Abstract
Background
Risks of pelvic organ prolapse and urinary incontinence rise after the first vaginal delivery. During the early postpartum period, a time of active regeneration and healing of the pelvic floor, women may be particularly vulnerable to greater pelvic floor loading.
Objectives
This prospective cohort study aimed to determine whether objectively measured moderate-to-vigorous physical activity (MVPA) in the early postpartum period predicts pelvic floor support and symptoms 1 year following first vaginal birth.
Study design
We enrolled nulliparous women in the third trimester, later excluding those delivered via cesarean or preterm. Participants wore triaxial wrist accelerometers at 2-3 and 5-6 weeks postpartum for ≥ 4 days. Primary outcomes, assessed 1 year postpartum, included 1) pelvic floor support on Pelvic Organ Prolapse Quantification examination , dichotomized as maximal vaginal descent < 0 cm (better support) versus ≥ 0 cm (worse support) and 2) pelvic floor symptom burden, considered positive with report of ≥1 bothersome symptom in ≥2 of 6 domains, assessed using the Epidemiology of Prolapse and Incontinence Questionnaire. The primary predictor was average daily MVPA. As we could not eliminate women with pelvic floor changes before pregnancy, we modeled prevalence, rather than risk, ratios (PR) for each outcome using modified Poisson regression.
Results
Of 825 participants eligible after delivery, 611 completed accelerometry and 1-year follow-up; 562 completed in-person visits and 609, questionnaires. Mean age was 28.9 years (SD 5.01). Mean (SD) MVPA was 57.3 (25.4) and 68.1 (28.9) minutes/day at 2-3 and 5-6 weeks, respectively. One year postpartum, 53/562 (9.4%) demonstrated worse vaginal support and 330/609 (54.2%) met criteria for pelvic floor symptom burden. 324 (53.1%), 284 (46.6%), 144 (23.6%), and 25 (4.1%) reported secondary outcomes of stress urinary incontinence, overactive bladder, anal incontinence, and constipation, respectively, and 264 (43.4%), 250 (41.0%) and 89 (14.6%) reported no, mild, or moderate to severe urinary incontinence, respectively.
The relationship between MVPA and outcomes was not linear. Based on plots, we grouped quintiles of MVPA into 3 categories: quintiles 1, 2 vs 3,4, vs 5. In final multivariable models, compared to women in MVPA quintiles 3 and 4, those in the lower 2 (PR 0.55 (95% CI 0.31, 1.00) and upper quintile (PR 0.70 (95% CI 0.35, 1.38)) trended towards lower prevalences of worse support. However, we observed the reverse for symptom burden: compared to women in quintiles 3, 4 those in the lower 2 (PR 1.20 (95% CI 1.02, 1.41) and upper quintile PR 1.34 (95% CI 1.11, 1.61) demonstrated higher prevalences of symptom burden.
MVPA did not predict any of the secondary outcomes. Presence of a delivery factor with potential to increase risk for levator muscle injury did not modify the effect of MVPA on outcomes.
Conclusion
Except for support, which was worse in women with moderately high levels of activity, early postpartum MVPA was either protective or had no effect on other parameters of pelvic floor health. Few women did substantial vigorous activity and thus these results do not apply to women doing strenuous exercise shortly after delivery.
Keywords: physical activity, pelvic floor disorder, postpartum, pelvic organ prolapse, urinary incontinence, accelerometry
INTRODUCTION
Up to 1 in 5 women undergoes surgery for pelvic organ prolapse (POP) or urinary incontinence (UI) in her lifetime.1-3 The path to future end-stage POP for most women and UI for many women begins at the first vaginal delivery. 4-14.15-17 While few young women require surgery, they do demonstrate a range of pelvic floor support and symptoms postpartum.18-32 Specific delivery events increase risk of traumatic levator ani muscle defects and impaired vaginal support.19,33-38 Other than childbirth, modifiable non-obstetric risk factors for pelvic floor disorders (PFDs) pertinent to reproductive aged women include obesity, lifestyle factors, constipation, and physical activity.31,39-44
Vaginal delivery is one of the larger soft tissue injuries most women sustain over their lives. However, while vaginal delivery increases the risk for end stage PFDs, it is not sufficient, in that most parous women don’t have surgery for these conditions. Women experience different injury and recovery courses, both affected by their inherent regenerative capabilities and potentially by mechanical stressors placed on the pelvic floor. Women in the early postpartum period, a time of active regeneration and healing, may be particularly vulnerable to such stressors. It is possible that a high amount of moderate to vigorous physical activity (MVPA) during this recovery period may impair regeneration of damaged tissues.
The primary aims of this prospective cohort study are to determine whether MVPA, measured objectively using accelerometry, in the early postpartum period predicts pelvic floor support and symptom burden 1 year following first vaginal birth. We hypothesized that greater MVPA during this period will predict worse pelvic floor support and greater symptom burden one year postpartum. In planned secondary aims, we explored whether MVPA in the early postpartum period predicts urinary incontinence severity, stress urinary incontinence (SUI), overactive bladder (OAB), constipation or anal incontinence (AI) at one year postpartum. Finally, in a planned exploratory aim, we estimated whether the presence of a delivery variable that places women at higher risk for levator ani muscle (LAM) injury modifies the effect of MVPA on pelvic floor outcomes.
MATERIALS AND METHODS
Local institutional review boards approved this study. All participants completed an informed consent process.
Recruitment
Overall study methods are reported in more detail elsewhere45 and summarized here. Research staff recruited women from 7 prenatal clinics in the Salt Lake Valley between 09/01/15 and 07/11/18. Participants were ≥ 18 years, English or Spanish speaking, nulliparous with a singleton gestation, ≥ 28 weeks gestation, planning vaginal delivery, not planning to move to a location precluding follow-up, and living within 60 miles of the research facility. Other exclusion criteria included medical conditions precluding physical activity, prior surgical treatment for POP or UI, and no email or telephone access. As our intent was to study women who underwent term vaginal delivery, after enrollment, women that delivered by cesarean or before 37 weeks gestation were excluded from further participation.
Outcome measures
The primary outcome measures were two dichotomous measures of pelvic floor health: 1) Pelvic floor support, assessed using the Pelvic Organ Prolapse Quantification (POP-Q) examination46-48, and categorized as maximal vaginal descent (the most distal point of C, Ba or Bp) of < 0 cm (above the hymen; better support) versus ≥ 0 cm (at or below the hymen; worse support) and 2) Pelvic floor symptom burden, assessed using the Epidemiology of Prolapse and Incontinence Questionnaire (EPIQ)49, and categorized as positive when a participant reported at least one symptom with bother > 0 on the visual analogue scale (VAS) in ≥ 2 domains.
We assessed the secondary outcomes of symptoms of SUI, OAB, and AI using the EPIQ and categorized each as positive when a participant reported ≥ 1 symptom with VAS > 0 in the pertinent domain. We categorized constipation as present with positive responses to both “Do you have < three bowel movements per week?” and “Do you have to strain > 25% of the time to have a bowel movement?” on the Defecation Distress Inventory.50,51 We compared MVPA by Incontinence Severity Index (ISI) categories of 0 (no UI), 1-2 (slight) and ≥3 (moderate to severe).52,53
Primary predictor
Our a priori primary predictor, MVPA, was average daily minutes of MVPA based upon total accrual of MVPA, to best reflect all potential forces on the pelvic floor.
Study procedures
Pertinent to this analysis, during the third trimester and one year postpartum, trained research staff performed the POP-Q examination with participants in the lithotomy position, backs elevated to 45 degrees, and during maximal strain, except for vaginal length. At these timepoints and at 5-10 weeks postpartum, participants completed questionnaires in English or Spanish using RedCap, a web-based electronic data capture tool.54 In addition to the study instruments noted above, others included demographic characteristics and medical history, the Rapid Assessment of Physical Activity questionnaire55, and checklists of physical activity types modeled after the Bone Loading History Questionnaire.56 We extracted delivery details from the electronic medical record.
To objectively measure physical activity, participants wore a GT9X triaxial accelerometer (Actigraph, Inc., Pensacola, FL) on the wrist at 2-3 and 5-6 weeks postpartum (operationalized as 12-25 days and 33-46 days postpartum, respectively). Details of the accelerometry protocol have been published.57 To provide context for the accelerometer results, participants completed physical activity questionnaires after each wear period. For this analysis, we included women with valid (analyzable) data at either or both time frames. To be analyzable, participants must have worn the accelerometer within the correct time frame with adequate wear time. Consistent with prior studies, we required ≥4 days of wear, with ≥1 weekend day, and ≥10 and 8 hours of wear on weekdays and a weekend day, respectively, to consider the accelerometry data valid.58,59 As some recent studies require ≥16 hours of daily wear when wrist, rather than waist, accelerometry is used60, we also calculated the proportion of participants that met this more stringent wear criterion and tested the effect of accelerometer wear time(≥16 hours/day versus 10 to <16 hours/day) in sensitivity analyses. We used GGIR V1.5, an R-package designed to process multi-day raw accelerometer data and applied quality control measures as per van Hees et al.61,62
The GT9X accelerometer provides vector magnitude, in units of gravities, as a summary metric of acceleration from three axes. The Euclidean Norm Minus One (ENMO) metric subtracts the contribution of gravity (1 g) from vector magnitude, resulting in acceleration values that reflect human movement in mili-gravities (mgs).63,64,65 The greater movement intensity and activity duration, averaged over 5-second epochs, the greater the ENMO value. An ENMO intensity threshold of ≥ 100.6 mg corresponds to MVPA.64,66,67
Based on work by others68, we considered a woman to be at higher risk for LAM injury if one of the following risk factors were present: age ≥ 33 years, second stage of labor duration ≥ 150 minutes, birthweight > 4000 grams, forceps delivery or anal sphincter tear (3rd or 4th degree laceration).
Statistical methods
In this cohort study, we cannot remove prevalent cases occurring before pregnancy.Thus, as we could not estimate a risk ratio, we calculated prevalence ratios (PR) with 95% confidence intervals (CI) for each outcome using modified Poisson regression with variance correction by generalized estimating equations (GEE).69,70
The adjustment variables in our multivariable analyses (provided in tables) were informed by directed acyclic graphs (DAG) completed prior to inspection of the data.71,72,73 Adjustment variables included symptoms and support during pregnancy which were categorized in the same way as at 1 year with 2 exceptions: For OAB,because most women would be categorized as positive during pregnancy, given the preponderance of nocturia and urinary frequency in pregnancy,74 we adjusted for UUI. For the symptom burden outcome, we adjusted for the SUI, UUI or AI, demonstrated to increase subsequent risk of the same symptoms 75-79,80-83. Our primary predictor, average daily minutes of MVPA, reflects the mean of daily MVPA across the 2 time-points. This approach was suupported by preliminary analyses, in which the predicted reliability of total MVPA between the 2 timepoints was moderately high(intraclass correlation=0.79), suggesting that both timepoints measure a single underlying construct. We conducted a planned sensitivity analysis, limiting the population to those with valid data at both timepoints, to assess whether MVPA at either timepoint independently predicted the primary outcomes. Finally, we conducted planned sensitivity analyses testing associations between outcomes and MVPA that occurred in at least 5-minute bouts, suggestive of sustained activity such as exercise.
Using SAS version 9.4, we checked models for model assumptions, multicollinearity, effects of sparseness, influential observations, and goodness of fit using standard regression diagnostics. For continuous variables, we used a graphical method of Hosmer and Lemeshow84 and the quasi-information criterion (QIC) to check linearity of their relationship with each outcome on the log-prevalence scale. If not linear, we combined adjacent quintiles with similar coefficients. Thus, different outcomes have different cut-points for predictor variables. As a final step, we performed multiple imputation on missing values in the covariates using Stata version 16.85,86 To estimate prevalence ratios, we used modified Poisson regression rather than log-binomial models as the latter did not always converge, particularly in multivariable models.69,87
We tested the primary outcomes, support and symptom burden, at the 5% significance level. For secondary outcomes, we adjusted the significance level based on the total number of secondary outcomes, degrees of freedom of the main exposure, and number of levels in the outcome of interest.
Sample size
Sample size was based on calculations for logistic regression, as no power calculation formulae are available for modified generalized linear models designed to estimate the prevalence ratio. We considered women at the mean for daily average MVPA as “lower risk” and those at the mean plus one standard deviation as “higher risk”. We assumed a frequency of MVD≥0 of 15% in the lower-risk group45 and that R2 of MVPA regressed on other predictors is 0.5. Therefore, a sample size of 585 women at 1- year follow-up provides 90% power to detect a minimal odds ratio of 1.70 for women whose daily average MVPA is 1 standard deviation higher than other women whose value is at the mean.
RESULTS
Participant flow is summarized in Figure 1. Of 1078 women enrolled, 825 remained eligible after delivery,739 had valid accelerometer data at 2-3 and/or 5-6 weeks postpartum and 611 of 825 (74.1%) completed 1-year follow-up. Of these, 562 of 825 eligible (68.8%) completed the pelvic floor support outcome assessment and 609 (73.8%) the symptom burden outcome assessment.
Fig. 1.
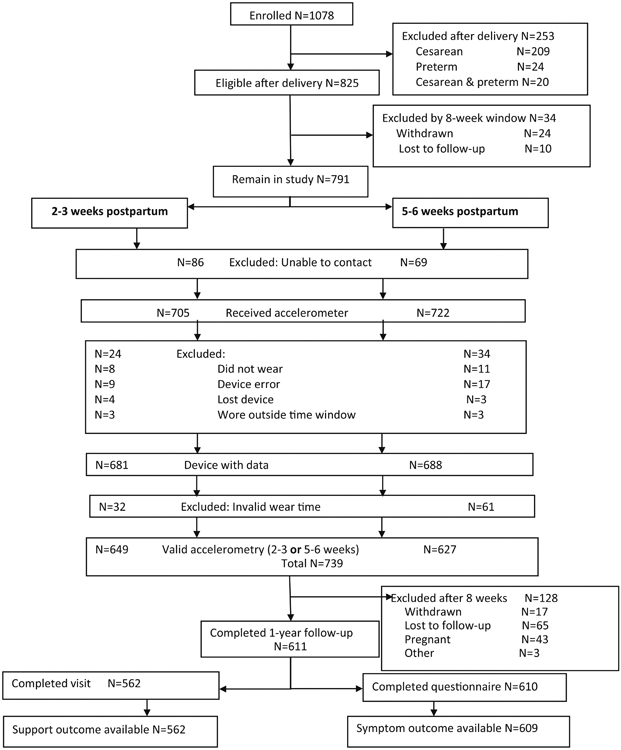
Participant flow
Participant characteristics are summarized in Table 1. Mean age of the sample at enrollment was 28.9 years (SD 5.01). There were no differences in age, ethnicity or insurance type between eligible women that agreed or that declined study participation. Of women still eligible after delivery, those that did not complete 1-year follow-up were younger (26.0 vs 28.8 years, p<0.001), more likely to report Hispanic ethnicity (32.6% vs 17.8%, p<0.0001), and less likely to have private insurance (61.2% vs 83.2%, p<0.0001), have attended college (62.0% vs 88.3%, p<0.0001) and be categorized as high-risk for LAM injury (18.5% vs 26.4%, p=0.03) than participants that did complete 1-year follow-up.
Table 1.
Participant Characteristics
| Characteristic | Missing* | N=611 |
|---|---|---|
| Age at enrollment, years; mean (SD) | 1 | 28.9 (5.0) |
| Gestational age at enrollment, weeks; mean (SD) | 4 | 33.7 (2.5) |
| Ethnicity | 0 | |
| Hispanic | 104 (17.0%) | |
| Non-Hispanic | 507 (83.0%) | |
| Race | 0 | |
| Caucasian/White | 562 (92.0%) | |
| Other/do not wish to identify | 49 (8.0%) | |
| Education | 2 | |
| High school or less | 66 (10.8%) | |
| Some college/completed college | 362 (59.4%) | |
| Graduate or professional degree | 181 (29.7%) | |
| Work status | 2 | |
| Working full-time (≥30 hours per week) | 408 (67.0%) | |
| Working part-time (< 30 hours per week) | 67 (11.0%) | |
| Other (student, homemaker, disabled) | 134 (22.0%) | |
| Heavy lifting or heavy work in 3rd trimester | 3 | 197 (32.2%) |
| Smoking in 3rd trimester | 5 | 7 (1.2%) |
| Diabetes, preexisting or gestational | 1 | 26 (4.3%) |
| Hypertension, preexisting or gestational | 1 | 9 (1.5%) |
| Family history of pelvic organ prolapse or urinary incontinence | 39 | 214 (37.4%) |
| Maximal vaginal descent ≥0 cm at 3rd trimester | 0 | 19 (3.1%) |
| SUI at 3rd trimester | 3 | 299 (49.2%) |
| UUI at 3rd trimester | 4 | 53 (8.7%) |
| AI at 3rd trimester | 4 | 152 (25.0%) |
| AT DELIVERY | ||
| Mode of delivery | 0 | |
| Normal spontaneous vaginal delivery | 550 (90.0%) | |
| Vacuum assisted delivery | 13 (2.1) | |
| Forceps assisted delivery | 47 (7.7%) | |
| Anal sphincter laceration | 4 | 23 (3.8%) |
| Epidural anesthesia | 0 | 487 (79.7%) |
| Labor augmentation or induction | 2 | 379 (62.0%) |
| Duration of 2nd stage of labor, minutes; median (IQR) | 154 | 83.0 (48.0, 142.0) |
| Birth weight, grams; mean (SD) | 11 | 3318.4 (396.3) |
| Presence of high-risk delivery variable | 0 | 163 (26.7%) |
| AT 5-10 WEEKS POSTPARTUM** | ||
| Body mass index, kg/m2; mean (SD) | 17 | 26.2 (4.9) |
| Work status | 28 | |
| Working full-time (≥30 hours per week) | 52 (8.9%) | |
| Working part-time (< 30 hours per week) | 43 (7.4%) | |
| Other | 488 (83.7%) | |
| Heavy lifting (other than baby) or heavy work in past 7 days | 29 | 158 (27.2%) |
| Performs pelvic floor muscles exercises, n (%) IF YES: | 28 | 296 (50.8%) |
| IF YES: | ||
| More than once a day/every day | 85 (28.9%) | |
| Less than daily but more than weekly | 185 (62.9%) | |
| Less than weekly | 24 (8.2%) | |
| Currently breastfeeding | 29 | 521 (89.5%) |
| UTI since delivery | 29 | 27 (4.6%) |
| Hormonal contraception | 28 | 290 (47.5%) |
| Chronic cough** | 28 | 8 (1.4%) |
| AT 1 YEAR POSTPARTUM ** | ||
| Body mass index, kg/m2; mean (SD) | 49 | 24.84 (5.62) |
| Heavy lifting (other than baby) or heavy work in past 7 days | 3 | 267 (43.9%) |
| Work status | 2 | |
| Working full-time (≥30 hours per week) | 308 (50.6%) | |
| Working part-time (< 30 hours per week) | 127 (20.9%) | |
| Other | 174 (28.6%) | |
| Performs pelvic floor muscles exercises, n (%) | 5 | 174 (28.7%) |
| IF YES: | ||
| More than once a day/every day | 31 (17.8%) | |
| Less than daily but more than weekly | 106 (60.9%) | |
| Less than weekly | 37 (21.3%) | |
| Currently breastfeeding | 2 | 278 (45.7%) |
| Chronic cough | 4 | 13 (2.1%) |
Percentages reflect the denominators with complete data for each variable. Family history of prolapse or urinary incontinence was added to the initial questionnaire after enrollment began, and one of the participating hospitals did not record duration of 2nd stage of labor, accounting for larger number of missing responses for these variables.
28 women did not complete the 5-10 week questionnaire. 49 women completed only the 1-year questionnaire and not the examination.
All participants in this analysis met the wear time criteria described in Methods necessary for inclusion. Almost all also met more stringent wear time criteria of ≥16 hours/day for at least 3 weekdays and 1 weekend day: 531/ 537 (98.9%) at 2-3 weeks and 513/527 (97.3%) at 5-6 weeks. Mean (SD) wear times were 23.39 (1.50) hours and 23.13 (1.95) hours/day at each timepoint, respectively. Mean (SD) MVPA was 57.3 (25.4) and 68.1 (28.9) minutes/day at 2-3 and 5-6 weeks, respectively. Figure 2 demonstrates the shift to increasing MVPA between the two time-points. All women did at least some (>0 minutes) moderate and over 99% did at least some vigorous activity (>428.8 mg) at each time point. At 2-3 weeks, 376 (70.0%) and at 5-6 weeks, 402 (76.3%) did at least one 5-minute bout of of MVPA. Only 23 (4.3%) at 2-3 weeks and 49 (9.3%) at 5-6 weeks did at least one 5-minute bout of vigorous activity. Table 2 summarizes activity types self-reported on checklists at each time point according to quintile of MVPA by accelerometry.
Fig. 2.
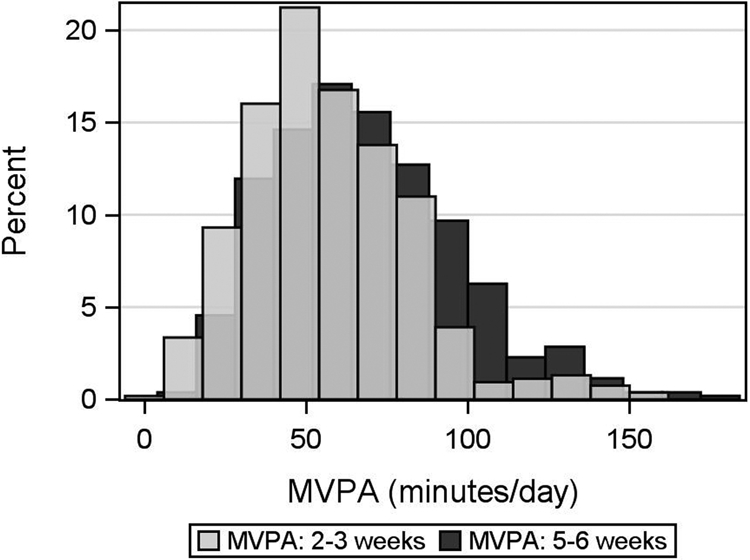
Moderate to Vigorous Physical Activity (MVPA) at 2-3 weeks and 5-6 weeks postpartum
Table 2.
Types of self-reported activities according to quintile of average daily minutes of Moderate to Vigorous Physical Activity (MVPA) at each time point*
| Time | MVPA Quintile (minutes per day) |
Activity that does not involve bouncing, jumping, straining or heavy lifting |
Activity that involves some bouncing or easy running and or greater effort |
Activity that involves hard running or straining |
Activity that involves heavy lifting or work |
Lifting weights at the gym |
|---|---|---|---|---|---|---|
| 2-3 weeks | 4.8 - 35.5 | 67 (17.2) | 6 (7.1) | 1 (5.9) | 6 (8.6) | 1 (5.6) |
| 35.5 – 48.4 | 77 (19.8) | 11 (13.1) | 1 (5.9) | 13 (18.6) | 4 (22.2) | |
| 48.4 - 60.5 | 88 (22.6) | 24 (28.6) | 2 (11.8) | 17 (24.3) | 2 (11.1) | |
| 60.5 - 77.5 | 74 (19) | 22 (26.2) | 6 (35.3) | 16 (22.9) | 3 (16.7) | |
| 77.5 - 153.1 | 83 (21.3) | 21 (25) | 7 (41.2) | 18 (25.7) | 8 (44.4) | |
| 5-6 weeks | 11.2 - 42.3 | 61 (16.1) | 25 (13.4) | 7 (9.1) | 21 (15) | 6 (10.9) |
| 42.3 - 58.6 | 71 (18.7) | 31 (16.6) | 12 (15.6) | 26 (18.6) | 10 (18.2) | |
| 58.6 - 72.5 | 84 (22.2) | 43 (23) | 12 (15.6) | 22 (15.7) | 7 (12.7) | |
| 72.5 - 90.5 | 82 (21.6) | 40 (21.4) | 20 (26) | 35 (25) | 13 (23.6) | |
| 90.5 - 177.1 | 81 (21.4) | 48 (25.7) | 26 (33.8) | 36 (25.7) | 19 (34.5) |
The population for this analysis includes participants with both valid accelerometry and completed physical activity questionnaires at each time-point (537 at 2-3 weeks postpartum and 527 at 5-6 weeks postpartum). Quintiles are shown for average daily minutes of MVPA according to wrist accelerometry at each time-point. Participants reported activity types performed for at least 10 minutes in the last 7 days, according to the modified Bone Loading Questionnaire. Participants could report more than one, or no, types of activities. Column percentages reflect the proportion of women that reported each activity type according to their objectively measured quintile of MVPA. Some column counts do not add up to 100% due to missing responses in some individual activity questions.
One year postpartum, 53 of 562 women (9.4%) demonstrated worse vaginal support. Mean (SD) points C (apex), Ba (anterior vagina) and Bp (posterior vagina) were −6.2 (1.09), −1.5 (0.77) and −2.1 (0.74) cm, respectively. About half, 330 of 609 (54.2%) met criteria for pelvic floor symptom burden; 119 (19.5%) reported no symptoms and 160 (26.2%) reported symptoms in only 1 domain. For secondary outcomes, 324 (53.1%) reported SUI, 284 (46.6%) OAB, 144 (23.6%) AI, and 25 (4.1%) constipation Incontinence severity was mild in 250 (41.0%) and moderate to severe in 89 (14.6%), while 264 (43.3%) reported no UI.
As demonstrated by Figure 3, MVPA did not demonstrate a linear association with either primary outcome (or, not shown, with any secondary outcomes). Therefore we grouped quintiles of MVPA as noted in Methods. For the primary outcomes, we grouped MVPA into 3 categories: first and second quintiles combined, third and fourth quintiles combined, and fifth quintile.
Fig. 3.
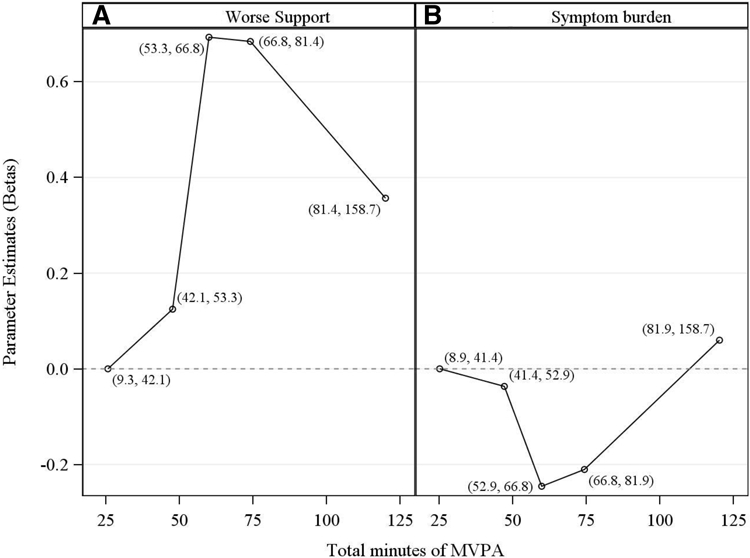
Figures demonstrate plots of relationships between each primary outcome and MVPA. On the y-axis is displayed the log of the prevalence ratio for each quintile versus the reference quintile (1st). The log of the prevalence ratio is the parameter estimate beta from an unadjusted modified Poisson regression. The x-axis displays total minutes of MVPA averaged over the two early postpartum time-points at which these data were collected. Open circles represent betas plotted at the mid-point of each quintile. The range for each quintile, in minutes, is shown next to each mid-point.
In final multivariable models, compared to women in quintiles 1 and 2 for MVPA, those in quintiles 3 and 4 had a marginally significant higher prevalence of worse support (PR 1.80 (95% CI 1.00,3.26); p=0.052) and a lower prevalence of greater symptom burden (PR 0.83 (95% CI 0.71, 0.98); p=0.03) 1 year postpartum (Tables 3a and 3b), while those in quintile 5 demonstrated no significant difference in prevalence ratios for either primary outcome.
Table 3a.
Univariate and final multivariable models summarizing prevalence of worse support at 1 year postpartum
| Univariate PR (95% CI) |
P | Multivariable PR (95% CI)* |
P | |
|---|---|---|---|---|
| MVPA postpartum | ||||
| Q (Quintiles) 3,4 vs Q1, 2 (reference) | 1.87 (1.03, 3.4) | 0.041 | 1.80 (1.00, 3.26) | 0.052 |
| Q5 vs Q1, 2 (reference) | 1.34 (0.62, 2.88) | 0.456 | 1.26 (0.58, 2.72) | 0.554 |
| Q 5 vs Q 3, 4 (reference) | 0.72 (0.36, 1.42) | 0.343 | 0.70 (0.35, 1.38) | 0.304 |
| Age, ≥30.4 (age quintiles 4,5) vs <30.4 (age quintiles 1,2,3) |
2.12 (1.26, 3.56) | 0.005 | 2.18 (1.30, 3.66) | 0.003 |
| High-risk delivery factor (yes vs no) | 1.43 (0.86, 2.39) | 0.173 | 1.02 (0.65, 1.59) | 0.933 |
| Ethnicity (Hispanic vs non-Hispanic)# | 0.78 (0.36, 1.67) | 0.518 | 1.00 (0.48, 2.08) | 1.000 |
| Support in 3rd trimester (worse vs better**)# | 4.09 (2.03, 8.24) | <.001 | 3.37 (1.80, 6.29) | <0.001 |
| Education (professional vs college or less) # | 1.36 (0.8, 2.29) | 0.257 | 0.77 (0.42, 1.42) | 0.406 |
| Chronic cough at 5-10 weeks postpartum (yes vs no)*** | 1.3 (0.2, 8.29) | 0.780 | N/A | N/A |
| Breastfeeding at 5-10 weeks postpartum (yes vs no) # | 5 (0.71, 35.35) | 0.107 | 3.55 (0.51, 24.79) | 0.202 |
| Body mass index (BMI) at 5-10 weeks postpartum# | ||||
| 25- <30 vs < 25 kg/m2 | 0.77 (0.44, 1.35) | 0.364 | 0.89 (0.50, 1.58) | 0.692 |
| ≥30 vs < 25 kg/m2 | 0.34 (0.12, 0.94) | 0.037 | 0.38 (0.14, 1.08) | 0.070 |
Final model (n=562) includes multiple imputation on missing values. Prior to imputation, n=533. There were no statistically significant differences in MVPA category according to the frequency of missing responses for any variable.
Worse=maximal vaginal descent ≥ 0 cm; Better = maximal vaginal descent < 0 cm.
Omitted from multivariable model due to small cell counts
The multivariable model based on the directed acyclic graph designed to test the total effect of MVPA on worse support is also appropriate to test the total effect of age and high-risk delivery factor on worse support, but reflects direct or ‘partial’ effects for the other variables after MVPA, a downstream intermediate variable, has been adjusted. The total effects of these variables include indirect effects that may be mediated via MVPA. Therefore, guided by separate DAGs to determine adjustment factors for the other variables in the above model, we created additional models designed to test the total effect of each of the other variables after adjustment. The total effects of these other variables are as follows: Ethnicity: PR 0.96 (95% CI 0.45, 2.03), p=0.91 (adjusted for age, high-risk delivery factor, antenatal support, education, breastfeeding at 5-10 weeks postpartum, BMI at 5-10 weeks postpartum). Antenatal support: PR 3.81 (95% CI 1.99, 7.33), p<0.0001 (adjusted for age, high-risk delivery factor, ethnicity, education, breastfeeding at 5-10 weeks postpartum, BMI at 5-10 weeks postpartum). Education: PR 0.83 (95% CI 0.46, 1.51), p=0.55 (adjusted for age, high-risk delivery factor, antenatal support, ethnicity, breastfeeding at 5-10 weeks postpartum, BMI at 5-10 weeks postpartum). Breastfeeding at 5-10 weeks postpartum: PR 3.4 (95% CI 0.48, 23.91), p=0.22 (adjusted for age, high-risk delivery factor, antenatal support, ethnicity, education, BMI at 5-10 weeks postpartum). BMI at 5-10 weeks postpartum: PR 0.76 (95% CI 0.37, 1.59), p=−.47 for 25- <30 vs < 25 kg/m2, and PR 0.26 (95% CI 0.07, 0.95), p=0.04 for ≥30 vs < 25 kg/m2 (adjusted for age, high-risk delivery factor, antenatal support, ethnicity, breastfeeding at 5-10 weeks postpartum, education, pre-pregnancy BMI).
Table 3b.
Univariate and final multivariable models summarizing prevalence of symptom burden outcome at 1 year postpartum
| Univariate PR (95% CI) |
P | Multivariable PR (95% CI)* |
P | |
|---|---|---|---|---|
| MVPA postpartum | ||||
| Q (Quintiles) 3,4 vs Q1,2 (reference) | 0.81 (0.68, 0.96) | 0.017 | 0.83 (0.71, 0.98) | 0.028 |
| Q5 vs Q1,2 (reference) | 1.08 (0.91, 1.29) | 0.382 | 1.12 (0.94, 1.32) | 0.202 |
| Q 5 vs Q 3, 4 (reference) | 1.33 (1.10, 1.62) | 0.003 | 1.34 (1.11, 1.61) | 0.002 |
| Age, ≥30.4 (age quintiles 4,5) vs <30.4 (age quintiles 1,2,3) |
1.03 (0.96, 1.1) | 0.468 | 1.00 (0.98, 1.01) | 0.686 |
| High-risk delivery factor (yes vs no) | 1.19 (1.03, 1.37) | 0.021 | 1.21 (1.03, 1.42) | 0.019 |
| Ethnicity (Hispanic vs non-Hispanic)# | 0.77 (0.61, 0.97) | 0.026 | 0.80 (0.65, 0.99) | 0.042 |
| SUI, UUI or AI in 3rd trimester (present vs absent)** , # | 1.93 (1.61, 2.33) | <.001 | 1.92 (1.60, 2.30) | <0.001 |
| Education (professional vs college or less) # | 0.95 (0.81, 1.12) | 0.565 | 0.90 (0.76, 1.07) | 0.222 |
| Chronic cough at 5-10 weeks postpartum (yes vs no)*** | 0.52 (0.16, 1.69) | 0.277 | N/A | N/A |
| Breastfeeding at 5-10 weeks postpartum (yes vs no) # | 1.04 (0.81, 1.34) | 0.754 | 0.96 (0.75, 1.23) | 0.754 |
| Body mass index (BMI) at 5-10 weeks postpartum# | ||||
| 25- <30 vs < 25 | 1.05 (0.89, 1.24) | 0.540 | 1.02 (0.87, 1.20) | 0.827 |
| ≥30 vs < 25 | 1.05 (0.86, 1.28) | 0.629 | 1.04 (0.87, 1.25) | 0.647 |
Final model (n=609) includes multiple imputation on missing values. Prior to imputation, n=575. There were no statistically significant differences in MVPA category according to the frequency of missing responses for any variable.
SUI=Stress urinary incontinence; UUI= Urgency urinary incontinence; AI=Anal incontinence
Omitted from multivariable model due to small cell counts
The multivariable model based on the directed acyclic graph designed to test the total effect of MVPA on symptom burden is also appropriate to test the total effect of age and high-risk delivery factor on worse support, but reflects direct or ‘partial’ effects for the other variables after MVPA, a downstream intermediate variable, has been adjusted. The total effects of these variables include indirect effects that may be mediated via MVPA. Therefore, guided by separate DAGs to determine adjustment factors for the other variables in the above model, we created additional models designed to test the total effect of each of the other variables after adjustment. The total effects of these other variables are as follows: Ethnicity: PR 0.80 (95% CI 0.64, 0.99), p=0.04 (adjusted for age, high-risk delivery factor, antenatal SUI, UUI or AI, education, breastfeeding at 5-10 weeks postpartum, BMI at 5-10 weeks postpartum). Antenatal SUI, UUI or AI: PR 1.93 (95% CI 1.60, 2.32), p<0.0001 (adjusted for age, high-risk delivery factor, antenatal support, ethnicity, education, breastfeeding at 5-10 weeks postpartum, BMI at 5-10 weeks postpartum).
Education: PR 0.90 (95% CI 0.76, 1.06), p=0.20 (adjusted for age, high-risk delivery factor, antenatal SUI, UUI or AI, ethnicity, breastfeeding at 5-10 weeks postpartum, BMI at 5-10 weeks postpartum). Breastfeeding at 5-10 weeks postpartum: PR 0.97 (95% CI 0.77, 1.22), p=0.81 (adjusted for age, high-risk delivery factor, antenatal SUI, UUI or AI, ethnicity, education, BMI at 5-10 weeks postpartum). BMI at 5-10 weeks postpartum: PR 1.01 (95% CI 00.84, 1.21), p=0.93 for 25- <30 vs < 25 kg/m2, and PR 1.02 (95% CI 0.75, 1.37), p=0.92 for ≥30 vs < 25 kg/m2 (adjusted for age, high-risk delivery factor, antenatal SUI, UUI or AI, ethnicity, breastfeeding at 5-10 weeks postpartum, education, pre-pregnancy BMI).
In final models, there were no statistical interactions between MVPA and higher-risk for LAM delivery variable on either primary outcome (p=0.85 for support and p=0.31 for symptom burden). There were also no statistical interactions between MVPA and age (p=0.38 for support and p=0.16 for symptom burden).
In multivariablesensitivity analyses, there were no significant associations between MVPA in 5-minute bouts and either worse support or symptom burden (Supplementary Tables 1a and 1b). In additional sensitivity analyses, MVPA at either timepoint did not independently predict either primary outcome (data not shown).
Given the primary findings, we explored variables that might explain the differences in the direction of effect for quintiles 3 and 4 versus quintile 5 of MVPA. As seen in Supplementary Table 2, there were some differences among the 3 groups but women in quintiles 3 and 4 did not report more pelvic loading activities, changes in activity pattern or factors that in theory might dissuade them from doing greater amounts of MVPA.
Final multivariable models revealed no statistically significant associations between MVPA and any of the secondary outcomes (data not shown).
COMMENT
Principal findings
We had hypothesized that greater MVPA in the early postpartum, a period of active healing and regeneration of muscles, ligaments and nerves, would increase the prevalence of worse pelvic floor health one year postpartum. Instead, in this prospective cohort study, we found differential effects of early postpartum MVPA on the primary outcomes. Multivariable analyses of quintiles of MVPA demonstrated an inverted U-shaped pattern of prevalence ratio for the support outcome and a shallow U-shaped pattern for the symptom burden outcome. Compared to women in the lowest two quintiles of MVPA (representing 8.9 to 52.8 minutes/day), those in quintiles 3 and 4 (52.9 to 81.9 minutes/day) had nearly 200% of the prevalence of worse support (though with marginal significance, with p= 0.051) and a 20% lower prevalence of worse symptom burden, while those in the highest quintile (81.9-158.7 minutes/day) demonstrated no significant difference in either outcome. The presence or absence of a high-risk delivery variable did not change the effect of MVPA on either primary outcome. Additionally, MVPA had no significant effect on any of the secondary outcomes.
There are several possible explanations for these findings. First, pelvic floor support tends to be poorly correlated with non-bulge pelvic floor symptoms88 and thus, physical activity may be deleterious in terms of support for some women but not in terms of symptoms, even in the face of acute healing postpartum. Indeed, in a recent report, women that reported exercising ≥ 3 times/week at 6 weeks postpartum were not at increased risk of vaginal bulge symptoms compared to non-exercisers.89 Second, even 2-3 weeks postpartum might be too late to fully test the effect, as substantial wound healing may have already occurred by this point. Third, the amount and intensity of activity may not have been high enough to have an effect. Consistent with other literature on postpartum women, few women engaged in vigorous activity, such as fast running (which would produce ENMO values ≥ the vigorous intensity cutpoint of 428.8 mg). However, given that even elite athletes are unlikely to resume their full volume of pre-pregnancy activities in the first 2-4 weeks postpartum,90,91 if our testing interval was too late after delivery to see an effect, this would have little clinical relevance.
The finding, that compared to the least active women, the most active women do not have an increased prevalence of worse support while those moderately active do, was surprising as one might anticipate a dose-response type of effect. Yet, further exploration of pelvic loading activities and other characteristics did not explain differences in outcomes between women in the 3 MVPA groups. Women in quintile 5 may differ from those in quintiles 3 and 4 in some factor that we were not able to measure that influences both ability to be more active and protection against worse support.
To quantify how strong an uncontrolled variable would have to be, in order to overturn our results, we calculated E-values for confounding92,93 and E-values for selection bias related to drop-outs.94 The E-value for confounding is the strength of association that an uncontrolled confounder must have with both early MVPA and the outcome in order to completely explain away our findings. For example, the E-value for confounding for the effects of MVPA quintiles 3 and 4 vs 1 and 2 on worse support is 3.0, and for symptom burden, 1.67. The E-value for selection bias related to drop-outs (that is, the bias that arises when a risk ratio in a population differs from that risk ratio in the subset of the population available for analysis95 ) addresses the strength of the association that an uncontrolled factor must have with both drop-out and the outcome, at each level of MVPA, in order to completely explain away our findings.94,95 The E-value for selection bias for the effects of MVPA quintiles 3 and 4 vs 1 and 2 on worse support at 1 year postpartum is 2.02; and for symptom burden, 1.41. Thus an uncontrolled factor would have to have considerable strength to overturn our finding for worse support, but only moderate strength to overturn it for worse symptom burden.
Strengths
Our 1-year follow-up rate was similar to or higher than other published studies that followed women between late pregnancy and 1 year postpartum, with rates from 37% to 77% with the exception of Chen who reported 100% adherence between 3rd trimester and 1-year postpartum.22,23,44,68,96,97 We objectively measured physical activity and adherence was high. We assessed total MVPA to better represent total loads on the pelvic floor, but also found no differences in sensitivity analyses of 5-minute bouts of MVPA, which might be more reflective of sustained exercise. By enrolling women during pregnancy, we were able to include pelvic floor symptoms and objectively measured support before delivery in our multivariable models. Consistent with studies of prevention strategies, our a priori analysis plan did not include adjusting for downstream events that occurred after the prevention period (early postpartum).
Weaknesses
While women that enrolled in the study were similar to those eligible who declined participation, there were key differences between participants that completed both accelerometry and 1-year follow-up versus those that did not. Those factors would have to have considerable strength to cause enough selection bias to overturn our finding for worse support, but only moderate strength to overturn our finding for symptom burden. In addition, our results may not be generalizable to other populations, including multiparas or other racial and ethnic groups. At 5-6 weeks postpartum, only about one-third of women in the highest quintile of MVPA reported doing strenuous exercise (Table 2) and thus, our results are not applicable to those competitively active women who seek guidance on resuming high-duration, high-impact and strenuous activity in the very early postpartum. However, the vast majority of women are not highly active or elite athletes and rather need guidance on whether common activities, such as jogging, cycling, swimming, or heavy housework or gardening after vaginal childbirth are safe in terms of pelvic floor health.
While the EPIQ provides thresholds validated for predicting women at high risk of being diagnosed with POP, SUI, OAB and AI, we used symptoms from the EPIQ as outcome measures to investigate experiences of young, postpartum women rather than urogynecologic conditions in middle-aged and older women. Indeed, studying selected pelvic floor symptoms extracted from validated condition-specific questionnaires is common.98-101 However, this approach and in particular our use of a non-validated measure of symptom burden, chosen as no single measure appropriate for our population exists, are limitations of this study.
Though the step output differs between wrist-and waist-worn accelerometers worn during daily life, the correlations between the two are good to high in adults and in postpartum women.102-105 Because compliance is better with accelerometers worn on the wrist compared to the waist or hip, wrist-worn devices are now preferentially used in large population-based studies.106 Accelerometry is likely to under-measure some loads on the pelvic floor. Body acceleration correlates highly with intra-abdominal pressure for dynamic activities, such as running, walking and jumping, but poorly for loading activities with little body movement, such as heavy lifting.107 However, most participants reported no heavy lifting types of activities at either time-point and so it is likely that accelerometry in our population captured most activities.
We used selected concepts and tools of modern causal analytic methods in executing and interpreting our analyses, such as directed acyclic graphs and E-values. In theory, a randomized masked trial, preferably stratifying women with and without levator ani muscle injuries, would provide the highest quality evidence about the effect of MVPA on the pelvic floor, but this approach has both ethical and logistical obstacles. If one hypothesizes, as we did, that higher MVPA during this vulnerable period increases the risk of pelvic floor problems, it would be unethical to randomize women to this group. However, logistical concerns are great as well. It is not possible to mask participants randomized to MVPA vs limited activity to their assigned group. Adherence to physical activity intervention and retention in trials is poor in healthy, inactive postpartum women.108
We based our sample size calculation on the prediction that 15% of the population would demonstrate worse pelvic floor support 1 year postpartum but the proportion that did so was one-third lower. Thus, this study may have been underpowered to explore all comparisons for the support outcome.
Meaning of the study
Our results provide objective data about pelvic floor health that in general support the most recent ACOG guidelines on postpartum PA, which state, “In the absence of medical or surgical complications, rapid resumption of (physical) activities has not been found to result in adverse effects”.109 With the exception of support, which was worse in women with moderately high activity levels in our population, MVPA was either protective or had no effect on other parameters of pelvic floor health.
Unanswered questions
Further research is needed to understand why moderate amounts of MVPA affect support and symptom burden differently in postpartum women. Larger populations are necessary to test whether the effects of MVPA in the early postpartum period differ in women with an objectively measured levator ani injury, pre-existing worse support, pre-existing high levels of fitness, or other factors.
Supplementary Material
CONDENSATION: Early postpartum moderate to vigorous physical activity had variable effects on pelvic floor support and symptoms at one year postpartum.
AJOG AT A GLANCE.
Why was this study conducted?
Risks of pelvic organ prolapse and urinary incontinence rise after the first vaginal delivery.
The early postpartum period, a time of active regeneration and healing of the pelvic floor, may be particularly vulnerable to high volumes of pelvic floor loading.
We aimed to determine whether objectively measured moderate-to-vigorous physical activity (MVPA) in the early postpartum period predicts pelvic floor support and symptom burden (bothersome symptoms in at least 2 domains) 1 year following first vaginal birth.
What are the key findings?
Early postpartum MVPA had differential effects on support and symptoms.
Women with a moderately high amount of physical activity trended toward higher prevalence of worse support but lower prevalence of symptom burden compared to both least active and most active women.
What does this study add to what is already known?
These results provide objective data about the effect of early postpartum physical activity on pelvic floor health amongst primiparous women delivered vaginally.
Further research is needed to understand the differential effect of moderate amounts of MVPA on support versus symptom burden in postpartum women.
Acknowledgment:
We gratefully acknowledge Jingye Yang, MSTAT, whose efforts assisted in producing the E-value analyses.
Sources of funding: The project described was supported by Grant Number 1P01HD080629 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the University of Utah Population Health Research (PHR) Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-05 (formerly 8UL1TR000105 and UL1RR025764). The computational resources used were partially funded by the NIH shared Instrumentation Grant 1S10OD021644-01A1. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Ingrid Nygaard received an honorarium from Elsevier during part of the time that this research was carried out. NONE of the other investigators report competing interests to disclose.
Contributor Information
Ingrid E. NYGAARD, Department of Obstetrics and Gynecology, University of Utah School of Medicine.
Ali WOLPERN, Department of Health and Kinesiology, University of Utah College of Health.
Tyler BARDSLEY, Study Design and Biostatistics Center, University of Utah Health Center for Clinical and Translational Science.
Marlene J. EGGER, Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine.
Janet M. SHAW, Department of Health and Kinesiology, University of Utah College of Health.
References
- 1.Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116(5):1096–1100. [DOI] [PubMed] [Google Scholar]
- 2.Løwenstein E, Ottesen B, Gimbel H. Incidence and lifetime risk of pelvic organ prolapse surgery in Denmark from 1977 to 2009. Int Urogynecol J. 2014. [DOI] [PubMed] [Google Scholar]
- 3.Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risk factors for genital prolapse in non-hysterectomized women around menopause. Results from a large cross-sectional study in menopausal clinics in Italy. Progetto Menopausa Italia Study Group. Eur J Obstet Gynecol Reprod Biol. 2000;93(2):135–140. [PubMed] [Google Scholar]
- 5.Foldspang A, Mommsen S, Lam GW, Elving L. Parity as a correlate of adult female urinary incontinence prevalence. Journal of epidemiology and community health. 1992;46(6):595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006;107(6):1253–1260. [DOI] [PubMed] [Google Scholar]
- 7.Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. British journal of obstetrics and gynaecology. 1997;104(5):579–585. [DOI] [PubMed] [Google Scholar]
- 8.Tegerstedt G, Maehle-Schmidt M, Nyren O, Hammarstrom M. Prevalence of symptomatic pelvic organ prolapse in a Swedish population. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(6):497–503. [DOI] [PubMed] [Google Scholar]
- 9.Moalli PA, Jones Ivy S, Meyn LA, Zyczynski HM. Risk factors associated with pelvic floor disorders in women undergoing surgical repair. Obstet Gynecol. 2003;101(5 Pt 1):869–874. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson EC, Victor FT, Tibblin G, Svardsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299–305. [DOI] [PubMed] [Google Scholar]
- 11.Gürel H, Gürel SA. Pelvic relaxation and associated risk factors: the results of logistic regression analysis. Acta Obstet Gynecol Scand. 1999;78(4):290–293. [DOI] [PubMed] [Google Scholar]
- 12.Rinne KM, Kirkinen PP. What predisposes young women to genital prolapse? Eur J Obstet Gynecol Reprod Biol. 1999;84(1):23–25. [DOI] [PubMed] [Google Scholar]
- 13.Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG. 2013;120(2):152–160. [DOI] [PubMed] [Google Scholar]
- 14.Volløyhaug I, Mørkved S, Salvesen Ø, Salvesen K. Pelvic organ prolapse and incontinence 15-23 years after first delivery: a cross-sectional study. BJOG. 2015;122(7):964–971. [DOI] [PubMed] [Google Scholar]
- 15.Wesnes SL, Hunskaar S, Bo K, Rortveit G. The effect of urinary incontinence status during pregnancy and delivery mode on incontinence postpartum. A cohort study. BJOG. 2009;116(5):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. The prevalence of urinary incontinence 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG. 2013;120(2):144–151. [DOI] [PubMed] [Google Scholar]
- 17.Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjälmås K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62(4 Suppl 1):16–23. [DOI] [PubMed] [Google Scholar]
- 18.Dannecker C, Lienemann A, Fischer T, Anthuber C. Influence of spontaneous and instrumental vaginal delivery on objective measures of pelvic organ support: assessment with the pelvic organ prolapse quantification (POPQ) technique and functional cine magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol. 2004;115(1):32–38. [DOI] [PubMed] [Google Scholar]
- 19.Handa VL, Nygaard I, Kenton K, et al. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(12):1407–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sze EH, Sherard GB, Dolezal JM. Pregnancy, labor, delivery, and pelvic organ prolapse. Obstet Gynecol. 2002;100(5 Pt 1):981–986. [DOI] [PubMed] [Google Scholar]
- 21.O'Boyle AL, O'Boyle JD, Calhoun B, Davis GD. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(1):69–72; discussion 72. [DOI] [PubMed] [Google Scholar]
- 22.Diez-Itza I, Arrue M, Ibañez L, Paredes J, Murgiondo A, Sarasqueta C. Influence of mode of delivery on pelvic organ support 6 months postpartum. Gynecol Obstet Invest. 2011;72(2):123–129. [DOI] [PubMed] [Google Scholar]
- 23.Elenskaia K, Thakar R, Sultan AH, Scheer I, Onwude J. Effect of childbirth on pelvic organ support and quality of life: a longitudinal cohort study. Int Urogynecol J. 2012. [DOI] [PubMed] [Google Scholar]
- 24.Wai CY, McIntire DD, Atnip SD, Schaffer JI, Bloom SL, Leveno KJ. Urodynamic indices and pelvic organ prolapse quantification 3 months after vaginal delivery in primiparous women. Int Urogynecol J. 2011;22(10):1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Delft K, Sultan A, Thakar R, Schwertner-Tiepelmann N, Kluivers K. The relationship between postpartum levator ani muscle avulsion and signs and symptoms of pelvic floor dysfunction. BJOG. 2014;121(9):1164–1172. [DOI] [PubMed] [Google Scholar]
- 26.Bortolini MA, Drutz HP, Lovatsis D, Alarab M. Vaginal delivery and pelvic floor dysfunction: current evidence and implications for future research. Int Urogynecol J. 2010;21(8):1025–1030. [DOI] [PubMed] [Google Scholar]
- 27.Brown S, Gartland D, Perlen S, McDonald E, MacArthur C. Consultation about urinary and faecal incontinence in the year after childbirth: a cohort study. BJOG. 2014. [DOI] [PubMed]
- 28.Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89(12):1511–1522. [DOI] [PubMed] [Google Scholar]
- 29.Reimers C, Staer-Jensen J, Siafarikas F, Saltyte-Benth J, Bø K, Ellström Engh M. Change in pelvic organ support during pregnancy and the first year postpartum: a longitudinal study. BJOG. 2016;123(5):821–829. [DOI] [PubMed] [Google Scholar]
- 30.Handa VL, Blomquist JL, Roem J, Muňoz A. Longitudinal study of quantitative changes in pelvic organ support among parous women. Am J Obstet Gynecol. 2018;218(3):320.e321–320.e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers RG, Leeman LM, Borders N, et al. Contribution of the second stage of labour to pelvic floor dysfunction: a prospective cohort comparison of nulliparous women. BJOG. 2014;121(9):1145–1153; discussion 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallock JL, Handa VL. The Epidemiology of Pelvic Floor Disorders and Childbirth: An Update. Obstet Gynecol Clin North Am. 2016;43(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLancey JO, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. [DOI] [PubMed] [Google Scholar]
- 35.Heilbrun ME, Nygaard IE, Lockhart ME, et al. Correlation between levator ani muscle injuries on magnetic resonance imaging and fecal incontinence, pelvic organ prolapse, and urinary incontinence in primiparous women. American journal of obstetrics and gynecology. 2010;202(5):488 e481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kearney R, Miller JM, Ashton-Miller JA, DeLancey JO. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107(1):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caudwell-Hall J, Kamisan Atan I, Martin A, et al. Intrapartum predictors of maternal levator ani injury. Acta Obstet Gynecol Scand. 2017;96(4):426–431. [DOI] [PubMed] [Google Scholar]
- 38.Rahmanou P, Caudwell-Hall J, Kamisan Atan I, Dietz HP. The association between maternal age at first delivery and risk of obstetric trauma. Am J Obstet Gynecol. 2016;215(4):451.e451–457. [DOI] [PubMed] [Google Scholar]
- 39.Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;109(6):1396–1403. [DOI] [PubMed] [Google Scholar]
- 40.Lonnée-Hoffmann RA, Salvesen Ø, Mørkved S, Schei B. Self-reported pelvic organ prolapse surgery, prevalence, and nonobstetric risk factors: findings from the Nord Trøndelag Health Study. Int Urogynecol J. 2015;26(3):407–414. [DOI] [PubMed] [Google Scholar]
- 41.Miedel A, Tegerstedt G, Maehle-Schmidt M, Nyren O, Hammarstrom M. Nonobstetric risk factors for symptomatic pelvic organ prolapse. Obstet Gynecol. 2009;113(5):1089–1097. [DOI] [PubMed] [Google Scholar]
- 42.Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG. 2013;120(2):152–160. [DOI] [PubMed] [Google Scholar]
- 43.Bradley CS, Erickson BA, Messersmith EE, et al. Evidence of the Impact of Diet, Fluid Intake, Caffeine, Alcohol and Tobacco on Lower Urinary Tract Symptoms: A Systematic Review. J Urol. 2017;198(5):1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urbankova I, Grohregin K, Hanacek J, et al. The effect of the first vaginal birth on pelvic floor anatomy and dysfunction. Int Urogynecol J. 2019;30(10):1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nygaard IE, Clark E, Clark L, et al. Physical and cultural determinants of postpartum pelvic floor support and symptoms following vaginal delivery: a protocol for a mixed-methods prospective cohort study. BMJ Open. 2017;7(1):e014252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobak WH, Rosenberger K, Walters MD. Interobserver variation in the assessment of pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7(3):121–124. [DOI] [PubMed] [Google Scholar]
- 47.Hall AF, Theofrastous JP, Cundiff GW, et al. Interobserver and intraobserver reliability of the proposed International Continence Society, Society of Gynecologic Surgeons, and American Urogynecologic Society pelvic organ prolapse classification system. American journal of obstetrics and gynecology. 1996;175(6):1467–1470; discussion 1470-1461. [DOI] [PubMed] [Google Scholar]
- 48.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. American journal of obstetrics and gynecology. 1996;175(1):10–17. [DOI] [PubMed] [Google Scholar]
- 49.Lukacz ES, Lawrence JM, Buckwalter JG, Burchette RJ, Nager CW, Luber KM. Epidemiology of prolapse and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):272–284. [DOI] [PubMed] [Google Scholar]
- 50.Drossman DA, Sandler RS, McKee DC, Lovitz AJ. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology. 1982;83(3):529–534. [PubMed] [Google Scholar]
- 51.Roovers JP, van der Bom JG, van der Vaart CH, Group HS. Hysterectomy does not cause constipation. Dis Colon Rectum. 2008;51(7):1068–1072; discussion 1072-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanley J, Capewell A, Hagen S. Validity study of the severity index, a simple measure of urinary incontinence in women. BMJ. 2001;322(7294):1096–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):520–524. [DOI] [PubMed] [Google Scholar]
- 54.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 56.Dolan SH, Williams DP, Ainsworth BE, Shaw JM. Development and reproducibility of the bone loading history questionnaire. Med Sci Sports Exerc. 2006;38(6):1121–1131. [DOI] [PubMed] [Google Scholar]
- 57.Wolpern AE, Sherwin KJ, Moss WD, et al. Compliance with wrist-worn accelerometers in primiparous early postpartum women. Heliyon. 2019;5(1):e01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. Br J Sports Med. 2014;48(13):1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003-2006. Prev Chronic Dis. 2012;9:E113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowlands AV, Harrington DM, Bodicoat DH, et al. Compliance of Adolescent Girls to Repeated Deployments of Wrist-Worn Accelerometers. Med Sci Sports Exerc. 2018;50(7):1508–1517. [DOI] [PubMed] [Google Scholar]
- 61.van Hees VT, Gorzelniak L, Dean León EC, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS One. 2013;8(4):e61691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Hees VT, Fang Z, Langford J, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol (1985). 2014;117(7):738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rowlands AV, Cliff DP, Fairclough SJ, et al. Moving Forward with Backward Compatibility: Translating Wrist Accelerometer Data. Med Sci Sports Exerc. 2016;48(11):2142–2149. [DOI] [PubMed] [Google Scholar]
- 64.Bakrania K, Yates T, Rowlands AV, et al. Intensity Thresholds on Raw Acceleration Data: Euclidean Norm Minus One (ENMO) and Mean Amplitude Deviation (MAD) Approaches. PLoS One. 2016;11(10):e0164045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabia S, van Hees VT, Shipley MJ, et al. Association between questionnaire- and accelerometer-assessed physical activity: the role of sociodemographic factors. Am J Epidemiol. 2014;179(6):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hildebrand M, VAN Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46(9):1816–1824. [DOI] [PubMed] [Google Scholar]
- 67.Rowlands AV, Yates T, Davies M, Khunti K, Edwardson CL. Raw Accelerometer Data Analysis with GGIR R-package: Does Accelerometer Brand Matter? Med Sci Sports Exerc. 2016;48(10):1935–1941. [DOI] [PubMed] [Google Scholar]
- 68.Low LK, Zielinski R, Tao Y, Galecki A, Brandon CJ, Miller JM. Predicting Birth-Related Levator Ani Tear Severity in Primiparous Women: Evaluating Maternal Recovery from Labor and Delivery (EMRLD Study). Open J Obstet Gynecol. 2014;4(6):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 70.Cummings P Methods for estimating adjusted risk ratios. The Stata Journal. 2009;9(2):175–196. [Google Scholar]
- 71.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. [DOI] [PubMed] [Google Scholar]
- 72.Sung VW. Reducing bias in pelvic floor disorders research: using directed acyclic graphs as an aid. Neurourol Urodyn. 2012;31(1):115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saltzman HM, Egger MJ, Bardsley T, Ding Q, Shaw JM, Nygaard IE. Differences in Pelvic Floor Symptoms During Pregnancy Between Hispanic and Non-Hispanic White Women. Female pelvic medicine & reconstructive surgery. 2018. [DOI] [PMC free article] [PubMed]
- 75.Viktrup L, Lose G. The risk of stress incontinence 5 years after first delivery. Am J Obstet Gynecol. 2001;185(1):82–87. [DOI] [PubMed] [Google Scholar]
- 76.Gartland D, MacArthur C, Woolhouse H, McDonald E, Brown SJ. Frequency, severity and risk factors for urinary and faecal incontinence at 4 years postpartum: a prospective cohort. BJOG. 2016;123(7):1203–1211. [DOI] [PubMed] [Google Scholar]
- 77.Viktrup L, Rortveit G, Lose G. Risk of stress urinary incontinence twelve years after the first pregnancy and delivery. Obstet Gynecol. 2006;108(2):248–254. [DOI] [PubMed] [Google Scholar]
- 78.Durnea CM, Khashan AS, Kenny LC, et al. What is to blame for postnatal pelvic floor dysfunction in primiparous women-Pre-pregnancy or intrapartum risk factors? Eur J Obstet Gynecol Reprod Biol. 2017;214:36–43. [DOI] [PubMed] [Google Scholar]
- 79.Jelovsek JE, Chagin K, Gyhagen M, et al. Predicting risk of pelvic floor disorders 12 and 20 years after delivery. Am J Obstet Gynecol. 2018;218(2):222.e221–222.e219. [DOI] [PubMed] [Google Scholar]
- 80.Johannessen HH, Wibe A, Stordahl A, Sandvik L, Backe B, Mørkved S. Prevalence and predictors of anal incontinence during pregnancy and 1 year after delivery: a prospective cohort study. BJOG. 2014;121(3):269–279. [DOI] [PubMed] [Google Scholar]
- 81.Nordenstam J, Altman D, Brismar S, Zetterström J. Natural progression of anal incontinence after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(9):1029–1035. [DOI] [PubMed] [Google Scholar]
- 82.Johannessen HH, Stafne SN, Falk RS, Stordahl A, Wibe A, Mørkved S. Prevalence and predictors of anal incontinence 6 years after first delivery. Neurourol Urodyn. 2019;38(1):310–319. [DOI] [PubMed] [Google Scholar]
- 83.Chan SS, Cheung RY, Yiu KW, Lee LL, Chung TK. Prevalence of urinary and fecal incontinence in Chinese women during and after their first pregnancy. Int Urogynecol J. 2013;24(9):1473–1479. [DOI] [PubMed] [Google Scholar]
- 84.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: Wiley; 2000. [Google Scholar]
- 85.Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol. 2010;171(5):624–632. [DOI] [PubMed] [Google Scholar]
- 86.van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. [DOI] [PubMed] [Google Scholar]
- 87.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 88.Broekhuis SR, Fütterer JJ, Hendriks JC, Barentsz JO, Vierhout ME, Kluivers KB. Symptoms of pelvic floor dysfunction are poorly correlated with findings on clinical examination and dynamic MR imaging of the pelvic floor. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(10):1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tennfjord MK, Engh ME, Bo K. The Influence of Early Exercise Postpartum on Pelvic Floor Muscle Function and Prevalence of Pelvic Floor Dysfunction 12 Months Postpartum. Phys Ther. 2020. [DOI] [PubMed]
- 90.Sundgot-Borgen J, Sundgot-Borgen C, Myklebust G, Sølvberg N, Torstveit MK. Elite athletes get pregnant, have healthy babies and return to sport early postpartum. BMJ Open Sport Exerc Med. 2019;5(1):e000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tenforde AS, Toth KE, Langen E, Fredericson M, Sainani KL. Running habits of competitive runners during pregnancy and breastfeeding. Sports Health. 2015;7(2):172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web Site and R Package for Computing E-values. Epidemiology. 2018;29(5):e45–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 94.Smith LH, VanderWeele TJ. Bounding Bias Due to Selection. Epidemiology. 2019;30(4):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hernán MA. Invited Commentary: Selection Bias Without Colliders. Am J Epidemiol. 2017;185(11):1048–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Delft K, Thakar R, Sultan AH, IntHout J, Kluivers KB. The natural history of levator avulsion one year following childbirth: a prospective study. BJOG. 2015;122(9):1266–1273. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y, Li FY, Lin X, Chen J, Chen C, Guess MK. The recovery of pelvic organ support during the first year postpartum. BJOG. 2013;120(11):1430–1437. [DOI] [PubMed] [Google Scholar]
- 98.Handa VL, Muňoz A, Blomquist JL. Temporal relationship between posterior vaginal prolapse and defecatory symptoms. Am J Obstet Gynecol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rutledge TL, Heckman SR, Qualls C, Muller CY, Rogers RG. Pelvic floor disorders and sexual function in gynecologic cancer survivors: a cohort study. Am J Obstet Gynecol. 2010;203(5):514.e511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gustilo-Ashby AM, Paraiso MF, Jelovsek JE, Walters MD, Barber MD. Bowel symptoms 1 year after surgery for prolapse: further analysis of a randomized trial of rectocele repair. Am J Obstet Gynecol. 2007;197(1):76.e71–75. [DOI] [PubMed] [Google Scholar]
- 101.Karjalainen PK, Mattsson NK, Nieminen K, Tolppanen AM, Jalkanen JT. The relationship of defecation symptoms and posterior vaginal wall prolapse in women undergoing pelvic organ prolapse surgery. Am J Obstet Gynecol. 2019;221(5):480.e481–480.e410. [DOI] [PubMed] [Google Scholar]
- 102.Tudor-Locke C, Barreira TV, Schuna JM. Comparison of step outputs for waist and wrist accelerometer attachment sites. Med Sci Sports Exerc. 2015;47(4):839–842. [DOI] [PubMed] [Google Scholar]
- 103.Ozemek C, Kirschner MM, Wilkerson BS, Byun W, Kaminsky LA. Intermonitor reliability of the GT3X+ accelerometer at hip, wrist and ankle sites during activities of daily living. Physiol Meas. 2014;35(2):129–138. [DOI] [PubMed] [Google Scholar]
- 104.Kamada M, Shiroma EJ, Harris TB, Lee IM. Comparison of physical activity assessed using hip- and wrist-worn accelerometers. Gait Posture. 2016;44:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hesketh KR, Evenson KR, Stroo M, Clancy SM, Østbye T, Benjamin-Neelon SE. Physical activity and sedentary behavior during pregnancy and postpartum, measured using hip and wrist-worn accelerometers. Prev Med Rep. 2018;10:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doherty A, Jackson D, Hammerla N, et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS One. 2017;12(2):e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Gennaro JD, de Gennaro CK, Shaw JM, Petelenz TJ, Nygaard IE, Hitchcock RW. The Relationship Between Intra-Abdominal Pressure and Body Acceleration During Exercise. Female Pelvic Med Reconstr Surg. 2019;25(3):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gilinsky AS, Dale H, Robinson C, Hughes AR, McInnes R, Lavallee D. Efficacy of physical activity interventions in post-natal populations: systematic review, meta-analysis and content coding of behaviour change techniques. Health Psychol Rev. 2015;9(2):244–263. [DOI] [PubMed] [Google Scholar]
- 109.ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol. 2015;126(6):e135–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


