Abstract
BACKGROUND:
Although 1% to 2% of the general population carries a cephalosporin allergy label (CAL), we lack validated testing strategies and predictors of true allergy.
OBJECTIVE:
To identify cross-reactivity patterns and predictors of skin test positive (STP) in geographically disparate patients with a CAL.
METHODS:
A total of 780 adult patients labeled with a CAL or penicillin allergy label (PAL) with unknown tolerance of cephalosporins identified from the Austin Hospital (Melbourne, Australia) (n = 410) and Vanderbilt University Medical Center (Nashville, TN) (n = 370) between 2014 and 2018 underwent a standardized skin testing.
RESULTS:
Of 328 patients with a CAL, 29 (8.8%) tested STP to ≥1 cephalosporin(s). There were no cefazolin or ceftriaxone STP, 0 of 452 (0%), in patients with a PAL only. Of 328 patients with a CAL, 16 (4.8%) were ampicillin STP. Eleven of 16 of these patients had an initial allergy label to cephalexin. Twenty of 29 cephalosporin STP patients demonstrated tolerance to a cephalosporin with a different R1 side chain, and 8 of 14 ampicillin STP patients demonstrated tolerance to ≥1 non-amino R1 group cephalosporin. Eleven of 13 patients STP to cefazolin were skin and ingestion challenge negative to all other penicillins and cephalosporins predicted by its distinct R1/R2 groups. Seven of 15 ceftriaxone STP patients demonstrated cross-reactivity with R1-similar cephalosporins. Time since the original reaction predicted STP testing to both penicillins, adjusted odds ratio (aOR) per year 0.93 (95% confidence interval [CI]: 0.90, 0.97), and cephalosporins, aOR per year 0.71 (95% CI: 0.56, 0.90).
CONCLUSIONS:
Cephalosporin cross-reactivity is based on shared R1 groupings. Increasing time since the original reaction and the presence of a PAL with unknown cephalosporin tolerance predict a lower likelihood of cephalosporin STP.
Keywords: Cephalosporin, Beta-lactam, Immediate hypersensitivity, Allergy, Penicillin, Skin testing, Cross-reactivity
Between 1% and 2% of the general population are labeled as cephalosporin allergic,1 but specific and validated testing strategies for both immediate (IgE-mediated) and delayed (T-cell-mediated) cephalosporin allergy are less well defined.2 Current drug allergy guidelines from the American Academy of Allergy, Asthma, and Immunology2 and European Academy of Allergy and Clinical Immunology3 each note the need for additional evidence on cephalosporin cross-reactivity patterns when using nonirritating drug concentrations and systematic skin testing approaches.3,4 To date, immediate cephalosporin hypersensitivity testing strategies using specific IgE and basophil activation, although safe, have both lacked sensitivity and specificity for diagnosing cephalosporin allergy.5–8 Furthermore, although there is general agreement as to the highest nonirritating concentrations for most cephalosporins, in vivo testing such as prick and intradermal testing (IDT) has varied widely as to the types and concentrations of cephalosporins employed.9–11 Studies suggest that cephalosporin skin test positivity is predictable primarily based on known R1 side chain cross-reactivity patterns such as those reported in European populations by Romano et al;12–17 however, the generalizability and prevalence of positive skin testing across different populations is unknown particularly in the United States where these testing practices are not common.14,18,19
We reviewed 2 large and geographically disparate adult drug allergy cohorts using similar approaches to cephalosporin skin testing and ingestion challenge, with the specific purpose of evaluating the safety and efficacy of testing and the cross-reactivity between cephalosporins sharing similar side chains. Time since the original reaction has been reported as an important predictor for skin test positivity to beta-lactams in the past.13 We therefore aimed to evaluate the extent to which positive cephalosporin allergy skin testing decreases over time since the original reaction in a cohort of consecutively tested patients. Furthermore, cephalosporin allergy cross-reactivity with penicillins has been hypothesized to be restricted to drugs that share R1 side chains, such as the aminopenicillin side chain shared by ampicillin, amoxicillin, cefadroxil, cefaclor, cephalexin, and cefprozil.20 Other cephalosporin R1 side chain families are not shared with penicillins. Examples include the methoxyimino side chain family shared by cefuroxime, ceftriaxone, cefotaxime, cefepime, and cefpodoxime; the R1 side chain of cefazolin that is shared by no other drug; and the R1 side chain of ceftazidime that is shared with aztreonam. Hence, we sought to evaluate the extent to which patients who are history or skin test positive (STP) to cephalosporins would have positive skin testing and/or ingestion challenge to penicillins. Finally, although it is not universally standard practice, in view of previously published higher cross-reactivity patterns between penicillins and cephalosporins, our original protocols tested for cephalosporin allergy in patients with penicillin allergy and unknown tolerance of cephalosporins. This was done to evaluate whether a penicillin allergy history alone would result in positive cephalosporin skin tests other than the shared aminopenicillin/aminocephalosporin R1 side chain.
METHODS
We performed a retrospective 2 center cohort study evaluating cephalosporin testing strategies. From 2014 to 2018, across 2 geographically disparate beta-lactam testing cohorts, the Austin Hospital (Melbourne, Australia) and Vanderbilt University Medical Center (VUMC), Nashville, TN, all patients reporting a cephalosporin or penicillin allergy with unknown tolerance of cephalosporins underwent a standardized skin testing and ingestion challenge protocol (Figures 1 and 2). Of a total of 1301 patients seen at these 2 sites from 2014 to 2018 for any drug allergy, 780 (60%) had a history of beta-lactam allergy consisting of either a reported cephalosporin allergy (n = 328) or penicillin allergy with unknown tolerance of cephalosporins (n = 452) and were subject to the retrospective review. A standardized chart review was performed to obtain patient age, sex, race, penicillin and cephalosporin labels, reaction history, which was classified as immediate or delayed, time since reaction, and testing outcomes from standardized skin testing and ingestion challenge protocols.
FIGURE 1.
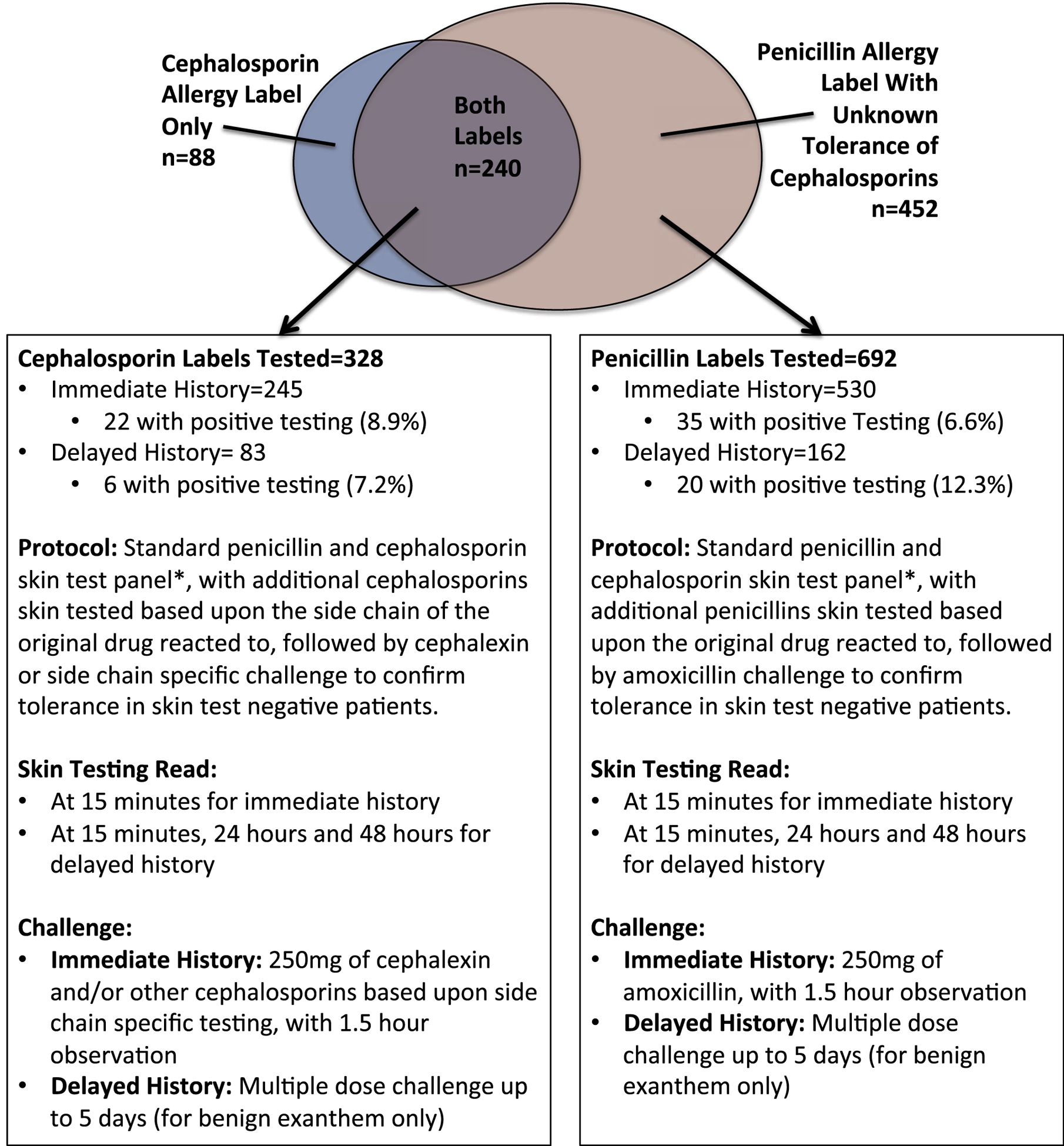
Flow diagram for selection of penicillin/cephalosporin testing as described in this article. The standard panel of testing included Pre Pen21 or DAP major determinant (Diater),22 minor determinant mix23 either Diater (Austin) or an in-house stock prepared solution of benzyl penicilloate (VUMC), ampicillin (25 mg/mL), and penicillin G (1000 and 10,000 IU/mL), cefazolin (1 mg/mL), and ceftriaxone (2.5 mg/mL) via skin prick and intradermal tests. Every skin test negative penicillin allergy was challenged with amoxicillin. Every skin test negative cephalosporin allergy was challenged with cephalexin. Additional skin test reagents and additional drugs were used to disprove side chainespecific allergies by selecting either the exact same drug as the patient’s index reaction, or a drug with the same side chain, based on availability in oral and sterile IV preparation, respectively. Note that 0 of 452 patients tested for a penicillin allergy label and unknown tolerance of cephalosporins had positive testing to cefazolin or ceftriaxone. *Concentrations for testing additional specific cephalosporins based on the R side chain structure are found in Table E1 (available in this article’s Online Repository at www.jaci-inpractice.org). IV, XXX; VUMC, Vanderbilt University Medical Center.
FIGURE 2.

Examples of side chain—specific cephalosporin skin tests, added in addition to the standard protocol in Figure 1, derived from published side chain patterns.20 *Concentrations for testing additional specific cephalosporins are found in Table E1 (available in this article’s Online Repository at www.jaci-inpractice.org). +Challenges using the same R1 side chain should be considered when index reactions are of low-moderate severity when skin testing is negative, or when the patient has a specific clinical need for the implicated drug or same R1 side-chain drugs. Note: there are currently no oral drugs containing the ceftazidime/aztreonam R1.
Skin testing reagents and protocols
Skin testing was defined as a standardized combined skin prick testing (SPT) and IDT approach that we employed. All 780 penicillin or cephalosporin allergy—labeled patients received a standard testing panel. The standard protocol consisted of Pre Pen21 or DAP major determinant (Diater),22 minor determinant mix23 either Diater (Austin) or an in-house stock prepared solution of benzyl penicilloate (VUMC), ampicillin (25 mg/mL), and penicillin G (1000 and 10,000 IU/mL), cefazolin (1 mg/mL), and ceftriaxone (2.5 mg/mL) via SPT and IDT using nonirritating concentrations as per previously published protocols.24,25 Concentrations of additional cephalosporins tested based on an individual’s history or initial testing results can be found in Table E1 (available in this article’s Online Repository at www.jaci-inpractice.org).
Skin testing methods
Penicillin and cephalosporin testing was performed by SPT and IDT using nonirritating concentrations (Table E1, available in this article’s Online Repository at www.jaci-inpractice.org).20 Prick testing was performed first when a higher severity immediate hypersensitivity to a beta-lactam such as immediate multisystem involvement on history or administration of epinephrine consistent with anaphylaxis was reported. Otherwise, patients only received IDTs.
All patients with cephalosporin allergy labels (CALs) were tested to 2 standard cephalosporins, as outlined in Figure 1, which were cefazolin and ceftriaxone. When the cephalosporin that the patient originally reacted to, their “label,” was not cefazolin or ceftriaxone, the patient was also tested against the implicated cephalosporin when it was available in sterile IV preparation (Figure 2; Table E1, available in this article’s Online Repository at www.jaci-inpractice.org).
In cases where the standard approach above was positive to any penicillin or cephalosporin, additional testing with cephalosporins that had shared R1/R2 side chains was performed when possible (Figure 2; Table E1, available in this article’s Online Repository at www.jaci-inpractice.org). This was done with prick testing, followed by immediate IDTs, in 2 separate testing steps, for immediate hypersensitivity, and in a single delayed IDT and/or patch testing for delayed hypersensitivity. The potential for structural cross-reactivity amongst other penicillins and cephalosporins was defined by previously published reports on R-group side chain similarity.12–17,20
Patients with a penicillin-only reaction history and unknown tolerance of cephalosporins were tested for cephalosporin reactivity via IDT to cefazolin and ceftriaxone only, without preceding SPTs.
Delayed readings of IDTs were performed at 24 and 48 hours in settings where the history suggested a delayed hypersensitivity.
Ingestion challenges
Ingestion drug challenges were performed in cases of negative skin testing as the gold standard procedure to rule out allergy and lead to removal of the cephalosporin and/or penicillin allergy label. Our overarching premises in challenges were as follows: (1) after negative skin testing, any cephalosporin can be used to rule out an allergy directed against the cephalosporin ring, and (2) when a cephalosporin skin test was positive to agents with R-side chain structural similarity but not to cephalosporins with different R-side chains, oral challenges with the skin test negative R-side chain containing cephalosporins should be performed when possible/available. Standard challenges included 250 mg of amoxicillin to rule out a penicillin allergy, and 250 mg of cephalexin to rule out a primary cephalosporin ring allergy, when the respective skin testing was negative. A 250 mg penicillin VK challenge step was used in selected cases where skin tests were selectively positive to ampicillin and negative to other determinants. At the Austin Hospital Australia, where penicillin is still the primary drug used for community-acquired infections including community-acquired pneumonia, a penicillin VK 250 mg as an oral ingestion challenge was also used where the primary implicated penicillin was penicillin VK or penicillin G or if the reaction was before the widespread use of amoxicillin in 1972.
This article reflects an institutional review boardeapproved retrospective review on real clinical practice and not an interventional study. No ingestion challenges were conducted to the identical cephalosporin drug in the case of a positive skin test to that drug, either by the prick test or IDT, such as might be done in the context of a prospective study. No intravenous or intramuscular challenges were performed due to the outpatient clinic setting of both cohorts. Therefore, when skin testing and cephalexin challenge were negative, or skin testing was positive in a pattern suggestive of an R-side chain specific allergy, additional selective ingestion challenges of other oral cephalosporins beyond cephalexin were used to ascertain tolerance of cephalosporins with different R1 and/or R2 side chains, as needed for the patient’s treatment. For example, cefpodoxime challenge (which shares an R1 side chain with ceftriaxone) was sometimes used (particularly when patient need for the drug dictated this in the context of real clinical practice) in addition to cephalexin to determine tolerance of cephalosporins and tolerance of the shared ceftriaxone R1 side chain in immediate reactions to ceftriaxone of high index severity, when skin testing was negative. Cefuroxime challenge was commonly used in Austin in patients whose testing pattern was suggestive of an aminopenicillin/aminocephalosporin allergy, as it has a dissimilar R1 side chain. This was a minor variation in protocol between our 2 testing sites that was necessitated by the higher rate of selective aminopenicillin and immediate anaphylactic aminocephalosporin reactions.
Statistical analysis
Descriptive statistics assessing differences between the 2 cohorts were performed using Fisher’s exact test for comparisons of categorical variables and the Wilcoxon rank-sum test for comparisons amongst continuous variables. Comparisons amongst the number of positive skin tests to beta-lactam type by site were performed using a 2-sided test of proportions (Table I).
TABLE I.
Combined and separate characteristics of 2 beta-lactam skin testing cohorts in Nashville, TN, and Melbourne, Australia
| Combined cohort (n = 780) | Vanderbilt (n = 370) | Austin (n = 410) | P value comparing subcohorts | |
|---|---|---|---|---|
| Age (y) | ||||
| Median [IQR] | 59 [46, 69] | 59 [45,69] | 58 [47, 69] | .81 |
| 50 or older | 538 (69%) | 251 (68%) | 287 (70%) | |
| Between 25 and 50 | 190 (24%) | 95 (26%) | 95 (23%) | |
| 25 and younger | 52 (7%) | 24 (6%) | 28 (7%) | |
| Sex (female) | 527 (67%) | 280 (76%) | 247 (60%) | <.005 |
| Race | <.005 | |||
| Black | 18 (2%) | 17 (5%) | 1 (0%) | |
| White | 728 (93%) | 340 (92%) | 388 (95%) | |
| Asian | 27 (3%) | 6 (2%) | 21 (5%) | |
| Other/mixed | 7 (1%) | 7 (2%) | 0 | |
| Hispanic ethnicity | 5 (1%) | 5 (1%) | 0 | .02 |
| By cephalosporin allergy history | ||||
| Anaphylaxis | 37 (5%) | 23 (6%) | 14 (3%) | |
| Angioedema/swelling | 31 (4%) | 21 (6%) | 10 (2%) | |
| Blistering rash/skin sloughing | 7 (1%) | 6 (2%) | 1 (0%) | |
| Diffuse itchy rash or disseminated itching | 59 (8%) | 34 (9%) | 25 (6%) | |
| Diffuse, nonitchy rash | 57 (7%) | 35 (9%) | 22 (5%) | |
| DRESS syndrome | 8 (1%) | 5 (1%) | 3 (1%) | |
| Drug fever | 7 (1%) | 3 (1%) | 4 (1%) | |
| Fixed drug eruption | 5 (1%) | 3 (1%) | 2 (0%) | |
| Nausea/vomiting/diarrhea only | 13 (2%) | 6 (2%) | 7 (2%) | |
| Respiratory symptoms only | 12 (2%) | 11 (3%) | 1 (0%) | |
| Urticaria only | 76 (10%) | 48 (13%) | 28 (7%) | |
| Other/unknown | 16 (2%) | 11 (3%) | 5 (1%) | |
| Penicillin allergy label with unknown tolerance of cephalosporins | 452 (58%) | 164 (44%) | 288 (70%) | |
| Original cephalosporin allergy labels (some patients report multiple labels) | ||||
| Cephalexin | 136 (17%) | 78 (21%) | 58 (14%) | |
| Ceftriaxone | 61 (8%) | 39 (11%) | 22 (5%) | |
| Cefazolin | 43 (6%) | 18 (5%) | 25 (6%) | |
| Cefaclor | 33 (4%) | 24 (6%) | 9 (2%) | |
| Cefuroxime | 23 (3%) | 21 (6%) | 2 (0%) | |
| Cefdinir | 21 (3%) | 21 (6%) | 0 (0%) | |
| Cefepime | 18 (2%) | 16 (4%) | 2 (0%) | |
| Ceftazidime | 10 (1%) | 8 (2%) | 2 (0%) | |
| Cefpodoxime | 3 (0%) | 3 (1%) | 0 (0%) | |
| Cefoxitin | 3 (0%) | 3 (1%) | 0 (0%) | |
| Cefotaxime | 2 (0%) | 2 (1%) | 0 (0%) | |
| Patient reports a previous reaction to an unknown cephalosporin | 36 (5%) | 33 (9%) | 3 (1%) |
DRESS, Drug reaction with eosinophilia and systemic symptom; IQR, interquartile range.
The specific time that had elapsed since the original reaction to a penicillin or cephalosporin leading to allergy labeling was known and reported by 427 of 530 (81%) of patients with a history of immediate penicillin allergy and 217 of 245 (89%) of patients with a history of immediate cephalosporin allergy. We therefore modeled the relationship between time since the original reaction and the outcome of skin test positivity in patients with a reported immediate reaction to penicillins or cephalosporins using univariate logistic regression. We then adjusted for covariates of age (continuous), sex, race, and testing site. These covariates were selected a priori to examine their potential as predictors of skin test positivity based on clinical experience during the performance of the challenges, and to avoid overfitting. We also performed an analysis stratified by site. Our multivariable logistic regression therefore used either 6 or 7 degrees of freedom depending on whether testing site was included. The minimum sample size analyzed in this way, with 6 degrees of freedom, was 55 participants. Statistical analysis was performed using STATA statistical software version 15.0.26
RESULTS
A total of 780 patients received standardized skin testing for a label of penicillin or cephalosporin allergy from 2014 to 2018. Of our investigated cohort, 692 of 780 (89%) had a history of penicillin allergy and 328 of 780 (42%) had a reported history of cephalosporin allergy, with a total of 396 CALs reported amongst these 328 patients (Table I). In terms of cephalosporin allergy, 88 reported cephalosporin allergy only, 240 of 780 (34%) reported allergy to both penicillins and cephalosporins, and 452 of 780 (58%) reported a penicillin allergy with unknown tolerance of cephalosporins. Amongst our cohort, 69% were 50 or older (median age, 59 years [interquartile range: 46, 69]), 93% were white, and 67% were female (Table I). The VUMC US cohort had a higher proportion of patients referred with CALs (56%) than the Australian cohort (30%) (P < .0005). Amongst cephalosporin allergy—labeled patients, only 29 of 328 (8.8%) demonstrated positive allergy skin testing to a cephalosporin. There were no positive cefazolin or ceftriaxone skin tests in patients with a penicillin allergy and an unknown tolerance of cephalosporins, 0 of 452 (0%) (Figure 3).
FIGURE 3.
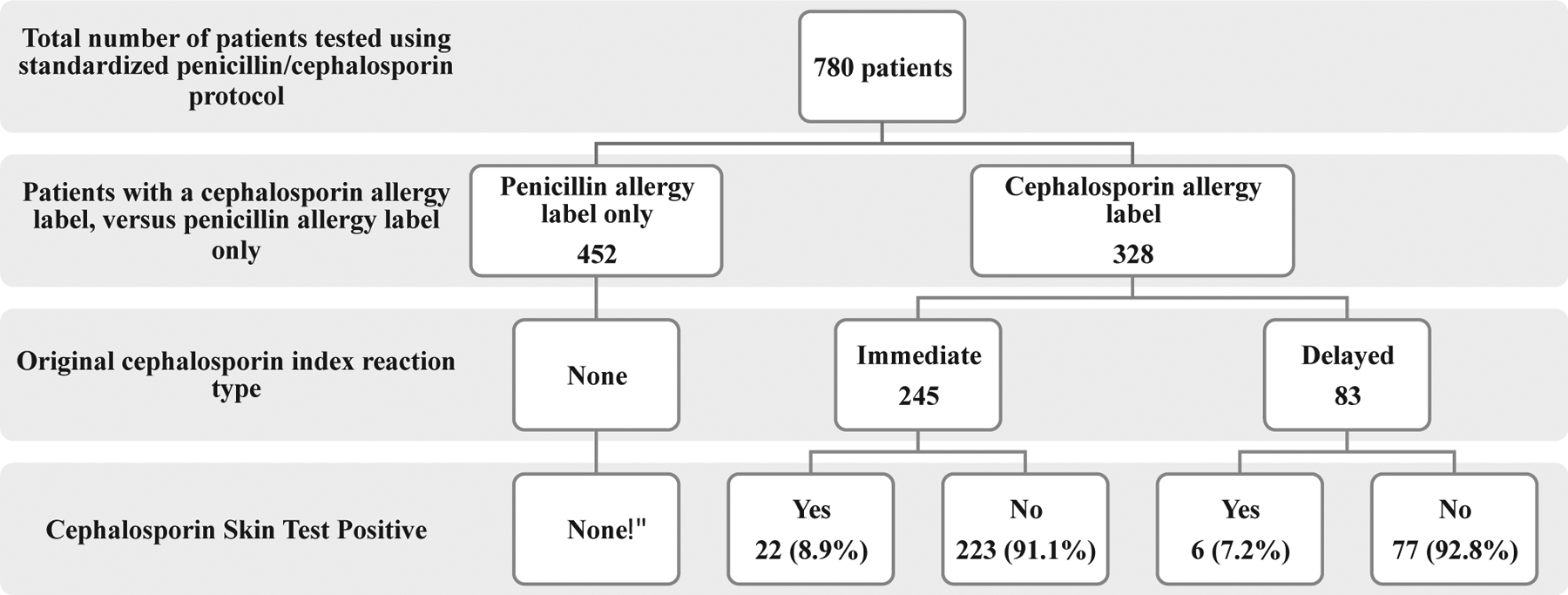
Flow diagram outlining cephalosporin testing results in patients with penicillin and cephalosporin allergy histories. *Patients with penicillin allergy label were tested with cefazolin and ceftriaxone only.
Immediate hypersensitivity cephalosporin labels
There were 245 patients with a CAL with a history of immediate hypersensitivity. Twenty-two of 245 (9.0%) had positive immediate hypersensitivity skin testing to a cephalosporin (Figure 4; Table E2, available in this article’s Online Repository at www.jaci-inpractice.org). This rate increased to 16 of 59 (27%) among patients with a history of an immediate hypersensitivity to a cephalosporin and a reported reaction with 1 year of testing. Among 22 patients with a confirmed allergy to either cefazolin, cefuroxime, ceftriaxone, ceftazidime, or cefepime, 17 tolerated an ingestion challenge to cephalexin, which has a non—cross-reactive R1 side chain with all of these cephalosporins. Another patient with a confirmed cefazolin allergy tolerated an ingestion challenge to cefuroxime. These patients were advised that they could take all cephalosporins in the future except those sharing an R1 or R2 group with the implicated cephalosporin (Figure 4; Table E2, available in this article’s Online Repository at www.jaci-inpractice.org). Representative skin test images can be found in Figures E1 and E2 (available in this article’s Online Repository at www.jaci-inpractice.org).
FIGURE 4.

Outcomes of immediate and delayed cephalosporin testing in this cohort. For individual-level data on labels, testing, and outcomes, please see Tables E2 and E5 (available in this article’s Online Repository at www.jaci-inpractice.org).
The other 223 of 245 patients with a history of immediate reaction to a cephalosporin had negative SPT and IDT to both penicillins and cephalosporins. This negative skin testing included the standard panel along with at least 1 drug containing the index reaction drug’s R1 side chain, when it was known. Of 223 of these patients, 139 (62%) underwent oral challenge to 250 mg penicillin VK or amoxicillin, followed by oral challenge to 250 mg cephalexin or another cephalosporin, and tolerated these challenges uneventfully, leading to delabeling of their cephalosporin allergy. A further 75 of 223 (34%) uneventfully tolerated oral challenge with penicillins but for logistical reasons have not yet not undergone oral challenge to a cephalosporin. These patients were advised it was safe to take all penicillins and advised to avoid cephalosporins pending further testing. Six of 223 (3%) have yet to undergo any oral challenge and have been advised to avoid both penicillins and cephalosporins pending further testing. There were no reactions observed during skin testing or ingestion challenges, either immediate or delayed, which were consistent with a true allergic reaction.
Delayed hypersensitivity cephalosporin labels
There were 83 patients with a history of delayed hypersensitivity to a cephalosporin; 7 of 83 (8%) had positive delayed IDT to a cephalosporin (Figure 4; Table E2, available in this article’s Online Repository at www.jaci-inpractice.org). Among 7 patients with a confirmed allergy to either cefazolin, cefoxitin, ceftriaxone, or cefepime, 2 underwent an ingestion challenge with cephalexin and both tolerated it. Representative skin test images can be found in Figure E3 (available in this article’s Online Repository at www.jaci-inpractice.org).
Skin testing results in patients labeled allergic to aminocephalosporins
Among patients who were initially labeled as allergic to aminocephalosporins (cefaclor, cefadroxil, cephalexin, cefprozil) and tested, 11 of 174 (6%) had positive skin testing to ampicillin, all of whom had carried cephalexin allergy labels, and 2 had positive skin testing to ceftriaxone (Figure 4). Sixteen patients had a STP to ampicillin with a primary reaction history to a cephalosporin. Of these, 10 (62.5%) had an immediate history and were positive on immediate SPT or IDT, with 8 of 10 (80%) reporting a primary reaction to cephalexin (an aminocephalosporin) and 1 to cefuroxime only. Another patient had a labeled history of immediate hypersensitivity reactions to both ceftriaxone and amoxicillin, but was positive to ampicillin skin testing only, and subsequently tolerated penicillin, flucloxacillin, cephalexin, and cefuroxime on test challenge in clinic and a subsequent therapeutic regimen of ceftriaxone (Tables E4 and E5, available in this article’s Online Repository at www.jaci-inpractice.org). Six of 16 (37.5%) had a delayed reaction history and were positive on delayed IDT to ampicillin within 24 and 48 hours of application. Of these, 3 (60%) reported a primary reaction to cephalexin, 1 to ceftriaxone only, and 1 to cefazolin only. The final patient reported separate delayed onset reactions to amoxicillin (drug reaction with eosinophilia and systemic symptom [DRESS]) and ceftriaxone (maculopapular exanthem), with positive tests to both drugs. Of these 16 cephalosporin-labeled patients who tested positive to ampicillin, only 2 had positive skin testing to a cephalosporin. In both of these cases, the patient reported an additional ceftriaxone allergy and tested positive to ceftriaxone. Testing strategies and specific modalities employed for each case are described in Tables E5 and E6 (available in this article’s Online Repository at www.jaci-inpractice.org).
There was notable geographic variation in positive ampicillin testing. Among all patients with ampicillin positive testing regardless of their initial penicillin or cephalosporin label, 32 of 420 (7.6%) ampicillin tests resulted positive in Melbourne versus 8 of 370 (2.2%) in Nashville, Fisher’s exact test P value < .005 (Table E3, available in this article’s Online Repository at www.jaci-inpractice.org). Within the subgroup of cephalosporin-labeled, ampicillin-positive patients with delayed and immediate reaction histories, 14 of 16 (93%) of these were Australian cases and only 2 were from VUMC.
Results from penicillin and cephalosporin allergy challenges
In total, of 328 patients with an initial reported label of cephalosporin allergy, 305 (80%) underwent an uneventful penicillin allergy challenge and 192 (59%) underwent an uneventful cephalosporin allergy challenge, leading to delabeling of their cephalosporin allergy. Of a total of 678 amoxicillin challenges and 230 cephalosporin challenges performed, only 1 beta-lactam challenge performed during our study elicited any significant symptoms: a patient with a history of cephalexin allergy and negative skin testing was challenged with amoxicillin and developed symptoms of pruritus, which was attributed to a nonimmunological effect (Austin Case 413, Table E5, available in this article’s Online Repository at www.jaci-inpractice.org) due to the absence of other clinical findings and resolution without treatment. The rest were tolerated uneventfully.
Characteristics of cefazolin and ceftriaxone positive cases
The most common STP cephalosporins in our cohort were cefazolin (Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org) and ceftriaxone (Figure E2, available in this article’s Online Repository at www.jaci-inpractice.org), which comprised 25 of 29 (86%) of all positive cases. Cefazolin was the most common drug to have positive immediate hypersensitivity skin testing, with 12 immediate cases observed and 1 delayed case observed. Of 43 cefazolin allergy labels tested, 30% resulted in positive testing. Ceftriaxone skin testing demonstrated 9 cases with positive immediate hypersensitivity skin testing and 6 with positive delayed hypersensitivity skin testing, making ceftriaxone the most common cephalosporin in our cohort to have a positive delayed hypersensitivity skin test. Of 61 ceftriaxone allergy labels tested, 9 (15%) resulted in positive testing, with 6 ceftriaxone positive skin tests in patients with primary labels to other cephalosporins (Figure 4; Table E2, available in this article’s Online Repository at www.jaci-inpractice.org). Seven of 15 (46%) of ceftriaxone STP patients demonstrated historical or skin test—based evidence of cross-reactivity with R1-similar cephalosporins such as cefepime, cefuroxime, or ceftazidime. There were 2 cases, both from Melbourne, who did not follow the typical pattern of side-chain cross-reactivity: patient 574, who had immediate skin test positivity to cefazolin, ceftriaxone, and penicillins in the absence of dermatographism, and patient 114, with a history of a delayed exanthem to cefazolin and meropenem DRESS that had a delayed skin test response to cefazolin, ceftriaxone, and meropenem. A more extensive panel of IDT and patch testing to other beta-lactams in these cases was not done due to lack of availability of the patients to return for further testing (Tables E2 and E3, available in this article’s Online Repository at www.jaci-inpractice.org).
Eleven of 11 cases who had clear isolated immediate skin test positivity to cefazolin also had clear histories of immediate reactions to cefazolin and were all negative on skin testing and oral challenge to other cephalosporins and penicillins, demonstrating the selective nature of immediate reactions to cefazolin consistent with the findings of other groups.13,14,19,27 Nine of 11 (82%) of these selective immediate cefazolin cases had their index reaction within the year before testing. An additional 2 cases with positive cefazolin skin testing had positive skin testing to other cephalosporins and/or beta-lactams as described above.
Predictive modeling: time since reaction
In univariate logistic regression analysis, increasing time since the original reaction in patients reporting immediate reactions was associated with decreased odds for a positive skin test to both penicillins (odds ratio [OR], 0.93 per year; 95% confidence interval [CI]: 0.89, 0.96) and cephalosporins (OR, 0.71 per year; 95% CI: 0.57, 0.90) (Figure 5). We observed that adjustment for age, sex, race, and site did not alter these findings significantly (Table E6, available in this article’s Online Repository at www.jaci-inpractice.org), except for a less significant relationship between time since reaction and penicillin testing at the Vanderbilt site. Our observed decrease in the probability of positive testing over time was consistent when stratified across both the US and Australian sites (Table E6, available in this article’s Online Repository at www.jaci-inpractice.org). More details about individual STP penicillin cases can be found in Table E5 (available in this article’s Online Repository at www.jaci-inpractice.org).
FIGURE 5.

Probability of obtaining positive immediate hypersensitivity skin testing in patients with an immediate reaction history (penicillins in the top panel, cephalosporins in the bottom panel). Predicted probabilities obtained via multivariable logistic regression modeling with adjustment for age, sex, race, and site.
DISCUSSION
Similar to penicillins, adult patients referred with a label of cephalosporin allergy can safely have their risk of a suspected IgE-mediated reaction or T-cel-mediated reaction assessed using a combination of skin testing and ingestion challenges. Only 1 patient with negative skin testing using our protocol had note-worthy symptoms during a challenge; challenges were otherwise well tolerated with rare reports of self-limited GI discomfort or subjective self-resolving pruritus. In our 2-site study, only 28 of 328 (8.5%) patients referred for a labeled cephalosporin allergy had positive cephalosporin skin testing, with 22 immediate hypersensitivity and 7 delayed hypersensitivity cases detected. Immediate skin test positivity was largely driven by time since the original reaction, with 18 of 22 (81%) immediate cephalosporin STP patients having their reaction within the last year (Table E3, available in this article’s Online Repository at www.jaci-inpractice.org). Cefazolin and ceftriaxone were the most common cephalosporins to test positive, which corresponded in every case but one with the suspected cephalosporin based on history, with an average time since index reaction of 0.57 years for cefazolin and 1.57 years for ceftriaxone (more delayed reactions to ceftriaxone were observed). There were no patients with a suspected false-positive immediate reaction to cephalosporins in our cohort, based on the recency and high severity of the index reactions, and our pretest causality assessment. In keeping with previous reports, 11 of 11 patients with a combination of a reported immediate reaction to cefazolin and positive skin testing to cefazolin were 100% selective to cefazolin with negative skin testing to ceftriaxone and ingestion challenges to other penicillins and cephalosporins (cephalexin) because cefazolin does not share a side chain with these drugs, a finding consistent with the reports of other groups.13,14,19,27
Our data do not support the idea that patients with a history of penicillin allergy and unknown tolerance of cephalosporins should be routinely tested for cephalosporin allergy, with 419 of 452 (92.7%) of patients being negative on skin testing and oral challenge to penicillins and 452 of 452 (100%) of such patients having negative cephalosporin skin testing to cefazolin and ceftriaxone. The exception to this might be patients with recent (within last 5 years) and immediate reactions to aminopenicillins where the risk of cross-reactivity to aminocephalosporins is still significant and may be geographically driven as reinforced by our study’s finding of 32 of 420 (7.6%) ampicillin positive skin tests in Melbourne versus 8 of 370 (2.2%) in Nashville despite our cohorts being of similar size. There is currently no validated strategy for skin testing aminocephalosporins that are only available as oral preparations; skin testing with a filtered preparation in at least 1 study has been shown to have low sensitivity,19 even though this has shown a high negative predictive value for other cephalosporins.13,17,28 Therefore, in these cases, we would recommend skin testing to parenterally available aminopenicillins and cephalosporins followed by oral challenge to cephalosporins not sharing the R1 aminocephalosporin side chain when aminopenicillin testing is positive. The flipside of this is that 16 of 328 (4.5%) patients with a history of cephalosporin allergy tested STP to ampicillin, 10 immediate and 6 delayed. Of all 16 ampicillin positive tests in patients with a CAL, 11 (69%) corresponded with an aminocephalosporin allergy history (cephalexin), suggesting that although the cross-reactivity may be low that this is a relevant strategy to pick up aminocephalosporin allergy. This phenomenon was predominantly observed in our Australian site cohort and is likely reflective of patterns reported previously in previous and Australian and European studies,14,17,24,29 where cross-reactivity between aminopenicillins such as ampicillin and aminocephalosporins such as cephalexin is more frequently observed.13,30 In the United States, because of differences in skin testing patterns and infrequent use of ampicillin as a testing reagent as well as lack of commercially available sterile reagents to perform IDT with aminocephalosporins, there is limited information from contemporary practice on the epidemiology of aminocephalosporin allergy and true cross-reactivity between aminocephalosporins and cephalosporins such as cefazolin and ceftriaxone. Similar to our study, however, previous studies have also suggested that the aminopenicillin/aminocephalosporin pattern of sensitization is infrequent in the United States.31
Our study was limited by the absence of universal oral challenge for all cephalosporin tested patients and focused on testing strategies using commercially available sterile IV cephalosporin reagents, specifically cefazolin and ceftriaxone. We used single-dose oral challenges, which are effective to rule out anaphylaxis, but a single-dose challenge has the potential to miss delayed onset reactions. We were further limited by the absence of an oral challenge strategy to account for the ceftazidime/aztreonam R1 side chain. In cases where skin testing was negative, we delabeled patients of allergy to intravenous drugs such as ceftriaxone based on oral tolerance of identical R1-containing oral drugs such as cefpodoxime, without direct intravenous or intramuscular challenge to the primary agent. Because this was a real-world retrospective cohort study at 2 clinical sites, the return of patients to complete follow-up testing was based in part on the clinical need of the patient as well as their ability to return. Not every patient with an initial positive skin test was able to return and complete follow-up testing to every relevant cephalosporin in our protocol. All patients with positive immediate or delayed skin testing had convincing recent histories of more recent immediate or delayed reactions, and in view of this high pretest probability and the high probability of inducing a clinical reaction, we did not directly challenge the specific cephalosporins where skin testing was positive. Given the severity of the immediate or delayed clinical reaction history many of these cases and high pretest probability, they were not challenged to confirm a systemic response. Contrary to previous studies, however,32 using criteria for a positive immediate skin test of a wheal ≥3 mm baseline and flare ≥5 mm baseline we did not see any STP to cephalosporins in patients with low probability or remote histories, and whenever possible all patients went onto ingestion challenge. Therefore, both the false negative and positive rates from skin testing would be predicted to be low using clinical history and ingestion challenge as the gold standard. For patients with a history of certain severe delayed reactions (eg, DRESS and acute granulomatous exanthematous pustulosis), we did not perform alternative cephalosporin challenges even when skin or patch testing was negative to an implicated drug, but would have considered doing so in the presence of a compelling clinical need where the benefit to be gained by treatment appeared to outweigh the risk.
However, the strengths of our study are that we observed that all our cases with positive immediate or delayed skin tests to cephalosporins already carried a preexisting label of cephalosporin allergy, typically acquired within 1 year of testing 22 of 29 (76%) had side chain—specific skin testing usually related to the R1 side chain, and 82% were proven to tolerate structurally dissimilar cephalosporins and penicillins. The strength of our study also lies in the overall similarity between 2 geographically distinct cohorts using the same testing protocols. Our findings are in keeping with previous studies that have reported loss of STP penicillin and cephalosporin allergy over time, and are limited for the time being to the risk factors that we report.33 In addition, although previous studies have reported loss of skin test reactivity over time, they have not followed patients with ingestion challenge. A number of questions remain unanswered such as risk factors for reactions to specific cephalosporins and the likelihood of positive cephalosporin testing. In addition, although previous studies have suggested significant loss of skin test reactivity over time, these have not been paired to demonstrated loss of ingestion challenge reactivity; therefore, longitudinal follow-up of our cohort is planned and will be important. Our retrospective 2-site study demonstrated that testing was safe and no patients experienced a systemic or severe reaction from immediate or delayed IDT or patch testing. Therefore, we believe that allergist knowledge of cephalosporin side chain groupings, specific testing protocols, and time since reaction are crucial elements of cephalosporin allergy management and provide insights into the mechanisms of these important drug allergies.
CONCLUSIONS
We demonstrate that cephalosporin skin testing and ingestion challenge is a safe mechanism to both delabel patients with cephalosporin allergy and identify patients with R1 side chain selective cross-reactivity who will tolerate non—R1 side chain sharing cephalosporins and penicillins. Although cefazolin and ceftriaxone were the most common cephalosporins associated with positive testing in both the Australian and US cohorts, geographical differences appeared for selective aminopenicillin allergy presenting with aminocephalosporin cross-reactivity. Selectively positive aminopenicillin skin testing, both immediate and delayed, and the potential for cross-reactivity with aminocephalosporins such as cephalexin appear to be much more common in Australia than the United States and may reflect differing utilization of parenteral aminopenicillins such as amoxicillin and amoxicillin-clavulanate that are widely used in Europe and Australia but not available in the United States.
Supplementary Material
What is already known about this topic?
Testing strategies for cephalosporin allergy have been poorly defined. Previous studies have suggested that cephalosporin cross-reactivity was primarily based on common shared R1 groupings.
What does this article add to our knowledge?
In 2 separate cohorts, allergy to ceftriaxone with R1 cross-reactivity and selective cefazolin allergy were the most common. Selective ampicillin intradermal test positivity in cephalexin-allergic patients was limited to the Australian cohort, and this was associated with aminopenicillin-aminocephalosporin R1 cross-reactivity.
How does this study impact current management guidelines?
Cephalosporin skin testing and ingestion challenge using approaches that account for structural similarities in R side chains is a safe and efficacious mechanism to evaluate cephalosporin allergy.
Acknowledgment
The authors would like to acknowledge Roger Yu for his assistance in supplementary data collection.
C. A. Stone receives funding from AHRQ 1K12HS026395-01, and was also funded by NIH/NHLBI T32 HL87738 and NIH/NIGMS T32 GM007569 during this project. J. A. Trubiano receives funding from National Health and Medical Research Council (NHMRC) postgraduate scholarship (GNT 1139902) and a postgraduate scholarship from the National Centre for Infections in Cancer, National Health and Medical Research Council, and BHF Centre of Research Excellence, Oxford (GNT 1116876). E. J. Phillips receives funding from National Institutes of Health (1P50GM115305-01, R21AI139021, and R34AI136815) and the National Health and Medical Research Foundation of Australia.
Abbreviations used
- CAL
Cephalosporin allergy label
- CI
Confidence interval
- DRESS
Drug reaction with eosinophilia and systemic symptom
- IDT
Intradermal test
- OR
Odds ratio
- SPT
Skin prick testing
- STP
Skin test positive
- VUMC
Vanderbilt University Medical Center
Footnotes
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
This study was done under IRB approved protocols from Vanderbilt University #161455 and the Austin Health HREC15/AUSTIN/75.
REFERENCES
- 1.Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep 2014;14:476. [DOI] [PubMed] [Google Scholar]
- 2.Joint Task Force on Practice Parameters. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010;105:259–73. [DOI] [PubMed] [Google Scholar]
- 3.Brockow K, Garvey L, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo M, et al. Skin test concentrations for systemically administered drugs—an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2013;68: 702–12. [DOI] [PubMed] [Google Scholar]
- 4.Demoly P, Adkinson N, Brockow K, Castells M, Chiriac A, Greenberger P, et al. International Consensus on drug allergy. Allergy 2014;69:420–37. [DOI] [PubMed] [Google Scholar]
- 5.Leysen J, Sabato V, Verweij M, De Knop K, Bridts C, De Clerck L, et al. The basophil activation test in the diagnosis of immediate drug hypersensitivity. Expert Rev Clin Immunol 2011;7:349–55. [DOI] [PubMed] [Google Scholar]
- 6.Hausmann O, Gentinetta T, Bridts C, Ebo D. The basophil activation test in immediate-type drug allergy. Immunol Allergy Clin North Am 2009;29:555–66. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Kim J, Jang Y, Choi J, Park S, Hwang Y, et al. The basophil activation test is safe and useful for confirming drug-induced anaphylaxis. Allergy Asthma Immunol Res 2016;8:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontaine C, Mayorga C, Bousquet P, Arnoux B, Torres M, Blanca M, et al. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate beta-lactam allergy. Allergy 2007;62:47–52. [DOI] [PubMed] [Google Scholar]
- 9.Johansson SG, Adedoyin J, van Hage M, Gronneberg R, Nopp A. False-positive penicillin immunoassay: an unnoticed common problem. J Allergy Clin Immunol 2013;132:235–7. [DOI] [PubMed] [Google Scholar]
- 10.Yoo H, Kim S, Kwon H, Kim T, Nam Y, Ye Y, et al. Immunologic evaluation of immediate hypersensitivity to cefaclor. Yonsei Med J 2014;55:1473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romano A, Gaeta F, Valluzzi R, Zaffiro A, Caruso C, Quaratino D. Natural evolution of skin-test sensitivity in patients with IgE-mediated hypersensitivity to cephalosporins. Allergy 2014;69:806–9. [DOI] [PubMed] [Google Scholar]
- 12.Van Gasse A, Ebo D, Faber M, Elst J, Hagendorens M, Bridts C, et al. Cross-reactivity in IgE-mediated allergy to cefuroxime: focus on the R1 side chain. J Allergy Clin Immunol Pract 2020;8:1094–10996.e1. [DOI] [PubMed] [Google Scholar]
- 13.Romano A, Valluzzi RL, Caruso C, Maggioletti M, Quaratino D, Gaeta F. Cross-reactivity and tolerability of cephalosporins in patients with IgE-mediated hypersensitivity to penicillins. J Allergy Clin Immunol Pract 2018;6:1662–72. [DOI] [PubMed] [Google Scholar]
- 14.Romano A, Gaeta F, Valluzzi R, Maggioletti M, Zaffiro A, Caruso C, et al. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of alternative cephalosporins. J Allergy Clin Immunol 2015;136:685–691.e3. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Sancho F, Perez-Inestrosa E, Suau R, Montanez MI, Mayorga C, Torres MJ, et al. Synthesis, characterization and immunochemical evaluation of cephalosporin antigenic determinants. J Mol Recognit 2003;16:148–56. [DOI] [PubMed] [Google Scholar]
- 16.Romano A, Torres M, Fernandez J, Vega J, Mayorga C, Garcia J, et al. Allergic reactions to ampicillin. Studies on the specificity and selectivity in subjects with immediate reactions. Clin Exp Allergy 1997;27:1425–31. [PubMed] [Google Scholar]
- 17.Romano A, Gaeta F, Valluzzi RL, Maggioletti M, Caruso C, Quaratino D. Cross-reactivity and tolerability of aztreonam and cephalosporins in subjects with a T cell-mediated hypersensitivity to penicillins. J Allergy Clin Immunol 2016;138:179–86. [DOI] [PubMed] [Google Scholar]
- 18.Romano A, Gaeta F, Arribas Poves M, Valluzzi R. Cross-reactivity among beta-lactams. Curr Allergy Asthma Rep 2016;16:24. [DOI] [PubMed] [Google Scholar]
- 19.Yuson C, Kumar K, Le A, Ahmadie A, Banovic T, Heddle R, et al. Immediate cephalosporin allergy. Intern Med J 2019;49:985–93. [DOI] [PubMed] [Google Scholar]
- 20.Trubiano J, Stone C, Grayson M, Urbancic K, Slavin M, Thursky K, et al. The 3 Cs of antibiotic allergy-classification, cross-reactivity, and collaboration. J Allergy Clin Immunol Pract 2017;5:1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. FDA Approved Drug Products. Washington, DC: US Food and Drug Administration: FDA; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/050114s008ltr.pdf. [Google Scholar]
- 22.Fernandez J, Torres M, Campos J, Arribas-Poves F, Blanca M, DAP-Diater Group. Prospective, multicenter clinical trial to validate new products for skin tests in the diagnosis of allergy to penicillin. J Investig Allergol Clin Immunol 2013;23:398–408. [PubMed] [Google Scholar]
- 23.Adkinson NF Jr, Mendelson LM, Ressler C, Keogh JC. Penicillin minor determinants: history and relevance for current diagnosis. Ann Allergy Asthma Immunol 2018;121:537–44. [DOI] [PubMed] [Google Scholar]
- 24.Bourke J, Pavlos R, James I, Phillips E. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract 2015;3 365–34.e1. [DOI] [PubMed] [Google Scholar]
- 25.Trubiano JA, Thursky KA, Stewardson AJ, Urbancic K, Worth LJ, Jackson C, et al. Impact of an integrated antibiotic allergy testing program on antimicrobial stewardship: a multicenter evaluation. Clin Infect Dis 2017;65:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP; 2017. [Google Scholar]
- 27.Uyttebroek AP, Decuyper II, Bridts CH, Romano A, Hagendorens MM, Ebo DG, et al. Cefazolin hypersensitivity: toward optimized diagnosis. J Allergy Clin Immunol Pract 2016;4:1232–6. [DOI] [PubMed] [Google Scholar]
- 28.Romano A, Gueant-Rodriguez RM, Viola M, Amoghly F, Gaeta F, Nicolas JP, et al. Diagnosing immediate reactions to cephalosporins. Clin Exp Allergy 2005;35:1234–42. [DOI] [PubMed] [Google Scholar]
- 29.Romano A, Gueant-Rodriguez RM, Viola M, Pettinato R, Gueant JL. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16–22. [DOI] [PubMed] [Google Scholar]
- 30.Miranda A, Blanca M, Vega JM, Moreno F, Carmona MJ, Garcia JJ, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671–7. [DOI] [PubMed] [Google Scholar]
- 31.Macy E, Blumenthal KG. Are cephalosporins safe for use in penicillin allergy without prior allergy evaluation? J Allergy Clin Immunol Pract 2018;6:82–9. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Kang D, Seo B, Park H, Park S, Kim M, et al. Incidence of cephalosporin-induced anaphylaxis and clinical efficacy of screening intradermal tests with cephalosporins: a large multicenter retrospective cohort study. Allergy 2018;73:1833–41. [DOI] [PubMed] [Google Scholar]
- 33.Trubiano JA, Adkinson NF, Phillips EJ. Penicillin allergy is not necessarily forever. JAMA 2017;318:82–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


