Abstract
Objective:
Describe NICU admission rate variation among hospitals in infants with birthweight ≥2500g and low illness acuity and describe factors that predict NICU admission.
Study Design:
Retrospective study from the Vizient Clinical Data Base/Resource Manager®. Support vector machine methodology was used to develop statistical models using 1) patient characteristics 2) only the indicator for the inborn hospital and 3) patient characteristics plus indicator for the inborn hospital.
Results:
NICU admission rates of 427,449 infants from 154 hospitals ranged from 0–28.6%. C-statistics for the patient characteristics model: 0.64 (Confidence Interval (CI) 0.62–0.65), hospital only model: 0.81 (CI, 0.81–0.82) and patient characteristic plus hospital variable model: 0.84 (CI, 0.83–0.84).
Conclusion/Relevance:
There is wide variation in NICU admission rates in infants with low acuity diagnoses. In all cohorts, birth hospital better predicted NICU admission than patient characteristics alone.
Introduction
Admission rates to the Neonatal Intensive Care Unit (NICU) among all birthweight categories have risen over the past decade.1 Near-term and term infants account for the largest percentage of infants admitted to the NICU.2 It is known that the majority of near-term and term infants admitted to the NICU do not have diagnoses associated with high illness acuity.2,3 A recent investigation from the California Perinatal Quality Care Collaborative (CPQCC) showed that near-term and term infants with high illness acuity accounted for only 11.9% of NICU admissions, but that there was a 34-fold variation in NICU admission rates among inborn neonates across participating institutions.2 Although variability in NICU admission criteria for high acuity conditions has been well studied, little is known about NICU admission practices for healthy near-term or term infants or those with common low acuity conditions.2–4
Because a wide range of illness severity is associated with NICU admission in the near-term and term infant population, there is growing concern that the decision to admit these infants is driven by differences in hospital practices and may represent overuse of NICU resources.2,3,5–7 The objective of this study is to describe variation in NICU admission in near-term and term neonates with common low acuity conditions and identify patient and hospital-level characteristics associated with NICU admission in this population.
Methods
Data
Data was obtained from the Vizient (formerly University HealthySystem Consortium) Clinical Data Base/Resource Manager® (CDB/RM). This database contains patient-level and discharge abstract (hospitalization-level) data from over 95% of academic medical centers and affiliated hospitals across the country. Vizient data are used to provide benchmarking measures to participating institutions to monitor healthcare resource utilization. Because all infants at participating hospitals are included in this dataset, data is available for healthy infants and infants that required admission to the NICU. Further details of the Vizient database are available from Loehrer et al.8 Vizient data has also been used in several previous investigations to study neonatal and pediatric care.9–13 The Institutional Review Board (IRB) at the University of California, San Francisco approved this study. Need for informed consent was waived by the IRB.
Code availability
Data was directly downloaded from the Vizient website and was cleaned and analyzed using Rstudio version 1.1.463 and Python-Spyder version 2.7. Computer code used in this study may be available from the authors after approval by the University of California, San Francisco IRB.
Study Population
We extracted data on infants with birthweight ≥2500g who were born between 2/1/2013 and 8/1/2017 and discharged home alive.14,15 This birthweight cutoff was used because the World Health Organization defines low birthweight as <2500g.16,17 We were unable to use gestational age because this information was not available for all infants. Hospitals were excluded if they reported fewer than 100 total inborn neonates, no NICU admissions, if NICU admission rates were less than the 1st percentile or greater than 99th percentile, or if over 5% of birthweights reported were extreme (defined as birthweight < 500g or >5000g) over the entire study period (Figure 1). All Patient Refined Diagnosis Related Groups Severity of Illness (APRSOI) scores range from 1 (lowest illness severity) to 4 (highest illness severity) and were generated by Vizient by applying 3M’s APR-DRG® grouper software. This software assigns severity of illness scores based on a combination of the patient’s principal diagnosis, secondary diagnoses (as defined by ICD diagnosis codes), age, and non-operative procedures performed. The APRSOI is currently a standard for benchmarking inpatient severity of illness, hospital performance, and hospital quality and has been validated to adjust for severity of illness in clinical settings.18–20 APR-DRGs were used because these diagnosis codes have been shown to have the best statistical performance when compared to other commonly used diagnosis codes.21 We excluded the 81 infants with an APRSOI over 2 because we felt that a high severity of illness in such a small number of patients in this low illness acuity cohort was likely due to inaccurate diagnosis coding.
Figure 1:
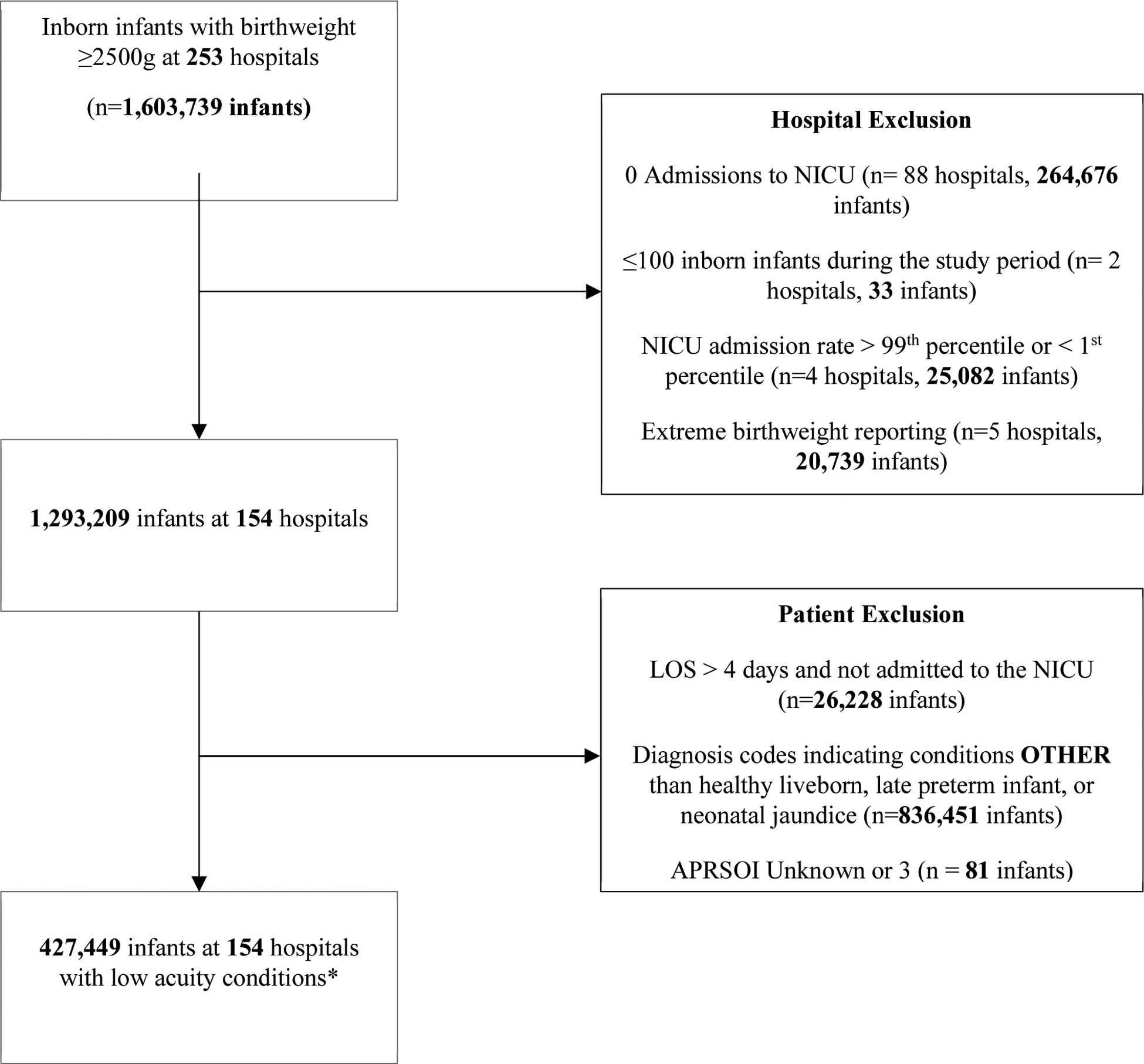
Flowchart for Inclusion and Exclusion Criteria
*Low acuity conditions defined in Table 1.
We identified low acuity conditions using categories from the Clinical Classifications Software (CCS) developed by the Agency for Healthcare Research and Quality.22 Individual CCS diagnosis categories are comprised of groups of ICD 9 or ICD 10 diagnosis codes. Because all infants had a primary diagnosis and many infants had secondary diagnoses, primary and secondary CCS categories were included for analysis. Infants having only a CCS category of being a “liveborn” were included in the low acuity conditions cohort because it was assumed the infant was a healthy liveborn (CCS code 218). However, based on literature and clinical experience, we also included patients who had only perinatal jaundice (CCS code 222) or late preterm birth (CCS code 219) (Table 1).23,24 Within the CCS categories of interest (i.e., liveborn, perinatal jaundice, or preterm birth) were some ICD diagnosis codes suggesting higher acuity conditions (Table 1). These included diagnosis codes possibly indicating birthweight less than 2500g, gestational age less than 34 weeks, or pathologic jaundice. We excluded infants who had these codes. A full list of included ICD diagnosis codes is shown in Supplemental Table 1. We also excluded newborns who were never admitted to the NICU or neonatal stepdown unit and had a length of stay greater than 4 days. This cutoff was chosen because even healthy babies and their mothers are permitted, under federal law, stays of up to 4 days if the delivery was via C-section.25
Table 1:
Study Population
| Inclusion Criteria | Exclusion Criteria | ||
|---|---|---|---|
| Cohort Names | CCS Category | CCS Description | Description |
| Healthy Liveborn | 218 | Liveborn | Assignment of any other CCS code |
| Low Acuity Neonatal Jaundice | 222 | Hemolytic Jaundice and Perinatal Jaundice | Assignment of any other CCS code or ICD9/10 diagnosis codes indicating pathologic jaundice1 |
| Low Acuity Late Preterm | 219 | Short gestation; low birthweight; and fetal growth retardation | Assignment of any other CCS code or ICD9/10 diagnosis codes indicating birthweight < 2500g or gestational age < 34 weeks2 |
| Total Low Acuity Infants | 218,222,219 | Above combination of above descriptions | Assignment of any other CCS code other than 218,219,222 or ICD9/10 codes indicating pathologic jaundice1 or ICD 9/10 codes indicating birthweight < 2500g or gestational age < 34 weeks2 |
Pathologic jaundice ICD9 and 10 codes include: 7744, 7747, 7733, 7734, P5929, P578, P579, P570, P5690, P560, P5699
Birthweight < 2500g or gestational age < 34 weeks ICD9 and 10 codes include: 76404,76414, P0726,P0506, P0700, 76496, 76405, 76493, 76422, P0731, P059, V2132, 76498,76409,76418, P0733, P0724, P0511, 76502, 76495, 76501, 76505, V2134, P0734, P0512, P0503, 76516, 76517, 76522, 76524, 76525, 76526, 76503, 76513, 76492, 76512, 76523, 76511, 76406, 76402,76403, 76504, 76521, 76401, 76491, 76494,76412, 76416, 76506, V2133, 76415, 76413, 76426, 76424, 76425, 76423, P0735, P0508, P0734, 76525, 76517, 76498, P0717, P0733, 76524, P0517, 76515,76497,V2135, P0717, P0508, 76518, P0718, P0518, 76527, 76411, 76421, P0702, P0715, P0720, P0716, P0735, P0736, P0514, P0516, P0703, P0714, P0725, P0732, P0723, P0701, P0721, P0515, P0722, P0722, P0505, P0501, P0502, P0504, 76527, P0736, P0518, P0718, 76526, 76518
Outcome
The outcome of interest was whether an infant was admitted to the inborn NICU. Contributing hospitals specified whether an infant was admitted to a N2ICU based on the number of days the patient was admitted to the intensive care unit.
Statistical Analysis
Descriptive statistics were used to characterize patient demographics. Data summaries are presented with median [interquartile range] for data that are not normally distributed. NICU admission rates at each hospital were calculated for each population and then stratified by geography as defined by census regions and hospital teaching status. Chi-squared tests were used to compare hospital characteristics between the cohorts.
Machine learning methodologies have been shown to produce more accurate predictive models than traditional statistical models. In particular the support vector machine (SVM) algorithm is a machine learning algorithm for estimating linear classifiers that is often more accurate than logistic regression.26 We used SVM methodology using the “scikit-learn” package in Python to develop the statistical models predicting NICU admissions in each of the populations.26 The coefficients of the SVM models were converted to odds ratios using Platt scaling. The Platt scaling converts the SVM model coefficients into odds ratios by fitting a logistic regression model to the score of the SVM model.27 Confidence intervals for the point estimates were computed using bootstrap, which is a machine learning methodology that uses Monte Carlo resampling to construct confidence intervals. Models using traditional logistic regression methodology were also used to compute odds ratios and confidence intervals of predictor variables in order to act as a sensitivity analysis. The results of the SVM and logistic regression models were quantitatively (in terms of C-statistic and odds ratios) and qualitatively (in terms of increased versus decreased odds for particular variables) compared.
Three machine learning SVM models were developed for each patient cohort. The first “Patient Characteristic” model used sex, birthweight, night admission, weekend admission, and birth year as predictors. The second “Hospital Only” model used only identifiers specifying the hospital in which the infant was born as a predictor variable. The third “Patient Characteristic Plus Hospital” model used all predictor variables from the first and second models. We calculated c-statistics and plotted receiver operating curves (ROCs) to assess model discrimination in predicting whether an infant would be admitted to the NICU. Hospital odds ratios are summarized with the median odds ratio of NICU admission between two randomly selected hospitals with clusters compared in descending order so that the odds ratios were always greater than 1.28–30 A sensitivity analysis using machine learning SVM models was also performed on all cohorts, where patients only with an APRSOI of 1 (lowest illness severity) were included. To adjust for possible coding practice variation, a second sensitivity analysis using SVM models was also performed on a cohort of patients that included any patient that had a low acuity diagnosis, including infants previously excluded due to hospital level factors. Additionally, in this analysis, we re-defined presence of a NICU admission as having a length of stay (LOS) > 2 days for patients delivered vaginally, > 4 days for patients delivered via cesarean section or if method of delivery was unknown.
Results
After applying exclusion criteria, a total of 154 hospitals were included in the study (Figure 1); 97 (63%) hospitals were teaching hospitals. All census regions are represented in the cohort of 154 hospitals. These hospitals reported 1,293,209 births. Among these births, 427,449 infants had CCS categories associated with low acuity conditions (healthy liveborn, neonatal jaundice, or late preterm) (Figure 1). Infants had a range of diagnoses within each diagnosis category (e.g., some infants with a CCS code of 218 (“Healthy Liveborn” cohort) had ICD diagnosis codes associated with observation for suspected infection or had ICD procedure codes associated with assistance with respiratory ventilation for less than 24 hours). The demographic and clinical characteristics of patients by CCS category are shown in Table 2. Consistent with our objective to define a cohort of low acuity infants, the APRSOI was 1 (the lowest illness severity) for 96.9% of the cohort, and APRSOI was 2 for 3.1%. Among all low acuity infants, 358,793 (84%) infants only had a CCS category of healthy liveborn.
Table 2:
Demographics
| Variable | Healthy Liveborn | Neonatal Jaundice | Late Preterm | Total Low Acuity Infants |
|---|---|---|---|---|
| Total | 358,793 | 58,191 | 10,465 | 427,449 |
| Sex (Male) | 159,848 (44.6%) | 25,250 (43.3%) | 5,076 (48.5%) | 190,172 (%) |
| Birthweight (grams) | 3320[3062–3590], 3332 | 3300[3045–3569], 3311 | 2755[2610–2948], 2809 | 3310 [3040–3578], 3317 |
| APRSOI | 1[1–1], 1.0 | 1[1–1], 1.2 | 1[1–1],1.2 | 1[1–1], 1.0 |
| Percent NICU Admit (Total) | 1,569 (0.4%) | 1,180 (2.0%) | 454 (4.3%) | 3,203 (0.75%) |
| APRSOI | ||||
| APRSOI = 1 | 1,557/358,353 (0.4%) | 673/47,563 (1.4%) | 400/8,126 (4.9%) | 2,630/ 414,047(0.6%) |
| APRSOI = 2 | 12/435 (2.8%) | 507/10,628 (4.8%) | 54/2,339 (2.3%) | 573/13,402(4.3%) |
| Hospital LOS (total) | 2[2–2], 2.1 | 2[2–3], 2.4 | 2[2–3], 2.4 | 2[2–2], 2.1 |
| Hospital LOS (never admitted to ICU) | 2[2–2],2.1 | 2[2–3], 2.3 | 2[2–3], 2.3 | 2[2–2], 2.1 |
| Hospital LOS (admitted to NICU) | 2[2–3], 2.7 | 3[3–4], 3.6 | 3[2–4], 3.3 | 3[2–4], 3.1 |
| Race/ Ethnicity | ||||
| White | 162,152 (45.2%) | 24,395 (41.9%) | 4,035 (38.6%) | 190,582 (44.6%) |
| Black | 55,782 (15.5%) | 7,286 (12.5%) | 2,208 (21.1%) | 65,276 (15.3%) |
| Hispanic | 57,395 (16.0%) | 12,858 (22.1%) | 1,828 (17.5%) | 72,081 (16.9%) |
| Asian | 17,388 (4.9%) | 4,038 (6.9%) | 493 (4.7%) | 21,919 (5.1%) |
| Unknown | 66,076 (18.4%) | 9,614 (16.5%) | 1,901 (18.2%) | 77,591 (18.2%) |
| Insurance | ||||
| Commercial | 191,618 (53.4%) | 30,323 (52.1%) | 4,767 (45.5%) | 226,708 (53.0%) |
| Government | 148,754 (41.5%) | 25,380 (43.6%) | 5,078 (48.5%) | 179,212 (41.9%) |
| Other | 18,421 (5.1%) | 2,488 (4.3%) | 620 (5.9%) | 21,529 (5.0%) |
| Number of Hospitals | 146 | 146 | 151 | 154 |
APRSOI = All Patient Refined Diagnosis Related Groups Severity of Illness, LOS = Length of Stay. Due to rounding totals of percentages in some categories equals 99.9. Sex, percent NICU admit, race/ethnicity, and insurance data presented as total number of patients (percentage of patients). Birthweight, APRSOI, LOS data presented as median [interquartile range], mean.
There was a statistically significant difference in rates of NICU admission by geographic region for all patient cohorts (Supplemental Table 2). Admission of late preterm infants to the NICU was also statistically significant when comparing teaching hospitals to non-teaching hospitals (4.1% vs 5.2%, respectively) (Supplemental Table 2). In the total low acuity infant cohort, there were 22 hospitals that had no NICU admissions for infants with any of the low acuity diagnoses. Of hospitals that admitted any low acuity patients to the NICU, admission rates ranged from 0.02% to 28.6% (median NICU admission rate of 0.5%). The late preterm cohort had the largest range of NICU admission rates across hospitals (0% – 47.4%) while the healthy liveborn cohort had the narrowest range of NICU admissions rates (0%–7.5%). The neonatal jaundice cohort also had wide variation in NICU admission rates (0%–33.5%). Distribution of hospital NICU admission rates by cohort is shown in Supplemental Figure 1.
In machine learning models for NICU admission, discrimination (measured by the c-statistic) among total low acuity infants using the patient characteristic model was 0.64 (95% Confidence Interval (CI), 0.62–0.65) and 0.81 (CI, 0.81–0.82) for the hospital model (Figure 2). Addition of patient characteristics to the hospital only model improved model discrimination to 0.84 (CI, 0.83–0.84) (Table 3). Sex did not substantially increase risk for NICU admission (Table 3). Similarly, models using only the hospital variable had higher c-statistics than the models using patient characteristics in each low acuity cohort, with minimal improvement after addition of patient characteristics (Table 3, Supplemental Table 3). Model discrimination for the hospital model was highest (0.89, CI 0.88–0.90) in the neonatal jaundice cohort (Supplemental Table 3). A sensitivity analysis of infants with an APRSOI of 1 also demonstrated that model discrimination was best for models that included inborn hospitals as a variable (Supplemental Tables 4 and 5). The SVM algorithm had equivalent discrimination (measured by the c-statistic) when compared to a statistical model computed with logistic regression in all cohorts (Supplemental Tables 6 and 7). The sensitivity analysis done to adjust for possible coding practice variation demonstrated the same trend described above where the AUC for the hospital only model was better than the AUC for the patient characteristic model for all cohorts (Supplemental Tables 8–10).
Figure 2:
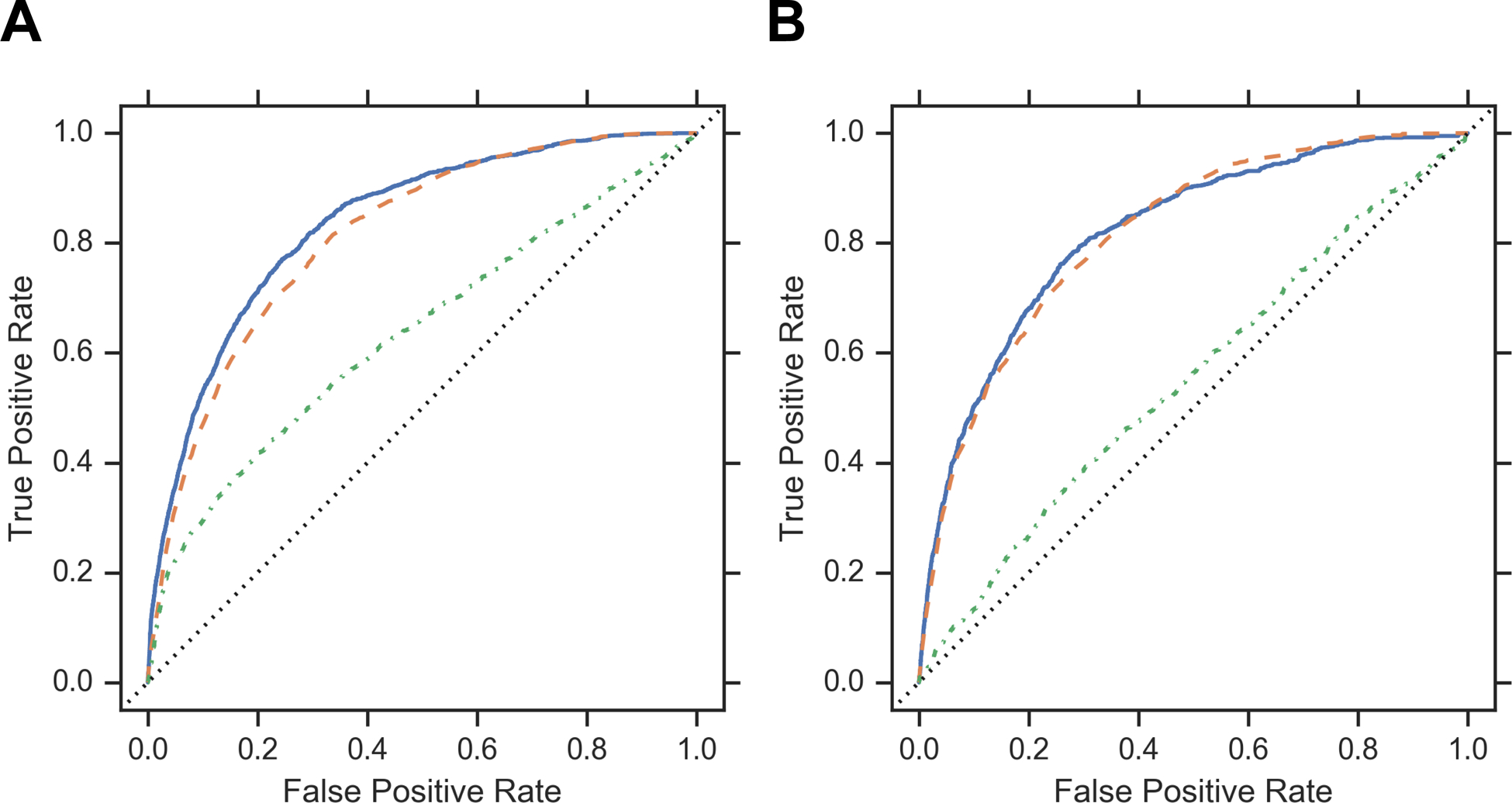
ROC Curves
Panel A: ROC Curves for All Low Acuity Infants. C-Statistics for the following models: “Clinical Variables plus Hospital Variable” model is 0.83 (0.83–0.83), “Hospital Only” model is 0.80 (0.80–0.81), and “Clinical Variables” model is 0.65 (0.65–0.66). Panel B: ROC Curves for Healthy Liveborn Infants. C-Statistics for the following models: “Clinical Variables plus Hospital Variable” model is 0.81 (0.80–0.82), “Hospital Only” model is 0.81 (0.80–0.82), “Clinical Variables” model is 0.54 (0.52–0.56)
Table 3:
Characteristics Associated with NICU Admission and Model Discrimination in Healthy Liveborn and Total Low Acuity Infants (Machine Learning Model)
| Healthy Liveborn | Total Low Acuity Infants | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Patient Characteristics | Hospital Only | Patient Characteristics Plus Hospital | Patient Characteristics | Hospital Only | Patient Characteristics Plus Hospital | |
| APRSOI 1 OR [CI] |
Ref = 1 | - | Ref = 1 | Ref = 1 | - | Ref = 1 | |
| APRSOI 2 | 3.95 (1.81–7.35) | 11.6 (3.13–46.9) | 7.04 (6.44–7.78) | 6.95 (5.82–9.15) | |||
| Birthweight OR [CI] |
1.00 (0.99–1.00) | - | 1.00 (0.99–1.00) | 0.99 (0.99–0.99) | - | 0.99 (0.98–0.99) | |
| Male OR [CI] |
1.05 (1.02–1.08) | - | 1.09 (1.05–1.16) | 1.10 (1.07–1.14) | - | 1.13 (1.08–1.18) | |
| Weekend OR [CI] |
1.01 (0.98–1.04) | - | 1.03 (0.98–1.08) | 0.98 (0.95–1.02) | - | 0.99 (0.95–1.04) | |
| Year OR [CI] |
1.02 (1.01–1.04) | - | 1.56 (1.03–1.09) | 0.99 (0.99–0.99) | - | 1.02 (1.01–1.04) | |
| Median Hospital OR [IQR] |
- | 1.57 [1.22–2.29] | 1.77 [1.27–4.67] | - | 1.31 [1.06–1.95] | 2.01 [1.30–6.88] | |
| C-statistic [CI] | 0.55 (0.53–0.57) | 0.82 (0.81–0.82) | 0.82 (0.81–0.83) | 0.64 (0.62–0.65) | 0.81 (0.81–0.82) | 0.84 (0.83–0.84) | |
After adjusting for patient characteristics, NICU admission rates strongly correlated with the inborn hospital in all cohorts. Hospital odds ratio for NICU admission in late preterm infants was the strongest factor associated with NICU admission (median odds ratio 1.52). Severity of illness had the highest odds associated with NICU admission in all cohorts but the late preterm cohort. Odds ratio for APRSOI in the late preterm cohort was 0.40 (CI 0.27–0.57); however, NICU admission rates for APRSOI 1 was 0.6%, compared to 4.3% for APRSOI 2 in this cohort (Table 2, Supplemental Table 3).
Discussion
This study describes NICU admission rates among near-term and term infants with low illness acuity diagnoses among a large cohort of hospitals in the United States. For all populations, the statistical model that only included the hospital in which the infant was born had a higher c-statistic than models using all available patient characteristics but not an indicator for hospital. The impact of hospital was sufficiently strong that adding patient characteristics to the model with hospital alone did not increase c-statistics in a meaningful way. This also held true in the sensitivity analysis for illness acuity, which included only infants with APRSOI of 1.
Although low acuity conditions were selected, some infants had higher APRSOI scores than others because elements such as procedures and combination of secondary diagnoses also factored into APRSOI calculations. Among patient characteristics, APRSOI had high calculated odds ratios (e.g., 6.95, CI (5.82–9.15) among total low acuity infants), making it the patient characteristic most strongly associated with NICU admission. However, because only 3.7% of the total population had an APRSOI score above 1, this variable only influenced predicted probability of NICU admission for a small minority of infants. For this reason, even though APRSOI had a very large coefficient, the models with patient characteristics only had low overall predictive power.
These findings are consistent with previous findings that wide variations of NICU admission rates exist in higher acuity conditions for near-term and term infant populations.2–4 Supplemental Figure 1 shows that hospitals varied widely in NICU admission rates for each CCS category. This is similar to findings involving high acuity admissions from the Vermont Oxford Network, a database of patients that represents about 40% of NICU admissions in the United States. Studies using this database demonstrated that NICU admission rates ranged from 0–44% in term infants with 15% of admissions being classified as high acuity admissions.4
We extend these findings as our study shows wide variation in NICU admission practices in a group of infants who should have an even lower likelihood to be admitted to the NICU. This highlights the possibility that variation in NICU admission rates may reflect hospital related factors such as differences in clinicians’ comfort with or hospital policies about managing patients outside the ICU. Another hospital-specific issue could be the supply of NICU beds, with hospitals with few beds using them rarely for low acuity infants while hospitals with more beds use them because they are available. Additionally, we also found statistically significant differences in NICU admission rates across geographic regions among all patient cohorts.
These findings raise the question of whether variation in hospital NICU admission rates can be primarily explained by differences in hospital practices and workflow. We initially speculated that the neonatal jaundice cohort would have the least amount of variation for NICU admission because there are well established guidelines for the initiation of phototherapy.31 However, NICU admission rates in the neonatal jaundice cohort varied from zero to about one third of neonates being admitted.
We speculate that several factors contribute to this observation. First, there may be hospitals where a high proportion of infants receiving phototherapy are not admitted to the NICU and other hospitals where the opposite is true. This suggests that hospital practices may contribute to differences in NICU resource utilization. Because there are uncharacterized variations in procedure documentation and codes submitted by hospitals, we could not verify that phototherapy prescribed was reported for all patients that required it. However, because phototherapy is a billable procedure, we believe that hospitals are likely to report this accurately and that the trend in differences in NICU admission practices for phototherapy is real and warrants further investigation. Secondly, there are differences in clinician practice when it comes to following the recommended phototherapy guidelines in near-term and term infants. Wickremasinghe et al. demonstrated in a Northern California Kaiser database that 19.1% of infants were treated with phototherapy when their serum bilirubin levels were below threshold.32 Lastly, there were variables that were not captured in our dataset, such as maternal and social factors as well as breastfeeding status, that may also affect the decision to admit an infant to the NICU.
One of the unique strengths of the dataset used is that it contained information on all infants born at participating hospitals. This includes infants who were healthy and never admitted to the NICU, which made it possible to calculate NICU admission rates. However, because ICD codes for gestational age were not available for all infants and because we could not link maternal records with newborn infants, we could not adjust for factors that could contribute to variability in NICU admission beyond the variables we assessed. Additionally, we were only able to assess calculated illness severity based on reported diagnosis and procedure codes because we were unable to access electronic health record data for patients. Clinical illness severity scores commonly used in neonatology such as the Score for Neonatal Acute Physiology (SNAP) were not available in this data set. Information submitted to the Clinical Data Base/Resource Manager® contained only discharge abstract data linked to deidentified patients and hospitals. The lack of ability to adjust for these clinical factors may limit the conclusions we can draw from the current analyses. Because only administrative codes were used to identify patient cohorts, it is possible that our findings reflect differences in coding practices rather than clinical practice. This is a limitation of our study. However, we believe this is unlikely. While coding of specific problems in a complicated problem list can be challenging, it is generally not difficult for coders when physicians are documenting either no problems at all or just a few, as with this cohort. Additionally, it would be very unusual for a patient to have many significant problems that physicians did not document, or coders did not record. Thus, we believe the absence of diagnoses typically associated with NICU admissions in this cohort of infants with low acuity conditions is a trend that is worth further study. Because no standardized definition of NICU level exists across states, we could not use this hospital characteristic as a predictor variable. Finally, we cannot definitively state that NICU admissions associated with high NICU utilization rates were inappropriate because we were unable to analyze physiologic data for the infants or mothers, peripartum obstetric history, or determine the level of care infants could receive outside the NICU in each hospital. However, it seems unlikely that differences in patient populations explain the wide variation we observed in NICU admission rates because patient cohorts were restricted to a homogenous group of diagnoses. This is further supported by results from the sensitivity analysis of illness severity that show that the effect sizes for the hospital variable do not change substantially. This suggests that hospital factors and not patient factors contribute most to NICU admission in this cohort of patients.
Conclusion:
Decreasing unnecessary NICU admission is important to prevent potential physical and emotional harm to patients and families and to avoid provision of low value care. There is wide variation in NICU admission rates in infants with low acuity diagnoses, and the strongest factor associated with NICU admission is the hospital in which the infant is born. Our findings, combined with similar observations among more severely ill neonates, suggest that further investigation is warranted to determine whether hospital practice patterns can be adjusted to avoid unnecessary NICU admissions.
Supplementary Material
Funding:
Support for this study was received from an NIH T32 award (HD049303-11). This work was performed at Benioff Children’s Hospital, San Francisco and the University of California, San Francisco
Footnotes
Conflict of Interest: The authors declare they have no conflict of interest
Supplementary information is available at JPER’s website.
References
- 1.Harrison W, Goodman D. Epidemiologic trends in neonatal intensive care, 2007–2012. JAMA Pediatr. 2015;169(9):855–862. doi: 10.1001/jamapediatrics.2015.1305 [DOI] [PubMed] [Google Scholar]
- 2.Schulman J, Braun D, Lee HC, et al. Association between neonatal intensive care unit admission rates and illness acuity. JAMA Pediatr. 2018;172(1):17–23. doi: 10.1001/jamapediatrics.2017.3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler KA, Paul DA, Hoffman M, Locke R. Variation in NICU Admission Rates Without Identifiable Cause. Hosp Pediatr. 2016;6(5):255–260. doi: 10.1542/hpeds.2015-0058 [DOI] [PubMed] [Google Scholar]
- 4.Edwards EM, Horbar JD. Variation in Use by NICU Types in the United States. Pediatrics. 2018;142(5):e20180457. doi: 10.1542/peds.2018-0457 [DOI] [PubMed] [Google Scholar]
- 5.Freedman S. Capacity and utilization in health care: The effect of empty beds on neonatal intensive care admission. Am Econ J Econ Policy. 2016;8(2):154–185. doi: 10.1257/pol.20120393.Capacity [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison WN, Wasserman JR, Goodman DC. Regional Variation in Neonatal Intensive Care Admissions and the Relationship to Bed Supply. J Pediatr. 2018;192:73–79.e4. doi: 10.1016/j.jpeds.2017.08.028 [DOI] [PubMed] [Google Scholar]
- 7.Carroll AE. The Concern for Supply-Sensitive Neonatal Intensive Care Unit Care If You Build Them , They Will Come. 2015:11–12. doi: 10.1377/hlthaff.w4 [DOI] [PubMed] [Google Scholar]
- 8.Loehrer AP, Chang DC, Scott JW, et al. Association of the affordable care act medicaid expansion with access to and quality of care for surgical conditions. JAMA Surg. 2018;153(3). doi: 10.1001/jamasurg.2017.5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McHugh KE, Hillman DG, Gurka MJ, Gutgesell HP. Three-stage palliation of hypoplastic left heart syndrome in the university healthSystem consortium. Congenit Heart Dis. 2010;5(1):8–15. doi: 10.1111/j.1747-0803.2009.00367.x [DOI] [PubMed] [Google Scholar]
- 10.Dean PN, Hillman DG, McHugh KE, Gutgesell HP. Inpatient Costs and Charges for Surgical Treatment of Hypoplastic Left Heart Syndrome. Pediatrics. 2011;128(5):e1181–e1186. doi: 10.1542/peds.2010-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu SK, Fernandez ID, Fisher SG, Asselin BL, Lyman GH. Length of stay and mortality associated with febrile neutropenia among children with cancer. J Clin Oncol. 2005;23(31):7958–7966. doi: 10.1200/JCO.2005.01.6378 [DOI] [PubMed] [Google Scholar]
- 12.Kane JM, Harbert J, Hohmann S, Pillai S, Behal R, selip D JT. Case Volume and Outcomes of Congenital Diaphragmatic Hernia Surgery in Academic Medical Centers. Am J Perinatol. 2015;32(9):845–852. doi: 10.1055/s-0034-1543980 [DOI] [PubMed] [Google Scholar]
- 13.Kane JM, Scalcucci J, Hohmann SF, Johnson T, Behal R. Using Administrative Data for Mortality Risk Adjustment in Pediatric Congenital Cardiac Surgery. Pediatr Crit Care Med. 2013;14(5):491–498. doi: 10.1097/pcc.0b013e31828a87ea [DOI] [PubMed] [Google Scholar]
- 14.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical Outcomes of Near-Term Infants. Pediatrics. 2004;114(2):372–376. doi: 10.1542/peds.114.2.372 [DOI] [PubMed] [Google Scholar]
- 15.Sarici SU, Serdar M a, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113(4):775–780. doi: 10.1542/peds.113.4.775 [DOI] [PubMed] [Google Scholar]
- 16.Organization WH. World Health Organization. Global nutrition targets 2025: low birth weight policy brief 2014.
- 17.Serdar MA, Korkmaz A, Oran O, Yurdako M, Yigit S. Incidence, Course, and Prediction of Hyperbilirubinemia in Near-Term and Term Newborns ¨. 2018;113(4):5–10. [DOI] [PubMed] [Google Scholar]
- 18.Klugman D, Berger JT, Sable CA, He J, Khandelwal SG, & Slonim AD. Pediatric patients hospitalized with myocarditis: a multi-institutional analysis. Pediatr Cardiol. 2010;31(2):222–228. doi: 10.1007/s00246-009-9589-9. [DOI] [PubMed] [Google Scholar]
- 19.McCormick PJ, Lin HM, Deiner SG LM. Validation of the All Patient Refined Diagnosis Related Group (APR-DRG) Risk of Mortality and Severity of Illness Modifiers as a Measure of Perioperative Risk. J Med Syst. 2018;22(5). doi: 10.1007/s10916-018-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bratton SL, Odetola FO, McCollegan J, Cabana MD, Levy FH KH. Regional variation in icu care for pediatric patients with asthma. J Pediatr. 2005;147(3):355–361. [DOI] [PubMed] [Google Scholar]
- 21.Muldoon JH. MEASUREMENT Structure and Performance of Different DRG Classification Systems for Neonatal Medicine. Pediatrics. 1999;103(January):302–318. [PubMed] [Google Scholar]
- 22.Clinical Classifications Software (CCS) for ICD-9-CM. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- 23.Horgan MJ. Management of the Late Preterm Infant. Pediatr Clin North Am. 2015;62(2):439–451. doi: 10.1016/j.pcl.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 24.Johnson LH, Phibbs C, Jeffrey Maisels M, et al. Kernicterus: Epidemiological Strategies for Its Prevention through Systems-Based Approaches. J Perinatol. 2004;24(10):650–662. doi: 10.1038/sj.jp.7211152 [DOI] [PubMed] [Google Scholar]
- 25.Benitz WE. Hospital Stay for Healthy Term Newborn Infants. Pediatrics. 2015;135(5):948–953. doi: 10.1542/peds.2015-0699 [DOI] [PubMed] [Google Scholar]
- 26.Hastie T, Tibshirani R FJ. The Elements of Statistical Learning Data Mining, Inference, and Prediction. 2nd ed. New York, NY: Springe; 2016. [Google Scholar]
- 27.Platt J Probabilistic outputs for support vector machines and comparisons to regularized likelihood methods. Adv large margin Classif. 1999;10(3):61–74. [Google Scholar]
- 28.Chen CL, Lin GA, Bardach NS, et al. Preoperative Medical Testing in Medicare Patients Undergoing Cataract Surgery. N Engl J Med. 2015;372(16):1530–1538. doi: 10.1056/NEJMsa1410846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol 2005; 161: 81–8. [DOI] [PubMed] [Google Scholar]
- 30.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006; 60: 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the Newborn Infant ≥35 Weeks’ Gestation: An Update With Clarifications. Pediatrics. 2009;124(4):1193–1198. doi: 10.1542/peds.2009-0329 [DOI] [PubMed] [Google Scholar]
- 32.Wickremasinghe AC, Kuzniewicz MW, McCulloch CE, Newman TB. Efficacy of subthreshold newborn phototherapy during the birth hospitalization in preventing readmission for phototherapy. JAMA Pediatr. 2018;172(4):378–385. doi: 10.1001/jamapediatrics.2017.5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


