Abstract
A true understanding of the distribution and functional correlates of Alzheimer’s disease pathology in dementia-free older adults requires a population-based perspective. Here we report initial findings from a sample of 102 cognitively unimpaired participants (average age 77.2 years, 54.9% women, 13.7% APOE*4 carriers) recruited for neuroimaging from a larger representative population-based cohort participating in an ongoing longitudinal study of aging, the Monongahela-Youghiogheny Healthy Aging Team (MYHAT). All participants scored <1.0 on the Clinical Dementia Rating (CDR) Scale, with 8 participants (7.8%) scoring CDR=0.5. Participants completed a positron emission tomography scan using the tracers [C-11]Pittsburgh Compound-B (PiB) and [F-18]AV-1451 to estimate amyloid and tau deposition. PiB positivity was defined on a regional basis using established standardized uptake value ratio cutoffs (SUVR; cerebellar gray matter reference), with 39 participants (38.2%) determined to be PiB(+). Health history, lifestyle, and cognitive abilities were assessed cross-sectionally at the nearest annual parent MYHAT study visit. A series of adjusted regression analyses modeled cognitive performance as a function of global PiB SUVR and [F-18]AV-1451 SUVR in Braak associated regions 1, 3/4, and 5/6. In comparison to PiB(−) participants (n=63), PiB(+) participants were older, less educated, and were more likely to be APOE*4 carriers. Global PiB SUVR was significantly correlated with [F-18]AV-1451 SUVR in all Braak-associated regions (r=0.38 – 0.53, p<.05). In independent models, higher Global PiB SUVR and Braak 1 [F-18]AV-1451 SUVR were associated with worse performance on a semantic interference verbal memory test. Our findings suggest that brain amyloid is common in a community-based setting, and is associated with tau deposition, but both pathologies show few associations with concurrent cognitive performance in a dementia-free sample.
Keywords: amyloid, tau, neuroimaging, population neuroscience, Alzheimer’s disease
INTRODUCTION
Dementia is a syndrome characterized by cognitive and functional decline, with an estimated 50 million prevalent cases worldwide. Recent reports suggest that the incidence rate of dementia may be on the decline in higher income countries (Derby, Katz, Lipton, & Hall, 2017; Satizabal, Beiser, & Seshadri, 2016; Sullivan et al., 2019). However, expanding life expectancy and the continuing demographic shift of the worldwide population towards older age will keep prevalence high, and therefore warrants increased attention towards early diagnosis and prevention efforts. Additionally, it is critical to better characterize the risk factors, distribution, and impact of dementia in population-based samples of older adults, to maximize external validity and generalizability beyond typical research settings such as Alzheimer Disease Research Centers (ADRCs) (Ganguli et al., 2018). High among these goals is a better understanding of biomarkers for Alzheimer’s disease (AD) in the broader population, including distribution and relationship to function in cognitively unimpaired (CU) older adults.
AD is the single most common pathological basis for dementia and is characterized by two neuropathological hallmarks: Amyloid beta (Aβ)-containing plaques and hyperphosphorylated tau-containing neurofibrillary tangles (Hyman et al., 2012). Advancements in neuroimaging have made it possible to assess the extent and distribution of both of these biomarkers in the brain in vivo. Aβ can be measured using positron emission tomography (PET) with numerous tracers including [C-11]Pittsburgh Compound-B (PiB) (Klunk et al., 2004). Relatively recently, PET tracers, including [F-18]AV-1451, have been developed to estimate tau pathology in vivo (Lois, Gonzalez, Johnson, & Price, 2019). Both biomarkers measure primary components of the recently proposed National Institute of Aging – Alzheimer’s Association research framework for defining AD biologically (Jack et al., 2018). Additionally, assessment of Aβ and tau is pivotal to early diagnosis and AD prevention efforts, as the accumulation of these pathologies, particularly Aβ, is thought to occur long before clinically significant functional impairment develops (Jack et al., 2013). However, most of what is known about the distribution and functional correlates of brain Aβ and tau in older adults comes from convenient clinic-based samples, where in-depth assessments can be carried out, but which may lack external validity. This challenge is a focus of the emerging research perspective termed “population neuroscience”.
Population neuroscience approaches emphasize the importance of employing traditional neuroscience methods, which have become more affordable and accessible in recent years, to more representative study populations (Falk et al., 2013; Ganguli et al., 2018). Among other goals outlined in a proposed population neuroscience framework by Falk et al. was an effort to integrate neuroimaging into subsamples of existing population-based cohort studies. The Monongahela Youghiogheny Healthy Aging Team (MYHAT) is a population-based prospective cohort study of cognitive decline among older adults representative of a small-town region in southwestern Pennsylvania. Here we present an initial report from the MYHAT Neuroimaging Study (MYHAT-NI), an ongoing effort to collect neuroimaging data from a sample of dementia-free members of the MYHAT cohort. Specifically, we aimed to 1) report the distribution of PET-measured Aβ and tau deposition; 2) explore distributions of sample characteristics by Aβ status; 3) examine associations between Aβ and tau deposition; and 4) examine cross-sectional associations between Aβ, tau, and cognitive performance as assessed with an extensive neuropsychological test battery.
METHODS
Study Design
Monongahela Youghiogheny Healthy Aging Team
MYHAT is a prospective population-based study of mild cognitive impairment (MCI) and dementia in a low socioeconomic status, formerly heavy manufacturing industrial small-town area of southwestern Pennsylvania. MYHAT has been ongoing since 2006 with annual assessments of an initial cohort of 1982 older adults and replenished with an additional 703 participants ages 65–74 recruited from 2016–2019. The cohort was recruited with age-stratified random sampling using publicly available voter registration lists. Inclusion criteria included age 65+, no significant vision or hearing impairment, not institutionalized at study entry, and having decisional capacity. Additionally, due to the primary study focus on MCI as an outcome, the MYHAT study required participants at study entry to score at least 21/30 on an age-education corrected Mini-Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975; Mungas, Marshall, Weldon, Haan, & Reed, 1996). Participants undergo neuropsychological testing (described below) and complete numerous self-report assessments with trained interviewers, including but not limited to health history, lifestyle, depressive symptoms, family history of memory problems, and a subjective memory questionnaire. They are then rated on the Clinical Dementia Rating (CDR) ®Staging Instrument (Morris, 1993). Blood is collected for APOE genotyping following methods previously reported (Kamboh et al., 2019). Additional details regarding MYHAT recruitment and assessment procedures have been described previously (Ganguli et al., 2010; Ganguli, Fu, Snitz, Hughes, & Chang, 2013).
Monongahela Youghiogheny Healthy Aging Team Neuroimaging Study
The MYHAT-NI study enrolled 102 participants (ages 67–96) from 2017 to 2019. Inclusion criteria were participation in the parent MYHAT study and CDR sum-of-box score of < 1.0. Eight participants (7.8%) in the current analysis sample were scored as global CDR=0.5, and the remaining 94 participants (92.2%) were scored as global CDR=0. Sixty-three participants were recruited from the original MYHAT cohort (2006-Present) and 39 participants were recruited from the replenishment cohort (2016-present). Exclusion criteria were contraindications for MRI or PET neuroimaging. Compared to the parent MYHAT cohort at the initial study visits in 2006–2008, the MYHAT-NI sample was younger (69.8 vs 75.6 average age), more highly educated (60.8% vs 47.5% >high school education), but comparable in sex distribution (54.9% vs 60.4% women). All participants gave written informed consent for all procedures, as approved by the Institutional Review Board of the University of Pittsburgh.
Neuroimaging Methods
Before the PET imaging session, a T1-weighted structural MRI series (MPRAGE) was acquired using a 3T Siemens PRISMA scanner. All PET images were acquired using a Siemens Biograph mCT Flow 64–4R PET/CT scanner.
[C-11]PiB (15 mCi) or [F-18]AV-1451 (7–10 mCi) were administered as slow bolus injections via the antecubital vein. PET emission data were collected in a series of 5 min frames spanning 50–70 min post-injection for [C-11]PiB and 80–100 min for [F-18]AV-1451. A low-dose CT scan (16 mrem) was acquired for attenuation and scatter correction. All PET scans were acquired in 3D-mode and reconstructed using filtered back projection.
PET images were inspected for inter-frame motion and, if required, framewise registration was performed using PMOD software (PMOD Technologies, Zurich, Switzerland). Subsequently, [C-11]PiB and [F-18]AV-1451 acquisition frames were summed into a single 20 min frame and registered to the participant’s T1 MR image using the normalized mutual information algorithm.
Each participant’s MR image was parcellated into a set of regions of interest (ROIs) using the default FreeSurfer v5.3 pipeline and atlas with the exception of striatal subregions, which were substituted by components from the Imperial College London Clinical Imaging Centre (CIC) atlas (Tziortzi et al., 2011) as previously described (Tudorascu et al., 2018). All FreeSurfer ROIs were visually inspected and manually edited where appropriate.
FreeSurfer ROIs were used to sample radioactivity concentrations in the summed PET images. For [C-11]PiB, nine composite regional outcomes were computed (anterior cingulate, posterior cingulate, insula, superior frontal cortex, orbitofrontal cortex, lateral temporal cortex, parietal, precuneus, and ventral striatum) by volume-weighted averaging of standard FreeSurfer and CIC ROIs (See Supplemental Methods). A global [C-11]PiB retention index was computed by volume-weighted averaging of all nine composite [C-11]PiB regions. For [F-18]AV-1451, three composite regional outcomes reflecting Braak pathologic staging (Braak 1, Braak 3/4, and Braak 5/6) were computed for [F-18]AV-1451 PET scans from a volume-weighted average of standard FreeSurfer ROIs, as previously described (Baker, Maass, & Jagust, 2017; Maass et al., 2017) (See Supplemental Methods). Striatal subregions (accumbens, caudate, putamen, and pallidum) were excluded from Braak 5/6 region due to frequent off-target binding. Similarly, Braak 2 was excluded from primary analyses due to potential for high off-target binding, but was averaged with Braak 1 in secondary analyses. Composite regional values were converted to standardized uptake value ratios (SUVRs) by normalizing to FreeSurfer cerebellar gray matter activity.
[C-11]PiB PET scans were classified as regionally PiB(+) or PiB(−) using a sparse k-means clustering method to define cut-points as previously described (Cohen et al., 2013). A participant was classified as PiB(+) if any one composite region SUVR exceeded the corresponding regional cutoff (See Supplemental Methods).
Cognitive Performance Measures
As part of baseline and annual MYHAT study visits, participants completed a neuropsychological test battery covering multiple domains: Attention/Processing Speed (Trail Making Test A, Digit Span Forward); Executive Functions (Trail Making Test B, Fluency for Initial Letters, Clock Drawing Test); Memory and Learning (Logical Memory, Immediate and Delayed; Visual Reproduction, Immediate and Delayed; Object Memory Evaluation); Language (Boston Naming Test, Fluency for Animals); and Visuospatial Function (Block Design). A global cognitive score was calculated by averaging performance on all domains.
Tests were grouped according to these conceptual domains, standardized into Z-scores, and averaged to create domain composite scores (Ganguli, Snitz, Vander Bilt, & Chang, 2009). Participants also completed two additional memory tests with the goal of increasing sensitivity to early AD pathology. The first was a 12-item paper-and-pencil version of the Face Name Associative Memory Exam (Rentz et al., 2011) administered during the MYHAT visit. Twelve face-name combinations were presented in an initial learning trial, then three delayed trials assessed recognition of faces, cued first-letter recall of names in response to faces, and multiple-choice for names in response to faces, respectively. A total score combining scores on all three trials of the test was used in analyses.
The second additional memory test was the Loewenstein-Acevedo Scale for Semantic Interference and Learning – II Edition (LASSI-L), administered during the MYHAT-NI study visit (Loewenstein et al., 2016). The LASSI-L uses controlled learning and cued recall over two trials (A1 and A2) to maximize storage of an initial list of 15 target words representing three semantic categories (List A). Then a semantically related list (List B) is presented with cued recall (B1), assessing proactive interference, followed by a second List B presentation and cued recall trial (B2), assessing recovery from proactive interference. Finally, a cued recall trial of List A again (A3) assessed retroactive interference. Delayed free recall of both lists was also included, with intrusion errors recorded. LASSI-L scores, each representing specific aspects of semantic interference, thus included cued recall of all trials of List A and B, as well as more traditional word-list learning scores of delayed free recall and delayed intrusion errors.
In summary, cognitive measures of interest in this analysis included 6 MYHAT neuropsychological domain scores, the total score of the Face-Name Association test, and 7 specific scores from the LASSI-L. The MYHAT-NI and MYHAT study visits were separated in time by an average of 22 (SD=13) weeks.
Statistical Analysis
We examined demographic variables, Braak regional [F-18]AV-1451 SUVR, and health status variables by PiB(+) vs. PiB(−) groups using t-test, chi-squared test or fisher’s exact test. We examined partial Pearson correlations between global PiB and regional [F-18]AV-1451 retention, adjusting for age. We examined associations between each cognitive performance variable and global PiB SUVR and regional [F-18]AV-1451 SUVR using linear regression and Poisson regression (intrusion errors), adjusting for age, sex, education and MYHAT cohort (original vs. replenishment). These regressions were repeated using Braak 1/2 [F-18]AV-1451 SUVR as the predictor in a secondary analysis. Each predictor was independently modeled with 14 different cognitive outcomes, and therefore a Bonferroni-correction was used to account for multiple comparisons.
RESULTS
Descriptive statistics are provided for the total sample (n=102) and by regional Aβ positivity status in Table 1. Thirty-eight % (39/102) were Aβ(+), specifically 25.7% under age 75 (9/35), 41.5% between age 75–84 (22/53), and 57.1% older than 85 (8/14). Aβ(+)participants were older on average (79.28 vs 75.90) than Aβ(−) participants, less likely to be college educated (48.7% vs 68.3%), and more likely to be APOE*4 carriers (28.2% vs 4.8%). Eight had global CDR=0.5; and of them, six were determined to be Aβ(+). There were no between-group differences for race, sex, MMSE, hypertension, diabetes, number of prescription medications, previous transient ischemic attack (TIA), depression, family history of memory problems, alcohol consumption, or smoking status. In comparison to Aβ(−) participants, Aβ(+) participants had higher average [F-18]AV-1451 SUVR in Braak-associated regions 1, 3/4, and 5/6 (see Figure 1). Table 2 presents correlations between age, global PiB SUVR, and [F-18]AV-1451 SUVR in Braak-associated regions 1, 3/4, and 5/6. Global PiB SUVR was significantly correlated with [F-18]AV-1451 SUVR in each of the tested regions (age-adjusted).
Table 1.
Demographics by Aβ Status and Total Sample
| Aβ(+) (n=39) | Aβ(−) (n=63) | Total (n=102) | p | |
|---|---|---|---|---|
| Average Age (SD) | 79.28 (6.40) | 75.90 (5.90) | 77.20 (6.29) | 0.008a |
| Female Sex, n(%) | 22 (56.4) | 34 (54.0) | 56 (54.9) | 0.810b |
| White, n(%) | 36 (92.3) | 59 (93.7) | 95 (93.1) | 1.000c |
| HS Education, n(%) | 20 (51.3) | 20 (31.7) | 40 (39.2) | 0.050 |
| >HS Education, n(%) | 19 (48.7) | 43 (68.3) | 62 (60.8) | |
| APOE*4 Carrier^, n(%) | 11 (28.2) | 3 (4.8) | 14 (13.7) | <0.001c |
| Average Global Aβ (SD) | 1.59 (0.36) | 1.10 (0.05) | 1.29 (0.33) | <0.001a |
| Average Braak 1 Tau (SD) | 1.21 (0.16) | 1.10 (0.12) | 1.14(0.14) | 0.001a |
| Average Braak 3_4 Tau (SD) | 1.15 (0.07) | 1.11 (0.07) | 1.13 (0.07) | 0.005a |
| Average Braak 5_6 Tau (SD) | 1.08 (0.08) | 1.04 (0.07) | 1.05 (0.08) | 0.020a |
| Average MMSE (SD) | 27.67 (2.29) | 28.19 (1.33) | 27.99 (1.77) | 0.199a |
| CDR=0.5, n(%) | 6 (15.4) | 2 (3.2) | 8 (7.8) | 0.051c |
| Subjective Memory Score >0, n (%) | 19 (48.7) | 30 (47.6) | 49 (48.0) | 0.914b |
| Current Hypertension, n(%) | 24 (61.5) | 43 (68.3) | 67 (65.7) | 0.488b |
| Current Diabetes, n(%) | 8 (20.5) | 12 (19.0) | 20 (19.6) | 0.856b |
| Previous TIA, n(%) | 5 (12.8) | 4 (6.3) | 9 (8.8) | 0.297c |
| Average Rx Medications (SD) | 5.03 (3.26) | 4.34 (2.52) | 4.61 (2.83) | 0.257a |
| High mCES-D, n(%) | 1 (2.6) | 4 (6.3) | 5 (4.9) | 0.647c |
| Family History of Memory Problems, n(%) | 9 (23.1) | 20 (31.7) | 29 (28.4) | 0.346b |
| Current Smoker, n(%) | 1 (2.6) | 7 (11.1) | 8 (7.4) | 0.150c |
| Current Drinker, n(%) | 28 (71.8) | 45 (71.4) | 73 (71.6) | 0.968b |
Note: Aβ=amyloid-beta, SD=standard deviation, HS=High School, MMSE=Mini-mental State Examination, CDR=Clinical Dementia Rating, TIA=Transient Ischemic Attack, mCES-D=modified Center for Epidemiologic Studies Depression Scale
Three participants missing APOE data
p-values reflect tests for significant differences between Aβ(+) and Aβ(−) groups using:
T-test
Pearsons Chi-Square
Fisher’s Exact Test
Figure 1.
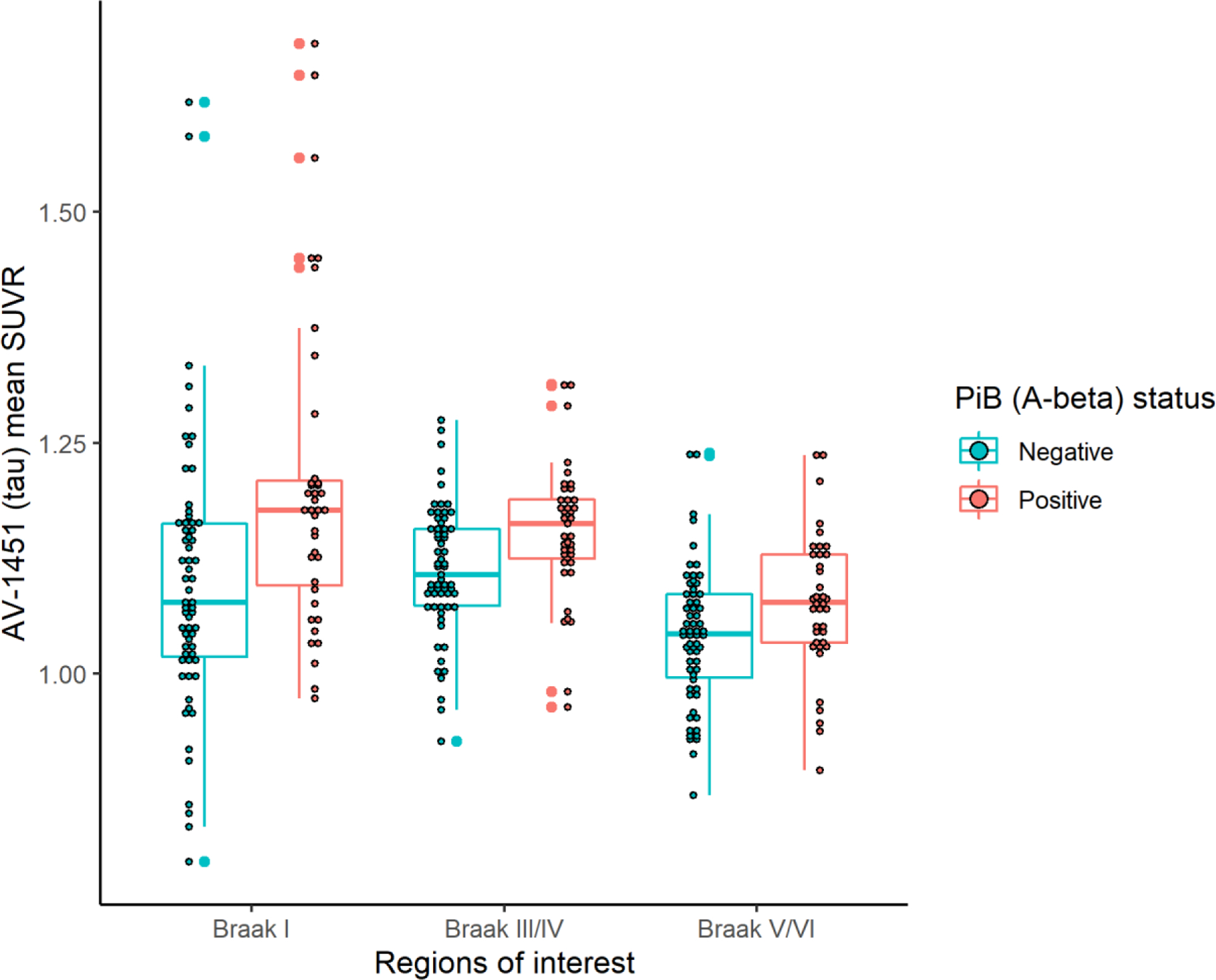
[F-18]AV-1451 (Tau) SUVR by Braak Region and Aβ Status
Table 2.
Correlation Matrix of Aβ, Tau and Age
| Age | Global SUVR Aβ | Braak 1 Tau | Braak 3/4 Tau | Braak 5/6 Tau | |
|---|---|---|---|---|---|
| Age | - | 0.145 | −0.073 | −0.024 | 0.039 |
| Global SUVR Aβ | - | - | 0.526* | 0.428* | 0.381* |
| Braak 1 Tau | - | - | - | 0.702* | 0.570* |
| Braak 3_4 Tau | - | - | - | - | 0.910* |
| Braak 5_6 Tau | - | - | - | - | - |
Note: Aβ=amyloid-beta, SUVR=Standardized Uptake Value Ratios
Cells contain Pearson correlation coefficients (r)
Correlations between global Aβ and regional Tau are age-adjusted
p<.01
Table 3 presents the results of a series of regression analyses modeling cross-sectional cognitive performance outcomes by global PiB SUVR and [F-18]AV-1451 in Braak-associated regions 1, 3/4, and 5/6, with adjustment for age, education, sex, and cohort. Higher global PiB SUVR was associated with worse performance on the delayed word recall component of the LASSI-L (β=−2.481, SE=1.229, p=.047), and higher count of delayed word recall intrusion errors (β=0.891, SE=0.333, p=.007), but neither of these associations met a Bonferroni-corrected α=.004. Braak 1 [F-18]AV-1451 SUVR was associated with worse performance on the A3 recall component of the LASSI-L, but better performance on the Visual-Spatial composite. However, neither association were robust to multiple comparison adjustment. We observed no other associations between global PiB or regional [F-18]AV-1451 retention with cross-sectional cognitive performance. In a secondary analysis, Braak 1/2 was not associated with any measure of cognitive performance (Supplement Table).
Table 3.
Adjusted Regression Analyses^ Predicting Cross Sectional Cognitive Performance with Aβ and Tau
| Primary Predictor | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global Aβ | Braak 1 Tau | Braak 3/4 Tau | Braak 5/6 Tau | |||||||||
| Cognitive Outcome | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p |
| Global Cognition | −0.017 | (−0.411, 0.377) | 0.931 | 0.188 | (−0.596, 0.972) | 0.635 | 1.405 | (−0.345, 3.155) | 0.114 | 1.560 | (−0.145, 3.264) | 0.072 |
| Attention domain | 0.004 | (−0.542, 0.550) | 0.988 | 0.304 | (−0.781, 1.389) | 0.579 | 2.073 | (−0.293, 4.440) | 0.085 | 2.062 | (−0.264, 4.388) | 0.082 |
| Executive Function domain | 0.045 | (−0.421, 0.511) | 0.848 | 0.313 | (−0.612, 1.238) | 0.503 | 1.261 | (−0.774, 3.297) | 0.222 | 1.383 | (−0.615, 3.381) | 0.173 |
| Language domain | −0.298 | (0.723, 0.127) | 0.168 | −0.073 | (−0.927, 0.781) | 0.866 | 1.325 | (−0.545, 3.196) | 0.163 | 1.599 | (−0.230, 3.429) | 0.086 |
| Memory domain | 0.071 | (−0.439, 0.581) | 0.784 | −0.012 | (−1.027, 1.004) | 0.982 | 1.268 | (−1.011, 3.548) | 0.272 | 1.513 | (−0.708, 3.734) | 0.18 |
| Visual Spatial Reasoning domain | 0.207 | (−0.449, 0.862) | 0.531 | 1.851 | (0.396, 3.306) | 0.013 | 1.710 | (−1.543, 4.963) | 0.298 | 0.999 | (−2.159, 4.157) | 0.53 |
| Face Name Association Test | 0.138 | (−2.787, 3.063) | 0.925 | 1.094 | (−4.734, 6.921) | 0.710 | 8.238 | (−4.523, 21.000) | 0.203 | 6.164 | (−6.502, 18.831) | 0.336 |
| LASSI-L Test Components | ||||||||||||
| Cued A1 Total | −0.125 | (−1.582, 1.332) | 0.865 | −0.754 | (−3.659, 2.152) | 0.608 | 0.654 | (−5.887, 7.194) | 0.843 | 0.683 | (−5.706, 7.073) | 0.832 |
| Cued A2 Total | −0.113 | (−1.204, 0.978) | 0.838 | 0.272 | (−1.907, 2.450) | 0.805 | 1.202 | (−3.692, 6.095) | 0.627 | 0.219 | (−4.567, 5.006) | 0.928 |
| Cued B1 Total | −0.238 | (−1.599, 1.124) | 0.73 | −0.979 | (−3.693, 1.736) | 0.476 | −1.423 | (−7.535, 4.689) | 0.645 | −0.047 | (−6.026, 5.931) | 0.988 |
| Cued B2 Total | −0.655 | (−1.790, 0.480) | 0.255 | −0.877 | (−3.153, 1.400) | 0.446 | −1.939 | (−7.058, 3.180) | 0.454 | −2.229 | (−7.224, 2.766) | 0.378 |
| Cued A3 Total | −1.125 | (−2.487, 0.237) | 0.104 | −2.967 | (−5.657, −0.278) | 0.031 | −2.704 | (−8.885, 3.477) | 0.387 | −1.589 | (−7.644, 4.466) | 0.603 |
| DR Correct | −2.481 | (−4.924, −0.038) | 0.047 | −2.728 | (−7.724, 2.268) | 0.281 | −4.898 | (−16.108, 6.313) | 0.388 | −2.250 | (−13.327, 8.827) | 0.687 |
| DR Intrusions | 0.891 | (0.210, 1.524) | 0.007 | −0.168 | (−1.826, 1.369) | 0.837 | −2.228 | (−5.614, 1.247) | 0.203 | −0.414 | (−3.836, 3.043) | 0.814 |
Note: Aβ=amyloid-beta, CI=Confidence Interval, LASSI-L= Loewenstein-Acevedo Scale for Semantic Interference and Learning – II Edition, DR=Delayed Recall.
Cells contain raw beta-coefficients
Bonferroni-corrected α=.004
Linear regression (Attention, Executive Function, Language, Memory, Visual Spatial Reasoning, Face Name, DR Correct), Poisson Regression (DR Intrusions), All model adjusted for age, sex, education, and cohort.
DISCUSSION
In this initial report from the MYHAT-NI study we describe a subsample of dementia-free older adults recruited from a representative population study in an economically depressed small-town area of southwestern Pennsylvania, who were eligible for and consented to neuroimaging. We report associations between PET indices of brain Aβ and tau with demographic and health status measures and with concurrent cognitive performance, as well as associations between Aβ and tau PET. Aβ(+) participants were older, had lower education, and were more likely to be APOE*4 carriers in comparison to Aβ(−) participants. Aβ and tau deposition were associated in all Braak-defined regions. Higher global Aβ deposition was associated with worse performance on two indices of a challenging semantic interference verbal memory paradigm (delayed recall and intrusions). We observed very few associations between tau deposition and any cognitive domain or test. Exceptions were 1) a negative association between Braak 1 (entorhinal cortex) tau and a verbal memory measure reflecting retroactive semantic interference, and 2) a positive association between Braak 1 tau and the visual spatial composite. The former finding is in line with predictions and a significant literature regarding the key role of entorhinal cortex in associative memory (Suh, Rivest, Nakashiba, Tominaga, & Tonegawa, 2011), as well as a recent study of [F-18]AV-1451 imaging showing signal in the posterior entorhinal cortex as most strongly correlated with episodic memory, among medial temporal lobe subregions, in normal aging (Maass et al., 2018). The latter finding is unexpected in its direction (better visual spatial performance associated with higher tau Braak 1 tau signal), and does not seem to be easily explained as a selection bias due to high cognitive reserve. The finding may simply be due to chance; replication can address this in future analyses. Given that our sample was population-based, cognitively normal, and relatively small, the statistically significant associations for tau and amyloid with cognitive measures were relatively weak, and were not robust to multiple-comparison correction. It remains to be seen whether these associations will become stronger as we follow our participants over time with repeated scans and cognitive assessments.
Regarding Aβ deposition in cognitively unimpaired (CU) older adults, present results are consistent with a large literature showing the expected associations with age and with the APOE*4 allele (Jansen et al., 2015; Nadkarni et al., 2019). APOE*4 is the most significant genetic factor for Aβ deposition, as identified in the first PiB-PET genome-wide association study (Yan et al., 2018). Meta-analyses report associations of small effect sizes cross-sectionally with cognitive function and Aβ, primarily episodic memory, in studies of CU older adults (J. E. Baker et al., 2017; Hedden, Oh, Younger, & Patel, 2013). Interestingly, we did not observe associations with specific process measures of face-name associative memory, nor of proactive / retroactive semantic interference measures, unlike previous reports (Loewenstein et al., 2016; Rentz et al., 2011). Previous studies drew from academic centers, and had generally more stringent exclusion criteria in place than the current study. While it is not clear whether these factors can account for differences in findings, we have previously observed that results from our population-based cohort differ from those from academic medical settings in head-to-head comparisons (Beer, Snitz, Chang, Loewenstein, & Ganguli, 2018; Snitz et al., 2018), typically in the direction of lower clinical progression rates and smaller risk factor effects for AD. Of note, MYHAT-NI participants with lower education were more likely to be Aβ(+). This observation stands in contrast to literature showing the opposite trend, that Aβ positivity tends to be associated with higher education in older adults without dementia (Jansen et al., 2015). This has been presumed to reflect a cognitive reserve selection bias in studies requiring CU status, and may also be characteristic of biomarker studies recruiting in academic research settings. The current study, which used a more generalizable sampling frame, may be less vulnerable to this selection bias.
Regarding brain tau pathology, our findings are consistent with other reports since the recent development of tau PET tracers that [F-18]AV-1451 signal is detectable in widespread cortical areas in cognitively unimpaired samples of older adults (Lowe et al., 2018; Mishra et al., 2017). In the largest reported cohort, 58% (334/576) of participants in the Mayo Clinic Study of Aging (MCSA) had abnormal tau-PET findings, with medial temporal lobe (MTL) abnormalities present in 41% and even 17% with extra-MTL abnormalities but without concurrent MTL abnormalities, inconsistent with the expected neuropathological Braak staging sequence (Lowe et al., 2018). In the Mayo study, as in our findings and in other studies (Johnson et al., 2016; Vemuri et al., 2017), increased tau PET was associated with presence of Aβ deposition. In our current findings, however, tau PET was not associated with age, consistent with some reports (Brier et al., 2016; Schwarz et al., 2016), but in contrast to age associations in others (Johnson et al., 2016; Lowe et al., 2018). Finally, lack of association between tau PET and cognitive measures contrasts other reports (Johnson et al., 2016; Mishra et al., 2017). Even tau signal confined to the MTL in the Braak 1/2 region was not associated with any of multiple specific memory measures, contrary to some studies with CU participants (Marks, Lockhart, Baker, & Jagust, 2017; Scholl et al., 2016). Association with Aβ, but not with age or cognition, suggests the possibility of a relatively ‘silent’ early phase of tau accumulation, which is consistent with presumed AD pathways, and which may have implications for optimizing intervention timing.
This neuroimaging cohort, recruited directly from an active, randomly sampled population-based cohort, represents a fundamental application of the population neuroscience paradigm. Other neuroimaging examples of this approach include the MCSA described above, drawing from older adults in Rochester, MN, and the multi-site ARIC-PET Amyloid Imaging Study (Gottesman et al., 2016). The sampling framework of MYHAT-NI attempted to minimize the selection bias often present in clinic-based or convenience samples utilizing neuroimaging, and thus to maximize generalizability to the broader population of older adults in the region. Nonetheless, like all neuroimaging studies, requirement of consent to extensive procedures and restrictive eligibility requirements inherently results in some degree of systematic bias. Furthermore, the MYHAT-NI study began roughly nine years after MYHAT baseline, thus older participants tended to be recruited from a survivor sample of original MYHAT participants. A final consideration is that the sample is primarily white, reflecting the demographics of the older adult population of the MYHAT communities.
CONCLUSIONS
Optimal characterization of AD biomarkers in older adults requires population neuroscience approaches that address the considerable heterogeneity of aging. Initial findings from the MYHAT-NI study illustrate this approach and suggest that brain Aβ and tau deposition are not infrequent in a population setting, and are associated with each other, but have few associations with concurrent cognitive performance. Future directions from the MYHAT-NI study include: 1) investigating prediction of clinical and cognitive change from baseline and rates of change in Aβ and tau and 2) investigating long-term lifestyle and health-related predictors of AD biomarker outcomes.
Supplementary Material
Acknowledgements
The authors thank Erin Jacobsen, Amy Carper, and Keith Van Horn for administrative, data management, and study recruitment support for MYHAT-NI. We are grateful to all of the staff of the Pittsburgh PET Research Center, and the participants and staff of the MYHAT and MYHAT-NI studies.
Funding
This work was supported by the National Institute on Aging at the National Institute of Health (R01 AG052521, R01 AG023651, RF1 AG025516, R01 AG030650, R01 AG064877, and T32 AG000181).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
GE Healthcare holds a license agreement with the University of Pittsburgh based on the PiB PET technology used in this manuscript. Dr. Klunk is a co-inventor of PiB and, as such, has a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. All other authors have no conflicts of interest with this work, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Baker JE, Lim YY, Pietrzak RH, Hassenstab J, Snyder PJ, Masters CL, & Maruff P (2017). Cognitive impairment and decline in cognitively normal older adults with high amyloid-beta: A meta-analysis. Alzheimers Dement (Amst), 6, 108–121. doi: 10.1016/j.dadm.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SL, Maass A, & Jagust WJ (2017). Considerations and code for partial volume correcting [(18)F]-AV-1451 tau PET data. Data Brief, 15, 648–657. doi: 10.1016/j.dib.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JC, Snitz BE, Chang CH, Loewenstein DA, & Ganguli M (2018). Does a cognitive stress test predict progression from mild cognitive impairment to dementia equally well in clinical versus population-based settings? Int Psychogeriatr, 30(10), 1435–1445. doi: 10.1017/S1041610217002666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, … Ances BM (2016). Tau and Abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med, 8(338), 338ra366. doi: 10.1126/scitranslmed.aaf2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, … Klunk WE (2013). Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage, 71, 207–215. doi: 10.1016/j.neuroimage.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby CA, Katz MJ, Lipton RB, & Hall CB (2017). Trends in Dementia Incidence in a Birth Cohort Analysis of the Einstein Aging Study. JAMA Neurol, 74(11), 1345–1351. doi: 10.1001/jamaneurol.2017.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Hyde LW, Mitchell C, Faul J, Gonzalez R, Heitzeg MM, … Schulenberg J (2013). What is a representative brain? Neuroscience meets population science. Proc Natl Acad Sci U S A, 110(44), 17615–17622. doi: 10.1073/pnas.1310134110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12(3), 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Ganguli M, Albanese E, Seshadri S, Bennett DA, Lyketsos C, Kukull WA, … Hendrie HC (2018). Population Neuroscience: Dementia Epidemiology Serving Precision Medicine and Population Health. Alzheimer Dis Assoc Disord, 32(1), 1–9. doi: 10.1097/WAD.0000000000000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, & Lee CW (2010). Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry, 18(8), 674–683. doi: 10.1097/JGP.0b013e3181cdee4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Fu B, Snitz BE, Hughes TF, & Chang CC (2013). Mild cognitive impairment: incidence and vascular risk factors in a population-based cohort. Neurology, 80(23), 2112–2120. doi: 10.1212/WNL.0b013e318295d776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Snitz B, Vander Bilt J, & Chang CC (2009). How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry, 24(11), 1277–1284. doi: 10.1002/gps.2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Schneider AL, Zhou Y, Chen X, Green E, Gupta N, … Mosley TH Jr. (2016). The ARIC-PET amyloid imaging study: Brain amyloid differences by age, race, sex, and APOE. Neurology, 87(5), 473–480. doi: 10.1212/WNL.0000000000002914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, & Patel TA (2013). Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology, 80(14), 1341–1348. doi: 10.1212/WNL.0b013e31828ab35d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, … Montine TJ (2012). National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement, 8(1), 1–13. doi: 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, … Contributors. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement, 14(4), 535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … Trojanowski JQ (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol, 12(2), 207–216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, … Zetterberg H (2015). Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA, 313(19), 1924–1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, … Sperling R (2016). Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol, 79(1), 110–119. doi: 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh MI, Fan KH, Yan Q, Beer JC, Snitz BE, Wang X, … Ganguli M (2019). Population-based genome-wide association study of cognitive decline in older adults free of dementia: identification of a novel locus for the attention domain. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2019.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, … Langstrom B (2004). Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol, 55(3), 306–319. doi: 10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel RE, Greig MT, Bauer RM, Rosado M, Bowers D, … Duara R (2016). A Novel Cognitive Stress Test for the Detection of Preclinical Alzheimer Disease: Discriminative Properties and Relation to Amyloid Load. Am J Geriatr Psychiatry, 24(10), 804–813. doi: 10.1016/j.jagp.2016.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Gonzalez I, Johnson KA, & Price JC (2019). PET imaging of tau protein targets: a methodology perspective. Brain Imaging Behav, 13(2), 333–344. doi: 10.1007/s11682-018-9847-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe VJ, Bruinsma TJ, Min HK, Lundt ES, Fang P, Senjem ML, … Jack CR Jr. (2018). Elevated medial temporal lobe and pervasive brain tau-PET signal in normal participants. Alzheimers Dement (Amst), 10, 210–216. doi: 10.1016/j.dadm.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, … Alzheimer’s Disease Neuroimaging I (2017). Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage, 157, 448–463. doi: 10.1016/j.neuroimage.2017.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K, … Jagust WJ (2018). Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. J Neurosci, 38(3), 530–543. doi: 10.1523/JNEUROSCI.2028-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SM, Lockhart SN, Baker SL, & Jagust WJ (2017). Tau and beta-Amyloid Are Associated with Medial Temporal Lobe Structure, Function, and Memory Encoding in Normal Aging. J Neurosci, 37(12), 3192–3201. doi: 10.1523/JNEUROSCI.3769-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Gordon BA, Su Y, Christensen J, Friedrichsen K, Jackson K, … Benzinger TLS (2017). AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: Defining a summary measure. Neuroimage, 161, 171–178. doi: 10.1016/j.neuroimage.2017.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Mungas D, Marshall SC, Weldon M, Haan M, & Reed BR (1996). Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology, 46(3), 700–706. doi: 10.1212/wnl.46.3.700 [DOI] [PubMed] [Google Scholar]
- Nadkarni NK, Tudorascu D, Campbell E, Snitz BE, Cohen AD, Halligan E, … Klunk WE (2019). Association Between Amyloid-beta, Small-vessel Disease, and Neurodegeneration Biomarker Positivity, and Progression to Mild Cognitive Impairment in Cognitively Normal Individuals. J Gerontol A Biol Sci Med Sci, 74(11), 1753–1760. doi: 10.1093/gerona/glz088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, … Sperling RA (2011). Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia, 49(9), 2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal C, Beiser AS, & Seshadri S (2016). Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med, 375(1), 93–94. doi: 10.1056/NEJMc1604823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, … Jagust WJ (2016). PET Imaging of Tau Deposition in the Aging Human Brain. Neuron, 89(5), 971–982. doi: 10.1016/j.neuron.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, … Mintun MS (2016). Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain, 139(Pt 5), 1539–1550. doi: 10.1093/brain/aww023 [DOI] [PubMed] [Google Scholar]
- Snitz BE, Wang T, Cloonan YK, Jacobsen E, Chang CH, Hughes TF, … Ganguli M (2018). Risk of progression from subjective cognitive decline to mild cognitive impairment: The role of study setting. Alzheimers Dement, 14(6), 734–742. doi: 10.1016/j.jalz.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, & Tonegawa S (2011). Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science, 334(6061), 1415–1420. doi: 10.1126/science.1210125 [DOI] [PubMed] [Google Scholar]
- Sullivan KJ, Dodge HH, Hughes TF, Chang CH, Zhu X, Liu A, & Ganguli M (2019). Declining Incident Dementia Rates Across Four Population-Based Birth Cohorts. J Gerontol A Biol Sci Med Sci, 74(9), 1439–1445. doi: 10.1093/gerona/gly236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudorascu DL, Minhas DS, Lao PJ, Betthauser TJ, Yu Z, Laymon CM, … Cohen AD (2018). The use of Centiloids for applying [(11)C]PiB classification cutoffs across region-of-interest delineation methods. Alzheimers Dement (Amst), 10, 332–339. doi: 10.1016/j.dadm.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, … Gunn RN (2011). Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage, 54(1), 264–277. doi: 10.1016/j.neuroimage.2010.06.044 [DOI] [PubMed] [Google Scholar]
- Vemuri P, Lowe VJ, Knopman DS, Senjem ML, Kemp BJ, Schwarz CG, … Jack CR Jr. (2017). Tau-PET uptake: Regional variation in average SUVR and impact of amyloid deposition. Alzheimers Dement (Amst), 6, 21–30. doi: 10.1016/j.dadm.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Nho K, Del-Aguila JL, Wang X, Risacher SL, Fan KH, … Kamboh MI (2018). Genome-wide association study of brain amyloid deposition as measured by Pittsburgh Compound-B (PiB)-PET imaging. Mol Psychiatry. doi: 10.1038/s41380-018-0246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


