Abstract
Background
Neoadjuvant chemotherapy (NAC) is increasingly used for cN+ tumors with intact primary breast cancer (IPBC) to downstage the axilla, and those who convert to cN0 may be eligible for sentinel lymph node biopsy (SLNB). Rates of axillary downstaging in occult primary breast cancer (OPBC) are unknown. The aim of this study was to determine the frequency of nodal pCR following NAC in a cohort of patients with OPBC.
Methods
Twenty-eight patients with stage II/III OPBC treated between January 2008 and December 2019 were identified. Twenty had cN1–3 OPBC, pretreatment lymph node needle biopsy, and received NAC; these constituted the study population. Treatment factors and nodal pathologic complete response (pCR) rates were summarized by tumor subtype.
Results
Median age at diagnosis was 54 years. Most presented with cN1 (75%) disease and ductal histology (80%). Nodal pCR was seen in 16/20 (80%) patients. Eight (40%) were triple negative, 6 (30%) ER+/HER2- and 6 (30%) HER2+, with pCR rates of 88%, 50%, and 100%, respectively. Among the 15 patients who presented as cN1, 14 (93%) converted to cN0 following NAC. Of these, 9 underwent SLNB and all achieved nodal pCR (100%).
Conclusion
In this small series, 80% of OPBC patients achieved nodal pCR following NAC. pCR rates varied by receptor profile, being lowest in the ER+/HER2- and highest in the HER2+ (50–100%); however, they are excellent and numerically exceed those in the literature for IPBC. Given the pCR rate, SLNB may be an option in select OPBC patients who downstage following NAC.
Keywords: occult primary breast cancer, neoadjuvant chemotherapy, axilla, downstaging, axillary lymph node dissection, sentinel lymph node biopsy, breast cancer
Occult primary breast cancer (OPBC) involves axillary lymphadenopathy that is histologically consistent with metastatic breast cancer, although no primary breast lesion can be identified despite complete clinical and radiographic assessment. OPBC accounts for < 1% of all newly diagnosed breast cancers,1–6 and can remain a diagnostic dilemma and therapeutic challenge to those practitioners who encounter this rare entity. Recommendations for the management of patients with OPBC are generally based on retrospective studies involving small numbers of patients, lending these to inherent heterogenicity in both work-up and treatment. Despite these limitations, the local management of OPBC has evolved tremendously, from a modified radical mastectomy (MRM) to a breast-conserving approach, consisting of an axillary lymph node dissection (ALND) followed by whole-breast radiation therapy (WBRT). Due to the rarity of OPBC, the practice patterns for this disease continue to vary amongst practitioners. According to the 2020 National Comprehensive Cancer Network (NCCN) guidelines, options for OPBC include mastectomy plus ALND, or ALND plus WBRT with or without nodal irradiation. Systemic chemotherapy, endocrine therapy, and targeted anti-human epidermal growth factor 2 (HER2) therapy are given according to standard recommendations for stage II or III disease based on the tumor receptor profile.7
For women with an intact primary tumor and node-positive disease, neoadjuvant chemotherapy (NAC) is used with increasing frequency in attempts to downstage both the breast and the axilla. The utilization of NAC can potentially allow for a less-morbid operation, with growing use seen among node-positive patients in attempts to forgo an ALND and the associated lymphedema risk. While NAC is associated with the possibility of axillary downstaging, rates of nodal pathologic complete response (pCR) differ substantially by tumor subtype,8–10 and, therefore, the likelihood of minimizing the extent of axillary surgery remains heavily affected by tumor biology. The highest rates of nodal pCR are seen among HER2 positive (HER2+) tumors, and the lowest rates are seen among estrogen receptor positive (ER+)/ progesterone receptor positive (PR+), HER2 negative (HER2-) tumors.8,10–13 Multiple prospective trials have assessed the feasibility and accuracy of sentinel lymph node biopsy (SLNB) following NAC among node-positive patients.11,14–16 These trials bolstered interest in downstaging axillary surgery among women with clinically node-positive (cN+) disease who convert to clinically node negative (cN0) following NAC and obtain a nodal pCR. However, the trials assessing feasibility have been performed among women with an intact primary tumor, and, therefore, it is unknown whether similar rates of nodal pCR are seen among the OPBC population. The aim of this study was to determine the frequency of nodal pCR and, therefore, potential omission of ALND following NAC in a cohort of patients with OPBC.
METHODS
Following Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB) approval, women with OPBC were identified from a prospectively maintained surgical database. Twenty-eight patients with biopsy-proven cN+ stage II/III OPBC treated between January 2008 and December 2019 were identified. The electronic medical record was accessed, and the relevant data abstracted. Clinicopathologic characteristics, including age and method of diagnosis, histology, type of axillary surgery, number of lymph nodes removed, presence of nodal treatment effect, type of breast surgery, receptor profile, receipt of radiation, type of chemotherapy, and use of adjuvant hormone therapy were recorded. Clinical nodal status was identified on physical exam, radiographic imaging, and confirmatory tissue sampling with ultrasound-guided core needle biopsy (CNB) or fine-needle aspiration (FNA). All patients had a negative mammogram (MMG), ultrasound, and magnetic resonance imaging (MRI) to evaluate for the presence of a primary breast lesion. Most patients (70%) underwent positron emission tomography (PET) scan to identify a primary tumor site other than the breast or sites of distant metastasis. In order to preserve the purity of the OPBC diagnosis, patients with a history of ipsilateral or contralateral breast cancer were excluded.
Eight of the 28 patients were excluded from analysis; 5 did not receive NAC and 3 underwent lymph node excisional biopsy. The remaining 20 patients presented with nonmetastatic cN1–3 OPBC, had pretreatment lymph node needle biopsy, and received NAC; these patients constituted the study population. The NAC regimens included doxorubicin, cyclophosphamide followed by paclitaxel (AC-T); cyclophosphamide with methotrexate and fluorouracil (CMF); or carboplatin and Taxol (TC). Rates of nodal pCR were compared by tumor subtype broken down as ER+, HER2- disease (ER+/HER2-), HER2+ disease, and triple negative breast cancer (TNBC). Due to small numbers, all HER2+ patients were combined into one HER2+ group regardless of hormone receptor status. Estrogen receptor positivity was calculated as > 1%, in accordance to the American Society of Clinical Oncology/College of American Pathologists guidelines.17,18
The final axillary surgery recommendations were at the discretion of the treating physician following completion of chemotherapy. At our institution, when an SLNB is performed following NAC, all women are mapped with dual tracer using technetium-99m sulfur colloid and isosulfan blue dye. Grossly positive nodes identified intraoperatively are also considered sentinel nodes. The indications for ALND after SNLB following NAC include any residual nodal metastasis, including micrometastasis (≤ 2 mm) as well as isolated tumor cells (ITCs), or retrieval of < 3 negative sentinel lymph nodes. Routine placement of biopsy clips in nodes was not utilized. Women who convert from cN1 to cN0 are considered eligible for an optimized SLNB and possible ALND.
Continuous variables were summarized using mean (min, max), whereas categorical variables were summarized using number and percentage. Patient outcomes, including the last known alive date, the occurrence of ipsilateral or contralateral breast events, regional or distant metastasis, and survival were collected. Follow-up time was calculated from the date of definitive operation to the last known alive date.
RESULTS
Twenty eligible OPBC patients were identified, and their clinicopathologic features are shown in Table 1. The median age at diagnosis was 54 years (interquartile range [IQR] 46, 62) and all patients were female. Most (75%) presented with cN1 disease and were of ductal histology (80%). Although tumor biopsy demonstrated carcinoma consistent with breast origin, tumor specific histology was unavailable for 3 (15%) patients, and these were labeled as undefined. The tumor subtypes were as follows: 8 (40%) were triple negative, 6 (30%) were ER+/HER2-, and 6 (30%) were HER2+.
TABLE 1.
Clinicopathologic Features at Presentation of OPBC Patients Treated with NAC
| Clinicopathological Features (n = 20) | Total Group n (%) |
|---|---|
| Age, years, median (IQR) | 54 (46, 62) |
| cN status | |
| cN1 | 15 (75) |
| cN2 | 4 (20) |
| cN3 | 1 (5) |
| Histology | |
| Ductal | 16 (80) |
| Lobular | 1 (5) |
| Undefined | 3 (15) |
| Receptor Profile | |
| ER+/HER2- | 6 (30) |
| HER2+ | 6 (30) |
| TNBC | 8 (40) |
OPBC occult primary breast cancer, NAC neoadjuvant chemotherapy, cN clinical nodal stage, cN1 clinically N1, cN2 clinically N2, cN3 clinically N3, ER estrogen receptor, HER2 human epidermal growth factor receptor 2, TNBC triple negative breast cancer, IQR interquartile range
Table 2 shows the different NAC treatment regimens employed. Most patients received AC-T (90%), and the remaining 10% received either CMF or TC combination chemotherapy. All women with HER2+ tumors undergoing NAC received dual anti-HER2 therapy with trastuzumab and pertuzumab. Seventeen (85%) patients either did not undergo any breast surgery or underwent an excision of a benign lesion. Nine (45%) of the 20 patients underwent SLNB alone, and the remaining 11 (55%) underwent ALND as their definitive axillary surgery. Seventeen (85%) completed either WBRT or postmastectomy radiation therapy (PMRT) with regional nodal irradiation as of January 2020. Of the 3 patients who did not undergo breast, chest wall or regional nodal irradiation, 2 underwent mastectomy and nodal surgery with a pCR, and 1 patient had an ALND alone but declined any additional adjuvant therapy.
TABLE 2.
Neoadjuvant and Adjuvant Treatment of OPBC Patients
| Treatment | n (%) |
|---|---|
| Axillary Surgery | |
| SLNB Alone | 9 (45) |
| ALND | 11 (55) |
| Breast Treatment | |
| No breast surgery/benign excision | 17 (85) |
| Mastectomy | 3 (15) |
| Radiation Therapy | |
| RT Breast/CW + RNI | 17 (85) |
| No additional radiotherapy | 3 (15) |
| Chemotherapy Regimen | |
| ACT | 18 (90) |
| TC | 1 (5) |
| CMF | 1 (5) |
| Dual Anti-HER2 Therapy* | 6/6 (100) |
| Endocrine therapy | 8/8 (100) |
All HER2 positive patients received trastuzumab and pertuzumab
SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection, ACT doxorubicin (A) and cyclophosphamide (C) followed by paclitaxel (T), TC paclitaxel, carboplatin, CMF cyclophosphamide (C) with methotrexate (M) and fluorouracil (F), RT Breast/CW radiation therapy breast/ chest wall, RNI regional nodal irradiation
Nodal pCR was observed in 16 of the 20 patients (80%) in this cohort. Among the 15 patients who presented with cN1 disease, 14 (93%) converted to cN0 following NAC. Of these, 9 underwent an SLNB alone; all of whom achieved a nodal pCR (100%). An average of 4.67 (range 3–10) sentinel lymph nodes (SLNs) were removed at SLNB. All women presenting with cN2-cN3 disease (n = 5) underwent an ALND; 3 remained pathologically node positive (pN+) and 2 achieved nodal pCR. Table 3 shows pCR rates by receptor profile: 3/6 (50%) ER+/HER2-, 7/8 (88%) TNBC, and 6/6 (100%) HER2+. Fig. 1 and Fig. 2 illustrate response based on receptor subtype and axillary management by receptor subtype, respectively. Treatment effect was noted in 17 of the 20 OPBC patients: 10 of 11 women undergoing ALND, and 7 of 9 women undergoing SLNB.
TABLE 3.
Nodal Pathologic Complete Response Stratified by Receptor Profile for OPBC in Entire Cohort
| Nodal Status at Diagnosis n (%) | n = 20 | pCR (n = 16) | ypN+ (n = 4) |
|---|---|---|---|
| cN1 | 15 (75) | 14 (93) | 1 (7) |
| cN2 | 4 (20) | 2 (50) | 2 (50) |
| cN3 | 1 (5) | 0 (0) | 1 (100) |
| Receptor Profile, n (%) | |||
| ER+/HER2- | 6 (30) | 3 (50) | 3 (50) |
| HER2+ | 6 (30) | 6 (100) | 0 (0) |
| Triple negative | 8 (40) | 7 (88) | 1 (12) |
OPBC occult primary breast cancer, NAC neoadjuvant chemotherapy, pCR complete pathologic response after neoadjuvant chemotherapy, ypN+ pathologically node positive after neoadjuvant chemotherapy, ER estrogen receptor, HER2 human epidermal growth factor receptor 2
Fig. 1. Response based on subtype.
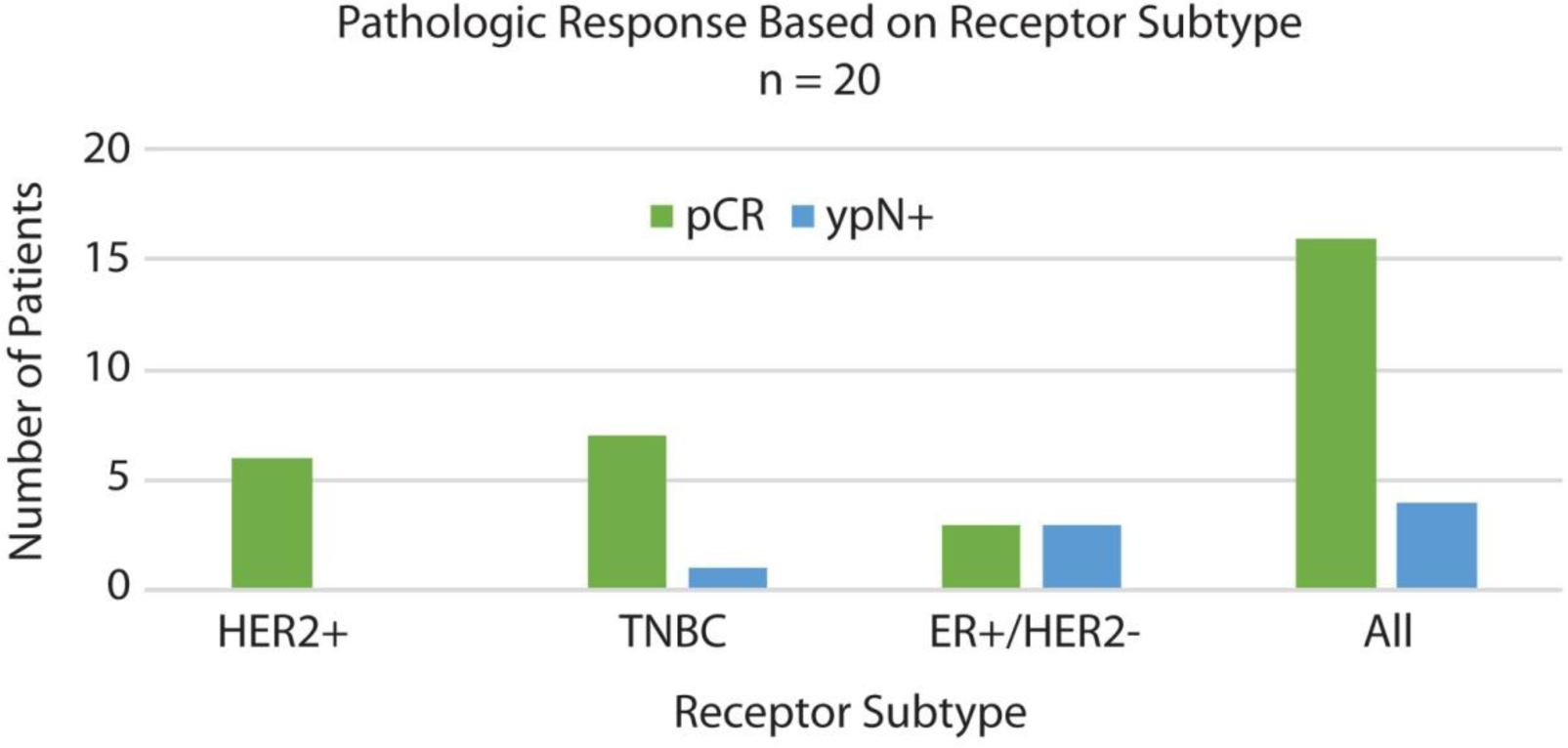
HER2 human epidermal growth factor receptor 2, TNBC triple negative breast cancer, ER estrogen receptor, pCR complete pathologic response after neoadjuvant chemotherapy, ypN+ pathologically node positive after neoadjuvant chemotherapy
Fig. 2. Axillary management by receptor subtype.
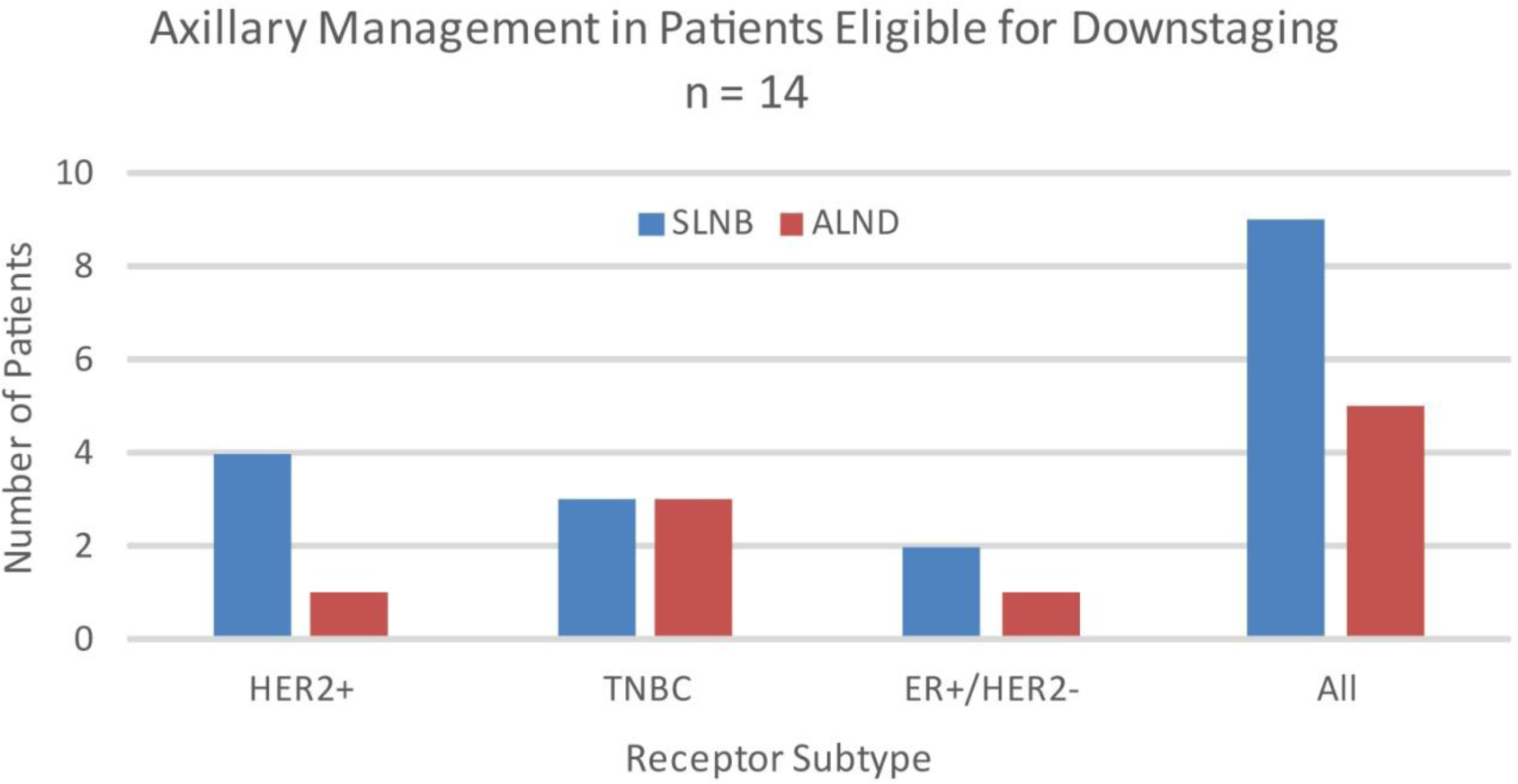
HER2 human epidermal growth factor receptor 2, TNBC triple negative breast cancer, ER estrogen receptor, SNLB sentinel lymph node biopsy, ALND axillary lymph node dissection
Nineteen of the 20 patients in this cohort are still alive at the time of last follow-up. The etiology of the 1 death is unknown. With a median follow up of 38.5 months (range 4.3–127.5), there have been no locoregional or distant recurrences, with 17 of the 20 patients having follow-up > 16.6 months.
DISCUSSION
The management of OPBC has evolved tremendously, although there continues to be variation in practice patterns when clinicians are faced with this rare diagnosis. Initial reports encouraging MRM in OPBC patients were partially due to the high rates (55–92%) of finding primary carcinoma within the pathological specimens, which justified the widespread use of mastectomy.6 However, these studies predated the use of MRI, which has become part of the standard work up for breast cancer patients presenting with axillary metastasis and no identifiable clinical or radiographic lesion utilizing traditional methods.19 A 2016 meta-analysis including 241 OPBC patients, of whom less than half underwent preoperative MRI, compared outcomes of those treated with ALND plus mastectomy versus ALND and WBRT, and found equivocal rates of local control and overall survival.20 A more contemporary Surveillance, Epidemiology and End Results (SEER) database review including 479 patients showed no difference in survival among the OPBC patients who underwent mastectomy with ALND with or without radiation therapy versus the patients who underwent ALND with radiation therapy.21 Though data now exist to support a less-morbid surgical approach to the breast, comparable evidence regarding the potential de-escalation of axillary surgery after NAC in this population has been limited.
The implementation of NAC to achieve nodal pCR and therefore potentially change axillary surgery is increasingly utilized among node-positive patients. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials were randomized controlled trials comparing adjuvant chemotherapy to NAC. Both reported significantly lower rates of nodal positivity following NAC compared to those undergoing upfront surgery, emphasizing the ability of NAC to downstage axillary disease.22–24 This concept has been broadened to potentially alter surgical management among those women achieving a nodal pCR following completion of NAC.23,24 Four prospective studies have since assessed the accuracy and feasibility of SLNB following NAC among women who presented with node-positive disease.11,14–16 These trials initially reported overall unacceptably high false-negative rates, although with use of dual-tracer mapping and retrieval of 3 or more SLNs, the false-negative rate of an SLNB in this population fell to clinically acceptable rates of < 10%.11,14–16 The 2020 NCCN guidelines support use of SLNB alone among women who convert from cN1 to cN0 and achieve a nodal pCR,7 and, in clinical practice, there is increasing use of NAC to attempt axillary surgery downstaging among this population. Though these guidelines apply to patients with known intact primary tumors, recent evidence suggests that this paradigm may be shifting into the OPBC setting as well. In 2020, Cohen et al reported on 684 OPBC patients, treated between 2004–2014 from the National Cancer Data Base (NCDB), in whom 214 (31%) patients received NAC. Following neoadjuvant therapy, 180 underwent ALND while 34 underwent SLNB; they reported a pCR of 26%, which did not differ significantly between those who underwent SLNB (32.4%) versus those who underwent ALND (24.4%).25 The series suggests that use of SLNB among this population may be non-compliant with current recommendations, as 9/34 (26.5%) women undergoing SLNB presented with cN2–3 disease and 21/34 (61.8%) remained pathologically node positive (ypN+) after NAC.25 Our data found that 14 of 15 women presenting with cN1 disease achieved a nodal pCR and, therefore, axillary surgery downstaging appears feasible among appropriately selected patients.
Studies among women with an intact breast primary report overall nodal pCR rates of 26–41%,11,14,15 with substantial variation by tumor subtype. Multiple studies have reported similar trends in rates of nodal pCR following NAC, with the lowest rates seen among hormone receptor positive, HER2- tumors (0–29%), and the highest rates seen among triple negative (47–73%) and HER2+ (49–82%) tumors.8,9,11–13 Studies among patients with OPBC, including ours, report variable nodal pCR rates ranging from 26–80%.25,26 In our small OPBC population, pCR was 80% and, expectedly, the rates of response varied by tumor profile. While the numbers are small in each subgroup, the pCR rates for triple negative and HER2+ tumors were impressively high, at 88% and 100%, respectively. In this cohort, 14 of the 20 (70%) women presented with triple negative or HER2+ disease, partially explaining the overall high pCR rate, although the ER+/HER2- patients obtained a nodal pCR rate in 50% of cases, a rate higher than that reported in the literature for women with an intact primary. Similarly, Rueth et al reported on a cohort of 36 OPBC patients, 55% of whom presented with triple negative or HER2+ disease. Of the 25 women treated with NAC, 15 obtained a nodal pCR.26
Aside from the greater number of treatment-sensitive subtypes, an additional hypothesis related to the exceptional nodal pCR rates observed in the OPBC population stems from the potential biology of this rare entity. While OPBC may represent a primary breast lesion that is too small to be detected with conventional breast imaging, it is also postulated that this clinical scenario is encountered when the primary tumor is eliminated by the immune system. Although breast cancer has not traditionally been considered susceptible to an anti-tumor immune response, several lines of recent evidence propose that this may not necessarily be true. Pathologic evaluation of primary breast cancer has revealed, at times, an abundance of infiltration by immune cells, which strongly correlated with outcome as well as the effectiveness of NAC.27,28 These results suggest that breast cancers can elicit an immune response and that this finding is relevant to the natural history of the tumor. In 1990, Rosen et al reported on a series of 48 women presenting with OPBC. In this pre-MRI imaging cohort, a primary tumor was pathologically identified in 75% of cases. They report that a striking characteristic of many of the primary lesions, particularly in those tumors too small to be palpable or in those with only an in situ component, was a prominent lymphocytic reaction in and around the lesion.5 Evidence of scar tissue within mastectomy specimens was noted, which could be remnants of an immune response to the invasive tumor.29 In this regard, our findings of an increased pCR rate in these patients may support the hypothesis that OPBC is a manifestation of an abscopal immune response. With the recently observed effectiveness of immunotherapy for breast cancer, it may be that OPBC patients would benefit from this approach.30
One argument against axillary surgery downstaging among OPBC patients is that this population has been shown to present with a heavier nodal disease burden and, therefore, may not be appropriate for SLNB. Comparing clinicopathological characteristics in a large contemporary group of OPBC patients using the SEER database, Ge at al found that OPBC patients were less likely to present as cN1 when compared to their staged-matched cohorts (60% versus 73%). Review of final pathology after upfront surgery demonstrated pN1 disease in 61% of OPBC group versus 71% in the non-occult patients, although interestingly, the OPBC group demonstrated superior survival compared to the stage matched non-OPBC group.21 While a greater nodal disease burden may be present, that does not equate to a lower response to systemic therapy. In 2016, Barrio et al assessed the likelihood of achieving pCR among locally advanced breast cancer patients and noted that tumor biology, not burden of disease, was predictive of response.31 These data suggest that OPBC patients, even with a potential greater nodal disease burden, should not be less likely to achieve nodal pCR following NAC. Although a smaller proportion of OPBC patients may present with cN1 disease and be eligible for axillary surgery downstaging post-NAC, a study is ongoing to assess the feasibility and accuracy of SLNB among locally advanced breast cancer patients, including those with cN2–3 disease, with excellent response to NAC.32 Future data will inform us on the safety of broadening use of SLNB among those presenting with a higher nodal stage.
This study is limited by its retrospective nature and small cohort size. While there was a small group of patients who underwent SLNB alone, axillary surgery was not standardized, but at the discretion of the treating surgeon. Furthermore, we are unable to comment on the FNR of SLNB among OPBC. Although there were no locoregional recurrences or distant metastases in our cohort of patients, longer-term follow-up is still needed to fully evaluate the safety of omitting an ALND in OPBC patients.
Conclusions
In this series of biopsy-proven OPBC patients evaluated and managed in a recent time period, 80% of patients achieved a nodal pCR following NAC. The rates of pCR varied by receptor profile, being lowest in the ER+/HER2- group and highest in the HER2+ group (50–100%); however, they are excellent and numerically exceed those in the literature for women with an intact breast primary tumor. Given the excellent nodal response rate seen among OPBC patients, SLNB alone may be a feasible surgical option in select OPBC patients who downstage following NAC.
Synopsis (limit 40 words):
Here we determine rate of nodal pCR post-NAC in occult primary breast cancer (OPBC) patients. We find that, given the excellent nodal response rate, SLNB alone may be a feasible surgical option in those OPBC patients who downstage following NAC.
ACKNOWLEDGMENTS
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30CA008748 to Memorial Sloan Kettering Cancer Center, and this study has been accepted for presentation in poster format at the 2020 Society of Surgical Oncology International Conference on Surgical Cancer Care. Jessica Lavery reports salary support for the Project Genomics Evidence Neoplasia Information Exchange (GENIE) through the American Association for Cancer Research. Dr. George Plitas reports scientific advisory board membership for Tizona Therapeutics and Merck, and research funding from Takeda Pharmaceutical Company, that has no relation to this study. All other authors have no conflict of interest disclosures to report.
Disclosures: The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30CA008748 to Memorial Sloan Kettering Cancer Center, and this study has been accepted for presentation in poster format at the 2020 Society of Surgical Oncology International Conference on Surgical Cancer Care. Jessica Lavery reports salary support for the Project Genomics Evidence Neoplasia Information Exchange (GENIE) through the American Association for Cancer Research. Dr. George Plitas reports scientific advisory board membership for Tizona Therapeutics and Merck, and research funding from Takeda Pharmaceutical Company, that has no relation to this study. All other authors have no conflict of interest disclosures to report.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Baron PL, Moore MP, Kinne DW, Candela FC, Osborne MP, Petrek JA. Occult breast cancer presenting with axillary metastases. Updated management. Arch Surg. February 1990;125(2):210–214. [DOI] [PubMed] [Google Scholar]
- 2.Montagna E, Bagnardi V, Rotmensz N, et al. Immunohistochemically defined subtypes and outcome in occult breast carcinoma with axillary presentation. Breast Cancer Res Treat. October 2011;129(3):867–875. [DOI] [PubMed] [Google Scholar]
- 3.Olson JA Jr., Morris EA, Van Zee KJ, Linehan DC, Borgen PI. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. July 2000;7(6):411–415. [DOI] [PubMed] [Google Scholar]
- 4.Patel J, Nemoto T, Rosner D, Dao TL, Pickren JW. Axillary lymph node metastasis from an occult breast cancer. Cancer. June 15 1981;47(12):2923–2927. [DOI] [PubMed] [Google Scholar]
- 5.Rosen PP, Kimmel M. Occult breast carcinoma presenting with axillary lymph node metastases: a follow-up study of 48 patients. Hum Pathol. May 1990;21(5):518–523. [DOI] [PubMed] [Google Scholar]
- 6.Vlastos G, Jean ME, Mirza AN, et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. June 2001;8(5):425–431. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology 2020. https://www.nccn.org/professionals/physician_gls/default.aspx (Accessed April 24, 2020).
- 8.Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. October 2016;23(11):3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilewskie M, Zabor EC, Mamtani A, Barrio AV, Stempel M, Morrow M. The Optimal Treatment Plan to Avoid Axillary Lymph Node Dissection in Early-Stage Breast Cancer Patients Differs by Surgical Strategy and Tumor Subtype. Ann Surg Oncol. November 2017;24(12):3527–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang GC, Zhang YF, Xu FP, et al. Axillary lymph node status, adjusted for pathologic complete response in breast and axilla after neoadjuvant chemotherapy, predicts differential disease-free survival in breast cancer. Curr Oncol. June 2013;20(3):e180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. October 2014;260(4):608–614; discussion 614–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diego EJ, McAuliffe PF, Soran A, et al. Axillary Staging After Neoadjuvant Chemotherapy for Breast Cancer: A Pilot Study Combining Sentinel Lymph Node Biopsy with Radioactive Seed Localization of Pre-treatment Positive Axillary Lymph Nodes. Ann Surg Oncol. May 2016;23(5):1549–1553. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Park HS, Kim S, Ryu J, Park S, Kim SI. Prognostic Nomogram for Prediction of Axillary Pathologic Complete Response After Neoadjuvant Chemotherapy in Cytologically Proven Node-Positive Breast Cancer. Medicine (Baltimore). October 2015;94(43):e1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. January 20 2015;33(3):258–264. [DOI] [PubMed] [Google Scholar]
- 15.Classe JM, Loaec C, Gimbergues P, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. January 2019;173(2):343–352. [DOI] [PubMed] [Google Scholar]
- 16.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. June 2013;14(7):609–618. [DOI] [PubMed] [Google Scholar]
- 17.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. July 2010;134(7):e48–72. [DOI] [PubMed] [Google Scholar]
- 18.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. June 1 2010;28(16):2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchanan CL, Morris EA, Dorn PL, Borgen PI, Van Zee KJ. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol. December 2005;12(12):1045–1053. [DOI] [PubMed] [Google Scholar]
- 20.Macedo FI, Eid JJ, Flynn J, Jacobs MJ, Mittal VK. Optimal Surgical Management for Occult Breast Carcinoma: A Meta-analysis. Ann Surg Oncol. June 2016;23(6):1838–1844. [DOI] [PubMed] [Google Scholar]
- 21.Ge LP, Liu XY, Xiao Y, et al. Clinicopathological characteristics and treatment outcomes of occult breast cancer: a SEER population-based study. Cancer Manag Res. 2018;10:4381–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. November 15 2003;21(22):4165–4174. [DOI] [PubMed] [Google Scholar]
- 23.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. July 1997;15(7):2483–2493. [DOI] [PubMed] [Google Scholar]
- 24.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. February 10 2008;26(5):778–785. [DOI] [PubMed] [Google Scholar]
- 25.Cohen BL, Collier AL, Kelly KN, et al. Surgical Management of the Axilla in Patients with Occult Breast Cancer (cT0 N+) After Neoadjuvant Chemotherapy. Ann Surg Oncol. June 2020;27(6):1830–1841. [DOI] [PubMed] [Google Scholar]
- 26.Rueth NM, Black DM, Limmer AR, et al. Breast conservation in the setting of contemporary multimodality treatment provides excellent outcomes for patients with occult primary breast cancer. Ann Surg Oncol. January 2015;22(1):90–95. [DOI] [PubMed] [Google Scholar]
- 27.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. January 2018;19(1):40–50. [DOI] [PubMed] [Google Scholar]
- 28.Savas P, Virassamy B, Ye C, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. July 2018;24(7):986–993. [DOI] [PubMed] [Google Scholar]
- 29.Hoda, Brogi, Koerner, Rosen, eds. 2014. Rosen’s Breast Pathology, 4th Edition Lippincott Williams & Wilkins. [Google Scholar]
- 30.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. November 29 2018;379(22):2108–2121. [DOI] [PubMed] [Google Scholar]
- 31.Barrio AV, Mamtani A, Edelweiss M, et al. How Often Is Treatment Effect Identified in Axillary Nodes with a Pathologic Complete Response After Neoadjuvant Chemotherapy? Ann Surg Oncol. October 2016;23(11):3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov. National Library of Medicine (US). (August 2017 - August 2022). Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy in Patients Presenting With Locally Advanced Breast Cancer: A Prospective Study. Identifier NCT03255577. https://clinicaltrials.gov/ct2/show/NCT03255577 (Accessed May 29, 2020). [Google Scholar]


