Abstract
Sarcopenia, defined as loss of muscle mass, can occur with aging. We conducted a single-center retrospective analysis to evaluate the impact of muscle quality in multiple myeloma (MM), a hematologic cancer of older adults, undergoing autologous hematopoietic cell transplantation (autoHCT). Healthy muscle was quantified by measuring the percent of high-density muscle within the L3 psoas muscle using a novel computed tomography method in 142 eligible patients. Early post-transplant complications were assessed in the first 100 days after transplant. Sarcopenia, defined as ≤80% high-density muscle, was found in 72 (51%) patients. Sarcopenic obesity, defined as sarcopenia and a BMI30, was found in 32 (23%) patients. One or more early complications occurred in 22 (16%) patients. Cardiovascular events accounted for 36% of all complications. Patients with sarcopenia had more cardiac complications (12.5%) than patients without (2.9%, p=0.03). Multivariate analysis revealed increased BMI at transplant, but not sarcopenia, was associated with worse OS (hazard ratio: 1.11, 95% confidence interval: 1.02–1.22, p=0.02). Our analysis suggests that sarcopenia is prevalent in MM and associated with increased early post-transplant cardiovascular complications in MM. Obesity, regardless of sarcopenia, is associated with worse survival in MM. Our study generates hypothesis-generating data to risk-stratify patients being considered for autoHCT.
Introduction
Sarcopenia, a reduction in muscle mass, composition, quality, and/or strength, is common in older adults1, 2 and is associated with chronic disease states, fatigue, falls and overall mortality.1, 3 Sarcopenic obesity is the combination of sarcopenia and increased fat mass3 and has been associated with poor clinical outcomes.4 The presence of sarcopenic obesity in cancer has been studied in several solid tumors,4 but its effect in hematologic cancers is underexplored.5, 6
Multiple myeloma (MM) is the second-most common hematologic malignancy affecting nearly 30,000 new individuals annually in the United States.7 New treatments, including novel therapies and the optimal use of autologous hematopoietic cell transplantation (autoHCT) have significantly improved the outcomes of MM patients.8 Nevertheless, these treatments can increase toxicity burden, sometimes precluding the ability of patients to complete therapies and/or tolerate future exposures to treatments. The median age at diagnosis of MM in the U.S. is 69 years with a majority of MM patients aged 65–74 years, which increases their risk of sarcopenia and makes symptom burden and management particularly important in this incurable chronic disease.7
Given that increasing age, a risk factor for sarcopenia, and obesity are risk factors for MM,9 we hypothesized that sarcopenia and sarcopenic obesity are prevalent in MM patients. The primary objectives of this study were to determine the prevalence of sarcopenia and sarcopenic obesity in MM patients undergoing autoHCT, to determine any significant correlations between sarcopenic obesity and baseline patient-, disease- and treatment-related characteristics, and to assess the impact of sarcopenia and obesity on early post-transplant course, progression-free, and overall survival.
Subjects and Methods:
Patients:
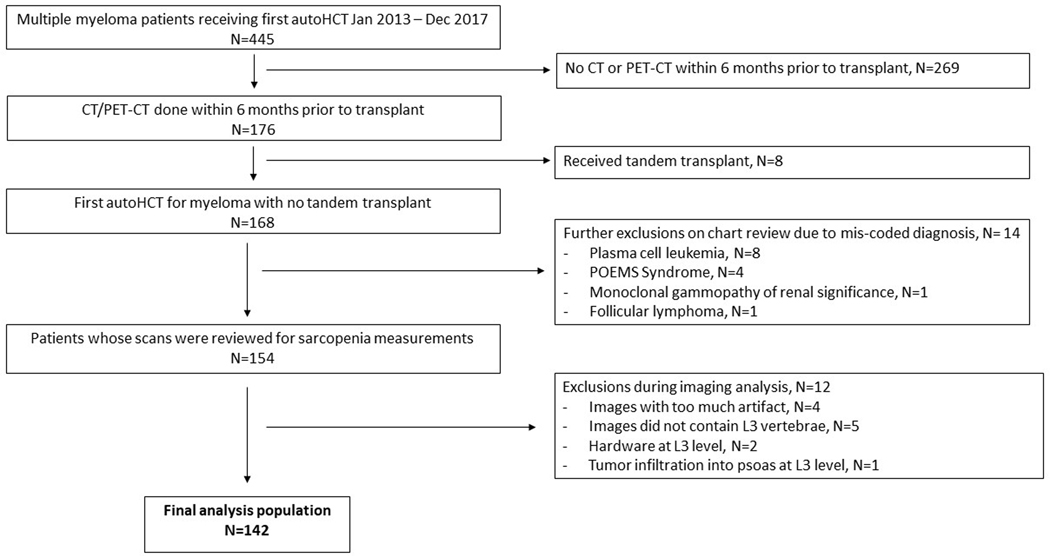
This IRB-approved single-center retrospective study included all MM patients who had a first autoHCT between January 2013 and December 2017 and underwent a computerized tomography (either CT or PET/CT) scan within 6 months prior to transplant. Figure 1 shows a flow chart of patient selection.
Figure 1.
Flow chart of study population
Measures:
Muscle mass and composition:
Sarcopenia was assessed employing a CT image containing the psoas muscle at the third lumbar vertebral level (L3). Previous studies have shown that evaluation of the psoas muscle at the L3 level well approximates overall body composition.10 The Advantage Workstation Volume Viewer software was used to calculate the cross-sectional area of the psoas muscle at L3 level and the histogram feature was used to assess the percentage of fat, low density muscle, and high-density muscle within the psoas. Fat was defined as attenuation less than −29 Hounsfield Units (HU), low-density muscle was defined as attenuation between −29 and +29 HU, high-density muscle attenuation was defined as attenuation >+29 HU.
Definitions of sarcopenia and sarcopenic obesity:
Patients with ≤ 80% high density muscle were categorized as sarcopenic. This cutoff was determined using a sensitivity analysis that provided the best cut-point by muscle density. Patients were classified as sarcopenic obese if sarcopenia was accompanied by BMI 30.
Clinical Variables of Interest:
Chart reviews were conducted for each patient in the study in order to collect baseline characteristics and post-transplant complications. The variables considered in this analysis included age at transplant, sex, BMI at transplant, stage of disease using International Staging System (ISS) and Revised International Staging System (R-ISS), presence of bone lesions, Hematopoietic Cell Transplant- Comorbidity Index (HCT-CI), and Karnofsky Performance Status (KPS).
Outcomes:
To assess early post-transplant outcomes, we chose the following transplant-related complications, all occurring within the first 100 days post-transplant as parameters for our evaluation: unplanned hospitalization, total days spent in hospital, discharge to a rehabilitation facility, ICU transfer, cardiovascular events defined by heart failure or arrhythmia, renal failure necessitating dialysis, respiratory failure requiring intubation, and sepsis. Overall survival (OS) was defined as time from transplant to last follow up or death. Progression free survival (PFS) was defined as time from transplant to last follow up or relapse. Age at transplany, BMI at transplant, Karnofsky performance status, and outpatient transplant were considered in the multivariate analysis for OS and PFS. Due to a relatively low number of events (relapse and death), we were unable to include variables of potential interest such as stage of disease, HCT-CI, pre-transplant disease status, and conditioning melphalan dose into the multivariate analysis. Alive patients were censored at last follow up.
Statistical Analysis:
Psoas area measurements were indexed to the total psoas area and presented as percent high-density muscle. Patients were divided into two cohorts distinguished by ≤80% and >80% high-density muscle. Demographic and disease characteristics were summarized using descriptive statistics and compared between the study cohorts using t-test and Wilcoxon-Mann-Whitney test for continuous and ordinal measures, respectively, and chi-square tests for categorical outcomes. Survival curves were estimated using the Kaplan-Meier method and compared between groups via the log-rank test. The cumulative incidence of engraftment was estimated using the Nelson-Aalen estimate with death without engraftment as competing risk. Cox proportional hazards model was fitted with percent high-density muscle (≤80 versus >80) as the main effect for the primary endpoint, overall survival (OS). Owing to the small sample size, only BMI at transplant, sarcopenia, KPS and outpatient transplant were considered in the multivariable analysis. Progression-free survival (PFS) was also analyzed similarly as a secondary endpoint. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics:
Baseline characteristics of the study population are described in Table 1. The median age at transplant was 62.4 years, ranging from 38.2 to 78.7 years, 65% were male. Median BMI at transplant was 28.9 kg/m2, ranging from 17.8 to 46.1 kg/m2. According to the BMI classification, 59 (42%) patients were obese (BMI 30), 56 (39%) were overweight (25 BMI < 30), 26 (18%) were normal weight (18.5 BMI < 25), and 1 (<1%) was underweight (BMI < 18.5).
Table 1.
Baseline characteristics and outcomes
| Sarcopenia N=72 | No Sarcopenia N=70 | P value | |
|---|---|---|---|
| Median Age at Transplant (range) | 63.3 (38.2–78.6) | 59.7 (43.8–78.7) | 0.7 |
| Male (%) | 46 (63.9%) | 46 (65.7%) | 0.8 |
| Race (White) (%) | 63 (87.5%) | 59 (84.3%) | 0.6 |
| Median BMI at Transplant (range) | 29.0 (21.5–46.1) | 28.8 (17.8–40.8) | 0.1 |
| Time from Diagnosis to Transplant | 5.4 (0.7–30.2) | 5.4 (3.3–27.6) | 0.6 |
| Stage (ISS) | 0.5 | ||
| ISS-I | 25 (36.8%) | 22 (33.3%) | |
| ISS-II | 27 (39.7%) | 25 (37.9%) | |
| ISS-III | 16 (23.5%) | 19 (28.8%) | |
| Bone Lesions | 63 (87.5%) | 56 (80.0%) | 0.2 |
| Median HCT-CI | 1.0 (0.0–8.0) | 1.0 (0.0–7.0) | 0.7 |
| KPS | 48 (66.7%) | 44 (62.9%) | 0.6 |
| ≥90 | |||
| Lines of Induction | 0.2 | ||
| 1 | 59 (81.9%) | 63 (90.0%) | |
| 2 | 13 (18.1%) | 7 (10.0%) | |
| Pre-transplant disease status | 0.3 | ||
| ≥VGPR | 44 (61.1%) | 37 (52.9%) | |
| ≤PR | 28(38.9%) | 33 (47.1%) | |
| Melphalan dose | 0.6 | ||
| 140 mg/m2 | 9 (12.5%) | 11 (15.7%) | |
| 200 mg/m2 | 63 (87.5%) | 59 (84.3%) | |
| Outpatient Transplant | 21 (29.2%) | 18 (25.7%) | 0.6 |
| Day 100 complications | |||
| Any Complication | 24 (33.3.%) | 15 (21.4%) | 0.3 |
| Any Adverse Event* | 14 (19.4%) | 8 (11.4%) | 0.2 |
| ICU Transfer | 3 (3.5%) | 2 (2.9%) | 0.7 |
| Cardiovascular Event | 9 (12.5%) | 2 (2.9%) | 0.03 |
| Renal Failure | 2 (2.9%) | 0 (0.0%) | 0.2 |
| Respiratory Failure | 0 (0.0%) | 0 (0.0%) | NA |
| Sepsis | 6 (8.3%) | 7 (10.0%) | 0.7 |
| Unplanned hospitalization | 17 (23.6%) | 11 (15.7%) | 0.2 |
| Total days spent in hospital | 12.0 (0.0–40.0) | 12.0 (0.0–43.0) | 0.8 |
| Discharge to rehab facility | 3 (4.2) | 4 (5.7) | 0.7 |
| Median f/u as survivors (months) | 27.2 (12.4–71.3) | 33.0 (12.8–74.3) | 0.09 |
Any adverse event describes any patient experiencing cardiovascular event, renal failure, respiratory failure, sepsis, or ICU transfer. If one patient experienced more than one adverse event, it is reported as one adverse event by this definition.
Similarly, any complication (CV, renal, respiratory, sepsis, ICU transfer, unplanned hospitalization) is reported as complication per patient. If one patient experienced more than one complication, it is reported as one in this category.
Sarcopenia:
Sarcopenia was found in 72 (51%) patients. Sarcopenic obesity was found in 32 (23%) patients. There were no significant baseline characteristics between the groups with and without sarcopenia.
Sarcopenia and Early Post-Transplant Events:
Early post-transplant complications among patients with and without sarcopenia are displayed in Table 1. One or more adverse events were found in 22 (16%) patients. Any complication (adverse events, ICU transfer, or unplanned hospitalization) occurred in 39 (27.5%) patients. Cardiovascular events were the most common, accounting for 36% of all adverse events. Patients with sarcopenia exhibited more cardiac complications (12.5%) than patients without sarcopenia (2.9%) (p=0.03). There were no significant differences in other complication rates between patients with and without sarcopenia.
Association with Survival:
The median follow-up of survivors with sarcopenia (27.2 months, range 12.4–71.3) or those without sarcopenia (33.0, range 12.8–74.3) was similar (p=.09). On multivariate analysis, sarcopenia was not associated with overall mortality (Table 2). However, increasing BMI at transplant was associated with worse OS (hazard ratio: 1.11, 95% confidence interval: 1.02–1.22, p=0.02). Sarcopenia, BMI at transplant, KPS, and outpatient transplant were tested in the multivariate analysis for OS. We additionally looked for an interaction between BMI at transplant and sarcopenia and this was not significant (hazard ratio 0.94, 95% confidence interval 0.77–1.13, p=0.50). Sarcopenia, BMI at transplant, nor age at transplant were associated with PFS, hazard ratio 1.20, 95% confidence interval 0.67–2.06, p=0.58, hazard ratio 1.02, 95% confidence interval 0.97–1.08, p=0.40, and hazard ratio 10.1, 95% confidence interval 0.97–1.08, p=0.68 respectively.
Table 2.
Multivariate analysis of overall survival
| Hazard Ratio | Confidence Interval | P value | |
|---|---|---|---|
| Sarcopenia | 1.28 | 0.47–3.48 | 0.63 |
| Outpatient Transplant | 0.47 | 0.13–1.73 | 0.26 |
| KPS | 1.24 | 0.43–3.56 | 0.69 |
| BMI at Transplant | 1.11 | 1.02–1.22 | 0.02 |
| Age at Transplant | 1.05 | 0.98–1.12 | 0.14 |
Discussion:
In this study of sarcopenia in MM patients undergoing autoHCT we make the following observations: 1) ) Sarcopenia was observed in 51% and sarcopenic obesity was observed in 23% of MM patients undergoing autoHCT, 2) despite the low rate of day 100 complications, autoHCT patients with sarcopenia were more likely to have cardiovascular complications, 3) Obesity was associated with reduced overall survival.
Sarcopenia and sarcopenic obesity have been associated with poorer outcomes in many clinical conditions.4, 5, 11, 12 Sarcopenia has been associated with dose-limiting toxicity, surgical complications, extended hospitalization, physical disability, and decreased survival.4 A recent study evaluating the significance of sarcopenia in lymphoma patients via CT analysis of muscle within the L3 vertebral level showed that sarcopenia was associated with higher non-relapse mortality, more complications, and more days spent in hospital among men who underwent autologous stem cell transplant for different types of lymphoma.5 Similarly, we report that sarcopenia is associated with higher incidence of cardiovascular complications in MM patients undergoing autologous stem cell transplant. Previous studies have reported that sarcopenia was independently associated with higher prevalence of cardiometabolic risk factors and established atherosclerotic cardiovascular disease, even in otherwise “healthy” populations.13–15 Although the detailed mechanism requires further investigation, it has been hypothesized that the decrease in myocytes, a cell type whose endocrine role involves the secretion of myokines with beneficial cardiovascular effects, seen in sarcopenia could contribute to the association with poorer cardiovascular status in these patients.14 Other studies hypothesize that both sarcopenia and cardiovascular disease share common pathways such as insulin resistance, decreased physical activity, and/or a high-inflammatory state that contribute to the development of both conditions.15 Similar pathology may be at play here, supporting the relationship between sarcopenia and post-transplant cardiovascular complication in MM patients.
We report a 51% prevalence of sarcopenia in our study. We did not find an association with sarcopenia and overall survival. A 2018 study evaluating sarcopenia in lymphoma patients undergoing autologous or allogeneic HCT by assessing muscle composition at the L3 indexed to height reported similar prevalence of sarcopenia (47–55%) similar to that reported here. Sarcopenia was also not related to clinical outcomes post-autologous transplant in that population.16 Within MM, the evidence regarding sarcopenia and survival is unclear. One study found that morphometric analysis of the psoas muscle using CT is predictive of OS in MM patients with spinal metastases.17 Another study evaluating sarcopenia in MM patients by assessing total psoas area found that sarcopenia did not predict overall survival in that cohort.18 The use of differing methods to evaluate sarcopenia may explain these inconsistent findings within the MM literature. Some studies have used total psoas area while others have used high-density area indexed to a standard (either height or total psoas area). These differences in methodology could account for the mixed evidence regarding the significance of sarcopenia in MM currently laid out in the literature.
Given that obesity and age are both risk factors common to both MM and to sarcopenia, we hypothesized there would be high rates of sarcopenia in MM patients.3, 9 Aging leads to changes in body composition such as increase in visceral fat and reduced muscle mass.3 MM is a cancer of older adults with 30% of patients being over the age of 75.9 Obesity is associated with increased incidence of MM and mortality from MM.9 Obesity may also potentiate the development of sarcopenia.3 The considerable roles that age and obesity play in disease processes led us to hypothesize that sarcopenia would be prevalent in MM. Contrasting our hypothesis are study results from Japan showing that low subcutaneous adipose tissue measured on CT was associated with decreased overall survival in a small study of newly diagnosed MM patients.19 These conclusions may stem from the fact that these studies are conducted in very different patient populations. For example, rates of obesity in Japan and obesity-related consequences differ greatly from those in the United States. While we did not find an association between sarcopenia and survival in MM, our data did show a negative association of obesity in MM. Similar to our study, Mishra et al report an increased fat index in patients undergoing allogeneic HCT was associated with worse overall survival.20 It has been postulated that obesity perpetuates a high-inflammatory state as adipocytes release inflammatory markers that could contribute to inferior OS.21 In contrast, other studies, report an association between obesity and improved survival, potentially due to larger energy stores. Specific to myeloma, Vogl et al describe an association between higher BMI and improved PFS, OS, and risk of progression thought to be potentially related to how anti-myeloma treatments distribute throughout the body in obese patients.22 This phenomenon has been described as the obesity paradox.23 These data suggest that body composition at either extreme could play a role in the disease process, response to treatment, and outcomes.
MM patients undergo rigorous treatment regimens including chemotherapy with steroids, autoHCT, as well as post-transplant continuous maintenance therapy. These interventions, though life prolonging, are likely to affect body composition. It is well known that steroids increase body fat as well as contribute to muscle atrophy, weakness, and fatigue.24 Recent literature identifies chemotherapy itself as a direct contributor to sarcopenia. Proposed mechanisms include: 1) impaired food intake with reduction in vitamin D, omega 3 fatty acids and protein, 2) reduced physical activity secondary to fatigue, 3) the direct effect of chemotherapy or targeted agents on muscle, and 4) malabsorption secondary to mucositis or treatment related pancreatic insufficiency.25 Lifestyle interventions can modify sarcopenia.26–29 The goals of these interventions are to decrease fat mass, improve fat-associated dysregulation of metabolism and inflammation, increase muscle mass, and improve muscle strength and physical function.27 Studies show that low-calorie, high-protein diets in conjunction with physical activity with a focus on aerobic exercise resistance training are well-established interventions that have significantly improved muscle strength and composition and also decrease fat mass.26–28 Other novel therapies under investigation include pharmacologic therapies such as myostatin inhibitors and testosterone, among others.26, 28 Ongoing studies are needed to see if pharmacological therapies also have a role in the treatment of sarcopenia.26 Although various studies have identified ways to modify sarcopenia, interventions aimed at modifying sarcopenia in patients with MM have not been explored.27
Our study is limited by a relatively small sample size, low incidence of post-transplant complications, and an arbitrary definition of sarcopenia. Further investigation is needed to better characterize the prognostic significance of sarcopenia and sarcopenic obesity in this population. Future directions to achieve this will incorporate gender and aged-matched controls to better define sarcopenia caused by MM, analyzing serial scans to assess how changes in body composition affect disease course in the longer term, and expanding our study population. We conclude that sarcopenia and sarcopenic obesity are prevalent in MM patients undergoing autoHCT. This study illustrates that even though MM patients undergoing autoHCT have an inherently low rate of early post-transplant complications, cardiovascular events, the most common early post-transplant complication, are significantly more common among MM patients with sarcopenia. Obesity, regardless of sarcopenia, is associated with worse overall survival in MM. Our study generates hypothesis-generating data to risk-stratify patients being considered for autoHCT.
Acknowledgements and Grant Support:
This work was funded by the 2019 American Society of Hematology Minority Medical Student Award Program (AW) and supported by a K23HL141445 from the National Heart, Lung, and Blood Institute (AD). Its contents are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: AW, DB, JP, AS, SC, SJ and MS do not report any conflict of interest. BD reports grants and/or fees from Celgene, Takeda, Jannsen, Amgen, GSK; PH reports grants and fees from Celgene/BMS, Takeda, Janssen, Amgen, Sanofi, Karyopharm; AD reports grants Takeda, Sanofi, TeneoBio, EDO Mundipharma and Prothena, and fees from Prothena, Pfizer, Akcea, Imbrium
References
- 1.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010; 1(2): 129–133. e-pub ahead of print 2011/04/09; doi: 10.1007/s13539-010-0014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walston JD. Sarcopenia in older adults. Current opinion in rheumatology 2012; 24(6): 623–627. e-pub ahead of print 2012/09/08; doi: 10.1097/BOR.0b013e328358d59b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi KM. Sarcopenia and sarcopenic obesity. The Korean journal of internal medicine 2016; 31(6): 1054–1060. e-pub ahead of print 2016/11/04; doi: 10.3904/kjim.2016.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carneiro IP, Mazurak VC, Prado CM. Clinical Implications of Sarcopenic Obesity in Cancer. Current oncology reports 2016; 18(10): 62. e-pub ahead of print 2016/08/20; doi: 10.1007/s11912-016-0546-5 [DOI] [PubMed] [Google Scholar]
- 5.Caram MV, Bellile EL, Englesbe MJ, Terjimanian M, Wang SC, Griggs JJ et al. Sarcopenia is associated with autologous transplant-related outcomes in patients with lymphoma. Leukemia & lymphoma 2015; 56(10): 2855–2862. e-pub ahead of print 2015/03/06; doi: 10.3109/10428194.2015.1014359 [DOI] [PubMed] [Google Scholar]
- 6.Lanic H, Kraut-Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leukemia & lymphoma 2014; 55(4): 817–823. e-pub ahead of print 2013/06/21; doi: 10.3109/10428194.2013.816421 [DOI] [PubMed] [Google Scholar]
- 7.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M et al. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019, 2019. [Google Scholar]
- 8.D’Souza A, Zhang MJ, Huang J, Fei M, Pasquini M, Hamadani M et al. Trends in pre- and post-transplant therapies with first autologous hematopoietic cell transplantation among patients with multiple myeloma in the United States, 2004–2014. Leukemia 2017; 31(9): 1998–2000. e-pub ahead of print 2017/07/01; doi: 10.1038/leu.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sergentanis TN, Zagouri F, Tsilimidos G, Tsagianni A, Tseliou M, Dimopoulos MA et al. Risk Factors for Multiple Myeloma: A Systematic Review of Meta-Analyses. Clinical lymphoma, myeloma & leukemia 2015; 15(10): 563–577 e561–563. e-pub ahead of print 2015/08/22; doi: 10.1016/j.clml.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 10.Patel JJ, Baruah D, Sobush D, Koester K, Aase J, Zellner S et al. Identifying High-Attenuating and Low-Attenuating Muscle Using Computerized Tomography and Exploring Its Impact on Physical Function and Muscle Strength in Obese Critically Ill Patients. Nutr Clin Pract 2020; 35(1): 133–141. e-pub ahead of print 2019/06/07; doi: 10.1002/ncp.10325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodewick TM, van Nijnatten TJ, van Dam RM, van Mierlo K, Dello SA, Neumann UP et al. Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB (Oxford) 2015; 17(5): 438–446. e-pub ahead of print 2014/12/17; doi: 10.1111/hpb.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2009; 15(22): 6973–6979. e-pub ahead of print 2009/11/06; doi: 10.1158/1078-0432.CCR-09-1525 [DOI] [PubMed] [Google Scholar]
- 13.Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PloS one 2013; 8(3): e60119. e-pub ahead of print 2013/03/28; doi: 10.1371/journal.pone.0060119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang DO, Park SY, Choi BG, Na JO, Choi CU, Kim EJ et al. Prognostic Impact of Low Skeletal Muscle Mass on Major Adverse Cardiovascular Events in Coronary Artery Disease: A Propensity Score-Matched Analysis of a Single Center All-Comer Cohort. J Clin Med 2019; 8(5). e-pub ahead of print 2019/05/22; doi: 10.3390/jcm8050712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko BJ, Chang Y, Jung HS, Yun KE, Kim CW, Park HS et al. Relationship Between Low Relative Muscle Mass and Coronary Artery Calcification in Healthy Adults. Arterioscler Thromb Vasc Biol 2016; 36(5): 1016–1021. e-pub ahead of print 2016/04/02; doi: 10.1161/ATVBAHA.116.307156 [DOI] [PubMed] [Google Scholar]
- 16.DeFilipp Z, Troschel FM, Qualls DA, Li S, Kuklinski MW, Kempner ME et al. Evolution of Body Composition Following Autologous and Allogeneic Hematopoietic Cell Transplantation: Incidence of Sarcopenia and Association with Clinical Outcomes. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018; 24(8): 1741–1747. e-pub ahead of print 2018/03/03; doi: 10.1016/j.bbmt.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 17.Zakaria HM, Elibe E, Macki M, Smith R, Boyce-Fappiano D, Lee I et al. Morphometrics predicts overall survival in patients with multiple myeloma spine metastasis: A retrospective cohort study. Surg Neurol Int 2018; 9: 172. e-pub ahead of print 2018/09/14; doi: 10.4103/sni.sni_383_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhamidipati PK, Carson KR, Wildes T. Psoas Cross-Sectional Area As Radiographic Measure Of Sarcopenia Does Not Predict Overall Survival In Multiple Myeloma Blood 2013; 122: 5326. doi: 10.1182/blood.V122.21.5326.5326 [DOI] [Google Scholar]
- 19.Takeoka Y, Sakatoku K, Miura A, Yamamura R, Araki T, Seura H et al. Prognostic Effect of Low Subcutaneous Adipose Tissue on Survival Outcome in Patients With Multiple Myeloma. Clinical lymphoma, myeloma & leukemia 2016; 16(8): 434–441. e-pub ahead of print 2016/06/06; doi: 10.1016/j.clml.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 20.Mishra A, Pidala JA, Betts B, Fernandez H, Nishihori T, Extermann M et al. Body Composition Predicts for Survival in Allogeneic (allo) Hematopoietic Stem Cell Transplantation (HCT) Recipients. Biol Blood Marrow Tr 2016; 22(3): S69–S69. doi: DOI 10.1016/j.bbmt.2015.11.361 [DOI] [Google Scholar]
- 21.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 2017; 13(4): 851–863. e-pub ahead of print 2017/07/20; doi: 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogl DT, Wang T, Perez WS, Stadtmauer EA, Heitjan DF, Lazarus HM et al. Effect of obesity on outcomes after autologous hematopoietic stem cell transplantation for multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2011; 17(12): 1765–1774. e-pub ahead of print 2011/06/01; doi: 10.1016/j.bbmt.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care 2015; 18(6): 535–551. e-pub ahead of print 2015/09/04; doi: 10.1097/MCO.0000000000000216 [DOI] [PubMed] [Google Scholar]
- 24.Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert opinion on drug safety 2016; 15(4): 457–465. e-pub ahead of print 2016/01/21; doi: 10.1517/14740338.2016.1140743 [DOI] [PubMed] [Google Scholar]
- 25.Davis MP, Panikkar R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. Ann Palliat Med 2019; 8(1): 86–101. e-pub ahead of print 2018/12/12; doi: 10.21037/apm.2018.08.02 [DOI] [PubMed] [Google Scholar]
- 26.Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes 2013; 20(5): 412–419. e-pub ahead of print 2013/08/27; doi: 10.1097/01.med.0000433071.11466.7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu KJ, Liao CD, Tsai MW, Chen CN. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019; 11(9). e-pub ahead of print 2019/09/12; doi: 10.3390/nu11092163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koliaki C, Liatis S, Dalamaga M, Kokkinos A. Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives. Curr Obes Rep 2019; 8(4): 458–471. e-pub ahead of print 2019/10/28; doi: 10.1007/s13679-019-00359-9 [DOI] [PubMed] [Google Scholar]
- 29.Smith L, McCourt O, Henrich M, Paton B, Yong K, Wardle J et al. Multiple myeloma and physical activity: a scoping review. BMJ Open 2015; 5(11): e009576. e-pub ahead of print 2015/11/29; doi: 10.1136/bmjopen-2015-009576 [DOI] [PMC free article] [PubMed] [Google Scholar]