Abstract
Background
The incidence of complex atypical hyperplasia and early stage endometrioid endometrial cancer is increasing, in part due to the epidemic of obesity, a risk factor tightly linked to development of endometrial hyperplasia and cancer. The standard upfront treatment for complex atypical hyperplasia and early stage endometrial cancer is hysterectomy. However, non-surgical treatment of early endometrial neoplasia may be necessary due to medical co-morbidities precluding surgery or desired future fertility.
Objective
We sought to evaluate efficacy of the Levonorgestrel Intrauterine Device to treat complex atypical hyperplasia and grade 1 endometrioid endometrial carcinoma.
Study Design
A single-institution, single-arm, phase II study of the Levonorgestrel Intrauterine Device (52 mg levonorgestrel, Mirena®) was conducted in patients with complex atypical hyperplasia or grade 1 endometrioid endometrial cancer. The primary endpoint was pathologic response rate at 12 months, including complete or partial response. Quality of life and toxicity were assessed. Molecular analyses for proliferation markers, hormone-regulated genes, and WNT pathway activation were performed at baseline and 3 months.
Results
Fifty-seven patients were treated (21 endometrial cancer, 36 complex atypical hyperplasia). Median age was 48.0 years, median body mass index was 45.5 kg/m2. Of 47 evaluable patients, 12-month response rate was 83% (90%CrI 72.7, 90.3); 37 complete responders (8 endometrial cancer; 29 complex atypical hyperplasia), 2 partial responders (2 endometrial cancer), 3 stable disease (2 endometrial cancer, 1 complex atypical hyperplasia), 5 progressive disease (3 endometrial cancer; 2 complex atypical hyperplasia). After stratification for histology, response rate was 90.6% for complex atypical hyperplasia and 66.7% for grade 1 endometrioid endometrial cancer. Four patients (9.5%) had relapse after initial response. Adverse events were mild, primarily irregular bleeding and cramping. Quality of life was not negatively impacted. At 3 months, exogenous progesterone effect was present in 96.9% of responders (31/32) versus 25% (2/8) of non-responders (p=0.001). Non-responders had higher baseline proliferation (Ki67) and lower DKK3 gene expression compared to responders (p=.023 and p=0.030). Non-responders had significantly different changes in sFRP1, FZD8, and RALDH2 compared to responders.
Conclusion
The Levonorgestrel Intrauterine Device has substantial activity in complex atypical hyperplasia and grade 1 endometrioid endometrial cancer, with a modest proportion demonstrating upfront progesterone resistance. Potential biomarkers were identified that may correlate with resistance to therapy, further exploration is warranted.
Keywords: Complex Atypical Hyperplasia, Conservative, Endometrial Cancer, Endometrial Hyperplasia Intrauterine Device, Predictive Biomarkers, Progesterone
Introduction
Endometrial cancer is the most common gynecologic cancer in the United States with an estimated 65,620 new cases and 12,590 deaths expected in 20201. Complex atypical hyperplasia (CAH) is a precursor to endometrioid endometrial cancer, the most common subtype2. Incidence of these entities is increasing, in part due to increasing obesity, a risk factor tightly linked to development of endometrial hyperplasia and cancer3.
The standard upfront treatment for CAH and early stage endometrial cancer is complete surgical resection3. However, this may not be the preferred option in a growing population of women with endometrial cancer who have not completed childbearing4. Furthermore, because of shared risk factors, women with endometrial cancer may be high-risk surgical candidates due to morbid obesity or multiple co-morbid conditions3, 5–7. As obesity increases worldwide, safe and effective conservative therapies are needed for this population, as incidence and mortality are predicted to continue to increase8.
Given the importance of unopposed estrogen in development of endometrial neoplasia, progesterone has been explored as primary therapy for CAH and an alternative to surgery in early stage, low grade endometrial cancer3, 7, 9, 10. Despite moderate success of oral progesterone treatment7, 9, 10, many women do not tolerate side effects of systemic progesterone, including significant weight gain, or are unable to maintain compliance with this therapy11. The levonorgestrel intrauterine device (LIUD, Bayer Health Care Pharmaceutical Inc, Wayne, NY) provides consistent localized progestin exposure. Preliminary prospective studies of this agent, alone and in combination with other hormonal therapies, suggest the LIUD reduces systemic symptoms and may provide superior efficacy compared to oral agents alone12–14. The LIUD has not been thoroughly studied as a single agent in CAH and grade 1 endometrioid endometrial carcinoma (EEC). Further, there exists a population for whom progesterone therapy of any modality is ineffective, manifesting with non-response or early relapse7, 9, 12, 13, 15–18. There are no existing clinical or molecular predictors for lack of clinical benefit to these treatments.
The purpose of this study was to prospectively evaluate response and safety of the LIUD to treat CAH and grade 1 EEC. In addition, association between response and clinical characteristics or molecular endpoints was explored. Patient-reported health-related quality of life was evaluated throughout the study period.
Note: According to ACOG and SGO Committee Opinion 631, the current preferred terminology for atypical hyperplasia is the endometrial intraepithelial neoplasia (EIN) schema. We agree with the importance of an improved system to distinguish between clinicopathologic entities. We have maintained the previous CAH terminology in the current study report, as treatment decisions and trial eligibility were determined based on the CAH terminology in use at that time.
METHODS
Study Oversight
The study (NCT00788671) was designed by the principal investigator and conducted in accordance with the provision of the Declaration of Helsinki and Good Clinical Practice Guidelines at The University of Texas MD Anderson Cancer Center and affiliated hospitals. Institutional Review Board approval was obtained at all participating hospitals.
Patients
Patients with histologically confirmed CAH or grade 1 EEC on endometrial biopsy or dilation and curettage (D&C) specimen were enrolled. All patients with CAH were eligible. Patients with EEC were eligible if they met one of the following criteria: 1) morbid obesity (BMI > 40kg/m2); 2) multiple co-morbidities (defined as American Society of Anesthesiologists (ASA) Score ≥ 3); or 3) desire to retain fertility. Patients were not eligible if they had prior treatment for CAH or EEC. Patients with medical contraindications to the LIUD were excluded, including pregnancy, active pelvic infection, immunodeficiency, liver disease, or structural uterine anomalies. Enrolled patients underwent D&C to sample the entire endometrium. Women who had previously undergone D&C for this diagnosis were not required to undergo a second D&C, but the previous pathology specimen was obtained. All pathologic diagnoses were reviewed and confirmed by central gynecologic pathology review. Written informed consent was obtained from all participants.
Study Design, Treatment, and End Points
This was a phase II, single-arm, open-label study of the LIUD (containing 52 mg levonorgestrel, Mirena®) in patients with CAH or grade 1 EEC. The LIUD was placed intra-operatively at time of D&C or in clinic for patients with recent D&C. Treatment was continued for 12 months or until evidence of unacceptable toxicity or disease progression. Patients with response to therapy at 12 months were offered continuation of LIUD versus other treatment options per physician discretion. The primary efficacy endpoint was pathologic response rate (RR) at 12 months defined as complete response (CR) or partial response (PR). CR was defined as no evidence of cancer or hyperplasia with atypia in CAH or EEC patients. PR was defined as CAH in EEC patients. Progressive disease (PD) was defined as grade 2 endometrioid endometrial cancer or above in EEC patients or presence of any cancer in CAH patients. Patients were considered evaluable for the primary endpoint if they completed 12 months of therapy or discontinued due to disease progression. Secondary endpoints included toxicity, quality of life and association between molecular profile or clinical factors and response to LIUD.
Clinical Assessments
Pretreatment evaluation included pregnancy test and cervical cultures. Patients with EEC underwent chest radiograph (CXR) and magnetic resonance imaging (MRI) to evaluate for myometrial invasion or extrauterine disease. Patients who were unable to undergo MRI due to weight or other contraindications (n=7) underwent computerized tomography (CT) of the abdomen and pelvis and/or transvaginal ultrasound (TVUS). Patients with initial diagnosis of CAH upgraded to EEC at time of D&C/ LIUD placement underwent post-placement CXR and MRI. One month after LIUD placement, patients returned for visual string check. Endometrial biopsies were performed every 3 months for 12 months. Biopsies were reviewed for exogenous progesterone effect, defined as small inactive glands and pseudodecidualized stroma.
Toxicity was monitored using an investigator-designed worksheet (Supplemental Figure 1). Patient health status was monitored at each visit utilizing the SF-36 instrument19. The SF-36 is a patient self-reported survey that measures health status across 8 subscales. The SF-36 is scored using weighted sums of the questions in the different subscales including physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. Each subscale score is transformed to a 0 to 100 scale. Higher scores reflect better functioning, while lower scores reflect poorer functioning.
Translational Studies
Tissue at baseline and 3-months was collected for exploratory biomarker analyses. Proliferation (Ki67) and progesterone receptor (PgR) were evaluated using immunohistochemistry (IHC) on formalin-fixed, paraffin-embedded tissue20, 21. Ki67 was scored as number of positively-stained nuclei in 10 high-power fields. PgR immunohistochemistry was scored as percentage of positively-stained epithelial cell nuclei.
Molecular markers of estrogen signaling and WNT pathway were evaluated for association with response to progesterone. We have previously discovered a cadre of genes that are significantly altered in the endometrium of postmenopausal women after estrogen treatment22. Given the antagonistic effect of progesterone, we predicted these changes would be reversed after effective progesterone treatment and have shown this association in normal endometrium from women at high-risk of developing cancer23. As several studies have described the impact of WNT/β-catenin signaling in endometrial cancer development and outcomes24–26, this pathway was also evaluated as a potential marker of progesterone response. The panel of estrogen-regulated and WNT pathway genes included PgR, EIG121 (aka ELAPOR1 or KIAA1324), IGF-1, IGF-2, IGFBP1, RALDH2 (aka ALDH1A2), SFRP1, SFRP4, survivin (aka BIRC5), DKK3, FZD8, FZD10, TCF7, and WNT5A. Expression was measured as previously described23 (details in Supplemental Methods). Gene expression data were normalized to 18SrRNA and reported as %18SrRNA. Gene expression results are displayed after linear scaling (x1000) to improve visualization.
Statistical Analyses
A Bayesian Phase II design was used to enroll a minimum of 20 and maximum of 50 evaluable subjects based on the following futility monitoring rule27: if Pr(RR ≥ 65% | data) < 0.05, (i.e., given the interim data from patients who have already completed the trial, there is less than a 5% chance that the RR is at least 65%), the trial would be stopped. The threshold of 65% was selected based on previous retrospective studies of progesterone7,9.
Summary statistics were calculated for demographic and clinical characteristics. We estimated RR with a 90% credible interval (CrI) assuming that RR followed a beta(1.3, 0.7) distribution. Exploratory analyses of the association between 12-month response and clinical and demographic characteristics were conducted using Wilcoxon rank-sum test, Fisher’s exact test and also logistic regression.
Baseline and 3-month biomarker values were compared with a Wilcoxon signed-rank test. Change from baseline was calculated for each biomarker and compared by 12-month response status using a Wilcoxon rank-sum test. Summary statistics were calculated for toxicity and SF-36 scores, at baseline and every three months until the patient completed the study. Wilcoxon rank-sum test was used to compare SF-36 scores between responders and non-responders. Statistical analyses were performed using Stata/MP v15.0 (College Station, TX) and IBM SPSS v23 (Armonk, NY).
RESULTS
Patients, Efficacy, and Safety
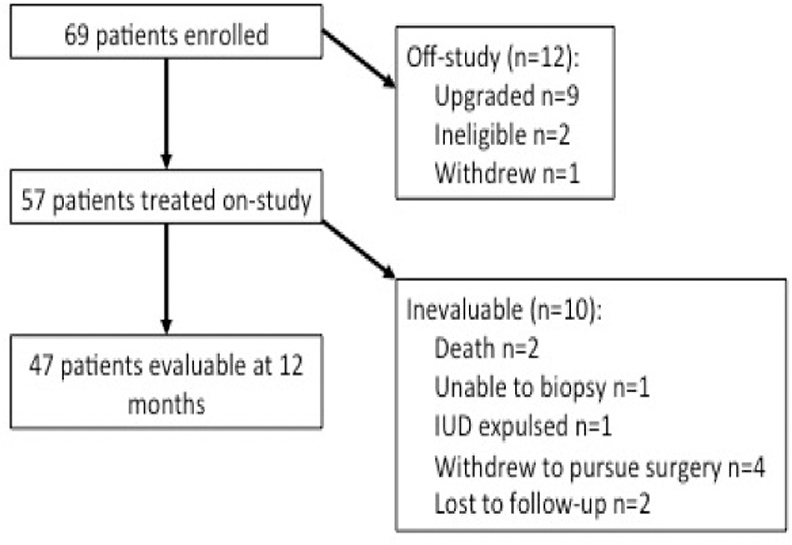
Sixty-nine patients were enrolled and 57 treated with the LIUD (Figure 1). Twelve patients were excluded from treatment, 9 had upgraded pathology at D&C, 1 patient withdrew, and 2 patients did not meet inclusion criteria (prior progesterone therapy n=1, EEC without required inclusion criteria n=1). Demographics and clinical characteristics are reported in Table 1. Of the 57 treated patients, 36 (63.2%) had CAH and 21 (36.8%) had grade 1 EEC. Median age was 48.0 years and median body mass index (BMI) was 45.5 kg/m2.
Figure 1. CONSORT Diagram.
Details describing the number of patients enrolled, treated with levonorgestrel intrauterine device (IUD) on-study, and evaluable at the 12 month time point, including reasons for patients coming off-study or deemed inevaluable.
Table 1.
Demographic and clinical characteristics of enrolled patients
| Patient characteristics (N=57) | |
|---|---|
| Median (range) | |
| Age at diagnosis, years | 48.0 (19.1 – 84.1) |
| BMI at diagnosis, kg/m2 | 45.5 (21 – 79) |
| Parity at diagnosis | 1 (0 – 13) |
| No. pts (%) | |
| Race | |
| White | 45 (78.9) |
| Black | 9 (15.8) |
| Asian | 2 (3.5) |
| Pacific Islander | 1 (1.8) |
| Ethnicity | |
| Hispanic | 20 (35.1) |
| Non-Hispanic | 37 (64.9) |
| Parity | |
| Parous | 27 (47.4) |
| Non-parous | 30 (52.6) |
| Menopausal Status | |
| Menopausal | 25 (43.9) |
| Pre-menopausal | 32 (56.1) |
| Diagnosis | |
| CAH | 36 (63.2) |
| G1EEC | 21 (36.8) |
| Indications | |
| CAH | |
| CAH alone | 8 (14.0) |
| Desires future childbearing | 6 (10.5) |
| BMI > 40 kg/m2 | 13 (22.8) |
| Desires future childbearing + BMI >40 kg/m2 | 4 (7.0) |
| BMI > 40 kg/m2 + multiple co-morbidities | 4 (7.0) |
| Desires future childbearing + BMI >40 kg/m2 + multiple co-morbidities | 1 (1.8) |
| G1EEC | |
| Desires future childbearing | 3 (5.3) |
| BMI > 40 kg/m2 | 5 (8.8) |
| Multiple co-morbidities | 2 (3.5) |
| Desires future childbearing + BMI >40 kg/m2 | 3 (5.3) |
| BMI > 40 kg/m2 + Multiple co-morbidities | 6 (10.5) |
| Desires future childbearing + BMI >40 kg/m2 + multiple co-morbidities | 2 (3.5) |
BMI, body mass index; CAH, complex atypical hyperplasia; G1EEC, grade 1 endometrioid endometrial cancer.
Overall 12-month RR was 83.0% (Table 2) with a 90% CrI 72.7%−90.3%. At 12 months, 37 patients had CR (78.7%, 8=EEC; 29=CAH); 2 patients had PR (4.3%, 2=EEC); 3 patients had SD (6.4%, 2=EEC, 1=CAH); 5 patients had PD (10.6%; 3=EEC, 2=CAH). After stratification for histology, RR was 90.6% for CAH (n=32 evaluable) and 66.7% for EEC (n=15 evaluable). The majority of patients responded to the LIUD in the first three months (n=36). However, there were new responses to therapy at the 6 (n=3), 9 (n=2) and 12 months (n=1) time points. Four patients (9.5%) had relapse of disease after initial response. One patient had CAH that initially resolved at 3 months, relapsed at 6 months and remained stable through 12 months. The other three patients had EEC with specific patterns of relapse. One patient had CR at 3 months then relapsed to CAH at 12 months. The second patient had CR at 3 months then relapsed to grade 1 EEC at 12 months. The third patient had PR at 6 months, then relapsed to grade 1 EEC at 9 and 12 months. Of the five patients that progressed, three underwent hysterectomy and two were unable to undergo surgery but received radiation therapy. All five patients are without evidence of disease.
Table 2.
Response rates to the levonorgestrel intrauterine device at 12 months
| No. Evaluable | CR | PR | SD | PD | Response rate (%) | 90% credible interval | |
|---|---|---|---|---|---|---|---|
| Overall | 47 | 37 (79%) | 2 (4%) | 3 (6%) | 5 (11%) | 83.0 | 72.7 – 90.3 |
| CAH | 32 | 29 (91%) | 0 (0%) | 1 (3%) | 2 (6%) | 90.6 | 79.3 – 96.3 |
| G1EEC | 15 | 8 (54%) | 2 (13%) | 2 (13%) | 3 (20%) | 66.7 | 47.2 – 83.6 |
CAH, complex atypical hyperplasia; G1EEC, grade 1 endometrioid endometrial cancer; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease
Ten patients were inevaluable for the 12-month response determination, for reasons detailed in Figure 1. Of 10 inevaluable patients, 3 women did not undergo further endometrial sampling after LIUD placement (one death unrelated to LIUD treatment, one LIUD expulsion, and one woman could not undergo biopsy). Of 7 inevaluable patients with post-LIUD biopsy, two were lost to follow-up (both CR at last biopsy), 1 woman died due to conditions unrelated to LIUD treatment (SD), and 4 women withdrew to pursue hysterectomy (3 SD and 1 CR). Noting that patients withdrawing to pursue surgery or not returning for follow-up could be influenced by early biopsy results and therefore introduce bias, an estimate of response rate using the last response available for all participants results in 78% response rate (42 CR or PR and 12 SD or PD).
Adverse events were self-limited and manageable (Supplemental Table 1). Abnormal vaginal bleeding was common, especially in the first 3 months (n=26, 56.5%). This primarily consisted of spotting (n=25) compared to moderate (n=7) or heavy bleeding (n=1). Other common adverse events included abdominal/pelvic pain and weight gain. There were no uterine perforations or pelvic infections reported. There were two deaths (3.5%) that occurred during the study, neither were attributed to the LIUD, but highlight the poor health status of patients with multiple co-morbidities. One patient died of respiratory failure due to a complicated pneumonia and the other died of heart failure in the setting of significant chronic obstructive pulmonary disorder.
Characteristics of Non-Response
Of the 47 patients evaluable at 12-months, there were no differences between responders and non-responders in age (44.5 vs 53.4y, p=.10) or BMI (43.4 vs 51.3, p=.16; Table 3). However, 96.9% (31/32) of responders had pathologic evidence of exogenous progesterone effect at 3 months compared to 25% (2/8) of non-responders (p=.001). Among evaluable patients with tissue for immunohistochemical analysis (n=17, Table 4), non-responders had higher baseline Ki67 compared to responders (p=0.023). Among evaluable patients with sufficient specimen for gene expression analysis (n=14), non-responders had significantly lower baseline gene expression of DKK3 (p=0.030, Table 4). When comparing 3 months to baseline, responders and non-responders showed statistically significant differences, with an opposite direction of change for RALDH2 (p=0.034), SFRP1 (p=0.024), and FZD8 (p=0.032).
Table 3.
Association of Clinical Factors with Response to Levonorgestrel Intrauterine Device at 12 Months
| Responders (CR+PR) n = 39 | Non Responder s (PD+SD) n = 8 | P Value* | OR (95% LB, 95% UB) | P Value** | |
|---|---|---|---|---|---|
| Mean Age, yrs (range) | 44.5 (19.1 – 76.9) | 53.4 (30.8 – 63.8) | 0.075 | 0.95 (0.89 – 1.01) | 0.100 |
| Mean BMI, kg/m2 (range) | 43.4 (21.0 – 79.0) | 51.3 (28.0 – 75.0) | 0.234 | 0.96 (0.90 – 1.02) | 0.156 |
| Mean Max Uterine Dimension, cm | 9.1 (6.5 – 14.8) | 9.4 (6.3 – 12.9) | 0.588 | 0.92 (0.63 – 1.34) | 0.666 |
| Metformin Use, n (%) | 33 (86.8) | 8 (100.0) | 0.569 | NA | NA |
| FinalDiagnosis of CAH, n (%) | 29 (74.4) | 3 (37.5) | 0.089 | 1.00 (1.00 – 1.00) | 0.054 |
| Exogenous Progesteron e Effect Present, n (%) | 31 (96.9) | 2 (25) | <0.001 | 93 (7.23 – 1196.69) | 0.001 |
BMI, body mass index; CAH, complex atypical hyperplasia; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; OR, odds ratio; LB, lower bound; UB, upper bound; NA, not applicable
Rank sum test for continuous measures and Fisher’s Exact Test statistic for categorical
Logistic regression modeling
Table 4.
Biomarker Expression in Baseline and 3-Month Post-Treatment Endometrial Biopsy Specimens
| Biomarker | Protein Expression | ||
|---|---|---|---|
| Responders (CR+PR) | Non Responders (PD+SD) | P Value* | |
| Ki67, # positive nuclei in 10 HPF, median (range)(N=17) | |||
| Baseline | 27.5 (4.0 – 73.0) | 65.5 (32.0 – 91.0) | 0.023 |
| 3 month change | −16.0 (−68.0 – 4.0) | −18.5 (−87.0 – 8.0) | 0.828 |
|
PgR, % positive nuclei, median (range) (n = 11) |
|||
| Baseline | 70.0 (50.0 – 90.0) | 80.0 (70.0 – 90.0) | 0.437 |
| 3 month change | −40.0 (−85.0 – 0) | −20.0 (−50 - −10) | 0.704 |
| Normalized Gene Expression (%18S rRNA) | |||
| Responders (CR+PR) (n = 10) | NonResponders (PD+SD) (n = 4) | P Value* | |
| PgR, median (range) | |||
| Baseline | 0.783 (0.046 – 2.136) | 0.541 (0.293 – 1.040) | 0.407 |
| 3 month change | −0.622 (−2.036 – 0.092) | −0.248 (−0.816 – 0.326) | 0.203 |
| EIG121, median (range) | |||
| Baseline | 0.039 (0.001 – 0.132) | 0.011 (0.004 – 0.074) | 0.238 |
| 3 month change | −0.036 (−0.110 – 0.005) | 0.003 (−0.049 – 0.057) | 0.090 |
| IGF-1, median (range) | |||
| Baseline | 0.039 (0.002 – 0.293) | 0.003 (0.000 – 0.118) | 0.176 |
| 3 month change | −0.043 (−0.284 – 0.019) | −0.018 (−0.0115 – 0.001) | 0.888 |
| IGF-2, median (range) | |||
| Baseline | 0.065 (0.002 – 5.673) | 0.046 (0.002 – 0.281) | 0.827 |
| 3 month change | 0.239 (−5.263 – 1.469) | −0.003 (−0.235 – 0.777) | 0.203 |
| IGFBP1, median (range) | |||
| Baseline | 0.004 (0.000 – 0.558) | 0.005 (0.001 – 0.019) | >0.999 |
| 3 month change | 36.351 (11.961 – 85.746) | 0.960 (0.003 – 55.487) | 0.066 |
| RALDH2, median (range) | |||
| Baseline | 0.102 (0.005 – 0.408) | 0.016 (0.006 – 0.184) | 0.176 |
| 3 month change | 0.073 (−0.077 – 0.170) | −0.017 (−0.080 – 0.007) | 0.034 |
| SFRP1, media (range) | |||
| Baseline | 0.747 (0.036 – 12.020) | 0.210 (0.029 – 0.655) | 0.089 |
| 3 month change | −0.628 (−3.625 – 0.368) | 0.372 (−0.128 – 1.080) | 0.024 |
| SFRP4, median (range) | |||
| Baseline | 0.871 (0.012 – 6.508) | 0.256 (0.009 – 2.579) | 0.150 |
| 3 month change | −0.891 (−6.504 – 0.050) | −0.429 (−2.567 – 0.029) | 0.396 |
| Survivin, median (range) | |||
| Baseline | 0.003 (0.000 – 0.010) | 0.007 (0.001 – 0.011) | 0.174 |
| 3 month change | −0.002 (−0.009 – 0.007) | −0.003 (−0.009 – 0.007) | 0.436 |
| DKK3, median (range) | |||
| Baseline | 0.081 (0.047 – 0.304) | 0.036 (0.021 – 0.051) | 0.030 |
| 3 month change | −0.042 (−0.212 – 0.103) | 0.002 (−0.016 – 0.019) | 0.667 |
| FZD8, median (range) | |||
| Baseline | 0.162 (0.118 – 0.390) | 0.245 (0.063 – 0.274) | >0.999 |
| 3 month change | −0.078 (−0.202 – 0.026) | 0.073 (0.033 – 0.112) | 0.032 |
| FZD10, median (range) | |||
| Baseline | 0.792 (0.365 – 3.670) | 1.639 (0.593 – 1.715) | 0.470 |
| 3 month change | −0.511 (−2.688 – 0.091) | 0.727 (−0.236 – 1.690) | 0.086 |
| TCF7, median (range) | |||
| Baseline | 0.124 (0.056 – 0.258) | 0.094 (0.080 – 0.116) | 0.248 |
| 3 month change | −0.064 (−0.160 – 0.179) | 0.109 (0.022 – 0.196) | 0.053 |
| Wnt5a, median (range) | |||
| Baseline | 1.882 (0.497 – 3.840) | 0.881 (0.611 – 1.919) | 0.194 |
| 3 month change | 2.282 (0.696 – 7.317) | 1.238 (0.493 – 1.984) | 0.197 |
HPF, high-power fields.
Quality of Life
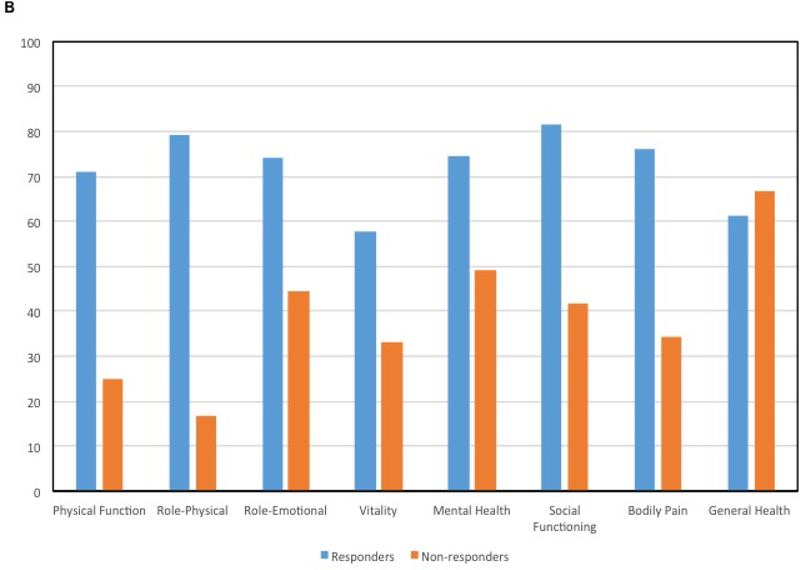
Table 5 shows scores for all patients across SF-36 subscales. Improvement in health status for all subscales was noted with higher mean scores at 12-months compared to baseline (Figure 2). Scores for Role-Physical improved (p=0.02) between baseline to 9 months and baseline to 12 months. At 6-months, responders reported significantly higher Vitality scores compared to non-responders (p=.04). At 9-months, responders reported better scores for Physical Function and Role-Physical (p=.03 and .02, respectively), as well as lower pain (p=.02) (Figure 2).
Table 5.
SF-36 scores for all patients treated on study
| SF-36 subscale | N | Time | Mean | Median | SD | Min | Max |
|---|---|---|---|---|---|---|---|
| Physical Functioning | 49 | Baseline | 61.1 | 70.0 | 31.2 | 0.0 | 100.0 |
| 46 | 3 months | 65.0 | 65.0 | 30.8 | 0.0 | 100.0 | |
| 42 | 6 months | 69.0 | 77.5 | 29.6 | 0.0 | 100.0 | |
| 40 | 9 months | 68.3 | 80.0 | 33.4 | 0.0 | 100.0 | |
| 37 | 12 months | 66.3 | 70.0 | 31.9 | 0.0 | 100.0 | |
| Role-Physical | 49 | Baseline | 55.1 | 50.0 | 41.8 | 0.0 | 100.0 |
| 47 | 3 months | 61.9 | 75.0 | 38.8 | 0.0 | 100.0 | |
| 42 | 6 months | 69.0 | 100.0 | 41.6 | 0.0 | 100.0 | |
| 40 | 9 months | 74.4 | 100.0 | 38.2 | 0.0 | 100.0 | |
| 37 | 12 months | 74.3 | 100.0 | 39.3 | 0.0 | 100.0 | |
| Role-Emotional | 50 | Baseline | 65.3 | 83.3 | 40.9 | 0.0 | 100.0 |
| 46 | 3 months | 61.6 | 66.7 | 40.3 | 0.0 | 100.0 | |
| 41 | 6 months | 72.4 | 100.0 | 37.2 | 0.0 | 100.0 | |
| 40 | 9 months | 71.7 | 100.0 | 39.6 | 0.0 | 100.0 | |
| 37 | 12 months | 72.1 | 100.0 | 40.5 | 0.0 | 100.0 | |
| Vitality | 50 | Baseline | 50.0 | 50.0 | 21.2 | 0.0 | 90.0 |
| 46 | 3 months | 50.1 | 50.0 | 25.0 | 0.0 | 90.0 | |
| 42 | 6 months | 57.5 | 60.0 | 20.8 | 15.0 | 90.0 | |
| 40 | 9 months | 56.9 | 60.0 | 21.5 | 0.0 | 100.0 | |
| 37 | 12 months | 51.9 | 60.0 | 26.1 | 0.0 | 90.0 | |
| Mental Health | 50 | Baseline | 67.7 | 72.0 | 21.7 | 12.0 | 100.0 |
| 46 | 3 months | 67.7 | 72.0 | 22.5 | 8.0 | 96.0 | |
| 42 | 6 months | 73.3 | 80.0 | 18.0 | 24.0 | 96.0 | |
| 40 | 9 months | 73.1 | 76.0 | 16.8 | 32.0 | 100.0 | |
| 37 | 12 months | 70.5 | 76.0 | 21.4 | 12.0 | 100.0 | |
| Social Functioning | 50 | Baseline | 68.5 | 75.0 | 27.9 | 12.5 | 100.0 |
| 47 | 3 months | 67.3 | 75.0 | 29.9 | 12.5 | 100.0 | |
| 42 | 6 months | 77.7 | 87.5 | 22.3 | 25.0 | 100.0 | |
| 40 | 9 months | 78.8 | 87.5 | 26.1 | 0.0 | 100.0 | |
| 37 | 12 months | 72.0 | 87.5 | 28.5 | 0.0 | 100.0 | |
| Pain | 50 | Baseline | 67.7 | 67.5 | 25.3 | 22.5 | 100.0 |
| 47 | 3 months | 70.5 | 77.5 | 24.0 | 22.5 | 100.0 | |
| 42 | 6 months | 72.0 | 77.5 | 24.3 | 10.0 | 100.0 | |
| 40 | 9 months | 73.5 | 77.5 | 24.9 | 0.0 | 100.0 | |
| 37 | 12 months | 70.7 | 67.5 | 23.4 | 10.0 | 100.0 | |
| General health | 50 | Baseline | 55.6 | 55.0 | 22.4 | 0.0 | 100.0 |
| 47 | 3 months | 56.8 | 60.0 | 22.2 | 5.0 | 95.0 | |
| 42 | 6 months | 60.8 | 65.0 | 21.1 | 5.0 | 100.0 | |
| 40 | 9 months | 62.0 | 70.0 | 20.0 | 5.0 | 100.0 | |
| 37 | 12 months | 60.1 | 65.0 | 19.6 | 0.0 | 85.0 |
Figure 2. Mean SF-36 Subscale Scores for All Patients.
A) Mean SF-36 scores across all time points and B) Comparison of mean SF-36 scores in responders and non-responders at the 9-month time point.
COMMENT
Principal Findings
The LIUD demonstrated promising durable response rates in women with CAH and grade 1 EEC. Overall RR was 83%, (90.6% CAH, 66.7% EEC). Four patients (9.5%) had relapse after initial response. Overall, adverse events were minimal.
Patient-reported health status scores demonstrated improvement in physical functioning over time, especially among responders. Improvements were across both physical functioning as well as energy levels.
Baseline endometrial biopsies from non-responders showed higher proliferation and lower DKK3 gene expression compared to responders. Endometrial biopsies at 3 months showed presence of exogenous progesterone effect was more common in responders (96.9%) versus non-responders (25%).
Results
The overall response rate of 83% observed in our study is consistent with literature regarding progesterone treatment in early endometrial neoplasia7, 9, 12–14, 16–18, 28. However, the majority of these studies are retrospective in nature and focus on oral progesterone agents14, 16, 18, 28, which have significant adverse events. Our study showed higher response rates for CAH compared to EEC, which is surprising given the difficulty in distinguishing these pathologic entities histologically29 and the frequency with which these diagnoses overlap30. Given these well-documented diagnostic challenges in differentiating CAH and grade 1 EEC, we have reported overall responses (combining the two groups) in addition to CAH and EEC separately. However, trials using the LIUD and GnRH agonists have also demonstrated a response differential between CAH and EEC12, 17.
Importantly, there is a subset of tumors that do not respond to progesterone-based therapy. We sought to assess clinical and molecular features associated with therapy resistance. We predicted BMI would play a role in response15, 31, 32. We found no difference in BMI between responders and non-responders; however, the cohort included only a small number of patients with lower BMI. In our retrospective study assessing LIUD in endometrial hyperplasia and cancer, we found that median uterine diameter was larger in non-responders to progesterone therapy33. We postulated that there could be limited progesterone for a larger surface area. This finding was not confirmed in the current study, where maximum uterine diameter was not associated with lack of response. Certainly, this is reassuring that the LIUD can be utilized as an anti-cancer agent for any uterine size.
In the absence of patient characteristics, use of pathologic markers would be valuable to determine if a patient is unlikely to respond to therapy. Identification of exogenous progesterone effect is a clinically straightforward way to determine therapeutic impact. We found absence of exogenous progesterone effect at 3 months was associated with lack of response to the LIUD therapy at 12 months. This supports findings of our retrospective study where 100% of responders had evidence of exogenous progesterone effect compared to only 50% of non-responders33. Identification of lack of exogenous progesterone effect early in treatment holds potential to identify need for additional systemic therapy or transition to alternative therapy.
Clinical implications
Given the increasing number of patients with endometrial cancer who are unable to undergo surgery due to medical co-morbidities or desire for future fertility, the LIUD has durable activity in CAH and EEC, with only a modest proportion (17%) demonstrating upfront progesterone resistance. There was no evidence of a negative impact on quality of life.
For patients with progestin-resistant disease or relapse after LIUD treatment, new therapeutic regimens are needed. Given the responses observed with anti-estrogen treatment combined with mTOR inhibition (letrozole and everolimus) in advanced/recurrent endometrial cancer20, the PI3K/AKT/mTOR pathway is an attractive target for early endometrial cancer. Multiple studies have shown potential crosstalk between estrogen signaling and PI3K/AKT signaling34, 35, which could support progesterone resistance. Further, in progesterone-resistant endometrial cancer cell lines, inhibition of PI3K/AKT signaling in combination with progesterone led to inhibition of cancer cell growth and up-regulation of PgR expression36. These studies led to the development of the ongoing LEVER trial (NCT02397083), which aims to assess the impact of the addition of everolimus, an mTOR inhibitor, to overcome progesterone resistance in patients with CAH and grade 1 EEC treated with LIUD.
Research Implications
The identification of other markers of resistance to progesterone is an unmet need. PgR expression has been evaluated extensively as a potential predictor. The majority of data have been reported in the setting of progesterone therapy for recurrent disease, where response is lower in tumors lacking PgR expression11. However, when evaluating early stage, low grade disease, the majority of tumors will have high levels of PgR expression. In our study, only a subset of patients had sufficient tissue available for IHC; however, PgR was expressed in all tumors and there was no difference in baseline or change in PgR expression after treatment between the responders and non-responders. Further prospective assessment is needed and assessment of functionality or subtypes of the progesterone receptor may be more predictive rather than just total expression.
Estrogen-regulated and WNT signaling genes were evaluated for statistically significant associations with treatment response based on previous studies defining the relevance of these genes22, 24. In our study, expression of FZD8 and sFRP1 increased following treatment in non-responders, but levels decreased in responders. Conversely, the inhibitory gene RALDH2 decreased after treatment in non-responders, but increased in responders. Baseline differences showed higher proliferation and lower expression of the tumor suppressor, DKK3, in non-responders. Although several studies suggested that the estrogen-regulated gene panel would be altered by progestin treatment, limited alterations were present in LIUD responders versus non-responders. This may reflect localized progestin exposure levels, exposure timing, heterogeneity of response, or microenvironment. We have observed that progestin response can occur directly adjacent to areas with persistent disease23. Because biomarkers were analyzed using large regions of endometrial tissue, a different pattern might be observed if tumor and endometrial glands were microdissected and evaluated separately from stroma. In addition, our results may indicate that lack of response to LIUD is not driven by estrogen or WNT signaling alone. Of course, our sample size is insufficient to make any bold conclusions, however, we are working to validate the use of these markers in ongoing prospective clinical trials of LIUD combination therapy (NCT02397083; NCT01686126).
Strengths and Limitations
Strengths of the current study include the prospective nature and long duration of data collection. Further, we were able to collect patient-reported health status over the entire duration of the study. The 12-month time point for response determination is stringent and allowed for identification of patients with early relapse. Our study population is also generalizable given inclusion of patients with multiple co-morbidities as well as those desiring future fertility. The limitations of the study include low numbers of patients with adequate tissue for molecular analysis. There were a small number of patients did not reach the 12-month time point.
Conclusions
This prospective trial confirms clear activity of the LIUD for treatment of CAH and early grade endometrial cancer with minimal adverse effects. Clinical features did not predict lack of response at baseline aside from the diagnosis of EEC, which had lower response rates overall. Thus, the LIUD may be considered across a broad patient population. The absence of exogenous progesterone effect after 3 months of treatment is associated with lack of response and may be considered to guide transition to alternative therapy. This trial provides insights into the molecular impact of progesterone and identifies candidate estrogen-regulated and WNT pathway markers of response and resistance. These findings must be explored further to improve the clinical care and outcomes of patients receiving uterine conserving therapy for early endometrial neoplasia.
Supplementary Material
Supplemental Figure 1. Investigator-designed worksheet. Toxicity and complication information was collected, including common adverse events and specific symptoms related to the levonorgestrel intrauterine device.
Condensation.
For individuals with early endometrial neoplasia for whom surgical treatment is not warranted, treatment with levonorgestrel intrauterine device has substantial activity with minimal adverse events
AJOG at a Glance.
A. Why was this study conducted?
A fertility-sparing, non-surgical approach is increasingly necessary for individuals diagnosed with early endometrial neoplasia due to increasing prevalence of significant comorbidities and proportion of premenopausal women diagnosed with this disease.
This study evaluates the levonorgestrel intrauterine device for treatment of complex atypical hyperplasia and early endometrial cancer, for which standard treatment is complete surgical resection.
B. What are the key findings?
Out of 57 treated patients, 47 were evaluable and 12-month response rate was 83% overall (90.6% for complex atypical hyperplasia and 66.7% for grade 1 endometrioid endometrial cancer).
9.5% had relapse after initial response.
Adverse events were mild, and quality of life was not negatively impacted.
C. What does this study add to what is already known?
This study used a rigorous 12-month endpoint to demonstrate durable response rates in women with early endometrial neoplasia and identified potential biomarkers for therapy resistance.
Acknowledgments
Funding: NIH SPORE in Uterine Cancer (NIH 5P50CA098258-13) (SW, CCS, RB, PS, KL, RC, MY), Uterine SPORE Career Development Grant (NIH 2P50 CA098258-06) (SW), M.D. Anderson Cancer Center Support Grant (NIH CA016672) supporting Biostatistics Resource and the Clinical Protocol and Data Management Group, NIH T32 Training Grant 5 T32 CA101642 02 (SW), Andrew Sabin Family Fellowship (SW), GOG Foundation Scholar Investigator Award (SW).The funding sources had no involvement in study design, data collection or interpretation, writing the manuscript, or decision to submit the article for publication.
Disclosure Statement: SNW reports personal fees for consulting from AstraZeneca, Circulogene, Clovis Oncology, Merck, Novartis, Pfizer, Roche/Genentech, GSK/Tesaro, Eisai, Zentalis and research funding to her institution from ArQule, AstraZeneca, Bayer, Clovis Oncology, Cotinga Pharmaceuticals, Novartis, Roche/Genentech, and GSK/Tesaro. PTS reports personal fees from GSK/Tesaro, Clovis Oncology, and research funding to her institution from Novartis and Incyte. NDF reports personal fees for consulting from Tesaro, Pfizer, and Bristol-Myers Squibb. YY reports personal fees for consulting from Abbvie, Amgen, Boehringer Ingelheim, Deciphera, Juno Therapeutics, Servier, Starpax, and Vertex. RLC reports personal fees for consulting from Abbvie, AstraZeneca, Clovis Oncology, Janssen, Immunogen, Tesaro, Array, Genmab, Gamamab, and research funding from Abbvie, AstraZeneca, Clovis Oncology, Roche/Genentech, Janssen, and Merck. All remaining authors have declared no conflicts of interest.
Footnotes
Clinicaltrials.gov registration # NCT00788671
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.KURMAN RJ, KAMINSKI PF, NORRIS HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer 1985;56:403–12. [DOI] [PubMed] [Google Scholar]
- 3.BURKE WM, ORR J, LEITAO M, et al. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol 2014;134:385–92. [DOI] [PubMed] [Google Scholar]
- 4.SOLIMAN PT, OH JC, SCHMELER KM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol 2005;105:575–80. [DOI] [PubMed] [Google Scholar]
- 5.OBERMAIR A, BRENNAN DJ, BAXTER E, ARMES JE, GEBSKI V, JANDA M. Surgical safety and personal costs in morbidly obese, multimorbid patients diagnosed with early-stage endometrial cancer having a hysterectomy. Gynecologic oncology research and practice 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GUNDERSON CC, JAVA J, MOORE KN, WALKER JL. The impact of obesity on surgical staging, complications, and survival with uterine cancer: a Gynecologic Oncology Group LAP2 ancillary data study. Gynecol Oncol 2014;133:23–7. [DOI] [PubMed] [Google Scholar]
- 7.RAMIREZ PT, FRUMOVITZ M, BODURKA DC, SUN CC, LEVENBACK C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecologic oncology 2004;95:133–8. [DOI] [PubMed] [Google Scholar]
- 8.HENLEY SJ, MILLER JW, DOWLING NF, BENARD VB, RICHARDSON LC. Uterine Cancer Incidence and Mortality - United States, 1999–2016. MMWR Morbidity and mortality weekly report 2018;67:1333–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GUNDERSON CC, FADER AN, CARSON KA, BRISTOW RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012;125:477–82. [DOI] [PubMed] [Google Scholar]
- 10.RANDALL TC, KURMAN RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol 1997;90:434–40. [DOI] [PubMed] [Google Scholar]
- 11.THIGPEN JT, BRADY MF, ALVAREZ RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1999;17:1736–44. [DOI] [PubMed] [Google Scholar]
- 12.MINIG L, FRANCHI D, BOVERI S, CASADIO C, BOCCIOLONE L, SIDERI M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2011;22:643–9. [DOI] [PubMed] [Google Scholar]
- 13.LAURELLI G, DI VAGNO G, SCAFFA C, LOSITO S, DEL GIUDICE M, GREGGI S. Conservative treatment of early endometrial cancer: preliminary results of a pilot study. Gynecol Oncol 2011;120:43–6. [DOI] [PubMed] [Google Scholar]
- 14.ORBO A, VEREIDE A, ARNES M, PETTERSEN I, STRAUME B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG : an international journal of obstetrics and gynaecology 2014;121:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GONTHIER C, WALKER F, LUTON D, YAZBECK C, MADELENAT P, KOSKAS M. Impact of obesity on the results of fertility-sparing management for atypical hyperplasia and grade 1 endometrial cancer. Gynecol Oncol 2014;133:33–7. [DOI] [PubMed] [Google Scholar]
- 16.SHAN BE, REN YL, SUN JM, et al. A prospective study of fertility-sparing treatment with megestrol acetate following hysteroscopic curettage for well-differentiated endometrioid carcinoma and atypical hyperplasia in young women. Arch Gynecol Obstet 2013;288:1115–23. [DOI] [PubMed] [Google Scholar]
- 17.PRONIN SM, NOVIKOVA OV, ANDREEVA JY, NOVIKOVA EG. Fertility-Sparing Treatment of Early Endometrial Cancer and Complex Atypical Hyperplasia in Young Women of Childbearing Potential. Int J Gynecol Cancer 2015;25:1010–4. [DOI] [PubMed] [Google Scholar]
- 18.BAKER WD, PIERCE SR, MILLS AM, GEHRIG PA, DUSKA LR. Nonoperative management of atypical endometrial hyperplasia and grade 1 endometrial cancer with the levonorgestrel intrauterine device in medically ill post-menopausal women. Gynecol Oncol 2017;146:34–38. [DOI] [PubMed] [Google Scholar]
- 19.WARE JE JR., SHERBOURNE CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 20.SLOMOVITZ BM, JIANG Y, YATES MS, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol 2015;33:930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.YATES MS, COLETTA AM, ZHANG Q, et al. Prospective Randomized Biomarker Study of Metformin and Lifestyle Intervention for Prevention in Obese Women at Increased Risk for Endometrial Cancer. Cancer Prev Res 2018;11:477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WESTIN SN, BROADDUS RR, DENG L, et al. Molecular clustering of endometrial carcinoma based on estrogen-induced gene expression. Cancer Biol Ther 2009;8:2126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LU KH, LOOSE DS, YATES MS, et al. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res (Phila) 2013;6:774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LIU Y, PATEL L, MILLS GB, et al. Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.JEONG JW, LEE HS, FRANCO HL, et al. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009;28:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GOAD J, KO YA, KUMAR M, JAMALUDDIN MFB, TANWAR PS. Oestrogen fuels the growth of endometrial hyperplastic lesions initiated by overactive Wnt/beta-catenin signalling. Carcinogenesis 2018;39:1105–16. [DOI] [PubMed] [Google Scholar]
- 27.THALL PF, SIMON RM, ESTEY EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med 1995;14:357–79. [DOI] [PubMed] [Google Scholar]
- 28.WILDEMEERSCH D, ANDERSON E, LAMBEIN K, PAUWELS P, DHONT M. Successful treatment of early endometrial carcinoma by local delivery of levonorgestrel: a case report. Obstetrics and gynecology international 2010;2010:431950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ZAINO RJ, KAUDERER J, TRIMBLE CL, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer 2006;106:804–11. [DOI] [PubMed] [Google Scholar]
- 30.TRIMBLE CL, KAUDERER J, ZAINO R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer 2006;106:812–9. [DOI] [PubMed] [Google Scholar]
- 31.GALLOS ID, GANESAN R, GUPTA JK. Prediction of regression and relapse of endometrial hyperplasia with conservative therapy. Obstet Gynecol 2013;121:1165–71. [DOI] [PubMed] [Google Scholar]
- 32.GRAUL A, WILSON E, KO E, et al. Conservative management of endometrial hyperplasia or carcinoma with the levonorgestrel intrauterine system may be less effective in morbidly obese patients. Gynecol Oncol Rep 2018;26:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.PAL N, BROADDUS RR, URBAUER DL, et al. Treatment of Low-Risk Endometrial Cancer and Complex Atypical Hyperplasia With the Levonorgestrel-Releasing Intrauterine Device. Obstet Gynecol 2018;131:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CUESTA R, GRITSENKO MA, PETYUK VA, et al. Phosphoproteome Analysis Reveals Estrogen-ER pathway as a modulator of mTOR activity via DEPTOR. Mol Cell Proteomics 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ZHANG J, XU H, ZHOU X, et al. Role of metformin in inhibiting estrogen-induced proliferation and regulating ERalpha and ERbeta expression in human endometrial cancer cells. Oncol Lett 2017;14:4949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GU C, ZHANG Z, YU Y, et al. Inhibiting the PI3K/Akt pathway reversed progestin resistance in endometrial cancer. Cancer science 2011;102:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Investigator-designed worksheet. Toxicity and complication information was collected, including common adverse events and specific symptoms related to the levonorgestrel intrauterine device.