Abstract
Racial disparities in colorectal cancer (CRC) incidence are widely documented. There are two potential mechanisms for these disparities: differences in access to screening, including screening follow-up, and differences in underlying risk of CRC. We reviewed the literature for evidence of these two mechanisms. We show that higher CRC incidence in blacks relative to whites emerged only after the dissemination of screening and describe evidence of racial disparities in screening rates. In contrast to the strong evidence for differences in CRC screening utilization, there is limited evidence for racial differences in adenoma prevalence. In general, black and white patients who are screened have similar adenoma prevalence, though there is some evidence that advanced adenomas and adenomas in the proximal colon are somewhat more likely in black than white patients. We conclude that higher rates of CRC incidence among black patients are primarily driven by lower rates of CRC screening. Our findings highlight the need to increase black patients’ access to quality screening to reduce CRC incidence and mortality.
Introduction
Racial disparities in colorectal cancer (CRC) incidence and mortality are well known and widely documented. Relative to non-Hispanic whites (‘whites’), non-Hispanic blacks (‘blacks’) tend to have higher rates of CRC incidence1, earlier age at diagnosis2–4, later stage at diagnosis5, and worse stage-specific CRC mortality6–9. These disparities have resulted in calls for earlier initiation of CRC screening for black patients10,11, especially black men12.
CRC screening first became available in 196713. In the late 1980s and early 1990s, randomized trials demonstrated the effectiveness of screening to reduce mortality14–16, leading to CRC screening guidelines in 199517,18. Currently available CRC screening tests include stool tests (guaiac-based fecal occult blood tests (FOBT), fecal immunochemical tests (FIT), and stool DNA tests), flexible sigmoidoscopy, colonoscopy, and computed tomographic colonography. Colonoscopy is both a screening test and a diagnostic test used to evaluate symptoms and follow-up abnormal results from other tests. Screening colonoscopy has been shown to reduce both incidence and mortality, with incidence reductions due to primary prevention via the removal of adenomas19. As recently as 2008, all average-risk individuals were recommended to begin screening at age 5020,21. In 2009, the American College of Gastroenterology recommended that average-risk African Americans begin screening at age 4522, citing the “high incidence and early onset of colorectal cancer in African Americans”10. The Institute for Clinical Systems Improvement made a similar recommendation in 201023. In 2012, the American College of Physicians recommended that African Americans begin screening at age 4023. In 2017, the U.S. Multi-Society Task Force on Colorectal Cancer suggested that African Americans begin screening at age 45, but noted that the evidence to support this recommendation was of low quality24. In 2018, the American Cancer Society issued a qualified recommendation that all individuals begin screening at age 4525.
Black-White Differences in CRC Incidence
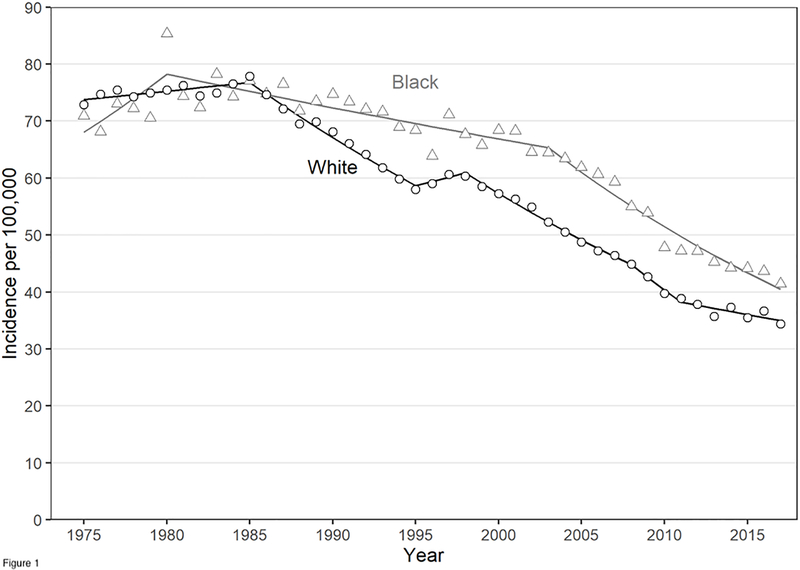
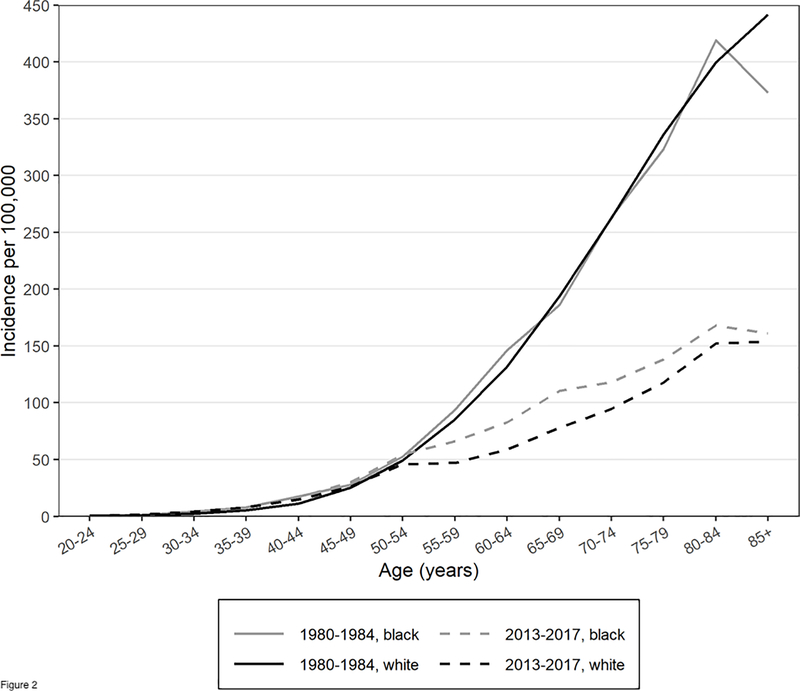
Before 1985, blacks and whites had similar overall CRC incidence rates and similar age-specific CRC incidence. Figure 1 shows overall CRC incidence rates for blacks and whites based on Surveillance, Epidemiology, and End Results (SEER) Program Data (estimation details are provided in the Appendix)26,27. In 1985, President Ronald Reagan’s diagnosis with CRC raised awareness about CRC28. After 1985, CRC incidence began to decline, especially in whites. The reasons for this decline are uncertain, but may be attributed to both changes in modifiable risk factors and increased screening29. Figure 2, which is similar to an analyses of SEER data by Murphy and colleagues30, shows that from 1980 to 1984 blacks and whites had similar age-specific CRC incidence rates, but in 2013–2017 black/white age-specific CRC incidence begins to diverge around age 50, the recommended age to initiate CRC screening.
Figure 1.
Colorectal cancer incidence per 100,000 by race from 1975–2017. Datapoints are from the SEER 9 Registry and the trend line is from a Joinpoint regression analysis.
Figure 2.
Colorectal cancer incidence per 100,000 by race and time period, from the SEER 9 Registry.
Two mechanisms could explain racial disparities in CRC incidence. Disparities could be attributable to differences in CRC screening utilization6,31,32, or differences in risk33,34. Because most CRC arises through the adenoma-carcinoma pathway35, we use adenoma prevalence as a proxy for CRC risk but do not examine mechanisms underlying risk differences. Differences in risk could be driven by racial differences in modifiable risk factors such as diet, smoking, exercise, and obesity36, by genetic differences10,33, or by the interplay between modifiable and genetic risk factors. In this paper we review the literature for evidence of these two mechanisms.
Materials and Methods
We searched the PubMed and Web of Science databases to identify articles focused on differential risk of CRC by race and ethnicity or utilization of CRC screening (details, including search terms, are provided in the Appendix). We also searched for citations to two articles focused on racial differences in risk37,38. Our search identified 2,085 articles. This review includes information from 17 articles that reported screening outcomes (16 articles) or behaviors (14 articles) separately for both black and white US study participants and included at least 100 participants of either black or white race. Screening outcomes included adenoma prevalence, screen-detected CRC rates, interval CRC rates, and screening behaviors (percent up-to-date with guidelines, percent who received a test, and percent who return for repeat testing). We excluded studies reporting only polyp prevalence, rather than adenoma prevalence, as polyps include lesions that may not harbor malignant potential.
Results
Evidence for Racial Differences in CRC Screening Utilization
CRC screening includes multiple steps: initiation of screening, receipt of a follow-up colonoscopy after an abnormal referral test (e.g., after an abnormal stool-based test or flexible sigmoidoscopy), repeat screening after a normal test, and colonoscopy surveillance after adenoma detection.
Table 1 compares screening rates of black and white participants at different steps in the screening process. In addition to observed percentages, Table 1 includes unadjusted odds ratios (ORs) and relative risks (RRs), and adjusted ORs (aORs) and adjusted RRs (aRR). Each study adjusted for somewhat different factors. When multiple adjusted estimates were provided, we focused on estimates that adjusted for factors common across studies: age, sex, income and education.
Table 1.
Evidence for Racial Differences in CRC Screening Utilization
| Unadjusted Percentage With the Screening Behavior | Odds Ratios and Risk Ratios* Black Relative to White With 95% Confidence Intervals |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Black |
White |
||||||||
| Outcome/data source | Data Type | Data Collection Years | Age Range (Years) | % | N | % | N | Unadjusted | Adjusted** |
| Up-to-date with CRC Screening Guidelines | |||||||||
| Based on receipt of FOBT | |||||||||
| NHIS (Anderson, 1995)39† | Survey | 1987 | >=50 | 15.8 | — | 22.4 | — | — | — |
| NHIS (Anderson, 1995)39† | Survey | 1992 | >=50 | 22.4 | — | 26.6 | — | — | — |
| MCBS (Doubeni, 2009)40 | Survey | 2000 | 65–80 | — | 761 | — | 6,705 | 0.56 (0.42,0.74) | 0.84 (0.62,1.13) |
| MCBS (Doubeni, 2009)40 | Survey | 2005 | 65–80 | — | 636 | — | 6,023 | 0.67 (0.45,1.01) | 0.80 (0.54,1.20) |
| Based on receipt of endoscopy | |||||||||
| MCBS (Doubeni, 2009)40 | Survey | 2000 | 65–80 | — | 761 | — | 6,705 | 0.70 (0.57,0.86) | 1.06 (0.88,1.30) |
| MCBS (Doubeni, 2009)40 | Survey | 2005 | 65–80 | — | 636 | — | 6,023 | 0.74 (0.57,0.96) | 1.11 (0.86,1.45) |
| Based on receipt of FOBT/FIT or endoscopy | |||||||||
| MEPS (Jerant, 2008)41 | Survey | 2001–2005 | >=50 | 48.2 | 2,809 | 57.2 | 14,823 | — | 0.86 (0.77,0.95) |
| VHA (Burgess, 2010)42 | Survey, EHR | 2005–2006 | 50–75 | 72 | 328 | 77 | 1,827 | 0.74 — | 0.91 — |
| BRFSS (Liss, 2014)43 | Survey | 2010 | 50–75 | 59.0 | — | 62.0 | — | 0.96 (0.94,0.98) | 1.02 (1.00,1.04) |
| NHIS (Sauer, 2018)44 | Survey | 2010–2013 | 50–75 | 56.8 | 3,567 | 59.8 | 14,527 | — | — |
| BRFSS (Sauer, 2018)44 | Survey | 2012–2014 | 50–75 | 67.5 | 34,191 | 67.1 | 362,721 | — | — |
| Health Center Patient Survey (Lee, 2020)45 | Survey | 2014 | >=50 | 61.3 | — | 58.6 | — | 1.07 — | 1.28 (1.02,1.60) |
| BRFSS (May, 2019)46 | Survey | 2008 | 50–75 | 60.6 | 13,830 | 63.9 | 157,150 | — | — |
| BRFSS (May, 2019) | Survey | 2010 | 50–75 | 64.4 | 16,871 | 66.2 | 180,961 | — | — |
| BRFSS (May, 2019) | Survey | 2012 | 50–75 | 65.8 | 17,978 | 67.4 | 180,654 | — | — |
| BRFSS (May, 2019) | Survey | 2014 | 50–75 | 67.8 | 15,943 | 68.4 | 180,150 | — | — |
| BRFSS (May, 2019) | Survey | 2016 | 50–75 | 66.4 | 17,518 | 70.4 | 186,331 | — | — |
| Receipt of CRC Screening | |||||||||
| Screening colonoscopy | |||||||||
| National Colonoscopy Study (Mendelsohn, 2017)32† | RCT | 2000–2002 2004–2007 |
40–69 | 77.9 | 245 | 82.9 | 240 | 0.94 (0.86,1.02) | 1.03 (0.96,1.11) |
| FOBT or endoscopy | |||||||||
| TRICARE (Changoor, 2018)48† | Claims | 2010–2013 | 50–53 | 56.5 | 5,062 | 53.5 | 17,258 | 1.14 (1.06,1.23) | 1.20 (1.11,1.29) |
| FIT | |||||||||
| ADVANCE (Huguet, 2019)49 | EHR | 2012–2013 | 50–64 | 10.5 | 32,101 | 9.2 | 76,344 | — | — |
| ADVANCE (Huguet, 2019)49 | EHR | 2014–2015 | 50–64 | 17.9 | 32,936 | 15.4 | 81,006 | — | — |
| Complete Screening Episode | |||||||||
| PROSPR (Burnett-Hartman, 2016)50 | EHR | 2010–2012 | 50–75 | 50.9 | 157,999 | 56.2 | 938,295 | — | 0.89 (0.88,0.91) |
| Follow-up Colonoscopy after Referral | |||||||||
| PLCO, 1st screening (Laiyemo, 2010)31 | RCT | 1993–2001 | 55–74 | 62.6 | 767 | 72.4 | 13,743 | — | 0.88 (0.83, 0.93) |
| PLCO, 2nd screening (Laiyemo, 2015)51 | RCT | 2009–2011 | 58–79 | 76.6 | 304 | 83.1 | 4,183 | — | 0.90 (0.84, 0.96) |
| VHA (Partin, 2017)52 | EHR | 2009–2011 | 50–85 | 55 | 13,618 | 48 | 49,692 | 1.33 (1.28,1.39) | 1.19 (1.14,1.25) |
| PROSPR (Burnett-Hartman, 2016)50 | EHR | 2010–2012 | 50–75 | 51.9 | 9,954 | 56.2 | 56,298 | — | 0.94 (0.88,1.00) |
| Repeat Screening | |||||||||
| PLCO, 2nd screening (Laiyemo, 2015)51 | RCT | 2009–2011 | 58–79 | 60.6 | 1609 | 69.2 | 31,117 | — | — |
| PROSPR, surveillance colonoscopy (Chubak, 2020)53 | EHR | 2010–2014 | 50–89 | 50.3 | — | 47.4 | — | — | 1.17 (0.99,1.39) |
Abbreviations: ADVANCE: Accelerating Data Value Across a National Community health center network; BRFSS: Behavioral Risk Factors Surveillance System; EHR: Electronic Health Record; FOBT: fecal occult blood test; FIT: fecal immunochemical test; NHIS: National Health Interview Survey; MCBS: Medicare Current Beneficiary Survey; PLCO: Prostate, Lung, Colorectal and Ovarian; PROSPR: Population-based Research to Optimize the Screening Process; RCT: Randomized Controlled Trial; SEER: VHA: Veterans Health Affairs.
Risk ratios are provided in italics to distinguish them from odds ratios.
Characteristics adjusted for in aOR and aRR estimates: MCBS40: age, sex, education, income, insurance type, usual place of health care, marital status, body mass index (BMI), census division, residence in a metropolitan service area, delayed care due to cost, language of the interview, self-reported general health status, and history of non–skin cancer; MEPS41: age, sex, education, and income; VHA (Burgess)42: age, education, income, family history of CRC, overall health, comorbidities, substance or psychiatric diagnoses; BRFSS (Liss)43: age, sex, education, income, insurance coverage, usual source of care, checkup in past year; National Colonoscopy Study31: age, sex, and education; TRICARE48: sex, marital status, beneficiary category (retired, dependent, active duty, other), self or sponsor rank (enlisted, officer, other), type of care facility (military or civilian), self or sponsor service (Air Force, Army, Navy, Coast Guard, Marine, other), region (South, Midwest, Northeast, West); PROSPR (Burnette-Hartman)50: age, sex, income, insurance type, length of prior enrollment, type of residence, comorbidity, and study site; PLCO, first screening31: age, sex, education, BMI, smoking status, family history of CRC, history of CRC within 3 years of enrollment, history of colon polyps, and screening center; PLCO, second screening51: age, sex, education, BMI, smoking status, family history of CRC, year of repeat flexible sigmoidoscopy and screening center; VA (Partin)52: age, comorbidity, prior colonoscopy, personal history of polyps, ordering physician, ordering facility. PROSPR (Chubak)53: age, sex, index colonoscopy findings, health system, insurance type, comorbidity index;
People of Hispanic ethnicity were included in both black and white groups.
Up-to-date with CRC Screening Guidelines
Most studies examined whether individuals were up-to-date with CRC screening guidelines in place for average-risk individuals at the time of the study, generally defined as having a FOBT or FIT in the last one to three years, receipt of flexible sigmoidoscopy in the last five years, or colonoscopy in the last ten years.
There is evidence that for many years, blacks were less likely than whites to be up-to-date with CRC screening guidelines, and that these differences may be narrowing. In 1987 when the National Health Interview Survey (NHIS) first asked about CRC screening, 15.8% of blacks and 22.4% of whites reported having completed a FOBT in the last 3 years39. Analysis of the 2000, 2003, and 2005 Medicare Current Beneficiary Surveys (MCBS) found that black beneficiaries were less likely than whites to be up-to-date with either FOBT or endoscopic screening, and that these differences could be accounted for by differences in education, income, marital status, type of health insurance, usual place of health care, and self-reported general health status40. Three studies of CRC screening in 2001 to 2010 also found that blacks were less likely to be up-to-date with screening than whites, and that these differences could largely be accounted for by differences in education, income, and health insurance40–43. Analysis of 2010–2013 NHIS data found that the percentage of adults 50 to 75 who were up-to-date with CRC screening was lower for blacks (56.8%, 95% CI (54.4%,59.0%)) compared to whites (59.8%, 95% CI (58.8,60.8))44. Analysis of 2012–2014 Behavioral Risk Factor Surveillance System (BRFSS) survey data found similar rates of being up-to-date with screening for blacks (67.5%, 95% CI (66.6%,68.5%)) and whites (67.1%, 95% CI (66.8%,67.4%) ) 44. A 2014 patient survey found that blacks were more likely than whites to be up-to-date with screening45. In this study, blacks were somewhat more likely to be insured than whites (77.8% versus 75.5%). Analysis of BRFSS data demonstrates steady increases in the percent of both blacks and whites who are up-to-date with screening, with black/white differences narrowing until 2014 when increases stalled among blacks but continued among whites46.
Receipt of CRC Screening
Receipt of CRC screening is a simpler outcome than being up-to-date with screening, because it is based solely on test completion and not on past testing. Studies examining receipt of screening were based on randomized controlled trials (RCT), claims, or electronic health record (EHR) data.
Evidence about black versus white differences in receipt of CRC screening is mixed. The National Colonoscopy Study47 found no significant difference in the likelihood of undergoing screening colonoscopy for black and white participants32. A study of patients insured by TRICARE in 2010–2013 found that blacks were more likely than whites to be screened48. Analysis of data from the Accelerating Data Value Across a National Community (ADVANCE) network of community health centers found no black/white differences in receipt of FOBT from 2012–201549. However, during approximately the same time period the Population-based Research to Optimize the Screening Process (PROSPR) study, which focused on members of a managed care organization, found that black patients were significantly less likely than white patients to complete CRC screening, including follow-up colonoscopy when indicated50.
Three studies estimated black/white differences in receipt of follow-up colonoscopy after an abnormal referral test and estimated differences after adjusting for age, sex, comorbidity, and personal history of colon polyps (among other factors). The Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial used flexible sigmoidoscopy as a referral test and found that black participants were significantly less likely than whites to return for follow-up colonoscopy within one year of referral at both the first31 and second51 screening rounds. In contrast, among Veterans Health Affairs (VHA) patients in 2009–2011, black patients were significantly more likely than white patients to have follow-up colonoscopy within 6 months of an abnormal stool test52. Finally, analysis of PROSPR data found that black patients were less likely than whites to have follow-up colonoscopy within 90 days of an abnormal stool test50.
Repeat Screening
We found only two studies that focused on racial differences in repeat screening. Black PLCO study participants were significantly less likely than white participants to return for screening 3 to 5 years after a normal screening flexible sigmoidoscopy (60.6% vs. 69.3%)51. Another study of patients in a managed care setting found no evidence of black/white differences in return for surveillance colonoscopy within 3.5 years of an index colonoscopy with high-risk findings53.
Summary
We found evidence that black people have had lower rates of CRC screening than whites. Survey data, which include repeated cross-sectional sampling of the US population across multiple years, indicate potential narrowing of black/white differences over time. Evidence of black/white differences in screening rates from studies of patient populations, primarily based on claims and EHR data, is mixed. The combined evidence across these studies indicates that at least until the recent past, blacks were less likely to be adequately screened for CRC than whites and that socioeconomic factors, including health insurance, factor into these disparities. For example, while the PLCO trial covered the cost of the initial screening flexible sigmoidoscopy, it did not cover the cost of follow-up colonoscopy after an abnormal screening test, and this may have contributed to lower rates of follow-up colonoscopy in black study participants31. Only studies that examined black and white patients who had similar rates of insurance found blacks more likely to be screened than whites45,48,52.
Evidence for Racial Differences in Adenoma Prevalence
The existence of racial disparities in CRC screening utilization does not rule out the potential for racial differences in CRC risk. If there are racial differences in CRC risk, then there should be evidence of differences in observable markers of the underlying disease process. These markers include adenoma prevalence and differences in adenoma size or histology, which could indicate differences in the rate of progression from a precursor lesion to cancer.
Table 2 compares findings at screening for black and white participants, including the unadjusted percentages of participants with outcomes of interest and ORs or RRs that estimate the strength of the relationship between race and screening outcomes. We include aORs and aRRs, though each study adjusted for somewhat different factors. Studies generally reported more than one outcome of interest. Findings by outcomes are summarized below. Consistent with the literature, we define advanced adenomas as adenomas that are ≥10mm or have high-grade dysplasia or villous histology.
Table 2.
Evidence for Racial Differences in Screening Outcomes
| Unadjusted Percentag With the Screening Outcome | Odds Ratios and Risk Ratios*Black Relative to White With 95% Confidence Intervals |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Black |
White |
||||||||
| Outcome/data source | Data Type | Data Collection Years | Age Range (Years) | % | N | % | N | Unadjusted | Adjusted** |
| Adenoma Prevalence | |||||||||
| At Screening Colonoscopy | |||||||||
| National Colonoscopy Study (Mendelsohn, 2017)32¶ | RCT | 2000–2002 2004–2007 |
40–69 | 25.1 | 191 | 26.1 | 199 | 0.93 (0.67,1.28) | 0.86 (0.60,1.25) |
| Stony Brook University (Stein, 2010)57 | Survey, Chart Review | 2006–2007 | >=40 | 35.8 | 356 | 35.7 | 67 | ||
| Kaiser (Corely, 2013)38 | EHR | 2006–2008 | >=50 | 27 | 892 | 24 | 13,266 | — | 1.15 (0.98,1.35) |
| Columbia University (Lebwohl, 2012)54 | EHR | 2006–2010 | >=50 | 25.9 | 591 | 19.1 | 3542 | 1.76 (1.52.2.04) | |
| Columbia University (Lebwohl, 2012)54 | EHR | 2006–2010 | 50–59 | 19.4 | 288 | 16.7 | 1494 | ||
| Columbia University (Lebwohl, 2012)54 | EHR | 2006–2010 | 60–69 | 28.0 | 186 | 17.2 | 1168 | ||
| Columbia University (Lebwohl, 2012)54 | EHR | 2006–2010 | >=70 | 38.5 | 117 | 24.9 | 880 | ||
| Temple University (Friedenberg, 2012)55 | Chart Review | 2007–2010 | 50–59 | 42.9 | 669 | 38.5 | 258 | ||
| Nassau University Medical Center (Zheng, 2014)58 | Chart Review | 2007–2011 | — | 21.8 | 635 | 24.0 | 283 | — | 0.86 (0.60,1.22) |
| Multitarget Stool DNA (Cooper, 2018)56 | Accuracy Study | 2012–2015 | 40–80 | 38.9 | 265 | 33.9 | 495 | ||
| CCPN (Eberth, 2018)59 | EHR | 2014–2016 | 45–64 | 32.2 | 483 | 39.5 | 335 | 0.73 (0.54,0.99) | 0.76 (0.54,1.06) |
| At Follow-up Colonoscopy | |||||||||
| PLCO, 1st screening (Laiyemo, 2010)31† | RCT | 1993–2001 | 55–74 | 50.8 | 480 | 54.2 | 9944 | — | 1.01 (0.92,1.11) |
| PLCO, 2nd screening (Laiyemo, 2015)51‡ | RCT | 2009–2011 | 58–79 | 37.3 | 233 | 41.9 | 3,477 | — | 1.06 (0.89,1.26) |
| At Surveillance Colonoscopy | |||||||||
| Pooled Chemoprevention Study Results (Wallace, 2015)60 | Meta-Analysis of RCTs | 1984–1998 | >50 | 47.7 | 172 | 47.4 | 2,022 | — | 1.08 (0.92,1.27) |
| Advanced Adenoma Prevalence | |||||||||
| At Screening Colonoscopy | |||||||||
| National Colonoscopy Study (Mendelsohn, 2017)32¶ | RCT | 2000–2002 2004–2007 |
40–69 | 7.3 | 191 | 5.5 | 199 | 1.19 (0.56,2.52) | 1.04 (0.39,2.74) |
| Stony Brook University (Stein, 2010)57 | Survey, Chart Review | 2006–2007 | >=40 | 6.0 | 356 | 7.3 | 67 | ||
| Columbia University (Lebwohl, 2012)54 | EHR | 2006–2010 | >=50 | 5.4 | 591 | 3.7 | 3542 | 1.91 (1.27,2.86) | |
| Temple University (Friedenberg, 2012)55 | Chart Review | 2007–2010 | 50–59 | 6.4 | 669 | 7.0 | 257 | ||
| Boston Medical Center (Schroy, 2013)61 | Survey, Chart Review | 2005–2012 | 50–79 | 5.0 | 1,681 | 6.8 | 1,172 | Women: | |
| 1.23 (0.70,2.18) | 1.32 (0.73,2.40) | ||||||||
| Men: | |||||||||
| 0.59 (0.40,0.88) | 0.59 (0.39,0.89) | ||||||||
| Multitarget Stool DNA (Cooper, 2018)56 | Accuracy Study | 2012–2015 | 40–80 | 6.8 | 265 | 6.7 | 495 | ||
| CCPN (Eberth, 2018)59 | EHR | 2014–2016 | 45–64 | 10.9 | 483 | 15.5 | 335 | ||
| At Follow-up Colonoscopy | |||||||||
| PLCO, 1st screening (Laiyemo, 2010)31† | RCT | 1993–2001 | 55–74 | 23.1 | 480 | 22.3 | 9,944 | — | 1.11 (0.94,1.30) |
| PLCO, 2nd screening (Laiyemo, 2015)51‡ | RCT | 1996–2006 | 58–79 | 11.6 | 233 | 13.7 | 3,477 | — | 1.27 (0.90,1.79) |
| At Surveillance Colonoscopy | |||||||||
| Pooled Chemoprevention Study Results (Wallace, 2015)60 | Meta-Analysis of RCTs | 1984–1998 | >50 | 13.4 | 172 | 14.2 | 2,022 | — | 1.05 (0.71,1.56) |
| Boston Medical Center (Kwah, 2014)62 | EHR | 2005–2012 | >=50 | 41.9 | 203 | 47.4 | 246 | 1.18 (0.65,2.16) | 1.30 (0.69,2.40) |
| Prevalence of Proximal Adenomas | |||||||||
| At Screening Colonoscopy | |||||||||
| Kaiser (Corley, 2013)38 | EHR | 2006–2008 | >=50 | 17.0 | 892 | 14.1 | 13,266 | — | 1.26 (1.04,1.54) |
| Temple University (Friedenberg, 2012)55 | Chart Review | 2007–2010 | 50–59 | 24.2 | 669 | 23.7 | 258 | ||
| Nassau University Medical Center (Zheng, 2014)58 | Chart Review | 2007–2011 | — | 16.8 | 635 | 20.5 | 283 | — | — |
| At Follow-up Colonoscopy | |||||||||
| PLCO, 1st screening (Laiyemo, 2010)31 | RCT | 1993–2001 | 55–74 | 21.0 | 480 | 19.0 | 9944 | — | 1.09 (0.91,1.29) |
| PLCO, 2nd screening (Laiyemo, 2015)51 | RCT | 1996–2006 | 58–79 | 18.8 | 233 | 18.9 | 3,477 | — | 1.11 (0.84,1.47) |
| Prevalence of Advanced Proximal Adenomas | |||||||||
| At Screening Colonoscopy | |||||||||
| Boston Medical Center (Schroy, 2013)61 | Survey, Chart Review | 2005–2012 | 50–79 | 2.6 | 1,681 | 2.6 | 1,172 | — | — |
| At Follow-up Colonoscopy | |||||||||
| PLCO, 1st screening (Laiyemo, 2010)31† | RCT | 1993–2001 | 55–74 | 8.5 | 480 | 5.5 | 9,944 | — | 1.56 (1.13,2.14) |
| PLCO, 2nd screening (Laiyemo, 2015)51‡ | RCT | 1996–2006 | 58–79 | 4.4 | 233 | 6.4 | 3,477 | — | 1.44 (0.84,2.48) |
| Screen Detected CRC | |||||||||
| At Screening Colonoscopy | |||||||||
| Boston Medical Center (Schroy, 2013)61 | Survey, Chart Review | 2005–2012 | 50–79 | 0.4 | 1,681 | 0.4 | 1,172 | — | — |
| At Follow-up Colonoscopy | |||||||||
| PLCO, 1st screening (Laiyemo, 2010)31† | RCT | 1993–2001 | 55–74 | 2.1 | 480 | 1.5 | 9,944 | 1.58 (0.80,3.12) | — |
| PLCO, 2nd screening (Laiyemo, 2015)51‡ | RCT | 1996–2006 | 58–79 | 1.3 | 233 | 0.5 | 3,477 | — | — |
| Interval CRC | |||||||||
| SEER-Medicare (Fedewa, 2017)67 | Claims | 2002–2013 | 66–75 | — | 4,196 | — | 51,313 | — | 1.31 (1.13,1.15)§ |
Abbreviations: BRFSS: Behavioral Risk Factors Surveillance System; CCPN: Colorectal Cancer Prevention Network; EHR: Electronic Health Record; NHIS: National Health Interview Survey; PLCO: Prostate, Lung, Colorectal and Ovarian; PROSPR: Population-based Research to Optimize the Screening Process; RCT: Randomized Controlled Trial; SEER: Surveillance, Epidemiology, and End Results; VA: Veterans Health Administration.
Risk ratios are provided in italics to distinguish them from odds ratios.
Characteristics adjusted for in aOR and aRR estimates: Columbia University54: age, sex, family history of CRC, insurance status (Medicaid vs. other), participation of a trainee in exam; Nassau University Medical Center58: age, sex, tobacco use, body mass index (BMI), indication for colonoscopy, alcohol use, dyslipidemia, hypertension; National Colonoscopy Study32: age, sex, and education; Kaiser38: age, family history of CRC; CCPN59: age, sex, education, rural residence, physician, language spoken; PLCO, first screening31: age, sex, smoking status, family history of CRC, BMI, education, history of CRC within 3 years of enrollment, history of colon polyps, and screening center; PLCO, second screening51: age, sex, smoking status, family history of CRC, BMI, education, year of repeat flexible sigmoidoscopy and screening center; Boston Medical Center61: age, smoking status, BMI, alcohol use, education, insurance type, NSAID use, aspirin use, use of birth control pills, use of hormone replacement therapy, red meat intake, multivitamin use, calcium intake, physical activity, diabetes, previous colonoscopy. Pooled Chemoprevention Studies60: age, sex, study treatment assignment, and follow-up time; SEER-Medicare67: age, sex, state of residence, poverty level, urban–rural classification, Charlson comorbidity score, diverticulitis, polyp removal at index colonoscopy, physician specialty, and physician polyp detection rate quartile;
People of Hispanic ethnicity were included in both black and white groups.
The PLCO study used flexible sigmoidoscopy as the reference screening test. During the first screening, the percentage of participants with a polyp detected at flexible sigmoidoscopy was 25.5% (N=3,011) and 23.9% (N=57,561) in black and white participants, respectively.
The PLCO study used flexible sigmoidoscopy as the reference screening test. During the second screening, the percentage of participants with a polyp detected at flexible sigmoidoscopy was 31.2% (N=975) and 19.4% (N=21,550) in black and white participants, respectively.
Estimated hazard ratio for interval CRC up to 59 months after colonoscopy.
Adenoma Prevalence
We found mixed evidence that adenoma prevalence differs for black and white people. Of eight studies that described racial differences in adenoma prevalence at screening colonoscopy, four estimated higher adenoma prevalence in blacks than whites38,54–56, two estimated similar adenoma prevalence in blacks and whites32,57, and two estimated lower adenoma prevalence in blacks than whites58,59. The study that found the strongest evidence of higher adenoma risk in blacks relative to whites (aRR=1.76), estimated that adenoma prevalence was 2.7 percentage points higher in blacks than whites among participants 50 to 59 years old, 10.3 points higher among participants 60 to 69 years old, and a 13.6 points higher among participants 70 and older54. The PLCO study found no detectable black versus white differences in adenoma prevalence among study participants who underwent follow-up colonoscopy, at either the first31 or second screen51, though only patients with abnormal findings at flexible sigmoidoscopy were referred to colonoscopy. Pooled analysis of data from three CRC chemoprevention studies, each with null findings, found no black versus white differences in adenoma prevalence at surveillance colonoscopy60.
Advanced Adenoma Prevalence
We found mixed evidence for racial differences in risk for advanced adenomas. Two of seven studies that described racial differences in advanced adenoma prevalence at screening colonoscopy observed a higher prevalence of advanced adenomas in black patients32, while five found that blacks had similar or lower risk of advanced adenomas than whites55–57,59,61. Two of three studies that reported aRRs (or aOR) of advanced adenomas in black versus white study participants at screening colonoscopy estimated an elevated risk of advanced adenomas in black participants54, including one study that found black women were at greater risk of advanced adenomas than white women, but black men were at lower risk of advanced adenomas than white men61. The PLCO study found no racial differences in the prevalence of advanced adenomas detected at the follow-up colonoscopy after the first flexible sigmoidoscopy screening31, but analysis of the second screening that controlled for screening center found evidence that blacks had a higher prevalence of advanced adenomas than whites51. Two studies of surveillance colonoscopy each found that blacks were more likely to have advanced adenomas than whites60,62.
Proximal Adenoma Prevalence
The location of adenomas is important because adenomas in the proximal colon may be more difficult to detect63 and proximal cancers may have a lower five-year survival rate than distal colon and rectal cancers due to differences in disease characteristics64,65. Consistent with the literature, we use proximal location to indicate the transverse colon, hepatic flexure, ascending colon, and cecum.
We found mixed evidence for racial differences in the prevalence of proximal adenomas based on results from three observational studies38,55,58. One study found similar proximal adenoma prevalence in black and white patients55. Another study found that blacks were significantly more likely to have proximal adenomas compared to whites, adjusting for only age and family history of CRC38. The third study found that proximal adenoma prevalence was lower in black patients than in white patients58. Finally, the PLCO study found that black and white study participants had similar odds of having a proximal adenoma detected at follow-up colonoscopy at both the first and second screening (3 to 5 years later), though only participants with findings at flexible sigmoidoscopy were referred to colonoscopy31,51.
Prevalence of Advanced Proximal Adenomas
Evidence about the relationship between race and prevalence of advanced proximal adenomas was limited and mixed. An observational study carried out in Boston Medical Center safety-net clinics found that black and white patients had the same prevalence of advanced proximal adenomas61. In contrast, the PLCO study found that black participants were more likely to have advanced proximal adenomas than white participants31,51. Estimated racial differences were observed at the first and second round of screening, but only reached statistical significance at the first round.
Interval CRC
Interval CRC refers to CRC detected after colonoscopy and before the next recommended screening exam66. Analysis of SEER-Medicare data, based on 66- to 75-year olds who underwent colonoscopy between 2002 and 2011, found that black patients were at significantly greater risk of interval cancer than white patients; the estimated probability of an interval cancer up to 54 months after colonoscopy was 0.071 for black patients and 0.051 for white patients67. Interval cancers can be attributed to provider characteristics (adenoma detection rates, rates of complete adenoma/serrated polyp resection), patient characteristics (adequate bowel cleansing), and disease characteristics (progression rates). Thus, it is unclear what drives differences in interval cancer rates.
Summary
We found mixed evidence for racial differences in the prevalence of adenomas and limited evidence that black people may be at greater risk for advanced and proximal adenomas compared to whites. Our findings are similar to results from a recent systematic review and meta-analysis of nine studies that found no difference in the prevalence of advanced adenomas in black (6.6%) and white (6.2%) people but did find evidence that blacks had a higher prevalence of advanced proximal adenomas than whites (3.3% versus 2.4%)68.
It is difficult to draw conclusions about racial differences in adenoma risk in the context of screening disparities. Colonoscopy with polypectomy has long-term effects on adenoma prevalence. If whites are more likely to be screened than blacks, this could result in underestimation of both adenoma and advanced adenoma prevalence in whites compared to blacks, even in studies that focused on screening outcomes. For example, in a study that described black/white differences in adenoma prevalence by age, differences were small before age 60, when people are less likely to be previously screened, and large after age 60 when the chance of previous screening increases, especially among whites54. On the other hand, if black people receive lower-quality colonoscopy than whites, this could result in underestimation of adenomas and advanced adenomas in blacks compared to whites. Within the PLCO study, racial differences in the prevalence of abnormal findings at the second screening flexible sigmoidoscopy were partly explained by black patients being more likely to have their colonoscopies at centers with lower adenoma detection rates51.
Discussion
Understanding what drives racial disparities will better equip us to address them. Disparities that are driven by disparate access to quality care point to the importance of improving access to and uptake of high-quality screening. Disparities that arise from disparate risk point to the need for more aggressive screening in higher-risk groups, including earlier initiation and shorter screening intervals, and further exploration of factors that drive increased risk. Based on available data, we found strong evidence for racial differences in CRC screening access, but limited evidence for racial differences in adenoma prevalence. In general, black and white patients who are screened have similar adenoma prevalence. While there is some indication that risk of advanced and proximal adenomas is higher in black than white people, these differences could be driven by differences in screening. Based on this review, we conclude that higher rates of CRC incidence among black patients is largely due to lower rates of screening.
Screening can reduce CRC incidence and mortality, but black Americans face greater barriers to screening than whites69. In 2017, black adults younger than 65 were less likely than whites to have health insurance (85.9% versus 91.5%)70, and people without health insurance are less likely to participate in CRC screening71. Racial barriers persist among the insured, where healthcare systems and physicians within these systems play an important role in promoting CRC screening. In spite of widespread knowledge that blacks have higher CRC incidence than whites, blacks are less likely than whites to receive a recommendation for CRC screening72,73. Physicians are less likely to recommend screening based on their perception of a patient’s ability to pay74. Physicians are also less likely to recommend CRC screening when they identify a shortage of specialists in the region, and blacks may be more likely than whites to live in such regions75. There is also evidence that black patients receive colonoscopies from physicians with lower adenoma detection rates than white patients 51,67,76. From the patient perspective, trust in an individual’s primary care provider is the most important factor in the decision to undergo CRC screening, and the degree to which trust plays a role in the decision varies across different racial and ethnic groups77.
Our report has several important limitations. This narrative review lacked the rigor of a systematic review and we do not provide pooled estimates of screening outcomes. We described differences in CRC screening and risk for black and white patients within the US and did not address differences across other racial or ethnic groups or in other countries. In addition, because of wide heterogeneity in genetics and ancestry for both “blacks” and “whites” in the US, comparisons between these groups could mask true risk differences in more homogeneous genetic subgroups. We relied primarily on adenoma prevalence as a proxy for underlying CRC risk. We did not address racial differences in the molecular characteristics of detected lesions which may be related to differences in disease progression and survival after diagnosis. Nor did we examine differences in the prevalence of sessile serrated polyps, which could be related to differences in the effectiveness of screening, and both CRC incidence and mortality78. Finally, there is potential for bias in study estimates, especially from observational studies. In particular, black/white differences in screening uptake could result in differential underestimation of adenoma and advanced adenoma prevalence, and people at increased risk of CRC due to modifiable risk factors, such as smoking, may be underrepresented in studies of screened populations. In spite of these limitations, we feel that the available research, taken together, provides a compelling argument that the higher CRC incidence observed in blacks relative to whites is primarily due to differences in screening utilization.
It is imperative that we seek ways to reduce racial disparities in CRC screening, but how? Multi-level solutions are needed, including national and state policies to improve access to screening and follow-up of abnormal screening tests, health systems solutions to promote screening, and patient education efforts79. Health care organization can ensure that testing is accessible by mailing FITs to all patients80 and using patient navigators to assist patients12,81. Overall, our review suggests that equal access to care can reduce screening disparities.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by Grant Number U01CA199335 (CMR, ABK) from the National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network (CISNET) and by a Research Scholar Grant from the American Cancer Society under award number RSG-15-002-01-CCE (ABK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the American Cancer Society. We also want to thank Jody Larkin for assistance with literature searches and Claudia Seguin for creating figures showing colorectal cancer incidence by race, age, and time period.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA: a cancer journal for clinicians. 2013;63(3):151–166. [DOI] [PubMed] [Google Scholar]
- 2.Rahman R, Schmaltz C, Jackson CS, Simoes EJ, Jackson-Thompson J, Ibdah JA. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer medicine. 2015;4(12):1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA: A Cancer Journal for Clinicians. 2019;69(3):211–233. [DOI] [PubMed] [Google Scholar]
- 4.Shavers VL. Racial/ethnic variation in the anatomic subsite location of in situ and invasive cancers of the colon. J Natl Med Assoc. 2007;99(7):733–748. [PMC free article] [PubMed] [Google Scholar]
- 5.Mandelblatt J, Andrews H, Kao R, Wallace R, Kerner J. The late-stage diagnosis of colorectal cancer: demographic and socioeconomic factors. Am J Public Health. 1996;86(12):1794–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975–2002). Cancer Epidemiol Biomarkers Prev. 2006;15(4):792–797. [DOI] [PubMed] [Google Scholar]
- 7.Rutter CM, Johnson EA, Feuer EJ, Knudsen AB, Kuntz KM, Schrag D. Secular Trends in Colon and Rectal Cancer Relative Survival. J Natl Cancer Inst. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams R, White P, Nieto J, Vieira D, Francois F, Hamilton F. Colorectal cancer in African Americans: an update. Clinical and translational gastroenterology. 2016;7(7):e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander DD, Waterbor J, Hughes T, Funkhouser E, Grizzle W, Manne U. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: an epidemiologic review. Cancer Biomarkers. 2007;3(6):301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100(3):515–523; discussion 514. [DOI] [PubMed] [Google Scholar]
- 11.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009;70(1):96–108, 108 e101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwaan MR, Jones-Webb R. Colorectal cancer screening in black men: recommendations for best practices. American journal of preventive medicine. 2018;55(5):S95–S102. [DOI] [PubMed] [Google Scholar]
- 13.Winawer SJ. A quarter century of colorectal cancer screening: progress and prospects. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(18 Suppl):6S–12S. [PubMed] [Google Scholar]
- 14.Hardcastle JD, Chamberlain JO, Moss SM, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1989:1160–1164. [DOI] [PubMed] [Google Scholar]
- 15.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–1471. [DOI] [PubMed] [Google Scholar]
- 16.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. [DOI] [PubMed] [Google Scholar]
- 17.Guide to clinical preventive services. Washington, DC: Department of Health and Human Services;1995. [Google Scholar]
- 18.Levin B, Bond JH. Colorectal cancer screening: Recommendations of the U.S. Preventive Services Task Force. Gastroenterology. 1996;111(5):1381–1384. [DOI] [PubMed] [Google Scholar]
- 19.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. New England Journal of Medicine. 2012;366(8):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin B, Lieberman DA, McFarland BG, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. [DOI] [PubMed] [Google Scholar]
- 21.U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. [DOI] [PubMed] [Google Scholar]
- 22.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104(3):739–750. [DOI] [PubMed] [Google Scholar]
- 23.Qaseem A, Denberg TD, Hopkins RH, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Annals of internal medicine. 2012;156(5):378–386. [DOI] [PubMed] [Google Scholar]
- 24.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. The American journal of gastroenterology. 2017;112(7):1016. [DOI] [PubMed] [Google Scholar]
- 25.Wolf AM, Fontham ET, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: a cancer journal for clinicians. 2018;68(4):250–281. [DOI] [PubMed] [Google Scholar]
- 26.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data with Delay-Adjustment, 9 Registries, Malignant Only, Nov 2019 Sub (1975–2017) - Linked To County Attributes - Time Dependent (1990–2017) Income/Rurality, 1969–2017 Counties. In: National Cancer Institute D, Surveillance Research Program, ed. [Google Scholar]
- 27.Joinpoint Regression Program [computer program]. Version Version 4.5.0.1: Statistical Research and Applications Branch, National Cancer Institute; June, 2017 [Google Scholar]
- 28.Sorensen RH, Farnsworth GS, Roberts JE, Welling DR, Rich NM. President Reagan’s Life Saving Colectomy and Subsequent Historical Implications. Military medicine. 2014;179(7):704–707. [DOI] [PubMed] [Google Scholar]
- 29.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2010;116(3):544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in incidence of colorectal cancer among individuals 50 years or older after recommendations for population-based screening. Clinical Gastroenterology and Hepatology. 2017;15(6):903–909. e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102(8):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendelsohn RB, Winawer SJ, Jammula A, et al. Adenoma Prevalence in Blacks and Whites Having Equal Adherence To Screening Colonoscopy: The National Colonoscopy Study. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2017;15(9):1469–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Digestive diseases and sciences. 2015;60(3):711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coughlin SS, Blumenthal DS, Seay SJ, Smith SA. Toward the elimination of colorectal cancer disparities among African Americans. Journal of racial and ethnic health disparities. 2016;3(4):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rex DK, Ahnen DJ, Baron JA, et al. Serrated Lesions of the Colorectum: Review and Recommendations From an Expert Panel. American Journal of Gastroenterology. 2012;107(9):1315–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kauh JB, Otis W; Berger Mitchell Z. Racial disparities in colorectal cancer. Current Problems in Cancer. 2007;31(3):123–133. [DOI] [PubMed] [Google Scholar]
- 37.Sonnenberg A, Turner KO, Genta RM. Ethnic variations in the occurrence of colonic neoplasms. United European gastroenterology journal. 2017;5(3):424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corley DA, Jensen CD, Marks AR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11(2):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson LM, May DS. Has the use of cervical, breast, and colorectal cancer screening increased in the United States? American Journal of Public Health. 1995;85(6):840–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2170–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168(12):1317–1324. [DOI] [PubMed] [Google Scholar]
- 42.Burgess DJ, Van Ryn M, Grill J, et al. Presence and correlates of racial disparities in adherence to colorectal cancer screening guidelines. Journal of general internal medicine. 2011;26(3):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. American journal of preventive medicine. 2014;46(3):228–236. [DOI] [PubMed] [Google Scholar]
- 44.Sauer AG, Liu B, Siegel RL, Jemal A, Fedewa SA. Comparing cancer screening estimates: Behavioral Risk Factor Surveillance System and National Health Interview Survey. Preventive medicine. 2018;106:94–100. [DOI] [PubMed] [Google Scholar]
- 45.Lee DC, Liang H, Chen N, Shi L, Liu Y. Cancer screening among racial/ethnic groups in health centers. Int J Equity Health. 2020;19(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.May FP, Yang L, Corona E, Glenn BA, Bastani R. Disparities in Colorectal Cancer Screening in the United States Before and After Implementation of the Affordable Care Act. Clin Gastroenterol Hepatol. 2019. [DOI] [PubMed] [Google Scholar]
- 47.Shaukat A, Church TR, Shanley R, et al. Development and validation of a clinical score for predicting risk of adenoma at screening colonoscopy. Cancer Epidemiology and Prevention Biomarkers. 2015;24(6):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Changoor NR, Pak LM, Nguyen LL, et al. Effect of an equal-access military health system on racial disparities in colorectal cancer screening. Cancer. 2018;124(18):3724–3732. [DOI] [PubMed] [Google Scholar]
- 49.Huguet N, Angier H, Rdesinski R, et al. Cervical and colorectal cancer screening prevalence before and after Affordable Care Act Medicaid expansion. Preventive Medicine. 2019;124:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnett-Hartman AN, Mehta SJ, Zheng Y, et al. Racial/ethnic disparities in colorectal cancer screening across healthcare systems. American journal of preventive medicine. 2016;51(4):e107–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laiyemo AO, Doubeni C, Pinsky PF, et al. Occurrence of distal colorectal neoplasia among whites and blacks following negative flexible sigmoidoscopy: An analysis of PLCO Trial. Journal of general internal medicine. 2015;30(10):1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Partin MR, Gravely AA, Burgess JF Jr, et al. Contribution of patient, physician, and environmental factors to demographic and health variation in colonoscopy follow-up for abnormal colorectal cancer screening test results. Cancer. 2017;123(18):3502–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chubak J, McLerran D, Zheng Y, et al. Receipt of Colonoscopy Following Diagnosis of Advanced Adenomas: An Analysis within Integrated Healthcare Delivery Systems. Cancer Epidemiol Biomarkers Prev. 2019;28(1):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lebwohl B, Capiak K, Neugut AI, Kastrinos F. Risk of colorectal adenomas and advanced neoplasia in Hispanic, black and white patients undergoing screening colonoscopy. Alimentary pharmacology & therapeutics. 2012;35(12):1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedenberg FK, Singh M, George NS, Sankineni A, Shah S. Prevalence and distribution of adenomas in black Americans undergoing colorectal cancer screening. Dig Dis Sci. 2012;57(2):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper GS, Markowitz SD, Chen Z, et al. Performance of multitarget stool DNA testing in African American patients. Cancer. 2018;124(19):3876–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein B, Anderson JC, Rajapakse R, Alpern ZA, Messina CR, Walker G. Body mass index as a predictor of colorectal neoplasia in ethnically diverse screening population. Dig Dis Sci. 2010;55(10):2945–2952. [DOI] [PubMed] [Google Scholar]
- 58.Zheng XE, Li T, Lipka S, et al. Location-dependent ethnic differences in the risk of colorectal adenoma: a retrospective multiethnic study. Journal of clinical gastroenterology. 2014;48(1):e1–e7. [DOI] [PubMed] [Google Scholar]
- 59.Eberth JM, Thibault A, Caldwell R, et al. A Statewide Program Providing Colorectal Cancer Screening to the Uninsured of South Carolina. Cancer. 2018;124(9):1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallace K, Burke CA, Ahnen DJ, et al. The Association of Age and Race and the Risk of Large Bowel Polyps. Cancer Epidem Biomar. 2015;24(2):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schroy PC 3rd, Coe A, Chen CA, O’Brien MJ, Heeren TC. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;159(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwah J, Schroy PC, Jacobson BC, Calderwood AH. Whites and blacks have similar risk of metachronous advanced colorectal neoplasia. Digestive diseases and sciences. 2014;59(9):2264–2271. [DOI] [PubMed] [Google Scholar]
- 63.Brenner H, Niedermaier T, Chen H. Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin. International journal of cancer. 2017;140(9):2015–2022. [DOI] [PubMed] [Google Scholar]
- 64.Chan JCY, Diakos CI, Engel A, et al. Tumor sidedness is not an independent prognostic marker of colorectal cancer patients undergoing curative resection: A retrospective cohort study. PLoS One. 2019;14(6):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995–2001. [DOI] [PubMed] [Google Scholar]
- 66.Sanduleanu S, le Clercq CM, Dekker E, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64(8):1257–1267. [DOI] [PubMed] [Google Scholar]
- 67.Fedewa SA, Flanders WD, Ward KC, et al. Racial and ethnic disparities in interval colorectal cancer incidence: a population-based cohort study. Annals of internal medicine. 2017;166(12):857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imperiale TF, Abhyankar PR, Stump TE, Emmett TW. Prevalence of advanced, precancerous colorectal neoplasms in black and white populations: a systematic review and meta-analysis. Gastroenterology. 2018;155(6):1776–1786. e1771 %@ 0016–5085. [DOI] [PubMed] [Google Scholar]
- 69.Warren Andersen S, Blot WJ, Lipworth L, Steinwandel M, Murff HJ, Zheng W. Association of Race and Socioeconomic Status With Colorectal Cancer Screening, Colorectal Cancer Risk, and Mortality in Southern US Adults. JAMA Network Open. 2019;2(12):e1917995–e1917995 %@ 1912574–1913805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agency for Healthcare Research and Quality. 2017 National Healthcare Quality and Disparities Report. Content last reviewed July 2019. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr17/index.html Accessed 8/23/2019. [PubMed]
- 71.de Moor JS, Cohen RA, Shapiro JA, et al. Colorectal cancer screening in the United States: trends from 2008 to 2015 and variation by health insurance coverage. Preventive medicine. 2018;112:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallace K, Hill EG, Lewin DN, et al. Racial disparities in advanced-stage colorectal cancer survival. Cancer Causes & Control. 2013;24(3):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.May FP, Almario CV, Ponce N, Spiegel BM. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. The American journal of gastroenterology. 2015;110(10):1388. [DOI] [PubMed] [Google Scholar]
- 74.Meissner HI, Klabunde CN, Breen N, Zapka JM. Breast and colorectal cancer screening: US primary care physicians’ reports of barriers. American journal of preventive medicine. 2012;43(6):584–589. [DOI] [PubMed] [Google Scholar]
- 75.Zapka J, Klabunde CN, Taplin S, Yuan G, Ransohoff D, Kobrin S. Screening colonoscopy in the US: attitudes and practices of primary care physicians. Journal of general internal medicine. 2012;27(9):1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.David Y, Ottaviano L, Park J, et al. Confounders in Adenoma Detection at Initial Screening Colonoscopy: A Factor in the Assessment of Racial Disparities as a Risk for Colon Cancer. J Cancer Ther. 2019;10(4):269–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S, Brenner AT, Ratanawongsa N, Inadomi JM. Patient trust in physician influences colorectal cancer screening in low-income patients. American Journal of Preventive Medicine. 2014;47(4):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nouraie M, Ashktorab H, Atefi N, et al. Can the rate and location of sessile serrated polyps be part of colorectal Cancer disparity in African Americans? BMC Gastroenterol. 2019;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and Possible Solutions to Colorectal Cancer Screening for the Underserved. JNCI: Journal of the National Cancer Institute. 2014;106(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Connor EA, Vollmer WM, Petrik AF, Green BB, Coronado GD. Moderators of the effectiveness of an intervention to increase colorectal cancer screening through mailed fecal immunochemical test kits: results from a pragmatic randomized trial. Trials. 2020;21(1):1–12 %@ 1745–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Percac-Lima S, López L, Ashburner JM, Green AR, Atlas SJ. The longitudinal impact of patient navigation on equity in colorectal cancer screening in a large primary care network. Cancer. 2014;120(13):2025–2031. [DOI] [PubMed] [Google Scholar]
- 82.Doubeni CA. The impact of colorectal cancer screening on the US population: is it time to celebrate? Cancer. 2014;120(18):2810–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.