Abstract
Background
Extreme thrombocytosis (EXT, platelet count >1000 × 103/μl) is an uncommon but potentially clinically significant finding. Primary EXT in the setting of myeloproliferative disorders is linked to thrombotic and/or bleeding complications more frequently than secondary EXT, which typically occurs in reaction to infection, inflammation, or iron deficiency. However, comorbidities have been reported in adults with secondary EXT. Clinical implications of EXT in children are not well-defined, as prior studies targeted small and/or specialized pediatric populations.
Objectives
Our objective was to determine etiologies and sequelae of extreme thrombocytosis in a hospitalized general pediatric patient population.
Patients and Methods
We retrospectively analyzed EXT cases from a single-center pediatric cohort of ~80,000 patients over 8 years.
Results
Virtually all cases (99.8%) were secondary in nature, and most were multifactorial. Many cases of EXT occurred in children under 2 years old (47%) and/or during critical illness (55%). No thrombotic or bleeding events directly resulted from EXT, confirming a paucity of clinical complications associated with EXT in pediatric patients. There were indications that neonatal hematopoiesis and individual genetic variation influenced some cases, in addition to certain diagnoses (e.g., sickle cell anemia) and clinical contexts (e.g., asplenia).
Conclusion
Our findings confirm that thrombotic events related to EXT are rare in pediatric patients, which can inform the use of empiric anti-platelet therapy.
Keywords: Anemia, critical illness, pediatrics, platelet count, thrombocytosis
Introduction
Extreme thrombocytosis (EXT, platelet count >1000 × 103/μl) is an uncommon clinical finding.[1] Primary EXT is associated with myeloproliferative disorders, such as essential thrombocythemia.[2] Secondary EXT is more common and occurs in reaction to infection, inflammation, or iron deficiency. High platelet counts are linked with arterial events, though not necessarily venous thrombosis.[3] Bleeding and thrombotic complications may occur more frequently in primary EXT cases,[1] although some studies have not associated primary EXT and thrombotic events.[4] Complications have also been reported in adults with secondary EXT.[5]
Etiologies and complications associated with EXT in children are less well-defined, as prior pediatric studies have been relatively small or restricted to specialized patient populations.[6,7] We characterized EXT in a large, single-center pediatric cohort over an 8 year period. Our findings, generalizable to all hospitalized pediatric patients, will help inform clinical interpretation and management related to this laboratory finding.
Patients and Methods
Patient identification
The Children’s Hospital of Philadelphia Institutional Review Board deemed this study exempt from review. Utilizing the electronic medical record, we identified patients with EXT admitted to our quaternary pediatric hospital from 2012 to March 2020. We retrospectively determined EXT etiologies in these patients, along with associated ICD-9 diagnoses, treatments, and complications. Each hospital admission in which a patient had EXT was considered an independent case. Most cases had multiple blood counts showing EXT-range platelet values, giving us confidence that cases did not result from laboratory error. Patient ages were assigned based on the first EXT-range platelet count value in each admission.
EXT case analysis
We defined one principal etiology for each EXT case. ‘Infection’ cases included those with culture-proven bacterial infections, laboratory-confirmed viral infections, and patients who received antimicrobials for suspected infection. Inflammation cases included those with clinical or laboratory evidence of inflammatory processes without confirmed infection (e.g., elevated C-reactive peptide values), as well as instances where malignancy, chemotherapy, trauma, or post-surgical changes were deemed principal causes. Iron deficiency cases were identified by ICD-9 diagnosis codes and/or supplemental iron prescriptions, in the absence of infection or signs of inflammation. ‘Other’ cases were those without a clear inciting cause. Many cases were potentially multifactorial, but typically involved one change (e.g., new infection) that was assigned as the principal etiology. For each EXT case, we ascertained information regarding central venous access lines, thromboembolic events, and hemorrhage in temporal relation to the development of EXT.
Results and Discussion
Etiologies and sequelae of EXT in a pediatric population
Analysis of 79,618 patients in 131,982 admissions identified EXT in 391 patients (0.5%) during 423 hospital admissions (0.3%, Table 1). Over the same time period, 15% of all patients admitted to our hospital had mild thrombocytosis >500 × 103/μl, and just 0.15% of patients were found to have EXT in outpatient settings.
Table 1.
Characteristics of patients analyzed for this study. A total of 79,618 patients and 131,982 admissions were queried to identify patients meeting inclusion criteria. Temporal associations were defined as ±2 weeks from the first laboratory identification of EXT.
| Characteristic | Value |
|---|---|
| Patients included (% of all patients) | 391 (0.5%) |
| Male patients included (%) | 195 (50%) |
| Age in years at time of EXT (median, 25th-75th percentile) | 2.2 (0.6 – 8.9)* |
| Admissions included (% of all admissions) | 423 (0.3%) |
| Principal etiology: | |
| Secondary/reactive thrombocytosis | 422 |
| Primary thrombocytosis (suspected) | 1 |
| Intensive care unit admissions (%) | 231 (55%) |
| Patients with temporally associated thrombotic event (%) | 21 (5%) |
| Occurred after EXT-range platelet count | 1 |
| Age at time of event (years, mean±SD) | 5.9 ± 7.2 |
| In ICU at time of event | 19 (90%) |
| Central venous access in place at time of event | 4 (19%) |
| Intercurrent infection at time of event | 16 (76%) |
| Documented sepsis at time of the event | 7 (33%) |
| Platelet count at time of the event (x 103/μl, mean±SD) | 744 ± 289 |
| Patients with temporally associated bleeding event (%) | 6 (1%) |
| Occurred after EXT-range platelet count | 0 |
| Age at time of event (years, mean±SD) | 5.2 ± 4.8 |
| In ICU at time of event | 5 (83%) |
| Central venous access in place at time of event | 1 (17%) |
| Intercurrent infection at time of event | 3 (50%) |
| Documented sepsis at time of the event | 0 (0%) |
| Platelet count at time of the event (x 103/μl, mean±SD) | 385 ± 292 |
| Empiric aspirin for EXT (%) | 16 (4%) |
| Platelet count at aspirin initiation (x 103/μl, mean±SD) | 1231 ± 438 |
One patient was followed from childhood to age 25 years.
At least 92% of our EXT cases were secondary to infection (47%), inflammation (40%), or iron deficiency anemia (5%, Fig. 1A). Other etiologies accounted for 8% of cases, including bone marrow reactivity after hemorrhage (n=2), cases with no clear cause, and 1 potential primary EXT case in the setting of a novel genetic mutation.[8] Overall, this etiologic distribution among our cohort was consistent with prior pediatric EXT reports.[6,7]
Figure 1.
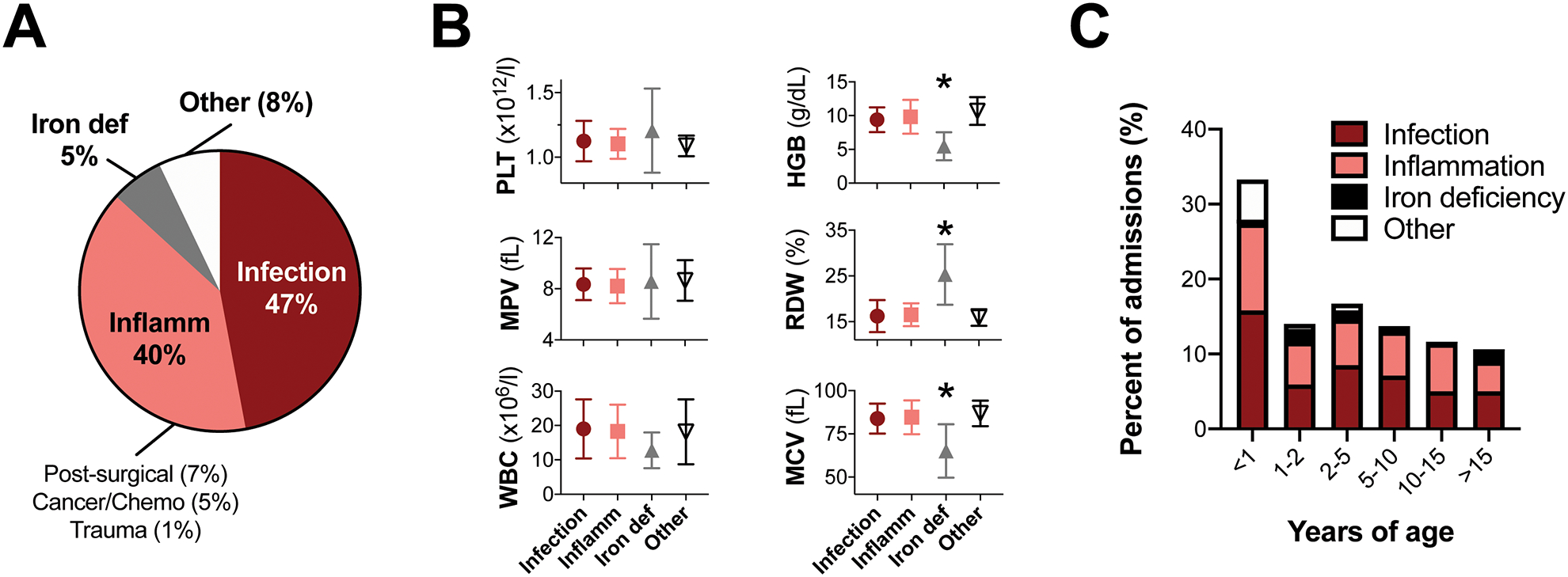
Etiologies, blood parameters and ages of patients with extreme thrombocytosis. A, Etiologies of EXT in our cohort. Cases were assigned to categories based on principal factors underlying EXT (infection, inflammation, iron deficiency anemia), or ‘other’ if there was no principal cause identified. B, Hematologic indices for cases in each category (mean±standard deviation). *p<0.05 by ANOVA versus all other categories. C, Ages at which patients had EXT. For each case, the first blood count showing EXT was used to calculate patient age. Colors within each bar depict etiology category breakdown within each age range.
Hematologic indices showed marked microcytic anemia in iron deficiency cases, and leukocytosis in infection or inflammation cases (Fig. 1B and Table 2). Indices in ‘other’ cases suggested robust multilineage hematopoiesis, with high red cell distribution width (RDW) and mean platelet volume (MPV) values indicating the presence of reticulocytes and immature platelets, but did not significantly differ from infection or inflammation cases. This could indicate hyperactive bone marrow following myelosuppression,[1] which we viewed as a diagnosis of exclusion.
Table 2.
Hematologic trait values for patients with EXT from the indicated principal etiologies. Values for the subset of patients hospitalized in the NICU are shown at bottom. The mean ± standard deviation for each parameter is shown.
| Trait | Normal Range | Infection | Inflammation | Iron def | Other |
|---|---|---|---|---|---|
| All patients | (n=200) | (n=168) | (n=23) | (n=32) | |
| PLT | 150 – 400 ×1000/μl | 1125 ± 156 | 1104 ± 115 | 1206 ± 326 | 1088 ± 80 |
| MPV | 9.0 – 10.9 fL | 8.3 ± 1.2 | 8.2 ± 1.3 | 8.6 ± 2.9 | 8.7 ± 1.6 |
| RBC | 3.1 – 4.5 ×106/μl | 3.5 ± 0.7 | 3.7 ± 0.9 | 3.1 ± 0.8 | 3.8 ± 0.7 |
| HGB | 9.5 – 13.5 g/dL | 9.4 ± 1.8 | 9.8 ± 2.5 | 5.5 ± 2.1* | 10.7 ± 2.1 |
| HCT | 29 – 41 % | 29.6 ± 5.6 | 31.0 ± 7.3 | 20.7 ± 5.7* | 32.4 ± 5.9 |
| MCV | 74 – 108 fL | 83.8 ± 8.7 | 84.6 ± 9.8 | 65.1 ± 15.5* | 86.9 ± 7.4 |
| MCH | 25 – 35 pg | 26.7 ± 3.6 | 26.9 ± 4.1 | 17.7 ± 6.5* | 28.6 ± 3.1 |
| MCHC | 30. – 36 g/dL | 31.8 ± 2.0 | 31.7 ± 2.4 | 26.6 ± 3.5* | 33.0 ± 1.8 |
| RDW | 12.2 – 14.3 % | 16.2 ± 3.5 | 16.5 ± 2.5 | 25.3 ± 6.6* | 15.9 ± 1.8 |
| WBC | 6.0 – 13.3 ×1000/μl | 19.0 ± 8.6 | 18.3 ± 7.8 | 12.8 ± 5.2 | 18.2 ± 9.4 |
| NICU patients | (n=12) | (n=19) | (n=0) | (n=17) | |
| PLT | 150 – 400 ×1000/μl | 1085 ± 72 | 1113 ± 104 | 1067 ± 27 | |
| MPV | 9.0 – 10.9 fL | 8.2 ± 1.4 | 9.0 ± 1.0 | 9.2 ± 1.6 | |
| RBC | 3.1 – 4.5 ×106/μl | 3.7 ± 0.4 | 3.5 ± 0.7 | 3.7 ± 0.6 | |
| HGB | 9.5 – 13.5 g/dL | 10.7 ± 0.8 | 10.4 ± 1.8 | 11.4 ± 1.8 | |
| HCT | 29 – 41 % | 32.9 ± 2.8 | 31.6 ± 5.5 | 33.8 ± 5.8 | |
| MCV | 74 – 108 fL | 89.4 ± 5.8 | 90.9 ± 7.1 | 90.6 ± 5.7 | |
| MCH | 25 – 35 pg | 29.2 ± 2.4 | 29.9 ± 3.3 | 30.5 ± 2.1 | |
| MCHC | 30. – 36 g/dL | 32.6 ± 1.1 | 32.9 ± 2.1 | 33.8 ± 1.4 | |
| RDW | 12.2 – 14.3 % | 15.2 ± 1.7 | 16.3 ± 1.4 | 15.2 ± 1.2 | |
| WBC | 6.0 – 13.3 ×1000/μl | 21.1 ± 7.6 | 18.6 ± 7.0 | 17.5 ± 7.1 | |
p<0.05 by ANOVA versus all other etiological categories.
PLT, platelet count. MPV, mean platelet volume. RBC, red blood cell count. HGB, hemoglobin. HCT, hematocrit. MCV, mean corpuscular volume. MCH, mean corpuscular hemoglobin. MCHC, mean corpuscular hemoglobin concentration. RDW, red cell distribution width. WBC, white blood cell count.
We also analyzed EXT on a per-patient basis, reasoning that certain diagnoses or clinical contexts may have been overrepresented in per-case analyses. Indeed, 32 patients out of 391 patients (8%) developed EXT during multiple inpatient admissions. However, when we considered just the first instance of EXT for each patient, the etiologic distribution did not show meaningful changes. Further, we noted that principal etiologies for EXT sometimes changed (e.g., a patient with surgery in the 1st instance who later developed an infection in the 2nd instance). Given the importance of retaining such information, estimates below represent per-case estimates except where noted.
Pediatric EXT is generally not associated with bleeding or thrombotic sequelae.[6,7] Despite this, aspirin was initiated for 16 patients in our cohort in response to laboratory values alone, at an average platelet count of 1231±438 × 103/μl (mean±SD). This likely underestimates aspirin use, since children with non-extreme thrombocytosis outside of the scope of our analysis may have also been treated empirically. This estimate does not include patients who received aspirin for other conditions (e.g., Kawasaki disease). There were 6 bleeding and 21 thrombotic events diagnosed within ±2 weeks of EXT, often in patients with bacterial infections requiring ICU hospitalization (Table 1). In all but one case, these complications preceded EXT onset, suggesting they were inciting factors as opposed to sequelae. For one patient, EXT preceded a central line-associated thrombus by 1 day. However, this child was medically complex, in acute respiratory failure from rhinovirus and E. coli sepsis, and was already taking aspirin for thrombocytosis. Therefore, it is impossible to establish causality or definitive attribution to EXT. Overall, our findings confirmed that bleeding or thrombosis from EXT are incredibly rare in pediatric patients.
Although hospital diagnoses varied within our cohort, EXT always resolved over several days without specific intervention, aside from treatment for associated etiologies (e.g., antimicrobials for infection). This may be relatively reassuring to those caring for pediatric patients who develop EXT, and help facilitate clinical interpretation when laboratory results identify EXT.
Young age and critical illness correlate with EXT
Infants and young children were overrepresented in our cohort, with 33% of patients less than 1 year old and almost half (47%) under 2 years old (Fig. 1C). By comparison, 30% of all admitted patients were under 2 years old in the queried time period (p=0.02 by two-sided Fisher’s exact test).
Interestingly, most EXT cases without clear etiology occurred in infants less than 1 year old (p=0.003 by two-sided Fisher’s exact test, Fig. 1C). It is possible that EXT occurred more frequently in infants whose hematopoietic systems responded differently to pathophysiologic processes than older children. The hyperproliferative capacity of neonatal megakaryocytes, relative to adult megakaryocytes [9], may also underlie the preponderance of infants in our cohort.
Exaggerated pathophysiology during critical illness might predispose to more extreme hematologic derangements. Indeed, 55% of our cohort required support in our pediatric (PICU), neonatal (NICU), and/or cardiac intensive care units. Most critically ill children with EXT were admitted to the PICU (73%), with a distribution of etiologies similar to our general patient population (Fig. 2A). This proportion of PICU patients with EXT (0.6%) was less than a prior report (~1.1%).[6] A further 21% of critically ill patients with EXT were cared for in the NICU, corresponding to 0.5% of all NICU patients in the queried time period.
Figure 2.
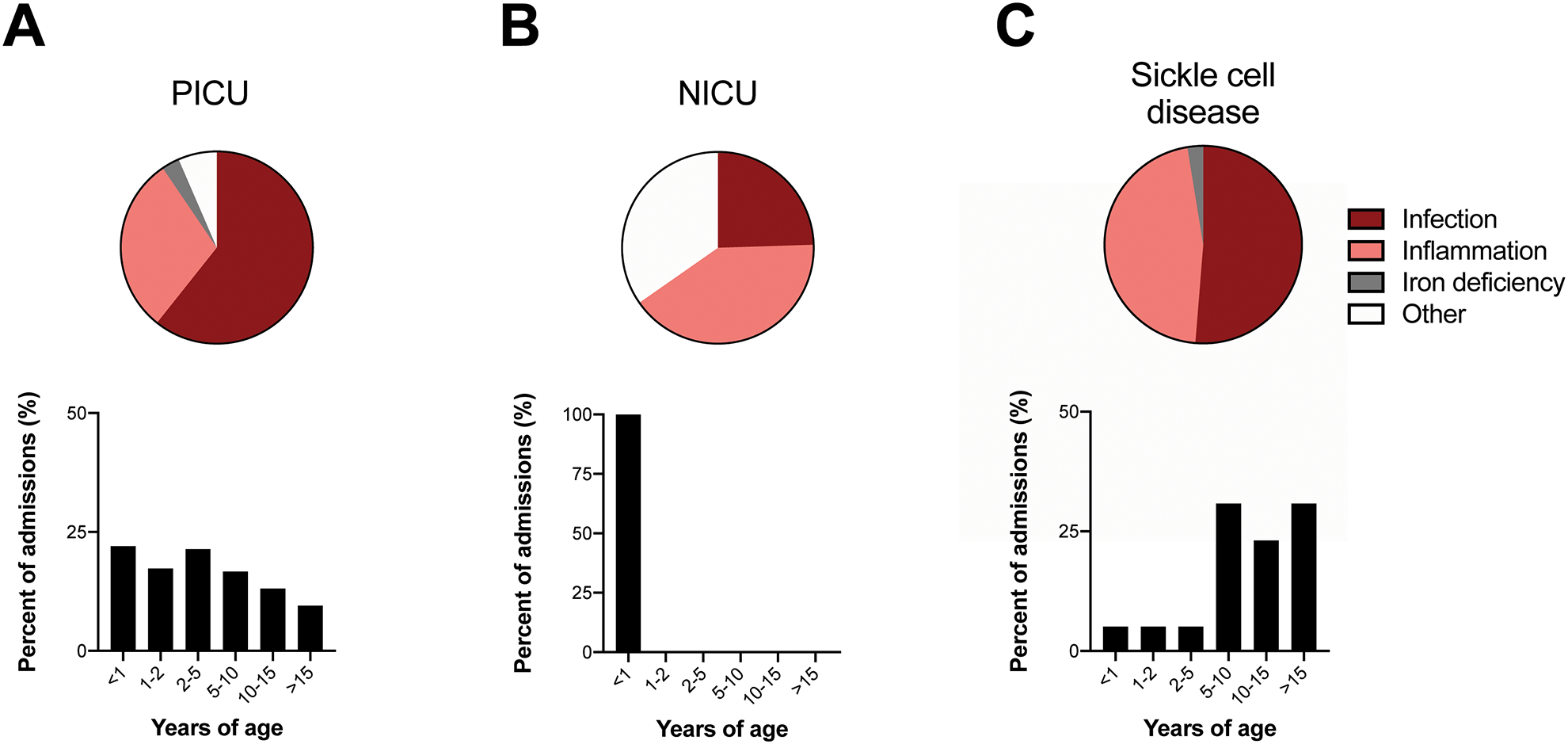
Etiologies, ages and blood parameters for exemplary patients with EXT, including those hospitalized in the PICU, NICU, or with sickle cell anemia. A, Pie chart shows principal etiologies for EXT in patients hospitalized in the PICU. Bar plot shows ages of PICU patients with EXT from any cause. B, Pie chart shows principal etiologies for EXT in patients hospitalized in the NICU. Bar plot shows ages of NICU patients with EXT from any cause. C, Pie chart shows principal etiologies for EXT in patients with sickle cell disease. Bar plot shows ages of sickle cell disease patients with EXT from any cause.
Given the medical complexity and severity of illness of many patients in our cohort, it was not surprising that many (11.5%) had thrombotic events diagnosed at some point in course of their illness. Indeed, we noted independent thromboembolic risk factors in many cases, including central venous lines (29% of our cases), severe infections, and/or ICU admission.[10] In the setting of these risk factors, this lifetime thrombotic event rate was consistent with published expectations.[10]
Remarkably, 35% of NICU patients had an unclear etiology for EXT (p<0.001 by two-sided Fisher’s exact test vs. all other ICU patients, Fig. 2B). In these patients, EXT frequently occurred just before discharge, without related clinical changes. We suspect these cases represented hyperactive bone marrow following myelosuppression due to critical illness, as suggested by robust multilineage hematopoiesis in these infants (Table 2). Blood count indices did not vary between etiological categories in these age-matched patients.
Diagnoses associated with EXT
Respiratory infections have been linked to EXT [7,11,12] and were common in our cohort. Bacterial and viral lung infections were diagnosed in 49% of infection-associated EXT cases. Although infants and young children may be at higher risk for hospitalization from respiratory infections (e.g., bronchiolitis), lung infections were not preferentially diagnosed in young patients (52% of children <2 years old vs. 48% of children >5 years old with EXT from infection). Thus, respiratory infections cannot account for the prevalence of infants in our cohort.
Asplenia can cause thrombocytosis due to reduced platelet storage and removal [7]. However, surgical splenectomy was relatively infrequent in our cohort (9 patients, 11 admissions). Sickle cell disease leads to autosplenectomy and functional asplenia by ~5 years of age.[13] In our cohort, 32 patients with sickle cell disease had EXT during 39 hospitalizations. This was somewhat lower than expected, given our large sickle cell program and their predisposition to encapsulated bacterial infections. However, as rebound thrombocytosis often follows acute illness resolution in sickle cell disease,[14] EXT may be more common outside of hospitalization in these patients. Principal EXT etiologies for hospitalized sickle cell patients virtually always involved vaso-occlusive crises, with or without infection (Fig. 2C). The age distribution for sickle cell patients presenting with EXT was consistent with functional asplenia onset, as 85% occurred after age 5 (Fig. 2C). These exemplary cases show principal events (e.g. infections) driving EXT only in a permissive context (e.g., underlying asplenia).
Thrombocytosis can also occur in the setting of inflammatory diseases (e.g., [15–18]). We identified many patients with inflammatory conditions, including 24 with inflammatory bowel disease (IBD),[15] 14 with Kawasaki disease,[16] 5 with nephrotic syndrome,[17] and 2 patients with juvenile idiopathic arthritis.[18] The relatively high number of patients with IBD may be due to disease prevalence among our patients, iatrogenic thrombocytosis from steroid use, and/or the potent thrombocytosis-inducing effects of cytokines produced during intestinal inflammation.[15]
In our cohort, 23 patients were treated with previous or concurrent chemotherapy (6%). Among these 23 oncology patients, 8 developed EXT during multiple admissions. This was most likely due to rebound bone marrow hyperactivity following chemotherapeutic myelosuppression, but some patients may have developed EXT due to inflammation secondary to malignancy and/or complications like infections. Overall, EXT was relatively infrequent among the many oncology patients treated in our hospital.
Our results also suggested that individual genetic variation may contribute to EXT, independent of clinical and environmental factors. It was unclear why certain individuals developed EXT, while others with identical conditions did not. For example, the reasons that a particular subset of 32 patients with sickle cell disease developed EXT were unknown. We also suspected underlying genetic contribution in 61 patients who had recurrent EXT (≥2 independent episodes). Some of these blood counts occurred as outpatients, so were not included in our primary analysis. Future studies to better understand such genetic factors may lead to novel insights into hematopoiesis, megakaryopoiesis, and/or thrombopoiesis.
Conclusions
There were few thrombotic or bleeding events temporally associated with EXT in our cohort, and no such events definitively resulted from EXT. In fact, temporal relationships suggested that thrombotic and bleeding events may have been inciting factors for some EXT cases, as opposed to sequelae. Therefore, EXT seems unlikely to independently or additively increase thromboembolic risk in pediatric patients.
These results contrasted the elevated risks of thrombosis and/or bleeding associated with high platelet count [3] and variably reported in adults with EXT.[4,19] These differences likely relate to underlying diagnoses in adults with EXT, including increased incidence of primary myeloproliferative disease and other oncologic diagnoses, and/or more prevalent cardiovascular comorbidities. This discrepancy underscores the importance of clarifying EXT etiologies and related complications in a generalizable pediatric population.
Guidelines for antiplatelet and anticoagulant agents require further study in the pediatric population.[20] Although our retrospective observational study should not substitute for dedicated prospective trials that assess utility and risks of empiric therapies, clinical decision-making regarding antiplatelet or anticoagulant treatments for thrombocytosis may be informed by our findings. Empiric treatment should be based on thromboembolic risk factors (e.g., central venous access, sepsis, and critical illness [10]) but not platelet counts.
Essentials.
Extreme thrombocytosis (EXT) etiologies and sequelae are unknown for general pediatric patients.
We retrospectively analyzed EXT in a single-center cohort of ~80,000 pediatric patients.
EXT did not definitively lead to any thrombotic or bleeding events.
Infants and critically ill patients were at higher risk for EXT than other patients.
Acknowledgements
The authors thank the patients and families for whom we are privileged to care at the Children’s Hospital of Philadelphia. We are grateful for incredible support from Scott Lorch, Heather French and the Children’s Hospital of Philadelphia Neonatal and Perinatal Medicine Fellowship program. We thank Russell Kesman and Kuan-Chi Lai for their thoughtful suggestions.
Funding
This work was supported by a grant from the National Institutes of Health, USA (T32HD043021 to CST), an American Academy of Pediatrics Marshall Klaus Neonatal‐Perinatal Research Award (CST), and the Children’s Hospital of Philadelphia Division of Neonatology.
Footnotes
Conflicts of interest
The authors declare no relevant conflicts of interest.
References
- 1.Bleeker JS, Hogan WJ. Thrombocytosis: Diagnostic Evaluation, Thrombotic Risk Stratification, and Risk-Based Management Strategies. Thrombosis 2011; 2011: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kucine N, Chastain KM, Mahler MB, Bussel JB. Primary thrombocytosis in children. Haematologica 2014; 99: 620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warny M, Helby J, Birgens HS, Bojesen SE, Nordestgaard BG. Arterial and venous thrombosis by high platelet count and high hematocrit: 108 521 individuals from the Copenhagen General Population Study. J Thromb Haemost 2019; 17: 1898–911. [DOI] [PubMed] [Google Scholar]
- 4.Campbell PJ, MacLean C, Beer PA, Buck G, Wheatley K, Kiladjian JJ, Forsyth C, Harrison CN, Green AR. Correlation of blood counts with vascular complications in essential thrombocythemia: Analysis of the prospective PT1 cohort. Blood 2012; 120: 1409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiwanitkit V Extreme thrombocytosis: What are the etiologies? Clin Appl Thromb 2006; 12: 85–7. [DOI] [PubMed] [Google Scholar]
- 6.Denton A, Davis P. Extreme thrombocytosis in admissions to paediatric intensive care: No requirement for treatment. Arch Dis Child 2007; 92: 515–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dame C, Sutor AH. Primary and secondary thrombocytosis in childhood. Br J Haematol 2005; 129: 165–77. [DOI] [PubMed] [Google Scholar]
- 8.Thom CS, Brandsma E, Lambert MP. Thrombocytosis in an infant with a TRPV4 mutation: a case report. Platelets 2020; 00: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrer-Marin F, Stanworth S, Josephson C, Sola-Visner M. Distinct differences in platelet production and function between neonates and adults: Implications for platelet transfusion practice. Transfusion 2013; 53: 2814–21. [DOI] [PubMed] [Google Scholar]
- 10.Witmer CM, Takemoto CM. Pediatric hospital acquired venous thromboembolism. Frontiers in Pediatrics 2017; 5: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlacha V, Feketea G. Thrombocytosis in Pediatric Patients Is Associated with Severe Lower Respiratory Tract Inflammation. Arch Med Res 2006; 37: 755–9. [DOI] [PubMed] [Google Scholar]
- 12.Zheng SY, Xiao QY, Xie XH, Deng Y, Ren L, Tian DY, Luo ZX, Luo J, Fu Z, Huang AL, Liu EM. Association between secondary thrombocytosis and viral respiratory tract infections in children. Sci Rep 2016; 6: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol 2014; 166: 165–76. [DOI] [PubMed] [Google Scholar]
- 14.Gardner K, Thein SL. Super-elevated LDH and thrombocytopenia are markers of a severe subtype of vaso-occlusive crisis in sickle cell disease. Am J Hematol 2015; 90: E206–7. [DOI] [PubMed] [Google Scholar]
- 15.Heits F, Stahl M, Ludwig D, Stange EF, Jelkmann W. Elevated Serum Thrombopoietin and Interleukin-6 Concentrations in Thrombocytosis Associated with Inflammatory Bowel Disease. J Interf Cytokine Res 1999; 19: 757–60. [DOI] [PubMed] [Google Scholar]
- 16.Nigrovic LE, Nigrovic PA, Harper MB, Chiang VW. Extreme thrombocytosis predicts Kawasaki disease in infants. Clin Pediatr (Phila) 2006; 45: 446–52. [DOI] [PubMed] [Google Scholar]
- 17.Eneman B, Levtchenko E, van den Heuvel B, Van Geet C, Freson K. Platelet abnormalities in nephrotic syndrome. Pediatr Nephrol 2016; 31: 1267–79. [DOI] [PubMed] [Google Scholar]
- 18.Iacono A, Sprocati M, Giuliani AL, Di Virgilio F, Borgna-Pignatti C, Maggiore G. Extreme thrombocytosis in systemic juvenile idiopathic arthritis. A case report. Ital J Pediatr 2019; 45: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh RW, Ravindran A, Hook CC, Begna KH, Ashrani AA, Pruthi RK, Marshall AL, Hogan W, Litzow M, Hoyer J, Oliveira JL, Vishnu P, Call TG, Al-Kali A, Patnaik M, Gangat N, Pardanani A, Tefferi A, Go RS. Etiologies of Extreme Thrombocytosis: A Contemporary Series. Mayo Clin Proc 2019; 94: 1542–50. [DOI] [PubMed] [Google Scholar]
- 20.Witmer C, Raffini L. Treatment of venous thromboembolism in pediatric patients. Blood 2020; 135: 335–43. [DOI] [PubMed] [Google Scholar]


