Abstract
Objective
To assess outcomes following post-hemorrhagic ventricular dilatation (PHVD) among infants born at ≤26 weeks of gestation.
Study design
Observational study of infants born 4/1/11–12/31/15 in the NICHD Neonatal Research Network and categorized into three groups: PHVD, intracranial hemorrhage without ventricular dilatation, or normal head ultrasound. PHVD was treated per center practice. Neurodevelopmental impairment at 18-26 months was defined by cerebral palsy, Bayley III cognitive or motor score <70, blindness or deafness. Multivariable logistic regression examined the association of death or impairment, adjusting for neonatal course, center, maternal education and parenchymal hemorrhage.
Results
Of 4216 infants, 815 had PHVD, 769 had hemorrhage without ventricular dilatation, and 2632 had normal head ultrasounds. Progressive dilatation occurred among 119/815 infants; the initial intervention in 66 infants was reservoir placement and 53 had ventriculoperitoneal shunt placement. Death or impairment occurred among 68%, 39%, and 28% of infants with PHVD, hemorrhage without dilatation and normal head ultrasound, respectively; adjusted odds ratios (aOR) 95% CI were 4.6 (3.8-5.7) PHVD vs. normal head ultrasound and 2.98 (2.3-3.8) for PHVD vs hemorrhage without dilatation. Death or impairment was more frequent with intervention for progressive dilatation vs. no intervention [80% vs. 65%; aOR 2.2 (1.38-3.8)]. Death or impairment increased with parenchymal hemorrhage, intervention for PHVD, male sex and surgery for retinopathy; odds decreased with each additional gestational week.
Conclusions
PHVD was associated with high rates of death or impairment among infants with gestational ages ≤26 weeks; risk was further increased among those with progressive ventricular dilation requiring intervention.
Keywords: Extremely low gestational age infants, post-hemorrhagic ventricular dilatation, outcome
Infants born at extremely low gestational ages (≤26 weeks) are at high risk for neurodevelopmental disabilities in childhood and adolescence. Risk factors associated with poor outcome include neonatal brain injury (intracranial hemorrhage in the intraventricular, parenchymal or cerebellar region, white matter injury, cystic periventricular leukomalacia, porencephaly and ventricular dilation), lung disease including bronchopulmonary dysplasia with or without postnatal corticosteroid steroid use, sepsis, necrotizing enterocolitis and exposure to surgery.1-6 Infants of any gestational age who develop posthemorrhagic ventricular dilation (PHVD) are also at high risk for cerebral palsy as well as cognitive, functional, attention and visual perception challenges at school age.7-10 The risk of neurodevelopmental impairment is higher if the PHVD is progressive and requires intervention in the neonatal period.11-13 Although there is insufficient evidence to recommend a specific weight or cerebrospinal fluid parameter to direct the timing of shunt placement in preterm infants with PHVD,14 the risk of deficits may be reduced by an early temporizing approach to treating PHVD.7,8,15 Data on the outcome of PHVD among extremely low gestational infants is sparse. The goal of our study was to examine outcomes of death or neurodevelopmental impairment among infants born at ≤26 weeks of gestation who developed PHVD. We hypothesized that infants requiring intervention for PHVD would be at highest risk for death or impairment compared with those with PHVD not requiring intervention, those with hemorrhage without PHVD and those with normal cranial head ultrasounds (HUS).
Methods
This study was conducted at 17 clinical centers in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network. Infants born at ≤26 weeks of gestation from 4/1/2011-12/31/2015 were included. Results of the most severe abnormality in HUS, including intracranial hemorrhage and ventricular size, evaluated by clinical center radiology readings closest to two time points, 28 days and 36 weeks postmenstrual age were documented as part of the on-going data registry. Blood in the germinal matrix/subependymal are was coded as grade I hemorrhage, blood within the ventricular system without ventricular enlargement as grade II, ventricular size enlarged with concurrent or prior blood was grade III and blood within the parenchyma was coded as grade IV hemorrhage. Information on follow-up was obtained from the NICHD NRN follow-up data registry. PHVD was diagnosed based on enlarged ventricles with preceding hemorrhage on serial HUS performed closest to 28 days and 36 weeks postmenstrual age. The intervention for progressive PHVD was per clinical center practice; ventricular reservoir placement with subsequent serial reservoir taps or insertion of a ventriculoperitoneal shunt (VPS). Infants with reservoir placement who did not have resolution of PHVD had insertion of a VPS prior to discharge. The age of the infant at reservoir and/or VPS insertion was recorded. Details of the frequency of cerebrospinal fluid removal or volumes removed from reservoir taps were not collected. Information on the perinatal and neonatal course until infant’s discharge home, transfer or death on or before 120 days, as well as death if hospitalized beyond 120 days was also collected. Neonatal data collected included surfactant receipt within 72 hours of birth, late-onset sepsis defined as a positive blood culture after 72 hours of age, necrotizing enterocolitis using modified Bell’s staging ≥IIA,16 bronchopulmonary dysplasia defined as need for oxygen at 36 weeks postmenstrual age and severe retinopathy of prematurity treated with surgery.
Study exclusion criteria were death within 12 hours of age, major anomaly, the decision to not provide intensive care at or following birth, and HUS data that was unavailable or missing for hemorrhage status. Infants were categorized into 3 groups based on the HUS data; PHVD, hemorrhage without PHVD and normal HUS. In the PHVD group, infants were classified as having no surgical intervention when PHVD resolved or stabilized as documented on the discharge diagnosis whereas those with progressive PHVD were categorized based on initial intervention, reservoir or VPS placement. Infants with reservoir placement who had VPS insertion as a second intervention remained in the reservoir group.
Outcome Measures
All infants were followed to 18-22 months of corrected age if they were born prior to July 1, 2012 and at 22-26 months of corrected age if born after July 1, 2012. Assessments at the follow-up study visit included a standardized neurologic examination17 and the Bayley Scales of Infant and Toddler Development 3rd edition (Bayley III) administered by trained and certified site examiners. The Bayley III cognitive, language and motor composite scores (normalized to a mean 100, SD 15) and scaled scores for fine and gross motor skills, and receptive and expressive language, (mean 10, SD 3) were obtained.18 Infants who were not successfully tested due to severe developmental delay, blindness or profound hearing loss were assigned a cognitive score of 54 and language and motor scores of 46.
Neurodevelopmental impairment was defined per the 2010 definition used in NICHD Neonatal Research Network as any of the following: moderate or severe cerebral palsy based on the gross motor functional classification system level ≥219, Bayley III cognitive or motor scores <70, bilateral blindness or deafness defined as profound hearing loss in spite of amplification. Normal survivors were defined as infants without any cerebral palsy, Bayley III cognitive and motor scores >85, and with normal vision and hearing assessments.
Statistical Analyses
To evaluate for bias, maternal and infant characteristics were compared among infants seen in follow up and those who survived to neonatal intensive care unit discharge but were lost to follow-up. The maternal and neonatal characteristics were then examined among infants seen in follow-up categorized by 3 study groups (PHVD, hemorrhage without PHVD and those with normal HUS) to evaluate for group differences, with chi-square tests and Fisher’s exact tests for categorical variables and Wilcoxon test for continuous outcomes. Similar comparisons were performed within the PHVD group among infants who received an intervention for progressive PHVD, either a ventricular reservoir as the first intervention for PHVD or insertion of a VPS, and those who had PHVD that stabilized or improved without any intervention.
The primary outcome of the study was death or neurodevelopmental impairment at the followup visit, because death is a competing outcome in this extremely low gestational age group. Secondary outcomes included components of the primary outcome, hospitalizations following discharge and measurement of growth at the follow-up evaluation. Risk for the primary outcome and components of impairment were assessed between the 3 study groups by odds ratios and 95% confidence intervals (OR, 95%CI) for categorical outcomes using Generalized Linear Mixed models. For continuous outcomes, adjusted estimates were obtained using Generalized Linear models. Covariates included in all models were center, male sex, antenatal steroids, histological chorioamnionitis and hypertension, mode of delivery, mother’s education, and gestational age. Center was included as a random effect for categorical outcomes. A similar analysis was conducted to evaluate risk for death or neurodevelopment impairment among infants with PHVD with and without intervention, adjusting for additional factors (delivery room intubation, receipt of surfactant, late onset sepsis, necrotizing enterocolitis, receipt of oxygen at 36 weeks, parenchymal hemorrhage and surgery for retinopathy of prematurity). Other than delivery room intubation and receipt of surfactant, the data of occurrence of the remaining variables was not available in the registry data, hence it is not known if they occurred prior to PHVD. A P value <.05 was considered significant. All statistical analyses were conducted by the data coordinating center at RTI International using SAS (version 9.3; SAS Institute, Cary, North Carolina) software. The birth and follow-up registries were approved by the institutional review board at each site and the study protocol was approved by the NICHD Neonatal Research Network.
Results
From 4/1/2011-12/31/2015 there were 5600 infants born at ≤26 weeks of gestation in the NICHD Neonatal Research Network centers. Of the 4216 infants in the study cohort, 815 (19.3%) infants had PHVD, 769 (18.2%) had intracranial hemorrhage without PHVD and 2632 (62.4%) had normal HUS (Figure 1; available at www.jpeds.com). Of the 3069 infants who attended the follow-up visit, data needed for evaluating neurodevelopmental impairment were incomplete for 177 infants (116 missing either Bayley cognitive or motor scores, 33 missing all the components of impairment and 28 missing some of the components of impairment). The majority of infants were seen at 22-26 months corrected age; 9% were evaluated at 18-22 months corrected age. Data for assessing neurodevelopmental impairment were available for 453 infants with PHVD (95.5%), 506 with hemorrhage without ventricular dilatation (92.2%) and 1933 infants (94.4%) with normal HUS. To evaluate for bias, baseline maternal and neonatal characteristics were evaluated for the 3069 infants who attended the follow-up visit and those who were lost to follow-up. Compared with mothers of infants seen in follow-up, mothers of those lost to follow-up were younger with infants of higher gestational age (Table I; available at www.jpeds.com).
Figure 1:
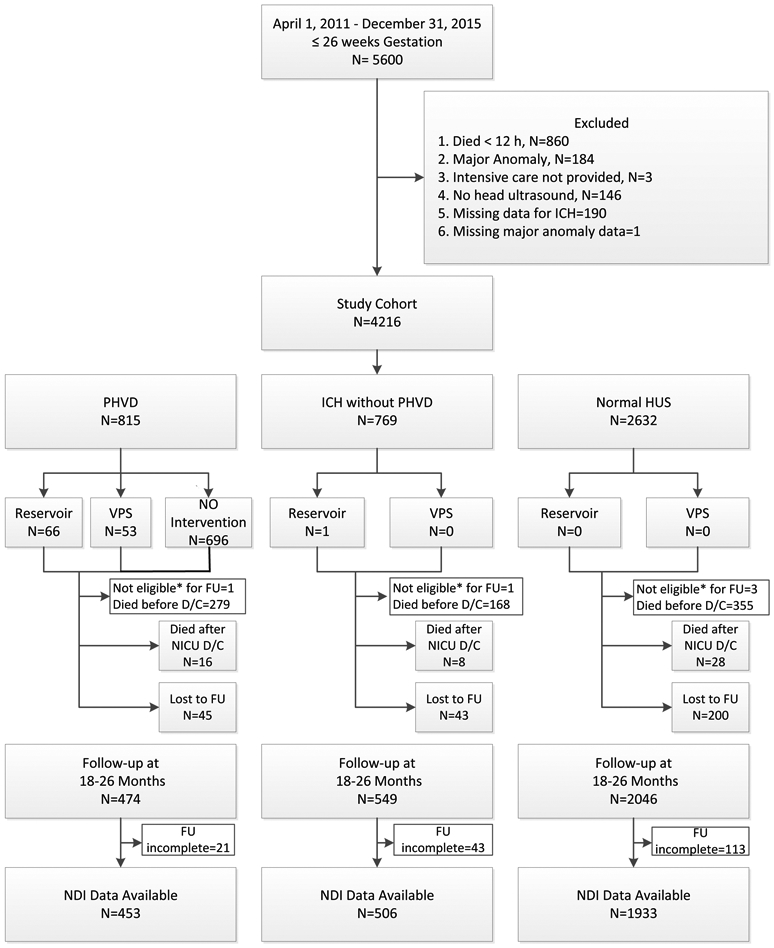
Flow of extremely low gestational age infants through a study of outcomes following post-hemorrhagic ventricular dilatation
* Not eligible for Follow-up because Infant not inborn.
Table I.
Maternal and neonatal characteristics of infants seen in follow-up versus those lost to follow-up
| Seen in Follow-up N=3069 |
Lost to Follow-up N=288 |
P-valuea | |
|---|---|---|---|
| Maternal variables | |||
| Age of mother (mean(SD))- years | N=3068 28.32(6.21) |
27.04(5.80) | <0.01 |
| Antenatal steroids –n (%) | 2756/3065(89.9) | 259/287(90.2) | 0.86 |
| Complete-n (%) | 2098/2747(76.4) | 200/259(77.2) | 0.76 |
| Partial-n (%) | 649/2747(23.6) | 59/259(22.8) | 0.82 |
| Delivery mode: Vaginal- n (%) | 1076/3069(35.1) | 107/288(37.2) | 0.48 |
| Histological chorioamnionitis- n (%) | 1599/2739(58.4) | 152/255(59.6) | 0.70 |
| Hypertension/preeclampsia-n (%) | 676/3064(22.1) | 53/287(18.5) | 0.16 |
| Maternal education < high school n (%) | 462/2437(19.0) | 40/214(18.7) | 0.92 |
| Race: Black- n(%) |
1310/2977(44.0) | 120/282(42.6) | 0.64 |
| White- n(%) | 1502/2977(50.5) | 140/282(49.7) | 0.80 |
| Other- n(%) | 165/2977(5.5) | 22/282(7.80) | 0.12 |
| Neonatal Variables | |||
| Outborn-n (%) | 106/3069(3.5) | 8/288(2.8) | 0.54 |
| Gestational age –wks (mean(SD)) | 24.91(1.04) | 25.05(1.02) | 0.02 |
| Birth weight g (mean(SD) ) | 757.09(159.92) | 776.27(160.33) | 0.07 |
| Male -n (%) | 1554/3065(50.7) | 143/288(49.7) | 0.73 |
| Delivery room intubation-n (%) | 2420/3069(78.9) | 226/288(78.5) | 0.88 |
| Surfactant use-n (%) | 2784/3069(90.7) | 261/288(90.6) | 0.96 |
| Postnatal steroids- n (%) | 688/2808(24.5) | 56/264(21.2) | 0.23 |
| Intraventricular hemorrhage- n (%) | 490/3059(16.0) | 40/287(13.9) | 0.36 |
| Parenchymal Hemorrhage- n (%) | 254/3059(8.30) | 24/287(8.36) | 0.97 |
| Oxygen at 36 weeks- n (%) | 1921/3067(62.6) | 171/288(59.4) | 0.27 |
| Necrotizing enterocolitis > Stage 2- n (%) | 267/3069(8.70) | 24/288(8.33) | 0.83 |
| Late onset sepsis - n (%) | 805/3069(26.2) | 69/288(24.0) | 0.40 |
| Retinopathy of prematurity requiring therapy- n (%) | 425/3040(14.0) | 32/279(11.5) | 0.24 |
P-values obtained using chi-square test or Fisher’s exact test for categorical and Nonparametric Wilcoxon test for continuous outcomes.
Maternal and Neonatal Characteristics
The maternal and neonatal characteristics of the infants in the study are presented in Table II. Mothers of infants with PHVD were younger, had lower rates of antenatal steroid administration, hypertension/preeclampsia and higher rates of histological chorioamnionitis. Vaginal delivery differed by group; 42% of PHVD, 45% hemorrhage without PHVD and 32% in the normal HUS groups (P < .0001). Compared with infants with hemorrhage without PHVD and those with normal HUS, infants with PHVD were younger in gestation, had lower birth weights, more neonatal interventions and higher rates of morbidity including delivery room intubation, surfactant use, oxygen need at 36 weeks postmenstrual age, necrotizing enterocolitis, late onset sepsis and retinopathy of prematurity requiring surgery. Male sex was similar in the PHVD and hemorrhage without PHVD groups; there were fewer males in the normal HUS group (P < 0.001). Among the 815 infants with PHVD, 119 (14.6%) infants had surgical intervention for progressive PHVD, based on center practice. The first intervention for progressive PHVD occurred at a median age of 29 days (at 29.1 weeks PMA) for placement of a ventricular reservoir or at a median age of 39 days (at 30.7 weeks PMA) for placement of a VPS. Among infants whose first intervention was a reservoir (n=66), 36 (54.5%) had a VPS inserted for continued drainage at a postmenstrual age of 36 ± 2 weeks at 84 ± 17 days (mean ± SD).
Table II.
Maternal and neonatal characteristics of infants by study group
| PHVD group N=815 |
Intracranial Hemorrhage without PHVD N=769 |
Normal HUS N=2632 |
P-valuea | |
|---|---|---|---|---|
| Maternal variables | ||||
| Age of mother (mean(SD))- years | N=813 27.55(6.38) |
N=768 28.07(6.42) |
28.21(6.13) | 0.02 |
| Antenatal steroids –n (%) | 650/813(80.0) | 660/767(86.0) | 2419/2629(92.0) | <0.01 |
| Complete-n (%) | 423/648(65.3) | 482/656(73.5) | 1922/2411(79.7) | <0.01 |
| Partial-n (%) | 225/648(34.7) | 174/656(26.5) | 489/2411(20.3) | <0.01 |
| Delivery mode: Vaginal- n (%) | 343/815(42.1) | 344/769(44.7) | 854/2632(32.4) | <0.01 |
| Histological chorioamnionitis- n (%) | 446/710(62.8) | 398/685(58.1) | 1296/2325(55.7) | <0.01 |
| Hypertension/preeclampsia-n (%) | 136/812(16.7) | 162/766(21.1) | 649/2626(24.7) | <0.01 |
| Maternal education < high school- n(%) | 137/616(22.2) | 127/609(20.9) | 389/2066(18.8) | 0.14 |
| Race: Black- n(%) |
357/779(45.8) | 334/751(44.5) | 1140/2563(44.5) | 0.79 |
| White- n(%) | 395/779(50.7) | 370/751(49.3) | 1266/2563(49.4) | 0.80 |
| Other- n(%) | 27/779(3.5) | 47/751(6.3) | 157/2563(6.1) | 0.01 |
| Neonatal Variables | ||||
| Outborn-n (%) | 38/815(4.66) | 29/769(3.77) | 83/2632(3.15) | 0.12 |
| Gestational age –wks (mean(SD)) | 24.32(1.16) | 24.65(1.12) | 24.95(1.03) | <0.01 |
| Birth weight g (mean(SD) ) | 706.31( 161.88) | 729.19(164.04) | 746.62( 168.62) | <0.01 |
| Male -n (%) | 456/815(56.0) | 430/767(56.1) | 1284/2630(48.8) | <0.01 |
| Delivery room intubation-n (%) | 734/815(90.1) | 630/768(82.0) | 2043/2632(77.6) | <0.01 |
| Surfactant use-n (%) | 792/815(97.2) | 716/769(93.1) | 2370/2632(90.0) | <0.01 |
| Postnatal steroids- n (%) | 178/745(23.9) | 160/718(22.3) | 558/2429(23.0) | 0.76 |
| Oxygen at 36 weeks- n (%) | 425/565(75.2) | 383/619(61.9) | 1414/2319(61.0) | <0.01 |
| Necrotizing enterocolitis > Stage 2- n (%) | 106/815(13.0) | 78/769(10.1) | 275/2632(10.4) | 0.09 |
| Late onset sepsis - n (%) | 253/761(33.2) | 240/740(32.4) | 642/2587(24.8) | <0.01 |
| Retinopathy of prematurity requiring therapy- n (%) | 134/580(23.1) | 104/621(16.7) | 261/2309(11.3) | <0.01 |
| Hemorrhage Grade Grade I |
22/815(2.7) | 343/769(44.6) | - | - |
| Grade II | 37/815(4.5) | 324/769(42.1) | - | - |
| Grade III | 354/815(43.4) | 0/769(0.0) | - | - |
| Grade IV | 402/815(49.3) | 102/769(13.3) | - | - |
| Days to first intervention for PHVD- median(IQR) overall | n=119 33(25,56) |
|||
| VPS | n=53 39(31,78) |
|||
| Ventricular reservoir | N=66 29(23,44) |
|||
| PMA at first intervention for PHVD (weeks)- median(IQR) overall | n=119 29.7(28.6,32.0) |
|||
| VPS | n=53 30.7(29.0,36.6) |
|||
| Ventricular reservoir | N=66 29.1(27.9,31.3) |
P-values obtained using chi-square test or Fisher’s exact test for categorical and Nonparametric Wilcoxon test for continuous outcomes.
Infants with PHVD who had an intervention for progressive PHVD were compared with those who did not have an intervention; those with an intervention had higher rates of parenchymal hemorrhage, oxygen use at 36 weeks postmenstrual age and necrotizing enterocolitis (Table III).
Table III.
Maternal and neonatal characteristics of infants who had no intervention versus Intervention in PHVD group
| No intervention N=696 |
Intervention N=119 |
P-valuea | |
|---|---|---|---|
| Maternal variables | |||
| Age of mother (mean(SD))- years | N=694 27.52(6.49) |
27.71(5.72) | 0.53 |
| Antenatal steroids –n (%) | 556/694(80.1) | 94/119(79.0) | 0.78 |
| Complete-n (%) | 367/555(66.1) | 56/93(60.2) | 0.27 |
| Partial-n (%) | 188/555(33.9) | 37/93(39.8) | 0.29 |
| Delivery mode: Vaginal- n (%) | 302/696(43.4) | 41/119(34.5) | 0.07 |
| Histological chorioamnionitis- n (%) | 382/608(62.8) | 64/102(62.7) | 0.99 |
| Hypertension/preeclampsia-n (%) | 119/693(17.2) | 17/119(14.3) | 0.44 |
| Maternal education < high school- n (%) | 119/533(22.3) | 18/83(21.7) | 0.90 |
| Race: Black- n(%) |
293/663(44.2) | 64/116(55.2) | 0.03 |
| White- n(%) | 346/663(52.2) | 49/116(42.2) | 0.05 |
| Other- n(%) | 24/663(3.6) | 3/116(2.6) | 0.78 |
| Neonatal Variables | |||
| Outborn-n (%) | 32/696(4.60) | 6/119(5.04) | 0.83 |
| Gestational age –wks (mean(SD)) | 24.32(1.17) | 24.33(1.13) | 0.91 |
| Birth weight g (mean(SD) ) | 703.32(165.20) | 723.81(140.21) | 0.10 |
| Male-n (%) | 397/696(57.0) | 59/119(49.6) | 0.13 |
| Delivery room intubation-n (%) | 622/696(89.4) | 112/119(94.1) | 0.11 |
| Surfactant use-n (%) | 675/696(97.0) | 117/119(98.3) | 0.56 |
| Postnatal steroids- n (%) | 145/637(22.8) | 33/108(30.6) | 0.08 |
| Intraventricular hemorrhage- n (%) | 351/696(50.4) | 40/119(33.6) | <0.01 |
| Parenchymal Hemorrhage- n (%) | 325/696(46.7) | 77/119(64.7) | <0.01 |
| Oxygen at 36 weeks- n (%) | 327/450(72.7) | 98/115(85.2) | 0.01 |
| Necrotizing enterocolitis > Stage 2- n (%) | 83/696(11.9) | 23/119(19.3) | 0.03 |
| Late onset sepsis - n (%) | 207/642(32.2) | 46/119(38.7) | 0.17 |
| Retinopathy od prematurity requiring therapy- n (%) | 101/463(21.8) | 33/117(28.2) | 0.14 |
P-values obtained using chi-square test or Fisher’s exact test for categorical and Nonparametric Wilcoxon test for continuous outcomes.
Outcomes
The follow-up outcomes of infants in the three groups are presented in Table IV. The primary outcome of death or neurodevelopmental impairment was observed among 68% of infants with PHVD compared with 39% of infants with hemorrhage without PHVD and 28% of the normal HUS group.
Table IV.
18-26 months corrected age outcome among infants with PHVD, intracranial hemorrhage without PHVD and normal HUS
| PHVD group | Intracranial Hemorrhage without PHVD |
Normal HUS | Adjusted odds ratio (95% CI)a or Adjusted estimates (95% CI)a |
|||
|---|---|---|---|---|---|---|
| PHVD vs. Normal |
Hemorrhage, NO PHVD vs. Normal |
PHVD vs. Hemorrhage without PHVD |
||||
| Death or NDI- n (%) | 506/748(67.7) | 269/682(39.4) | 649/2316(28.0) | 4.65(3.77,5.73)*** | 1.56(1.27,1.92)*** | 2.98(2.31,3.82)*** |
| Neurodevelop- mental impairmentb | 211/453(46.6) | 93/506(18.4) | 266/1933(13.8) | 4.96(3.84,6.41)*** | 1.34(1.01,1.78)* | 3.70(2.67,5.11)*** |
| Death-n(%) (beforec/after DC) | 295/814(36.2) | 176/768(22.9) | 383/2627(14.6) | 2.54(2.05,3.15)*** | 1.58(1.25,1.99)*** | 1.61(1.25,2.08)*** |
| Normal outcome- survivorsd- n (%) | 123/460(26.7) | 265/516(51.4) | 1105/1953(56.6) | 0.28(0.22,0.36)*** | 0.84(0.67,1.04) | 0.34(0.25,0.46)*** |
| Cerebral Palsy- n (%) | 202/471(42.9) | 68/540(12.6) | 178/2016(8.8) | 7.16(5.46,9.38)*** | 1.47(1.06,2.04)* | 4.86(3.44,6.88)*** |
| BSID III Cognitive > 85- n (%) | 150/452(33.2) | 283/523(54.1) | 1141/1966(58.0) | 0.37(0.29,0.47)*** | 0.85(0.69,1.06) | 0.43(0.32,0.58)*** |
| 70-85 -n (%) | 168/452(37.2) | 181/523(34.6) | 672/1966(34.2) | 1.05(0.82,1.33) | 1.00(0.80.1.25) | 1.05(0.79,1.41) |
| 69-55- n (%) | 111/452(24.6) | 58/523(11.1) | 135/1966(6.9) | 4.11(3.01,5.61)*** | 1.79(1.26,2.53)*** | 2.30(1.58,3.35)*** |
| < 55b - n (%) | 23/452(5.1) | 1/523(0.2) | 18/1966(0.9) | - | - | - |
| Motor composite score > 85 n (%) | 149/434(34.3) | 290/508(57.1) | 1168/1946(60.0) | 0.35(0.27,0.45)*** | 0.91(0.72,1.14) | 0.39(0.29,0.52)*** |
| 70-85 -n (%) | 125/434(28.8) | 154/508(30.3) | 605/1946(31.1) | 0.86(0.66,1.11) | 0.97(0.76,1.22) | 0.89(0.65,1.21) |
| 69-55 -n (%) | 77/434(17.7) | 41/508(8.1) | 110/1946(5.7) | 3.39(2.40,4.79)*** | 1.37(0.91,2.05) | 2.48(1.60,3.85)*** |
| <55b -n (%) | 83/434(19.1) | 23/508(4.5) | 63/1946(3.2) | 5.77(3.90,8.52)*** | 1.25(0.74,2.11) | 4.61(2.73,7.81)*** |
| Hearing impairment -n (%) | 25/469(5.3) | 11/534(2.1) | 48/2004(2.4) | 1.87(1.08,3.23)* | 0.87(0.43,1.76) | 2.15(0.99,4.68)* |
| Vision impairment - n (%) | 27/469(5.8) | 4/540(0.7) | 12/2019(0.6) | 9.04(4.28,19.29)*** | 0.92(0.25,3.34) | 9.83(2.91,33.28)*** |
| Rehospitalized (%) | 277/474(58.4) | 268/547(48.9) | 973/2040(47.7) | 1.33(1.06,1.66)** | 0.96(0.78,1.18) | 1.38(1.05,1.82)* |
| Rehospitalization for seizures or VPS revision- n (%) | 46/276(16.7) | 7/268(2.6) | 34/971(3.5) | 5.18(3.08,8.70)*** | 0.67(0.27,1.63) | 7.78(3.20,18.92)*** |
| Growth: | ||||||
| Weight kgs (mean(SD) ) | N=471 11.55(4.24) |
N=539 11.49(1.92) |
N=2009 11.43(1.68) |
0.12(−0.13,0.38) | 0.09(−0.14,0.33) | 0.03(−0.28,0.34) |
| Height cms, (mean(SD) ) | N=463 | N=535 | N=1995 | −0.41(−0.92,0.09) | 0.26(−0.20,0.72) | −0.68(−1.29,−0.07)* |
| 83.94(5.07) | 84.49(4.78) | 84.34(4.64) | ||||
| Head circumference cms (mean(SD)) | N=469 46.83(2.57) |
N=532 47.32(2.43) |
N=1992 47.34(2.10) |
−0.36(−0.60,−0.13)** | 0.07(−0.15,0.29) | −0.43(−0.72,−0.14)** |
| Weight<10th percentile | 110/471(23.4) | 99/539(18.4) | 358/2009(17.8) | 1.41(1.08,1.84)** | 1.05(0.81,1.37) | 1.34(0.96,1.86) |
| Height<10th percentile - n (%) | 181/463(39.1) | 159/535(29.7) | 592/1995(29.7) | 1.55(1.23,1.96)*** | 0.96(0.76,1.20) | 1.62(1.21,2.16)** |
| Head circumference<1 0th percentile - n (%) | 149/469(31.8) | 108/532(20.3) | 369/1992(18.5) | 1.82(1.41,2.36)*** | 0.99(0.76,1.30) | 1.84(1.33,2.54)*** |
| Corrected Age at Follow-up Months (mean(SD)) | N=474 23.88(3.52) |
N=545 23.77(3.39) |
N=2036 23.64(3.37) |
0.08(−0.27,0.43) | 0.17(−0.15,0.50) | −0.09(−0.52,0.33) |
Adjusted odds ratio (95% CI) for categorical outcomes were obtained using Generalized Linear Mixed models. For continuous outcomes, adjusted estimates (95% CI) were obtained using Generalized Linear models. Covariates adjusted in all models were center, male, antenatal steroids, histological chorioamnionitis, hypertension, mode of delivery, mother’s education, and gestational age. Center variations have been adjusted as a random effect for categorical outcomes.
Neurodevelopmental impairment is defined as moderate/severe cerebral palsy or Bayley III cognitive or motor score <70, blindness or deafness.
Withdrawal of respiratory support among those who died prior to hospital discharge was 23.1% among infants with PHVD, 14.1% among ICH no PHVD and 7.6% among those with normal HUS, P <.001
Normal outcome in survivors is defined as no cerebral palsy, Bayley III cognitive composite score ≥85, motor composite score ≥85, no blindness and no deafness.
P <.05
P <.01
P <.001
Neurodevelopmental impairment occurred in 47% of infants with PHVD, 18% of those with hemorrhage without PHVD and 14% of those with normal HUS. The rate of cerebral palsy was 43% in the PHVD group compared with 13% in the hemorrhage without PHVD group and 9% in the normal HUS group. Compared with the other two groups, more infants in the PHVD group had Bayley III cognitive scores <85 or motor scores <85 and more infants had hearing impairment and vision impairment.
Infants in the PHVD group had more hospitalizations after neonatal intensive care unit discharge, including those for seizures or VPS revisions than infants with hemorrhage without PHVD and those with normal HUS. The head circumference at follow-up was smaller among infants with PHVD compared with the other groups; more infants with PHVD had a head circumference below the 10th percentile.
The outcomes of the 91 infants with progressive PHVD who received an intervention was compared with the 383 infants with PHVD stabilizing without intervention (Table V). Death or neurodevelopmental impairment and impairment among survivors was higher among infants with PHVD with intervention than among infants with no intervention; 9.9% of infants with PHVD with intervention had normal outcomes.
Table V.
18-26 months corrected age outcome among infants with no intervention vs. intervention among infants with PHVD only.
| No intervention | With intervention (VPS or VR) |
Adjusted odds ratio (95% CI) a or Estimates (95% CI)a PHVD with intervention vs. PHVD with no Intervention. |
|
|---|---|---|---|
| Death or Neurodevelopmental impairment- n (%) | 420/641(65.5) | 86/107(80.4) | 1.97(1.15,3.38)* |
| Neurodevelopmental impairment | 142/363(39.1) | 69/90(76.7) | 4.28(2.44,7.51)*** |
| Death -n (%) (beforec/after DC) | 278/695(40.0) | 17/119(14.3) | 0.18(0.10,0.32)*** |
| Normal outcome in survivorsd- n (%) | 114/369(30.9) | 9/91(9.9) | 0.32(0.15,0.69)** |
| Cerebral Palsy- n (%) | 133/380(35.0) | 69/91(75.8) | 4.56(2.63,7.93)*** |
| BSID III Cognitive > 85- n (%) | 139/369(37.7) | 11/83(13.3) | 0.28(0.14,0.57)*** |
| 70-85 -n (%) | 142/369(38.5) | 26/83(31.3) | 0.79(0.47,1.36) |
| 69-55- n (%) | 73/369(19.8) | 38/83(45.8) | 2.85(1.66,4.88)*** |
| < 55 - n (%) | 15/369(4.07) | 8/83(9.64) | 2.50(0.95,6.62) |
| Motor composite score > 85 n (%) | 138/353(39.1) | 11/81(13.6) | 0.30(0.15,0.60)*** |
| 70-85 -n (%) | 111/353(31.4) | 14/81(17.3) | 0.48(0.26,0.92)* |
| 69-55 -n (%) | 52/353(14.7) | 25/81(30.9) | 2.23(1.24,4.00)** |
| <55 -n (%) | 52/353(14.7) | 31/81(38.3) | 3.17(1.75,5.73)*** |
| Hearing impairment -n (%) | 18/378(4.76) | 7/91(7.69) | 1.85(0.71,4.85) |
| Vision impairment - n (%) | 15/379(3.96) | 12/90(13.3) | 3.53(1.51,8.27)** |
| Rehospitalized-n (%) | 208/383(54.3) | 69/91(75.8) | 2.41(1.40,4.14)** |
| Rehospitalization for seizures or VPS revision- n (%) | 20/207(9.7) | 26/69(37.7) | 5.07(2.48,10.37)*** |
| Growth: | |||
| Weight kgs (mean(SD) | N=381 11.66(4.64) |
N=90 11.09(1.63) |
−0.34(−1.37,0.68) |
| Height cms, (mean(SD) ) | N=373 84.13(5.06) |
N=90 83.15(5.07) |
−0.88(−2.08,0.31) |
| Head circumference cms (mean(SD) ) | N=378 47.05(2.42) |
N=91 45.93(2.98) |
−1.07(−1.67,−0.48)*** |
| Weight<10th percentile - n (%) | 82/381(21.5) | 28/90(31.1) | 1.38(0.81,2.36) |
| Height< 10th percentile - n (%) | 139/373(37.3) | 42/90(46.7) | 1.37(0.84,2.23) |
| Head circumference < 10th percentile - n (%) | 102/378(27.0) | 47/91(51.6) | 2.50(1.52,4.11)*** |
Adjusted odds ratio (95% CI) for categorical outcomes has been obtained using Generalized Linear Mixed models. For continuous outcome, adjusted estimates (95% CI) were obtained using Generalized Linear Models. Covariates adjusted in all models are center, mothers’ education, and gestational age and parenchymal hemorrhage. Center variations have been adjusted as a random effect for categorical outcomes.
Neurodevelopmental impairment was defined as moderate/severe cerebral palsy or Bayley III cognitive or motor score <70, blindness or deafness.
Withdrawal of respiratory support among those who died prior to hospital discharge was 26.7% among infants with no intervention and 5.9% among those with intervention, P <.001
Normal outcome in survivors is defined as no cerebral palsy, Bayley III cognitive composite score ≥85, motor composite score ≥85, no blindness and no deafness.
P<.05
P <.01
P <.001
Predictors of Death or Neurodevelopmental Impairment
There was no association between age at the first intervention for PHVD and death or neurodevelopmental impairment following adjustment for center, gestational age of the infant and maternal education [aOR 0.98 (0.97-1.00)]; similarly, there was no association between age at first intervention and impairment among survivors, [aOR 0.99 (0.97-1.00)]. Factors associated with death or impairment among infants with PHVD with aOR (95% CI) were: intervention for progressive PHVD, 4.57 (2.34-8.94), surgery for retinopathy of prematurity, 2.61 (1.46-4.64), parenchymal hemorrhage 2.25 (1.32-3.84) and male sex, 1.70 (1.07-2.71); each advancing week of gestation decreased odds of death or impairment, 0.76 (0.61-0.94) (Figure 2; available at www.jpeds.com). More infants with intervention for PHVD were hospitalized and had lack of head growth at follow-up assessment compared with infants with no intervention for PHVD. Fewer infants in the PHVD group with intervention had Bayley III cognitive and motor scores in the normal range and more of them had very low cognitive and motor scores and impaired vision (Table V).
Figure 2:
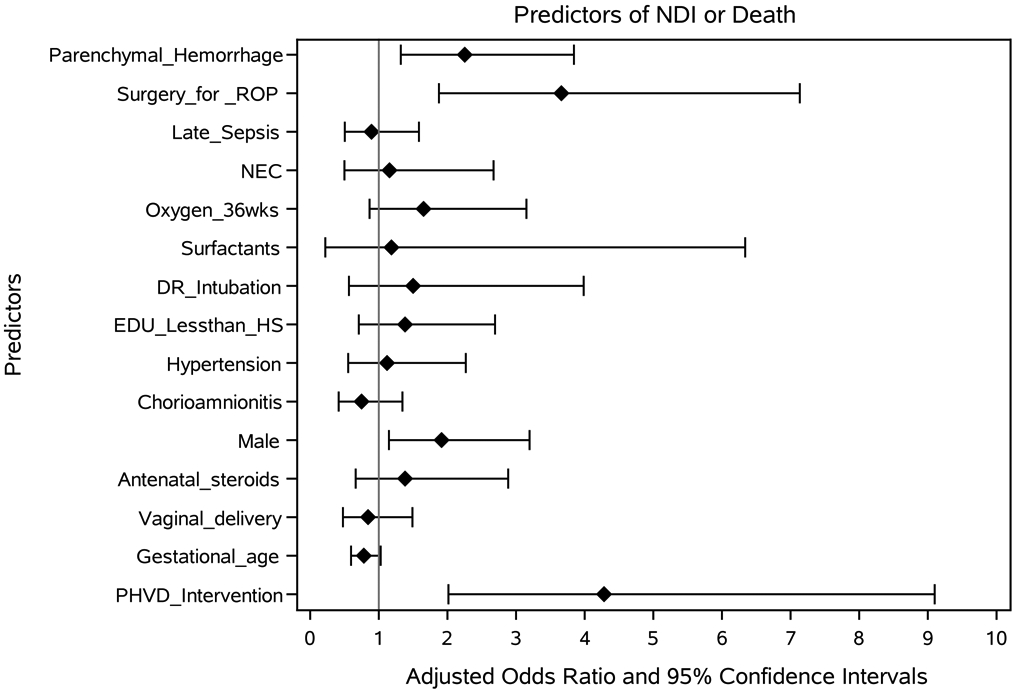
Factors associated with death or neurodevelopmental impairment after post-hemorrhagic ventricular dilatation among extremely low gestational age infants
Discussion
In this observational study of prospectively collected data, infants born at ≤26 weeks of gestation who had PHVD compared with those with those with hemorrhage without PHVD or those with a normal HUS, had a higher risk of death or neurodevelopmental impairment at follow-up at 18-22 months or 22-24 months corrected age and a lower likelihood of normal outcome. Within the PHVD group, infants requiring intervention for progressive ventricular dilatation with either a ventricular reservoir or VPS insertion had very high disability rates (cerebral palsy 76%, hearing impairment 8% and vision impairment 13%). Only 13% of infants receiving intervention had a normal Bayley III cognitive or motor composite score (>85).
A systematic literature review evaluated optimal treatment strategies for progressive PHVD in preterm infants.14 Level 1 evidence did not support use of intraventricular thrombolytic agents and systemic acetazolamide and furosemide as optimal methods to reduce the need for permanent drainage with VPS. Evidence was insufficient to recommend a specific weight or cerebrospinal fluid component to direct timing of shunt placement in preterm infants with progressive PHVD. However, the impact of early intervention using temporizing measures for progressive PHVD on outcomes of preterm infants is currently being investigated20. In the Early versus Late Ventricular Intervention Study (ELVIS), a multicenter randomized controlled trial of lower versus higher threshold for intervention for PHVD in 126 infants ≤34 weeks’ gestational age, de Vries et al noted there was no significant difference in the primary composite outcome of death or VPS placement in infants treated at a lower versus higher threshold for intervention.8 Low threshold was defined as a ventricular index ≤the 97th percentile and anterior horn width >6mm whereas the higher threshold was a ventricular index >the 97th percentile +4mm and anterior horn width >10mm. However, in a sub-study of ELVIS, neonatal magnetic resonance imaging at term-equivalent age demonstrated more brain injury and larger ventricular volumes in the high versus low threshold group.20 The neurodevelopmental outcome of ELVIS trial participants is ongoing. An observational study by Leijser et al at the Hospital for Sick Children (Canada) and two institutions in the Netherlands evaluated 127 infants with gestational ages <30 weeks, comparing an early approach (n=78) using ventricular measurements (standard of care in Dutch centers) and a late approach (n=49, standard of care in the Canadian center). The early approach with lumbar punctures or reservoir placement was initiated at a mean of 29.4 weeks postmenstrual age and the late approach was 2 weeks later; all late approach infants were outborn and transferred once there were signs of increased intracranial pressure and/or intervention was deemed necessary.7 The 18-24 month outcomes among those with early intervention for PHVD were within the normal range and indistinguishable from those without intervention. Ventricular measurements (as a basis for intervention) are not routinely performed in most US and Canadian centers and were not collected in the NICHD Neonatal Research Network Registry. The timing of the first intervention in our study was 29.7 (median) 28, 32 (IQR) weeks postmenstrual age; we found no association between time to first intervention and death or NDI at follow-up among infants with progressive PHVD requiring therapy.
The rate of conversion of temporary drainage with a reservoir to VPS insertion was 54.5% in our study which is higher than the study by Leijser7 (24%) but lower than that reported among <1500g neonates with PHVD from the Hydrocephalus Clinical Research Network (69%)21 or from the St. Louis Children’s Hospital report on infants born at ≤34 weeks of gestation with PHVD (77%).22
It is unclear whether the higher rates of neurodevelopmental impairment in our study are related to extreme prematurity. The rate of cerebral palsy was 27-32% in the study by Leijser among infants with gestational ages ≤30 weeks with PHVD without intervention compared to 25-94 % with intervention7, whereas in our study of infants with gestational ages ≤26 weeks, cerebral palsy rates were 35% without intervention and 76% among those with intervention. In a longitudinal study of 25 children with PHVD ranging in gestational age from 25-37 weeks, born between 1996 and 2003 and a control group matched for gestation, sex and year of birth, the rate of cerebral palsy at 10 years of age was 28% compared to 12% among the matched group. Within the PHVD group, IQ was significantly lower in those who had undergone surgical intervention (n=12).9
The strengths of our study are that data were collected prospectively in a multicenter setting, the focus was on the extremely low gestational age infants, the cohort with PHVD is the largest one evaluated to date and outcomes in infancy included the composite of death (a competing outcome) or neurodevelopmental impairment, as well as components of impairment. Similarly collected information on a comparison groups of infants with hemorrhage without PHVD and those with normal HUS is also a strength of our study. The limitations of our study include lack of central reading for HUS and assessment of rapidity of progression of ventricular dilatation. We did not have sonographic ventricular measurements nor have magnetic resonance imaging data. The details of clinical indications for intervention for progressive PHVD, indicators of intracranial pressure and differences in indications for intervention among centers is also a limitation. We did not collect details of VPS shunt obstructions and infections; factors known to influence outcome.23
The outcome in childhood of extremely low gestational age infants is influenced by their neonatal morbidity; Cheong et al followed 499 infants born at <28 weeks of gestation to 8 years of age; 241 (48%) had no major postnatal events and had the highest probability of survival without a major disability1. Four major morbidities were associated with disability; grade 3 or 4 hemorrhage, cystic periventricular leukomalacia, lung disease treated with postnatal steroids and any surgical condition. Hosti et al evaluated infants born at 23-25 weeks of gestation at 10 years of age; 21/132 adolescent survivors had a major disability that was associated with bronchopulmonary dysplasia, retinopathy of prematurity and neonatal brain injuries.2 Details of occurrence of PHVD are not mentioned in these two studies. Lastly, in a subset of extremely preterm infants with imaging data drawn from a randomized trial, cystic periventricular leukomalacia, porencephaly, moderate or severe ventricular dilatation, ventricular peritoneal shunts, and cerebellar injury were all associated with disability at age 6–7 years.3
Neonatal intensive care is now being offered to extremely preterm infants and their survival rates are improving.24 Our study demonstrates that infants born with gestational ages <26 weeks are at risk for severe intracranial hemorrhage, often with the complication of PHVD. Infants requiring intervention for progressive PHVD have a guarded prognosis. The interventions for infants with PHVD are evolving, with additional surgical interventions being attempted such as endoscopic third ventriculostomy with or without choroid plexus cauterization25; the impact of these interventions on outcomes needs to be evaluated. The challenge for clinicians is to optimize care for infants and to lower rates of brain injury, especially PHVD which is associated with the highest odds of death or survival with a poor outcome.
Supplementary Material
Acknowledgments
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s on-going Birth Registry and Follow-up Registry through cooperative agreements. There is no potential conflict of interest, real or perceived, with the study sponsor. The sponsor did not have a role in the study design, collection of data, analysis and interpretation of data, writing the report or decision to submit the manuscript for publication. The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (U10 HD21373, UG1 HD21364, UG1 HD21385, UG1 Funding Sources: The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (U10 HD21373, UG1 HD21364, UG1 HD21385, UG1 HD27851, UG1 HD27853, UG1 HD27856, UG1 HD27880,UG1 HD27904, UG1 HD34216, UG1 HD36790, UG1 HD40492, UG1 HD40689, UG1 HD53089, UG1 HD53109, UG1 HD68244, UG1 HD68270, UG1 HD68278, UG1 HD68263, UG1 HD68284; UG1 HD87226, UG1 HD87229) and the National Center for Advancing Translational Sciences (NCATS) (UL1 TR6, UL1 TR41, UL1 TR42, UL1 TR77, UL1 TR93, UL1 TR105, UL1 TR442, UL1 TR454, UL1 TR1117, provided grant support for the Neonatal Research Network, including for the Follow-up Study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov ID: Generic Database: NCT00063063
Data Sharing
Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
REFERENCES
- 1.Cheong JLY, Lee KJ, Boland RA, Spittle AJ, Opie GF, Burnet AC, et al. Changes in long-term prognosis with increasing postnatal survival and the occurrence of postnatal morbidities in extremely preterm infants offered intensive care: a prospective observational study. Lancet Child Adolesc Health 2018;872–9. [DOI] [PubMed] [Google Scholar]
- 2.Holsti A, Serenius F, Farooqi A. Impact of major neonatal morbidities on adolescents born at 23–25 weeks of gestation. Acta Paediatr 2018;107: 1893–901. [DOI] [PubMed] [Google Scholar]
- 3.Hintz SR, Vohr BR, Bann CM, Taylor HG, Das A, Gustafson KE et al. Preterm neuroimaging and school-age cognitive outcomes. Pediatrics 2018; 142: e20174058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cayam-Rand D, Guo T, Grunau RE, Benavente-Fernández I, Synnes A, Chau V et al. Predicting neurodevelopmental outcome in preterm infants A simple white matter imaging rule. Neurology 2019. September 24;93(13):e1231–e1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morriss FH Jr, Saha S, Bell EF, Colaizy TT, Stoll BJS, Hintz SR, et al. Surgery and neurodevelopmental outcome in very low-birth-weight infants. JAMA Pediatr 2014; 168:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fullerton BS, Hong CR, Velazco CS, Mercier CE, Morrow KA, Edwards EM et al. Severe neurodevelopmental disability and healthcare needs among survivors of medical and surgical necrotizing enterocolitis: a prospective cohort study. J Pediatr Surgery 2018;53: 101–07. [DOI] [PubMed] [Google Scholar]
- 7.Leijser LM, Miller SP, van Wezel-Meijler G, Brouwer A, Traubici JT, van Haastert IC, et al. Posthemorrhagic ventricular dilatation in preterm infants, when best to intervene? Neurology 2018,90:e698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries LS, Groenendaal F, Liem KD, Heep A, Brouwer AJ, Van’t Verlaat E, et al. The ELVIS study group. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomized controlled trial. Arch Dis Child Fetal Neonatal Ed 2018;0:F1–6. [DOI] [PubMed] [Google Scholar]
- 9.Holwerda JC, van Braeckel KNJA, Roze E, Hoving EW, Masthuis CGB, Brouwer O, et al. Functional outcome at school age of neonatal post-hemorrhagic ventricular dilatation. Early Human Devel 2016;96:15–20. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasakumar P, Limbrick D, Munro R, Mercer D, Rao R, Inder T, et al. Post hemorrhagic Ventricular dilatation-impact on early neurodevelopmental outcome. Am J Perinatal 2013; 30:207–14. [DOI] [PubMed] [Google Scholar]
- 11.Shankaran S, Koepke T, Woldt E, Bedard MP, Dajani R, Eisenbrey AB, Canady A. Outcome following post hemorrhagic ventriculomegaly in comparison with mild hemorrhage without ventriculomegaly. J Pediatr 1989;114: 109–14. [DOI] [PubMed] [Google Scholar]
- 12.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R for the NICHD Neonatal Research Network. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 2008;121:e1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwer A, Groenendaal F, van Haastert, Rademaker K, Hanlo P, de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr 2008;152:648–54. [DOI] [PubMed] [Google Scholar]
- 14.Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD Jr, Rogido M, Mitchell L, et al. for the Pediatric Hydrocephalus Systematic Review and Evidenced-Based Guidelines Task Force. J Neurosurg Pediatr 2014;1:8–23. [DOI] [PubMed] [Google Scholar]
- 15.Bassan H, Eshel E, Golan I, Kohelet D, Sira LB, Mandel D, et al. for the External Ventricular Drainage Study Investigators. Timing of external ventricular drainage and neurodevelopmental outcome in preterm infants with post hemorrhagic hydrocephalus. Eur J Pediatr Neurol 2012; 16:662–70. [DOI] [PubMed] [Google Scholar]
- 16.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978; 187 (1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman JE, Bann CM, Vohr BR, Dusick AM, Higgins RD; Follow-Up Study Group of Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Improving the Neonatal Research Network annual certification for neurologic examination of the 18-22 month child. J Pediatr 2012;161:1041–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayley N. Bayley scales of infant and toddler development, third edition. San Antonio, TX: Harcourt Assessment, Inc; 2006. [Google Scholar]
- 19.Palisano R, Rossenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997 [DOI] [PubMed] [Google Scholar]
- 20.Cizmeci MN, Khalili N, Claessens HP, Groenendaal F, Liem KD, Heep A, et al. Assessment of brain injury and brain volumes after posthemorrhagic ventricular dilatation: A nested substudy of the randomized ELVIS trial. J Pediatr 2019;208:191–7. [DOI] [PubMed] [Google Scholar]
- 21.Wellons JC 3rd, Shannon CN, Holubkov R, Riva-Cambrin J, Kulkarni J, Limbrick DD Jr et al. Shunting outcomes in posthemorrhagic hydrocephalus:results of a Hydrocephalus Clinical Research Network prospective cohort study. J Neurosurg Pediatr 2017; 20:19–29. [DOI] [PubMed] [Google Scholar]
- 22.Han RH, Berger D, Gabir M, Baksh BS, Morales DM, Mathur AM, et al. Time-to-event analysis of surgically treated post hemorrhagic hydrocephalus in preterm infants:a single- institution retrospective study. Childs Nerv Syst 2017;33:1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero L, Ros B, Rius F, Gonzalez L, Medina JM, Martin A, et al. Ventriculoperitoneal shunt as the primary neurosurgical procedure in newborn posthemorrhagic hydrocephalus:report of a series of 47 shunted patients. Childs Nerv Syst 2014; 1:91–7. [DOI] [PubMed] [Google Scholar]
- 24.Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and neurodevelopmental outcomes among periviable Infants. N Engl J Med 2017; 376: 617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koschnitzky JE, Keep RF, Limbrick DD, McAllister JP, Morris JA, Strahle J, et al. Opportunities in posthemorrhagic hydrocephalus research: outcomes of the Hydrocephalus Association Posthemorrhagic Hydrocephalus Workshop. Fluids Barriers CNS 2018; 15:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


