Abstract
Cancer-related cognitive dysfunction is an important issue for breast cancer survivors. Previous research has identified both cross-sectional and longitudinal alterations in brain function related to cancer status and treatment. In this study, we prospectively collected functional magnetic resonance imaging data in breast cancer cases treated with adjuvant chemotherapy and in controls with no cancer history during a working memory task. Data and blood specimens were collected immediately prior to the start of treatment (baseline) and following completion of treatment (follow-up), and at yoked intervals for controls. In secondary analysis we assessed the levels of oxidative DNA damage in peripheral blood lymphocytes of cases and controls using the Comet assay. A significant group*time interaction revealed reduced deactivation in the superior frontal gyrus in the controls at follow-up, in contrast to cases, who exhibited similar magnitude of deactivation at baseline and follow-up. Working memory performance indicated a significant improvement in the controls at follow-up, and no change in performance in cases. In secondary analyses, oxidative DNA damage levels were elevated in the cases at follow-up compared to controls, but no associations were found between the Comet assay variables and functional imaging at either time-point or group. In light of previous reports on task induced deactivations, our findings reflect continuing effortful processing at follow-up in the breast cancer group, with relatively less effortful processing in the control group given the reduced novelty and practice effects from the baseline to follow-up.
Keywords: working memory, fMRI, cancer
Introduction
Attention and working memory dysfunction is increasingly recognized as one feature of cognitive difficulties following breast cancer diagnosis and treatment. Previous work from our lab suggests a pattern of working memory and attentional dysfunction that may partly explain self-reported memory difficulties (Root, Andreotti, Tsu, Ellmore, & Ahles, 2015; Root, Ryan, et al., 2015). These analyses revealed a pattern of inefficient learning of novel information that was compensated for by repetition of learning trials, yielding normal memory functioning following a delay. Attentional dysfunction is additionally indicated by intra-individual variability in breast cancer survivors (Bernstein, Catton, & Tannock, 2014) and electroencephalogram studies that report alterations in P300 amplitude (Datta et al., 2007), a component associated with attention and cognitive load (Kam et al., 2015; Kreukels, Hamburger, et al., 2008; Kreukels et al., 2006; Kreukels et al., 2005; Kreukels, van Dam, Ridderinkhof, Boogerd, & Schagen, 2008; Schagen, Hamburger, Muller, Boogerd, & van Dam, 2001)
Findings of attentional/working memory dysfunction are consistent with structural imaging studies in survivors following treatment, as evidenced by alterations in prefrontal structure, including in anterior white matter integrity, and in dorsal and medial prefrontal cortex, that support efficient attention, working memory and initial encoding of information (Conroy, McDonald, Smith, et al., 2013; Deprez et al., 2010; Inagaki et al., 2007; McDonald, Conroy, Ahles, West, & Saykin, 2010; McDonald, Conroy, Smith, West, & Saykin, 2013; McDonald & Saykin, 2013). Several other studies demonstrated alterations in prefrontal recruitment in survivors performing executive, attention and working memory tasks (Conroy, McDonald, Smith, et al., 2013; de Ruiter et al., 2011; Ferguson, McDonald, Saykin, & Ahles, 2007; Kesler, Bennett, Mahaffey, & Spiegel, 2009; McDonald, Conroy, Ahles, West, & Saykin, 2012) as well as alterations in regional cerebral blood flow (Nudelman et al., 2014; Silverman et al., 2007). Functional alterations have also been found to overlap with structural changes in middle and superior frontal gyri (McDonald et al., 2010, 2012).
We aimed to investigate alterations in task-related activations and deactivations that may be altered from pre- to post-treatment. We utilized a variant of the working memory task described above (the n-back task) and contrasted functional brain alterations from baseline to follow-up between cases and controls. Given previous findings (Conroy, McDonald, Ahles, West, & Saykin, 2013), along with assessment of task positive regions (middle frontal gyrus), we also focused on task-negative regions which consisted of rostral prefrontal, precuneus, and posterior cingulate cortex. Task-induced deactivation (TID) in these regions has been interpreted as reflecting a shift in resource allocation from internal, ongoing cognitive processing to externally directed stimulus processing (Fox et al., 2005). Relative task difficulty has been previously suggested and studied as a potential contributor to the magnitude of task-positive activation and task-negative deactivation (Shulman et al., 1997). McKiernan, Kaufman, Kucera-Thompson, and Binder (2003) parametrically manipulated task difficulty to assess the relationship of relative levels of effort with magnitude of deactivation, and found that increasing task difficulty led to greater deactivation of regions that support internally directed processing. Of specific interest to the current study, Zou, Gu, Wang, Gao, and Yang (2011) found a relationship of increasing deactivation with manipulation of working memory loads. We hypothesized that the chemotherapy exposed group would exhibit hypoactivation in dorsolateral prefrontal cortex and persistent or increased deactivation in task-negative regions following treatment. As a secondary objective, building on prior reports finding higher levels of oxidative DNA damage following cancer treatment (Conroy, McDonald, Smith, et al., 2013; Scuric et al., 2017; Tomasello et al., 2017), we tested the hypothesis that higher levels of oxidative DNA damage measured in peripheral lymphocytes would be associated with functional recruitment in the chemotherapy-exposed group.
Methods
Design.
This was a longitudinal design contrasting breast cancer patients (cases) and individuals with no history of cancer (controls) at two time-points: for cases, after surgical resection and prior to initiation of adjuvant therapy (baseline) and following chemotherapy treatment (follow-up), and for controls, baseline and follow-up assessments at yoked intervals.
Study Subjects.
Cases were recruited at Memorial Sloan Kettering Cancer Center (MSK) and controls through local advertising. All participants signed written informed consent to participate in this research study approved by the MSK Institutional Review Board. Cases were eligible if they had a diagnosis of breast cancer, were post-resection, and were scheduled to undergo adjuvant treatment. Potential participants were excluded if they had been diagnosed with any central nervous system disease, or had a history of neurological or psychiatric disorders. Controls were matched with patients on age and education.
In all, a total of 23 and 18 subjects were recruited for the case and control groups, respectively. Of these, ten cases were excluded due to: lost to follow-up (n=3), excessive movement (n=2), scanner malfunction (n=3), and contraindication to MRI (n=2). Four controls were excluded due to lost to follow-up (n=3), and claustrophobia (n=1). After these exclusions, the analysis included 13 breast cancer cases and 14 controls.
fMRI Activation Paradigm.
The functional activation paradigm consisted of a working memory n-back task used previously (McDonald et al., 2012). Subjects were presented a series of single letters in four blocks representing four conditions repeated three times for a total of 12 blocks (three blocks of each condition) in pseudo-random order. Subjects were to press their index finger for all non-matches, and their thumb for all matches. For each condition, a letter was considered a match if it was the same as the one presented three letters before (3-back), two letters before (2-back), one letter before (1-back), or matched the letter “J” (0-back). The focus for analysis was the 3-back versus fixation contrast. We chose this contrast to specifically examine task-induced deactivation more clearly in this limited sample by focusing on a condition with high ;task-demand versus fixation, during which no task is performed.
Imaging Data Acquisition and Pre-processing.
Image data were acquired with a GE Signa 3-Tesla MRI scanner (max gradient strength 40 mT/m, max gradient slew rate 150 T/m/s; General Electric Company, Waukesha, Wisconsin, USA) with pre-processing following previously published methods (Pergolizzi et al., 2019) (supplemental material).
Processing of Imaging Data.
Modified SPM software (including elements from spm99 to spm12, Wellcome Department of Imaging Neuroscience) was used for processing the data (for details see Pan, Epstein, Silbersweig, & Stern, 2011; Pergolizzi et al., 2019) (Supplemental Material).
Biospecimens and Assessment of Endogenous and Oxidative DNA Damage Levels.
Blood samples were drawn at baseline and follow-up. Heparinized blood samples were light-protected and immediately transported at room temperature to the Molecular Epidemiology Laboratory at MSK. Peripheral blood lymphocytes were isolated using standard methods and cryopreserved in liquid nitrogen to keep viability until batch-tested with the Comet assay (Supplemental Material).
Statistical Analyses:
fMRI:
Following preprocessing, statistical analyses were conducted using customized fmristat software (Worsley et al., 2002). A two-level voxel-wise linear mixed-effects model was utilized to examine the key group, condition, and time contrasts of interest. First, a whole-brain voxel-wise multiple linear regression model was employed at the individual subject level which comprised the regressors of interest, consisting of stimulus onset times (contrast: 3-back versus fixation; baseline versus follow-up) convolved with a prototypical hemodynamic response function, the covariates of no interest (the first-order temporal derivative of the regressor of interest, global and physiological fluctuations, realignment parameters, scanning period means, and baseline drift up to the third order polynomials) and a first order auto-regression model of the residual time series to accommodate temporal correlation in consecutive scans. Second, at the group level, a mixed-effects model was used (contrast: 3-back versus fixation; baseline versus follow-up), which accounts for intra- and inter-subject variability, and allows for population-based inferences to be drawn. Age was used as a covariate of no interest in an analysis of covariance setting. The statistical inference at the group level was then drawn according to Gaussian random field theory. Initial voxel-wise threshold was p < 0.001; all comparisons reported were considered significant at family-wise error corrected (FWE) p < 0.05 in whole brain correction with a minimum cluster extent of k = 10 voxels (27mm3 per voxel).
fMRI Task Performance:
N-back performance accuracy was assessed using a 2 (group: Case; Control) X 2 (time: Baseline; Follow-up) X 4 (working memory load: 0-back, 1-back, 2-back, 3-back) repeated measures ANOVA.
DNA damage.
For each participant (at baseline and follow-up), viable peripheral blood lymphocytes were tested with the alkaline Comet Assay to establish (a) endogenous damage (untreated) and (b) oxidative damage by treating cells with (b1) endonuclease III (EndoIII) to recognize oxidized pyrimidines, and (b2) formamidopyrimidine DNA glycosylase (FpG) to recognize 8-OH Guanine and other oxidised purines (details in Supplemental Material). The ‘net’ oxidative damage levels were estimated by subtracting pre-existing endogenous damage from the total damage obtained post EndoIII and Fpg treatments. Tail Moment (TM), Tail Intensity (TI), and the net oxidative DNA damage levels were referred to as TMEndoIII-net, TIEndoIII-net; and TMFpg-net, TIFpg-net and are expressed in arbitrary units. Values were entered into a 2 (group: Case; Control) X 2 (time: Baseline; Follow-up) repeated measures ANOVA. Comparisons were conducted using independent samples t-tests contrasting groups at baseline and follow-up, and paired samples t-test to assess change within groups from baseline to follow-up. TMFPG-net, TMEndoIII-net, TIFPG-net, TIEndoIII-net were entered into a regression model with functional activation maps and assessed for associations between magnitude of oxidative damage and functional recruitment.
Results
Average time between baseline and follow-up scan sessions was 152 days for cases and 167 days for controls. Average time between surgery and baseline was 46 days. Average time between completion of chemotherapy treatment and follow-up was 39 days. At follow-up, one case was on hormonal treatment and two cases had completed radiation therapy (14 and 32 days from completion to follow-up) due to scheduling conflicts. There were no between group differences in age (p = 0.87) or years of education (p = 0.39). Self-reported depression as measured by the CES-D was not different between groups at either baseline (p = 0.103) or follow-up (p = 0.672). Self-reported state-related anxiety as measured by the STAI-S was not different between groups at either baseline (p = 0.194) or follow-up (p = 0.494).
Functional Imaging Task-Induced Activations and Deactivations (Figure 1; Table 2):
Figure 1.
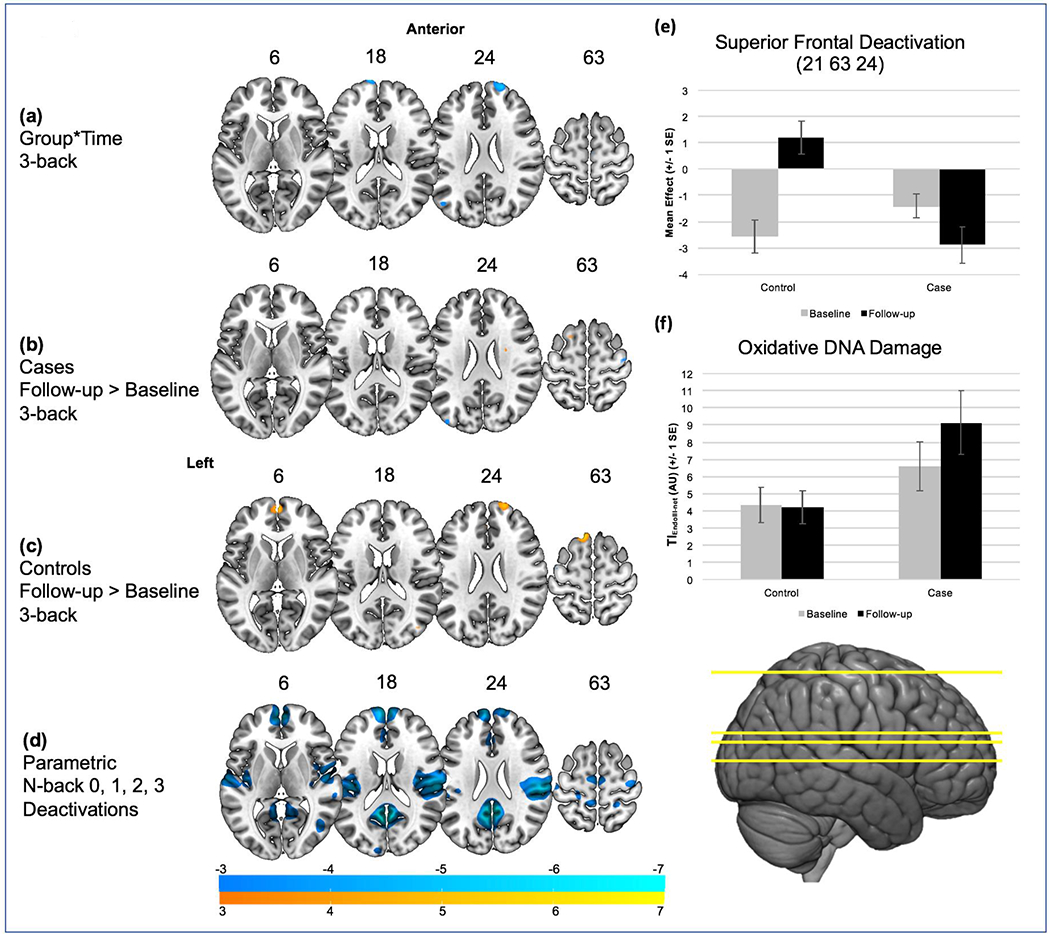
The number above each slice indicates the MNI z coordinate. Warm colors indicate increased activation while cool colors indicate decreased activation for the breast cancer versus healthy control group. a) Group x Time interaction in superior medial frontal gyrus and superior frontal gyrus for the 3-back condition; b) for cases, no significant change in recruitment for the 3-back condition; c) for controls, significantly decreased task induced deactivation in medial and superior frontal gyrus at follow-up compared to baseline for the 3-back condition; d) parametric weighting of 0, 1, 2, 3 back and associated task induced deactivations to contextualize changes in task-induced deactivation with increasing working memory load from 0- to 3-back conditions; e) BOLD signal magnitudes for cases and controls from baseline to follow-up in superior frontal gyrus (21 63 24); f) Endonuclease III Treated Tail Intensity (TIEndoIII) minus endogenous damage for cases and controls from baseline to follow-up; AU, arbitrary units.
Table 2.
Imaging Results
| Region | Hem | K | FWE- p | Max-Z | (x y z) | |||
|---|---|---|---|---|---|---|---|---|
| Group x Time | ||||||||
| Cases (n=13) | Frontal Superior | L | 945 | 0.044 | 4.13 | −12 | 72 | 18 |
| Controls (n=14) | 0.057 | 4.06 | −15 | 75 | 21 | |||
| Frontal Superior | R | 1107 | 0.052 | 4.09 | 21 | 63 | 24 | |
| 0.183 | 3.71 | 15 | 66 | 21 | ||||
| Within Groups | ||||||||
| Baseline> Time 2 | ||||||||
| Cases | Cerebellum 6 | L | 648 | 0.029 | 4.68 | −27 | −60 | −18 |
| Controls | Vermis 8 | R | 270 | 0.076 | 3.97 | 6 | −69 | −36 |
| Time 2>Baseline | ||||||||
| Cases | n.s | |||||||
| Controls | Frontal Superior | L | 1026 | 0.009 | 4.55 | −12 | 27 | 63 |
| Frontal Superior Medial | L | 594 | 0.026 | 4.27 | 0 | 60 | 6 | |
| Frontal Superior | R | 837 | 0.047 | 4.11 | 18 | 63 | 27 | |
| Between Groups | ||||||||
| Baseline | ||||||||
| Cases>Controls | n.s. | |||||||
| Controls>Cases | n.s. | |||||||
| Time 2 | ||||||||
| Cases>Controls | n.s. | |||||||
| Controls>Cases | n.s. | |||||||
Hem: Hemisphere; K: Cluster extent; FWE-p: Family-wise Error Corrected p-value; Max-Z: maximal z-score; (X Y Z): MNI coordinates; L, left; R, right. Gray cells highlight non-significant activations (n.s.).
A significant group*time interaction was found in superior medial frontal gyrus (−12 72 18) (p=0.04corrected) and a trend in superior frontal gyrus (21 63 24) (p=0.052corrected) (Figure 1(a)). Within group analysis assessing changes in functional activation from baseline to follow-up found significantly decreased deactivation in these regions in controls at follow-up, driving the group*time interaction, including superior medial frontal gyrus (0 60 6) (p=0.003corrected), superior frontal gyrus (18 63 27) (p=0.04corrected), as well as in more posterior regions of superior frontal gyrus (−12 27 63) (p=0.009corrected) (Figure 1(c)). A significant decrease from baseline to follow-up was exhibited in left cerebellum for cases, (−27 −60 −18) (p=0.029corrected) and a trend decrease in right cerebellum for controls (6 −69 −36) (p=0.076corrected). No significant or trend differences were exhibited between groups at either baseline or follow-up. To contextualize the group*time interaction of TID, areas of increasing deactivation associated with task difficulty were derived by parametric weighting of 0-, 1, 2, and 3-back as regressors on functional maps for controls at baseline (Figure 1(d)) to examine regions that exhibit increasing task-induced deactivation with increasing task difficulty.
Functional Imaging Task Performance:
N-back performance was analyzable for 12 controls and 11 cases due to missing data. Results of the repeated measures ANOVA found significant main effects for time F (1, 21) = 9.06, p = 0.007, and working memory load F (3, 63) = 43.25, p = <0.001, and significant interaction of time*group F(1, 21) = 5.48, p = 0.029. Table 4 presents within-group longitudinal differences at each load condition; as can be seen, aggregating n-back performance across all working memory load conditions (0, 1, 2, 3-back), controls exhibited improved performance t(11) = −3.313, p = 0.008, in contrast to cases t(10) = −0.621, p = 0.549 from baseline to follow-up. This finding appears to be driven by significant and trend improvements in 1-back, 2-back, and 3-back condition performance, while cases exhibited improvement in only the 2-back condition. In between-group, cross-sectional analyses, no significant differences were found at either baseline or follow-up.
Table 4.
Within group changes in n-back performance by group and load
| Controls (n=12) | Mean (SD) | t (Df) | Sig (2-tailed) |
|---|---|---|---|
| 0-back | 0.47 (7.89) | 0.20 (11) | 0.839 |
| 1-back | −13.07 (13.55) | −3.34 (11) | 0.007 |
| 2-back | −6.42 (12.24) | −1.82 (11) | 0.096 |
| 3-back | −4.65 (8.56) | −1.88 (11) | 0.086 |
| All | −5.91 (6.39) | −3.20 (11) | 0.008 |
| Cases (n=13) | |||
| 0-back | −0.85 (6.74) | −0.42 (10) | 0.682 |
| 1-back | 0.48 (6.40) | 0.25 (10) | 0.808 |
| 2-back | −3.35 (4.97) | −2.23 (10) | 0.049 |
| 3-back | 0.77 (10.08) | 0.25 (10) | 0.805 |
| All | −0.74 (3.73) | −0.66 (10) | 0.526 |
Endogenous and Oxidative DNA Damage Levels (Figure 1f; Table 3):
Table 3.
Endogenous and net oxidative DNA damage as per Comet assay by case-control group and timepoint
| Endogenous DNA damage | Oxidative DNA damage | |||||
|---|---|---|---|---|---|---|
| TM Mean (SD) | TMFPG-net Mean (SD) | TMEndoIII-net Mean (SD) | ||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Controls (n=12) | 1.26 (0.45) | 1.05 (0.55) | 7.88 (4.18) | 7.12 (3.45) | 0.92 (0.98) | 0.84 (0.75) |
| Cases (n=13) | 1.22 (1) | 1.22 (1.01) | 9.36 (2.68) | 9 (5.83) | 1.27 (1.29) | 2.03 (2.13)† |
| TI Mean (SD) | TIFPG-net Mean (SD) | TIEndoIII-net Mean (SD) | ||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Controls (n=12) | 9.14 (1.98) | 8.04 (1.87) | 21.81 (9.5) | 20.58 (8.63) | 4.37 (3.58) | 4.22 (3.3) |
| Cases (n=13) | 8.77 (3.44) | 8.69 (3.86) | 27.11 (7) | 26.09 (10.87) | 6.61 (5.08) | 9.15 (6.73)*† |
TM and TI, Tail moment and Tail intensity, mean values expressed in arbitrary units; TMEndo-net and TIEndo-net, net oxidative DNA damage measured after EndoIII treatment; TMFPG-net and TIFPG-net, net oxidative DNA damage after FpG treatment.
p < 0.05 between group significance;
p < 0.05 within group significance.
The Comet assay was informative for 12 controls and 13 cases. Samples were not collected for two controls. No significant interaction was exhibited between groups or time-points measured as endogenous TM or TI. Significant group*time interactions were found for TMEndoIII-net, F (1, 23) = 4.67, p = 0.041, and TIEndoIII-net, F (1, 23) = 4.50, p = 0.045. There were no significant differences between groups for TMEndoIII-net or TIEndoIII-net at baseline. At follow-up, cases exhibited significantly greater TIEndoIII-net t(23)= −2.29, p = 0.03 and greater levels of TMEndoIII-net t(23) = −1.831, p = 0.08) compared to controls that did not reach statistical significance. No significant differences were found from baseline to follow-up for controls for either TMEndoIII-net or TIEndoIII-net, while cases exhibited a significantly greater TMEndoIII-net t(12)= −2.28, p = 0.04 and TIEndoIII-net t(12) = −2.81, p = 0.02 from baseline to follow-up. No significant interaction, cross-sectional, or within subject findings were exhibited for TMFPG-net or TIFPG-net. No significant association was found between Comet assay values and functional imaging activations or deactivations at either time-point or group that survived comparison for multiple corrections.
Discussion
The primary finding in this prospective study is persistent task-induced deactivation (TID) in task negative prefrontal regions in breast cancer cases from baseline to follow-up, in contrast to controls, who exhibited reduced TID and improved working memory performance at follow-up. Prefrontal areas of reduced TID in controls at follow-up exhibited significant overlap with prefrontal areas of increasing TID in relation to working memory load as can be seen in comparisons of Figure 1(a), Figure 1(c) and Figure 1(d). Lack of behavioral improvement and persistent TID in cases are interpreted as a failure to benefit from previous exposure to the scanner environment and cognitive task, in contrast to controls who exhibited both reduced TID and improved performance at follow-up. Contrary to hypothesized hypoactivation of dorsolateral prefrontal cortex in cases post-treatment, we failed to find this effect, and only found hypothesized persistent TID. This may be due to our relatively limited sample size which may limit power to detect hypothesized hypoactivation in DLPFC. While theoretical, if persistent TID is reflective of compensatory resource allocation, this may be expected to influence recruitment of task-positive regions.
Task difficulty may be manipulated by increasing task demands or following CNS insult. Most consistent with our findings, Conroy, McDonald, Ahles, et al. (2013) found increased deactivation in task-negative regions in subjects with chemotherapy-induced amenorrhea (i.e., post-treatment). This increased deactivation in task-negative regions was interpreted as reflective of increased difficulty in performing the same 3-back task following treatment relative to baseline, while the other comparison groups exhibited either the same or reduced deactivation. TID is increasingly recognized as an important correlate in healthy cognition of the efficient engagement in various cognitive processes including working memory (Newton, Morgan, Rogers, & Gore, 2011).
These findings are interpreted in light of previous research that indicates that breast cancer patients recruit neural resources in a manner that reflects greater experienced task difficulty. Neuroimaging studies in healthy populations have manipulated task difficulty by increasing cognitive load, i.e., the amount of processing resources required to complete a task. Increasing cognitive load during working memory reveals an inverted U-function on brain activation – brain regions increase activation until working memory load reaches capacity limitations whereupon decreased activation is observed (Callicott et al., 1999). Similarly, the same regions that show increased activation during a single task showed decreased activation during dual task performance (Fletcher et al., 1995). Chemotherapy-treated breast cancer patients have exhibited hyperactivation on functional imaging of cognition, suggested to reflect compensation via reserve neural resources (Ferguson et al., 2007; Kesler et al., 2009; McDonald et al., 2012; Silverman et al., 2007). Other studies, however, have shown relative decreased activations (de Ruiter et al., 2011; Kam et al., 2015; Kesler, Kent, & O’Hara, 2011). One possible interpretation for these discrepant findings is that treatment taxes processing limitations within cognitive domains. Consistent with increased cognitive load, patients could require increased activations to adapt to diminished memory-related processing resources until utilizing executive functions exceeds capacity limitations, leading to relative activation decreases. Consistent with this notion, prospective examinations from before to after chemotherapy treatment showed increased activations with increased load over time (Menning et al., 2017), but decreased activation over time when comparing multitasking to single task performance (Deprez et al., 2014). This emphasizes the need for future work to assess relative processing limitations of chemotherapy treated survivors, such as comparing single versus dual cross-domain tasks.
Alkylating chemotherapy agents such as cyclophosphamide --frequently used for treatment of breast cancer-- can induce DNA damage (Seigers & Fardell, 2011). This, in turn, has been also implicated in brain aging (Badiola et al., 2015). We found levels of oxidative DNA damage were significantly increased in cases after treatment with chemotherapy relative to controls (Conroy, McDonald, Smith, et al., 2013), and that oxidative damage is more prevalent in pyrimidines than in 8-OH Guanine and other purines, as evidenced by the net EndoNI-induced damage levels. Although we did not find any association between DNA damage and functional recruitment in either time-point or group, the finding of greater oxidative DNA damage levels may suggest a mechanistic explanation for alterations to brain activity seen in chemotherapy treated breast cancer patients that might be tested in the future in a larger sample.
The interpretation of these findings is limited by certain factors. While the longitudinal design suggests no significant differences between groups at baseline, the lack of a cancer control group not treated with chemotherapy, or not treated with hormonal therapy post-adjuvant treatment, limits interpretation of potential etiologies of post-treatment changes, including effects of cancer itself, psychological effects of diagnosis, and effects of hormonal treatments. The challenge of recruiting participants for multiple time-points led to a smaller sample size that may have obscured detection of more subtle neural activity changes. The only region to show differences in TID was the rostral portion of the superior frontal gyrus, a region thought to bias processing toward or away from stimulus-independent thought (Gilbert et al., 2006). TID of this region may be interpreted as suppression of off-task thinking, but we did not assess subjective reports from participants to better understand the role of TID in this region in particular.
Supplementary Material
Table 1.
Demographic and treatment characteristics
| Cases (n=13) | Controls (n=14) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age at baseline (years) | 47.76 (6.4) | 48.21 (7.7) |
| Education (years) | 17 (6.4) | 16 (7.8) |
| N (%) | N (%) | |
| Race & ethnicity | ||
| Caucasian | 5 (38) | 12 (85) |
| African-American | 1 (7) | 2 (14) |
| Asian | 5 (38) | 0 (0) |
| Hispanic | 2 (15) | 0 (0) |
| Handedness | ||
| Right | 13 (100) | 14 (100) |
| Left | 0 (0) | 0 (0) |
| Menopausal Status (baseline) | ||
| Pre | 6 (46) | 5 (35) |
| Peri | 2 (15) | 3 (21) |
| Post | 5 (38) | 6 (43) |
| Time from surgery to baseline scan | 46 (17) | |
| Cancer stage | ||
| i | 3 (21) | |
| ii | 1 (7) | |
| iia | 4 (28) | |
| iib | 1 (7) | |
| iiia | 2 (14) | |
| No stage recorded | 2 (14) | |
| Chemotherapy type | ||
| Adriamycin, Cytoxan, and Taxol | 9 (69) | |
| Cyclophosphamide, methotrexate, fluorouracil | 4 (28) | |
| Hormone Therapy | 10 (78) | |
| Tamoxifen | 1 (10) | |
| Arimedex | 8 (80) | |
| Femara | 1 (10) | |
| Radiation Therapy | 12 (92) | |
| Mean (range) | Mean (range) | |
| STAI-S | ||
| Baseline | 31 (21-46) | 27 (21-47) |
| Followup | 30 (20-50) | 33 (22-68) |
| CES-D | ||
| Baseline | 10 (2-20) | 6 (0-20) |
| Followup | 8 (2-14) | 9 (1-29) |
Note. No differences were found for age or education or handedness between cases and controls
Acknowledgements:
Authors acknowledge Himali Patel and Kenneth Cheung in the Molecular Epidemiology Laboratory, Department of Epidemiology and Biostatistics, for their contribution through the handling of biospecimens and comet assay experiments on samples and internal laboratory controls.
Funding Sources: Starr Cancer Consortium (protocol #1-A17) and Amgen, Inc. NIH/NCI Cancer Center Support Grant P30 CA008748 and the NCI award number T32 CA009461. The content is solely responsible of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Standards: IRB review and approval was obtained for this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest: James Root declares that he has no conflict of interest. Denise Pergolizzi declares that she has no conflict of interest. Hong Pan declares that he has no conflict of interest. Irene Orlow declares that she has no conflict of interest. Steven Passik declares that he has no conflict of interest. David Silbersweig declares that he has no conflict of interest. Emily Stern declares that she has no conflict of interest. Tim Ahles declares that he has no conflict of interest.
References Cited
- Badiola I, Santaolalla F, Garcia-Gallastegui P, Ana SD, Unda F, & Ibarretxe G (2015). Biomolecular bases of the senescence process and cancer. A new approach to oncological treatment linked to ageing. Ageing Res Rev, 23(Pt B), 125–138. doi: 10.1016/j.arr.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Bernstein LJ, Catton PA, & Tannock IF (2014). Intra-individual variability in women with breast cancer. J Int Neuropsychol Soc, 20(4), 380–390. doi: 10.1017/S1355617714000125 [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, … Weinberger DR (1999). Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex, 9(1), 20–26. [DOI] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Ahles TA, West JD, & Saykin AJ (2013). Chemotherapy-induced amenorrhea: a prospective study of brain activation changes and neurocognitive correlates. Brain Imaging Behav, 7(4), 491–500. doi: 10.1007/s11682-013-9240-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, … Saykin AJ (2013). Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat, 137(2), 493–502. doi: 10.1007/s10549-012-2385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Cusack R, Hawkins K, Heutink J, Rorden C, Robertson IH, & Manly T (2007). The p300 as a marker of waning attention and error propensity. Comput Intell Neurosci, 93968. doi: 10.1155/2007/93968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, … Schagen SB (2011). Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp, 32(8), 1206–1219. doi: 10.1002/hbm.21102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, … Sunaert S. (2010). Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Vandenbulcke M, Peeters R, Emsell L, Smeets A, Christiaens MR, … Sunaert S. (2014). Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. J Clin Oncol, 32(19), 2031–2038. doi: 10.1200/JCO.2013.53.6219 [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, & Ahles TA (2007). Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol, 25(25), 3866–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RS, & Dolan RJ (1995). Brain systems for encoding and retrieval of auditory-verbal memory. An in vivo study in humans. Brain, 118 (Pt 2), 401–416. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102(27), 9673–9678. doi: 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, & Burgess PW (2006). Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci, 18(6), 932–948. doi: 10.1162/jocn.2006.18.6.932 [DOI] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, … Uchitomi Y. (2007). Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer, 109(1), 146–156. [DOI] [PubMed] [Google Scholar]
- Kam JW, Brenner CA, Handy TC, Boyd LA, Liu-Ambrose T, Lim HJ, … Campbell KL (2015). Sustained attention abnormalities in breast cancer survivors with cognitive deficits post chemotherapy: An electrophysiological study. Clin Neurophysiol. doi: 10.1016/j.clinph.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Kesler SR, Bennett FC, Mahaffey ML, & Spiegel D (2009). Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res, 15(21), 6665–6673. doi: 10.1158/1078-0432.CCR-09-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, & O’Hara R (2011). Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol, 68(11), 1447–1453. doi: 10.1001/archneurol.2011.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreukels BP, Hamburger HL, de Ruiter MB, van Dam FS, Ridderinkhof KR, Boogerd W, & Schagen SB (2008). ERP amplitude and latency in breast cancer survivors treated with adjuvant chemotherapy. Clin Neurophysiol, 119(3), 533–541. doi:S1388-2457(07)00696-7 [pii] 10.1016/j.clinph.2007.11.011 [DOI] [PubMed] [Google Scholar]
- Kreukels BP, Schagen SB, Ridderinkhof KR, Boogerd W, Hamburger HL, Muller MJ, & van Dam FS (2006). Effects of high-dose and conventional-dose adjuvant chemotherapy on long-term cognitive sequelae in patients with breast cancer: an electrophysiologic study. Clin Breast Cancer, 7(1), 67–78. [DOI] [PubMed] [Google Scholar]
- Kreukels BP, Schagen SB, Ridderinkhof KR, Boogerd W, Hamburger HL, & van Dam FS (2005). Electrophysiological correlates of information processing in breast-cancer patients treated with adjuvant chemotherapy. Breast Cancer Res Treat, 94(1), 53–61. doi: 10.1007/s10549-005-7093-3 [DOI] [PubMed] [Google Scholar]
- Kreukels BP, van Dam FS, Ridderinkhof KR, Boogerd W, & Schagen SB (2008). Persistent neurocognitive problems after adjuvant chemotherapy for breast cancer. Clin Breast Cancer, 8(1), 80–87. doi: 10.3816/CBC.2008.n.006 [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, & Saykin AJ (2010). Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, & Saykin AJ (2012). Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol, 30(20), 2500–2508. doi: 10.1200/JCO.2011.38.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Smith DJ, West JD, & Saykin AJ (2013). Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behavior and Immunity, 30 Suppl, S117–125. doi: 10.1016/j.bbi.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, & Saykin AJ (2013). Alterations in brain structure related to breast cancer and its treatment: chemotherapy and other considerations. Brain Imaging Behav, 7(4), 374–387. doi: 10.1007/s11682-013-9256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, & Binder JR (2003). A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci, 15(3), 394–408. doi: 10.1162/089892903321593117 [DOI] [PubMed] [Google Scholar]
- Menning S, de Ruiter MB, Veltman DJ, Boogerd W, Oldenburg HS, Reneman L, & Schagen SB (2017). Changes in brain activation in breast cancer patients depend on cognitive domain and treatment type. PLoS One, 12(3), e0171724. doi: 10.1371/journal.pone.0171724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, & Gore JC (2011). Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Hum Brain Mapp, 32(10), 1649–1659. doi: 10.1002/hbm.21138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman KN, Wang Y, McDonald BC, Conroy SK, Smith DJ, West JD, … Saykin AJ (2014). Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: a prospective study using pulsed arterial spin labeling MRI perfusion. PLoS One, 9(5), e96713. doi: 10.1371/journal.pone.0096713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Epstein J, Silbersweig DA, & Stern E (2011). New and emerging imaging techniques for mapping brain circuitry. Brain Res Rev, 67(1–2), 226–251. doi: 10.1016/j.brainresrev.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Pergolizzi D, Root JC, Pan H, Silbersweig D, Stern E, Passik SD, & Ahles TA (2019). Episodic memory for visual scenes suggests compensatory brain activity in breast cancer patients: a prospective longitudinal fMRI study. Brain Imaging Behav. doi: 10.1007/s11682-019-00038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root JC, Andreotti C, Tsu L, Ellmore TM, & Ahles TA (2015). Learning and memory performance in breast cancer survivors 2 to 6 years post-treatment: the role of encoding versus forgetting. J Cancer Surviv. doi: 10.1007/s11764-015-0505-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root JC, Ryan E, Barnett G, Andreotti C, Bolutayo K, & Ahles T (2015). Learning and memory performance in a cohort of clinically referred breast cancer survivors: the role of attention versus forgetting in patient-reported memory complaints. Psychooncology, 24(5), 548–555. doi: 10.1002/pon.3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagen SB, Hamburger HL, Muller MJ, Boogerd W, & van Dam FS (2001). Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function. J Neurooncol, 51(2), 159–165. [DOI] [PubMed] [Google Scholar]
- Scuric Z, Carroll JE, Bower JE, Ramos-Perlberg S, Petersen L, Esquivel S, … Schiestl R. (2017). Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer, 3, 50. doi: 10.1038/s41523-017-0050-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, & Fardell JE (2011). Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev, 35(3), 729–741. doi: 10.1016/j.neubiorev.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, & Petersen SE (1997). Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci, 9(5), 648–663. doi: 10.1162/jocn.1997.9.5.648 [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, … Ganz PA. (2007). Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat, 103(3), 303–311. doi: 10.1007/s10549-006-9380-z [DOI] [PubMed] [Google Scholar]
- Tomasello B, Malfa GA, Strazzanti A, Gangi S, Di Giacomo C, Basile F, & Renis M (2017). Effects of physical activity on systemic oxidative/DNA status in breast cancer survivors. Oncol Lett, 13(1), 441–448. doi: 10.3892/ol.2016.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, & Evans AC (2002). A general statistical analysis for fMRI data. Neuroimage, 15(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Zou Q, Gu H, Wang DJ, Gao JH, & Yang Y (2011). Quantification of Load Dependent Brain Activity in Parametric N-Back Working Memory Tasks using Pseudo-continuous Arterial Spin Labeling (pCASL) Perfusion Imaging. J Cogn Sci (Seoul), 12(2), 127–210. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


