Abstract
Purpose:
For high-risk prostate cancer, standard treatment options include radical prostatectomy (RP) or radiation therapy plus androgen-deprivation therapy (ADT). Despite definitive therapy, many patients will have disease recurrence. Imaging has the potential to better define characteristics of response and resistance. In this study, we evaluated prostate multiparametric MRI (mpMRI) before and after neoadjuvant enzalutamide plus ADT.
Experimental Design:
Men with localized intermediate- or high-risk prostate cancer underwent a baseline mpMRI and mpMRI-targeted biopsy followed by a second mpMRI after 6 months of enzalutamide and ADT prior to RP. Specimens were sectioned in the same plane as mpMRI using patient-specific 3D-printed molds to permit mpMRI-targeted biopsies to be compared to the same lesion from the RP. Specimens were analyzed for imaging and histologic correlates of response.
Results:
Of 39 patients enrolled, 36 completed imaging and RP. Most patients (92%) had high-risk disease. Fifty-eight lesions were detected on baseline mpMRI, of which 40 (69%) remained measurable at 6-month follow-up imaging. 55/59 lesions (93%) demonstrated >50% volume reduction on post-treatment mpMRI. 3/59 lesions (5%) demonstrated growth in size at follow-up imaging, with 2 lesions increasing more than threefold in volume. On whole-mount pathology, 15 patients demonstrated minimal residual disease (MRD) of <0.05cc or pathologic complete response. Low initial mpMRI relative tumor burden was most predictive of MRD on final pathology.
Conclusions:
Low relative lesion volume at baseline mpMRI was predictive of pathologic response. A subset of patients had limited response. Selection of patients based on these metrics may improve outcomes in high-risk disease.
Keywords: prostate cancer, enzalutamide, androgen-deprivation therapy, multiparametric MRI, radical prostatectomy
Introduction
Prostate cancer is the second leading cause of cancer death in individuals who are born biologically male in the United States, with an estimated 191,930 new cases and 33,330 deaths in 2020 (1). While radical prostatectomy (RP) or radiation therapy (RT) are potentially curative modalities for patients with localized disease, 20–40% of patients treated with RP or RT will ultimately develop castration-resistant prostate cancer (2). It is imperative to evaluate new treatment strategies, particularly in individuals with high-risk disease. Androgen deprivation therapy (ADT), combined with newer generation androgen receptor (AR)-axis inhibitors such as abiraterone and enzalutamide are widely used in metastatic castration-resistant prostate cancer (3,4), metastatic castration-sensitive prostate cancer (5) and non-metastatic castration-resistant prostate cancer (6). More recent studies have shown promise in reducing tumor burden and cancer stage in the neoadjuvant setting, prior to RP (7–10).
In particular, neoadjuvant systemic therapy has been evaluated in multiple clinical trials as a primary intervention to reduce morbidity and mortality of prostate cancer in intermediate- and high-risk patients. These studies have focused on the intensification of ADT with more potent AR-axis inhibitors. A recurrent outcome measure in these trials has been the determination of the pathologic complete response (pCR) rate, which implies the complete elimination of disease, although a threshold for specifying minimal residual disease (MRD) similarly identifies exceptional responders. In an exploratory pooled analysis of 3 neoadjuvant studies (11), neoadjuvant treatment prior to RP showed a positive impact on time to biochemical recurrence (BCR). Patients below the MRD threshold did not experience BCR in the timeframe of the meta-analysis, suggesting that complete response (CR) or MRD is a short-term surrogate outcome for BCR.
Utilizing intense ADT with enzalutamide in the neoadjuvant setting in combination with a dedicated imaging modality, such as multiparametric MRI (mpMRI), is a potentially promising strategy in high-risk localized disease. There is a critical need to incorporate newer imaging modalities in order to better define the initial tumor burden and subsequent response. While mpMRI has been successfully used to identify high-risk disease for sampling by biopsy (12), the role of mpMRI in the setting of neoadjuvant therapy is unclear. This feasibility trial was designed to evaluate tumors on mpMRI both before and after treatment with enzalutamide plus ADT prior to robot-assisted radical prostatectomy (RARP) and to correlate imaging findings with final pathology findings.
Patients and Methods
Study approval
This trial was approved by the Institutional Review Board of the Center for Cancer Research, National Cancer Institute (ClinicalTrials.gov identifier: NCT02430480). This study has been conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with the International Council on Harmonization guidelines on Good Clinical Practice, all applicable laws and regulatory requirements, and all conditions required by a regulatory authority and/or institutional review board. Written informed consent was obtained for all patients prior to performing study-related procedures in accordance with federal and institutional guidelines.
Study design
In this study, men with newly diagnosed intermediate- or high-risk localized prostate cancer were treated for 6 months with complete androgen blockade with enzalutamide, a potent AR antagonist, plus ADT with goserelin, leuprolide, or degarelix (based on discretion of the treating physician), followed by RARP. Patients underwent 2 mpMRIs: one at baseline and one after 6 months of treatment. Each patient underwent subsequent RARP with whole-mount histopathological analysis to assess lesion response.
Eligible patents had pathologically confirmed intermediate- or high-risk localized prostate cancer with no prior history of treatment for prostate cancer. Intermediate-risk disease was defined as a prostate-specific antigen (PSA) level of 10–20 ng/mL or a Gleason score of 7 or clinical stage T2b or T2c. High-risk disease was defined as PSA >20 ng/mL at diagnosis or Gleason score of 8–10 or seminal vesicle involvement or possible extracapsular extension (on mpMRI). Patients were required to have an Eastern Cooperative Oncology Group performance status of 0–1 and baseline testosterone levels of ≥100 ng/dL. Patients with distant metastatic disease beyond N1 (regional) lymph nodes on conventional imaging studies (CT, MRI, or bone scan) were excluded.
This was an open-label feasibility study of enzalutamide 160 mg orally daily × 28 days for a total of 6 cycles plus ADT. Thirty-seven patients received goserelin 10.8 mg subcutaneously at weeks 0 and week 12 on treatment. One patient had leuprolide acetate depot 22.5 mg intramuscularly × 2 doses. One patient received the loading dose of degarelix (240 mg subcutaneously at week 0), followed by degarelix 80 mg subcutaneously × 1 dose at week 4 and was transitioned to goserelin at week 8. Patients underwent 2 separate mpMRIs of the prostate, at baseline and after 24 weeks, followed by RARP (Fig. 1). Patients who underwent MRI-targeted, combined biopsy at the NCI for prostate cancer diagnosis did not require an additional biopsy prior to study enrollment. If a patient had not undergone an MRI-targeted biopsy previously, a second biopsy was required.
Figure 1. Study schema.
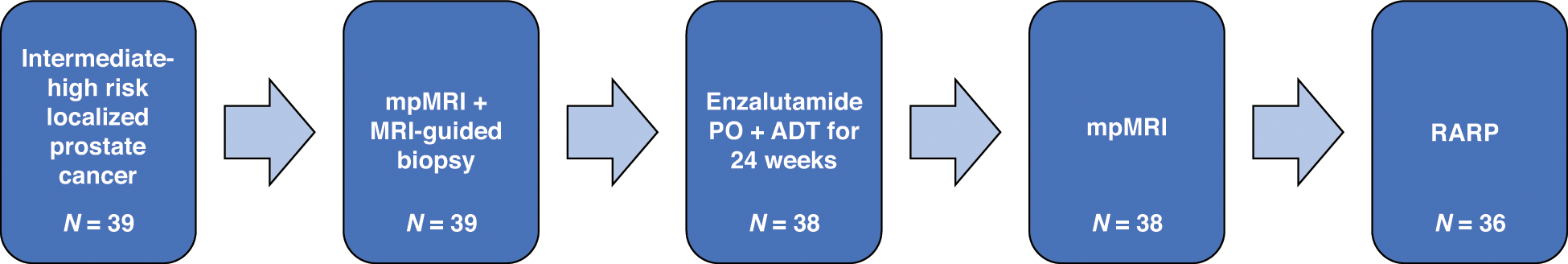
mpMRI: multiparametric MRI; ADT: androgen deprivation therapy; RARP: robot-assisted radical prostatectomy.
Surgery and pathologic analysis
Initial pathology was determined by transrectal ultrasound (TRUS)-guided systematic biopsy, if there was reasonable assurance that the biopsy was from the index lesion, and/or TRUS-MRI fusion-guided biopsy prior to initiation of neoadjuvant enzalutamide plus ADT. The index lesion by mpMRI was defined as the lesion with the largest size and highest PI-RADS score. All biopsy specimens were reviewed and assigned Gleason scores by a genitourinary pathologist.
RARP was performed on 36 patients after 24 weeks of complete androgen blockade and final mpMRI. A single urologist performed all but one RARP. All patients underwent extended lymph node dissection. Whole-mount prostate pathology slides were prepared using 3D-printed custom prostate molds to correspond with slices from each patient’s final mpMRI scan (13).
All post-treatment specimens were analyzed by 2 non-blinded genitourinary pathologists. Post-operative pathologic staging was assigned to each patient; however, Gleason tumor grade could not be assigned to residual tumors due to treatment effect. Presence of residual tumor was noted and annotated on H&E whole-mount pathology slides. For three patients who demonstrated substantial pathologic resistance to treatment, the NCI Laboratory of Pathology selected FFPE tissue blocks for panel-based Oncomine Comprehensive Assay v3 (Thermo Fisher Scientific) sequencing to determine potential eligibility for ongoing clinical trials.
Image analysis of mpMRI
Prostate and lesion volumes on baseline and post-treatment mpMRI were calculated from T2W-MRI sequences using software embedded in the PACS after manual contouring by the same radiologist (B.T.). mpMRI at baseline and after 24 weeks of neoadjuvant treatment were acquired with a 3-Tesla scanner (Achieva; Philips Healthcare, Best, The Netherlands). Patients underwent imaging with integrated external (surface) phased array coil and endorectal coil. Imaging studies were interpreted by a single genitourinary radiologist with over 10 years of experience in prostate imaging. Evaluated imaging sequences included T2W-MRI, apparent diffusion coefficient (ADC) maps of diffusion-weighted imaging, high-b value (b = 2000 s/mm2), and dynamic contrast-enhanced MRI (temporal resolution = 5.6s). Lesions at baseline mpMRI were assigned Prostate Imaging Reporting and Data Systems (PI-RADS) categories based on stringent interpretation of PI-RADSv2.0 guidelines (14) (PI-RADSv2.1 was published near the end of this study).
All MRI-visible lesions at baseline were documented and followed over the course of the study. Evidence of extra-prostatic extension (EPE), seminal vesical invasion, and bulky nodal disease were noted in mpMRI reports. Each mpMRI scan was assigned an EPE grade from 0–3, as previously described (15): 0 = no evidence of EPE, 1 = large lesion-capsule contact length or capsular bulge, 2 = large lesion-capsule contact length and capsular bulge, 3 = frank EPE. Only EPE grade 0 was considered “organ-confined disease.” Per PI-RADSv2 guidelines, up to 4 MRI-visible lesions per patient were targeted for biopsy and were included in lesion-level analyses, irrespective of biopsy Gleason score. Biopsy targeting was performed using a commercially available TRUS/MRI-fusion biopsy platform, UroNav (InVivo, Philips, Gainsville, FL). Prior to biopsy, whole prostate gland MRI and MRI-visible lesions were manually segmented using the DynaCad platform (InVivo, Gainsville, FL).
In addition to these qualitative mpMRI characteristics, higher-order quantitative metrics were obtained from baseline mpMRI scans, including lesion volume, ADCs, and perfusion (Ktrans; calculated using a 2-compartment Tofts model with standardized arterial input function). In patients with multiple contoured lesions at baseline, their volumes were added to produce a patient-level mpMRI lesion volume. To assess MRI evidence of neoadjuvant treatment response, post-treatment mpMRI scans were acquired with the same parameters; however, PI-RADS categories were not assigned due to treatment effects on MRI. On both mpMRI scans, total prostate volume was measured. Relative tumor burden (RTB) volumes were calculated by dividing the combined volumes of each lesion divided by the prostate volume, such that RTB is expressed as the percentage of prostate volume taken by the tumor.
Residual cancer burden calculations
Residual tumor was concordantly identified by 3 board-certified genitourinary pathologists (M.M., R.L., and H.Y.). Pathologic analysis was performed independently from imaging analysis, and pathologists did not have access to imaging data to inform tissue analysis. All prostate specimens were completely submitted for identification of residual disease. Residual cancer burden (RCB) was measured and calculated by multiplying the number of slices through which each residual tumor extended by the largest cross-sectional width and length and block thickness (0.6 cm). Volume was further corrected by multiplying by 0.4 and the estimated tumor cellularity. Minimal residual disease (MRD) was considered < 0.05 cc, and non-response (NR) was > 0.05 cc. Each mpMRI-visible lesion was similarly classified based on the volume of the evaluable tumor remaining in the anatomical region the lesion was in. If a patient had more than one residual lesion at final pathology, the largest cross-sectional dimension of tumor was used to determine RCB for the purpose of classifying the patient as MRD or NR.
Statistics on mpMRI
Logistic regression analysis was performed to correlate clinical variables and MRI parameters with patient response categorized as CR/MRD vs NR. Variables which were significant at the 0.05 level in the univariable analysis were considered for multivariable analysis. The performance of predictors was evaluated by the area under the curve (AUC) with its 95% confidence interval determined by the Delong method. The Youden index was calculated to determine the cutoffs that gave the optimal combination of sensitivity and specificity for patient response. A two-sided P value <0.05 was considered statistically significant for all analyses. All analyses were conducted using R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Between June 2015 and September 2018, 39 patients with newly diagnosed localized prostate cancer were enrolled on this study and treated with enzalutamide plus ADT (see Fig. 1). The median baseline PSA was 9.56 ng/mL (1.18–985.9 ng/mL). The median grade group was 4 (Gleason score 8). Thirty-six patients had high-risk disease and 3 had intermediate-risk disease (Table 1). Three patients did not complete RARP. One patient died from an recreational drug overdose during the first month of treatment. A second patient was unable to undergo RARP due to anesthesia concerns, and one patient progressed on-treatment. A third patient exhibited tumor extension to the bladder while on treatment and underwent palliative transurethral resection of the prostate/transurethral resection of a bladder tumor (TURP/TURBT) due to the extent of the prostate cancer and thus did not under RARP, so final pathology volumes are not available. He went on to receive RT to the pelvis.
Table 1.
Baseline patient characteristics for 39 patients with localized prostate cancer. Data are presented as N (IQR) or % (N). IQR: interquartile range.
| Demographics | ||
| yr | (IQR) | |
| Median age | 61 | (58–69) |
| Race | % | (N) |
| White | 69 | (27) |
| Black | 15 | (6) |
| Other | 15 | (6) |
| Clinical | ||
| # | (IQR) | |
| Median PSA (ng/ml) | 9.56 | (1.18–985.9) |
| Median PSA density | 0.22 | (0.15–0.39) |
| Median prostate volume (cc) | 42.0 | (33.0–49.0) |
| Median MRI tumor burden (cc) | 3.47 | (1.9–9.8) |
| Risk group | % | (N) |
| Intermediate | 8 | 3 |
| High | 92 | 36 |
| Histology | ||
| Pathologic stage | % | (N) |
| T2 | 15 | (6) |
| T3a | 44 | (17) |
| T3b | 28 | (11) |
| T4 | 12 | (5) |
| N1 | 28 | (11) |
| Gleason score (Grade group) | % | (N) |
| 3 + 4 (2) | 15.4 | (6) |
| 4 + 3 (3) | 12.8 | (5) |
| 4 + 4 (4) | 28.2 | (11) |
| 4 + 5; 5 + 4; 5 + 5 (5) | 46.6 | (17) |
Of 39 patients enrolled on-study, three had disease progression with enlargement of lesions on mpMRI (Fig. 2a). All three non-responders recurred during the first year. One patient progressed during treatment (as above) and two progressed shortly after RARP. Additionally, to date, 9 patients had recurred biochemically; 2 patients in the first year, 5 patients in the second and 2 patients during the third year. The median follow-up is 3 years. Two of the non-responders went on to develop metastatic disease and 1 has died.
Figure 2. Response to neoadjuvant enzalutamide plus ADT.
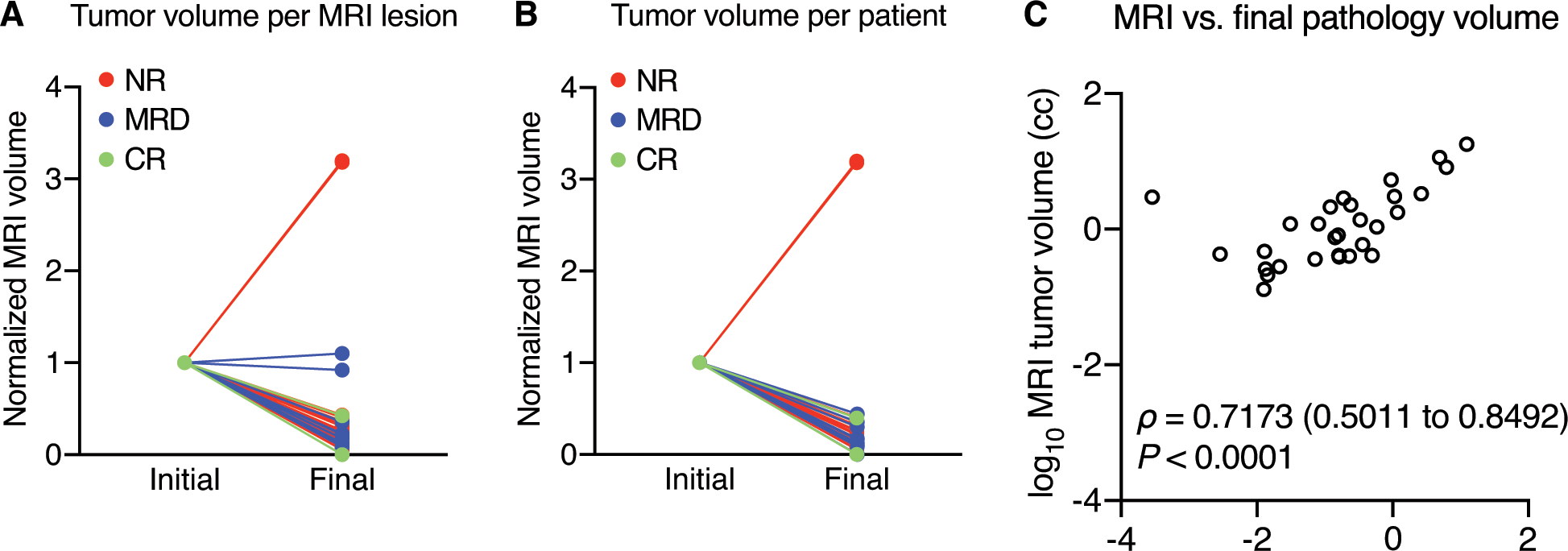
(A) 55/59 lesions demonstrated >50% volume reduction. (B) 36/38 patients demonstrated >50% volume reduction. Lesions and patients are color-coded based on pathologic response to treatment at RP. One patient completed baseline and post-enzalutamide plus ADT mpMRI but did not undergo RARP, thus he was included to demonstrate MRI evidence of extreme non-response but was excluded from all analyses involving pathologic response to treatment. (C) Spearman correlation of final imaging volumes per patient with final pathologic residual cancer burden, logarithmically transformed. Values of zero were transformed to nominal values to preserve their rank within the dataset but omitted from the graph.
Adverse events
Treatment was well tolerated, with no grade 4 events and one grade 5 event not attributable to study treatment (Supplementary Table 1). The most common adverse events (AEs) were grade 1 hot flashes (34/39, 87%), grade 1 fatigue (20/39, 51%), grade 1 decrease in libido (15/39, 38%), grade 1 insomnia (12/39, 31%), and grade 1 erectile dysfunction (9/39, 23%). Grade 2 and grade 3 hypertension (18% each) was manageable with effective antihypertensive medication. No patients discontinued therapy due to toxicity. Sexual function assessment was captured through the Common Terminology Criteria for Adverse Events (CTCAE) and not through sexual function assessment.
Response to neoadjuvant treatment on mpMRI
Within the study population, 59 lesions were detected at baseline mpMRI and 40 remained measurable at follow-up mpMRI after 24 weeks of enzalutamide plus ADT. 55/59 lesions (93%) demonstrated greater than 50% volume reduction on post-treatment mpMRI. 3/59 lesions (5%) demonstrated growth in size at follow-up imaging, with 2 of the lesions increasing more than threefold in volume (see Fig. 2a). At baseline mpMRI, all patients had measurable disease, with a median tumor burden of 3.44 cc. Median prostate volume decreased from 39.5 cc to 20.0 cc after treatment, and median PSA decreased from 9.56 ng/mL to undetectable (<0.02 ng/mL). On post-treatment MRI, 4/36 patients (11%) had no visible disease, and on final pathology, 2/36 patients (6%) were staged as a complete pathologic response (ypT0N0). Six patients of 36 (17%) had no evidence of EPE on baseline mpMRI, and this number increased to 21 patients (58%) without EPE at mpMRI after treatment. Twenty-three patients (64%) had organ-confined disease on final pathology. On baseline mpMRI, 4 patients (11%) had evidence of bladder or rectal invasion; only one patient had visible bladder or rectal invasion on follow-up imaging and on final pathology. Table 2 summarizes mpMRI evidence of pathologic features before and after treatment, including EPE, SVI lymph node involvement, etc., and corresponding findings at final pathology.
Table 2.
mpMRI detection of pathologic features before and after complete androgen blockage and corresponding findings on whole-mount RARP specimens (N = 36). mpMRI: multiparametric MRI; RARP: robot-assisted radical prostatectomy
| Feature | Baseline | Post-treatment | Final Pathology |
|---|---|---|---|
| No visible disease | 0 (0%) | 4 (11%) | 2 (6%) |
| Organ-confined disease | 6 (17%) | 21 (58%) | 23 (64%) |
| Seminal vesical invasion | 10 (28%) | 6 (17%) | 8 (22%) |
| Regional lymph node involvement | 10 (28%) | 7 (19%) | 5 (14%) |
| Bladder/rectal invasion | 4 (11%) | 1 (3%) | 1 (3%) |
Upon final pathologic analysis, 9 lesions (15%) demonstrated CR, 30 lesions (52%) demonstrated MRD, and 19 lesions (33%) demonstrated NR (see Fig. 2a). At the patient level, two patients (6%) demonstrated CR, 13 patients (36%) demonstrated MRD, and 22 patients (61%) demonstrated NR, including one patient who underwent TURP/TURBT for prostate cancer (Fig. 2b). Two patients with NR were determined by IHC of post-treatment tissue to have decreased AR and PSA expression in combination for positive staining for synaptophysin and Ki-67, which indicated neuroendocrine differentiation (16,17). The combined volumes of each imaging lesion per patient demonstrated a statistically significant correlation with final pathology residual cancer burdens with Spearman ρ = 0.7173 (Fig. 2c).
For the three NR patients (Supplementary Table 3) who demonstrated substantial volumes of residual disease or progression on treatment, we performed clinical cancer panel sequencing on post-treatment surgical tissue using the Oncomine platform to assess potential drivers and determine eligibility for additional trials. Germline alterations to homologous recombination genes were observed in two of the three patients (BRCA2 frameshift and PALB2 nonsense), with loss of function alterations to RB1 observed in all three cases. In one patient whose radical prostatectomy specimen demonstrated focal neuroendocrine features, biallelic inactivation of TP53, PTEN, and RB1 were observed in conjunction with the TMPRSS2-ERG fusion.
Pathology and immunohistochemistry results
As enzalutamide plus ADT synergize by reducing the concentration of testosterone, dihydrotestosterone, and androgens binding to the AR, the effect of intense androgen suppression and inhibition was measured on tumors using anti-AR and anti-PSA immunostains of biopsy and surgical specimens (Fig. 3a and 3b). Median nuclear AR levels were substantially higher in diagnostic biopsy specimens vs. residual tumor (staining indices of 0.7568 and 0.1661, respectively, P < 0.0001 by Welch’s t test) (Fig. 3c). As the KLK3 gene encoding the PSA protein is a primary target of AR-driven transcription, anti-PSA IHC was performed to assess AR activity in situ. Like AR, median cytoplasmic PSA histology scores of residual tumors were substantially lower compared to pre-treatment tumor biopsies (0.1380 and 0.7062, respectively, P < 0.0001 by Welch’s t test), demonstrating reduced but persistent AR activity (Fig. 3d).
Figure 3. Histologic analysis of in situ tissue phenotypes.
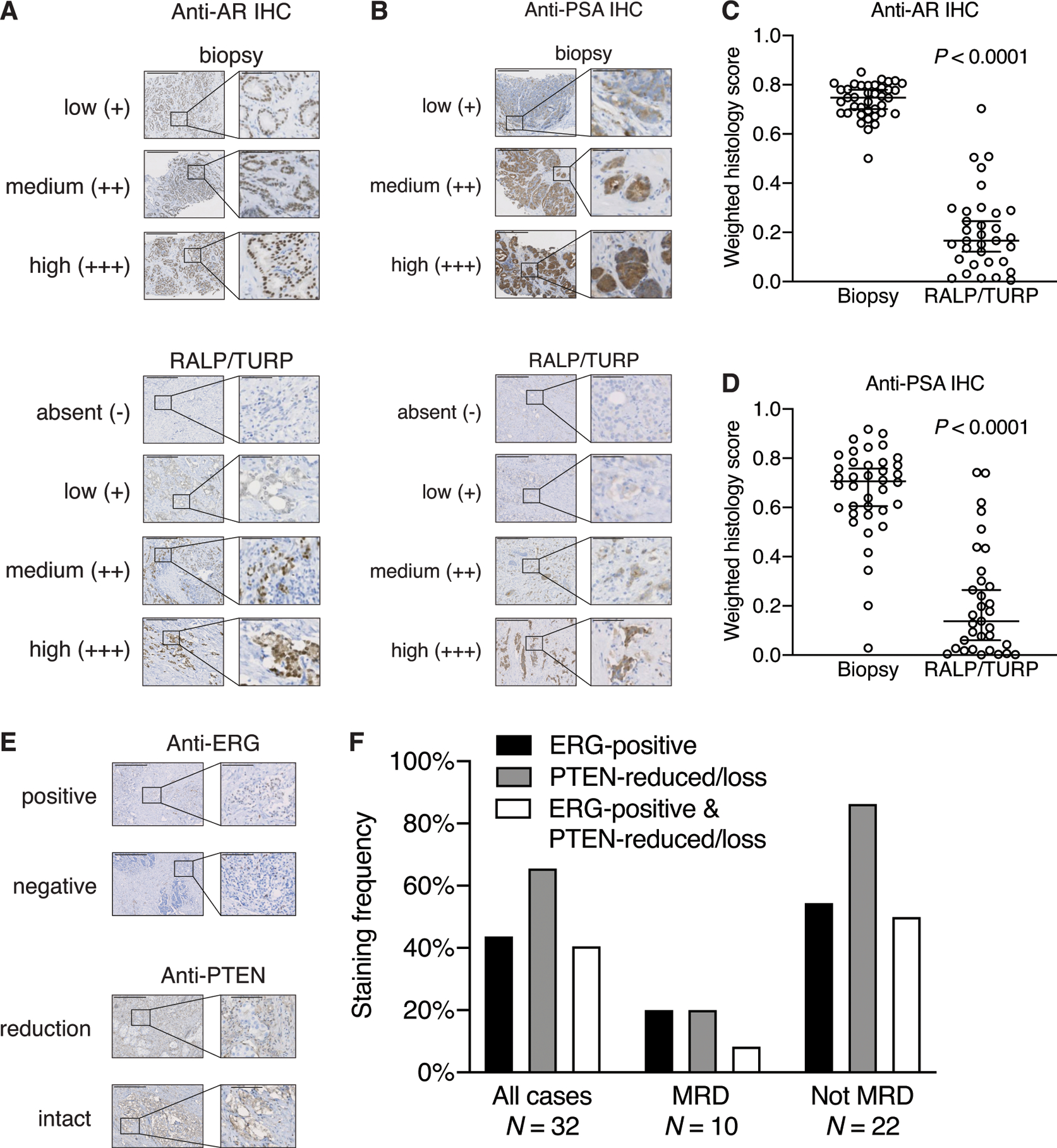
Representative micrographs of low, medium, and high histology scores for anti-AR (A) and anti-PSA (B) immunostaining of biopsies (top) and absent, low, medium, and high histology scores for post-treatment surgical RALP or TURP specimens (bottom). There are no examples of absent anti-AR or PSA staining in pre-treatment biopsied tumor tissue. Scale bar: 500 μm. Inset scale bar: 100 μm. Tissue sections immunostained with (C) anti-AR from biopsies (N = 39 cases representing 82 different tissue blocks) and post-treatment surgical specimens (N = 33 cases representing 61 different tissue blocks) or (D) anti-PSA from biopsies (N = 38 cases representing 79 different tissue blocks) and post-treatment surgical specimens (N = 33 cases representing 64 different tissue blocks) were quantified with Definiens to measure stain intensity. Histology scores were reported on a weighted index of 0 to 1, and multiple slides from the same individual were added before computing the index. Each circle represents a single patient. Bars represent median and 95% confidence interval. P < 0.0001 for both AR and PSA staining, by Welch’s t test. (E) Representative micrographs of positive (overexpressed) and negative nuclear ERG immunostaining (left) and reduced or intact PTEN (right) in post-treatment tissue. Scale bar: 500 μm. Inset scale bar: 100 μm. Note that nuclear ERG intensity is reduced relative to endogenous ERG expression in endothelial cells on account of its expression being driven by (reduced) AR activity. (F) Post-treatment tissue sections were immunostained with anti-ERG and anti-PTEN antibodies and the frequency of ERG-positive tumor cells (any percentage positive), or PTEN-reduced or PTEN-deficient (at least 5% of tumor cells) are shown. pCR/MRD vs. NR for PTEN was different at P = 0.0006 by Fisher’s exact test.
TMPRSS2-ERG fusion occurs in approximately 50% of prostate cancers and frequently co-occurs with the loss of PTEN, which in turn is associated with more aggressive prostate cancer (18). Therefore, ERG protein overexpression and PTEN reduction was evaluated using IHC in post-treatment specimens (Fig. 3e). Of 37 patients who completed treatment and underwent surgery, evaluable residual tumor was available in 32 cases, of which 14 (43.8%) harbored ERG-positive tumor cells and 21 (65.6%) demonstrated PTEN staining reduction or loss (Fig. 3f). Comparing pCR/MRD vs. NR cases, reduction of PTEN showed significant differences (P = 0.0006, Fisher’s exact test) between the pCR/MRD group (2 cases, 20%) vs. the NR group (19 cases, 86.4%). However, the difference between groups was less with respect to ERG-positive tumor cell staining (20% vs. 54.5%, respectively; P = 0.073, Fisher’s exact test) and the combined reduction of PTEN with ERG-positive staining (8.3% vs. 50%, respectively; P = 0.0931, Fisher’s exact test). Therefore, reduction of PTEN levels is significantly associated with resistance to intense ADT driving greater residual tumor volumes.
Predicting patient-level response from baseline imaging
Although final imaging volumes correlated well with residual cancer burdens (see Fig. 1c), we performed extensive univariate analyses on baseline imaging features to determine whether final pathologic response could be predicted by mpMRI. Patient-level imaging characteristics associated with patient-level treatment response are listed in Supplementary Table 3. As depicted in Fig. 4a, low relative tumor burden (RTB, i.e. the combined volume of each tumor lesion divided by the total prostate volume) was the only predictor in the final multivariate logistic regression model (OR 0.77 [0.63–0.94], P = 0.001). Figure 4b shows that the AUC for RTB was 0.89 (0.79–0.99) at the optimal cut-off of 8.1% for RTB specificity (81%) and sensitivity (87%). Representative responder and nonresponder cases are shown in Figure 4c, which were both high-risk, cT3a cases. The responder initial lesion volume was 1.56 cc, so with a baseline prostate volume of 46 cc, the RTB was 3.39%. In contrast, the nonresponder lesion volume was 7.13 cc at the start of treatment, and with a baseline prostate volume of 33 cc, the RTB was 21.6%.
Figure 4. Low relative tumor burden is a statistically significant predictor of final pathologic outcome.
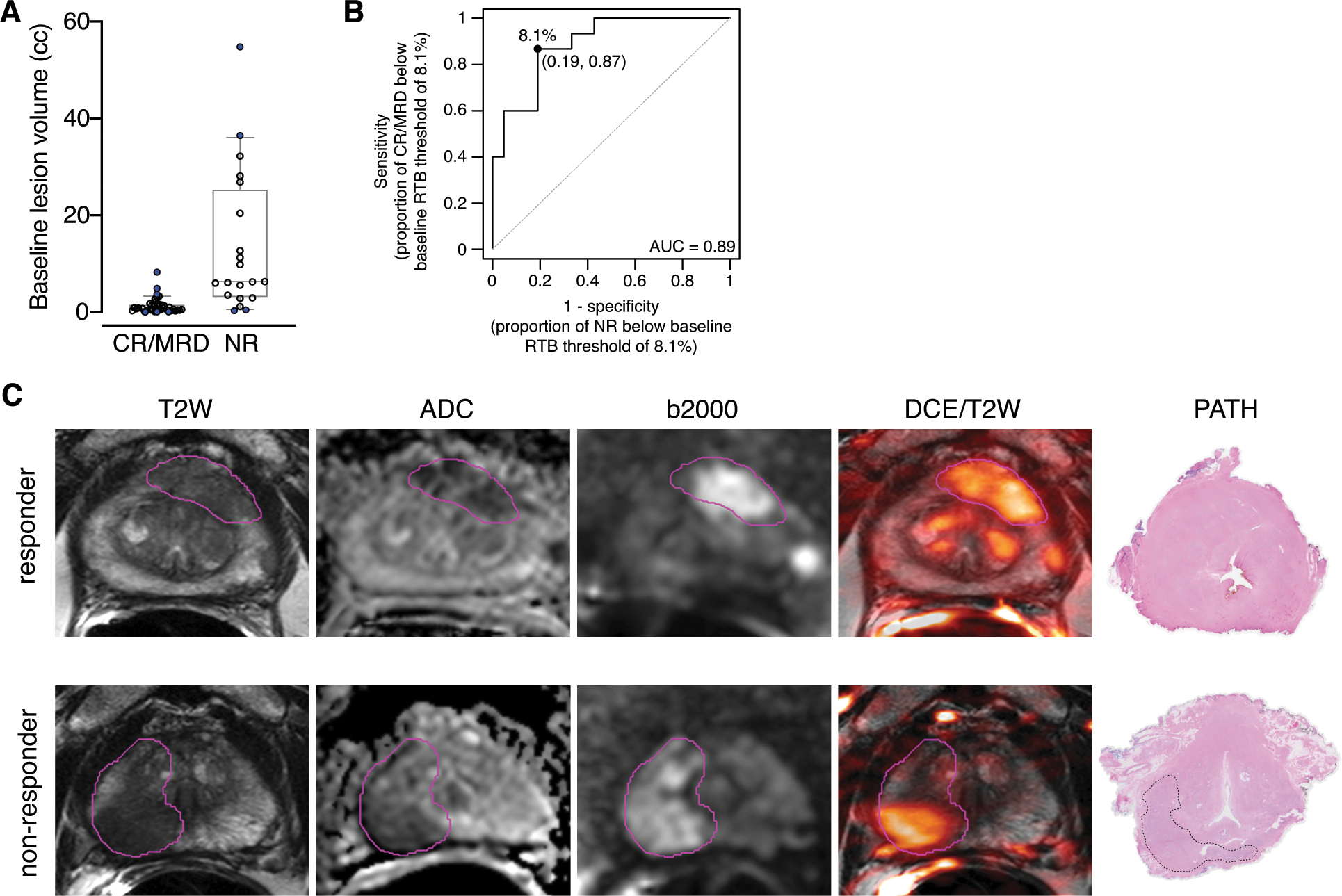
(A) Baseline lesion volumes in cc, based on the final pathology status for each patient. N = 39 for CR/MRD lesions and N = 20 for NR lesions. P < 0.001 by two-sided Mann-Whitney test. (B) Receiver operating characteristic curve for pre-treatment relative tumor burden (RTB) yielding an AUC of 0.89 and optimal threshold of 8.1%. (C) Baseline mpMRI of 2 different patients with high-risk localized prostate cancer and corresponding final pathology. Responder: 72-year-old with a PSA of 5.81 ng/ml had a PI-RADS 5 lesion in the left apical-mid anterior peripheral zone which revealed Gleason 4+4=8 cancer at targeted biopsy. Baseline relative tumor burden was 3.4% of the prostate. Final pathology revealed pathologic complete response. Non-responder: 66-year-old with a PSA of 5.53 ng/ml had a large PI-RADS 5 lesion in the right apical-base peripheral zone which revealed Gleason 4+5=9 cancer at targeted biopsy. Baseline relative tumor burden was 21.5% of the prostate. Final pathology revealed the patient was a non-responder with RCB of 0.24 cc.
Discussion
The effects of neoadjuvant therapies on prostate tumors are not uniform among patients or even distinct cancer lesions within a patient’s prostate (19). Information from mpMRI of the prostate after intense androgen deprivation can be used to assess treatment response on a per-lesion and per-patient basis, as well as for surgical planning. Predicting which patients will respond to neoadjuvant therapies based on baseline mpMRI characteristics could allow for better patient selection and overall responses to treatment in subsequent clinical trials, while patients who would not benefit from treatment would be spared from toxicities (20). mpMRI may also be able to identify patients with intrinsic resistance despite declines in PSA. This intrinsic resistance, as seen with androgen independence leading to tumor cell lineage plasticity, may play a role in resistance to AR-targeted therapy (21). Thus, perturbations in AR-regulated lineage characteristics observed phenotypically by less PSA expression and genomic loss of tumor suppressors such as RB1 and TP53 may represent aggressive prostate variants. Early identification of this high-risk population, aided by imaging, is paramount to treatment of this subset of patients.
We found that low relative tumor burden on mpMRI was the best predictor of response and was the only baseline characteristic that showed statistically significant predictive performance in multivariate analysis at both the lesion and patient levels. This was the strongest predictor of pathologic response, with an optimal cutoff of 8.1%, and may be a consideration when selecting patients for future neoadjuvant studies. Contouring of the prostate and lesions within the prostate on mpMRI in order to obtain a volume can be performed by radiologists with readily available software, and automated lesion segmentation software is becoming available. This makes baseline mpMRI an important prognostic tool for predicting tumor- and patient-level response to neoadjuvant intense ADT.
Post-treatment mpMRI revealed a significant decrease in tumor volume after therapy in an overwhelming majority of patients, although at baseline and after 6 months of treatment, it is not possible to determine the kinetics of tumor reduction during treatment. It is important to note that while 4/36 patients had no visible disease on mpMRI, on final pathology only 2/36 patients had complete pathologic response. However, studies suggest lesions undetected by mpMRI may be lower in grade and size than detected lesions, making mpMRI an important tool in risk-stratifying prostate cancer (22). Nonetheless, amongst all patients the residual cancer volumes estimated by post-treatment mpMRI correlated well with final pathology at ρ = 0.7173, making the serial mpMRI a meaningful measure for assessing organ-wide response when comprehensive pathologic analyses are not routinely feasible.
Previous studies have demonstrated that the positive staining for the TMPRSS2-ERG fusion and PTEN loss by IHC are enriched in patients with increased volumes of residual disease (8,23). We similarly observed that NR patients exhibited increased rates of positive ERG staining and negative PTEN staining than CR/MRD, although only PTEN reduction was statistically significant (P = 0.0006). In three patients who demonstrated minimal tumor reductions on post-treatment mpMRI, we performed additional genomic analysis to assess eligibility for biomarker driven studies; in particular, loss of homologous recombination genes would indicate eligibility for treatment with olaparib. In two of these three patients, germline pathologic variants to BRCA2 and PALB2 observed. Interestingly, 1- or 2-copy loss of RB1 were found in all three patients, and biallelic inactivation of TP53 was also observed in two patients. Although these findings are anecdotal, they emphasize the consequence of bypassing the AR via RB1 loss (often in conjunction with loss of tumor suppressors or due to a defect in DNA damage response). As these genomic events are more frequently associated with the “AR low” phenotypes of localized prostate cancer (23), it aligns with our finding that the vast majority of all post treatment specimens expressed less AR and PSA despite variable volumes of residual disease. Because AR indifference, including neuroendocrine transdifferentiation (24), can result from treatment with AR-axis inhibitors like enzalutamide and abiraterone, the use of genomic analysis to infer resistance mechanisms is needed in the neoadjuvant setting. Furthermore, earlier evaluation with imaging and molecular characteristics might better predict resistance, enabling discontinuation in patients unlikely to benefit from neoadjuvant therapy.
The results of our study reveal a significant increase in the number of patients with organ-confined disease at imaging prior to RARP. It has been suggested that patients receiving neoadjuvant ADT prior to RP are more likely to have negative surgical margins at RP (25,26). The effect of negative margins on BCR has not been well characterized in patients who undergo neoadjuvant ADT prior to surgery, and some studies have described a lack of significant decreases in rates of BCR in this population compared to those who undergo RP alone (27,28). While this study and other similar phase II studies have shown favorable imaging and pathologic responses to neoadjuvant AR antagonists plus ADT in patients with high-risk localized disease, long-term follow-up for overall survival needs to be considered (8,29).
There are several limitations to this study. First, a small sample size of 36 patients limits the power of our statistical analyses. Second, all mpMRI scans were interpreted and contoured by a single radiologist who specializes in prostate mpMRI, which could limit reproducibility of results. However, new lesion-segmenting software may soon mitigate this problem. Third, mpMRIs were acquired using an endorectal coil which may have influenced the results. Most mpMRIs are increasingly obtained without an endorectal coil. However, the endorectal coil was used here to ensure the highest quality images. Fourth, although clinical grade Oncomine sequencing identified three patients with RB loss, only one of these utilized a pre-treatment biopsy sample, and were not confirmed using anti-RB IHC on any of the specimens. Finally, the short-term follow-up of the current study precludes using time to BCR as an outcome to assess neoadjuvant intense ADT. However, the finding that low residual tumor volumes (i.e. CR and MRD) were associated with freedom from BCR (11) implies that the 15 patients in our study who exhibited CR/MRD are less likely to experience BCR within 3 years than the 22 patients who did not.
To our knowledge, this is one of the first studies to combine serial mpMRI imaging with neoadjuvant intense ADT in prostate cancer. Based on our findings, imaging with mpMRI may enhance patient selection for neoadjuvant treatment. Although most (but not all) of patients with the RTB had CR/MRD, nearly every patient experienced tumor shrinkage regardless of initial tumor burden, and this finding was consistent with previous neoadjuvant intense ADT studies (8–10). Thus, patients with higher relative tumor burdens at baseline were less likely to benefit from the regimen in this study, they might benefit from either longer a duration of intense ADT or an alternative or precision-guided treatment, with such decision being guided by a combination of clinical, pathology, and imaging assessments. Neoadjuvant treatment in our patient population was tolerable, and extension of treatment beyond 6 months may be feasible.
Neoadjuvant enzalutamide with ADT prior to RARP significantly reduces tumor burden at mpMRI in most patients with localized high-risk prostate cancer. Baseline low relative tumor burden at mpMRI was the strongest predictor of pathologic response with an optimal cutoff of 8.1%, a consideration when selecting patients for future neoadjuvant studies. Additionally, imaging can enhance evaluation for high-risk molecular signatures where combination with imaging can be combined to identify patients who are unlikely to benefit from intensive neoadjuvant ADT. In this study, we found mpMRI can be used to assess treatment responses, and three patients with adverse pathologic features and multiple deleterious genomic alterations also harbored experienced RCB. Deeper molecular analysis of this and other neoadjuvant studies (i.e., ClinicalTrials.gov Identifier NCT03860987 involves mpMRI at mid-treatment along with 18F-DCFPyL [PSMA] imaging) will facilitate patient selection and development of appropriate endpoints for neoadjuvant therapies in prostate cancer.
Supplementary Material
Translational Relevance.
In high-risk localized prostate cancer, despite definitive therapy with either radical prostatectomy or radiation therapy plus androgen deprivation therapy (ADT), many patients will have recurrence of disease. Androgen receptor (AR) pathway inhibitors are increasingly being studied in men prior to prostatectomy. Studies suggest intense ADT prior to prostatectomy may have a positive impact on time to biochemical recurrence. Incorporating imaging modalities, such as multiparametric MRI (mpMRI), to neoadjuvant AR-directed therapies may help with selection of patients who would benefit from the most from this type of treatment. In this study, men with localized intermediate- or high-risk prostate cancer underwent a baseline mpMRI and mpMRI targeted biopsy followed by a second mpMRI after 6 months of enzalutamide and ADT prior to robot-assisted radical prostatectomy. We found that low relative lesion volume at baseline mpMRI was predictive of pathologic response and may be a consideration when selecting patients for future neoadjuvant studies.
Acknowledgments
The authors thank all the patients who participated in this study and their families. Enzalutamide, goserelin, leuprolide acetate, and degarelix were provided through the Center for Cancer Research, National Institutes of Health. This project was supported by the Prostate Cancer Foundation (Young Investigator Awards to F.K., S.W., R.A.M., S.A.H., D.J.V., H.Y. and A.G.S.), the Department of Defense Prostate Cancer Research Program (W81XWH-19-1-0712 to S.W., W81XWH-16-1-0433 to A.G.S.), the National Cancer Institute (HHSN261200800001E), and the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services or the Department of Defense, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors also thank Bonnie L. Casey for editorial assistance in the preparation of this manuscript.
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
ClinicalTrials.gov Identifier: NCT02430480
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7–30 doi 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Eastham JA, Cronin AM, Fuks Z, Zhang Z, Yamada Y, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 2010;28(9):1508–13 doi 10.1200/jco.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367(13):1187–97 doi 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 4.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371(5):424–33 doi 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol 2019;37(32):2974–86 doi 10.1200/jco.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2018;378(26):2465–74 doi 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoun F, Bourgi A, Ayoub E, El Rassy E, van Velthoven R, Peltier A. Androgen deprivation therapy in the treatment of locally advanced, nonmetastatic prostate cancer: practical experience and a review of the clinical trial evidence. Ther Adv Urol 2017;9(3–4):73–80 doi 10.1177/1756287217697661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay RR, Ye H, Xie W, Lis R, Calagua C, Zhang Z, et al. Evaluation of Intense Androgen Deprivation Before Prostatectomy: A Randomized Phase II Trial of Enzalutamide and Leuprolide With or Without Abiraterone. J Clin Oncol 2019;37(11):923–31 doi 10.1200/jco.18.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery B, Tretiakova MS, Joshua AM, Gleave ME, Fleshner N, Bubley GJ, et al. Neoadjuvant Enzalutamide Prior to Prostatectomy. Clin Cancer Res 2017;23(9):2169–76 doi 10.1158/1078-0432.CCR-16-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 2014;32(33):3705–15 doi 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKay RR, Montgomery B, Xie W, Zhang Z, Bubley GJ, Lin DW, et al. Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis 2018;21(3):364–72 doi 10.1038/s41391-017-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 2020;382(10):917–28 doi 10.1056/NEJMoa1910038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011;186(5):1818–24 doi 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69(1):16–40 doi 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehralivand S, Shih JH, Harmon S, Smith C, Bloom J, Czarniecki M, et al. A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI. Radiology 2019;290(3):709–19 doi 10.1148/radiol.2018181278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci U S A 1986;83(10):3500–4 doi 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. Prostate 1999;39(2):135–48 doi . [DOI] [PubMed] [Google Scholar]
- 18.Sowalsky AG, Ye H, Bubley GJ, Balk SP. Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res 2013;73(3):1050–5 doi 10.1158/0008-5472.Can-12-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson S, Harmon SA, Terrigino NT, Karzai F, Pinto PA, Madan RA, et al. A case report of multiple primary prostate tumors with differential drug sensitivity. Nat Commun 2020;11(1):837 doi 10.1038/s41467-020-14657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology 2003;61(2 Suppl 1):32–8 doi 10.1016/s0090-4295(02)02397-x. [DOI] [PubMed] [Google Scholar]
- 21.Beltran H, Hruszkewycz A, Scher HI, Hildesheim J, Isaacs J, Yu EY, et al. The Role of Lineage Plasticity in Prostate Cancer Therapy Resistance. Clin Cancer Res 2019;25(23):6916–24 doi 10.1158/1078-0432.Ccr-19-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norris JM, Carmona Echeverria LM, Bott SRJ, Brown LC, Burns-Cox N, Dudderidge T, et al. What Type of Prostate Cancer Is Systematically Overlooked by Multiparametric Magnetic Resonance Imaging? An Analysis from the PROMIS Cohort. Eur Urol 2020. doi 10.1016/j.eururo.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sowalsky AG, Ye H, Bhasin M, Van Allen EM, Loda M, Lis RT, et al. Neoadjuvant-Intensive Androgen Deprivation Therapy Selects for Prostate Tumor Foci with Diverse Subclonal Oncogenic Alterations. Cancer Res 2018;78(16):4716–30 doi 10.1158/0008-5472.CAN-18-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol 2018;15(5):271–86 doi 10.1038/nrurol.2018.22. [DOI] [PubMed] [Google Scholar]
- 25.Cookson MS, Sogani PC, Russo P, Sheinfeld J, Herr H, Dalbagni G, et al. Pathological staging and biochemical recurrence after neoadjuvant androgen deprivation therapy in combination with radical prostatectomy in clinically localized prostate cancer: results of a phase II study. Br J Urol 1997;79(3):432–8 doi 10.1046/j.1464-410x.1997.00022.x. [DOI] [PubMed] [Google Scholar]
- 26.McClintock TR, von Landenberg N, Cole AP, Lipsitz SR, Gild P, Sun M, et al. Neoadjuvant Androgen Deprivation Therapy Prior to Radical Prostatectomy: Recent Trends in Utilization and Association with Postoperative Surgical Margin Status. Ann Surg Oncol 2019;26(1):297–305 doi 10.1245/s10434-018-7035-z. [DOI] [PubMed] [Google Scholar]
- 27.Fair WR, Cookson MS, Stroumbakis N, Cohen D, Aprikian AG, Wang Y, et al. The indications, rationale, and results of neoadjuvant androgen deprivation in the treatment of prostatic cancer: Memorial Sloan-Kettering Cancer Center results. Urology 1997;49(3A Suppl):46–55 doi 10.1016/s0090-4295(97)00169-6. [DOI] [PubMed] [Google Scholar]
- 28.Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology 2005;66(6):1245–50 doi 10.1016/j.urology.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 29.Caffo O, Maines F, Donner D, Veccia A, Chierichetti F, Galligioni E. Impact of enzalutamide administration on primary prostate cancer volume: a metabolic evaluation by choline positron emission tomography in castration-resistant prostate cancer patients. Clin Genitourin Cancer 2014;12(5):312–6 doi 10.1016/j.clgc.2014.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


