Abstract
Purpose:
Although intensity-modulated radiation therapy (IMPT) is dosimetrically superior to passive scattering proton therapy (PSPT) for locally advanced non-small cell lung cancer (LA-NSCLC), direct comparisons of clinical outcomes are lacking. Here, we compare toxicity profiles and clinical outcomes after IMPT vs. PSPT for LA-NSCLC.
Patients and Methods:
This is a non-randomized, comparative study of two independent cohorts with LA-NSCLC (stage II-IIIB, stage IV with solitary brain metastasis) treated with concurrent chemotherapy and proton beam therapy. Toxicity (CTCAE v4.0) and outcomes were prospectively collected as part of a clinical trial (NCT00915005) or prospective registry (NCT00991094).
Results:
Of 139 patients, 86 (62%) received PSPT and 53 (38%) IMPT; median follow-up times were 23.9 and 29.0 months, respectively. IMPT delivered lower mean radiation doses to the lungs (PSPT 16.0Gy vs. IMPT 13.0Gy, P < 0.001), heart (10.7Gy vs. 6.6Gy, P = 0.004) and esophagus (27.4Gy vs. 21.8Gy, P = 0.005). Consequently, the IMPT cohort had lower rates of grade ≥3 pulmonary (17% vs. 2%, P=0.005) and cardiac (11% vs. 0%, P = 0.01) toxicities. Six (7%) PSPT patients and 0 (0%) IMPT patients experienced grade 4 or 5 toxicity. Lower rates of pulmonary (28% vs. 3%, P = 0.006) and cardiac (14% vs. 0%, P = 0.05) toxicities were observed in the IMPT cohort even after propensity score matching for baseline imbalances. There was also a trend toward longer median overall survival in the IMPT group (23.9 months vs. 36.2 months, P = 0.09). No difference was found in 3-year rates of local (25% vs. 20%, P = 0.44), local-regional (29% vs. 36%, P = 0.56) and distant (52% vs. 51%, P = 0.71) recurrences.
Conclusion:
IMPT is associated with lower radiation doses to the lung, heart and esophagus, and lower rates of grade ≥3 cardiopulmonary toxicity; additional clinical studies will be needed to assess potential differences in survival between the two techniques.
INTRODUCTION
Due to recent advances in the treatment of locally advanced non-small cell lung cancer (LA-NSCLC) we have observed improvements in overall survival, and the current standard of care for inoperable patients is concurrent chemotherapy and radiation therapy (RT) followed by adjuvant immunotherapy.1,2 With improvements in survival, mitigating treatment toxicity has become imperative.3
Regarding radiation therapy, initial efforts to improve local tumor control with dose escalation experienced a setback after the NRG Oncology clinical trial RTOG-0617 showed worse overall survival (OS) with higher RT dose.4 Subset analyses suggested that treatment-induced toxicity, including increased radiation dose to the heart, may have contributed to the unexpectedly poor survival seen in the higher dose cohort, underscoring the importance of minimizing RT-induced toxicity in extending patient survival.5 Subsequent studies have sought to identify the most effective RT modality to deliver the prescribed dose to the tumor while minimizing the dose to healthy organs at risk (OARs) and, consequently, mitigating RT-induced toxicity. Intensity-modulated radiation therapy (IMRT) has since been established as the standard of care, demonstrating both improved oncologic outcomes and reduced toxicity over traditional 3D conformal therapy.3,6,7
Proton beam therapy (PBT) has recently emerged as an appealing alternative to IMRT for thoracic malignancies. The physical characteristics of proton particles, and the Bragg peak properties of proton beams, translate into superior dosimetric profiles for PBT in terms of sparing OARs, and preliminary findings on toxicity were promising.8–10 However, the only randomized clinical trial to date directly comparing PBT with IMRT showed no improvement in toxicity or survival.11 The trial, however, suffered from the limitations of passive scattering proton therapy (PSPT), which lacks the conformality of dose intensity modulation enabled by IMRT. Intensity-modulated proton therapy (IMPT, pencil beam scanning), on the other hand, is a type of PBT that effectively combines the dosimetric advantages of the proton’s Bragg peak with the advantages of dose intensity modulation.12 The dosimetric advantages of IMPT over PSPT have been previously documented,13 but direct clinical comparisons between these two modalities are lacking. Here, we report toxicity, disease-related outcomes, and survival in a comparison of two independent patient groups with LA-NSCLC, one treated with IMPT and the other with PSPT, both given with concurrent chemotherapy.
PATIENTS AND METHODS
Design, Setting, and Participants
This study consists of two independent patient cohorts from whom toxicity and oncologic outcome data were collected prospectively from 2009–2016. The PSPT cohort data were collected as part of a randomized trial comparing PSPT with IMRT (clinicaltrials.gov, NCT00915005), and the IMPT patient cohort data was extracted from a prospective registry study, Normal Tissue Toxicity for Proton Therapy (clinicaltrial.gov, NCT00991094), conducted at MD Anderson Cancer Center.
Inclusion criteria for both cohorts were the same, and consisted of: biopsy-proven diagnosis of NSCLC; Karnofsky Performance Score of ≥70; stage II-IIIB disease or stage IV disease with a solitary brain metastasis, or recurrent tumor after surgical resection that could be treated definitively with concurrent chemoradiation.11 Disease was staged according to the American Joint Commission on Cancer (AJCC) staging system, 7th (2010) edition. The diagnostic work-up included computed tomography (CT) and positron emission tomography (PET-CT), and bronchoscopy with endobronchial ultrasound or mediastinoscopy. Magnetic resonance imaging of the brain was performed in accordance with National Comprehensive Cancer Network (NCCN) guidelines and obtained for all patients with AJCC 7 stage ≥II disease.
Treatments/Interventions: Chemotherapy, IMPT and PSPT
Treatment consisted of concurrent chemoradiotherapy (chemoRT). Chemotherapy most often involved weekly intravenous infusions of carboplatin (area under the curve of 2 units) plus paclitaxel (50 mg/m2). Induction chemotherapy was allowed at the discretion of the treating medical oncologist for both groups. Intravenous hydration and anti-emetic therapy were routinely given to all patients according to the institutional standard of care.
Before treatment, each patient underwent standard RT planning procedures that included 4-dimensional CT (4DCT) scanning for motion assessment, with findings from 10 breathing phases used to reconstruct a maximum intensity projection scan. These images were then used to define an internal gross tumor volume (iGTV), which was used as the basis for the clinical target volume (defined as the iGTV with a 7–8 mm isotropic expansion, excluding anatomic boundaries); a subsequent 5-mm volumetric expansion was used to define the final, planning treatment volume (PTV). Treatment plans were designed on the average CT data sets derived from 4DCT. For IMPT, a simultaneous integrated boost was used to push the dosimetric hot spots to inside the iGTV. Daily patient positioning and set-up were verified by using orthogonal kilovoltage imaging; cone beam CT (CBCT) was not used for set-up verification in either cohort due to facility limitations. Elective nodal irradiation was not used routinely for either group. Dosimetric planning for both PSPT and IMPT was done with an Eclipse treatment planning system (Varian Medical Systems) using 3 or 4 radiation beams. The IMPT plans were further optimized on an in-house built robustness optimization system, which was protocolled to analyze the plan against 3-mm set-up and 3.5% range uncertainties, converted into DICOM files, and transferred into Eclipse for dose recalculations and treatment delivery, as previously described in detail.14–16 Verification simulation was performed weekly for the PSPT cohort as mandated by the protocol in which they were treated on.11 Repeat simulation for the IMPT cohort and adaptive treatment re-planning for both cohorts were done the discretion of the treating physician. The radiation was prescribed so that ≥99% of the PTV received ≥95% of the prescription dose (i.e. V99 ≥ 95%) while respecting OAR dose constraints (Suppl. Table e1).
Treatment Evaluation and Data Collection
After completing chemoRT, patients were evaluated every 3 months for the first 2 years and every 6 months thereafter. At each visit, an interim history, physical examination, lab work, and axial chest CT were obtained. Interval PET-CT scans and biopsy of suspicious lesions were obtained at the discretion of the treating physician. Toxicity was assessed by a dedicated research nurse and scored according to the Common Terminology Criteria for Adverse Effects v4.0 weekly during treatment and at follow-up visits. All clinically meaningful (grade ≥3) cardiac and pulmonary toxicities were reviewed with a dedicated cardiologist and pulmonologist, respectively, to confirm toxicity type and severity. Patients underwent chest CT imaging routinely, and cardiac echocardiography or electrocardiograms examination when clinically indicated. Local recurrence was defined as treatment failure within the PTV plus a ≤1-cm margin; regional recurrence was defined as recurrence of intrathoracic disease outside the local recurrence region; and distant recurrence as recurrence outside the thorax.
Statistical Considerations
Patient characteristics, dosimetry details, and toxicity rates are presented in tabular format, with means compared by using Pearson’s χ2 or two-sided Fisher’s exact test for categorical variables and 2-sided t tests for continuous variables. A propensity score-matched (PSM) analysis was also performed between the two cohorts matching for age, tumor stage and location, type of systemic therapy, adaptive planning, and prescribed radiation dose with a ratio of 1:1 to reduce bias. The match tolerance was set at 0.02. The Kaplan-Meier survival function was used to estimate survival times, and the log-rank test was used to compare the equality of the survival functions. Endpoints assessed include OS and local, local-regional, and distant recurrence. Time to failure was measured from the last day of chemoRT. A P < 0.05 was considered statistically significant. All analyses were done with SPSS statistical software version 24.0 (IBM Corp, Armonk, NY, USA).
RESULTS
A total of 139 patients were included in this analysis; the PSPT cohort consisted of 86 patients (62%) who received proton therapy as part of a direct comparison of IMRT and PSPT (Figure 1).11 The IMPT cohort consisted of 53 patients (38%), 51 from the MD Anderson Normal Tissue Toxicity for Proton Therapy prospective registry, and 2 patients who enrolled in the aforementioned trial but were ultimately treated off-protocol with IMPT because normal tissue radiation dose constraints could not be met with PSPT (Figure 1). The two cohorts were relatively well-balanced in terms of patient and tumor characteristics, with non-significant differences in sex (P = 0.88), smoking status (P = 0.13), tumor histology (P = 0.84), stage (P = 0.52), location (P = 0.35), gross tumor volume (P = 0.99), or rate of adaptive planning (P = 0.90). The patients in the PSPT group trended toward a younger age (median [range]: PSPT 67 years [39–80] vs. 70 years [43–83], P = 0.06), exhibit a higher median radiation dose (PSPT 74Gy [54–78] vs. IMPT 67Gy [59–78], P = 0.03), and higher utilization of induction chemotherapy (49% vs. 25%, P = 0.004) (Table 1). After PSM, baseline characteristics between the cohorts were well-balanced in terms of age (PSPT 70 years [48–80] vs. IMPT 68 years [49–81], P = 0.84) and radiation dose (PSPT 74Gy [54–75] vs. IMPT 70Gy [60–78], P = 0.83) (Suppl. Table e2). Additional baseline characteristics were also well-distributed with no differences in sex (P = 0.81), smoking status (P = 1.00), tumor location (P = 0.54) and stage (P = 0.88), and type of systemic therapy (P = 1.00) (Suppl. Table e2).
Figure 1. CONSORT diagram.
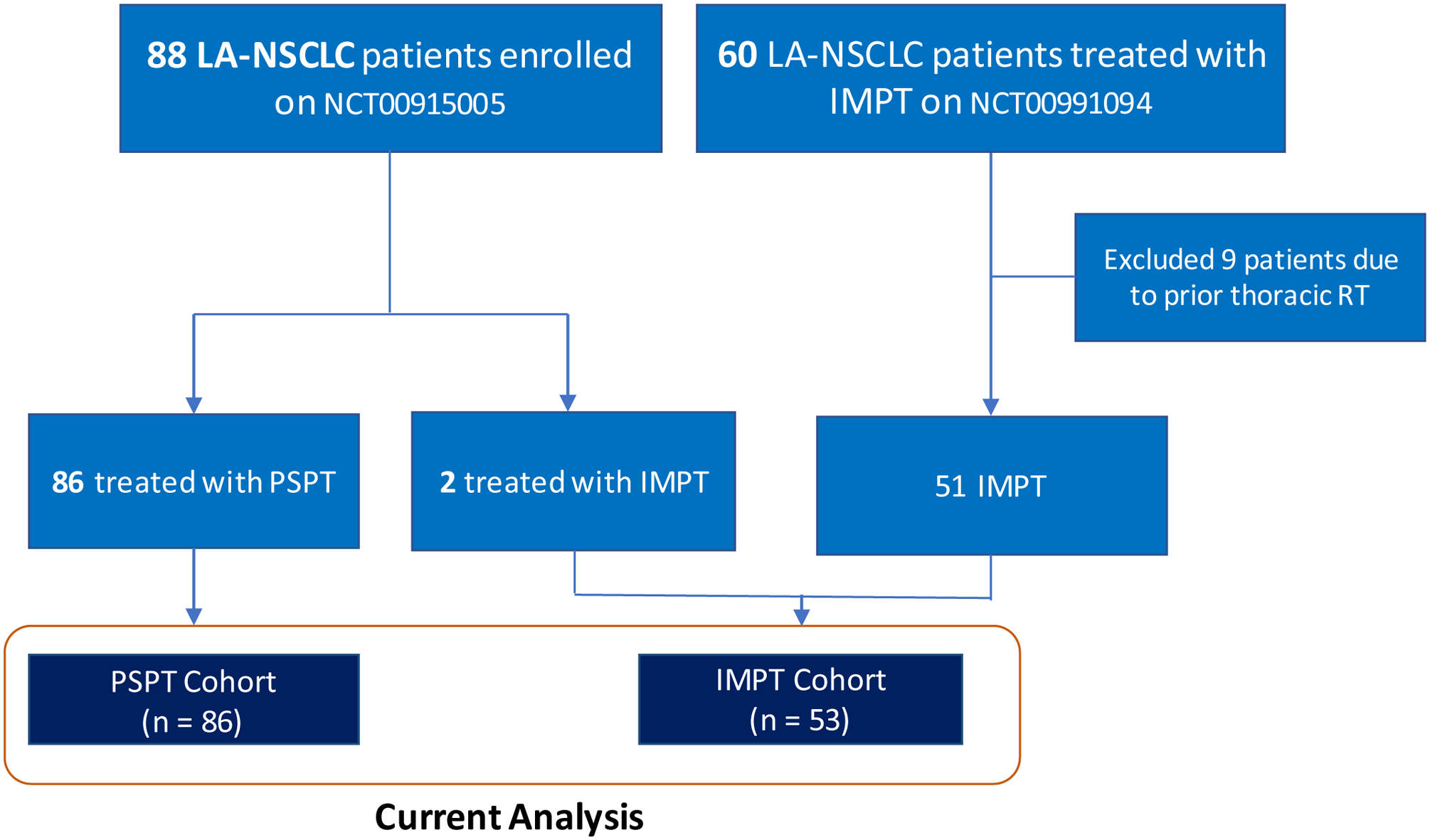
This study was a comparison of 86 patients with locally advanced non-small cell lung cancer (LA-NSCLC) treated with passive scattering proton therapy (PSPT) as part of a randomized clinical trial (clinicaltrials.gov NCT00915005). The intensity-modulated proton therapy (IMPT) group included 51 patients with LA-NSCLC who received IMPT at MD Anderson while enrolled in the prospective patient registry Normal Tissue Toxicity for Proton Therapy (clinicaltrial.gov NCT00991094), plus 2 patients from the NCT00915005 who ultimately received IMPT treatment.
Table 1.
Patient Characteristics
| Characteristic | All Patients | PSPT Group | IMPT Group | P Value |
|---|---|---|---|---|
| Sex | 0.88 | |||
| Female | 64 (46) | 40 (47) | 24 (45) | |
| Male | 75 (54) | 46 (53) | 29 (55) | |
| Age, median, years | 68 (39–83) | 67 (39–80) | 70 (43–83) | 0.06 |
| Age group, No. (%) | ||||
| <70 | 79 (57) | 54 (63) | 25 (48) | 0.09 |
| ≥70 | 59 (43) | 32 (37) | 27 (52) | |
| Smoking history, No. (%) | ||||
| Never | 12 (9) | 5 (6) | 7 (13) | 0.13 |
| Ever | 127 (91) | 81 (94) | 46 (87) | |
| Tumor histology, No. (%) | ||||
| Adenocarcinoma | 73 (53) | 45 (52) | 28 (53) | 0.84 |
| Squamous cell carcinoma | 43 (31) | 26 (30) | 17 (32) | |
| NSCLC, unspecified | 19 (14) | 12 (14) | 7 (13) | |
| Large cell | 2 (1) | 2 (3) | 0 (0) | |
| Other | 2 (1) | 1 (1) | 1 (2) | |
| Disease stage, No. (%) | 0.52 | |||
| II | 16 (12) | 10 (12) | 6 (11) | |
| IIIA | 55 (40) | 33 (38) | 22 (42) | |
| IIIB | 63 (45) | 38 (44) | 25 (47) | |
| IV | 3 (2) | 3 (3) | 0 (0) | |
| Recurrent | 2 (1) | 2 (2) | 0 (0) | |
| Nodal status, No. (%) | 0.42 | |||
| N0 | 9 (7) | 4 (5) | 5 (9) | |
| N1 | 16 (12) | 12 (14) | 4 (8) | |
| N2 | 64 (46) | 42 (49) | 22 (42) | |
| N3 | 41 (30) | 24 (28) | 17 (32) | |
| Nx | 9 (6) | 4 (5) | 5 (9) | |
| Tumor Location, No. (%) | 0.35 | |||
| RUL | 48 (35) | 30 (35) | 18 (34) | |
| RML | 10 (7) | 3 (4) | 7 (13) | |
| RLL | 17 (12) | 12 (14) | 5 (9) | |
| LUL | 32 (23) | 20 (23) | 12 (23) | |
| LLL | 18 (13) | 13 (15) | 5 (9) | |
| Mediastinum only | 13 (9) | 7 (8) | 6 (11) | |
| Adaptive Planning, No. (%) | 0.90 | |||
| Yes | 28 (20) | 18 (21) | 10 (19) | |
| No | 111 (80) | 68 (79) | 43 (81) | |
| Induction Chemotherapy, No. (%) | 0.004 | |||
| Yes | 55 (40) | 42 (49) | 13 (25) | |
| No | 84 (60) | 44 (51) | 40 (75) | |
| iGTV, cm3, mean (SD) | 140 (143) | 139 (148) | 141 (136) | 0.99 |
| Radiation dose, No. (%) | 0.02 | |||
| <66 Gy(RBE) | 18 (13) | 12 (14) | 6 (11) | |
| 66–68 Gy(RBE) | 39 (28) | 17 (20) | 22 (42) | |
| 68–78 Gy(RBE) | 82 (59) | 57 (66) | 25 (47) | |
| Radiation dose, median (range) | 70 (54–78) | 74 (54–75) | 67 (59–78) | 0.03 |
Abbreviations: iGTV, internal gross tumor volume; SD, standard deviation; RUL, right upper lube; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
The mean doses to three critical OARs (lung, heart, and esophagus) are shown in Figure 2A. IMPT was associated with lower mean dose to the lungs (16.5Gy vs. 13.0Gy, P < 0.001), heart (10.7Gy vs. 6.6Gy, P = 0.004), and esophagus (27.4Gy vs. 21.8Gy, P = 0.005) relative to PSPT (Figure 2A). Compared with PSPT, IMPT was associated with similar lung V10 (the volume of lung exposed to ≥10Gy) but significantly lower V20-V70 (Figure 2B). IMPT was also associated with similar heart V10, but significantly lower V20-V70, than did PSPT (Figure 2C), and significantly lower esophageal V30-V70 (Figure 2D).
Figure 2. Dosimetric analysis.
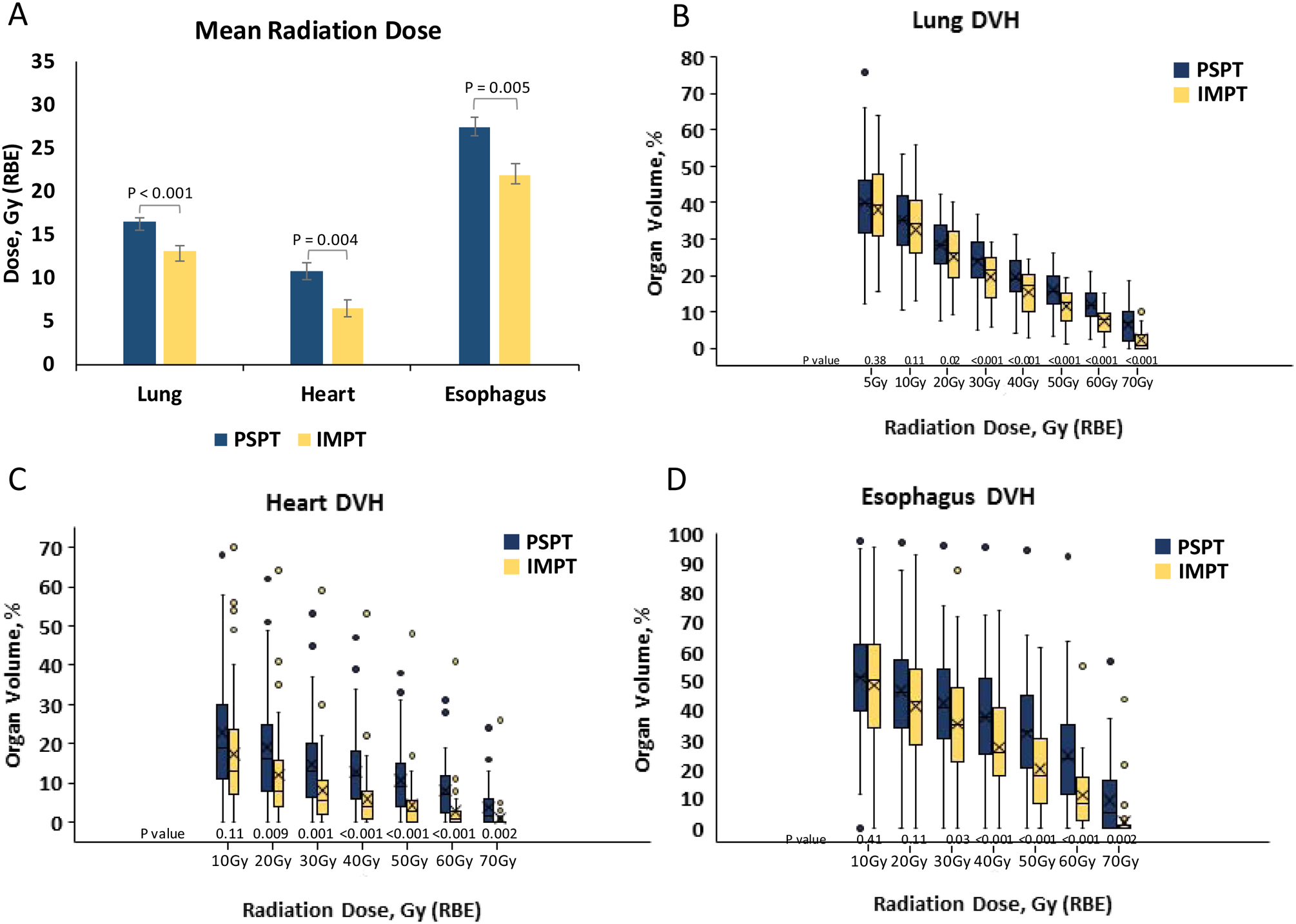
Mean radiation dose to critical organs at risk from passive scattering proton therapy (PSPT) and intensity-modulated proton therapy (IMPT) (A). Box plots of dose-volume histogram (DVH) data on radiation dose and organ volume for the lung (B), heart (C) and esophagus (D). Whiskers indicate 1.5 times the interquartile range above and below the median; dots represent outliers. P < 0.05 was the threshold for statistical significance.
Detailed toxicity findings for each cohort before and after PSM, separated by grade and OAR, are provided in Suppl. Table e3. In the PSPT arm, 15 patients (17%) experienced grade ≥3 pulmonary toxicity (composite of pneumonia, radiation pneumonitis, and fibrosis) compared with only 1 (2%) in the IMPT arm (P = 0.005) (Figure 3; Suppl. Table e3). The PSPT group also experienced more cardiac toxicity (4 grade 3 [5%], 2 grade 4 [2%], and 4 grade 5 [5%]) than did the IMPT group (0 [0%] grade ≥3 cardiac toxicity, P = 0.01) (Figure 3; Suppl. Table e3). The rate grade ≥3 pulmonary (28% vs 2%, P = 0.006) and cardiac (14% vs. 0%, P = 0.05) toxicity remained significantly higher in the PSPT arm even after PSM (Suppl. Figure e1; Suppl. Table e3). No other grade 4 or 5 toxicity was experienced by either group. The rates of grade ≥3 esophageal toxicity (16% vs. 8%, P = 0.11) was not different between PSPT and IMPT cohorts before PSM (Figure 3, Suppl. Table e3), but it was significantly higher in the PSPT cohort after PSM (28% vs. 6%, P = 0.02) (Suppl. Figure e1, Suppl. Table 3). Additionally, the incidence of grade ≥3 fatigue was significantly higher in PSPT cohort (13% vs. 2%, P = 0.03), and remained significant after PSM (22% vs. 3%, P = 0.03).
Figure 3. Toxicity profiles.
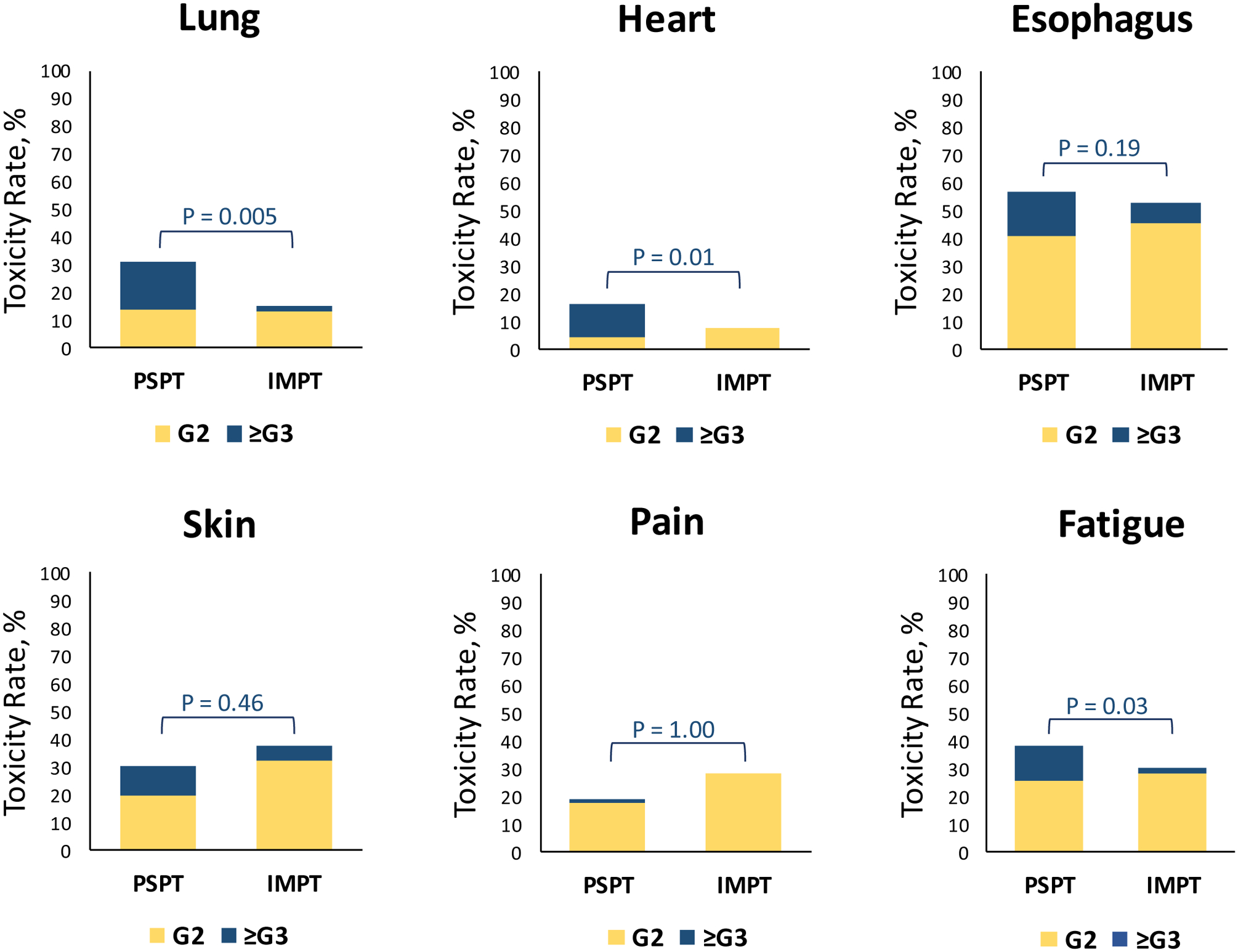
Rates of grade 2 (yellow) and grade ≥3 (blue) toxicity to critical organs at risk for patients treated with passive scattering proton therapy (PSPT) or intensity-modulated proton therapy (IMPT). The P values above each set of bars correspond to differences in the rates of grade ≥3 toxicity only. P < 0.05 was the threshold for statistical significance.
The median follow-up times were 23.9 months for the PSPT group (range 1.5−109.0) and 29.0 months for the IMPT group (range 1.0−84.1). The 3-year rates of local (25% vs. 20%, P = 0.44), local-regional (29% vs. 36%, P = 0.56) and distant (52% vs. 51%, P = 0.71) recurrences were not statistically different between PSPT vs. IMPT, respectively (Figure 4A–C). Median overall survival times were 23.9 months for PSPT (95% confidence interval [CI] 18.9−28.8) and 36.2 months for IMPT (95% CI 16.0−56.5) (P = 0.09) (Figure 4D). After PSM, the median survival times were 27.5 months for IMPT (95% CI 9.2–45.9) and 21.0 months for PSPT (95% CI 12.6–29.5) (P = 0.39) (Suppl. Figure e2).
Figure 4. Disease Control and Survival Analyses.
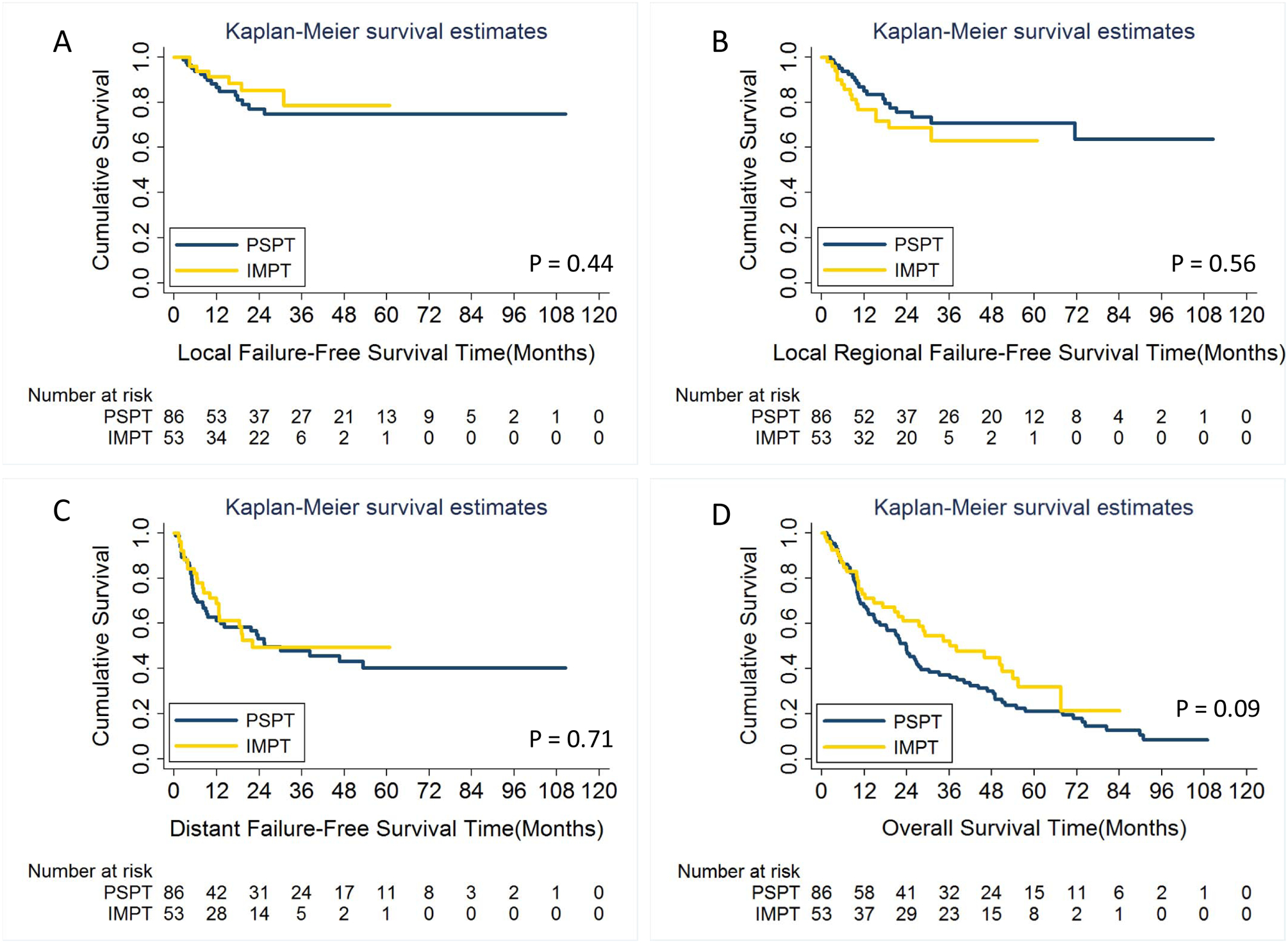
No differences were found between treatment groups in local failure-free survival (A), local-regional failure-free survival (B), distant failure-free survival (C), or overall survival (D). PSPT, passive scattering proton therapy; IMPT, intensity-modulated proton therapy.
DISCUSSION
Optimizing RT delivery for patients with LA-NSCLC continues to remain a high priority, given the morbidity and mortality associated with this disease and its treatment. Herein, we provide the first direct comparison of clinical outcomes after IMPT vs. PSPT for patients with LA-NSCLC from whom data on toxicity and outcomes were prospectively collected. We found that IMPT was associated with significantly lower rates of grade ≥3 pulmonary and cardiac toxicity that resulted from more favorable dosimetric profiles without compromising local disease control. In addition, despite having similar disease control rates, patients who received IMPT had a trend toward longer OS compared to patients treated with PSPT, although this did not meet statistical significance (P = 0.09).
Dosimetrically, PBT has significant advantages over photon (X-ray) therapy. By exploiting the Bragg peak dose distribution, we can deliver high doses of radiation to the tumor while minimizing exit dose through critical thoracic OARs.17 Of the two PBT modalities, IMPT has several advantages over PSPT, because the pencil beam scanning technology allows still more conformal “dose painting” of the high-dose region.18 Despite these theoretical advantages and few reports on the dosimetric superiority of IMPT for lung cancer, no information on the clinical superiority of this technique existed until the present report. Here we showed that cardiac, lung, and esophageal dose-volume characteristics were statistically improved with IMPT over PSPT. These advantages were more pronounced in higher-dose regions (V≥20 for lung and heart, and V≥30 for esophagus) but were not significant for low-dose regions (V≤10 for lung and for heart, and V≤20 for esophagus). Notably, the low-dose region resulting from either PSPT or IMPT is negligible compared with that resulting from photon therapy, and further improvements in these volumes with IMPT (relative to PSPT) is neither a goal nor a likely outcome.
The dosimetric superiority of proton to photons, or IMPT to PSPT is well established.8,13 However, evidence that improved dosimetric profiles translates into improvement in clinical outcomes is lacking. In the PSPT vs. IMRT randomized trial,11 PSPT did not reduce rates of radiation pneumonitis or cardiac toxicity despite all expectations from anteceding retrospective and dosimetric studies. The current report is the first comparative study demonstrating that the superior cardiac and pulmonary dosimetric profiles resulting from IMPT were associated with markedly reduced toxicity for both organs. Impressively, no grade ≥4 toxicity of any type was experienced in the IMPT group, underscoring the promise of this modality. As noted above, most of the dosimetric gains achieved by IMPT were in high-dose regions while providing no measurable benefit in the low-dose regions, suggesting that the former is better predictor of cardiopulmonary toxicity in the setting of proton beam therapy given the notable difference in grade ≥3 toxicity observed in our cohorts. This is also consistent with prior reports suggesting that high-dose regions are better predictors of cardiac and pulmonary toxicity in clinical and model case studies.5,16,19 Indeed, one potential explanation for the higher rate of pneumonitis after PSPT relative to IMRT in the randomized trial was the relatively larger pulmonary high-dose volume in the PSPT group11; the findings in this report suggest that IMPT may be able to overcome this disadvantage of PSPT while simultaneously offering other advantages in the low-dose regions, perhaps making IMPT preferable even over IMRT. The answer to these questions will ultimately be answered by the currently active phase III randomized clinical trial, RTOG 1308, which is comparing proton vs. photon for non-small cell lung cancer (NCT01629498), and by protocol NCT01629498 of “Image-Guided, Intensity-Modulated Photon or Proton Beam Radiation Therapy in Treating Patients With Stage II-IIIB Non-small Cell Lung Cancer.”
Interestingly, IMPT did not significantly reduce the risk of esophagitis despite having lower mean and high-dose region esophageal doses. This finding may result from the anatomic position of the esophagus in the posterior mediastinum, which can be spared with PSPT as effectively as IMPT. It is reasonable to deduce that the threshold dose needed to cause clinically significant esophageal toxicity was avoided with either modality effectively. Also notable was that the rate of grade ≥3 esophageal toxicity in the IMPT group was half that in the PSPT group, and did ultimately show a significant difference after propensity score matching, potentially indicating that the lack of statistical difference in the initial cohorts may be due to inadequate power rather than lack of clinical benefit.
Verification simulation was carried out weekly in the PSPT study and at the discretion of the physician for the IMPT cohort. Despite this, there was no difference between the rates of adaptive planning applied in the two cohorts. This was not unexpected, as during the initial proton beam therapy trials at our institution, adaptive planning was only performed in a minority of patients, commonly mid-way through treatment, despite routine use of weekly verification simulaton.7 As a result, our institutional practice has evolved to recommend one verification simulation for proton therapy plans at the mid-chemoRT treatment point, at which time adaptive planning is only done if dosimetry has been significantly altered by tumor and anatomical changes. IMPT is also more sensitive to positional uncertainties and anatomical changes during treatment. The lower rate of toxicity in the IMPT cohort and similar tumor control rates despite utilizing similar adaptive planning speak in support of the in-house built robustness optimization system utilized for multi-filed optimization plans as a method of addressing the various positional and range uncertainties inherent with IMPT.15,16
The median survival of 23.9 months in the PSPT cohort is similar to that reported in other recent prospective studies investigating chemoradiation with proton or photon therapy in the setting of unresectable LA-NSCLC, where median survival has ranged between 26 to 30 months.1,2,7,9 The median survival of 36.2 months in the IMPT arm is higher than we had anticipated and is potentially explained by the reduced toxicity associated with IMPT. This is in line with RTOG-0617, which demonstrated that radiation and the way it is delivered can significantly affect long-term survival.20 In the current study, survival time seemed to be longer in the IMPT group than in the PSPT group, despite the lack of difference in local and distant disease control rates. One might speculate that the reduced toxicity from IMPT may have positively affected OS. In fact, a series of other studies have linked increased radiation dose to the heart with worse OS.5,16 Even though it is possible that the high radiation doses to the heart in these studies may simply be a surrogate for higher tumor burden, which is what actually conferred the worse survival, this is unlikely to be the case in the current study. The two groups had similar tumor target volumes and the group with the lower cardiac dose (IMPT) trended toward longer survival. Furthermore, it is unlikely that a median time difference of 3 years sufficiently explains the differences in survival, especially since both groups were treated prior to the publication of the PACIFIC trial, and the use if immunotherapy was not commonplace in either cohort with only 7 patients treated with immunotherapy in the salvage setting.
This study had several limitations. As is true for any non-randomized study, the groups were subject to selection bias. Indeed, the PSPT cohort consisted predominantly of patients who were randomized to PSPT and whose PSPT plan was deemed acceptable by a radiation oncologist, which excluded a significant number of patients who were randomized to PSPT but whose plans were deemed not safe to deliver.11 However, such a step is expected to skew the toxicity profile in favor of PSPT, further strengthening the argument that IMPT causes less toxicity than PSPT. Furthermore, there were certain imbalances between the two cohorts, including age, stage, systemic chemotherapy, and total prescribed dose. However, the comprehensive propensity score-matched analysis accounting not only for baseline imbalances, but also clinically meaningful factors, showed that the differences in cardiac and pulmonary toxicity remained significant even when accounting for these factors. Unlike the group who received PSPT, the IMPT patients in the current study were not treated on a clinical trial, which may raise the concern of incomplete data collection; however, data on toxicity and outcomes for this cohort were collected as part of the MD Anderson Proton Toxicity registry, which was designed to provide a comprehensive set of such data to use as the basis for future studies. Lastly, both cohorts underwent only daily online orthogonal kilovoltage imaging but not CBCT scanning, which has since become more widely utilized in this setting. It is conceivable that the use of CBCT for alignment and target verification may further reduce the absolute toxicity rates for both these techniques below what is reported in this study.
In summary, this is the largest comparison undertaken to date of IMPT and PSPT, for patients with LA-NSCLC. IMPT led to lower mean and high-dose volumes for lung, heart, and esophagus, and caused significantly less grade ≥3 cardiopulmonary toxicity. However, neither disease control nor OS were different between groups, even though there was a trend toward improved survival with IMPT. This may have resulted from small numbers of patients and hence inadequate statistical power. Additional, larger studies await the continuing penetration of IMPT into clinical practice for LA-NSCLC.
Supplementary Material
Grant funding:
Supported in part by grants from the National Cancer Institute (1R21 CA222749-01A1, Proton Therapy to Reduce Heart Damage for Lung Cancer Patients; and Cancer Center Support [Core] Grant P30 CA016672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
Stephen G Chun, MD serves as a consultant to AstraZeneca.
Saumil Gandhi, MD, PhD reports an AstraZeneca Inc: Research grant..
Carl M Gay, MD, PhD reports Research Grant funding from AstraZeneca not pertient to current work..
Zhongxing Liao, MD reports grants from National Cancer Institute, during the conduct of the study; .Remaining authors have nothing to disclose.
References
- 1.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. [DOI] [PubMed] [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377(20):1919–1929. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer. Version 3.2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 04/09/2020.
- 4.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao ZX, Komaki RR, Thames HD Jr., et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(3):775–781. [DOI] [PubMed] [Google Scholar]
- 8.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(4):1087–1096. [DOI] [PubMed] [Google Scholar]
- 9.Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117(20):4707–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen QN, Ly NB, Komaki R, et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol. 2015;115(3):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Z, Lee JJ, Komaki R, et al. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(18):1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohan R, Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev. 2017;109:26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang JY, Li H, Zhu XR, et al. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2014;90(4):809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardar L, Li Y, Li X, et al. Evaluation and mitigation of the interplay effects of intensity modulated proton therapy for lung cancer in a clinical setting. Pract Radiat Oncol. 2014;4(6):e259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39(2):1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elhammali A, Blanchard P, Yoder A, et al. Clinical outcomes after intensity-modulated proton therapy with concurrent chemotherapy for inoperable non-small cell lung cancer. Radiother Oncol. 2019;136:136–142. [DOI] [PubMed] [Google Scholar]
- 17.Gjyshi O, Liao Z. Proton therapy for locally advanced non-small cell lung cancer. Br J Radiol. 2020;93(1107):20190378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks ED, Ning MS, Verma V, Zhu XR, Chang JY. Proton therapy for non-small cell lung cancer: the road ahead. Transl Lung Cancer Res. 2019;8(Suppl 2):S202–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker SL, Xu T, Paganetti H, et al. Validation of Effective Dose as a Better Predictor of Radiation Pneumonitis Risk Than Mean Lung Dose: Secondary Analysis of a Randomized Trial. Int J Radiat Oncol Biol Phys. 2019;103(2):403–410. [DOI] [PubMed] [Google Scholar]
- 20.Bradley JD, Hu C, Komaki RR, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard-Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2020;38(7):706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


