Abstract
Background:
Distal radius fractures (DRF) in the elderly are treated with volar locking plates (VLP), external fixation (ex-fix), percutaneous pinning (CRPP), or casting. This study performs an economic analysis of these treatments in elderly patients with closed DRFs.
Methods:
This is a secondary analysis of the Wrist and Radius Injury Surgical Trial (WRIST), a randomized multicenter international clinical trial with a parallel nonoperative casted group of patients over the age of 60 with surgically indicated, extra-articular closed DRFs. SF-36-converted utilities and total costs from Medicare were used to calculate quality-adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER).
Results:
Casted patients were self-selected and older (p<0.001) than the randomized surgical cohorts, but otherwise similar in comorbidities and sociodemographic characteristics. QALYs for CRPP were highest at 9.17 and ex-fix lowest at 8.81. Total cost expended were $16,354 for VLP, $16,012 for ex-fix, $11,329 for CRPP, and $6,837 for casting. The ICER for VLP and ex-fix were dominated by CRPP and casting. The ICER for CRPP compared to casting was $28,717 and VLP compared to ex-fix was $2,117. One-way sensitivity analysis demonstrated robustness of the results. Probabilistic sensitivity analysis revealed a 10%, 5%, 53%, and 32% chance of VLP, ex-fix, CRPP, and casting being cost-effective at the willingness-to-pay threshold of $100,000/QALY.
Conclusions:
Casting is the most cost-effective treatment modality in the elderly with closed extra-articular DRFs and should be considered before operative intervention. In unstable closed fractures, CRPP, which is the most cost-effective surgical intervention, may be considered before VLP or ex-fix.
INTRODUCTION
Distal radius fracture (DRF) is the most common acute fracture encountered in the emergency room with 640,000 cases reported yearly in the United States.1 DRF is a prevalent injury across all age groups, with heightened incidence among children between 5 and 14 years old, males less than 50, and females greater than 40.2 Treatment options for DRFs vary from closed reduction and casting to surgical fixation with volar locking plates (VLP), external fixators (ex-fix), and percutaneous pinning (CRPP). Each intervention has its strengths and weaknesses. For example, CRPP is less invasive and cheaper, but may not reliably maintain reductions for comminuted fractures.3–7 VLP is the most expensive and invasive treatment, but provides a stable construct even for comminuted fractures and earlier functional recovery.7–13 Despite numerous treatments, there are limited DRF management guidelines leading to variations in practice pattern. Such variations and uncertainty in practice behavior cause higher cost yet lower quality of care.14,15
Cost-effectiveness studies based on previous randomized controlled trials in Europe compared two different surgical interventions such as VLP and CRPP.10,12 Other cost-effectiveness studies performed in the United States relied on these model parameters on literature review.11,16 There are noticeable differences in conclusions among these studies: although those applying randomized controlled trials concluded that VLP is not cost-effective compared to CRPP10,12, others showed that VLP is a cost-effective treatment compared to casting11 or that it is difficult to distinguish VLP or CRPP as the more cost-effective intervention.16 Given these conflicting results of cost-effectiveness in managing an extremely prevalent injury, more studies based on robust evidence are needed to clarify the best treatment strategies for different patient populations.
We aim to perform a cost-effectiveness study among casting, VLP, CRPP, and ex-fix in the elderly patient with extra-articular distal radius fracture leveraging data from the largest randomized clinical trial, WRIST. WRIST is a 24 center, over 100 investigator trial funded by the National Institutes of Health over 8 years to compare for the first time 4 existing treatments for DRFs focused on the growing elderly population. To our knowledge, this is the only cost-effectiveness study in the elderly comparing all four DRF treatments using evidence from an international multi-center randomized clinical trial and United States’ costs. We evaluated the most germane factors associated with cost-effectiveness to aid in the decision-making process of treating DRFs in the elderly.
METHODS
Study Population and Data Source
Data from the Wrist and Radius Injury Surgical Trial (WRIST) were leveraged to conduct this cost-effectiveness analysis. WRIST is a multi-center randomized clinical trial of treatment for closed, displaced, surgically-indicated extra-articular DRFs in patients above 60 years of age. All fractures in enrolled patients met a set of pre-defined radiographic criteria. Patients with significant medical comorbidities that prevented surgical intervention were excluded from the study. The surgical arm (VLP, CRPP, ex-fix) were randomized whereas the non-surgical arm consisted of patients who declined surgery. WRIST participants were recruited from 24 sites in the United States (20 sites), Canada (3 sites), and Singapore (1 site) from April 10, 2012 to December 31, 2016. Patients with open fractures, bilateral fractures, prior DRF on the studied wrist, stable fractures amenable to conservative management, and other severe trauma were excluded from the trial. Detailed study design and protocols of the trial and the full consort diagram has been illustrated previously.17 The research protocol was approved by the institutional review board at the coordinating center and at all participating sites (University of Michigan Medical School IRBMED HUM28291). Written consent was obtained from all study participants.
Model Design
We performed a model-based economic analysis from a societal perspective and healthcare sector perspective as recommended by the Second Panel on Cost Effectiveness in Health and Medicine.18 A decision-tree was constructed based on procedure type, complications, and stages of recovery with a life-time horizon. The Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) results for patients who underwent each treatment type in the WRIST trial were converted to derive health utilities. This analysis adhered to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines.
The base case scenario was a 71.1-year-old adult (based on the average participant of WRIST) who underwent VLP surgery for closed, displaced, extra-articular DRF. Patients first incur costs of the initial procedure, rehabilitation, and recovery which varied by procedure type. (Table 1) The postoperative time off work and complication rates were determined from the average reported rates in the literature. Only complications that might be treated with re-operation or re-admission to the hospital were included in the model because it was assumed minor complications that are temporary and resolve spontaneously do not incur much cost or affect health utilities. The complications included in the model are: carpal tunnel release, ulnar nerve release, arthritis leading to wrist fusion, tendon adhesion or rupture, early reduction loss requiring VLP conversion, symptomatic malunion requiring revision, post-operative admission, and re-admission for infection. Average treatment-specific complication rates reported in the literature are shown in Table 1.19–42
Table 1:
List of Model Variables for Base Case Scenario
| Variable | Base Case Value | Lowƚ | Highƚ | Distribution | Source |
|---|---|---|---|---|---|
| Age at time of injury | 71.1 years | 58 years | 97 years | Uniform | WRIST Study |
| Life Expectancy | 86 years | 82 years | 98 years | U.S. Center for Disease Control44 | |
| Complication Rates | |||||
| VLP | |||||
| Carpal Tunnel Release | 0.0% | 0.0% | 5.0% | Beta | Rhee et al.30 2012, Al-Amin et al.19 2018, Navarro et al.28 2015, Quadlbauer et al.29 2018, Bentohami et al.22 2014, Esenwein et al.24 2013, Diaz-Garcia et al.34 2011, |
| Severe Ulnar Neuropathy | 0.0% | 0.0% | 2.0% | Beta | Assumption (insufficient literature evidence) |
| Wrist Fusion | 0.0% | 0.0% | 2.0% | Beta | Assumption (insufficient literature evidence) |
| Tendon Adhesion/Rupture | 1.3% | 0.0% | 15.0% | Beta | Mathews et al.27 2015, Navarro et al.28 2015, Quadlbauer et al.29 2018, Alter et al.20 2019, Mathews et al.27 2015, Rhee et al.30 2012, Arora et al.21 2007, Bentohami et al.22 2014, Esenwein et al.24 2013, Johnson et al.25 2014, Thorninger et al.41 2017, Diaz-Garcia et al.34 2011, Grewal et al.36 2011 |
| Early Reduction Loss (VLP Conversion) | 1.3% | 0.0% | 10.0% | Beta | Rozental et al.31 2006, Quadlbauer et al.29 2018, Esenwein et al24 2013, Bentohami et al.22 2014, Navarro et al.28 2015, |
| Post-op Admission | 2.7% | 0.0% | 3.0% | Beta | Assumption (insufficient literature evidence) |
| Re-admission for infection | 0.0% | 0.0% | 8.0% | Beta | Bentohami et al.22 2014, Thorninger et al.41 2017, Diaz-Garcia et al.34 2011, Curtin et al.23 2014, |
| Symptomatic Malunion Requiring Revision Surgery | 0.0% | 0.0% | 0.0% | Beta | Mulders et al. 2019, WRIST Study |
| Ex-fix | |||||
| Carpal Tunnel Release | 1.7% | 0.0% | 6.0% | Beta | Navarro et al28 2015, Anderson et al.33 2004, Leung et al.37 2008, Margaliot et al.38 2005, Sanders et al.40 1991, Diaz-Garcia et al.34 2011 |
| Severe Ulnar Neuropathy | 1.7% | 0.0% | 2.0% | Beta | Assumption (insufficient literature evidence) |
| Wrist Fusion | 1.7% | 0.0% | 2.0% | Beta | Assumption (insufficient literature evidence) |
| Tendon Adhesion/Rupture | 1.7% | 0.0% | 2.0% | Beta | Navarro et al.28 2015, Margaliot et al.38 2005, Diaz-Garcia et al.34 2011 |
| Early Reduction Loss (VLP Conversion) | 3.3% | 0.0% | 3.0% | Beta | Leung et al.37 2008, Margaliot et al.38 2005, Sanders et al.40 1991, Anderson et al.33 2004, Navarro et al.28 2015, |
| Post-op Admission | 1.7% | 0.0% | 2.0% | Beta | Assumption (insufficient literature evidence) |
| Re-admission for infection | 0.0% | 0.0% | 10.0% | Beta | Diaz-Garcia et al.34 2011, Leung et al.37 2008, Margaliot et al.38 2005, Sanders et al.40 1991, Curtin et al.23 2014 |
| Symptomatic Malunion Requiring Revision Surgery | 1.7% | 0.0% | 5.0% | Beta | WRIST Study |
| CRPP | |||||
| Carpal Tunnel Release | 2.0% | 0.0% | 2.0% | Beta | Navarro et al.28 2015, |
| Severe Ulnar Neuropathy | 2.0% | 0.0% | 2.0% | Beta | Assumption (insufficient literature evidence) |
| Wrist Fusion | 0.0% | 0.0% | 0.0% | Beta | Assumption (insufficient literature evidence) |
| Tendon Adhesion/Rupture | 2.0% | 0.0% | 2.0% | Beta | Navarro et al.28 2015, Diaz-Garcia et al.34 2011, |
| Early Reduction Loss (VLP Conversion) | 2.0% | 0.0% | 5.0% | Beta | Glickel et al.35 2008, Navarro et al.28 2015, Subramanian et al.32 2012 |
| Post-op Admission | 0.0% | 0.0% | 2.0% | Beta | Assumption (insufficient literature evidence) |
| Re-admission for infection | 16.7% | 0.0% | 30.0% | Beta | Curtin et al.23 2014 |
| Symptomatic Malunion Requiring Revision Surgery | 2.0% | 0.0% | 5.0% | Beta | WRIST Study |
| Casting | |||||
| Carpal Tunnel Release | 2.8% | 0.0% | 5.0% | Beta | Lutz et al.26 2014 |
| Severe Ulnar Neuropathy | 0.0% | 0.0% | 0.0% | Beta | Assumption (insufficient literature evidence) |
| Wrist Fusion | 0.9% | 0.0% | 1.0% | Beta | Assumption (insufficient literature evidence) |
| Tendon Adhesion/Rupture | 0.9% | 0.0% | 2.0% | Beta | Alter et al.20 2019, Diaz-Garcia et al.34 2011 |
| Early Reduction Loss (VLP Conversion) | 6.4% | 0.0% | 35.0% | Beta | Martinez-Mendez et al.39 2018, Mathews et al.27 2015 |
| Post-op Admission | 0.0% | 0.0% | 0.0% | Beta | Assumption (insufficient literature evidence) |
| Re-admission for infection | 0.0% | 0.0% | 0.0% | Beta | Assumption (insufficient literature evidence) |
| Symptomatic Malunion Requiring Revision Surgery | 4.5% | 0.9% | 13.6% | Beta | Mulders et al. 2019, WRIST Study |
| Costs | |||||
| Direct Costs | |||||
| VLP | |||||
| Physician Fee‡ | $762.00 | $647.70 | $876.30 | Normal | 2019 National Physician Fee Schedule |
| Anesthesia Fee | $167.00 | $142.15 | $192.31 | Normal | 2019 National Physician Fee Schedule |
| Facility Fee | $5,700.00 | $4,844.65 | $6,554.53 | Normal | Medicare and HCUP |
| Hand Therapy Cost† | $9,099.00 | $7,734.15 | $10,463.85 | Normal | Medicare and HCUP |
| Ex-Fix | |||||
| Physician Fee‡ | $620.00 | $527.00 | $713.00 | Normal | 2019 National Physician Fee Schedule |
| Anesthesia Fee | $146.00 | $124.41 | $168.31 | Normal | 2019 National Physician Fee Schedule |
| Facility Fee | $7,371.00 | $6,265.37 | $8,476.67 | Normal | Medicare and HCUP |
| Hand Therapy Cost† | $6,614.00 | $5,621.90 | $7,606.10 | Normal | Medicare and HCUP |
| CRPP | |||||
| Physician Fee‡ | $687.00 | $583.95 | $790.05 | Normal | 2019 National Physician Fee Schedule |
| Anesthesia Fee | $116.00 | $98.40 | $133.14 | Normal | 2019 National Physician Fee Schedule |
| Facility Fee | $2,623.00 | $2,229.84 | $3,016.84 | Normal | Medicare and HCUP |
| Hand Therapy Cost† | $6,614.00 | $5,621.90 | $7,606.10 | Normal | Medicare and HCUP |
| Casting | |||||
| Physician Fee‡ | $77.00 | $77.00 | $120.00 | Normal | 2019 National Physician Fee Schedule |
| Anesthesia Fee | $0.00 | $0.00 | $0.00 | Normal | 2019 National Physician Fee Schedule |
| Facility Fee | $136.00 | $34 | $323.95 | Normal | Medicare and HCUP |
| Hand Therapy Cost† | $4,682.00 | $3,979.70 | $5,384.30 | Normal | Medicare and HCUP |
| Imaging | |||||
| VLP | $251.00 | $213.07 | $288.27 | Normal | Medicare and HCUP |
| Ex-fix | $280.00 | $238.14 | $322.18 | Normal | Medicare and HCUP |
| CRPP | $249.00 | $211.40 | $286.02 | Normal | Medicare and HCUP |
| Casting | $221.00 | $188.00 | $254.36 | Normal | Medicare and HCUP |
| Complication Costs | |||||
| Carpal Tunnel Release | |||||
| Physician fee | $444.00 | $399.60 | $488.40 | Normal | Medicare and HCUP |
| Anesthesia fee | $95.00 | $85.84 | $104.91 | Normal | Medicare and HCUP |
| Hospital fee | $1,631.00 | $1,468.33 | $1,794.63 | Normal | Medicare and HCUP |
| Ulnar Nerve Release | |||||
| Physician fee | $614.00 | $552.70 | $675.52 | Normal | Medicare and HCUP |
| Anesthesia fee | $125.00 | $112.25 | $137.20 | Normal | Medicare and HCUP |
| Hospital fee | $1,631.00 | $1,468.33 | $1,794.63 | Normal | Medicare and HCUP |
| Wrist Fusion | |||||
| Physician fee | $758.00 | $681.79 | $833.29 | Normal | Medicare and HCUP |
| Anesthesia fee | $198.00 | $178.28 | $217.90 | Normal | Medicare and HCUP |
| Hospital fee | $5,700.00 | $5,129.63 | $6,269.55 | Normal | Medicare and HCUP |
| Tenolysis | |||||
| Physician fee | $545.00 | $490.10 | $599.01 | Normal | Medicare and HCUP |
| Anesthesia fee | $154.00 | $138.66 | $169.48 | Normal | Medicare and HCUP |
| Hospital fee | $2,623.00 | $2,361.01 | $2,885.67 | Normal | Medicare and HCUP |
| Tendon Repair | |||||
| Physician fee | $822.00 | $739.85 | $904.26 | Normal | Medicare and HCUP |
| Anesthesia fee | $125.00 | $112.25 | $137.20 | Normal | Medicare and HCUP |
| Hospital fee | $2,623.00 | $2,361.01 | $2,885.67 | Normal | Medicare and HCUP |
| Early Reduction Loss (VLP Conversion) | |||||
| Physician fee | $902.00 | $811.80 | $992.20 | Normal | Medicare and HCUP |
| Anesthesia fee | $167.00 | $150.51 | $183.95 | Normal | Medicare and HCUP |
| Hospital fee | $5,700.00 | $5,129.63 | $6,269.55 | Normal | Medicare and HCUP |
| Hand Therapy Cost | $9,099.00 | $7,734.15 | $10,463.85 | Normal | Medicare and HCUP |
| Re-admission | |||||
| Hospital fee | $11,940.00 | $10,746.00 | $13,134.00 | Normal | Medicare and HCUP |
| Overnight Observation | |||||
| Hospital fee | $2,919.00 | $2,627.41 | $3,211.27 | Normal | Medicare and HCUP |
| Symptomatic Malunion (VLP Conversion) | |||||
| Physician fee | $1,893.00 | $1,703.70 | $2,082.30 | Normal | Medicare and HCUP |
| Anesthesia fee | $242.00 | $217.80 | $266.20 | Normal | Medicare and HCUP |
| Hospital fee | $5,700.00 | $5,130.00 | $6,270.00 | Normal | Medicare and HCUP |
| Hand Therapy Cost | $9,099.00 | $7,734.15 | $10,463.85 | Normal | Medicare and HCUP |
| Wage Calculations | |||||
| Average annual mean wage | $51,960 | $10,000 | $250,000 | Normal | WRIST Database and U.S. Census Bureau38 |
| Time off for recovery and rehab | |||||
| VLP | 11.9 days | 0 days | 30 days | Lognormal | WRIST Study |
| Ex-fix | 28.4 days | 0 days | 75 days | Lognormal | WRIST Study |
| CRPP | 11.7 days | 0 days | 30 days | Lognormal | WRIST Study |
| Casting | 6.9 days | 0 days | 21 days | Lognormal | WRIST Study |
| Retirement age | 66 years | 60 years | 70 years | Normal | Social Security |
| Discount Rate | 3.0% | 0.0% | 5.0% | Assumption | |
| Utilities | |||||
| Baseline | |||||
| VLP | 0.52 | 0.52 | 0.49 | Normal | WRIST Study (and 95% CI) |
| Ex-fix | 0.52 | 0.52 | 0.49 | Normal | WRIST Study (and 95% CI) |
| CRPP | 0.53 | 0.53 | 0.50 | Normal | WRIST Study (and 95% CI) |
| Casting | 0.56 | 0.56 | 0.54 | Normal | WRIST Study (and 95% CI) |
| 2 week | |||||
| VLP | 0.55 | 0.55 | 0.53 | Normal | WRIST Study (and 95% CI) |
| Ex-fix | 0.54 | 0.54 | 0.52 | Normal | WRIST Study (and 95% CI) |
| CRPP | 0.53 | 0.53 | 0.49 | Normal | WRIST Study (and 95% CI) |
| Casting | 0.56 | 0.56 | 0.55 | Normal | WRIST Study (and 95% CI) |
| 6 week | |||||
| VLP | 0.60 | 0.60 | 0.58 | Normal | WRIST Study (and 95% CI) |
| Ex-fix | 0.56 | 0.56 | 0.54 | Normal | WRIST Study (and 95% CI) |
| CRPP | 0.58 | 0.58 | 0.55 | Normal | WRIST Study (and 95% CI) |
| Casting | 0.61 | 0.61 | 0.59 | Normal | WRIST Study (and 95% CI) |
| 3 month | |||||
| VLP | 0.69 | 0.69 | 0.66 | Normal | WRIST Study (and 95% CI) |
| Ex-fix | 0.65 | 0.65 | 0.62 | Normal | WRIST Study (and 95% CI) |
| CRPP | 0.67 | 0.67 | 0.64 | Normal | WRIST Study (and 95% CI) |
| Casting | 0.67 | 0.67 | 0.64 | Normal | WRIST Study (and 95% CI) |
| 6 month | |||||
| VLP | 0.72 | 0.72 | 0.69 | Normal | WRIST Study (and 95% CI) |
| Ex-fix | 0.68 | 0.68 | 0.65 | Normal | WRIST Study (and 95% CI) |
| CRPP | 0.70 | 0.70 | 0.66 | Normal | WRIST Study (and 95% CI) |
| Casting | 0.68 | 0.68 | 0.65 | Normal | WRIST Study (and 95% CI) |
| 12 month | |||||
| VLP | 0.70 | 0.70 | 0.66 | Normal | WRIST Study (and 95% CI) |
| Ex-fix | 0.69 | 0.69 | 0.65 | Normal | WRIST Study (and 95% CI) |
| CRPP | 0.72 | 0.72 | 0.67 | Normal | WRIST Study (and 95% CI) |
| Casting | 0.71 | 0.71 | 0.68 | Normal | WRIST Study (and 95% CI) |
| Decrement utility VLP revision surgery | |||||
| VLP | 0.000 | 0.000 | 0.000 | Normal | WRIST Study (and 95% CI) |
| Ex-fix | 0.010 | 0.003 | 0.017 | Normal | WRIST Study (and 95% CI) |
| Pinning | −0.019 | −0.032 | −0.006 | Normal | WRIST Study (and 95% CI) |
| Casting | −0.007 | −0.011 | −0.003 | Normal | WRIST Study (and 95% CI) |
| Decrement utility Wrist Fusion | |||||
| VLP | −0.042 | −0.077 | 0 | N/A | |
| Ex-fix | −0.042 | −0.077 | 0 | N/A | |
| Pinning | −0.042 | −0.077 | 0 | N/A | |
| Cast | −0.042 | −0.077 | 0 | N/A |
for sensitivity analysis
based on CPT codes: 25607
based on CPT codes: 97165, 97110, 97535, 97760, 97530, 97763, 97140, 97010, 97018
based on CPT codes: 25400, 25607, 20902
The distributions for the probabilistic sensitivity analysis are parameterized as follows: All distributions are set so as to have roughly 95% of the distribution lie between the low and high values. Beta distribution are based on 100 data points informing the estimate and using a non-informative prior. All Normal distributions are truncated so they are > 0 (and < 1 for the reduction in wages and utilities). All distributions are assumed to be independent
Health States
Health utility for each DRF treatment type was derived from SF-36 surveys at different recovery time points in the WRIST trial. SF-36 surveys were administered to trial participants at enrollment before surgery and 2 weeks, 6 weeks, 3 months, 6 months, and 12 months postoperatively. SF-36 scores were converted to utilities according to published methods.43 The utility at 12 months were assumed to remain stable for the remainder of life. Utilities at different postoperative times were weighed according to the length of time spent with the utility value to calculate an aggregate quality-adjusted life year (QALY). Among the complications, only revision surgery to VLP for failure of reduction, wrist fusion from arthritis, and symptomatic malunion requiring revision surgery were considered long-term complications that may adversely affect long-term quality of life. The utility decrement for VLP revision surgery was assumed to be equal to the difference between 12-month utility of the original intervention (e.g. casting, CRPP, ex-fix) and the 12-month utility after VLP. Because there are no published utility values for complications resulting from DRF treatment, we assumed a base case utility decrement of −0.063 (0 for slight limitation in moderate activities and −0.063 for role limitation) based on the SF-36 to utility conversion regression.43 Prior studies have made similar assumptions in utility decrements while studying musculoskeletal conditions.44 Life expectancy and remaining years of life were derived from the United States CDC life tables.45 Depending on the patient’s age at time of injury, the remaining years of life were varied accordingly. Age at time of injury was varied from 58 and 97, slightly larger than the age range of participants in WRIST, to better simulate patients at extremes of ages.
Costs
The model included the following direct costs: physician fees, anesthesia fees, facility fees, hand therapy costs, imaging costs, and additional costs incurred by complications. Indirect costs included the patient’s wages lost from recovery. Costs were determined using Medicare reimbursement rates from the Healthcare Cost and Utilization Project.46 Physician and facility fees were derived from 2019 National Physician Fee Schedule using CPT codes. CPT code 25606 for CRPP, 20690 for ex-fix, and 25607 were used for VLP. For symptomatic malunions requiring revision surgery with VLP, an aggregate of CPT code 25400 (osteotomy), 25607 (VLP), and 20902 (bone graft) were used. Anesthesia costs for the initial procedure and surgical complications were calculated using 2019 Medicare reimbursement and anesthesia conversion factors. Hand therapy costs were estimated by Michigan Medicine hand therapists using the CPT codes listed in Table 1.
Patient’s wages lost from recovery time after the procedures were accounted for as indirect costs for sensitivity analysis. The base case scenario in our study was a 71-year-old retired patient; therefore, wages were not considered in the reference case. Lengths of time off work for recovery after each procedure were derived from the WRIST study. (Table 1) The base case scenario estimated 11.9 days, 28.4 days, 11.7 days, and 6.9 days off work for VLP, ex-fix, CRPP, and casting respectively. We assumed that there is equal prevalence of distal radius fractures regardless of employment type; therefore, the 2018 national mean salary from the Bureau of Labor and Statistics were used as the base case salary.47 Even though many patients will return to one-handed duty for 6 weeks after DRF surgery, we did not discount the salary because it was assumed that all patients will return to full-duty after recovery until retirement in all treatments. Both cost and health outcomes were discounted by 3%. The Second Panel on Cost-Effectiveness Analysis recommends performing cost-effectiveness studies from both a societal and healthcare sector perspective.18 But because the base case scenario was a 71-year-old patient who is past the average U.S. retirement age, the two perspectives yielded the same result.
Statistical Analysis
The primary outcome of this study was the incremental cost-effectiveness ratio (ICER) between the four DRF treatments. ICER represents the economic value of an intervention compared to an alternative and is calculated from dividing the difference in total expended cost between two interventions by the difference in total health benefit gained between two interventions. The primary analysis was first conducted using the base case model parameters denoted in Table 1. We then performed sensitivity analyses by varying key model parameters to determine the factors that are most influential. All direct costs were varied by 15% from the Medicare amount. Utilities were varied between the low and high bounds of the 95% confidence interval of those in the WRIST study. Remaining model parameters including age at time of injury, life expectancy, complication rates, wages, time off work, retirement age, and discount rate were varied as listed in Table 1. One-way and two-way sensitivity analyses were conducted with the variables that most affected the ICER. A probabilistic sensitivity analysis using Monte Carlo Simulation was conducted to quantify the level of uncertainty in the results from overall uncertainty from the model inputs.48 This method randomly samples all variables in the model from their respective distribution many times to demonstrate how robust the conclusions of the cost-effectiveness analysis are to the combined uncertainty in all parameters. 95% credible intervals were calculated for cost and QALY outcomes using the Monte Carlo simulation results. Uncertainty in cost-effectiveness is described in terms of cost-effectiveness acceptability curves. We used a willingness-to-pay threshold of $100,000/QALY as a definition of cost-effectiveness.49,50 Statistical package R version 3.6.0 (R Foundation for Statistical Computing) and Excel Office 365 (Microsoft Inc.) were used for modelling and analysis.
RESULTS
A total of 296 patients in the WRIST trial were included for analysis. There were 75, 60, 51, and 110 as treated patients who underwent VLP, ex-fix, CRPP, and casting respectively. Most demographic characteristics including race, number of comorbidities (diabetes, hypertension, congestive heart failure, chronic obstructive pulmonary disease, osteoarthritis), smoking status, education level, income level, dominant hand injuries, and proportion of retirees were not different among the four treatment groups. (see Table, Supplemental Digital Content 1, which illustrates descriptive statistics of demographic variables of patients, INSERT HYPER LINK) On average, patients who were casted were older (age in years [SD], 75.7 [9.6]) than those who underwent surgery. (age in years [SD], VLP 67.4 [6.4]; ex-fix 69.7 [8.1]; CRPP 68.2 [6.6]; p<0.001) Also, casted patients had a shorter time off work (days [SD], 6.9 [3.1]) than VLP (days [SD], 11.9 [4.7]), ex-fix (days [SD], 28.4 [10.7]), CRPP (days [SD], 11.7 [4.3]). (see Table, Supplemental Digital Content 1, p=0.04, INSERT HYPER LINK)
The 12-month utility scores were highest for CRPP patients, but the scores were not significantly different among the four treatment groups (utility [95%CI], VLP 0.70 [0.66,0.74], ex-fix 0.69 [0.65,0.73], CRPP 0.72 [0.67,0.77], cast 0.71 [0.68,0.74]). Corresponding lifetime quality-adjusted life years (QALY) were highest in the CRPP group (absolute number [95%CI], 9.17 [2.66–12.98]) and lowest in the ex-fix group (absolute number [95%CI], 8.80 [2.55–12.48]), but they were not significantly different. (Table 2) VLP and ex-fix were dominated by CRPP and casting, because VLP and ex-fix were both more expensive and yielded fewer QALYs than CRPP or casting. CRPP was cost-effective compared to casting with an ICER of $28,717.
Table 2:
Quality-adjusted Life Year and Incremental Cost-effectiveness Ratio by Distal Radius Fracture Treatment
| Treatment Type | 12-month SF-36 Converted Health Utility (95% CI2) | QALYs3 (95% CrI5*) | Total Cost (95% CrI5*) | Incremental Costs (95% CrI5*) | ICER($/QALY)3ƚ |
|---|---|---|---|---|---|
| Casting | 0.71 (0.68 – 0.74) | 9.01 (2.61 – 12.73) | $6,837 ($5,357 – $8,078) | Lowest cost | -- |
| CRPP1 | 0.72 (0.67 – 0.77) | 9.17 (2.66 – 12.98) | $11,329 ($9,994 – $13,164) | CRPP vs. Casting: $4,492 ($3,318 – $6,497) | $28,717 |
| Ex-fix1 | 0.69 (0.65 – 0.73) | 8.81 (2.55 – 12.48) | $16,012 ($14,135 – $17,977) | Ex-fix vs. Casting: $9,175 ($7,526 – $11,388) | Dominated |
| Ex-fix vs. CRPP: $4,683 ($2,472 – $6,627) | Dominated | ||||
| VLP1 | 0.70 (0.66 – 0.74) | 8.96 (2.62 – 12.68) | $16,354 ($14,651 – $18,465) | VLP vs. casting: $9,517 ($8,062 – $11,923) | Dominated |
| VLP vs. CRPP: $5,025 ($2,993 – $7,165) | Dominated | ||||
| VLP vs. Ex-fix: $342 (−$1,847 – $2,857) | $2,117 |
VLP: Volar locking plate; Ex-fix: External fixation; CRPP: Closed reduction percutaneous pinning
CI: Confidence Interval
QALY: Quality-adjusted Life Year
ICER: Incremental Cost-effectiveness Ratio
CrI: Credible Interval
Based on model and probabilistic sensitivity analysis
See Figure 3 for uncertainty in cost-effectiveness
Sensitivity Analysis
Because CRPP and casting were found to be dominant over the other two interventions for most extremes of model parameters (see Table, Supplemental Digital Content 2, which demonstrates one-way sensitivity analyses varying all model parameters, INSERT HYPER LINK), we focused our sensitivity analysis to those interventions. We conducted one-way sensitivity analyses on all model parameters to assess the uncertainties in the cost-effectiveness estimates. (see Table, Supplemental Digital Content 2, INSERT HYPER LINK) 12-month utility after casting, 12-month utility after CRPP, and age at time of injury were the most significant model parameters that influenced the cost-effectiveness of these two therapies based on one-way sensitivity analyses. (Figure 1) Because CRPP is more expensive than casting, when the CRPP 12-month utility is less than 0.71 or casting 12-month utility is greater than 0.71, CRPP is no longer cost-effective compared to casting. If the age at time of injury is under 83, the ICER of CRPP compared to casting stayed under the willingness-to-pay threshold of $100,000/QALY. However, when patients were older than 83, the ICER rose above $100,000/QALY.
Figure 1:
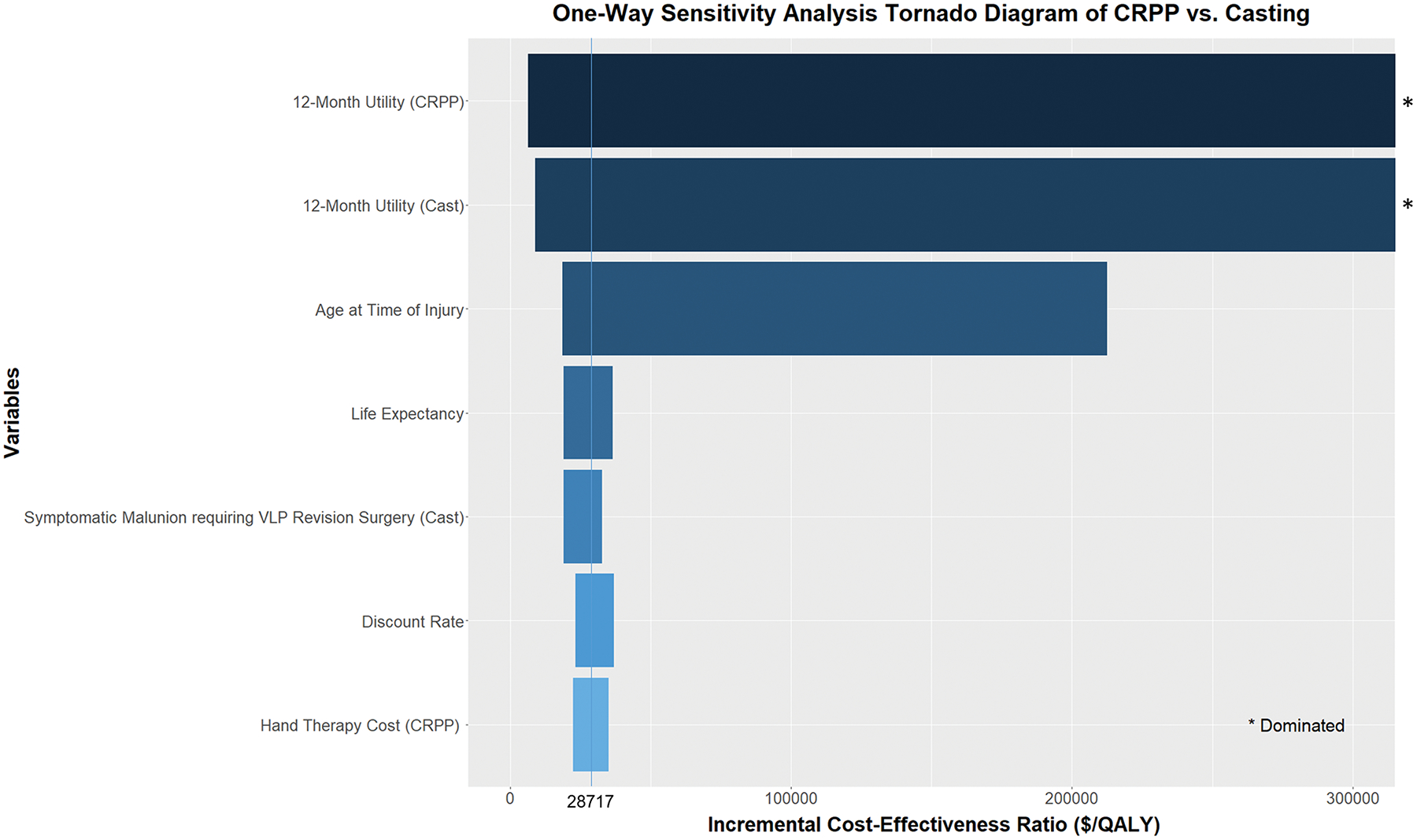
Tornado diagram illustrating one-way sensitivity analyses of ICER between closed reduction percutaneous pinning (CRPP) and casting. The 12-month utilities and age at time of injury were the most important factors that affect cost-effectiveness.
Two-way sensitivity analysis varying age at time of injury and the difference in utility between CRPP and casting were conducted. (Figure 2) When the utility after CRPP is greater than 0.04 compared to casting, CRPP is cost-effective compared to casting at all ages. If the patient is below the age of 83, even a 0.01 utility gain of CRPP over casting makes CRPP a cost-effective intervention. (Figure 3) Probabilistic sensitivity analysis revealed at a willingness-to-pay threshold of $100,000/QALY, there is a 10%, 5%, 53%, and 32% chance of VLP, ex-fix, CRPP, and casting being cost-effective respectively.
Figure 2:
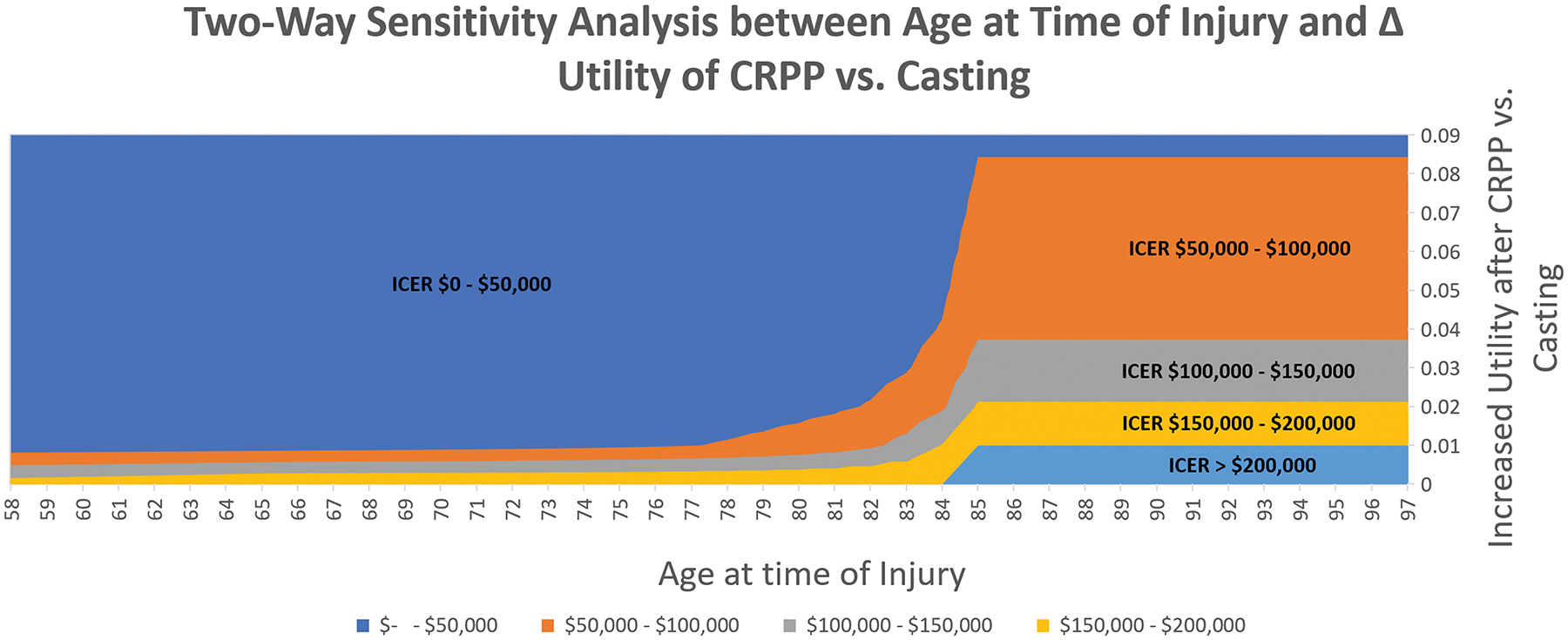
Two-way sensitivity analysis between age at time of injury and difference in utility between closed reduction percutaneous pinning (CRPP) and casting. If the patient is below the age of 83, even a 0.01 utility gain of CRPP over casting makes CRPP a cost-effective intervention.
Figure 3:
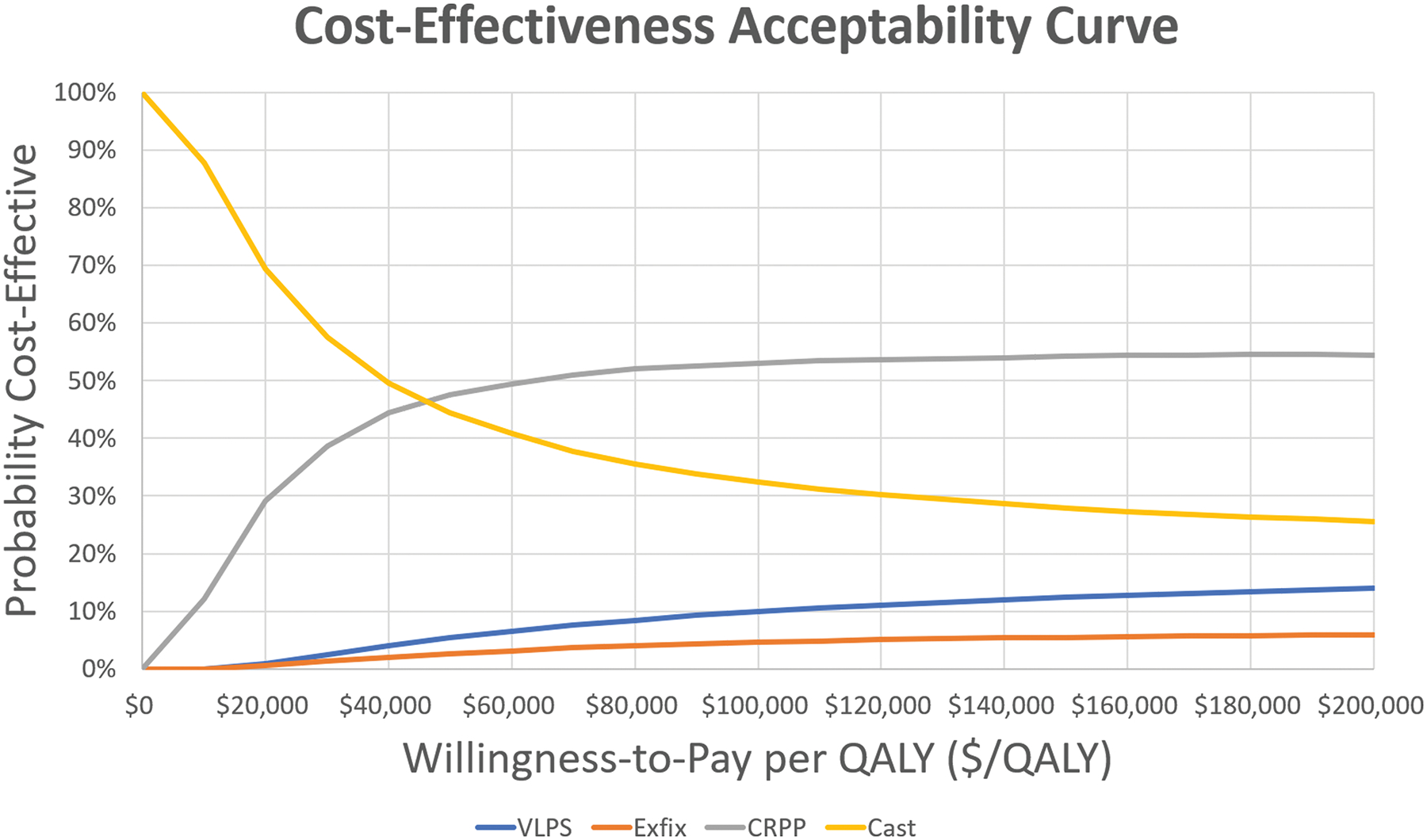
Probabilistic sensitivity analysis with 10,000 iterations by treatment type. At a willingness-to-pay threshold of $100,000/QALY, there is a 10%, 5%, 53%, and 32% chance of volar locking plate (VLP), external fixation (ex-fix), closed reduction percutaneous pinning (CRPP), and casting being cost-effective respectively.
DISCUSSION
This study suggests that treatment type does not significantly affect utilities after extra-articular distal radius fracture management in the elderly. Because of that, the more expensive treatments such as VLP and ex-fix were dominated by less expensive modalities such as CRPP and casting. The robustness of this finding was confirmed with one-way sensitivity analyses of all model parameters. CRPP was the only surgical intervention that was cost-effective when compared to casting. Sensitivity analyses between CRPP and casting revealed that CRPP continued to be a cost-effective modality for older patients until the age of 83. CRPP had a higher chance of being cost-effective than casting in a given patient, but these results were sensitive to the variability in post-surgical utility scores. Our findings highlight that CRPP and casting are both cost-effective treatment for the elderly patient with closed extra-articular DRF.
Our study findings agree with previous cost-effectiveness studies performed in Europe based on randomized clinical trials10,12 that CRPP was a more cost-effective intervention for DRF treatment in adults. However, possibly because our study population was in elderly adults, our findings differed with these analyses in that CRPP not only was cheaper, but also resulted in more QALY gains long-term making it a cost-saving intervention compared to VLP. Our findings partly agreed with the study by Rajan et al. who found that from a healthcare perspective CRPP dominated VLP and ex-fix. But their societal perspective analysis found that VLP was more cost-effective than CRPP.16 This is likely because the model started with a 50-year-old working adult, while most of our participants were retired elderly. This study did not include non-operative casting as a treatment arm.
DRF treatment is reported to be inconsistent according to surgeon preference, experience, and practice setting.51 This may be attributable to the lack of clear treatment guidelines for distal radius fractures. For instance, 21 of 29 recommendations from the American Academy of Orthopaedic Surgeons (AAOS) guideline for distal radius fracture treatment are based on inconclusive or limited evidence.52 The guideline specifies that it is unable to recommend for or against operative treatment for patients over the age of 55. Lack of evidence-guided treatment protocol can perpetuate clinical inconsistencies that negatively burden quality of care and healthcare expenditure.14,15 Given the prevalence of distal radius fractures and lack of consensus guidelines, more high-quality studies must be performed to elucidate the optimal treatment for distal radius fracture in different patient populations.
There are reports of VLP resulting in earlier functional recovery in adults compared to other DRF treatments.5,7,13 The WRIST trial only included elderly patients with nearly two-thirds of the study population already retired. In the younger or middle-aged patients who are more active and are part of the workforce that incur substantial monetary loss from being off work may have different utilities and indirect costs after VLP compared to other treatments that may change cost-effectiveness; therefore, it is important to emphasize that our study findings pertain only to the elderly population. In addition, our study conclusions only apply to extra-articular distal radius fractures based on the WRIST trial inclusion criteria. For comminuted intra-articular fractures that cannot hold reduction with CRPP, VLP may have better outcomes37, and thus be cost-effective. Furthermore, intra-articular fractures managed conservatively are reported to have appreciable rates of symptomatic malunion that require revision surgery53,54, which may increase the cost-effectiveness of VLP compared to conservative treatment or CRPP in those fracture patterns. Evidence for true symptomatic malunion rate in extra-articular distal radius fracture requiring revision surgery after both non-operative and operative managements were scarce in the literature.42
A potential limitation of this study is that it is not known whether SF-36 accurately captures all specific aspects of postoperative quality of life. But traditional methods of eliciting utilities such as the time trade-off survey and standard gamble tend to yield overinflated utilities in non-life-threatening injuries.55 Previous studies have demonstrated that global health outcome measures such as the SF-36 or Euroqol-5D (EQ-5D) accurately reflect the decreased quality of life from upper extremity afflictions and subsequently normalize to general population norms with recovery.56,57 Other more specific patient-reported upper extremity measures have not been validated to be converted to utilities. Future research should investigate in new methodologies that elicit general health utilities from upper extremity patient-reported outcomes. Another potential limitation is that the casting group was on average older than the operative group, but all four intervention groups did not differ in other baseline characteristics including medical comorbidities. There are other demographic characteristics not assessed in this study, such as psychosocial differences, that may have influenced SF-36 scores and potentially have introduced a selection bias. However, we calculated the baseline SF-36 mental component summary (MCS) of the surgical and non-surgical cohorts and found that they were not significantly different (average[SD], 49.7 [13.7] vs. 49.4 [13.8] respectively, p=0.88). Although there are no clear guidelines for distal radius fracture surgical indications52, the trial mitigated this by only including patients meeting a pre-defined standard radiographic criteria. The degree of missingness of 12-month SF-36 scores may be another source of selection bias. We excluded non-operative minor complications because we assumed they did not incur much cost and did not affect long-term quality of life. As in all cohort-based models, the results of this study may not be applicable to patients whose characteristics are outside of the ranges in the sensitivity analysis.
To our knowledge, this is the only U.S. cost-effectiveness study of DRF treatments based on a real-world sample of multi-center randomized clinical trial. In addition, this analysis investigates both operative and non-operative treatments in the elderly to better represent all treatment options available; given the current DRF treatment guidelines do not favor surgical or non-surgical interventions for patients over the age of 55.52 Lastly, the diverse regional and practice settings of participating WRIST institutions increase the generalizability of this study that may be applicable to future treatment guidelines.
CONCLUSIONS
Closed-reduction percutaneous pinning and casting are the most cost-effective interventions for closed extra-articular distal radius fractures in the elderly. When the reduction is stable after closed reduction and casting, it should be considered as a treatment option based on cost-effectiveness. When the reduction is not stable without surgical intervention based on fracture pattern, closed-reduction percutaneous pinning should be considered first as a potential treatment. Open reduction internal fixation should be reserved for complex fracture patterns not amenable to closed reduction percutaneous pinning in the elderly.
Supplementary Material
Table, Supplemental Digital Content 1: Descriptive statistics of demographic variables of patients by injury pattern.
Table, Supplemental Digital Content 2: One-way sensitivity analyses varying all model parameters demonstrating the uncertainty of the ICER around the base case scenario.
Financial Disclosures:
Dr. Chung receives funding from the National Institutes of Health and book royalties from Wolters Kluwer and Elsevier. He has received financial support from Axogen to attend conferences. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging of the National Institutes of Health under Award Number R01 AR062066 and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 2 K24-AR053120-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Trial registration: ClinicalTrials.gov NCT01589692
References:
- 1.Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908–915. [DOI] [PubMed] [Google Scholar]
- 2.Rockwood C, Bucholz R. Court-Brown CM, Heckman JD, and Tornetta P., 2006, Rockwood and Green’s Fractures in Adults. Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 3.Barton T, Chambers C, Lane E, Bannister G. Do Kirschner wires maintain reduction of displaced Colles’ fractures? Injury. 2005;36(12):1431–1434. [DOI] [PubMed] [Google Scholar]
- 4.Costa ML, Achten J, Parsons NR, et al. Percutaneous fixation with Kirschner wires versus volar locking plate fixation in adults with dorsally displaced fracture of distal radius: randomised controlled trial. BMJ. 2014;5(349). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karantana A, Downing ND, Forward DP, et al. Surgical treatment of distal radial fractures with a volar locking plate versus conventional percutaneous methods: a randomized controlled trial. J Bone Joint Surg Am. 2013;95(19):1737–1744. [DOI] [PubMed] [Google Scholar]
- 6.Marcheix P-S, Dotzis A, Benkö P-E, Siegler J, Arnaud J-P, Charissoux J-L. Extension fractures of the distal radius in patients older than 50: a prospective randomized study comparing fixation using mixed pins or a palmar fixed-angle plate. J Hand Surg Eur. 2010;35(8):646–651. [DOI] [PubMed] [Google Scholar]
- 7.Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837–1846. [DOI] [PubMed] [Google Scholar]
- 8.Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen NC, Jupiter JB. Management of distal radial fractures. J Bone Joint Surg Am. 2007;89(9):2051–2062. [DOI] [PubMed] [Google Scholar]
- 10.Karantana A, Scammell BE, Davis TR, Whynes DK. Cost-effectiveness of volar locking plate versus percutaneous fixation for distal radial fractures: Economic evaluation alongside a randomised clinical trial. Bone Joint J. 2015:35560. [DOI] [PubMed] [Google Scholar]
- 11.Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912–1918. [DOI] [PubMed] [Google Scholar]
- 12.Tubeuf S, Yu G, Achten J, et al. Cost effectiveness of treatment with percutaneous Kirschner wires versus volar locking plate for adult patients with a dorsally displaced fracture of the distal radius: analysis from the DRAFFT trial. Bone Joint J. 2015:35234. [DOI] [PubMed] [Google Scholar]
- 13.McFadyen I, Field J, McCann P, Ward J, Nicol S, Curwen C. Should unstable extra-articular distal radial fractures be treated with fixed-angle volar-locked plates or percutaneous Kirschner wires? A prospective randomised controlled trial. Injury. 2011;42(2):162–166. [DOI] [PubMed] [Google Scholar]
- 14.Smith R. Where is the wisdom…? : BMJ 1991. October 5;303(6806):798–9. doi: 10.1136/bmj.303.6806.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Nash D. Health care myth busters: Is there a high degree of scientific certainty in modern medicine. Sci Am. 2011;25. [Google Scholar]
- 16.Rajan PV, Qudsi RA, Dyer GSM, Losina E. The Cost-Effectiveness of Surgical Fixation of Distal Radial Fractures: A Computer Model-Based Evaluation of Three Operative Modalities. J Bone Joint Surg Am. 2018;100(3):00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reflections 1 year into the 21-Center National Institutes of Health--funded WRIST study: a primer on conducting a multicenter clinical trial. J Hand Surg Am. 2013;38(6):1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 19.Al-Amin Z, Senyurek SA, Van Lieshout EMM, Wijffels MME. Systematic review and pooled analysis of the rate of carpal tunnel syndrome after prophylactic carpal tunnel release in patients with a distal radius fracture. Hand Surg Rehabil. 2018;37(3):155–159. [DOI] [PubMed] [Google Scholar]
- 20.Alter TH, Sandrowski K, Gallant G, Kwok M, Ilyas AM. Complications of Volar Plating of Distal Radius Fractures: A Systematic Review. J Wrist Surg. 2019;8(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora R, Lutz M, Hennerbichler A, Krappinger D, Espen D, Gabl M. Complications following internal fixation of unstable distal radius fracture with a palmar locking-plate. J Orthop Trauma. 2007;21(5):316–322. [DOI] [PubMed] [Google Scholar]
- 22.Bentohami A, de Burlet K, de Korte N, van den Bekerom MP, Goslings JC, Schep NW. Complications following volar locking plate fixation for distal radial fractures: a systematic review. J Hand Surg Eur. 2014;39(7):745–754. [DOI] [PubMed] [Google Scholar]
- 23.Curtin CM, Hernandez-Boussard T. Readmissions after treatment of distal radius fractures. J Hand Surg Am. 2014;39(10):1926–1932. [DOI] [PubMed] [Google Scholar]
- 24.Esenwein P, Sonderegger J, Gruenert J, Ellenrieder B, Tawfik J, Jakubietz M. Complications following palmar plate fixation of distal radius fractures: a review of 665 cases. Arch Orthop Trauma Surg. 2013;133(8):1155–1162. [DOI] [PubMed] [Google Scholar]
- 25.Johnson NA, Cutler L, Dias JJ, Ullah AS, Wildin CJ, Bhowal B. Complications after volar locking plate fixation of distal radius fractures. Injury. 2014;45(3):528–533. [DOI] [PubMed] [Google Scholar]
- 26.Lutz K, Yeoh KM, MacDermid JC, Symonette C, Grewal R. Complications associated with operative versus nonsurgical treatment of distal radius fractures in patients aged 65 years and older. J Hand Surg Am. 2014;39(7):1280–1286. [DOI] [PubMed] [Google Scholar]
- 27.Mathews AL, Chung KC. Management of complications of distal radius fractures. Hand Clin. 2015;31(2):205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro CM, Pettersson HJ, Enocson A. Complications after distal radius fracture surgery: results from a Swedish nationwide registry study. J Orthop Trauma. 2015;29(2):0000000000000199. [DOI] [PubMed] [Google Scholar]
- 29.Quadlbauer S, Pezzei C, Jurkowitsch J, et al. Early complications and radiological outcome after distal radius fractures stabilized by volar angular stable locking plate. Arch Orthop Trauma Surg. 2018;138(12):1773–1782. [DOI] [PubMed] [Google Scholar]
- 30.Rhee PC, Dennison DG, Kakar S. Avoiding and treating perioperative complications of distal radius fractures. Hand Clin. 2012;28(2):185–198. [DOI] [PubMed] [Google Scholar]
- 31.Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359–365. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian P, Kantharuban S, Shilston S, Pearce OJ. Complications of Kirschner-wire fixation in distal radius fractures. Tech Hand Up Extrem Surg. 2012;16(3):120–123. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JT, Lucas GL, Buhr BR. Complications of treating distal radius fractures with external fixation: a community experience. Iowa Orthop J. 2004;24:53–59. [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glickel SZ, Catalano LW, Raia FJ, Barron OA, Grabow R, Chia B. Long-term outcomes of closed reduction and percutaneous pinning for the treatment of distal radius fractures. J Hand Surg Am. 2008;33(10):1700–1705. [DOI] [PubMed] [Google Scholar]
- 36.Grewal R, MacDermid JC, King GJ, Faber KJ. Open reduction internal fixation versus percutaneous pinning with external fixation of distal radius fractures: a prospective, randomized clinical trial. J Hand Surg Am. 2011;36(12):1899–1906. [DOI] [PubMed] [Google Scholar]
- 37.Leung F, Tu YK, Chew WY, Chow SP. Comparison of external and percutaneous pin fixation with plate fixation for intra-articular distal radial fractures. A randomized study. J Bone Joint Surg Am. 2008;90(1):16–22. [DOI] [PubMed] [Google Scholar]
- 38.Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185–1199. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Mendez D, Lizaur-Utrilla A, de-Juan-Herrero J. Intra-articular distal radius fractures in elderly patients: a randomized prospective study of casting versus volar plating. J Hand Surg Eur. 2018;43(2):142–147. [DOI] [PubMed] [Google Scholar]
- 40.Sanders RA, Keppel FL, Waldrop JI. External fixation of distal radial fractures: results and complications. J Hand Surg Am. 1991;16(3):385–391. [DOI] [PubMed] [Google Scholar]
- 41.Thorninger R, Madsen ML, Waever D, Borris LC, Rolfing JHD. Complications of volar locking plating of distal radius fractures in 576 patients with 3.2 years follow-up. Injury. 2017;48(6):1104–1109. [DOI] [PubMed] [Google Scholar]
- 42.Mulders MA, Walenkamp MM, van Dieren S, Goslings JC, Schep NW, Collaborators VT. Volar Plate Fixation Versus Plaster Immobilization in Acceptably Reduced Extra-Articular Distal Radial Fractures: A Multicenter Randomized Controlled Trial. J Bone Joint Surg Am. 2019;101(9):787–796. [DOI] [PubMed] [Google Scholar]
- 43.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–859. [DOI] [PubMed] [Google Scholar]
- 44.Brazzelli M, Cruickshank M, Tassie E, et al. Collagenase clostridium histolyticum for the treatment of Dupuytren’s contracture: systematic review and economic evaluation. Health Technol Assess. 2015;19(90):1–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention: Life Expectancy. 2016; https://www.cdc.gov/nchs/fastats/life-expectancy.htm.
- 46.HCUPnet. Healtchare Cost and Utilization Project (HCUP). Agency for HealthCare Research and Quality 2015; http://hcupnet.ahrq.gov/ Accessed July 22, 2018. [PubMed]
- 47.Bureau of Labor Statistics. 2017; https://www.bls.gov/bls/proghome.htm#wages.
- 48.Hatswell AJ, Bullement A, Briggs A, Paulden M, Stevenson MD. Probabilistic Sensitivity Analysis in Cost-Effectiveness Models: Determining Model Convergence in Cohort Models. Pharmacoeconomics. 2018;36(12):1421–1426. [DOI] [PubMed] [Google Scholar]
- 49.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness — the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 50.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641. [DOI] [PubMed] [Google Scholar]
- 51.Bruce KK, Merenstein DJ, Narvaez MV, et al. Lack of Agreement on Distal Radius Fracture Treatment. J Am Board Fam Med. 2016;29(2):218–225. [DOI] [PubMed] [Google Scholar]
- 52.AAOS. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. 2009. Accessed October 21, 2019.
- 53.Sharma H, Khare GN, Singh S, Ramaswamy AG, Kumaraswamy V, Singh AK. Outcomes and complications of fractures of distal radius (AO type B and C): volar plating versus nonoperative treatment. J Orthop Sci. 2014;19(4):537–544. [DOI] [PubMed] [Google Scholar]
- 54.Young BT, Rayan GM. Outcome following nonoperative treatment of displaced distal radius fractures in low-demand patients older than 60 years. J Hand Surg Am. 2000;25(1):19–28. [DOI] [PubMed] [Google Scholar]
- 55.Gu NY, Botteman MF, Gerber RA, et al. Eliciting health state utilities for Dupuytren’s contracture using a discrete choice experiment. Acta Orthop. 2013;84(6):571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Putter C, Selles R, Haagsma J, et al. Health-related quality of life after upper extremity injuries and predictors for suboptimal outcome. Injury. 2014;45(11):1752–1758. [DOI] [PubMed] [Google Scholar]
- 57.Slatkowsky-Christensen B, Mowinckel P, Loge JH, Kvien TK. Health-related quality of life in women with symptomatic hand osteoarthritis: a comparison with rheumatoid arthritis patients, healthy controls, and normative data. Arthritis Rheum. 2007;57(8):1404–1409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table, Supplemental Digital Content 1: Descriptive statistics of demographic variables of patients by injury pattern.
Table, Supplemental Digital Content 2: One-way sensitivity analyses varying all model parameters demonstrating the uncertainty of the ICER around the base case scenario.


