Abstract
Introduction
Pulmonary complications (PC) are the most common adverse event after injury and second greatest cause of failure to rescue (FTR-P, death after PC). It is not known if readily accessible trauma center data can be used to stratify center level performance for different complications. Performance variation between trauma centers would allow sharing of best practices amongst otherwise similar hospitals. We hypothesized that high, average and low-performing centers for PC and FTR-P could be identified and that hospital factors associated with success and failure could be discovered.
Methods
Pennsylvania state trauma registry data (2007–2015) were abstracted for PC. Burns and age<17yrs were excluded. Multivariable logistic regression models were developed for PC and FTR-P using demographics, comorbidities and injuries/physiology. Expected event rates were compared to observed rates to identify outliers. Center-level variables associated with outcomes of interest were taken from the American Hospital Association Annual Survey Database and assessed for inclusion.
Results
283,121 mostly male (60%) blunt trauma (92%) patients were included. 3% (8,381/283,121) developed PC (center-level range 0.18–5.8%). FTR-P was 13.4% (1,120/8,381, center-level range 0.0–22.6%). For PC, 13/27 centers were high performers (95% CI for O:E ratio<1) and 7/27 were low (95% CI for O:E ratio>1). For FTR-P, 2/27 were low performers and the remainder average. There was little concordance between performance for PC/FTR-P. Research programs, large non-teaching hospitals, those with advanced practice providers and those with health maintenance organizations had reduced FTR-P.
Conclusions
Factors associated with complications were distinct from those affecting FTR and center level success in reducing complications often did not translate into success in preventing death once they occurred. Our data demonstrate that high and low performing centers and the factors driving success or failure are identifiable. This work serves as a guide for comparing practices and improving outcomes with readily available data.
Keywords: Failure to rescue, Outcome Assessment, Traumatology, Pulmonary Complications
We have demonstrated a reproducible road map for comparing trauma center outcomes using readily available state registry data. We have uncovered significant strengths and weaknesses of both specific and omnibus failure to rescue rates as an outcome metric and provide guidance for future research.
Complication and mortality rates have long been the bellwether of hospital performance. Their utility is hindered by reliance on potentially non-modifiable patient level factors which limit center-to-center comparison. That inadequacy has led to increasing interest in failure to rescue (FTR), death after a complication, as a measure of hospital quality. 1 Unlike complication and mortality rates, FTR is associated more strongly with center level attributes; potentially modifiable targets for quality improvement.1–3 Identifying such hospital features could illuminate a pathway to reduced institutional mortality.
Robust study of FTR has led to application of the metric to a variety of specific diseases and patient populations, including trauma. 3–17 FTR is traditionally measured by looking at overall major complications and overall death in the same cohort. While this is a sensitive measure of overall performance, it may lack the specificity required to address the most important aspect of any quality measure: the ability to identify best practices and replicate them. 17 In trauma centers, which are often similar in structure and capability - and a world away from the elective population that FTR was designed for - a more refined approach may be needed to identify differences in performance. To this end, FTR metrics with emphasis on specific complications, especially those that disproportionately contribute to death, are an important area of trauma research.
We previously demonstrated that it is possible to identify high and low performing centers for cardiac complications (CC) and failure to rescue after cardiac complications (FTR-C).18 Pulmonary complications (PC), though, are even more common and comprise almost 40% of adverse events after injury and up to 32% of FTR deaths.19 This identifies them as a priority for quality improvement efforts aimed at increasing in-hospital survival after injury.
As a first step in this process we sought to identify high and low performing centers for PC and FTR-P. We hypothesized that using our comprehensive state trauma registry database would allow us to identify high and low performing trauma centers for both pulmonary complications (PC) and FTR from them (FTR-P). Further, we hypothesized that we could identify center level structural factors from the American Hospital Association Annual Survey of Hospitals underlying success and failure. Such factors would allow a reproducible guide for states to evaluate their trauma center performance and compare practices for success.
Methods
Study Design
We utilized data from the Pennsylvania Trauma Outcome Study (PTOS) database from 2007–2015 and excluded burn patients and those under 17 years old. PTOS is comprehensive, includes all trauma centers in the Commonwealth of Pennsylvania and is prospectively maintained by specialized registrars. All trauma patients admitted for at least 48 hours or admitted for at least 36 hours with injury severity score ≥9, all intensive care unit and step-down admissions regardless of duration, all deaths and all transfers are entered into this dataset. Patients with isolated hip fractures or suffering drowning, poisoning, asphyxiation or in hospital injury are not included in PTOS.
For this study, pulmonary complications (PC) were included only if they were the index complication and were defined as acute respiratory distress syndrome, acute respiratory failure, aspiration and non-aspiration pneumonia, pleural effusion and atelectasis in keeping with our previously published definitions. 19,20 Definitions for these conditions were based upon the Pennsylvania Trauma Systems Foundation 2014 Data Manual (Appendix A, www.ptsf.org/upload/2014_PTOS_Manual_Tab_1_through_4_Final.doc). Failure to rescue is not an existing PTOS variable. Failure to rescue was defined as in hospital death after a sentinel pulmonary complication (FTR-P).
Statistical Analysis
We compared attributes of patients with and without PC utilizing t-tests or Mann-Whitney tests for continuous variables and chi-square tests for categorical variables. Multiple imputation by chained equations was used to impute missing values for key variables. This was done to avoid introducing bias from complete case analysis.21,22
Next, we created a risk-adjusted model for the development of PC. Univariate logistic regression was used first to test the association between each patient exposure variable and PC. These variables included age, sex, race, mechanism of injury, maximum chest and head abbreviated injury score (AIS), injury severity score (ISS) and revised trauma score (RTS, reflecting physiology). Variables were examined for linearity using LOWESS plots. Candidate comorbidities were also considered and included (full list of 44 variables found in Appendix A, PTOS 2014 Data Manual).
Variables associated with the development of PC with p<0.2 in the univariate model were included as candidate variables in the multivariable model. A final model was generated using stepwise selection. Our final multivariable model was a logistic regression robust clustered for standard errors. The risk-adjusted model for FTR-P used the same modeling strategy but restricted to only those patients with PC. Model discrimination was evaluated using receiver operating characteristic (ROC) curves.
Observed rates of PC and FTR-P were summarized by center and expected rates of PC and FTR-P were calculated based on the multivariable logistic regression models. The expected number of events at each hospital was calculated as the sum of the predicted probabilities for each patients within that hospital, which was then compared to the observed number of events and represented as an observed to expected (O:E) ratio. Caterpillar plots were created by plotting the 95% confidence intervals (CI) of the O:E ratios for each center for both PC and FTR-P. The Rothman-Greenland method was used to create CIs.23
If the entire CI remained below 1, a center was termed a high performer (HP, fewer observed events than expected) and if the CI remained entirely above 1, the center was a low performer (LP, more observed events than expected). If the CI crossed one, the center was termed average in performance (AP) with an expected number of PC or FTR-P.
To facilitate our evaluation of center-level risk factors, the American Hospital Association (AHA) Annual Survey Database and PTOS datasets were linked. The AHA Annual Survey Database is a hospital dataset which gathers data from the AHA Annual Survey of Hospitals and from the U.S. Census Bureau, American Hospital Association Registration Database, accrediting agencies and numerous others.24 1,174 hospital level variables were considered for each center but those with more than a third of their values missing were excluded. This was also the case for binary variables where >90% of centers had the same outcome. Center level variables were added one at a time to the previous multivariable logistic regression models for presence or absence of PC and FTR-P that included only patient-level predictors. Hospital level variables with p<0.2 were considered as representing center level complexity and technology, geography, payor structure, staffing, teaching status or volume based on prior previously utilized methodology and remained in the final multivariable models.18 All analyses were accomplished with R version 3.5.1.
Results
Demographics (Tables 1 and 2)
Table 1:
Demographics and Injury Patterns
| Subject | Non-PC (274,740) | PC (8,381) | p | Non-FTR (7,261) | FTR (1,120) | p |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Median Age (IQR) | 55.00 [34.00, 76.00] | 55.00 [36.00, 74.00] | 0.248 | 53.00 [34.00, 71.00] | 74.00 [55.00, 84.00] | <0.001 |
| Sex | ||||||
| Male | 162,873 (59.3%) | 6,153 (73.4%) | <0.001 | 5,379 (74.1%) | 774 (69.1%) | 0.001 |
| Race | ||||||
| White | 220,490 (80.3%) | 6,871 (82.0%) | 0.001 | 5,904 (81.3%) | 967 (86.3%) | 0.001 |
| Black | 35,922 (13.1%) | 1,031 (12.3%) | 931 (12.8%) | 100 (8.9%) | ||
| Asian | 2,395 (0.9%) | 64 (0.8%) | 56 (0.8%) | 8 (0.7%) | ||
| Other | 4,462 (1.6%) | 130 (1.6%) | 113 (1.6%) | 17 (1.5%) | ||
| Unknown | 11,471 (4.2%) | 285 (3.4%) | 257 (3.5%) | 28 (2.5%) | ||
| Injury Patterns | ||||||
| Blunt | 252,551 (91.9%) | 7,801 (93.1%) | <0.001 | 6,718 (92.5%) | 1,083 (96.7%) | <0.001 |
| Penetrating | 22,189 (8.1%) | 580 (6.9%) | 543 (7.5%) | 37 (3.3%) | ||
| Median ISS (IQR) | 9.00 [5.00, 14.00] | 21.00 [11.00, 29.00] | <0.001 | 21.00 [12.00, 29.00] | 21.00 [10.00, 29.00] | 0.984 |
| Median RTS (IQR) | 7.84 [7.84, 7.84] | 7.84 [5.68, 7.84] | <0.001 | 7.84 [5.68, 7.84] | 7.84 [4.74, 7.84] | 0.086 |
| Max AIS Chest | ||||||
| 0 | 199,551 (72.6%) | 3,865 (46.1%) | <0.001 | 3,226 (44.4%) | 639 (57.1%) | <0.001 |
| 1 | 16,094 (5.9%) | 420 (5.0%) | 350 (4.8%) | 70 (6.2%) | ||
| 2 | 15,132 (5.5%) | 572 (6.8%) | 517 (7.1%) | 55 (4.9%) | ||
| 3 | 33,482 (12.2%) | 2,185 (26.1%) | 1,956 (26.9%) | 229 (20.4%) | ||
| 4 | 8,372 (3.0%) | 1,121 (13.4%) | 1,009 (13.9%) | 112 (10.0%) | ||
| 5 | 1,533 (0.6%) | 210 (2.5%) | 197 (2.7%) | 13 (1.2%) | ||
| 6 | 576 (0.2%) | 8 (0.1%) | 6 (0.1%) | 2 (0.2%) | ||
| Max AIS Head | ||||||
| 0 | 154,716 (56.3%) | 3,075 (36.7%) | <0.001 | 2,737 (37.7%) | 338 (30.2%) | <0.001 |
| 1 | 27,850 (10.1%) | 732 (8.7%) | 644 (8.9%) | 88 (7.9%) | ||
| 2 | 31,407 (11.4%) | 733 (8.7%) | 673 (9.3%) | 60 (5.4%) | ||
| 3 | 27,834 (10.1%) | 1,166 (13.9%) | 1,013 (14.0%) | 153 (13.7%) | ||
| 4 | 20,451 (7.4%) | 1,094 (13.1%) | 959 (13.2%) | 135 (12.1%) | ||
| 5 | 12,313 (4.5%) | 1,578 (18.8%) | 1,233 (17.0%) | 345 (30.8%) | ||
| 6 | 169 (0.1%) | 3 (0.0%) | 2 (0.0%) | 1 (0.1%) |
Demographics of patients with and without pulmonary complications and failure to rescue from them.
Abbreviations: ISS = Injury Severity Score; RTS = Revised Trauma Score, AIS =Abbreviated Injury Score, IQR = Interquartile Range
Table 2:
Comorbidities and Relation to PC and FTR-P
| Comorbidity | Non-PC (274,740) | PC (8,381) | p | Non-FTR(7,261) | FTR (1,120) | p |
|---|---|---|---|---|---|---|
| Psychiatric History | 57,161 (20.8%) | 1,670 (19.9%) | 0.052 | 1,447 (19.9%) | 223 (19.9%) | 1.000 |
| Thyroid Disease | 28524 (10.4%) | 719 (8.6%) | <0.001 | 575 (7.9%) | 144 (12.9%) | <0.001 |
| Stroke | 14,515 (5.3%) | 540 (6.4%) | <0.001 | 417 (5.7%) | 123 (11.0%) | <0.001 |
| Obesity | 18,571 (6.8%) | 805 (9.6%) | <0.001 | 687 (9.5%) | 118 (10.5%) | 0.280 |
| Alcohol Use | 19,819 (7.2%) | 1,234 (14.7%) | <0.001 | 1,134 (15.6%) | 100 (8.9%) | <0.001 |
| Current Smoking | 28,556 (10.4%) | 858 (10.2%) | 0.657 | 796 (11.0%) | 62 (5.5%) | <0.001 |
| Arrival Creatinine >2 | 3,364 (1.2%) | 163 (1.9%) | <0.001 | 102 (1.4%) | 61 (5.4%) | <0.001 |
| Cirrhosis | 2,862 (1.0%) | 180 (2.1%) | <0.001 | 138 (1.9%) | 42 (3.8%) | <0.001 |
| Seizures | 5,779 (2.1%) | 228 (2.7%) | <0.001 | 196 (2.7%) | 32 (2.9%) | 0.839 |
| Dialysis | 2,371 (0.9%) | 87 (1.0%) | 0.101 | 55 (0.8%) | 32 (2.9%) | <0.001 |
| Drug Use | 13,213 (4.8%) | 600 (7.2%) | <0.001 | 572 (7.9%) | 28 (2.5%) | <0.001 |
| Peripheral Vascular Disease | 2,136 (0.8%) | 89 (1.1%) | 0.004 | 68 (0.9%) | 21 (1.9%) | 0.007 |
| Parkinson’s Disease | 1,775 (0.6%) | 64 (0.8%) | 0.211 | 51 (0.7%) | 13 (1.2%) | 0.145 |
| Cognitive Impairment | 1,511 (0.5%) | 78 (0.9%) | <0.001 | 66 (0.9%) | 12 (1.1%) | 0.719 |
| HIV or AIDS | 1,273 (0.5%) | 47 (0.6%) | 0.227 | 40 (0.6%) | 7 (0.6%) | 0.925 |
| Ascites | 123 (0.0%) | 18 (0.2%) | <0.001 | 12 (0.2%) | 6 (0.5%) | 0.032 |
| History of Organ Transplant | 829 (0.3%) | 37 (0.4%) | 0.029 | 32 (0.4%) | 5 (0.4%) | 1.000 |
| Congenital Disorders | 562 (0.2%) | 29 (0.3%) | 0.008 | 25 (0.3%) | 4 (0.4%) | 1.000 |
| Lupus | 608 (0.2%) | 25 (0.3%) | 0.176 | 21 (0.3%) | 4 (0.4%) | 0.925 |
| Cerebral Palsy | 251 (0.1%) | 12 (0.1%) | 0.176 | 9 (0.1%) | 3 (0.3%) | 0.447 |
| Spinal Cord Injury | 260 (0.1%) | 13 (0.2%) | 0.114 | 13 (0.2%) | 0 (0.0%) | 0.313 |
| Asthma | 2 (0.0%) | 1 (0.0%) | 0.161 | 1 (0.0%) | 0 (0.0%) | 1.000 |
| Chest Pain/Angina | 26 (0.0%) | 2 (0.0%) | 0.454 | 2 (0.0%) | 0 (0.0%) | 1.000 |
Comorbidities of patients with and without PC and FTR-P.
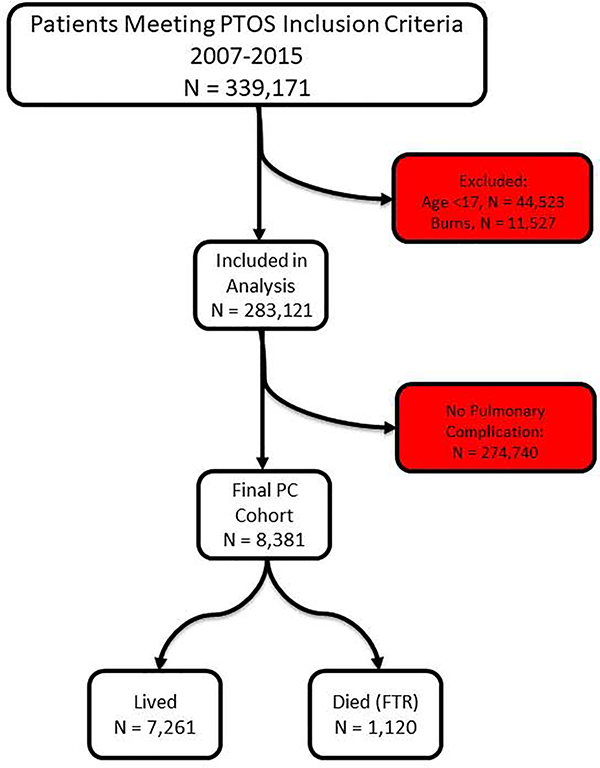
283,121 patients were captured in the study period. They were primarily white (80%), male (60%) and victims of blunt trauma (92%). A consort diagram of included patients can be seen in Figure 1. Further characteristics of our population including demographics, injury patterns and comorbidities can be seen in Tables 1 and 2. Rates of missingness ranged from <0.001% (age) to 9.5% (revised trauma score).
Figure 1:
Consort flow diagram of all inclusion and exclusion criteria.
There were 8,381 patients who developed PC, for an overall rate of 3.0% in the population (center level range 0.18–5.8%). The most frequent PC was pneumonia (3,470/8,381; 41% of PC), followed by acute respiratory failure (2,847/8,381, 34% of PC) and aspiration (927/8,381, 11% of PC). Of the patients who developed PC, 1,120 died for an overall FTR-P rate of 13.4% (center level range 0.0–22.6%).
Center Level Performance (Figures 2 and 3)
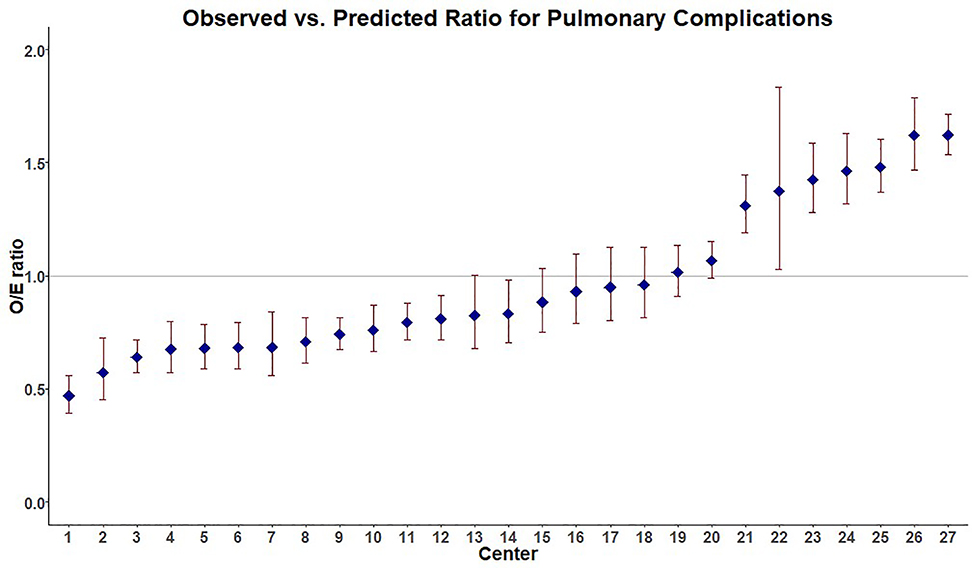
Figure 2.
Performance with regard to development of PC for individual Pennsylvania trauma centers showing 13/27 as high performers (HP, 95%CI for O:E ratio<1) and 7/27 as low performers (LP, 95%CI for O:E ratio>1). 3 centers were excluded due to small sample size.
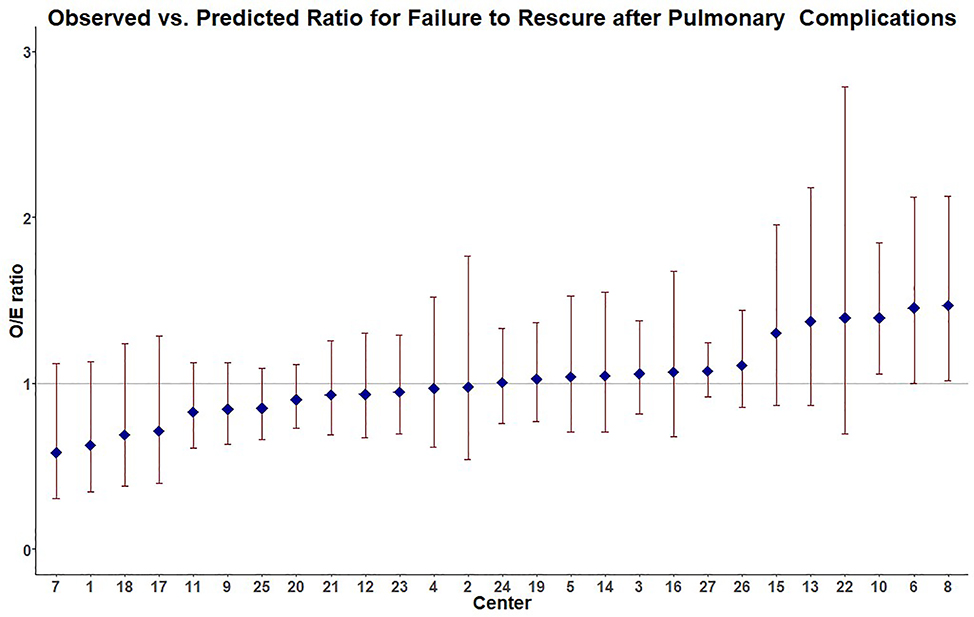
Figure 3:
Performance for FTR-P of individual Pennsylvania trauma centers showing 2/27 hospitals as low performers (LP, 95%CI for O:E ratio>1) and the remainder average performers with no higher performers (95% CI for O:E ratio<1) identified. 3 centers were excluded for low sample size.
In evaluating center level performance for PC while accounting for patient factors, 13/27 centers were high performers (HP, 95%CI for O:E ratio<1), 7/27 were (LP, 95%CI for O:E ratio>1) and the remaining 7/27 centers were average performers (AP, 95% CI for O:E ratio crossed 1). Our patient level model showed good discrimination (area under the curve (AUC) 0.81, 95% CI 0.80–0.81). Three centers were excluded for low enrollment. Center level performance for PC can be seen in Figure 2.
The same analysis was performed for center level performance with FTR-P while accounting for patient level factors. Our model again showed acceptable discrimination (area under the curve (AUC) 0.77, 95% CI 0.76–0.79). 2/27 centers were LP (95%CI for O:E ratio>1) and the remainder AP with no HP centers identified. The same 3 centers were excluded for low enrollment. Center level performance for FTR-P can be seen in Figure 3.
Comparison of Center Performance for PC and FTR-P (Figure 4)
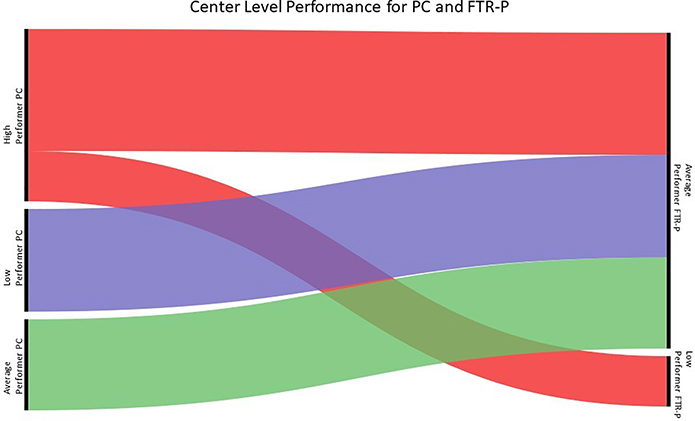
Figure 4:
There was little concordance for performance from PC to FTR-P. The majority of hospitals were AP for FTR-P and there were no HP centers. Both LP centers for FTR-P were HP for PC.
While HP, AP and LP were identified for PC, only AP and LP centers were identified for FTR-P. 11/13 HP centers for PC became AP centers for FTR-P and the other 2/13 HP centers for PC became the only LP centers for FTR-P. All 7 LP and 7 AP centers for PC were AP centers with respect to FTR-P.
Pulmonary Complications (Table 3, Appendix B1)
Table 3:
Multivariable model results for pulmonary complications.
| Variable | OR for PC | 95% CI | p | |
|---|---|---|---|---|
| Patient Level Factors | ||||
| Demographics | ||||
| Male (Ref) | ||||
| Female | 0.626 | 0.584 | 0.670 | <0.001 |
| White (Ref) | ||||
| Black | 0.842 | 0.760 | 0.934 | 0.001 |
| Asian | 0.864 | 0.673 | 1.111 | 0.254 |
| Other Race | 1.073 | 0.917 | 1.255 | 0.378 |
| Injury Patterns | ||||
| ISS | 1.066 | 1.058 | 1.074 | <0.001 |
| RTS | 0.888 | 0.857 | 0.921 | <0.001 |
| Penetrating Injury | 0.763 | 0.678 | 0.858 | <0.001 |
| Max AIS Chest | ||||
| 1 | 1.290 | 1.126 | 1.476 | <0.001 |
| 2 | 1.578 | 1.393 | 1.787 | <0.001 |
| 3 | 1.942 | 1.730 | 2.179 | <0.001 |
| 4 | 2.129 | 1.791 | 2.529 | <0.001 |
| 5 | 0.912 | 0.709 | 1.175 | 0.477 |
| 6 | 0.010 | 0.004 | 0.028 | <0.001 |
| Max AIS Head | ||||
| 1 | 1.156 | 1.048 | 1.275 | 0.004 |
| 2 | 0.971 | 0.887 | 1.064 | 0.530 |
| 3 | 1.405 | 1.282 | 1.540 | <0.001 |
| 4 | 1.135 | 0.982 | 1.313 | 0.087 |
| 5 | 1.333 | 1.018 | 1.746 | 0.037 |
| 6 | 0.008 | 0.002 | 0.034 | 0.000 |
| Comorbidities | ||||
| Cardiac Surgery | 1.083 | 0.979 | 1.197 | 0.121 |
| Coronary Artery Disease | 1.248 | 1.136 | 1.370 | <0.001 |
| Congestive Heart Failure | 1.312 | 1.149 | 1.498 | <0.001 |
| Myocardial Infarction | 1.137 | 1.019 | 1.269 | 0.022 |
| Hypertension | 1.550 | 0.743 | 3.232 | 0.243 |
| Peptic Ulcer Disease | 1.378 | 1.039 | 1.827 | 0.026 |
| Bariatric Surgery | 0.542 | 0.347 | 0.848 | 0.007 |
| Acquired Coagulopathy | 1.252 | 0.759 | 2.063 | 0.379 |
| Reversible Antithrombotic | 1.189 | 1.059 | 1.335 | 0.003 |
| Anemia | 1.205 | 1.005 | 1.446 | 0.044 |
| Antiplatelet Use | 0.884 | 0.785 | 0.994 | 0.040 |
| Psychiatric Condition | 1.068 | 1.019 | 1.120 | 0.006 |
| Intellectual Disability | 1.735 | 1.155 | 2.607 | 0.008 |
| HIV/AIDS | 1.354 | 0.967 | 1.894 | 0.077 |
| Transplant Recipient | 1.151 | 0.732 | 1.811 | 0.542 |
| Steroid Use | 1.633 | 1.404 | 1.898 | <0.001 |
| Arrival Bilirubin >2 | 1.201 | 0.582 | 2.479 | 0.619 |
| Cirrhosis | 1.438 | 1.217 | 1.700 | <0.001 |
| Metastatic Cancer | 1.216 | 1.001 | 1.477 | 0.048 |
| Arthritis | 0.954 | 0.847 | 1.075 | 0.437 |
| Lupus | 2.067 | 1.459 | 2.928 | <0.001 |
| Spinal Cord Injury | 1.968 | 0.957 | 4.047 | 0.066 |
| Seizures | 1.127 | 1.013 | 1.254 | 0.028 |
| Dementia | 1.005 | 0.829 | 1.218 | 0.962 |
| Parkinson’s | 1.377 | 1.096 | 1.731 | 0.006 |
| Cerebrovascular Accident | 1.351 | 1.205 | 1.514 | <0.001 |
| Cerebral Palsy | 2.301 | 1.467 | 3.608 | <0.001 |
| Obesity | 1.730 | 1.551 | 1.930 | <0.001 |
| Asthma | 0.007 | 0.001 | 0.045 | <0.001 |
| Arrival Creatinine >2 | 1.526 | 1.128 | 2.064 | 0.006 |
| Dialysis | 0.982 | 0.746 | 1.292 | 0.896 |
| Drug Abuse | 1.140 | 1.066 | 1.219 | <0.001 |
| Alcohol Abuse | 1.889 | 1.720 | 2.073 | <0.001 |
| Pregnancy | 0.411 | 0.157 | 1.079 | 0.071 |
| Thyroid Disease | 1.094 | 0.996 | 1.202 | 0.062 |
| Ascites | 3.511 | 1.980 | 6.225 | <0.001 |
| Current Smoker | 1.081 | 0.973 | 1.201 | 0.146 |
| Chest Pain | 3.977 | 0.793 | 19.944 | 0.093 |
| Peripheral Vascular Disease | 1.381 | 1.001 | 1.906 | 0.050 |
| Advanced Directive Order | 1.048 | 0.896 | 1.225 | 0.559 |
| Congenital Conditions | 1.408 | 0.994 | 1.994 | 0.054 |
| Hospital Level Factors | ||||
| Complexity/Technology | ||||
| Research Program | 1.242 | 0.905 | 1.704 | 0.180 |
| Cardiac Intensive Care Unit | 0.797 | 0.588 | 1.082 | 0.146 |
| Medical Surgical ICU Beds | 0.992 | 0.988 | 0.996 | <0.001 |
| Location | ||||
| Rank in 100 Largest Cities | 0.997 | 0.991 | 1.002 | 0.237 |
| Payor Status | ||||
| Physicians Salaried | 1.508 | 1.289 | 1.763 | <0.001 |
| Fee for Service Plan | 1.389 | 1.171 | 1.649 | <0.001 |
| HMO Hospital | 0.851 | 0.571 | 1.269 | 0.429 |
| Teaching Status | ||||
| Full Time Trainees | 1.010 | 1.006 | 1.015 | <0.001 |
| No Medical School Affiliation | 0.521 | 0.407 | 0.668 | <0.001 |
| No ACGME Programs | 1.087 | 0.819 | 1.442 | 0.564 |
| Staffing | ||||
| Full Time Respiratory Therapists | 1.006 | 0.999 | 1.014 | 0.097 |
| Full Time Registered Nurses | 0.998 | 0.997 | 0.999 | 0.001 |
| Full Time Licensed Practical Nurses | 0.995 | 0.992 | 0.998 | 0.003 |
| Volume | ||||
| Total Inpatient Days | 1.001 | 1.000 | 1.001 | 0.003 |
| Medical/Surgical Beds | 1.001 | 1.000 | 1.001 | 0.166 |
| Total Licensed Beds | 0.997 | 0.995 | 0.999 | 0.006 |
Multivariable model outputs for development of PC including patient demographics, injury patterns, comorbidities and structural variables of hospitals. Variables with significance <0.2 were considered for entry into this model. Abbreviations: ISS = Injury Severity Score; RTS = Revised Trauma Score, AIS =Abbreviated Injury Score, OR = Odds Ratio.
In multivariable logistic regression modelling including structural variables, we found that male sex, blunt injury mechanism, elevated injury severity scores (ISS), varying degrees of chest and head injury and a number of comorbidities increased the likelihood of PC (see Table 3 for a full listing of point estimates with 95% CI). The addition of structural variables only minimally improved our model discrimination for PC (AUC 0.82, 95% CI 0.81–0.82).
However, a salaried physician or indemnity fee for service payment model were associated with increased PC as were higher numbers of non-physician trainees and a higher volume of inpatient care. Higher numbers of medical and surgical ICU beds, not being associated with a medical school, higher numbers of registered and licensed practical nurses and increasing licensed bed numbers were associated with reduced PC.
Pulmonary Failure to Rescue (Table 4, Appendix B2)
Table 4:
Multivariable model outputs for failure to rescue from pulmonary complications.
| Variable | OR FTR-P | 95% CI | p | |
|---|---|---|---|---|
| Patient Level Factors | ||||
| Demographics | ||||
| Age | 1.035 | 1.029 | 1.042 | <0.001 |
| Male (Reference) | ||||
| Female | 0.988 | 0.850 | 1.149 | 0.875 |
| White (Reference) | ||||
| Black | 0.996 | 0.777 | 1.277 | 0.975 |
| Asian | 1.168 | 0.547 | 2.490 | 0.689 |
| Other Race | 1.240 | 0.761 | 2.020 | 0.388 |
| Injury Patterns | ||||
| ISS | 1.011 | 1.000 | 1.022 | 0.052 |
| RTS | 0.844 | 0.820 | 0.868 | <0.001 |
| Penetrating Injury | 1.445 | 0.880 | 2.372 | 0.146 |
| Max AIS Chest | ||||
| 1 | 1.272 | 0.915 | 1.768 | 0.152 |
| 2 | 0.730 | 0.506 | 1.053 | 0.092 |
| 3 | 0.848 | 0.721 | 0.998 | 0.047 |
| 4 | 0.834 | 0.642 | 1.084 | 0.175 |
| 5 | 0.589 | 0.288 | 1.204 | 0.147 |
| 6 | 0.606 | 0.056 | 6.558 | 0.680 |
| Max AIS Head | ||||
| 1 | 1.184 | 0.900 | 1.556 | 0.227 |
| 2 | 0.962 | 0.686 | 1.349 | 0.821 |
| 3 | 1.178 | 0.916 | 1.517 | 0.202 |
| 4 | 1.028 | 0.783 | 1.350 | 0.843 |
| 5 | 2.468 | 1.800 | 3.384 | <0.001 |
| 6 | 9.882 | 5.192 | 18.811 | <0.001 |
| Comorbidities | ||||
| Day of Complication | 0.988 | 0.961 | 1.017 | 0.422 |
| Cardiac Surgery | 0.782 | 0.620 | 0.985 | 0.037 |
| Coronary Artery Disease | 1.117 | 0.895 | 1.393 | 0.328 |
| Congestive Heart Failure | 1.373 | 0.964 | 1.956 | 0.079 |
| Myocardial Infarction | 1.012 | 0.716 | 1.431 | 0.945 |
| Hypertension | 1.011 | 0.810 | 1.264 | 0.920 |
| Reversible Antithrombotic | 1.411 | 1.105 | 1.802 | 0.006 |
| Anemia | 0.870 | 0.637 | 1.188 | 0.381 |
| Antiplatelet Use | 1.457 | 1.176 | 1.806 | 0.001 |
| Non-Reversible Antithrombotic | 1.296 | 0.944 | 1.780 | 0.109 |
| Attention Deficit Disorder | 1.216 | 0.470 | 3.144 | 0.686 |
| Diabetes | 1.112 | 0.903 | 1.370 | 0.317 |
| Steroid Use | 0.848 | 0.551 | 1.307 | 0.456 |
| Chemotherapy | 4.075 | 0.831 | 19.981 | 0.083 |
| Cirrhosis | 2.481 | 1.552 | 3.967 | <0.001 |
| Transplant Recipient | 1.256 | 0.741 | 2.128 | 0.397 |
| Metastatic Cancer | 1.418 | 0.980 | 2.052 | 0.064 |
| Arthritis | 1.304 | 0.860 | 1.979 | 0.212 |
| Alzheimer’s | 1.152 | 0.717 | 1.851 | 0.559 |
| Dementia | 1.638 | 1.157 | 2.318 | 0.005 |
| Parkinson’s | 1.059 | 0.485 | 2.308 | 0.886 |
| Cerebrovascular Accident | 0.976 | 0.789 | 1.208 | 0.825 |
| Respiratory Condition | 0.938 | 0.788 | 1.117 | 0.473 |
| Arrival Creatinine >2 | 2.352 | 1.354 | 4.085 | 0.002 |
| Dialysis | 1.737 | 0.911 | 3.309 | 0.093 |
| Drug Abuse | 0.737 | 0.509 | 1.067 | 0.107 |
| Alcohol Abuse | 0.605 | 0.464 | 0.790 | <0.001 |
| Thyroid Disease | 0.841 | 0.680 | 1.040 | 0.109 |
| Ascites | 1.432 | 0.417 | 4.919 | 0.569 |
| Smoker | 0.819 | 0.685 | 0.980 | 0.029 |
| Advanced Directive Order | 1.542 | 1.035 | 2.298 | 0.033 |
| Dependent on Care of Others | 1.202 | 0.782 | 1.848 | 0.401 |
| Peripheral Vascular Disease | 1.132 | 0.613 | 2.090 | 0.691 |
| Cardiopulmonary Resuscitation | 3.686 | 1.928 | 7.049 | <0.001 |
| Hospital Attributes | ||||
| Complexity/Technology | ||||
| Single Photon Emission Computerized Tomography | 0.833 | 0.532 | 1.303 | 0.423 |
| Stereotactic Radiology Capabilities | 1.096 | 0.971 | 1.237 | 0.139 |
| Research Program | 0.534 | 0.437 | 0.654 | <0.001 |
| Payor Status | ||||
| Part of HMO Hospital System | 0.994 | 0.896 | 1.104 | 0.916 |
| Management Service Organization | 1.639 | 1.367 | 1.966 | <0.001 |
| HMO Hospital | 0.668 | 0.501 | 0.891 | 0.006 |
| Staffing | ||||
| Employs Advanced Practice Providers | 0.714 | 0.648 | 0.786 | <0.001 |
| Employs Nursing Home Personnel | 1.000 | 0.997 | 1.003 | 0.755 |
| Open Physician Staffing Model | 0.929 | 0.785 | 1.099 | 0.388 |
| Teaching Status | ||||
| No Medical School Affiliation | 0.518 | 0.449 | 0.599 | <0.001 |
| Full Time Trainees | 1.000 | 0.999 | 1.002 | 0.848 |
| No Nursing School | 1.017 | 0.889 | 1.163 | 0.804 |
| Volume | ||||
| 200–299 Beds | 0.970 | 0.804 | 1.170 | 0.751 |
| 300–399 Beds | 0.769 | 0.665 | 0.889 | <0.001 |
| 400–599 Beds | 0.721 | 0.671 | 0.776 | <0.001 |
| Total Number of Beds | 0.999 | 0.999 | 0.999 | <0.001 |
Multivariable outputs for FTR-P including patient demographics, injury patterns, comorbidities and structural variables of hospitals. Variables with significance <0.2 were considered for entry into this model. Abbreviations: ISS = Injury Severity Score; RTS = Revised Trauma Score, AIS =Abbreviated Injury Score, OR = Odds Ratio.
In multivariable logistic regression modeling for FTR-P, advanced age was the only demographic variable significantly associated with FTR. A higher RTS (improved physiology) was associated with reduced FTR-P while the most severe head injuries (AIS 5 and 6) were associated with increased FTR-P. With regard to comorbidities, a history of cardiac surgery, alcohol use and smoking were associated with reduced FTR-P while antithrombotic use, cirrhosis, dementia, elevated creatinine, an active advanced directive order and prehospital CPR were associated with increased FTR-P. See Table 4 for a full listing of point estimates with 95% CI. The addition of structural variables only minimally improved our model discrimination for FTR-P, as was the case in our PC model (AUC 0.77, 95% CI 0.76–0.79).
In evaluation of structural characteristics, a management service organization was correlated to increased FTR-P while the presence of research programs, employment of advance practice providers, the lack of medical school affiliation, increased number of hospital beds and the presence of a research program were all associated with reduced FTR-P.
Discussion
Utilizing our state trauma registry, we were able to identify high, average and low performing trauma centers in Pennsylvania for PC and average and low performers for FTR-P. In unveiling such differences, we have demonstrated a reproducible means for comparing trauma center outcomes and enhancing quality improvement efforts. Although we identified specific hospital attributes associated with both PC and FTR-P, they contributed very little to the discriminatory ability of our models for PC or FTR-P. In completing this study, we add to our prior work and show that center level performance can be readily identified using registry datasets for multiple specific complications.
One of our most significant findings echoed our prior study on cardiac complications (CC) and FTR from cardiac complications (FTR-C) after injury in the same population; centers that perform well for PC are not necessarily high performers for FTR-P.18 In other words, the hospitals that may be successful in limiting the occurrence of complications may not be the same hospitals that successfully rescue patients from complications once they occur. Indeed, in review of our past work we note that Pennsylvania trauma centers performing well for CC, FTR-C, PC and FTR-P over the same time frame were discordant, suggesting that factors driving performance for one complication and its FTR rate may be different from one another.18 This may be a product of differing patient populations suffering certain adverse events or different disease severities. However, this may also be from yet to be discovered differences in processes of care, such as the availability of disease specific entity like a lung rescue program, from center to center.25
Importantly, our findings suggest a weakness in both specific and omnibus measurement of FTR. Study of global FTR rates may be skewed by the most common or most lethal adverse events while hiding successful, or unsuccessful, practices in some complications. Should a center perform well in one complication, a measure of only that FTR rate could obscure significant weaknesses in another area. Similarly, proficiency in the most common complications in an omnibus FTR measurement would indicate high overall performance while ignoring other potentially significant areas for improvement. Most known factors associated with FTR reduction, including those that we studied, might be expected to improve FTR from diverse complications but that was not what occurred here when comparing our CC and FTR-C data.18 This suggests additional variables exist that require more study for detection.
By incorporating hospital level structural factors in our modeling, we attempted to shine a light on these additional factors. FTR is known to be associated with center level attributes and study has shown that FTR rates are particularly impacted by factors relating to hospital complexity and technology, staffing, teaching status and payment models.1–3,25,26 Specifically, past work has demonstrated that being a teaching hospital, being high volume and having high rates of insured patients reduced FTR.4, 27–29 We echoed this methodology and incorporated hospital location as an additional metric. We found a number of significant variables for both PC and FTR-P, many of which were similar to those found for cardiac complications (CC) and FTR from them (FTR-C) in study of the same centers.18
Hospitals with more ICU beds and higher rates of full time nurses had lower rates of PC, re-demonstrating past findings from other authors but conflicting with findings for cardiac complications (CC) in the same trauma center cohort. Larger hospitals had both lower CC and PC.18 Not being affiliated with a medical school was associated with lower PC and FTR-P, although other factors relating to being a teaching hospital were insignificant and, curiously, having a research program – which is a frequent hallmark of an academic medical center – was associated with a reduction in FTR-P. The reason for this is elusive as many hospitals without a direct medical school affiliation may still be teaching hospitals and may have research programs. While many of these factors may not be easily modifiable, others certainly are. It is possible that bed numbers, minimum hospital volume, nurse staffing levels and research programs may become mandated criteria for trauma center accreditation in America. Ultimately, our identified structural variables added little to our model performance as compared to patient level data – a significant finding that may be a stumbling block that trauma related FTR is prone to.
It is quite likely that the homogenous nature of trauma populations - and indeed the trauma centers themselves – may limit variation and cause less striking differences within structural variables than past non-trauma research on FTR has identified.18 This may also be why many of our significant factors for both PC and FTR-P related to the injury severity and presenting physiology of the patients themselves. The fact that addition of structural variables had minimal effect on our multivariable model suggests that development of PC and death from it may be much more heavily reliant on the trauma patient and their injury than the institution they present to. Trauma center homogeneity, as demanded by rigid accreditation standards, may also be a reason that no high performers were identified for FTR-P. The contributions of injury severity and physiology are logical as the injury itself would be a driving force of the complication and mortality outcomes, perhaps irrespective of the similar care they may receive at any of the state’s trauma centers. Additionally, many key structural differences that may impact the outcomes of specific complications, such as the presence of a lung rescue program impacting pulmonary complications, are outside the scope of our dataset but may also explain the variability in FTR-P.24
It is our hope that our methodology and findings give trauma centers across the country a road map to compare their practices and identify these other factors, many of which may be subtle. We anticipate that after delineating high and low performers, direct hospital to hospital comparison of best practices will illuminate structural differences impacting care that may be outside the scope of database study. Such factors may be less tangible than lung rescue programs, less studied than nurse staffing and more modifiable than payment models and location. In particular, hospital level initiatives, culture and protocols would often elude database capture. These could include protocols for intubation, respiratory therapy interventions, ventilator bundles and airway emergency teams.
Limitations
Utilization of a large dataset such as the PTOS database is critical to evaluating trauma centers across the state, but it is not without disadvantages. For instance, while we are able to account for the presence or absence of a given comorbidity, there is no information in the dataset that allows us parse the severity of these conditions. However, unless patients with more severe comorbid conditions or complications were clustered at specific hospitals this lack of severity measurement would be unlikely to influence our findings. Second, our definition of what constitutes a ‘pulmonary’ complication is somewhat arbitrary and may group together conditions with different causes and pathophysiology together. However, on some level any grouping of complications would be subject to the same critique. We believe that although imperfect, our definition of pulmonary complications based on their manifestations and treatments is sound. Finally, while we successfully identified outlier centers and some structural variables, we are ultimately unable to determine why each variable was significant. Additional structural datapoints thought to be important to FTR rates including safety culture, rapid intervention programs and early care escalation are not readily measured with database level study.30–35 Alternatively, FTR may not always be able to be predicted, no matter the number of variables at our disposal. More detailed study prospective study of the process of care in place out outlier centers will be needed.
Conclusions
High and low performing trauma centers for multiple specific complications can be identified using state registry data, but centers that perform well or perform poorly for PC are not the same as those for FTR-P nor for other singular adverse events. Given that we have identified such variable performance within different complications for the same centers in a homogenous trauma system, evaluation of multiple individual complications and their contributions to FTR may yield more actionable data than the omnibus sum of all parts in trauma related FTR research. Additional study, ideally prospective, is needed to determine why such variation exists in an effort to improve care. We encourage identification of failure to rescue performance and best practice comparison at local and state meetings to improve processes of care affecting our admitted trauma patients.
Supplementary Material
Appendix B1: ROC of our final multivariable model including structural variables. Structural variables minimally improved our model performance (AUC 0.82, 95% CI 0.81–0.82)
Appendix B2: ROC of our final multivariable model for FTR-P including structural variables. Structural variables minimally improved our model performance (AUC 0.77, 95% CI 0.76–0.79).
Acknowledgements
Funding/Support
This work was funded by a grant from the National Heart, Lung and Blood Institute (grant #K08HL131995).
Footnotes
COI/Disclosure
No author has a conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silber JH, Williams S V, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care 1992;30:615–9. [DOI] [PubMed] [Google Scholar]
- 2.Silber JH, Arriaga AF, Niknam BA, Hill A, Ross A, Romano PS. Failure-to-Rescue after Acute Myocardial Infarction. Med Care 2018;56:416–23. [DOI] [PubMed] [Google Scholar]
- 3.Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg 2010;211:325–30. [DOI] [PubMed] [Google Scholar]
- 4.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med 2009;361:1368–75. [DOI] [PubMed] [Google Scholar]
- 5.Almoudaris AM, Burns EM, Mamidanna R, Bottla A, Aylin P, Vincent C, et al. Value of failure to rescue as a marker of the standard of care following reoperation for complications after colorectal resection. Br J Surg 2011;98:1775–83. [DOI] [PubMed] [Google Scholar]
- 6.Scarborough JE, Pappas TN, Bennett KM, Lagoo-Deenadayalan S. Failure-to-pursue rescue: explaining excess mortality in elderly emergency general surgical patients with preexisting “do-not-resuscitate” orders. Ann Surg 2012;256:453–61. [DOI] [PubMed] [Google Scholar]
- 7.Mehta A, Efron DT, Stevens K, Manukyan M, Joseph B, Sakran J. Hospital variation in mortality after emergent bowel resections. J Trauma Acute Care Surg 2018;84:702–10. [DOI] [PubMed] [Google Scholar]
- 8.Khan M, Azim A, O’Keeffe T, Jehan F, Kulvatunyou N, Santino C, et al. Geriatric rescue after surgery (GRAS) score to predict failure-to-rescue in geriatric emergency general surgery patients. Am J Surg 2018;215:53–7. [DOI] [PubMed] [Google Scholar]
- 9.Glance LG, Dick AW, Meredith JW, Mukamel DB. Variation in hospital complication rates and failure-to-rescue for trauma patients. Ann Surg 2011;253:811–6 [DOI] [PubMed] [Google Scholar]
- 10.Haas B, Gomez D, Hemmila MR, Nathens AB. P Haas B, Gomez D, et al. Prevention of complications and successful rescue of patients with serious complications: characteristics of high-performing trauma centers. J Trauma 2011;70:575–82. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman EJ, Earl-Royal E, Barie PS, Holena DN. Failure to Rescue after Infectious Complications in a Statewide Trauma System. Surg Infect 2016; 18: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holena DN, Earl-Royal E, Delgado MK, Sims CA, Pascual JL, Hsu J et al. Failure to rescue in trauma: Coming to terms with the second term. Injury 2015;47:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holena DN, Kaufman EJ, Delgado MK, Wiebe DJ, Carr B, Christie J, et al. A metric of our own: Failure to rescue after trauma. J Trauma Acute Care Surg 2017;83:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph B, Zangbar B, Khalil M, Kulvatunyou N, Haider A, O’Keeffe T, et al. Factors associated with failure-to-rescue in patients undergoing trauma laparotomy. Surgery 2015;158:393–8. [DOI] [PubMed] [Google Scholar]
- 15.Kuo LE, Kaufman E, Hoffman RL, Pascual JL, Martin ND, Kelz R, et al. Failure-to-rescue after injury is associated with preventability: The results of mortality panel review of failure-to-rescue cases in trauma. Surgery 2017;161:782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coates M, Pillado E, Kim J, Vasak R, Yule A, Kim D. Role of Preventability in Redefining Failure to Rescue Among Major Trauma Patients. JAMA Surg 2017;152:1083–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silber JH, Romano PS, Rosen AK, Wang Y, Even-Shoshan O, Volpp KG. Failure-to-rescue: comparing definitions to measure quality of care. Med Care 2007;45:918–25. [DOI] [PubMed] [Google Scholar]
- 18.Scantling D, Hatchimonji J, Kaufman EJ, Xiong A, Yang P, Christie JD, et al. Cardiac Complications and Failure to Rescue After Injury in a Mature State Trauma System: Towards Identifying Opportunities for Improvement. Injury 2020; 51: 1216–23. [DOI] [PubMed] [Google Scholar]
- 19.Chung JJ, Earl-Royal EC, Delgado MK, Pascual JL, Reilly PM, Wiebe DL, et al. Where we fail: Location and timing of failure to rescue in trauma. Am Surg 2017;83:250–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Sharoky CE, Martin ND, Smith BP, Pascual JL, Kaplan LJ, Reilly PM, et al. The Location and Timing of Failure-to-Rescue Events Across a Statewide Trauma System. J Surg Res 2019;235:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newgard CD. The validity of using multiple imputation for missing out-of-hospital data in a state trauma registry. Acad Emerg Med 2006;13:314–24. [DOI] [PubMed] [Google Scholar]
- 22.Royston P, White I. Multiple Imputation by Chained Equations (MICE): Implementation in Stata . J Stat Softw 2015; 45: 1–20. [Google Scholar]
- 23.Rothman Rothman KJ Greenland S. Modern epidemiology 2nd ed. Philadelphia (PA) Lippincott-Raven Publishers; 1998. [Google Scholar]
- 24.American Hospital Association. American Hospital Association annual survey database. www.ahadata.com/aha-annual-survey-database-asdb/ Accessed May 3, 2020.
- 25.Menaker J, Dolly K, Rector R, Kufera J, Lee E, Tabatabai A, et al. The lung rescue unit — Does a dedicated intensive care unit for venovenous extracorporeal membrane oxygenation improve survival to discharge? J Trauma Acute Care Surg 2017;83: 428–42. [DOI] [PubMed] [Google Scholar]
- 26.Hatchimonji JS, Kaufman EJ, Sharoky CE, Ma L, AG Garcia-Whitlock, Holena DN. Failure to rescue in surgical patients : A review for acute care surgeons. J Trauma Acute Care Surg 2019;87:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheetz KH, Dimick JB, Ghaferi AA. Impact of hospital characteristics on failure to rescue following major surgery. Ann Surg 2016;263:692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finlayson SRG, Weissman JS, Hevelone ND, Maine R, Swain J, Lipsitz S, et al. Failure to Rescue in Safety-Net Hospitals. JAMA Surg 2014;149:229–35. [DOI] [PubMed] [Google Scholar]
- 29.Bell TM, Zarzaur BL. Insurance status is a predictor of failure to rescue in trauma patients at both safety net and non-safety net hospitals. J Trauma Acute Care Surg 2013;75:728–33. [DOI] [PubMed] [Google Scholar]
- 30.Taenzer AH, Pyke JB, McGrath SP. A review of current and emerging approaches to address failure-to-rescue. Anesthesiology 2011;115:421–31. [DOI] [PubMed] [Google Scholar]
- 31.Johnston MJ, Arora S, King D, Bouras G, Almoudaris A, Davis R, et al. A systematic review to identify the factors that affect failure to rescue and escalation of care in surgery. Surgery 2015;157:752–63. [DOI] [PubMed] [Google Scholar]
- 32.Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques T, Norman SL, et al. Antecedents to hospital deaths. Intern Med J 2001;31:343–8. [DOI] [PubMed] [Google Scholar]
- 33.Schein RM, Hazday N, Pena M, Ruben B, Sprung C. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest 1990;98:1388–92. [DOI] [PubMed] [Google Scholar]
- 34.MMcQuillan P, Pilkington S, Allan A, Taylor B, Short A, Morgan G, et al. Confidential inquiry into quality of care before admission to intensive care. BMJ 1998;316:1853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buist M, Bernard S, Nguyen T, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation 2004;62:137–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix B1: ROC of our final multivariable model including structural variables. Structural variables minimally improved our model performance (AUC 0.82, 95% CI 0.81–0.82)
Appendix B2: ROC of our final multivariable model for FTR-P including structural variables. Structural variables minimally improved our model performance (AUC 0.77, 95% CI 0.76–0.79).