Abstract
Optical coherence tomographic angiography (OCTA) is a novel technology capable of imaging retinal vasculature three-dimensionally at capillary scale without the need to inject any extrinsic dye contrast. However, projection artifacts cause superficial retinal vascular patterns to be duplicated in deeper layers, thus interfering with the clean visualization of some retinal plexuses and vascular pathologies. Projection-resolved OCTA (PR-OCTA) uses post-processing algorithms to reduce projection artifacts. With PR-OCTA, it is now possible to resolve up to 4 distinct retinal vascular plexuses in the living human eye. The technology also allows us to detect and distinguish between various retinal and optic nerve diseases. For example, optic nerve diseases such as glaucoma primarily reduces the capillary density in the superficial vascular complex, which comprises the nerve fiber layer plexus and the ganglion cell layer plexus. Outer retinal diseases such as retinitis pigmentosa primarily reduce the capillary density in the deep vascular complex, which comprises the intermediate capillary plexus and the deep capillary plexus. Retinal vascular diseases such as diabetic retinopathy and vein occlusion affect all plexuses, but with different patterns of capillary loss and vascular malformations. PR-OCTA is also useful in distinguishing various types of choroidal neovascularization and monitoring their response to anti-angiogenic medications. In retinal angiomatous proliferation and macular telangiectasia type 2, PR-OCTA can trace the pathologic vascular extension into deeper layers as the disease progress through stages. Plexus-specific visualization and measurement of retinal vascular changes are improving our ability to diagnose, stage, monitor, and assess treatment response in a wide variety of optic nerve and retinal diseases. These applications will be further enhanced with the continuing improvement of the speed and resolution of the OCT platforms, as well as the development of software algorithms to reduce artifacts, improve image quality, and make quantitative measurements.
1. Introduction
With approximately 30 million examinations being performed per year worldwide (Fujimoto and Huang, 2016), optical coherence tomography (OCT) (Huang et al., 1991) is the most commonly performed imaging procedure in ophthalmology (Swanson and Huang, 2011). The frequency of its use reflects the merits of OCT imaging: the modality provides micron-scale depth resolution and is uniquely able to resolve internal retinal structures non-invasively. These traits make OCT an important technology for the treatment of several diseases (Brand, 2012), where it is used to diagnose pathology, identify patients that would benefit from treatment, and assess treatment efficacy. In just the treatment of age-related macular degeneration (AMD) alone, OCT has been estimated to provide more than $1.0 billion a year in Medicare savings (Windsor et al., 2018). Since vascular changes can precede clinical developments such as exudation, and some diseases are primarily vascular in character, it is natural to wonder if OCT could be similarly useful in vascular imaging. Unfortunately, since the contrast of conventional structural OCT is based on tissue reflectance alone, it cannot provide visualization of capillaries, which have similar reflectivity to the tissues in which they are embedded. Optical coherence tomographic angiography (OCTA) is an important extension of OCT imaging that overcomes this contrast limitation and allows for imaging of retinal and choroidal microcirculation with capillary detail (Fig. 1). To do so OCTA relies on the inherent flow-related motion contrast between consecutive OCT cross-sectional scans.
Figure 1.
Multimodal imaging of macular circulation in a patient matched with postmortem examination of the same eye: (A) histology, (B) OCT angiography (OCTA). The OCTA image represents a projection of all retinal vascular plexuses. Reprinted with permission from (Balaratnasingam et al., 2019).
In addition to providing high-resolution, depth-resolved vascular imaging, OCTA is also non-invasive and avoids the need for intravenous injection of fluorescent dyes, as in traditional fundus angiography, which could cause patient discomfort and potentially serious side effects (Bearelly et al., 2009; Xu et al., 2016). Furthermore, dye injections can be dangerous for patients with allergies and high blood pressure (Kornblau and El-Annan, 2019). The bright blue light used in fluorescein angiography (FA) can also be difficult to tolerate for some patients, contrasting with the invisible infrared light used in OCTA. The non-invasive nature of OCTA also allows the technique to be used more frequently – potentially at every visit when the screening and follow-up of retinal diseases is indicated. OCTA can be performed on many commercial high-speed OCT platforms without hardware modification. Thus, the angiography functionality could be added as a simple software upgrade, making it readily available. Because leading causes of irreversible blindness - including glaucoma (Quigley and Broman, 2006; Tham et al., 2014), AMD (Wong et al., 2014) and diabetic retinopathy (DR) (Yau et al., 2012)- are all associated with changes in ocular circulation, the increased functionality provided by OCTA is important.
Similar to any new imaging modality, the interpretation of OCTA came with many pitfalls that required learning on the part of clinicians and many improvements to the image processing software on the part of the developers. Blood vessels with very slow flow may not appear on OCTA but could still be visualized by dye filling. And the leakage of dyes is an important marker for neovascularization, exudation, and inflammation. While neovascularization and exudation can be inferred by other means using OCTA and structural OCT, there is no clear means for OCTA to assess vasculitic inflammation.
Furthermore, as originally described, OCTA images tended to be cluttered by motion and projection artifacts (Enders et al., 2019; Spaide et al., 2015a). Projection artifacts appeared as tails on blood vessels on cross-sectional OCTA that made vessels look like long lines instead of single dots. On en face OCTA images, projection artifacts map superficial vascular structures onto deeper anatomic slabs, blending features from different plexuses together. Projection artifact can be removed by resolving the ambiguity between the in situ and projected flow signal using post-processing algorithms. Projection-resolved optical coherence tomographic angiography (PR-OCTA) algorithms (Wang et al., 2017; Zhang et al., 2016a) remove tails from cross-sectional OCTA images and provide clean images of individual retinal plexuses on en face views. This capability enables clinicians to improve the classification and staging of various optic nerve and retinal diseases by providing a plexus-specific images of developing pathology.
2. Principles of optical coherence angiography
Optical coherence tomographic angiography is an extension of OCT that uses motion contrast to visualize blood vessels down to the capillary level. OCT is a three-dimensional (3D), interferometric imaging modality that utilizes coherence gating to resolve tissue depth. Modern OCT systems achieve very high imaging speeds and efficient signal detection by using the Fourier-domain OCT (FD-OCT) principle. In FD-OCT, a spectral interferogram is obtained by combining tissue backscattering with a stationary reference reflection in an interferometer. A Fourier transform calculation converts the spectral interferogram into an axial line (A-line) that relates reflectance to axial depth. The spectral interferogram is generated by either a rapidly tuned laser source (swept-source OCT, or SS-OCT) or a spectrometer (spectral-domain OCT, or SD-OCT). Optical coherence tomographic angiography uses these high-speed OCT platforms to obtain repeated cross-sectional images (B-frames) at the same tissue location and measure the signal change caused by motion. Since the scans are obtained rapidly, bulk tissue motion is usually small and motion contrast is primarily generated by the motion of blood cells in both large and small vessels, including capillaries. The motion contrast principle is illustrated in Fig. 2 using flying tennis balls as an analogy to flowing red blood cells. Subtraction of consecutive video frames removes the static background and accentuates the moving tennis balls. The shadows projected by the moving tennis balls on the wall is also accentuated, illustrating the generation of projection artifacts.
Figure 2.
The principle of motion contrast in optical coherence tomographic angiography (OCTA) is explained using video frames. In frames 1 and 2, tennis balls falling out of a tube represent red blood cells. Motion contrast is generated by subtracting the two frames from each other, which accentuates the moving parts in the image while removing the stationary parts (right). Note both the tennis balls (representing flowing blood) and the shadows they cast on the wall (representing projection artifacts) are highlighted.
The actual generation of OCTA is a bit more complicated than the tennis ball analogy in several ways. One is that red blood cells are much smaller–smaller than the OCT beam focal spot and small enough for forward scattering to predominate over shadowing (the OCT beam is not completely blocked). Another is the importance of speckle, because OCT is a coherent imaging technique. The OCT image contrast at the spatial scale of blood cells is dominated by speckle generated by interference between blood cells and adjacent structures. Relative motion between blood cells and adjacent structures changes the speckle pattern. Thus the flow-related variation in OCT signal is also called speckle variance in some literature (Zhang et al., 2015a). The OCT signal is described by complex numbers with both magnitude (amplitude) and phase components, both of which change with speckle variation. Flow signal could be obtained by calculating either the amplitude, phase, or both. Thus there are three major types of OCTA algorithms: phase-based, amplitude-based, and complex-signal-based. In any of these algorithms, speckle contrast and the flow detection signal-to-noise ratio can be enhanced by the split-spectrum technique, which is also reviewed below.
2.1. Phase-based
Attempts to measure aspects of retinal circulation using OCT are almost as old as the technology itself. Chen et al. and Izatt et al. made efforts in this direction by measuring the Doppler phase shift (Chen et al., 1997; Izatt et al., 1997) between consecutive axial scans (A-lines). However, Doppler OCT is not ideal as an angiography approach because it only detects the flow vector component parallel to the beam and therefore poorly detects retinal blood vessels, which are generally perpendicular to OCT beams. Fortunately, algorithms that measured phase variation (called “phase variance” or “Doppler variance”) rather than phase shift could detect flow in any direction (Fingler et al., 2008; Fingler et al., 2007; Makita et al., 2006) and became the basis of early OCTA exploration. With algorithmic and hardware advances, phase-based OCTA achieved volumetric imaging of retinal circulations (Kim et al., 2013; Schwartz et al., 2014). However, while phase change is a very sensitive detector of small sub-wavelength motion and therefore slow flow (Szkulmowski et al., 2009), it is consequently also susceptible to noise from bulk motion and system phase noise. For this reason, phase-based OCTA is not currently used in commercial instruments.
2.2. Amplitude-based
The first amplitude-based approaches to imaging vasculature were attempts to circumvent the insensitivity of Doppler OCT to transverse flow. Barton and Stromski achieved amplitude-based OCTA using a time-domain OCT system and demonstrated its potential by imaging a hamster’s skin (Barton and Stromski, 2005). Fourier-domain instruments made it possible to compare amplitude between consecutive B-frames instead of A-lines, enabling volumetric OCTA (Mariampillai et al., 2010; Mariampillai et al., 2008). Because amplitude-based OCTA ignores the phase component of the OCT signal, it is not affected by the phase noise caused by bulk motion or system noise. This makes it practically easier to obtain cleaner OCTA images. Most commercial OCTA systems (Optovue, Topcon, and Heidelberg) are amplitude-based. In Heidelberg instruments, the amplitude variance is measured. Both Topcon and Optovue produce flow signal using measurements that are less dependent on the OCT signal strength. In Topcon machines, this is the amplitude ratio. In Optovue systems, the decorrelation (equivalent of variance divided by the average) is calculated. Spectral splitting is also used by Optovue systems to enhance the flow detection signal-to-noise ratio (see Section 2.1.4).
2.3. Complex-signal-based
Complex-signal-based OCTA leverages all of the flow information available in the OCT data by using both the magnitude and phase components. Theoretically, this maximizes the sensitivity to low-velocity flow. However, since the complex signal includes the phase component, this approach is still susceptible to phase noise generated by bulk motion and system phase fluctuation, though to a smaller extent than purely phase-based OCTA. Optical microangiography (OMAG) is a prominent example of complex signal OCTA. Wang et al. developed the essential technique in 2007 (Wang et al., 2007). The original OMAG required phase modulation between A-lines by moving the reference mirror (An and Wang, 2008) or offsetting the sample-arm scanning mirror (Wang, 2010). An et al. later introduced ultrahigh sensitive OMAG (An et al., 2010), which compares the complex signal between consecutive B-frames. This version of OMAG is commercialized in the Zeiss instruments (Tan et al., 2018).
2.4. Split-spectrum OCTA algorithms
Independent of the specific type of signal being processed, the speckle contrast for flow detection can be enhanced by splitting the OCT spectrum into narrower bands. This is because the spatial range of speckle interference is inversely proportional to the spectral bandwidth. The flow signal from the spectral splits can then be averaged to enhance the signal-to-noise ratio of detected flow. The narrower spectral splits decrease the axial resolution, but this is inconsequential as long as the resolution is sufficient to resolve the retinal plexuses. A side benefit of the reduced resolution is decreased sensitivity to axial bulk motion (e.g. from ocular pulsation due to heartbeat). Split-spectrum amplitude-decorrelation angiography (SSADA) (Jia et al., 2012) has been found to enhances the signal-to-noise ratio by a factor of 4 and enable the production of high-quality OCTA using only two B-frames at each tissue location (Gao et al., 2015). Optovue OCTA systems use the SSADA algorithm.
The split-spectrum approach has also been applied to the phase-gradient signal to further enhance flow detection while suppressing phase noise (Liu et al., 2016). This algorithm is not yet commercially available.
3. Visualization
Regardless of which OCTA algorithm or instrument is used to make measurements, the resulting data needs to be displayed in order to be interpreted by clinicians. While 3D rendering of fly-through videos are impressive presentations (Spaide, 2016), visual recognition is best facilitated by dividing the volumetric data into en face or cross-sectional slabs and then projecting the flow signal into a two-dimensional (2D) angiogram.
3.1. Cross-sectional angiograms
Cross-sections are the predominant ways in which structural OCT has been used to visualize pathologies such as edema, holes, and tractional membranes (Hood and Kardon, 2007; Mookiah et al., 2015; Schmidt-Erfurth et al., 2017). Cross-sectional OCTA is also useful in the detection and classification of a number of vascular pathologies such as retinal neovascularization (NV), choroidal neovascularization (CNV), polypoidal choroidal vasculopathy (PCV), and macular telangiectasia (MacTel), where the depth of abnormal vessels provides important specific diagnostic information. Although en face OCTA could also be used to diagnose these conditions, misclassification could occur if slab segmentation is in error.
Therefore, cross-sectional OCTA should always be used to determine the precise depth of the abnormal vessels visualized on en face OCTA. These pathologies are discussed in more detail below.
The retinal circulation is concentrated in four layers or plexuses that are apparent in cross-sectional OCTA (Fig. 3). The organizations of these plexuses are discussed in more detail later.
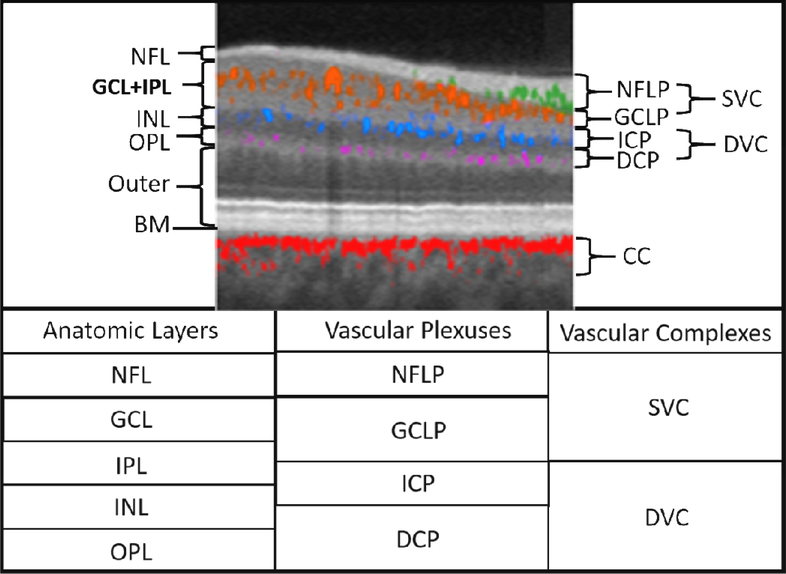
Figure 3.
Relationship between the retinal vascular plexuses and anatomic layers illustrated on a cross-sectional projection-resolved (PR) OCTA of a normal eye. Flow signals are color coded according to plexus and overlaid on a gray-scale reflectance image. Anatomic slabs (labels to the left of the image) are NFL = nerve fiber layer; GCL = ganglion cell complex, IPL = inner plexiform layer; INL = inner nuclear layer; OPL = outer plexiform layer; BM = Bruch’s membrane. Vascular plexuses (labels on the right of the image) are NFLP = nerve fiber layer plexus; GCLP = ganglion cell layer plexus; ICP = intermediate capillary plexus; DCP = deep capillary plexus. The GCLP extends from the NFL 67% of the way through the GCL and IPL. The vascular complexes are SVC = superficial vascular complex (NFLP + GCLP); DVC = deep vascular complex (ICP+DCP); CC = choriocapillaris. The table below gives the relative location of the retinal anatomic layers, vascular plexuses, and vascular complexes. Adapted with permission from (Campbell et al., 2017b).
Projection artifacts produce “tails” for blood vessels in cross-sectional view. These artifacts are particularly prominent in hyper-reflective layers such as the retinal pigment epithelium (RPE) and some pathologies such as drusen. They could be mistaken for CNV. Thus, to avoid misdiagnosis, it is important to use a projection-resolved OCTA algorithm to clean the cross-sectional view.
While cross-sectional OCT and OCTA are traditionally viewed as single B-frames, it can be advantageous to combine several adjacent B-frames into a thicker slab and average the signal to suppress speckle noise and enhance the continuity of retinal layers and vessels (Fig. 3) (Bhavsar et al., 2017; Campbell et al., 2017b). Unlike en face OCTA, in which only flow signal is displayed, cross-sectional OCTA images benefit from the simultaneous display of color-coded flow signal and gray-scale reflectance (structural OCT) signal (Fig. 3).
3.2. Slab-based en face angiograms
En face views are essential to OCTA for the visualization of capillary dropout within the continuous vascular network in the retinal plexuses. They are also useful for visualizing the extent of abnormal vascular networks in normally avascular layers, such as diabetic NV in the pre-retinal vitreous or CNV in the outer retina. En face OCTA also affords a perspective that can be compared to fundus photography and dye-based fundus angiography. For these reasons, the en face view has become the primary display for OCTA.
To construct en face images, the 3D OCTA data must first be segmented into anatomically meaningful slabs. The slab could be for one specific retinal plexus, a complex of plexuses, or a layer that is normally avascular in a healthy subject (Fig. 4). Automated computer image segmentation algorithms have made slab visualization feasible, with current approaches based on graph search (Guo et al., 2018; Yin et al., 2014; Zang et al., 2017; Zhang et al., 2015c), machine learning (Pekala et al., 2018), or both (Miri et al., 2017; Zang et al., 2017; Zang et al., 2019).
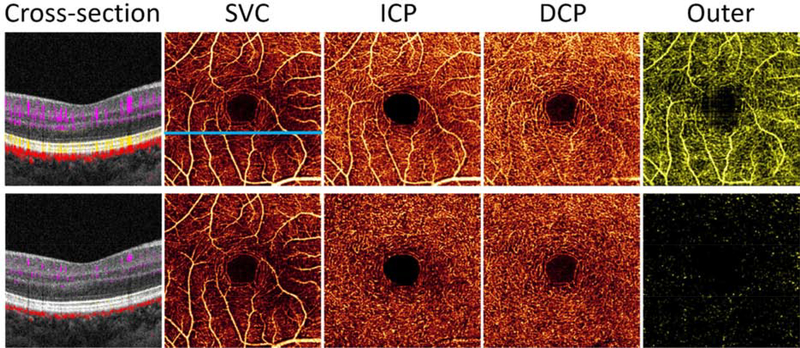
Figure 4.
Comparison of retinal OCTA (3×3mm) from a healthy volunteer without projection resolution (row 1), and with PR-OCTA (row 2). The en face OCTAs shown are for the superficial vascular complex (SVC), intermediate capillary plexus (ICP), deep capillary plexus (DCP), and outer retina (outer). Also shown are cross-sectional images from the location of the blue line in the original superficial angiogram. In the original en face OCTA, the projection artifacts are most obvious in the outer retinal slab due to the strongly reflective photoreceptor and RPE layers. Large superficial vessels also project bright flow signal in the ICP slab. These artifacts are cleanly removed in PR-OCTA. The projection artifact appears as bright vertical tails on blood vessels in the original cross-sectional OCTA (upper left). These tails are also removed by the PR-OCTA algorithm, restoring the blood vessels as distinct dots (lower left).
Both mean- and maximum-value projection methods have been used to produce en face OCTA images. Maximum-value projection emphasizes the single voxels with the brightest flow signal and ignores overlap of multiple vessels or projection artifacts. Mean-value projection emphasizes the cumulative flow signal over multiple voxels, so thicker vessels or overlap of multiple vessels are accentuated. Unfortunately for mean-value projection, projection artifacts are added together with real in situ flow signals. So, for retinal OCTA, maximum projection generally provides cleaner angiograms. It has been statistically demonstrated that maximum projection achieves better signal-to-noise ratio and image contrast for retinal OCTA (Hormel et al., 2018). These considerations could be different for the choriocapillaris, where mean-value projection could provide information on the cumulative perfusion provided by overlapping capillaries. But since this article focuses on retinal OCTA, all of the en face images shown are produced by maximum projection.
En face OCTA provides a familiar format to clinicians already familiar with fluorescein and indocyanine green angiography, while avoiding possible adverse reactions to dye injection. Another advantage of OCTA is that good capillary contrast can be consistently obtained from both eyes of the subject, thus facilitating the measurement of capillary density that can be compared between individuals and visits. While FA and ICGA can also provide good capillary contrast, this is only possible during a short period during dye transit, making it difficult to image capillary detail in both eyes.
4. Artifacts and their removal
4.1. Flow projection artifacts
The flow contrast in OCTA is generated by any change in OCT signal between consecutive B-frames taken at the same location. The change could be due to in situ blood flow (moving blood cells within the image voxel) or projected blood flow (variability in the OCT beam due to scattering by more superficial blood cells). In the tennis ball analogy (Fig. 2), the flow projection is represented by the moving shadow projected on the wall. Similarly, in OCTA images the projected signal is strongest in hyper-reflective layers such as the RPE in the outer retina (Fig. 4). A particularly important diagnostic problem arises when projected flow on hyper-reflective pigment epithelial detachments or drusen gives a false impression of neovascularization (Dansingani et al., 2015; Spaide et al., 2015a). Clinicians could recognize these artifacts by their characteristic appearance as tails on cross-sectional images and duplication of inner retinal flow patterns in the outer retinal slab (Fig. 4). But an even better solution is to use a post-processing algorithm to resolve the ambiguity between in situ and projected flow and produce clean projection-resolved OCTA (PR-OCTA) images.
An early approach to suppress the projection artifact was to simply subtract the superficial slab flow signal from deeper slabs (Jia et al., 2015; Jia et al., 2014a; Liu et al., 2015; Zhang et al., 2015b). However, slab subtraction algorithms replace the bright projection artifact with a dark shadow artifact and disrupt the continuity of vascular networks in the deeper slabs. Furthermore, it only applies to en face slabs and cannot be used to clean up cross-sectional OCTA images.
A better approach is projection-resolved OCTA (PR-OCTA) algorithms that provide voxel-by-voxel differentiation between in situ (real) and projected flow signal over the entire 3D dataset (Wang et al., 2017; Zhang et al., 2016a). The result is that artifactual projected flow is removed without removing true in situ flow from deeper layers. With PR-OCTA it is possible to visualize the continuous capillary networks in the intermediate and deep capillary plexuses while minimizing both bright projection or dark shadow artifacts from overlying vessels.
4.2. Bulk motion artifacts
Unlike dye-based angiography, OCTA is not a snapshot modality. Eye movements are unavoidable during the several seconds of acquisition time for OCTA. Even with good gaze fixation, it is normal to have 2–3 rapid microsaccadic movements per second with slow drift motion in between. Ocular microtremor, cardiac pulsation, and breathing are other sources of bulk motion.
Microsaccades are faster than capillary blood flow and appear as bright line artifacts on en face OCTA. It is not possible to compensate for such strong background noise and thus these line artifacts are dealt with by excluding entire B-frames. The excluded B-frames are replaced by re-scanning the same position using real-time eye tracking. Alternatively or in addition, two or more volumes could be merged in post-processing to avoid gaps left by removing microsaccadic frames (Camino et al., 2017).
Slower bulk motion appears as graded stripes of brighter flow signal that could nevertheless affect accurate measurement of vessel density and other quantitative parameters. Several types of algorithms have been developed to subtract the estimated contribution of bulk motion from the flow signal. The residual bulk motion on cross-sectional images can be removed by approaches that use averaging (Wang and An, 2009), histograms (Makita et al., 2006; Mariampillai et al., 2010), or standard deviation (Wei et al., 2018) in phase- and complex-signal-based OCTA. In amplitude-based OCTA, median subtraction was conventionally used (Jia et al., 2012). These older approaches ignore the dependence of bulk motion signal on tissue reflectance and the nonlinear addition of flow signal. A more accurate regression-based bulk motion subtraction algorithm has been developed to recover the flow signal on a voxel by voxel basis (Camino et al., 2017; Camino et al., 2018). Used as a processing step in vessel density calculations, this algorithm has shown improved quantification for this parameter in healthy eyes as well as eyes with diabetic retinopathy (Wang et al., 2019a). The regression-based bulk motion subtraction more completely removes background noise in the flow signal which could be seen in the foveal avascular zone as well as in areas of capillary dropout in an eye with diabetic retinopathy (Fig. 5)
Figure 5.
Bulk motion artifact removal. In the original OCTA of an eye with diabetic retinopathy (left), background is bright due to the presence of bulk motion artifacts. The bulk motion signal can be removed by subtracting the median signal value from the image, but this approach ignores the dependence of bulk motion signal on tissue reflectance and the nonlinear addition of bulk motion and flow signals. Better artifact compensation is achieved with a regression-based approach (Camino et al., 2017). Areas of capillary dropout are more clearly visualized with the bulk motion noise removed.
4.3. Signal strength bias and shadowing artifacts
OCTA signals derived from the variance of reflectance magnitude or complex amplitude are intrinsically proportional to the average reflectance magnitude (signal strength). Other types of OCTA signal, such as amplitude decorrelation, amplitude ratio (log variance), or phase variance, are theoretically unrelated to the signal strength. But in reality there is still a relationship between the flow signal and the reflectance signal strength due to digital signal processing filters to suppress background noise in low amplitude voxels in OCTA algorithms. The flow signal has been found to be proportional to the logarithm of signal strength (Jain et al., 2016) The measured retinal vessel density has also been found to be proportional to the logarithm of signal strength in two of the commonly used commercial OCTA instruments based on SSADA and ultrahigh sensitive OMAG (Yu et al., 2019). The signal strength can be measured by averaging the OCT reflectance signal in tissue (e.g. the signal strength index, or SSI parameter, on the AngioVue system; the signal strength, or SS parameter, on the AngioPlex system).
The signal strength of an OCTA image can be affected by beam attenuation (e.g. from cataract and other media opacity) and defocus (e.g. from spherical defocus, astigmatism, or higher order aberrations). The effects of beam attenuation and defocus on vessel density measured on OCTA images have been demonstrated experimentally (Yu et al.), where vessel density was shown to decline by approximately 20% over 4 diopters. This signal strength bias could present a problem in the clinical evaluation of diseases that reduce vessel density. For example, one would not want to misdiagnose glaucoma based on retinal vessel reduction caused by low signal strength associated with a cataract. Therefore, it is desirable to measure retinal vessel density using algorithms that compensate for signal strength variation. One approach is to compensate for the signal strength bias by taking into account the local measured tissue reflectance in select retinal layers (Gao et al., 2016a). This works well for diseases such as glaucoma, where there is no generalized disruption of retinal tissue structure. Another approach is to take into account the reflectance of individual vascular voxels (Camino et al., 2018), which has the merit of being unaffected by edema or exudates in nonvascular tissue.
Blockage of the OCT beam by the iris (vignetting) or vitreous floaters (shadows) could attenuate the signal so much that no vessel density measurement is possible in the locally affected area (Fig. 6). Shadows can be automatically recognized using artificial intelligence (AI) algorithms that examine both tissue reflectance and flow signal patterns. The algorithm can then block out the shadow area on the en face OCTA from further analysis so that the artifact would not be mistaken for capillary dropout (Guo et al., 2019).
Figure 6.
Shadow artifacts. Shown are 3×3 mm retinal angiograms from the same eye before (A) and after (B) vitrectomy surgery to remove vitreous floaters that caused shadow artifacts. The shadow artifact (blue arrow) caused an area within the angiogram to appear avascular; after vitrectomy, the intact vasculature is apparent. Modified with permission from (Camino et al., 2019).
5. Quantification
An important advantage of OCTA is the consistent contrast for blood vessels at the capillary level. This differs from traditional fluorescein angiography (FA), in which retinal capillaries can be visualized only during a short phase of dye transit, and could be obscured by dye leakage and the bright choroidal background. OCTA is used to measure nonperfusion area and foveal avascular zone dimensions, as FA has been used to do. In addition, OCTA is also used to measure vessel density, capillary density, and low-perfusion areas. Using PR-OCTA, it is now possible to make these measurements on individual retinal plexuses, thus gaining additional specificity in the assessment of disease classification and severity.
5.1. Vessel and capillary densities
Reduction in in vessel density is an important marker in diseases such as glaucoma and diabetic retinopathy (Jenkins et al., 2015; Nesper et al., 2017; Triolo et al., 2017; Yarmohammadi et al., 2016a). In OCTA, vessel density (VD) can be measured by separating the vascular and nonvascular pixels on en face OCTA of the relevant vascular plexus or complex. The percent area occupied by vascular pixels is the most common definition for vessel density. This is called the “vessel density” in AngioVue (Optovue, Inc.) and “perfusion density” in AngioPlex OCTA systems (Carl Zeiss Meditec, Inc.). Vascular and nonvascular pixels are distinguished by a threshold flow signal value. This threshold could be a fixed value defined by the flow noise level in a known avascular area such as the foveal avascular zone (Hwang et al., 2016a).This has the advantage of a well characterized distribution of flow noise in nonvascular pixels, but ignores the distribution of flow values in vascular tissues. Alternatively, the threshold could be based on the global flow signal statistics in the image such as the average (Rabiolo et al., 2018) or intergroup variance (i.e., Otsu’s method) (Chidambara et al., 2016). Local adaptive thresholding, random field models, and morphological operations have also been used (Eladawi et al., 2017; Lei et al., 2017; Yousefi et al., 2015). These algorithms make assumptions about the flow signal distribution and shapes of blood vessels that may not be valid across all regions and diseases states, so careful empirical validation is needed.
There are some limitations for measuring VD by % area. One is that the transverse spatial resolutions of commercial OCT systems, as defined by the full-width 1/e2 focal spot diameter, are approximately 15–20 μm, larger than the typical capillary width of 5–10 μm (Shimizu and Ujiie, 1978). The caliber of capillaries on OCTA therefore appear 2–3 time wider than their true width. This is obvious when OCTA is compared side-by-side with histology (Fig. 1). So the percent area VD reported by commercial OCTA systems are larger than the actual values by a certain multiplying factor. OCTA is partially sensitive to changes in vessel width that occur below system resolution – physical flow phantom measurements have demonstrated that flow signal is influenced by both the flow velocity and the width of the channel (Su et al., 2016; Tokayer et al., 2013). Thus it is possible to detect dilated capillaries by the increase in flow signal amplitude (Dongye et al., 2017b), even if the pixel width is minimally affected. Overall, a reduction in vessel area density as measured by OCTA primarily reflects the loss or shutdown of capillaries, but could also be due to the reduction in capillary flow velocity or width to such a degree that flow signal falls below detection threshold. Therefore VD is useful in detecting pathological reduction in perfusion, even if the area value is not physically exact.
An alternative metric for vessel density measures vascular length divided by the image area. This metric is called the “vessel density” on the AngioPlex and is measured in units of inverse mm. To distinguish it from vessel area density, a more specific term would be “vessel length density.” Theoretically, length density could be more accurate because it is not affected by the fact that the OCT beam focal spot size and OCTA pixel widths are larger than capillary diameters. This solution is not a panacea, however, since it requires accurate skeletonization of the capillary network that depends on near-perfect vessel continuity. This requires high sampling density (pixel width < focal spot diameter) and image quality. These requirements are not met on many OCTA scan patterns, which rely on undersampling to achieve larger fields of view.
A reduction in VD can be used to detect retinal diseases (Jia et al., 2015; Nesper et al., 2017) and glaucoma (Chen et al., 2016; Liu et al., 2019; Takusagawa et al., 2017; Yarmohammadi et al., 2016a; Yarmohammadi et al., 2016b). Empirical studies have demonstrated high repeatability and reproducibility of such measurements in some OCTA systems (Al-sheikh et al., 2017; Lei et al., 2017). However, measurements from different devices are generally not interchangeable (Rabiolo et al., 2018). In part, this may be attributable to variation in how issues such as signal strength reduction and bulk motion artifacts, which can bias these measurements, are handled. It is desirable to use vessel density measurement algorithms that reduce the effect of these artifacts (Camino et al., 2018; Gao et al., 2016a).
In addition to density, morphological changes such as microaneurysms (Schreur et al., 2019) or dilated capillaries could also provide diagnostic information. These could be assessed by human grading or automated algorithms (Dongye et al., 2017a).
In the peripapillary region, large retinal vessels dominate vessel density measurements and can mask the detection of focal capillary loss in glaucoma. In this situation, it is desirable to remove large vessels from the analysis. This results in the capillary density (CD) (Liu et al., 2019).
5.2. Avascular, non-perfusion, and low-perfusion areas
Some diseases such as diabetic retinopathy (DR) are characterized by patchy capillary drop out that is better captured by measuring the avascular area (AA) than vessel density. Measuring AA was already used to detect DR in the days of fluorescein angiography (FA) (ETDRSRG, 1991). With OCTA, AA has been found to have higher sensitivity than VD in detecting mild DR (Schottenhamml et al., 2016).
On en face OCTA, AA is defined by gaps between blood vessels that are larger than the typical separation between capillaries. Automated algorithms can detect such large gaps by looking for nonvascular pixels with a distance to the nearest vascular pixel that exceeds a certain characteristic threshold for the plexus and anatomic region (Schottenhamml et al., 2016; Zhang et al., 2016b).
Not all AA are pathological – the foveal avascular zone (FAZ) is a normal part of healthy eyes. Because there is a large variation in the FAZ area among the normal healthy population, it is helpful to exclude the fovea from analysis when the goal is to detect abnormality (Hwang et al., 2018). The extrafoveal AA can be called “non-perfusion area” (NPA), which is always pathological. Since the FAZ area is not a sensitive detector of pathology, other metrics have been developed to improve the measurement of foveal ischemia based on FAZ shape and irregularity (Lu et al., 2018), nonperfused capillaries (Lynch et al., 2018), and VD measured around a theoretical baseline FAZ defined by structural criteria (Wang et al., 2019a).
Areas of reduced signal strength caused by iris vignetting or vitreous floater shadowing could appear indistinguishable from nonperfusion areas on en face OCTA. However, they can be identified by examining the structural OCT images to see if the reflectance of choroidal and retinal layers are also attenuated. A convolutional neural network, a type of deep learning artificial intelligence, has proven capable of reliably making this distinction between NPA and signal reduction artifacts (Guo et al., 2019).
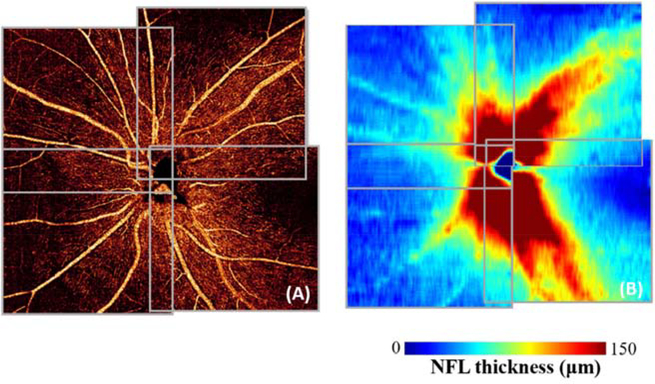
In some diseases, such as glaucoma, capillary density is reduced in characteristic regions, but patches of capillary dropout are not large enough to be identified as NPA. In these cases, it is better to identify areas of relatively low CD on a CD map, which can be constructed by low pass filtering the en face OCTA. The low perfusion area (LPA) is defined by local CD below the normal range established in a group of healthy subjects (Fig. 7). The LPA has been found to be a powerful way to both detect glaucoma and visualize the area of damage with excellent correlation to the visual field (Liu et al., 2019).
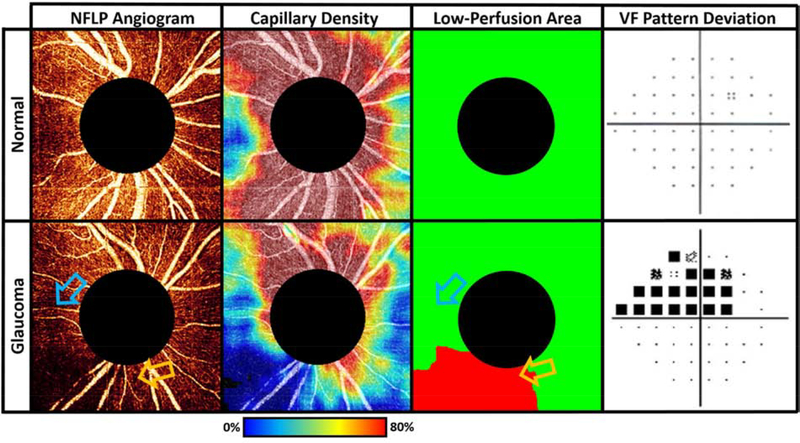
Figure 7.
Low-perfusion area (LPA) in the peripapillary NFLP distinguishes glaucomatous eyes from normal. The glaucomatous eye (bottom row) has capillary loss identified in the LPA map. The LPA map identifies areas with significantly lower capillary density than that of the same location in a normal eye, providing a more accurate perfusion loss localization than either the raw angiogram or the capillary density map. In the bottom row, the blue arrow marks a region of low capillary density that is normal, while the orange arrow indicates a region that retains high capillary density but that is nonetheless relatively low in comparison to healthy populations. The orange arrow, but not the blue arrow, therefore indicates a low perfusion area, even though the capillary density is higher at the location of the orange arrow. The LPA locations correlate well with the locations of visual field loss in the glaucomatous eye.
5.3. Retinal or choroidal neovascularization area
While OCTA slabs corresponding to retinal plexuses are used to detect loss of capillaries, normally avascular slabs are used to detect abnormal vascular formation. The preretinal vitreous slab is used to detect retinal neovascularization (RNV) (Hwang et al., 2015), while the outer retinal slab is used to visualize choroidal neovascularization (CNV) (Jia et al., 2014a).
Automated algorithms have been developed to measure the area of RNV and CNV membranes as well as the vessel area within these membranes (Gao et al., 2017b; Liu et al., 2015; Xue et al., 2018). The measurement of CNV area has been used to characterize the pharmacodynamics of anti-angiogenic treatment, and the growth of untreated, nonexudative CNV (Bailey et al., 2019b).
6. Vascular anatomy of the normal retina
Projection artifacts interfered with the clean separation of retinal plexuses in early OCTA. But with the development of PR-OCTA algorithms, it is now possible to clearly visualize distinct retinal plexuses. This section reviews the organization of these plexuses as background information to aid later discussion on pathologies.
6.1. Four plexuses and two complexes
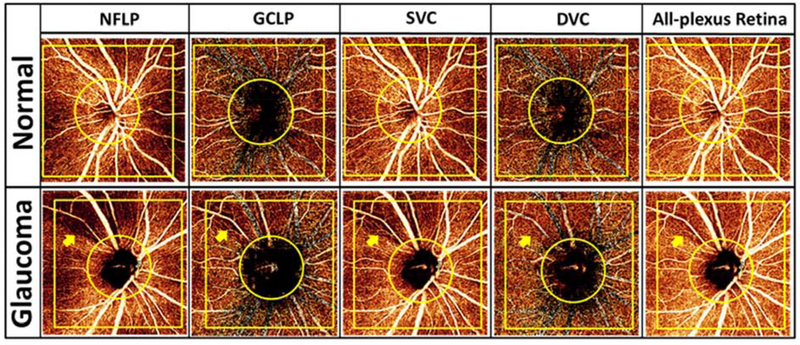
Histology (Fig. 8) (Balaratnasingam et al., 2019; Stone et al., 2008), ex vivo confocal microscopy (Chan et al., 2012; Tan et al., 2012), and in vivo studies using research instruments (Chan et al., 2012; Kurokawa et al., 2012) all support the existence of up to four vertically stratified retinal vascular networks in the primate macula. Contrary to this identification, many early studies performed with OCTA identified only two networks, one superficial and one deep (Spaide et al., 2015b). The reason is that projection artifacts mingled the flow signal from three plexuses - the nerve fiber layer plexus (NFLP), ganglion cell layer plexus (GCLP), and intermediate capillary plexus (ICP) – into one vascular pattern (Fig. 4). With PR-OCTA, it is now possible to accurately distinguish all four plexuses (Fig 3, 9).
Figure 8.
Retinal layer co-localization and histologic characteristics of the macular capillary plexuses in the left eye of an 84-year old female. Flat mount (A) and cross-sectional (B) histologic images of a donor eye with no known eye disease, depicting all vascular plexuses, demonstrate the density and complexity of the macular capillary circulation. The region where the cross-sectional image has been attained is represented by a white-fenestrated line on Panel A. On the tissue cross-section, nuclei appear in red stain and the vascular endothelium appears in green stain. The cross-section demonstrates that the superficial vascular complex is predominantly localized to the level of the ganglion cell layer, the intermediate plexus to the inner aspect of the inner nuclear layer and the deep plexus to the outer aspect of the inner nuclear layer. (C – E) Capillary plexuses on histologic flat-mounts have been false-colored blue (superficial complex), yellow (intermediate plexus) and red (deep plexus). Reprinted with permission from (Balaratnasingam et al., 2019).
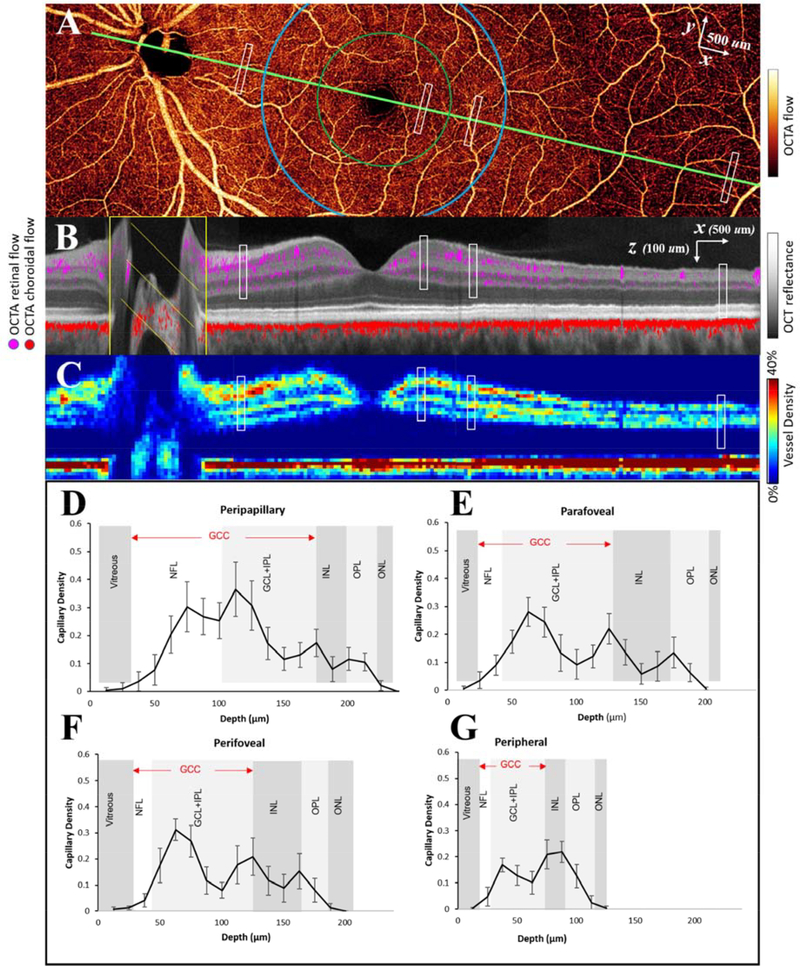
Figure 9.
Vascular anatomy of a healthy retina as imaged by OCTA. (A) Montaged high-definition en face OCTA of the inner retina. The green line shows the maculopapillary axis and the green and blue circles demarcate the parafovea and perifovea, respectively. (B) Cross-sectional view along the maculopapillary axis. Retinal (violet) and choroidal (red) flow signal are overlaid on the structural OCT image (reflectance in grayscale). (C) Cross-sectional image showing color-coded vascular density. (E-G) Capillary density by depth in 4 distinct regions, marked by the white rectangles in (A-C), support the existence of 4 distinct plexuses in some parts of the retina. NFL: nerve fiber layer; GCL: ganglion cell layer; IPL: inner plexiform layer; OPL: outer plexiform layer; ONL: outer nuclear layer. Adapted with permission from Campbell et al. (Campbell et al., 2017b).
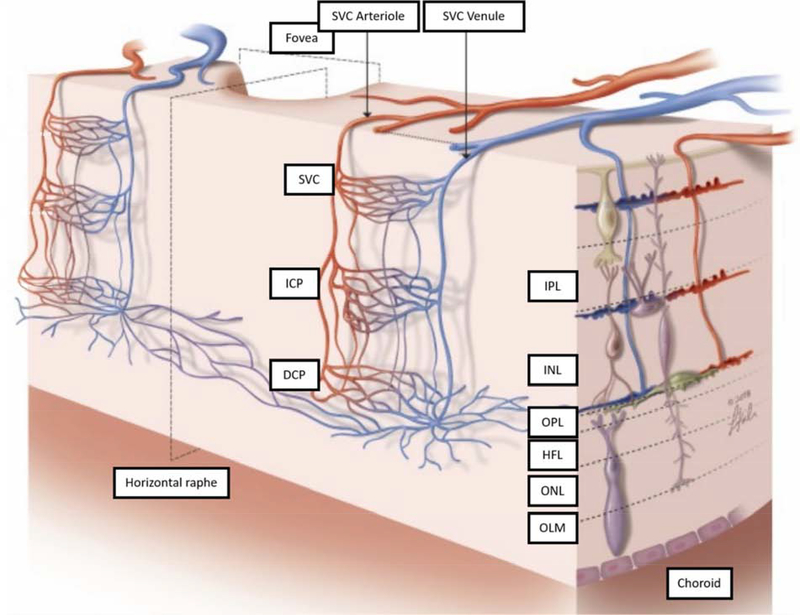
The most superficial of the four plexuses is the NFLP, which perfuses the nerve fiber layer (NFL). The NFLP forms a distinct concentrated layer of capillaries only in the peripapillary region (Fig. 9), and elsewhere appears continuous with the slightly deeper GCLP that resides in the ganglion cell layer (GCL). Thus in most of the macula the NFLP and GCLP together form a continuous superficial vascular complex (SVC) that supplies the ganglion cell complex (GCC) formed by the combination of the NFL, GCL, and inner plexiform layer (IPL), representing the axons, cell bodies, and dendrites of the retinal ganglion cells. The SVC is dominated by the NFLP near the optic nerve head and by the GCLP elsewhere; it includes the retinal arterioles and venules as well as interconnecting capillaries that form a complex mesh.
The NFLP exists in the peripapillary region and along the arcuate nerve fiber bundles, where the NFL is thickest. The capillaries are distributed within the volume of the NFL, and in general the capillaries run parallel to the nerve fibers. Where the NFL is thick, the capillaries stack in overlapping layers approximately every 47 μm. Where the NFL is thin (below 18 μm), the NFLP is absent (Jia et al., 2017). The SVC is a network. The NFLP includes the radial peripapillary capillary plexus (RPCP) (Campbell et al., 2017b; Jia et al., 2017; Spaide et al., 2015b) that had been described as radially oriented capillaries specific to the peripapillary region. The new term NFLP is more general and includes the extension of the RPCP along the arcuate nerve fiber bundles around the macula, far beyond the peripapillary region (Fig. 10) (Jia et al., 2017). Along the arcuate bundles the capillaries within the NFLP are arcuately, rather than radially, oriented. Finally, this plexus is not purely composed of capillaries, since retinal arterioles and venules often partially reside in this layer. For all of these reasons, we believe that NFLP is a useful novel term to incorporate our new understanding that the capillary network in the nerve fiber layer extends beyond the peripapillary region.
Figure 10.
Nerve fiber layer plexus (NFLP) angiogram (A) and nerve fiber layer (NFL) thickness map (B). Vessel density is proportional to NFL thickness. The capillaries are mostly parallel to nerve fiber trajectory, which is radial next to the optic disc and arcuate along the superior and inferior nerve fiber bundles. Reprinted with permission from (Jia et al., 2017).
Beneath the NFLP, the GCLP occupies the GCL and extends through the inner 67% of GCL/IPL. The upper border of the GCLP is naturally the NFL-GCL boundary. The lower border of the GCLP has been described as 80% of the GCC thickness below the ILM (Campbell et al., 2017b). While this definition works in most of the macula, it leads to gross errors near the disc, where the GCC is almost entirely made up of the NFL. We have found a more accurate lower border to be 67% of the GCL+IPL thickness below the NFL-GCL boundary. This depth corresponds to the watershed between the GCLP and the intermediate capillary plexus where vascular density reaches a minimum. The combined GCL+IPL thickness is used in this definition because the boundary between the GCL and IPL is indistinct and difficult to segment.
Below the SVC lies the deep vascular complex (DVC) that is comprised of the intermediate capillary plexus (ICP) at the IPL / inner nuclear layer (INL) boundary, and the deep capillary plexus (DCP) at the INL / outer plexiform layer (OPL) boundary. The ICP and DCP are primarily capillary networks (Fig. 4). On this background of a uniform capillary network, it is relatively easy to detect malformations such as microaneurysms and dilated vessels (Schreur et al., 2019). In contrast to the SVC, where capillaries are distributed in the volume of the NFL and GCL, the ICP and DCP form two narrow laminar planes in the macula (Fig. 8 and 9). More peripherally, where the INL is too thin to separate them, the ICP and DCP merge into one network, (Fig. 9).
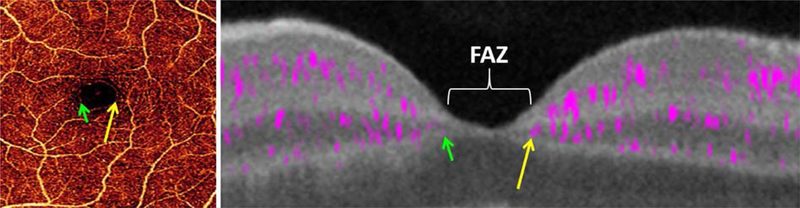
In the fovea, the retinal plexuses merge into a single capillary ring that encircles the foveal avascular zone (FAZ) (Fig. 11). Thus the FAZ area should be measured on the en face OCTA of the entire inner retina. Some publications separately measure a superficial FAZ area and deep FAZ area (Dimitrova et al., 2017; Samara et al., 2017; Takagi et al., 2018). Such superficial/deep distinction is not anatomically meaningful as applied to the FAZ, and the resulting area measurements are likely to reflect alterations in segmentation boundary due to tissue edema as much as actual perfusion changes.
Figure 11.
En face retinal OCTA (left) and cross-sectional (right) PR-OCTA of the fovea. The retinal plexuses merge into a single ring of capillaries (green and yellow arrows) around the foveal avascular zone (FAZ). Adapted with permission from (Campbell et al., 2017b).
6.2. Connections between plexuses: serial versus parallel
The retinal arterioles and venules are located in the SVC (primarily centered in the GCLP), which can be clearly seen on OCTA. The organization of the capillary network in between, particularly that of deeper plexuses, is a still a matter of some controversy. There is evidence that retinal venous drainage occurs predominantly through the DCP, in which centripetal capillaries feed into central venules that empty directly into larger venules in the SVC (Fouquet et al., 2017). Some have argued that this indicates a serial organization of the retinal circulation, wherein the SVC capillaries are on the arterial side of the circulation, with subsequent serial flow through the ICP and DCP before draining into the venous circulation (Au and Sarraf, 2019; Fouquet et al., 2017). In this model, venous drainage only occurs through the DCP, and arterial inflow only occurs through the SVC. However, a strictly serial organization would imply that (1) oxygen concentration should get progressively lower as blood flows through the SVC, ICP, and DCP, and (2) flow should be equal across plexuses. These consequences are not observed. Oxygen electrode measurements in macaques do not consistently show a reduction in oxygen content with increasing depth in the retina, and sometimes show either little change or even increasing concentration (Ahmed et al., 1993; Birol et al., 2007). In addition, PR-OCTA has shown that flow alteration in the retinal plexuses can occur independently. Breathing oxygen-enriched air reduces flow only in the DVC (Hagag et al., 2018). Optic nerve diseases such as glaucoma primarily reduce capillary density in the SVC but not deeper plexuses (Liu et al., 2019; Takusagawa et al., 2017). Outer retinal diseases, such as retinitis pigmentosa and choroideremia, primarily reduce capillary density in the DCP (Gao et al., 2017a; Hagag et al., 2019; Jain et al., 2016) Overall, evidence supports a view of retinal capillary organization as neither strictly serial nor parallel, but a composite network of complex horizontal and vertical interconnections (Fig. 12) (Nesper and Fawzi, 2018).
Figure 12.
Schematic depiction of the retinal circulation. The ICP and DCP receive arteriolar supply (red) from SVC arterioles, and drain (blue) to SVC venules. Direct anastomotic connections between each of these layers are seen on both the arteriolar and venular sides of the capillary beds. The DCP includes vortices that drain centrally into a venule, and also connect to other venules and arterioles through radially oriented capillaries. The DCP contains channels that traverse the horizontal raphe. HFL, Henle fiber layer; OLM, outer limiting membrane; ONL, outer nuclear layer; IPL, inner plexiform layer. Reprinted with permission from (Nesper and Fawzi, 2018).
7. Plexus-specific retinal vascular pathologies
Plexus-specific examination by PR-OCTA provides a novel method to classify and stage retinal pathologies. In general, optic nerve diseases, of which glaucoma is the prime example, affect the superficial vascular complex (SVC), which perfuses the ganglion cell complex (GCC) comprising the axons, cell bodies, and dendrites of the retinal ganglion cells. Outer retinal diseases, such as retinitis pigmentosa, primarily affect the deep vascular complex (DVC), which supplies the retina external to the inner nuclear layer (INL). Primarily microvascular diseases such as diabetic retinopathy (DR) affect all retinal plexuses. In some diseases, such as macular telangiectasia type 2 (MacTel) and retinal angiomatous proliferation, the vascular pathology reaches deeper in more advanced stages. The disease specific patterns are detailed in this section.
One challenge for OCTA clinical management is an inability to directly visualize exudation. However, in choroidal neovacularization and diabetic macular edema, exudation is assessed by sub-retinal and intraretinal fluid space, which can be visualized on structural OCT images that are also produced by OCTA scans. Choroidal neovasulcarization and retinal neovascularization are highlighted by dye leakage in fluorescein angiography, but in OCTA they are highlighted by their location in the normally avascular outer retinal and vitreous layers. Treatment response could be assessment by change in the vessel area with active flow rather than change in dye leakage. In some vasculitides, dye leakage is a useful indicator of active inflammation which do not have a parallel finding in OCTA. Therefore OCTA could not replace the clinical information produced by fluorescein angiography (FA) in indicating vasculitic activity.
7.1. Glaucoma
Glaucoma comprises a group of optical neuropathies generally associated with intraocular pressure elevation, although what constitutes elevation varies widely in different groups of individuals. It is a leading cause of irreversible blindness worldwide (Tham et al., 2014). Using OCTA, it has been shown that glaucoma reduces optic nerve head vascular density both in the superficial disc tissue and deep in the lamina cribrosa (Jia et al., 2014b). In the peripapillary region, PR-OCTA has shown that glaucoma pathology can be best visualized in the NFLP slab (Fig. 13), while the ganglion cell layer plexus (GCLP) is also affected (Liu et al., 2019). In the macula (Takusagawa et al., 2017), glaucomatous damage can be best visualized in the SVC slab (Fig. 7). However, using PR-OCTA, the DVC has been shown to be unaffected by glaucoma (Takusagawa et al., 2017) (Liu et al., 2019).
Figure 13.
Comparison between a normal and glaucomatous eye imaged with PR-OCTA. Row 1: 4.5×4.5-mm en face projections of a healthy eye; row 2: equivalent views for an eye with glaucoma. The focal capillary dropout in the glaucomatous eye (arrow) is most easily observed in the nerve fiber layer plexus (NFLP). The deep vascular complex (DVC) appears unaffected. GCLP: ganglion cell layer plexus; SVC: superficial vascular complex. Reprinted with permission from Liu et al. (Liu et al., 2019).
One study has shown that glaucoma also affects the peripapillary choriocapillaris (Suh et al., 2016). However, projection artifacts were not algorithmically removed, so this observation should be interpreted with caution.
Findings from PR-OCTA show that perfusion loss occurs in the same layers (GCC, NFL) that undergo tissue thinning. So does OCTA have additional value besides demonstrating parallel changes that can already be seen on structural OCT? The answer appears to be “yes” at the two extremes of the spectrum of glaucoma severity–the early and late stages.
There is evidence that OCTA is more sensitive than structural OCT in detecting pre-perimetric glaucoma (Akil et al., 2017; Yarmohammadi et al., 2018), an early stage of glaucoma at which visual field (VF) damage cannot yet be clearly seen. One explanation for this may be that OCTA can detect reduced perfusion due to decreased metabolism in sick ganglion cells, before they undergo apoptosis and cause tissue loss and thinning.
In the later (moderate and severe) stages of glaucoma, structural OCT parameters such as NFL thickness approaches a floor value, where thinning is so advanced that further changes cannot be detected. Beyond this point, global NFL thickness correlates poorly with VF parameters (Ajtony et al., 2007; Bowd et al., 2017; Hood and Kardon, 2007; Wollstein et al., 2012), though qualitative evaluation of residual nerve fibers could still be performed with en face OCT (Hood et al., 2015). OCTA parameters (vessel and capillary density) have less of a floor limitation and appeared to be well correlated with VF parameters, even in later stages (Liu et al., 2019; Moghimi et al., 2019; Yarmohammadi et al., 2016b). Thus, OCTA has the potential to improve the staging and monitoring of glaucoma progression in these later stages (Shoji et al., 2017). Large longitudinal clinical studies are needed to demonstrate the practical value of this potential application.
7.2. Age-related macular degeneration and choroidal neovascularization
Another leading cause of irreversible blindness is age-related macular degeneration (AMD) (Flaxman et al., 2017). This neurodegenerative disease is projected to affect 288 million people by 2040 (Wong et al., 2014). Although choroidal neovascularization (CNV) occurs in only around 10–15% of all AMD cases, approximately 90% of vision loss from AMD is associated with the neovascular form of the disease (Mitchell et al., 2018).
CNV refers to pathologic angiogenesis from the choroid that perforates Bruch’s Membrane (BM) and gains access to the outer retina, between Bruch’s membrane and the outer plexiform layer (OPL), a region that is devoid of blood vessels in healthy eyes (Grossniklaus and Green, 2004). En face OCTA allows direct visualization of CNV vascular networks by observing the flow signal in the outer retina (Jia et al., 2014a). Cross-sectional OCTA improves CNV diagnostic accuracy because CNV can readily be detected even in the presence of segmentation errors that interfere with the en face outer retinal image (Faridi et al., 2017). A meta-analysis found OCTA to be of high diagnostic value for detecting CNV (Wang et al., 2019b). Prior to OCTA, intravenous dye-based (fluorescein or indocyanine green) angiograms detected CNV based on characteristic patterns of hyperfluorescence over 5–10 minutes following dye injection. By comparison, OCTA allows noninvasive angiograms that are acquired in several seconds, and it is capable of detecting CNV in cases that are ill-defined by fluorescein angiography(FA) (Malihi et al., 2017).
Projection artifacts in OCTA produce background noise in the outer retina that make it difficult to cleanly identify CNV. In fact, projection artifacts in pigment epithelial detachment or drusen can sometimes appear similar to CNV (Zheng et al., 2016). Initial attempts to address projection artifacts, such as the slab subtraction technique, resulted in inadvertent attenuation of the CNV flow signal as well (Zhang et al., 2017). Fortunately, more advanced PR-OCTA allows for efficient removal of projection artifacts while preserving the CNV flow signal, improving quantitative analysis and repeatability of CNV measurements (Patel et al., 2018a).
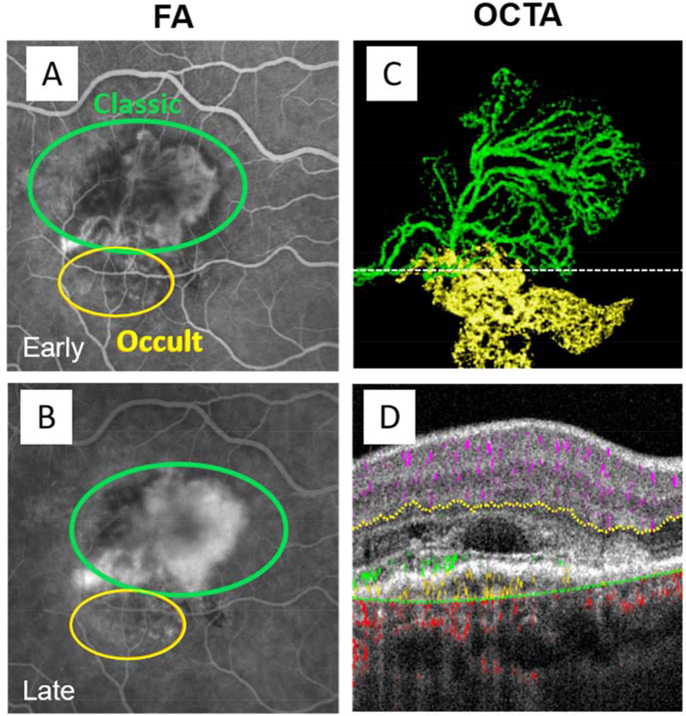
As an additional benefit, PR-OCTA significantly improves cross-sectional CNV depth resolution. CNV can be classified into 3 types, which carries implications for natural history as well as response to antiangiogenic treatment (Kim et al., 2019a). Type 1 CNV is located beneath the retinal pigment epithelium (RPE) and is associated with RPE detachment (Jia et al., 2014a; Xu et al., 2018). It corresponds to the occult component in FA. Type 1 CNV can sometime be non-exudative and not vision threatening – such lesions are discussed in a later section on non-exudative CNV. Type 2 CNV also originates in the choroid, but extends above the RPE, and corresponds to the classic component in FA (El Ameen et al., 2015; Lumbroso et al., 2015). Many type 2 CNVs also have a type 1 CNV component, and PR-OCTA allows clear discrimination of each component in such mixed lesions (Fig. 14). Finally, type 3 CNV, (Freund et al., 2008) also called retinal angiomatous proliferation, develops from neovascular tissue in the inner retina that subsequently extends to the sub-retinal, sub-RPE space, and in some cases can continue to anastomose with the choroidal circulation (Kuehlewein et al., 2015; Miere et al., 2015). Cross-sectional PR-OCTA allows visualization of type 3 CNV growth from the DCP with extension and connection to the choroid over several follow-up visits (Bhavsar et al., 2017) (Fig. 15).
Figure 14.
Projection-resolved OCTA allows depth-resolved discrimination of CNV components in mixed type CNV. (A) Fluorescein angiography shortly after injection (A) and after dye leakage (B). (C) En face OCTA, with type 1 CNV flow shown in green and type 2 in yellow. (D) Cross-sectional OCT located at the dotted line in (C) with OCTA flow signal overlaid, with type 1 CNV with flow signal below the RPE and type 2 CNV with flow signal above the RPE colored as in (C), and normal retinal and choroidal flow shown in violet and yellow, respectively. Type 1 CNV corresponds to occult CNV (yellow circles) in the FA images, and type 2 CNV corresponds classic CNV (green circles).
Figure 15.
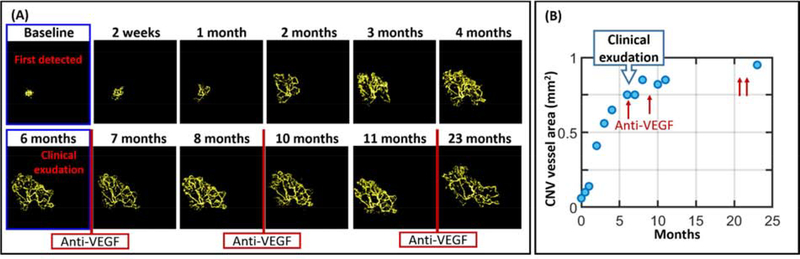
Serial projection-resolved OCTA images demonstrating evolution of a type 3 choroidal neovascularization (CNV), or retinal angiomatous proliferation (RAP), imaged with PR-OCTA. Top row: en face projection of the deep capillary plexus (DCP) showing dilated vessels (blue circles) at the origin of the RAP lesion. Middle row: Pathologic vessels from RAP lesion are visible with en face outer retinal angiogram. Bottom row: cross-sectional PR-OCTA with inner retinal (violet), choroidal (red), and pathological neovascular (yellow) flow overlaid, revealing vascular extension of the RAP lesion to the sub-RPE space and the choroid. Flow signal from several adjacent B-frames was combined by maximum projection to create these images in order to enhance the visibility of RAP features. The RAP lesion (blue circles, top row) began its outward extension (yellow flow signal in outer retina, bottom row) 5 months prior to clinical diagnosis, by which time it has extended into the subretinal space. Reprinted with permission from Bhavsar et al. (Bhavsar et al., 2017).
Treatment of CNV with injection of intravitreal anti-vascular endothelial growth factor is highly effective in preserving vision (Brown et al., 2006). Structural OCT is commonly used to monitor retinal fluid, a pharmacodynamic biomarker used to customize the schedule of intravitreal injections of anti-vascular endothelial growth factor (VEGF) medications in treat-and-extend and as-needed regimens (Group, 2011; Wykoff et al., 2015). With OCT monitoring, it is possible to give less frequent injections while keeping the disease under control. This use of OCT is estimated to save approximately $1 billion annually in reducing the cost of anti-VEGF injections (Windsor et al., 2018). With the development of OCTA, it became possible to directly visualize the CNV vascular network by observing the flow signal, as well as assess the presence retinal fluid using the reflectance signal from the same volumetric scan. Projection-resolved OCTA improves the ability to accurately distinguish active neovascular vessels with flow signal from non-flow areas in a neovascular membrane that may represent either inactive vessels or scar tissue. Cessation of flow in a treated CNV membrane can signal long-term remission without further treatment (McClintic et al., 2018).
Since OCTA is noninvasive it is possible to use it frequently in the monitoring of treatment outcome after anti-VEGF injection. Serial OCTA images of CNV under anti-VEGF therapy revealed pruning of small peripheral CNV branches and anastomosis along with ongoing vascular remodeling over time (Spaide, 2015). Daily to weekly measurement of CNV revealed rapid closure in the first 2 weeks after anti-VEGF treatment, followed by reopening and growth in CNV area (Huang et al., 2015). Several studies have found that increases in CNV vessel area preceded exudation under both treat-and-extend and treat-as-needed protocols (Huang et al., 2015; McClintic et al., 2018). Besides growth rate, CNV morphology has also been studied as a potential predictive factor for exudation and anti-VEGF treatment response (Coscas et al., 2018; Coscas et al., 2015; Miere et al., 2015). CNV vessel length, branching rate, and fractal dimension have all been investigated (Al-sheikh et al., 2018; Faatz et al., 2019; Gao et al., 2016b; Roberts et al., 2018; Xu et al., 2018). Using PR-OCTA, 3D CNV complexity (multi-layered structure) has been negatively correlated with treatment response (Nesper et al., 2018).
Treatment naïve CNV that is detected by OCTA but not on either FA (no dye leakage) or structural OCT (no intraretinal or subretinal fluid accumulation) has been called non-exudative CNV, subclinical macular neovascularization, or quiescent CNV (Bailey et al., 2019b; Carnevali et al., 2016; de Oliveira Dias et al., 2018). Although non-exudative CNV could also be visualized with indocyanine green (ICG) angiography (Querques et al., 2013; Roisman et al., 2016), it was rarely detected because ICG angiography is not usually indicated in these asymptomatic patients. With more frequent use of noninvasive OCTA, it is now clear that non-exudative CNV is not rare – it is found in 8–19% of eyes with intermediate CNV (Bailey et al., 2019a; de Oliveira Dias et al., 2018; Yanagi et al., 2018). Non-exudative CNV is most frequently type 1 and some have hypothesized that these lesions may be protective against the development of geographic atrophy (Capuano et al., 2017; Freund et al., 2010; Grossniklaus and Green, 2004). Eyes with non-exudative CNV detected by OCTA on routine checkup develop exudation at a 14–18 times greater rate than eyes with no non-exudative CNV (Bailey et al., 2019b; Carnevali et al., 2018; de Oliveira Dias et al., 2018; Yang et al., 2019). Thus, regular follow-up is indicated for non-exudative CNV – and monthly monitoring may be needed for newly discovered lesions (Bailey et al., 2019b; de Oliveira Dias et al., 2018).
Since clearance of retinal fluid is the current endpoint for anti-VEGF treatment, there is no current indication for treating non-exudative CNV. However, rapid growth of CNV area may precede exudation, and anti-VEGF treatment slows growth rate (Fig. 16) (Heiferman and Fawzi, 2019). Thus, slowing the growth rate (percent per month) of CNV area could potentially serve as an endpoint in a clinical trial for prophylactic treatment of non-exudative CNV (Bailey et al., 2019b). Outside of AMD, OCTA has also been used to detect and measure CNV associated with a number conditions, including myopic degeneration (Querques et al., 2017), pachychoroidal disorders (Chawla et al., 2016), central serous chorioretinopathy (Bonini Filho et al., 2015; McClintic et al., 2015; Quaranta-El Maftouhi et al., 2015) and inflammatory diseases (Astroz et al., 2018; Levison et al., 2016).
Figure 16.
Serial OCTA of a 71-year old eye with non-exudative CNV revealing rapid growth and leading to exudation after 6 months. (A) Time series of OCTA images over almost 2 years, and (B) corresponding measurements of CNV vessel area. Treatment with anti-vascular endothelial growth factor on an as needed basis slowed CNV growth rate.
7.3. Diabetic Retinopathy
Diabetic retinopathy is another leading cause of blindness (Yau et al., 2012). It is the most common microvascular complication due to diabetes (Antonetti et al., 2012), and its global prevalence is expected to increase markedly as the number of people living with diabetes grows (Guariguata et al., 2014). By identifying eyes with high-risk features prior to vision loss and intervening in timely fashion, the morbidity associated with the disease can be minimized. The Early Treatment of Diabetic Retinopathy Study (ETDRS) proposed a severity scale based on clinical findings that predicts the risk of progression to vision-threatening stages of retinopathy (Group, 1991). FA has been shown to be able to detect retinopathy prior to clinical detection (Rand et al., 1987) and add to the predictive value of the clinical examination (ETDRSRG, 1991). However, due to the cost and the risk associated with FA, it is not recommended for screening purposes (Flaxel et al., in press).
By contrast, the safety and low cost of OCTA make this a reasonable modality for both screening and routine clinical care. Many of the same angiographic features seen on fluorescein angiography can be seen on OCTA, including microaneurysms, neovascularization, capillary dilation, capillary nonperfusion, and focal arteriolar narrowing (Hwang et al., 2015; Ishibazawa et al., 2015). With PR-OCTA, plexus-specific features such as deep plexus capillary dilation have been identified as features strongly associated with more severe DR (Hwang et al., 2016b). Especially with extended field of view, OCTA can be useful in detecting clinically unsuspected neovascularization (Fig. 17) (You et al., 2019) and identifying most eyes with neovascularization (Russell et al., 2019). This capability is especially important as neovascularization represents a significantly increased risk for severe vision loss (Wong et al., 2018). In the era of anti-VEGF treatments for proliferative DR, the ability to objectively measure the change in neovascularization may be critical in optimizing the management of this disease (Gross et al., 2015).
Figure 17.
60-year old white man with type 2 diabetes mellitus. (A): Wide-field optical coherence tomography angiography (OCTA) revealed a small 0.26 mm2 patch of retinal neovascularization (arrow) in the vitreous slab (yellow). Color coding allows the viewer to locate the neovascularization relative to the retinal vasculature (purple inner retinal slab) along the inferior arcade. (B): In color fundus photo the retinal neovascularization (arrow) appeared as a retinal hemorrhage; the neovascularization was overlooked clinically. Adapted with permission from (You et al., 2019).
One potential area for OCTA to transform the management of this disease is development of quantitative biomarkers to objectively assess the risk of disease progression. Macular ischemia has a known association with the risk of progression to proliferative diabetic retinopathy (ETDRSRG, 1991; Ip et al., 2015). Numerous studies have examined this potential for OCTA, which, due to high-contrast, capillary level detail, and lack of leakage, is more suitable for quantification of capillary loss in the macula than fluorescein angiography. Biomarkers for measuring diabetic microvasculopathy include the vessel density, fractal dimensions of vessels, vessel length, intercapillary spaces, avascular areas, and the area and shape of the foveal avascular zone (Bhardwaj et al., 2017; Durbin et al., 2017; Hwang et al., 2018; Kim et al., 2016; Lu et al., 2018; Miwa et al., 2016; Zahid et al., 2016).
Earlier OCTA studies on macular ischemia have applied one or two-layer segmentation schemes to the retinal circulation (Spaide et al., 2015a). PR-OCTA now allows clean 3-layer segmentation of the retinal circulation to enhance the visualization and measurement of macular capillary dropout (Fig. 18) (Hwang et al., 2016b). Plexus-specific avascular area is a metric that can detect perfusion loss even in diabetics without clinically evident retinopathy. It is also better able to distinguish different levels of retinopathy severity (Durbin et al., 2017; Hwang et al., 2018; Samara et al., 2017).
Figure 18.
Avascular area (blue overlay) is best visualized in individual plexuses. Shown are sample en face images from four different DR severities, including a healthy control, diabetes without DR, mild/moderate NPDR, and severe NPDR or PDR.
7.4. Retinal vein occlusions and other retinal vascular diseases
Retinal vein occlusion is the most common retinal vascular disease after DR, with an estimated prevalence of 0.5% (Pulido et al., 2016). Central and branch retinal vein occlusions (CRVO and BRVO, respectively) can lead to both ischemia (Hayreh, 2014) and edema (Kadomoto et al., 2018). On OCTA, RVO is associated with dilated capillaries, telangiectasias, collateral vessel formation (Suzuki et al., 2016), and capillary nonperfusion (Kim et al., 2019b) (Fig. 19). The nonperfusion area (NPA) measured on OCTA correlates well with FA measurement (Nobre Cardoso et al., 2016) and is predictive of visual acuity outcomes (Kadomoto et al., 2018). Additionally, measurements of NPA from OCTA and plexus-specific evaluation has shown that RVO produces greater loss of capillary perfusion (Coscas et al., 2016) and collateral formation at the edge of the affected territory in the DCP (Tsuboi et al., 2019).
Figure 19.
An eye with branch retinal vein occlusion (BRVO) showing capillary dropout. The deep capillary plexus (DCP) is most severely affected. Dilated capillaries, most likely collaterals, are most prominent in the intermediate and deep capillary plexuses near the edge of the affected territory. The cross-sectional OCTA is taken at the location of the blue line in the SVC image. SVC: superficial vascular complex; ICP: intermediate capillary plexus.
Paracentral acute middle maculopathy (PAMM) is a clinical finding associated with RVO and other retinal vascular diseases. It is observable on structural OCT as hyperreflective lesions mostly confined to the inner nuclear layer (Rahimy et al., 2014; Sarraf et al., 2013) and is thus considered to represent focal ischemia involving the intermediate or deep capillary plexuses (Falavarjani et al., 2017; Nemiroff et al., 2016; Sridhar et al., 2015). A study using cross-sectional PR-OCTA found all PAMM lesions to be associated with focal capillary loss in the ICP and DCP, with occasional involvement of the GCLP (Chu et al., 2018).
Acute macular neuroretinopathy (AMN) is a rare disorder characterized by patchy reddish-brown lesions in the macula that are difficult to recognize clinically but are apparent on infrared imaging and OCT as focal areas of outer retinal abnormalities in young women (Bhavsar et al., 2016). Cross-sectional PR-OCTA found AMN lesions to be associated with focal capillary loss in the DCP (Chu et al., 2018). Thus both OCT and OCTA agree in the characterization of AMN as an outer retinal lesion, in contrast with the middle retinal location of PAMM.
Sickle cell retinopathy (SCR) is a consequence of sickle cell disease, which affects close to 100,000 Americans (Hassell, 2010). It can affect both the macula and the periphery, and can cause both occlusions and neovascularization (Falavarjani et al., 2016; Han et al., 2015); one systematic review found that the prevalence of SCR in the population of individuals with sickle cell disease was 45% (Leitão Guerra et al., 2019). Several studies have used OCTA to measure avascular area in SCR (Han et al., 2017; Han et al., 2015; Pahl et al., 2017). In SCR avascular area often coincides with retinal thinning, and while more separate avascular areas are found in the SVC, in the DVC the total avascular area is larger (Leitão Guerra et al., 2019). Other OCTA metrics such as FAZ size (Minvielle et al., 2016) or vessel density or tortuosity (Alam et al., 2017a, b; Khansari et al., 2017) can also distinguish SCR from healthy eyes.
Other retinal vascular diseases, including retinal artery occlusion (Wang et al., 2018) and retinopathy of prematurity (Campbell et al., 2017a), have also been studied with OCTA. They generally affect all retinal plexuses, though not always to the same degree.
Macular telangiectasia type 2
Macular telangiectasia type 2 (MacTel) is an acquired bilateral disease of unknown etiology characterized by changes in the macular capillaries, neurosensory atrophy, and neovascularization (Charbel et al., 2013). The classic stages of progression are defined by FA findings: Stage 1 is characterized by diffuse hyperfluorescence of capillaries temporal to the fovea; stage 2 with reduced parafoveal retinal transparency; stage 3 with right-angled venules that dive vertically into the outer retina; stage 4 with intraretinal pigment clumping; and stage 5, which is characterized by subretinal neovascularization and associated with the most severe vision loss (Gass and Blodi, 1993). OCTA has demonstrated a well-preserved GCLP in the earliest stages in the presence of dilated DVC vessels (Zeimer et al., 2015), loss of capillaries (Chidambara et al., 2016), and characteristic dilation and dendritic appearance of the DVC vessels and invasion of the abnormal vessels into the normally avascular outer retina (Spaide et al., 2015c; Thorell et al., 2014). PR-OCTA is further able to clarify the vertical course of the right angle venules (Patel et al) (Fig. 20) and formation of chorioretinal anastomosis in some cases of subretinal neovascularization. (Zhang et al., 2015d).
Figure 20.
Macular telangiectasia type 2 in the left eye of a 55-year-old woman. (A) Fluorescein angiography (FA) reveals leakage temporal to the fovea. The green box indicates a 3 × 3-mm region imaged by OCTA, and the yellow line corresponds to the location of the cross-section shown. (B) Cross-sectional uncorrected OCTA shows projection artifacts that are difficult to distinguish from the outer retinal pathology. The flow signal is color coded according to slab: inner retina (violet), outer retina (yellow), choriocapillaris (red). (C) Cross-sectional PR-OCTA shows neovascularization in the outer retina associated with focal inner retinal capillary loss. Note that the neovascularization is limited to its anatomic location in (C), while in (B) spurious flow signal appears outside of the afflicted region. Some disorganization of retinal layers can be seen to in the structural OCT at this same location. (D) En face PR-OCTA of the outer retinal slab shows the extent of the subretinal neovascular complex. (E-G) PR-OCTA of the individual retinal plexuses reveals vascular rarefication temporal to the fovea and a diving venule (white arrow in E). Reprinted with permission from Patel et al. (Patel et al., 2018b).
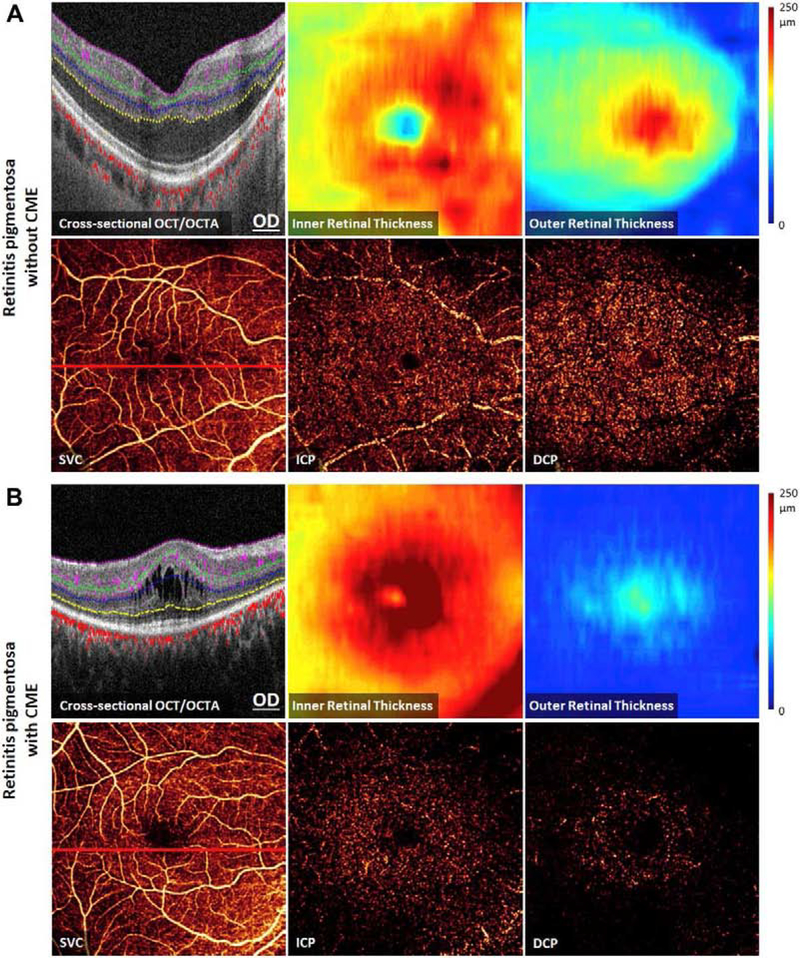
7.5. Retinitis Pigmentosa
Retinitis pigmentosa (RP) is a hereditary retinal disease (Hartong et al., 2006) and a leading cause of blindness for people under 60. Progression can be monitored by electroretinogram and VF (Grover et al., 2003; Holopigian et al., 1996), but both of these tests have low repeatability (Bittner et al., 2011; Grover et al., 2003). Measurements of the ellipsoidal zone area from structural OCT, which has better repeatability, is also be used to monitor RP progression (Hariri et al., 2016). Imaging with OCTA has the potential to improve monitoring by objectively quantifying vascular changes, since retinal perfusion in eyes with RP correlates with visual function (Murakami et al., 2014; Toto et al., 2016).
OCTA measurements have shown that RP-associated perfusion loss occurs primarily in the DCP (Koyanagi et al., 2018; Parodi et al., 2017; Takagi et al., 2018). Using PR-OCTA, it was found that capillary dropout occurs in both the DCP and ICP, but the SVC is spared except in cases with cystoid macular edema (Hagag et al., 2019). This dropout was strongly correlated with outer retinal thickness (Hagag et al., 2019; Toto et al., 2016). PR-OCTA (Fig. 21) also showed that vessel density loss is greater in the DCP compared to the ICP, and greater in the perifovea compared to the parafovea (Hagag et al., 2019). The OCTA results are consistent with a model for RP progression in which vascular attenuation is secondary to photoreceptor death. Specifically, the areas affected by RP appear more wide-spread in terms of loss of the ellipsoidal zone and thinning of the outer retina, followed by capillary dropout in the DCP, followed by loss in the ICP. This gradation suggests propagation of loss from the photoreceptor layer to the DCP and ICP by proximity. Furthermore, if the disease were primarily vascular, we would expect to find a strong correlation between vessel density and cystoid macular edema. This was not found (Hagag et al., 2019). Instead, both CME and vessel density loss were strongly correlated with outer retinal thinning. This suggests that CME and capillary loss are both subsequent to photoreceptor damage.
Figure 21.
Structural OCT and OCTA (6×6-mm) in eyes with retinitis pigmentosa (RP). The cross-sectional OCTA images showed segmentations for the superficial vascular complex (SVC, between magenta and green lines), intermediate capillary plexus (ICP, between the green and blue lines), and the deep capillary plexus (DCP, between the blue and yellow lines). Outer retinal thickness was calculated between the outer boundary of the outer plexiform layer (OPL) and Bruch’s membrane (BM). Inner retinal thickness was measured from the inner limiting membrane to the outer boundary of the OPL. (A) A 26 -year old with mild RP and no cystoid macular edema (CME). OCT showed outer retinal thinning in some perifoveal areas. OCTA showed DCP capillary dropout in some perifoveal areas. The parafovea and the ICP were minimally involved. The inner retina and the SVC appeared normal. (B) A 59-year old with moderate RP including CME. OCT showed severe perifoveal and parafoveal outer retinal thinning, with sparing of the photoreceptor ellipsoidal zone only in the fovea. OCTA showed severe perifoveal and moderate parafoveal capillary dropout in the DCP, with milder loss in the ICP. The inner retina and SVC appeared normal except in the foveal area affected by CME. Reprinted with permission from Hagag et al. (Hagag et al., 2019).
8. Future Directions
The OCTA images shown in this review were taken using commercial OCT systems with 15–20 μm transverse resolution. Although the PR-OCTA algorithm was able to suppress projection artifacts and recover the true depth distribution of the retinal circulation, it could not improve the resolution to recover the true caliber of the capillaries and the smallest arterioles and venules. The next big step in refining OCTA imaging of the retinal circulation is to overcome this limitation.
8.1. Adaptive-optics optical coherence tomographic angiography
Improving the transverse resolution of OCTA to the capillary level (5–10 μm) (Tan et al., 2015; Wang et al., 2011) will require the use of adaptive optics (AO) to reduce the optical aberrations of the human eye. Adaptive optics has been used in confocal scanning laser ophthalmoscopy to improve retinal imaging to the 1–2 μm level needed to resolve foveal photoreceptors (Dubra and Sulai, 2011; Dubra et al., 2011). The benefit of the AO has been extended to OCT and OCTA retinal imaging as well, enabling detailed volumetric visualization of various types of retinal cells and vascular networks (Jonnal et al., 2016; Kurokawa et al., 2017; Liu et al., 2017; Pircher and Zawadzki, 2017; Salas et al., 2017). The requirement for capillary imaging is less demanding and could be achieved using a simplified AO system without a wavefront sensor (Ju et al., 2017). We have used such a sensorless AO-OCT system to obtain OCTA images of the human retina (Fig. 22). The increased resolution and contrast produced en face OCTA images is closer to that of histology. An added bonus is the marked reduction of projection artifacts, so that the PR-OCTA algorithm was not necessary to cleanly visualize the ICP and DCP (Fig. 22). AO-OCTA has the potential to provide better visualization of the 3D retinal vascular network and small vascular pathologies such as microaneurysm, dilated capillaries, and “polyps” (as in polypoidal choroidal vaculopathy). More accurate measurements of vessel density, capillary density, and flow may also be possible.
Figure 22.
Visualization of the inner retinal vascular plexuses with a sensorless adaptive optics (SAO) OCT system with 6 μm transverse resolution and 3 μm axial resolution. Convergence of five low order Zernike modes (defocus, vertical and oblique astigmatism, and vertical and horizontal coma) was achieved by a hill-climbing algorithm. The 1.5×1.5 mm OCTA scan was obtained from a normal eye in a healthy human volunteer, and the aberration optimization took 2.8 seconds after which the subject was allowed to blink, and acquisition of OCTA images took 3 seconds. (A) Depth-resolved reflectance intensity and flow profile obtained by averaging B-frames located between the fovea and optic disc after the pixels are aligned at the inner retinal surface. (B) Cross-sectional structural OCT and (C) cross-sectional OCTA are taken at the same location between the fovea and optic disc. (D) En face OCTA and (E) en face OCT. Reflectance is highest in the nerve fiber layer (NFL), inner plexiform layer (IPL) and outer plexiform layer (OPL). Due to the high axial resolution, the trilaminar (3 reflectance peaks) structure of the IPL could be observed. The nuclear layers, ganglion cell layer (GCL) and inner nuclear layer (INL), have low internal reflectivity. Because the NFL is thin at this location, the flow density is lower in the nerve fiber layer plexus (NFLP) relative to the ganglion cell layer plexus (GCLP). The intermediate capillary plexus (ICP) and deep capillary plexus (DCP) show distinct flow signal peaks compared to the low level of flow in the IPL and INL. The higher resolution of the SAO OCT system suppressed projection artifacts so it was not necessary to apply the PR-OCTA algorithm to visualize the distinct capillary patterns in the ICP and DCP. The capillary patterns are also visible on the en face OCT images with much better contrast than standard OCT. More details of this work can be found in (Camino et al., 2020).
9. Summary
Optical coherence tomographic angiography is an important extension of OCT technology that enables visualization and measurement of retinal circulation down to the capillary level. It uses motion contrast, which is vulnerable to artifacts that interfere with the visualization of both normal vessels and pathologies. Fortunately, these artifacts can be suppressed by the use of real-time tracking, post-processing algorithms, and improved hardware. In particular, the PR-OCTA algorithm has allowed us to cleanly image the four retinal plexuses and plexus-specific pathologies. This allows us to distinguish between optic neuropathies (e.g. glaucoma) that affect the SVC, outer retinal diseases that affect the DVC, and primarily vascular diseases with various plexus-specific patterns. With further improvements in OCTA speed, resolution, and algorithms we can expect continuing improvements in our ability to diagnose, stage, and manage the many eye diseases that affect retinal circulation.
Acknowledgements
This research was supported by NIH/NEI grants R01 EY10145, R01 EY027833, R01 EY024544, R01 EY023285, P30 EY010572, Oregon Health & Science University (OHSU) foundation, and William & Mary Greve special scholar award and an unrestricted departmental fund from Research to Prevent Blindness (New York, NY).
Footnotes
Disclosures
David Huang, Yali Jia and OHSU have financial interest in Optovue Inc. and Yifan Jian has financial interests in Seymour Vision Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed J, Braun RD, Dunn RJ, Linsenmeier RA, 1993. Oxygen distribution in the macaque retina. Investigative Ophthalmology and Visual Science 34, 516–521. [PubMed] [Google Scholar]
- Ajtony C, Balla Z, Somoskeoy S, Kovacs B, 2007. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by optical coherence tomography. Investigative Ophthalmology and Visual Science 48, 258–263. [DOI] [PubMed] [Google Scholar]
- Akil H, Huang AS, Francis BA, Sadda SR, Chopra V, 2017. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS ONE 12, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-sheikh M, Sadda SR, Sarraf D, lafe NA, Phasukkijwatana N, Sadda SR, Sarraf D, 2018. Biomarkers of Neovascular Activity in Age-Related Macular Degeneration Using Optical Coherence Tomography Angiography. Retina 0, 1–11. [DOI] [PubMed] [Google Scholar]
- Al-sheikh M, Tepelus TC, Nazikyan T, Sadda SRVR, 2017. Repeatability of automated vessel density measurements using optical coherence tomography angiography. British Journal of Ophthalmology 101, 449–452. [DOI] [PubMed] [Google Scholar]
- Alam M, Thapa D, Lim JI, Cao D, Yao X, 2017a. Computer-aided classification of sickle cell retinopathy using quantitative features in optical coherence tomography angiography. Biomedical Optics Express 8, 4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Thapa D, Lim JI, Cao D, Yao X, 2017b. Quantitative characteristics of sickle cell retinopathy in optical coherence tomography angiography. Biomedical Optics Express 8, 1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L, Qin J, Wang RK, 2010. Ultrahigh sensitive optical microangiography for in vivo imaging of microcirculations within human skin tissue beds. Optics Express 18, 8220–8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L, Wang RK, 2008. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Optics Express 16, 11438–11452. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Gardner TW, 2012. Diabetic retinopathy. New England Journal of Medicine 366, 1227–1239. [DOI] [PubMed] [Google Scholar]
- Astroz P, Miere A, Mrejen S, Sekfali R, Souied EH, Jung C, Nghiem-Buffet S, Cohen SY, 2018. Optical Coherence Tomography Angiography to Distinguish Choroidal Neovascularization From Macular Inflammatory Lesions in Multifocal Choroiditis. Retina 38, 299–309. [DOI] [PubMed] [Google Scholar]
- Au A, Sarraf D, 2019. Vascular anatomy and its relationship to pathology in retinoschisis. Eye 33, 693–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey ST, Thaware O, Wang J, Hagag A, Zhang X, Flaxel CJ, Lauer AK, Hwang TS, Lin P, Huang D, Jia Y, 2019a. Detection of nonexudative choroidal neovascularization and progression to exudative choroidal neovascularization using OCT angiography. Ophthalmology Retina 3, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey ST, Thaware O, Wang J, Hagag AM, Zhang X, Flaxel CJ, Lauer AK, Hwang TS, Lin P, Huang D, Jia Y, 2019b. Detection of Nonexudative Choroidal Neovascularization and Progression to Exudative Choroidal Neovascularization Using OCT Angiography. Ophthalmology Retina 3, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaratnasingam C, An D, Freund KB, Francke A, Yu D-Y, 2019. Correlation between Histologic and OCT Angiography Analysis of Macular Circulation. Ophthalmology 126, 1588–1589. [DOI] [PubMed] [Google Scholar]
- Barton JK, Stromski S, 2005. Flow measurement without phase information in optical coherence tomography images. Optics Express 13, 5234–5239. [DOI] [PubMed] [Google Scholar]
- Bearelly S, Rao S, Fekrat S, 2009. Anaphylaxis following intravenous fluorescein angiography in a vitreoretinal clinic: Report of 4 cases. Canadian Journal of Ophthalmology 44, 444–445. [DOI] [PubMed] [Google Scholar]
- Bhardwaj S, Tsui E, Zahid S, Young E, Mehta N, Agemy S, Garcia P, Rosen RB, Young JA, 2017. Value of Fractal Analysis of Optical Coherence Tomography Angiography in Various Stages of Diabetic Retinopathy. Retina. [DOI] [PubMed] [Google Scholar]
- Bhavsar KV, Jia Y, Wang J, Patel RC, Lauer AK, Huang D, Bailey ST, 2017. Projection-resolved optical coherence tomography angiography exhibiting early flow prior to clinically observed retinal angiomatous proliferation. American Journal of Ophthalmology Case Reports 8, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, Cunningham ET, 2016. Acute macular neuroretinopathy: A comprehensive review of the literature. Survey of Ophthalmology 61, 538–565. [DOI] [PubMed] [Google Scholar]
- Birol G, Wang S, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA, 2007. Oxygen distribution and consumption in the macaque retina, American Journal of Physiology - Heart and Circulatory Physiology, pp. 1696–1704. [DOI] [PubMed] [Google Scholar]
- Bittner AK, Iftikhar MH, Dagnelie G, 2011. Test-Retest, within-visit variability of Goldmann visual fields in retinitis pigmentosa. Investigative Ophthalmology and Visual Science 52, 8042–8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini Filho MA, De Carlo TE, Ferrara D, Adhi M, Baumal CR, Witkin AJ, Reichel E, Duker JS, Waheed NK, 2015. Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. JAMA Ophthalmology 133, 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowd C, Zangwill LM, Weinreb RN, Medeiros FA, Belghith A, 2017. Estimating optical coherence tomography structural measurement floors to improve detection of progression in advanced glaucoma. American Journal of Ophthalmology 175, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand CS, 2012. Management of retinal vascular diseases: A patient-centric approach. Eye (Basingstoke) 26, S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, 2006. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. New England Journal of Medicine 355, 1432–1444. [DOI] [PubMed] [Google Scholar]
- Camino A, Jia Y, Liu G, Wang J, Huang D, 2017. Regression-Based Algorithm for Bulk Motion Subtraction in Optical Coherence Tomography Angiography. Biomedical Optics Express 8, 3053–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino A, Jia Y, Yu J, Wang J, Liu L, Huang D, 2019. Automated detection of shadow artifacts in optical coherence tomography angiography. Biomedical Optics Express 10, 1514–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino A, Ng R, Huang J, Guo Y, Ni S, Jia Y, Huang D, Jian Y, 2020. Depth-resolved optimization of a real-time sensorless adaptive optics optical coherence tomography, Optics Letters, pp. 2612–2615. [DOI] [PubMed] [Google Scholar]
- Camino A, Zhang M, Liu L, Wang J, Jia Y, Huang D, 2018. Enhanced quantification of retinal perfusion by improved discrimination of blood flow from bulk motion signal in OCTA. Translational Vision Science and Technology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Nudleman E, Yang J, Tan O, Chan RVP, Chiang MF, Huang D, Liu G, 2017a. Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. JAMA Ophthalmology 135, 977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, Huang D, 2017b. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Scientific Reports 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano V, Miere A, Querques L, Sacconi R, Carnevali A, Amoroso F, Bandello F, Souied EH, Querques G, 2017. Treatment-Naïve Quiescent Choroidal Neovascularization in Geographic Atrophy Secondary to Nonexudative Age-Related Macular Degeneration. American Journal of Ophthalmology 182, 45–55. [DOI] [PubMed] [Google Scholar]
- Carnevali A, Cicinelli MV, Capuano V, Corvi F, Mazzaferro A, Querques L, Scorcia V, Souied EH, Bandello F, Querques G, 2016. Optical Coherence Tomography Angiography: A Useful Tool for Diagnosis of Treatment-Naïve Quiescent Choroidal Neovascularization. American Journal of Ophthalmology 169, 189–198. [DOI] [PubMed] [Google Scholar]
- Carnevali A, Sacconi R, Querques L, Marchese A, Capuano V, Rabiolo A, Corbelli E, Panozzo G, Miere A, Souied E, Bandello F, Querques G, 2018. Natural History of Treatment-Naïve Quiescent Choroidal Neovascularization in Age-Related Macular Degeneration Using OCT Angiography. Ophthalmology Retina 2, 922–930. [DOI] [PubMed] [Google Scholar]
- Chan G, Balaratnasingam C, Yu PK, Morgan WH, McAllister IL, Cringle SJ, Yu DY, 2012. Quantitative morphometry of perifoveal capillary networks in the human retina. Investigative Ophthalmology and Visual Science 53, 5502–5514. [DOI] [PubMed] [Google Scholar]
- Charbel P, Gillies MC, Chew EY, Bird AC, Heeren TFC, Peto T, Holz FG, Scholl HPN, 2013. Progress in Retinal and Eye Research Macular telangiectasia type 2. Progress in Retinal and Eye Research 34, 49–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla R, Mittal K, Vohra R, 2016. Optical Coherence Tomography Angiography Study of Choroidal Neovascularization Associated With Focal Choroidal Excavation. Ophthalmic Surgery, Lasers and Imaging Retina 47, 969–971. [DOI] [PubMed] [Google Scholar]
- Chen C.-l.L., Zhang A, Bojikian KD, Wen JC, Zhang Q, Xin C, Mudumbai RC, Johnstone MA, Chen PP, Wang RK, 2016. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography–based microangiography. Investigative Ophthalmology and Visual Science 57, 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Milner TE, Srinivas S, Wang X, Malekafzali a., van Gemert MJ, Nelson JS, 1997. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography. Optics letters 22, 1119–1121. [DOI] [PubMed] [Google Scholar]
- Chidambara L, Gadde SGK, Yadav NK, Jayadev C, Bhanushali D, Appaji AM, Akkali M, Khurana A, Shetty R, 2016. Characteristics and quantification of vascular changes in macular telangiectasia type 2 on optical coherence tomography angiography. British Journal of Ophthalmology 100, 1482–1488. [DOI] [PubMed] [Google Scholar]
- Chu S, Nesper PL, Soetikno BT, Bakri SJ, Fawzi AA, 2018. Projection-resolved OCT angiography of microvascular changes in paracentral acute middle maculopathy and acute macular neuroretinopathy. Investigative Ophthalmology and Visual Science 59, 2913–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscas F, Cabral D, Pereira T, Geraldes C, Narotamo H, Miere A, Lupidi M, Sellam A, Papoila A, Coscas G, Souied E, 2018. Quantitative optical coherence tomography angiography biomarkers for neovascular agerelated macular degeneration in remission. PLoS ONE 13, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscas F, Glacet-Bernard A, Miere A, Caillaux V, Uzzan J, Lupidi M, Coscas G, Souied EH, 2016. Optical Coherence Tomography Angiography in Retinal Vein Occlusion: Evaluation of Superficial and Deep Capillary Plexa. American Journal of Ophthalmology 161, 160–171.e162. [DOI] [PubMed] [Google Scholar]
- Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH, 2015. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: A new diagnostic challenge. Retina 35, 2219–2228. [DOI] [PubMed] [Google Scholar]
- Dansingani KK, Naysan J, Freund KB, 2015. En face OCT angiography demonstrates flow in early type 3 neovascularization (retinal angiomatous proliferation). Eye 29, 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Dias JR, Zhang Q, Garcia JMB, Zheng F, Motulsky EH, Roisman L, Miller A, Chen CL, Kubach S, de Sisternes L, Durbin MK, Feuer W, Wang RK, Gregori G, Rosenfeld PJ, 2018. Natural History of Subclinical Neovascularization in Nonexudative Age-Related Macular Degeneration Using Swept-Source OCT Angiography, Ophthalmology. American Academy of Ophthalmology, pp. 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K, 2017. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investigative Ophthalmology and Visual Science 58, 190–196. [DOI] [PubMed] [Google Scholar]
- Dongye C, Zhang M, Hwang TS, Wang J, Gao SS, Liu L, Huang D, Wilson DJ, Jia Y, 2017a. Automated detection of dilated capillaries on optical coherence tomography angiography. Biomedical Optics Express 8, 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongye C, Zhang M, Hwang TS, Wang J, Gao SS, Liu L, Huang D, Wilson DJ, Jia Y, 2017b. Automated detection of dilated capillaries on optical coherence tomography angiography. Biomedical Optics Express 8, 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubra A, Sulai Y, 2011. Reflective afocal broadband adaptive optics scanning ophthalmoscope. Biomedical Optics Express 2, 1757–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubra A, Sulai Y, Norris JL, Cooper RF, Dubis AM, Williams DR, Carroll J, 2011. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomedical Optics Express 2, 1864–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin MK, An L, Shemonski ND, Soares M, Santos T, Lopes M, Neves C, Cunha-Vaz J, 2017. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmology 135, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ameen A, Cohen SY, Semoun O, Miere A, Srour M, Maftouhi MQE, Oubraham H, Blanco-Garavito R, Querques G, Souied EH, 2015. Type 2 neovascularization secondary to age-related macular degeneration imaged by optical coherence tomography angiography. Retina 35, 2212–2218. [DOI] [PubMed] [Google Scholar]
- Eladawi N, Elmogy M, Helmy O, Aboelfetouh A, Riad A, Sandhu H, Schaal S, El-Baz A, 2017. Automatic blood vessels segmentation based on different retinal maps from OCTA scans. Computers in Biology and Medicine 89, 150–161. [DOI] [PubMed] [Google Scholar]
- Enders C, Lang GE, Dreyhaupt J, Loidl M, Lang GK, Werner JU, 2019. Quantity and quality of image artifacts in optical coherence tomography angiography. PLoS ONE 14, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ETDRSRG, 1991. Fluorescein Angiographic Risk Factors for Progression of Diabetic Retinopathy: ETDRS Report Number 13. Ophthalmology 98, 834–840. [PubMed] [Google Scholar]
- Faatz H, Farecki ML, Rothaus K, Gunnemann F, Gutfleisch M, Lommatzsch A, Pauleikhoff D, 2019. Optical coherence tomography angiography of types 1 and 2 choroidal neovascularization in age-related macular degeneration during anti-VEGF therapy: evaluation of a new quantitative method. Eye 33, 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falavarjani KG, Phasukkijwatana N, Freund KB, Cunningham ET, Kalevar A, McDonald HR, Dolz- Marco R, Roberts PK, Tsui I, Rosen R, Jampol LMEEM, Sadda SR, Ghasemi Falavarjani K, Phasukkijwatana N, Freund KB, Cunningham ET, Kalevar A, McDonald HR, Dolz-Marco R, Roberts PK, Tsui I, Rosen R, Jampol LMEEM, Sadda SR, Sarraf D, 2017. En Face Optical Coherence Tomography Analysis to Assess the Spectrum of Perivenular Ischemia and Paracentral Acute Middle Maculopathy in Retinal Vein Occlusion. American Journal of Ophthalmology 177, 131–138. [DOI] [PubMed] [Google Scholar]
- Falavarjani KG, Scott AW, Wang K, Han IC, Chen X, Klufas M, Hubschman JP, Schwartz SD, Sadda SR, Sarraf D, Tsui I, 2016. Correlation of multimodal imaging in sickle cell retinopathy. Retina 36, S111–S117. [DOI] [PubMed] [Google Scholar]
- Faridi A, Jia Y, Gao SS, Huang D, Bhavsar KV, Wilson DJ, Sill A, Flaxel CJ, Hwang TS, Lauer AK, Bailey ST, 2017. Sensitivity and Specificity of OCT Angiography to Detect Choroidal Neovascularization. Ophthalmology Retina 1, 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingler J, Readhead C, Schwartz DM, Fraser SE, 2008. Phase-contrast OCT imaging of transverse flows in the mouse retina and choroid. Investigative Ophthalmology and Visual Science 49, 5055–5059. [DOI] [PubMed] [Google Scholar]
- Fingler J, Schwartz D, Yang C, Fraser SE, 2007. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. Optics Express 15, 12636–12653. [DOI] [PubMed] [Google Scholar]
- Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen J, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong T, Taylor H, Arditi A, Barkana Y, Bozkurt B, Bron A, Budenz D, Cai F, Casson R, Chakravarthy U, Choi J, Congdon N, Dana R, Dandona R, Dandona L, Dekaris I, Del Monte M, Deva J, Dreer L, Ellwein L, Frazier M, Frick K, Friedman D, Furtado J, Gao H, Gazzard G, George R, Gichuhi S, Gonzalez V, Hammond B, Hartnett ME, He M, Hejtmancik J, Hirai F, Huang J, Ingram A, Javitt J, Joslin C, Khairallah M, Khanna R, Kim J, Lambrou G, Lansingh VC, Lanzetta P, Lim J, Mansouri K, Mathew A, Morse A, Munoz B, Musch D, Nangia V, Palaiou M, Parodi MB, Pena FY, Peto T, Quigley H, Raju M, Ramulu P, Reza D, Robin A, Rossetti L, Saaddine J, Sandar M, Serle J, Shen T, Shetty R, Sieving P, Silva JC, Sitorus RS, Stambolian D, Tejedor J, Tielsch J, Tsilimbaris M, van Meurs J, Varma R, Virgili G, Wang YX, Wang NL, West S, Wiedemann P, Wormald R, Zheng Y, 2017. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. The Lancet Global Health 5, e1221–e1234. [DOI] [PubMed] [Google Scholar]
- Fouquet S, Vacca O, Sennlaub F, Paques M, 2017. The 3D retinal capillary circulation in pigs reveals a predominant serial organization. Investigative Ophthalmology and Visual Science 58, 5754–5763. [DOI] [PubMed] [Google Scholar]
- Freund KB, Van Ho I, Barbazetto IA, Koizumi H, Laud K, Ferrara D, Matsumoto Y, Sorenson JA, Yannuzzi L, 2008. Type 3 neovascularization: The expanded spectrum of retinal angiomatous proliferation. Retina 28, 201–211. [DOI] [PubMed] [Google Scholar]
- Freund KB, Zweifel SA, Engelbert M, 2010. Edirorial: Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 30, 1333–1349. [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Huang D, 2016. Foreword: 25 years of optical coherence tomography. Investigative Ophthalmology and Visual Science 57, OCTi–OCTii. [DOI] [PubMed] [Google Scholar]
- Gao SS, Jia Y, Liu L, Zhang M, Takusagawa HL, Morrison JC, Huang D, 2016a. Compensation for reflectance variation in vessel density quantification by optical coherence tomography angiography. Investigative Ophthalmology and Visual Science 57, 4485–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Liu G, Huang D, Jia Y, 2015. Optimization of the split-spectrum amplitude-decorrelation angiography algorithm on a spectral optical coherence tomography system. Optics Letters 40, 2305–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Liu L, Bailey ST, Flaxel CJ, Huang D, Li D, Jia Y, 2016b. Quantification of choroidal neovascularization vessel length using optical coherence tomography angiography. Journal of Biomedical Optics 21, 076010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Patel RC, Jain N, Zhang M, Weleber RG, Huang D, Pennesi ME, 2017a. Choriocapillaris evaluation in choroideremia using optical coherence tomography angiography. Biomedical Optics Express 8, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Bu W, Zheng Y, Wu X, 2017b. Automated layer segmentation of macular OCT images via graph-based SLIC superpixels and manifold ranking approach. Computerized Medical Imaging and Graphics 55, 42–53. [DOI] [PubMed] [Google Scholar]
- Gass JDM, Blodi BA, 1993. Idiopathic Juxtafoveolar Retinal Telangiectasis: Update of Classification and Follow-up Study. Ophthalmology 100, 1536–1546. [PubMed] [Google Scholar]
- Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW, 2015. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA - Journal of the American Medical Association 314, 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Green w.R., 2004. Choroidal neovascularization. Choroidal Neovascularization 137, 496–503. [DOI] [PubMed] [Google Scholar]
- Group, C.R., 2011. Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. The New England Journal of Medicine 364, 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, E.T.o.D.R.S.R., 1991. Fundus Photographic Risk Factors for Progression of Diabetic Retinopathy: ETDRS Report Number 12. Ophthalmology 98, 823–833. [PubMed] [Google Scholar]
- Grover S, Fishman GA, Birch DG, Locke KG, Rosner B, 2003. Variability of Full-field Electroretinogram Photoreceptor Cell Disease. Ophthalmology 110, 1159–1163. [DOI] [PubMed] [Google Scholar]
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, 2014. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice 103, 137–149. [DOI] [PubMed] [Google Scholar]
- Guo Y, Camino A, Zhang M, Wang J, Huang D, Hwang T, Jia Y, 2018. Automated segmentation of retinal layer boundaries and capillary plexuses in wide-field optical coherence tomographic angiography. Biomedical Optics Express 9, 4429–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Hormel TT, Xiong H, Wang B, Camino A, Wang J, Huang D, Hwang TS, Jia Y, 2019. Development and validation of a deep learning algorithm for distinguishing the nonperfusion area from signal reduction artifacts on OCT angiography. Biomedical Optics Express 10, 3257–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagag AM, Pechauer AD, Liu L, Wang J, Zhang M, Jia Y, Huang D, 2018. OCT angiography changes in the 3 parafoveal retinal plexuses in response to hyperoxia. Ophthalmology Retina 2, 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagag AM, Wang JIE, Lu K, Harman G, Weleber RG, Huang D, Yang P, Pennesi ME, Jia Y, 2019. Projection-Resolved Optical Coherence Tomographic Angiography of Retinal Plexuses in Retinitis Pigmentosa. American Journal of Ophthalmology 204, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han IC, Tadarati M, Pacheco KD, Scott AW, 2017. Evaluation of Macular Vascular Abnormalities Identified by Optical Coherence Tomography Angiography in Sickle Cell Disease. American Journal of Ophthalmology 177, 90–99. [DOI] [PubMed] [Google Scholar]
- Han IC, Tadarati M, Scott AW, 2015. Macular vascular abnormalities identified by optical coherence tomographic angiography in patients with sickle cell disease. JAMA Ophthalmology 133, 1337–1340. [DOI] [PubMed] [Google Scholar]
- Hariri AH, Zhang HY, Ho A, Francis P, Weleber RG, Birch DG, Ferris FL, Sadda SVR, Bernstein PS, Heckenlively J, Iannaccone A, Lam B, Pennesi ME, Chiostri J, Erker LR, Wilson DJ, McCormack JB, 2016. Quantification of ellipsoid zone changes in retinitis pigmentosa using en face spectral domain-optical coherence tomography. JAMA Ophthalmology 134, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP, 2006. Retinitis pigmentosa. The Lancet 368, 1795–1809. [DOI] [PubMed] [Google Scholar]
- Hassell KL, 2010. Population Estimates of Sickle Cell Disease in the U.S. American Journal of Preventive Medicine 38, S512–S521. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, 2014. Ocular vascular occlusive disorders: Natural history of visual outcome, Progress in Retinal and Eye Research. Elsevier Ltd, pp. 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiferman MJ, Fawzi AA, 2019. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS ONE 14, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopigian K, Greenstein V, Seiple W, Carr RE, 1996. Rates of change differ among measures of visual function in patients with retinitis pigmentosa. Ophthalmology 103, 398–405. [DOI] [PubMed] [Google Scholar]
- Hood DC, Fortune B, Mavrommatis MA, Reynaud J, Ramachandran R, Ritch R, Rosen RB, Muhammad H, Dubra A, Chui TYP, 2015. Details of glaucomatous damage are better seen on OCT en face images than on OCT retinal nerve fiber layer thickness maps. Investigative Ophthalmology and Visual Science 56, 6208–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Kardon RH, 2007. A framework for comparing structural and functional measures of glaucomatous damage, Progress in Retinal and Eye Research, pp. 688–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormel TT, Wang J, Bailey ST, Hwang TS, Huang D, Jia Y, 2018. Maximum value projection produces better en face OCT angiograms than mean value projection. Biomedical Optics Express 9, 6412–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Jia Y, Rispoli M, Tan O, Lumbroso B, 2015. Optical coherence tomography angiography of time course of choroidal neovascularization in response to anti-angiogenic treatment. Retina 35, 2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, Fujimoto JG, 1991. Optical Coherence Tomography. Science 22, 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, Wilson DJ, Huang D, Jia Y, 2016a. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmology 134, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TS, Hagag AM, Wang J, Zhang M, Smith A, Wilson DJ, Huang D, Jia Y, 2018. Automated Quantification of Nonperfusion Areas in 3 Vascular Plexuses With Optical Coherence Tomography Angiography in Eyes of Patients With Diabetes . JAMA Ophthalmology 97239, 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TS, Jia Y, Gao SS, Bailey ST, Lauer AK, Flaxel CJ, Wilson DJ, Huang D, 2015. Optical coherence tomography angiography features in diabetic retionopathy. Retina 35, 2371–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TS, Miao Z, Bhavsar K, Xinbo Z, Campbell JP, Lin P, Bailey ST, Flaxel CJ, Lauer AK, Wilson DJ, Huang D, Jia Y, 2016b. Visualization of 3 distinct retinal plexuses by projection-resolved optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmology 134, 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip MS, Domalpally A, Sun JK, Ehrlich JS, 2015. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology 122, 367–374. [DOI] [PubMed] [Google Scholar]
- Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, Yokota H, Yoshida A, 2015. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. American Journal of Ophthalmology 160, 35–44. [DOI] [PubMed] [Google Scholar]
- Izatt JA, Kulkarni MD, Yazdanfar S, Barton JK, Welch AJ, 1997. In vivo bidirectional color Doppler flow imaging of picoliter blood volumes using optical coherence tomography. Optics Letters 22, 1439–1441. [DOI] [PubMed] [Google Scholar]
- Jain N, Jia Y, Gao SS, Zhang X, Weleber RG, Huang D, Pennesi ME, 2016. Optical Coherence Tomography Angiography in Choroideremia Correlating Choriocapillaris Loss With Overlying Degeneration. JAMA Ophthalmology 134, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O’Neal DN, Januszewski AS, 2015. Biomarkers in diabetic retinopathy. Review of Diabetic Studies 12, 159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Bailey ST, Hwang TS, McClintic SM, Gao SS, Pennesi ME, Flaxel CJ, Lauer AK, Wilson DJ, Hornegger J, Fujimoto JG, Huang D, 2015. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proceedings of the National Academy of Sciences 112, E2395–E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, Potsaid B, Liu JJ, Lu CD, Kraus MF, Fujimoto JG, Huang D, 2014a. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 121, 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Simonett JM, Wang J, Hua X, Liu L, Hwang TS, Huang D, 2017. Wide-field OCT angiography investigation of the relationship between radial peripapillary capillary plexus density and nerve fiber layer thickness. Investigative Ophthalmology and Visual Science 58, 5188–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D, 2012. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Optics Express 20, 4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, Lombardi LH, Gattey DM, Armour RL, Edmunds B, Kraus MF, Fujimoto JG, Huang D, 2014b. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 121, 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnal RS, Kocaoglu OP, Zawadzki RJ, Liu Z, Miller DT, Werner JS, 2016. A review of adaptive optics optical coherence tomography: Technical advances, scientific applications, and the future. Investigative Ophthalmology and Visual Science 57, OCT51–OCT68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju MJ, Heisler M, Wahl D, Jian Y, Sarunic MV, 2017. Multiscale sensorless adaptive optics OCT angiography system for in vivo human retinal imaging. Journal of Biomedical Optics 22, 121703-121701-121712. [DOI] [PubMed] [Google Scholar]
- Kadomoto S, Muraoka Y, Ooto S, Miwa Y, Iida Y, Suzuma K, Murakami T, Ghashut R, Tsujikawa A, Yoshimura N, 2018. Evaluation of macular ischemia in eye with branch retinal vein occlusion: an optical coherence tomography angiography study. Retina 38, 272–282. [DOI] [PubMed] [Google Scholar]
- Khansari MM, O’Neill W, Lim J, Shahidi M, 2017. Method for quantitative assessment of retinal vessel tortuosity in optical coherence tomography angiography applied to sickle cell retinopathy. Biomedical Optics Express 8, 3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH, 2016. Quantifying Microvascular Density and Morphology in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography Angiography. Investigative Ophthalmology and Visual Science 57, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Fingler J, Zawadzki RJ, Park SS, Morse LS, Schwartz DM, Fraser SE, Werner JS, 2013. Optical imaging of the chorioretinal vasculature in the living human eye. Proceedings of the National Academy of Sciences 110, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Cho HJ, Kim Y, Jung SH, Lee DW, Kim JW, 2019a. Responses of Types 1 and 2 Neovascularization in Age-Related Macular Degeneration to Anti-Vascular Endothelial Growth Factor Treatment: Optical Coherence Tomography Angiography Analysis. Seminars in Ophthalmology 34, 168–176. [DOI] [PubMed] [Google Scholar]
- Kim JT, Chun YS, Lee JK, Moon NJ, Yi DY, 2019b. Comparison of Vessel Density Reduction in the Deep and Superficial Capillary Plexuses in Branch Retinal Vein Occlusion. Ophthalmologica 16, 1–9. [DOI] [PubMed] [Google Scholar]
- Kornblau IS, El-Annan JF, 2019. Adverse reactions to fluorescein angiography: A comprehensive review of the literature. Survey of Ophthalmology. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y, Murakami Y, Funatsu J, Akiyama M, Nakatake S, Fujiwara K, Tachibana T, Nakao S, Hisatomi T, Yoshida S, Ishibashi T, Sonoda KH, Ikeda Y, 2018. Optical coherence tomography angiography of the macular microvasculature changes in retinitis pigmentosa. Acta Ophthalmologica 96, e59–e67. [DOI] [PubMed] [Google Scholar]
- Kuehlewein L, Bansal M, Lenis TL, lafe NA, Sadda SR, Bonini Filho MA, De Carlo TE, Waheed NK, Duker JS, Sarraf D, 2015. Optical Coherence Tomography Angiography of Type 2 Neovascularization in Age-Related Macular Degeneration. American Journal of Ophthalmology 160, 52–56. [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Liu Z, Miller DT, 2017. Adaptive optics optical coherence tomography angiography for morphometric analysis of choriocapillaris. Biomedical Optics Express 8, 1803–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Sasaki K, Makita S, Hong Y-J, Yasuno Y, 2012. Three-dimensional retinal and choroidal capillary imaging by power Doppler optical coherence angiography with adaptive optics. Optics Express 20, 22796–22812. [DOI] [PubMed] [Google Scholar]
- Lei J, Durbin MK, Shi Y, Uji A, Balasubramanian S, Baghdasaryan E, Al-Sheikh M, Sadda SR, 2017. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmology 135, 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão Guerra RL, Leitão Guerra CL, Bastos MG, de Oliveira AHP, Salles C, 2019. Sickle cell retinopathy: What we now understand using optical coherence tomography angiography. A systematic review. Blood Reviews 35, 32–42. [DOI] [PubMed] [Google Scholar]
- Levison AL, Baynes K, Lowder CY, Srivastava SK, 2016. OCT angiography identification of choroidal neovascularization secondary to acute zonal occult outer retinopathy. Ophthalmic Surgery Lasers and Imaging Retina 47, 73–75. [DOI] [PubMed] [Google Scholar]
- Liu G, Jia Y, Pechauer AD, Chandwani R, Huang D, 2016. Split-spectrum phase-gradient optical coherence tomography angiography. Biomedical Optics Express 7, 2943–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Edmunds B, Takusagawa H, Tehrani S, Lombardi L, Morrison JC, Jia Y, Huang D, 2019. Projection-Resolved Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. American Journal of Ophthalmology 207, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Gao SS, Bailey ST, Huang D, Li D, Jia Y, 2015. Automated choroidal neovascularization detection algorithm for optical coherence tomography angiography. Biomedical Optics Express 6, 3564–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kurokawa K, Zhang F, Lee JJ, Miller DT, 2017. Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proceedings of the National Academy of Sciences of the United States of America 114, 12803–12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Simonett JM, Wang J, Zhang M, Hwang T, Hagag AM, Huang D, Li D, Jia Y, 2018. Evaluation of automatically quantified foveal avascular zone metrics for diagnosis of diabetic retinopathy using optical coherence tomography angiography. Investigative Ophthalmology and Visual Science 59, 2212–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbroso B, Rispoli M, Savastano MC, 2015. Longitudinal optical coherence tomography-angiography study of type 2 naïve choroidal neovascularization early response after treatment. Retina 35, 2242–2251. [DOI] [PubMed] [Google Scholar]
- Lynch G, Romo JSA, Linderman R, Krawitz B, Mo S, Zakik A, Carroll J, Rosen RB, Chui TYP, 2018. Within-subject assessment of foveal avascular zone enlargement in different stages of diabetic retinopathy using en face OCT reflectance and OCT angiography. Biomedical Optics Express 9, 5982–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita S, Hong Y, Yamanari M, Yatagai T, Yasuno Y, 2006. Optical coherence angiography. Optics Express 14, 7821–7840. [DOI] [PubMed] [Google Scholar]
- Malihi M, Jia Y, Gao SS, Flaxel C, Lauer AK, Hwang T, Wilson DJ, Huang D, Bailey ST, 2017. Optical coherence tomographic angiography of choroidal neovascularization ill-defined with fluorescein angiography. British Journal of Ophthalmology 101, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariampillai A, Leung MKK, Jarvi M, Standish BA, Lee K, Wilson BC, Vitkin A, Yang VXD, 2010. Optimized speckle variance OCT imaging of microvasculature. Optics Letters 35, 1257–1259. [DOI] [PubMed] [Google Scholar]
- Mariampillai A, Standish BA, Moriyama EH, Khurana M, Munce NR, Leung MKK, Jiang J, Cable A, Wilson BC, Vitkin IA, Yang VXD, 2008. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Optics Letters 33, 1530–1532. [DOI] [PubMed] [Google Scholar]
- McClintic SM, Gao S, Wang J, Hagag A, Lauer AK, Flaxel CJ, Bhavsar K, Hwang TS, Huang D, Jia Y, Bailey ST, 2018. Quantitative Evaluation of Choroidal Neovascularization under Pre Re Nata Anti-Vascular Endothelial Growth Factor Therapy with OCT Angiography. Ophthalmology Retina 2, 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintic SM, Jia Y, Huang D, Bailey ST, 2015. Optical Coherence Tomographic Angiography of Choroidal Neovascularization Associated With Central Serous Chorioretinopathy. JAMA Ophthalmology 133, 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miere A, Querques G, Semoun O, El Ameen A.a., Capuano V, Souied EH, 2015. Optical coherence tomography angiography in early type 3 neovascularization. Retina 35, 2236–2241. [DOI] [PubMed] [Google Scholar]
- Minvielle W, Caillaux V, Cohen SY, Chasset F, Zambrowski O, Miere A, Souied EH, 2016. Macular Microangiopathy in Sickle Cell Disease Using Optical Coherence Tomography Angiography. American Journal of Ophthalmology 164, 137–144.e131. [DOI] [PubMed] [Google Scholar]
- Miri MS, Abràmoff MD, Kwon YH, Sonka M, Garvin MK, 2017. A machine-learning graph-based approach for 3D segmentation of Bruch’s membrane opening from glaucomatous SD-OCT volumes. Medical Image Analysis 39, 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Liew G, Gopinath B, Wong TY, 2018. Age-related macular degeneration. The Lancet 392, 1147–1159. [DOI] [PubMed] [Google Scholar]
- Miwa Y, Murakami T, Suzuma K, Uji A, Yoshitake S, 2016. Relationship between Functional and Structural Changes in Diabetic Vessels in Optical Coherence Tomography Angiography. Nature Publishing Group, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi S, Bowd C, Zangwill LM, Penteado RC, Hasenstab K, Hou H, Ghahari E, Manalastas PIC, Proudfoot J, Weinreb RN, 2019. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 126, 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookiah MRK, Acharya UR, Fujita H, Tan JH, Chua CK, Bhandary SV, Laude A, Tong L, 2015. Application of different imaging modalities for diagnosis of Diabetic Macular Edema: A review. Computers in Biology and Medicine 66, 295–315. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Ikeda Y, Akiyama M, Fujiwara K, Yoshida N, Notomi S, Nabeshima T, Hisatomi T, Enaida H, Ishibashi T, 2014. Correlation between macular blood flow and central visual sensitivity in retinitis pigmentosa. Acta Ophthalmologica 93, 644–648. [DOI] [PubMed] [Google Scholar]
- Nemiroff J, Kuehlewein L, Rahimy E, Tsui I, Doshi R, Gaudric A, Gorin MB, Sadda S, Sarraf D, 2016. Assessing Deep Retinal Capillary Ischemia in Paracentral Acute Middle Maculopathy by Optical Coherence Tomography Angiography. American Journal of Ophthalmology 162, 121–132.e121. [DOI] [PubMed] [Google Scholar]
- Nesper PL, Fawzi AA, 2018. Human parafoveal capillary vascular anatomy and connectivity revealed by optical coherence tomography angiography. Investigative Ophthalmology and Visual Science 59, 3858–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesper PL, Roberts PK, Onishi AC, Chai H, Liu L, Jampol LM, Fawzi AA, 2017. Quantifying Microvascular Abnormalities With Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Investigative ophthalmology & visual science 58, BIO307–BIO315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesper PL, Soetikno BT, Treister AD, Fawzi AA, 2018. Volume-rendered projection-resolved OCT angiography: 3D lesion complexity is associated with therapy response in wet age-related macular degeneration. Investigative Ophthalmology and Visual Science 59, 1944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre Cardoso J, Keane PA, Sim DA, Bradley P, Agrawal R, Addison PK, Egan C, Tufail A, 2016. Systematic Evaluation of Optical Coherence Tomography Angiography in Retinal Vein Occlusion. American Journal of Ophthalmology 163, 93–107.e106. [DOI] [PubMed] [Google Scholar]
- Pahl DA, Green NS, Bhatia M, Lee MT, Chang JS, Licursi M, Briamonte C, Smilow E, Chen RWS, 2017. Optical Coherence Tomography Angiography and Ultra-widefield Fluorescein Angiography for Early Detection of Adolescent Sickle Retinopathy. American Journal of Ophthalmology 183, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi MB, Cicinelli MV, Rabiolo A, Pierro L, Gagliardi M, Bolognesi G, Bandello F, 2017. Vessel density analysis in patients with retinitis pigmentosa by means of optical coherence tomography angiography. British Journal of Ophthalmology 101, 428–432. [DOI] [PubMed] [Google Scholar]
- Patel R, Wang J, Campbell JP, Kiang L, Lauer AK, Flaxel CJ, Hwang TS, Lujan BJ, Huang D, Bailey ST, Jia Y, 2018a. Classification of Choroidal Neovascularization Using Projection-Resolved Optical Coherence Tomographic Angiography. Investigative Ophthalmology and Visual Science 59, 4285–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RC, Wang J, Hwang TS, Zhang M, Gao SS, Pennesi ME, Bailey ST, Lujan BJ, Wang X, Wilson DJ, Huang D, Jia Y, 2018b. Plexus-Specific Detection of Retinal Vascular Pathologic Conditions with Projection-Resolved OCT Angiography. Ophthalmology Retina 2, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala M, Joshi N, Freund DE, Bressler NM, DeBuc DC, Burlina PM, 2018. Deep Learning based Retinal OCT Segmentation. arXiv. [DOI] [PubMed] [Google Scholar]
- Pircher M, Zawadzki RJ, 2017. Review of adaptive optics OCT (AO-OCT): principles and applications for retinal imaging. Biomedical Optics Express 8, 2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido JS, Flaxel CJ, Adelman RA, Hyman L, Folk JC, Olsen TW, 2016. Retinal Vein Occlusions Preferred Practice Pattern® Guidelines. Ophthalmology 123, P182–P208. [DOI] [PubMed] [Google Scholar]
- Quaranta-El Maftouhi M, El Maftouhi A, Eandi CM, 2015. Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. American Journal of Ophthalmology 160, 581–587.e581. [DOI] [PubMed] [Google Scholar]
- Querques G, Srour M, Massamba N, Georges A, Ben Moussa N, Rafaeli O, Souied EH, 2013. Functional characterization and multimodal imaging of treatment-naïve “quiescent” choroidal neovascularization. Investigative ophthalmology & visual science 54, 6886–6892. [DOI] [PubMed] [Google Scholar]
- Querques L, Giuffrè C, Corvi F, Zucchiatti I, Carnevali A, Vitis LAD, Querques G, Bandello F,2017. Optical coherence tomography angiography of myopic choroidal neovascularisation. British Journal of Ophthalmology 101, 609–615. [DOI] [PubMed] [Google Scholar]
- Quigley H, Broman AT, 2006. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology 90, 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiolo A, Gelormini F, Sacconi R, Cicinelli MV, Triolo G, Bettin P, Nouri-Mahdavi K, Bandello F, Querques G, 2018. Comparison of methods to quantify macular and peripapillary vessel density in optical coherence tomography angiography. PLoS ONE, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimy E, Sarraf D, Dollin ML, Pitcher JD, Ho AC, 2014. Paracentral acute middle maculopathy in nonischemic central retinal vein occlusion. American Journal of Ophthalmology 158, 372–380.e371. [DOI] [PubMed] [Google Scholar]
- Rand LI, Davis MD, Hubbard LD, Segal P, Cleary PA, 1987. Color photography vs fluorescein angiography in the detection of diabetic retinopathy in the diabetes control and complications trial: The Diabetes Control and Complications Trial Research Group. Archives of Ophthalmology 105, 1344–1351. [DOI] [PubMed] [Google Scholar]
- Roberts PK, Nesper PL, Gill MK, Fawzi AA, 2018. A semi-automated quantittative approach to characterize treatment response in neovascular age-related macular degeneration: a real world study. Retina 37, 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman L, Zhang Q, Wang RK, Gregori G, Zhang A, Chen CL, Durbin MK, An L, Stetson PF, Robbins G, Miller A, Zheng F, Rosenfeld PJ, 2016. Optical Coherence Tomography Angiography of Asymptomatic Neovascularization in Intermediate Age-Related Macular Degeneration. Ophthalmology 123, 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JF, Flynn HW, Sridhar J, Townsend JH, Shi Y, Fan KC, Scott NL, Hinkle JW, Lyu C, Gregori G, Russell SR, Rosenfeld PJ, 2019. Distribution of Diabetic Neovascularization on Ultra-Widefield Fluorescein Angiography and on Simulated Widefield OCT Angiography. American Journal of Ophthalmology 207, 110–120. [DOI] [PubMed] [Google Scholar]
- Salas M, Augustin M, Ginner L, Kumar A, Baumann B, Leitgeb R, Drexler W, Prager S, Hafner J, Schmidt-Erfurth U, Pircher M, 2017. Visualization of micro-capillaries using optical coherence tomography angiography with and without adaptive optics. Biomedical Optics Express 8, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, Hsu J, Ho AC, 2017. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 124, 235–244. [DOI] [PubMed] [Google Scholar]
- Sarraf D, Rahimy E, Fawzi AA, Sohn E, Barbazetto I, Zacks DN, Mittra RA, Klancnik JM, Mrejen S, Goldberg NR, Beardsley R, Sorenson JA, Bailey Freund K, 2013. Paracentral acute middle maculopathy a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmology 131, 1275–1287. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Klimscha S, Waldstein SM, Bogunović H, 2017. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye 31, 26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenhamml J, Moult EM, Ploner S, Lee B, Novais EA, Cole E, Dang S, Lu CD, Husvogt L, Waheed NK, Duker JS, Hornegger J, Fujimoto JG, 2016. An automatic, intercapillary area-based algorithm for quantifying diabetes-related capillary dropout using optical coherence tomography angiography. Retina 36, S93–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreur V, Domanian A, Liefers B, Venhuizen FG, Klevering BJ, Hoyng CB, De Jong EK, Theelen T, 2019. Morphological and topographical appearance of microaneurysms on optical coherence tomography angiography. British Journal of Ophthalmology 103, 630–635. [DOI] [PubMed] [Google Scholar]
- Schwartz DM, Fingler J, Kim DY, Zawadzki RJ, Morse LS, Park SS, Fraser SE, Werner JS, 2014. Phase-variance optical coherence tomography: A technique for noninvasive angiography. Ophthalmology 121, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Ujiie K, 1978. Structure of Ocular Vessels. Igaku-Shoin. [Google Scholar]
- Shoji T, Zangwill LM, Akagi T, Saunders LJ, Yarmohammadi A, Manalastas PIC, Penteado RC, Weinreb RN, 2017. Progressive Macula Vessel Density Loss in Primary Open-Angle Glaucoma: A Longitudinal Study. American Journal of Ophthalmology 182, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, 2015. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. American Journal of Ophthalmology 160, 6–16. [DOI] [PubMed] [Google Scholar]
- Spaide RF, 2016. Volume-Rendered Optical Coherence Tomography of Retinal Vein Occlusion Pilot Study. American Journal of Ophthalmology 165, 133–144. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Fujimoto JG, Waheed NK, 2015a. Image artifacts in Optical Coherence Angiography. Retina 35, 2163–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Klancnik JM, Cooney MJ, 2015b. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmology 133, 45–50. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Klancnik JM, Cooney MJ, 2015c. Retinal vascular layers in macular telangiectasia type 2 imaged by optical coherence tomographic angiography. JAMA Ophthalmology 133, 66–73. [DOI] [PubMed] [Google Scholar]
- Sridhar J, Shahlaee A, Rahimy E, Hong BK, Khan MA, Maguire JI, Dunn JP, Mehta S, Ho AC, 2015. Optical Coherence Tomography Angiography and en Face Optical Coherence Tomography Features of Paracentral Acute Middle Maculopathy. American Journal of Ophthalmology 160, 1259–1268.e1252. [DOI] [PubMed] [Google Scholar]
- Stone J, van Driel D, Valter K, Rees S, Provis J, 2008. The locations of mitochondria in mammalian photoreceptors: Relation to retinal vasculature. Brain Research 1189, 58–69. [DOI] [PubMed] [Google Scholar]
- Su JP, Chandwani R, Gao SS, Pechauer AD, Zhang M, Wang J, Jia Y, Huang D, Liu G, 2016. Calibration of optical coherence tomography angiography with a microfluidic chip. Journal of biomedical optics 21, 86015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh MH, Zangwill LM, Manalastas PIC, Belghith A, Yarmohammadi A, Medeiros FA, Diniz-Filho A, Saunders LJ, Weinreb RN, 2016. Deep Retinal Layer Microvasculature Dropout Detected by the Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 123, 2509–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Hirano Y, Yoshida M, Tomiyasu T, Uemura A, Yasukawa T, Ogura Y, 2016. Microvascular Abnormalities on Optical Coherence Tomography Angiography in Macular Edema Associated with Branch Retinal Vein Occlusion. American Journal of Ophthalmology 161, 126–132e121. [DOI] [PubMed] [Google Scholar]
- Swanson EA, Huang D, 2011. Ophthalmic OCT Reaches $1Billion Per Year. Retinal Physician, 56–62. [Google Scholar]
- Szkulmowski M, Grulkowski I, Szlag D, Szkulmowska A, Kowalczyk A, Wojtkowski M, 2009. Flow velocity estimation by complex ambiguity free joint Spectral and Time domain Optical Coherence Tomography. Optics Express 17, 14281–14297. [DOI] [PubMed] [Google Scholar]
- Takagi S, Hirami Y, Takahashi M, Fujihara M, Mandai M, Miyakoshi C, Tomita G, Kurimoto Y, 2018. Optical coherence tomography angiography in patients with retinitis pigmentosa who have normal visual acuity. Acta Ophthalmologica 96, e636–e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takusagawa HL, Liu L, Ma KN, Jia Y, Gao SS, Zhang M, Edmunds B, Parikh M, Tehrani S, Morrison JC, Huang D, 2017. Projection-Resolved Optical Coherence Tomography Angiography of Macular Retinal Circulation in Glaucoma. Ophthalmology 124, 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ACS, Tan GS, Denniston AK, Keane PA, Ang M, Milea D, Chakravarthy U, Cheung CMG, 2018. An overview of the clinical applications of optical coherence tomography angiography. Eye (Basingstoke) 32, 262–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PEZ, Balaratnasingam C, Xu J, Mammo Z, Han SX, Mackenzie P, Kirker AW, Albiani D, Merkur AB, Sarunic MV, Yu DY, 2015. Quantitative comparison of retinal capillary images derived by speckle variance optical coherence tomography with histology. Investigative Ophthalmology and Visual Science 56, 3989–3996. [DOI] [PubMed] [Google Scholar]
- Tan PEZ, Yu PK, Balaratnasingam C, Cringle SJ, Morgan WH, McAllister IL, Yu DY, 2012. Quantitative confocal imaging of the retinal microvasculature in the human retina. Investigative Ophthalmology and Visual Science 53, 5728–5736. [DOI] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY, 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121, 2081–2090. [DOI] [PubMed] [Google Scholar]
- Thorell MR, Zhang Q, Huang Y, An L, Durbin MK, Laron M, Sharma U, Stetson PF, Gregori G, Wang RK, Rosenfeld PJ, 2014. Swept-source OCT angiography of macular telangiectasia type 2. Ophthalmic Surgery Lasers and Imaging Retina 45, 369–380. [DOI] [PubMed] [Google Scholar]
- Tokayer J, Jia Y, Dhalla A-H, Huang D, 2013. Blood flow velocity quantification using split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Biomedical Optics Express 4, 1909–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toto L, BorreMi E, Mastropasqua R, Senatore A, Di Antonio L, Di Nicola M, Carpineto P, Mastropasqua L, 2016. Macular features in retinitis pigmentosa: Correlations among ganglion cell complex thickness, capillary density, and macular function. Investigative Ophthalmology and Visual Science 57, 6360–6366. [DOI] [PubMed] [Google Scholar]
- Triolo G, Rabiolo A, Shemonski ND, Fard A, Di Matteo F, Sacconi R, Bettin P, Magazzeni S, Querques G, Vazquez LE, Barboni P, Bandello F, 2017. Optical coherence tomography angiography macular and peripapillary vessel perfusion density in healthy subjects, glaucoma suspects, and glaucoma patients. Investigative Ophthalmology and Visual Science 58, 5713–5722. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sasajima H, Kamei M, 2019. Collateral Vessels in Branch Retinal Vein Occlusion: Anatomic and Functional Analyses by OCT Angiography. Ophthalmology. Retina, 1–10. [DOI] [PubMed] [Google Scholar]
- Wang B, Camino A, Pi S, Guo Y, Wang J, Huang D, Hwang TS, Jia Y, 2019a. Three-dimensional structural and angiographic evaluation of foveal ischemia in diabetic retinopathy: method and validation. Biomedical Optics Express 10, 3522–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang M, Hwang TS, Bailey ST, Huang D, Wilson DJ, Jia Y, 2017. Reflectance-based projection-resolved optical coherence tomography angiography [ Invited ]. Biomedical Optics Express 8, 1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Kocaoglu OP, Cense B, Bruestle J, Jonnal RS, Gao W, Miller DT, 2011. Imaging retinal capillaries using ultrahigh-resolution optical coherence tomography and adaptive optics. Investigative Ophthalmology and Visual Science 52, 6292–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Liang Z, Liu X, 2019b. Diagnostic accuracy of optical coherence tomography for bladder cancer: A systematic review and meta-analysis. BMC Ophthalmology 19, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RK, 2010. Optical microangiography provides depth-resolved images of directional ocular blood perfusion in posterior eye segment. Journal of Biomedical Optics 15, 020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RK, An L, 2009. Doppler optical micro-angiography for volumetric imaging of vascular perfusion in vivo. Optics Express 17, 8926–8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A, 2007. Three dimensional optical angiography. Optics Express 15, 4083–4097. [DOI] [PubMed] [Google Scholar]
- Wang X, Sun B, Wang J, Jia Y, Huang D, Dong J, 2018. Quantitative evaluation of retinal artery occlusion using optical coherence tomography angiography: A case report. Medicine (United States) 97, 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Camino A, Pi S, Cepurna W, Huang D, Morrison JC, Jia Y, 2018. Fast and robust standard-deviation-based method for bulk motion compensation in phase-based functional OCT. Optics Letters 43, 2204–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor MA, Sun SJJ, Frick KD, Swanson EA, Rosenfeld PJ, Huang D, 2018. Estimating Public and Patient Savings From Basic Research—A Study of Optical Coherence Tomography in Managing Antiangiogenic Therapy. American Journal of Ophthalmology 185, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollstein G, Kagemann L, Bilonick RA, Ishikawa H, Folio LS, Gabriele ML, Ungar AK, Duker JS, Fujimoto JG, Schuman JS, 2012. Retinal nerve fibre layer and visual function loss in glaucoma: The tipping point. British Journal of Ophthalmology 96, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, Maia M, Mathenge W, Moreker S, Muqit MMK, Resnikoff S, Verdaguer J, Zhao P, Ferris F, Aiello LP, Taylor HR, 2018. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 125, 1608–1622. [DOI] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, Wong TY, 2014. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. The Lancet Global Health 2, e106–e116. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, Abdelfattah NS, Sadda SR, 2015. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 122, 2514–2522. [DOI] [PubMed] [Google Scholar]
- Xu D, Dávila JP, Rahimi M, Rebhun CB, Alibhai AY, Waheed NK, Sarraf D, 2018. Long-term Progression of Type 1 Neovascularization in Age-related Macular Degeneration Using Optical Coherence Tomography Angiography. American Journal of Ophthalmology 187, 10–20. [DOI] [PubMed] [Google Scholar]
- Xu K, Tzankova V, Li C, Sharma S, 2016. Intravenous fluorescein angiography-associated adverse reactions. Canadian Journal of Ophthalmology 51, 321–325. [DOI] [PubMed] [Google Scholar]
- Xue J, Camino A, Bailey ST, Liu X, Li D, Jia Y, 2018. Automatic quantification of choroidal neovascularization lesion area on OCT angiography based on density cell-like P systems with active membranes. Biomedical Optics Express 9, 3208–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y, Mohla A, Lee SY, Mathur R, Chan CM, Yeo I, Wong TY, Cheung CMG, 2018. Incidence of Fellow Eye Involvement in Patients With Unilateral Exudative Age-Related Macular Degeneration. JAMA Ophthalmology 136, 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang Q, Motulsky EH, Thulliez M, Shi Y, Lyu C, de Sisternes L, Durbin MK, Feuer W, Wang RK, Gregori G, Rosenfeld PJ, 2019. Two-Year Risk of Exudation in Eyes with Nonexudative Age-Related Macular Degeneration and Subclinical Neovascularization Detected with Swept Source Optical Coherence Tomography Angiography. American Journal of Ophthalmology 208, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalastas PI, Fatehee N, Yousefi S, Belghith A, Saunders LJ, Medeiros FA, Huang D, Weinreb RN, 2016a. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Investigative Ophthalmology and Visual Science 57, OCT451–OCT459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Yousefi S, Saunders LJ, Belghith A, Manalastas PIC, Medeiros FA, Weinreb RN, 2016b. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 123, 2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmohammadi A, Zangwill LM, Manalastas PIC, Fuller NJ, Diniz-Filho A, Saunders LJ, Suh MH, Hasenstab K, Weinreb RN, 2018. Peripapillary and Macular Vessel Density in Patients with Primary Open-Angle Glaucoma and Unilateral Visual Field Loss. Ophthalmology 125, 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BEK, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Zu L, Yasuda M, Zhang X, Mitchell P, Wong TY, 2012. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Chao JR, Wang RK, 2014. User-guided segmentation for volumetric retinal optical coherence tomography images. Journal of Biomedical Optics 19, 086020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You QS, Guo Y, Wang J, Wei X, Camino A, Zang P, Flaxel CJ, Bailey ST, Huang D, Jia Y, Hwang TS, 2019. Detection of Clinically Unsuspected Retinal Neovascularization With Wide-Field Optical Coherence Tomography Angiography. Retina, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S, Liu T, Wang RK, 2015. Segmentation and quantification of blood vessels for OCT-based micro-angiograms using hybrid shape/intensity compounding. Microvascular Research 97, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJ, Camino A, Liu L, Zhang X, Wang J, Gao SS, Jia Y, Huang D, Signal Strength Reduction Effects in OCT Angiography. Ophthalmology Retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJ, Camino A, Liu L, Zhang X, Wang J, Gao SS, Jia Y, Huang D, 2019. Signal Strength Reduction Effects in OCT Angiography. Ophthalmology Retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid S, Dolz-Marco R, Freund KB, Balaratnasingam C, Dansingani K, Gilani F, Mehta N, Young E, Klifto MR, Chae B, Yannuzzi LA, Young JA, 2016. Fractal Dimensional Analysis of Optical Coherence Tomography Angiography in Eyes With Diabetic Retinopathy. Investigative Opthalmology & Visual Science 57, 4940–4947. [DOI] [PubMed] [Google Scholar]
- Zang P, Gao SS, Hwang TS, Flaxel CJ, Wilson DJ, Morrison JC, Huang D, Li D, Jia Y, 2017. Automated boundary detection of the optic disc and layer segmentation of the peripapillary retina in volumetric structural and angiographic optical coherence tomography. Biomedical Optics Express 8, 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang P, Wang J, Hormel TT, Liu L, Huang D, Jia Y, 2019. Automated segmentation of peripapillary retinal boundaries in OCT combining a convolutional neural network and a multi-weights graph search. Biomedical Optics Express 10, 4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeimer M, Gutfleisch M, Heimes B, Spital G, Lommatzsch A, Pauleikhoff D, 2015. Association between changes in macular vasculature in optical coherence tomography- and fluorescein-angiography and distribution of macular pigment in type 2 idiopathic macular telangiectasia. Retina 35, 2307–2316. [DOI] [PubMed] [Google Scholar]
- Zhang A, Zhang Q, Chen C-L, Wang RK, 2015a. Methods and algorithms for optical coherence tomography-based angiography: a review and comparison. Journal of Biomedical Optics 20, 100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zhang Q, Wang RK, 2015b. Minimizing projection artifacts for accurate presentation of choroidal neovascularization in OCT micro-angiography. Biomedical Optics Express 6, 4130–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Hwang TS, Campbell JP, Bailey ST, Wilson DJ, Huang D, Jia Y, 2016a. Projection-resolved optical coherence tomographic angiography. Biomedical Optics Express 7, 816–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Hwang TS, Dongye C, Wilson DJ, Huang D, Jia Y, 2016b. Automated quantification of nonperfusion in three retinal plexuses using projection-resolved optical coherence tomography angiography in diabetic retinopathy. Investigative Ophthalmology and Visual Science 57, 5101–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang J, Pechauer AD, Hwang TS, Gao SS, Liu L, Liu L, Bailey ST, Wilson DJ, Huang D, Jia Y, 2015c. Advanced image processing for optical coherence tomographic angiography of macular diseases. Biomedical Optics Express 6, 4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang RK, Chen CL, Legarreta AD, Durbin MK, An L, Sharma U, Stetson PF, Legarreta JE, Roisman L, Gregori G, Rosenfeld PJ, 2015d. Swept source optical coherence tomography angiography of neovascular macular telangiectasia type 2. Retina 35, 2285–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang A, Lee CS, Lee AY, Rezaei KA, Roisman L, Miller A, Zheng F, Gregori G, Durbin MK, An L, Stetson PF, Rosenfeld PJ, Wang RK, 2017. Projection artifact removal improves visualization and quantitation of macular neovascularization imaged by optical coherence tomography angiography. Ophthalmology Retina 1, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Roisman L, Schaal KB, Miller AR, Robbins G, Gregori G, Rosenfeld PJ, 2016. Artifactual flow signals within drusen detected by OCT angiography. Ophthalmic Surgery Lasers and Imaging Retina 47, 517–522. [DOI] [PubMed] [Google Scholar]