM, Chen KL, Ren S, Zhang J. P2Y1R is involved in visceral hypersensitivity in rats with experimental irritable bowel syndrome. World J Gastroenterol. 2017;23(34):6339–6349. doi: 10.3748/wjg.v23.i34.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shigetomi E, Hirayama YJ, Ikenaka K, Tanaka KF, Koizumi S. Role of purinergic receptor P2Y1 in spatiotemporal Ca(2+) dynamics in astrocytes. J Neurosci. 2018;38(6):1383–1395. doi: 10.1523/JNEUROSCI.2625-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichenbach N, Delekate A, Breithausen B, Keppler K, Poll S, Schulte T, Peter J, Plescher M, Hansen JN, Blank N, Keller A, Fuhrmann M, Henneberger C, Halle A, Petzold GC. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer's disease model. J Exp Med. 2018;215(6):1649–1663. doi: 10.1084/jem.20171487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng W, Talley Watts L, Holstein DM, Wewer J, Lechleiter JD. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33(4):600–611. doi: 10.1038/jcbfm.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talley Watts L, Sprague S, Zheng W, Garling RJ, Jimenez D, Digicaylioglu M, Lechleiter J. Purinergic 2Y(1) receptor stimulation decreases cerebral edema and reactive gliosis in a traumatic brain injury model. J Neurotrauma. 2013;30(1):55–66. doi: 10.1089/neu.2012.2488. [DOI] [PubMed] [Google Scholar]
- 30.Baurand A, Raboisson P, Freund M, Leon C, Cazenave JP, Bourguignon JJ, Gachet C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412(3):213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- 31.Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y 1 receptor. Biochem Pharmacol. 2004;68(10):1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunne H, Cowman J, Kenny D. MRS2179: a novel inhibitor of platelet function. BMC Proc. 2015;9(Suppl 1):A2. doi: 10.1186/1753-6561-9-S1-A2. [DOI] [Google Scholar]
- 33.Chen J, Wang L, Zhang Y, Yang J. P2Y1 purinoceptor inhibition reduces extracellular signal-regulated protein kinase 1/2 phosphorylation in spinal cord and dorsal root ganglia: implications for cancer-induced bone pain. Acta Biochim Biophys Sin Shanghai. 2012;44(4):367–372. doi: 10.1093/abbs/gms007. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Holstein JD, Upadhyay G, Lin DT, Conway S, Muller E, Lechleiter JD. Purinergic receptor-stimulated IP3-mediated Ca2+ release enhances neuroprotection by increasing astrocyte mitochondrial metabolism during aging. J Neurosci. 2007;27(24):6510–6520. doi: 10.1523/JNEUROSCI.1256-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borrmann T, Hinz S, Bertarelli DC, Li W, Florin NC, Scheiff AB, Muller CE. 1-alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity. J Med Chem. 2009;52(13):3994–4006. doi: 10.1021/jm900413e. [DOI] [PubMed] [Google Scholar]
- 36.Behrenswerth A, Volz N, Torang J, Hinz S, Brase S, Muller CE. Synthesis and pharmacological evaluation of coumarin derivatives as cannabinoid receptor antagonists and inverse agonists. Bioorg Med Chem. 2009;17(7):2842–2851. doi: 10.1016/j.bmc.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Alnouri MW, Jepards S, Casari A, Schiedel AC, Hinz S, Muller CE. Selectivity is species-dependent: characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal. 2015;11(3):389–407. doi: 10.1007/s11302-015-9460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao ZG, Blaustein JB, Gross AS, Melman N, Jacobson KA. N6-substituted adenosine derivatives: selectivity, efficacy, and species differences at A3 adenosine receptors. Biochem Pharmacol. 2003;65(10):1675–1684. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yegutkin GG, Burnstock G. Steady-state binding of adenine nucleotides ATP, ADP and AMP to rat liver and adipose plasma membranes. J Recept Signal Transduct Res. 1999;19(1–4):437–448. doi: 10.3109/10799899909036663. [DOI] [PubMed] [Google Scholar]
- 40.Gao ZG, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. Structural determinants of A(3) adenosine receptor activation: nucleoside ligands at the agonist/antagonist boundary. J Med Chem. 2002;45(20):4471–4484. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao ZG, Jacobson KA. Partial agonists for A(3) adenosine receptors. Curr Top Med Chem. 2004;4(8):855–862. doi: 10.2174/1568026043450989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dias DA, de Barros PB, Dos Santos LD, Dos Santos PM, Arruda CCP, Schetinger MRC, Leal DBR, Dos Santos Jaques JA. Characterization of ectonucleoside triphosphate diphosphohydrolase (E-NTPDase; EC 3.6.1.5) activity in mouse peritoneal cavity cells. Cell Biochem Funct. 2017;35(7):358–363. doi: 10.1002/cbf.3281. [DOI] [PubMed] [Google Scholar]
- 43.Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem. 2003;278(15):13468–13479. doi: 10.1074/jbc.M300569200. [DOI] [PubMed] [Google Scholar]
- 44.Aslam M, Sedding D, Koshty A, Santoso S, Schulz R, Hamm C, Gunduz D. Nucleoside triphosphates inhibit ADP, collagen, and epinephrine-induced platelet aggregation: role of P2Y(1) and P2Y(1)(2) receptors. Thromb Res. 2013;132(5):548–557. doi: 10.1016/j.thromres.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Vetri F, Xu H, Mao L, Paisansathan C, Pelligrino DA. ATP hydrolysis pathways and their contributions to pial arteriolar dilation in rats. Am J Physiol Heart Circ Physiol. 2011;301(4):H1369–H1377. doi: 10.1152/ajpheart.00556.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caiazzo E, Morello S, Carnuccio R, Ialenti A, Cicala C. The ecto-5'-nucleotidase/cd73 inhibitor, alpha,beta-methylene adenosine 5′-diphosphate, exacerbates carrageenan-induced pleurisy in rat. Front Pharmacol. 2019;10:775. doi: 10.3389/fphar.2019.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maguire MH, Krishnakantha TP, Aronson DM. Human placental 5′-nucleotidase: purification and properties. Placenta. 1984;5(1):21–39. doi: 10.1016/s0143-4004(84)80046-6. [DOI] [PubMed] [Google Scholar]
- 48.Humphries RG, Tomlinson W, Clegg JA, Ingall AH, Kindon ND, Leff P. Pharmacological profile of the novel P2T-purinoceptor antagonist, FPL 67085 in vitro and in the anaesthetized rat in vivo. Br J Pharmacol. 1995;115(6):1110–1116. doi: 10.1111/j.1476-5381.1995.tb15925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingall AH, Dixon J, Bailey A, Coombs ME, Cox D, McInally JI, Hunt SF, Kindon ND, Teobald BJ, Willis PA, Humphries RG, Leff P, Clegg JA, Smith JA, Tomlinson W. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42(2):213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 50.Kochanek PM, Verrier JD, Wagner AK, Jackson EK (2013) The many roles of adenosine in traumatic brain injury. In: Masino S and Boison D (eds) Adenosine: A Key Link between Metabolism and Brain Activity. Springer, pp 307–322. 10.1007/978-1-4614-3903-5_15
- 51.Fox IH, Kelley WN. The role of adenosine and 2′-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- 52.Jensen K, Johnson LA, Jacobson PA, Kachler S, Kirstein MN, Lamba J, Klotz KN. Cytotoxic purine nucleoside analogues bind to A1, A2A, and A3 adenosine receptors. Naunyn Schmiedeberg's Arch Pharmacol. 2012;385(5):519–525. doi: 10.1007/s00210-011-0719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohno M, Gao ZG, Van Rompaey P, Tchilibon S, Kim SK, Harris BA, Gross AS, Duong HT, Van Calenbergh S, Jacobson KA. Modulation of adenosine receptor affinity and intrinsic efficacy in adenine nucleosides substituted at the 2-position. Bioorg Med Chem. 2004;12(11):2995–3007. doi: 10.1016/j.bmc.2004.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciancetta A, O'Connor RD, Paoletta S, Jacobson KA. Demystifying P2Y1 receptor ligand recognition through docking and molecular dynamics analyses. J Chem Inf Model. 2017;57(12):3104–3123. doi: 10.1021/acs.jcim.7b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. [32P]2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147(5):459–467. doi: 10.1038/sj.bjp.0706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bird JE, Wang X, Smith PL, Barbera F, Huang C, Schumacher WA. A platelet target for venous thrombosis? P2Y1 deletion or antagonism protects mice from vena cava thrombosis. J Thromb Thrombolysis. 2012;34(2):199–207. doi: 10.1007/s11239-012-0745-3. [DOI] [PubMed] [Google Scholar]
- 57.Mane N, Jimenez-Sabado V, Jimenez M. BPTU, an allosteric antagonist of P2Y1 receptor, blocks nerve mediated inhibitory neuromuscular responses in the gastrointestinal tract of rodents. Neuropharmacology. 2016;110(Pt a):376–385. doi: 10.1016/j.neuropharm.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 58.Bourdon DM, Mahanty SK, Jacobson KA, Boyer JL, Harden TK. (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J Thromb Haemost. 2006;4(4):861–868. doi: 10.1111/j.1538-7836.2006.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durnin L, Hwang SJ, Kurahashi M, Drumm BT, Ward SM, Sasse KC, Sanders KM, Mutafova-Yambolieva VN. Uridine adenosine tetraphosphate is a novel neurogenic P2Y1 receptor activator in the gut. Proc Natl Acad Sci U S A. 2014;111(44):15821–15826. doi: 10.1073/pnas.1409078111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, Rance DJ, Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283(1):46–58. [PubMed] [Google Scholar]
- 61.Conroy S, Kindon N, Kellam B, Stocks MJ. Drug-like antagonists of P2Y receptors-from Lead identification to drug development. J Med Chem. 2016;59(22):9981–10005. doi: 10.1021/acs.jmedchem.5b01972. [DOI] [PubMed] [Google Scholar]