Abstract
Invariant natural killer T (iNKT) cells are an innate-like population characterized by their recognition of glycolipid antigens and rapid cytokine production upon activation. Unlike conventional T cells, which require TCR ligation, iNKT cells can also be stimulated independently of their TCR. This feature allows iNKT cells to respond even in the absence of glycolipid antigens, for example during viral infections. Although the TCR-dependent and -independent activation of iNKT cells have been relatively well established, the exact contributions of IL-12, IL-18, and toll-like receptors (TLRs) remain unclear for these two activation pathways. To definitively investigate how these components affect the direct and indirect stimulation of iNKT cells, we used mice deficient for either MyD88 or the IL-12Rβ2 in the T cell lineage. Using these tools, we demonstrate that IL-12, IL-18, and TLRs are completely dispensable for the TCR activation pathway, when a strong agonist is used. In contrast, during murine cytomegalovirus (MCMV) infection when the TCR is not engaged, IL-12 signaling is essential and TLR signaling is expendable. Importantly, we discovered an intrinsic requirement for IL-18 signaling by splenic iNKT cells, but not liver iNKT cells, suggesting that there might be diversity even within the NKT1 population.
INTRODUCTION
CD1d-restricted T cells are a unique family of innate-like T cells, found in a number of species (including humans and rodents), which are activated by glycolipid antigens rather than traditional peptides (1). Owing to their expression of NK cell markers and a TCR, they are referred to as natural killer T (NKT) cells. iNKT cells, or type I NKT cells, express a semi-invariant TCR repertoire, where a single α-chain pairs to a limited number of β-chains. iNKT cells are further characterized by their ability to undergo rapid activation and cytokine production upon TCR ligation with a strong agonist, such as the glycolipid α-galactosylceramide (α-GalCer) (2, 3); this rapid activation is in part due to the expression of preformed mRNAs for IFN-γ and IL-4 (4, 5). Early work originally indicated that TCR engagement was not sufficient for this response to α-GalCer (6, 7). iNKT cells can also be uniquely activated independently of their TCR by external signals, either exclusively by inflammatory cytokines (e.g. IFN-α/β, IL-12, and IL-18) or in tandem with weaker antigens (8, 9). This requires the activation of dendritic cells, for example following TLR engagement (10–12). There is also work suggesting that iNKT cells can become activated through direct TLR engagement (13–15). Access to TCR-independent mechanisms of activation allows for iNKT cells to participate during viral infections (16, 17), even in the absence of virally-derived lipid antigens.
Murine cytomegalovirus (MCMV) is a β-herpesvirus, and well-characterized model of viral infection for human CMV infections. The acute cytotoxic response is spearheaded by natural killer (NK) cells, which are robust producers of IFN-γ, perforin, and granzymes (18, 19). iNKT cells also make large amounts of IFN-γ during the acute response. We previously found that IL-12, and to a lesser extent IFN-α/β, was necessary for iNKT cell activation and cytokine production, but that CD1d was dispensable (16). Others have shown that only IL-12 is required for in vivo iNKT cell activation during MCMV (17). However, this previous work from our lab and others has largely relied on global knockout mouse lines and in vitro studies (16, 17). More recently, the iNKT cell field has also shifted away from a linear model of development/maturation towards a lineage model. This categorization more accurately, and definitively, classifies iNKT cells into different subsets that parallel CD4 T helper cell nomenclature; these are based on iNKT cell transcription factor requirements (e.g. RORγt, T-bet, GATA3, and PLZF) and cytokine responses (e.g. IFN-γ, IL-4, and IL-17), and include NKT1, NKT2, and NKT17 cells, as well as specialized subsets such as NKT10 and NKTfh cells (9, 20). It is also interesting to note that different strains of mice can be enriched for certain iNKT cell lineages; for example C57BL/6 mice primarily have NKT1 cells in the thymus, spleen, and liver, while BALB/c mice have robust populations of NKT1, NKT2, and NKT17 cells (20).
Taking into account the changing iNKT cell field, including their previously unappreciated heterogeneity, and the lack of T cell lineage-specific mouse models, we revisited the response of iNKT cells during both TCR-dependent (a-Galcer) and TCR-independent (MCMV) stimulation. To elucidate this, and more accurately establish the signaling requirements for the contribution of iNKT cells, we began by generating mice conditionally deficient for either IL-12Rβ2 or MyD88 signaling in the T cell lineage. MyD88 not only acts down-stream of all the TLRs, except TLR3, but is also a component of the IL-1R/IL-18R superfamily. Using these new mice, we first demonstrated that IL-12Rβ2 and MyD88 signaling are dispensable for iNKT cell development and peripheral localization. We also determined that IL-12, IL-18, and TLR engagement are expendable when a strong agonist is used. Additionally, we confirmed that IL-12 is necessary for the activation of iNKT cells during MCMV infection and ruled out a role for TLRs. Somewhat unexpectedly, we found that iNKT cells have an intrinsic requirement for MyD88 during infection; splenic iNKT cells become hyporesponsive in the absence of MyD88 signaling, yet hepatic iNKT cells are unaffected. This is not because of an inability to appropriately produce IFN-γ upon activation, or a diminished population of cells capable of producing IFN-γ (NKT1 cells). Rather, we determined that this defect remains even after MyD88-deficient iNKT cells are adoptively transferred into a wild-type environment. We also ascertained that the specific component of MyD88 signaling important for splenic iNKT cells is the IL-18R. Together, these data illustrate a previously unappreciated outcome when iNKT cells are stimulated via their IL-18R; this is indicated by the requirement of IL-18 signaling of splenic iNKT cells during MCMV infection, but not hepatic iNKT cells.
MATERIALS & METHODS
Mice.
IL-12Rβ2fl/fl mice were generated at the Brown Transgenic Facility, as previously described (21). B6.129P2(SJL)-MyD88tm1Defr/J (MyD88fl/fl, Cat #: 008888), B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (eYFP, Cat #: 006148), IL-18−/− (Cat #: 004130), and C57BL/6 (B6, Cat #: 000664) mice were purchased from Jackson Laboratory. B6.Cg-Tg(CD4-cre)1Cwi N9 (CD4cre, Cat #: 4196), Balb/c (Cat #: BALB-F), and B6.SJL (Cat #: 002014) mice were purchased from Taconic. IL-12Rβ2fl/fl CD4cre+/− and MyD88fl/fl eYFP+/− CD4cre+/− mice were generated and maintained in-house, along with littermate controls (IL-12Rβ2fl/+ CD4cre+/− and MyD88fl/+ eYFP+/− CD4cre+/−, respectively). Both age- and sex-matched female and male mice (6–26 weeks) were used for these studies; littermates were used as controls for MyD88 cKO and IL-12βR2 cKO mice. All experiments were performed in accordance to the Guide for the Care of Use of Laboratory Animals, as defined by the NIH (PHS Assurance #A3284–01). The Institutional Animal Care and Use Committee (IACUC) of Brown University reviewed, and approved, the animal protocols performed in this study. Animals were housed in an AAALAC-accredited and centralized research facility.
Virus and infection protocol.
MCMV-RVG102 (referred to throughout as MCMV) expresses recombinant EGFP under the immediate early-1 promoter (22), and was a gift from Dr. John Hamilton (Duke University). MCMV stocks were prepared from salivary gland homogenate and their viral titers were determined via standard plaque assay using mouse embryonic fibroblast cells (18). Infections were performed with 5 × 104 or 1 × 105 PFU i.p. All infections examining the iNKT cell response were for 36 hours, and all infections for CD8+ T cells were for 7 days.
α-GalCer treatment.
Mice were treated with 2 μg of α-GalCer (KRN7000, Avanti Polar Lipids, Inc.) in 0.5% PBS-Tween i.p. for two hours, and compared to a 0.5% PBS-Tween vehicle control.
Lymphocyte isolation.
Spleens were dissociated in 1% PBS-serum, filtered, and underlayed with lympholyte-M (Cedarlane Laboratories) or dissociated in 150 mM NH4Cl for 10 minutes, filtered, and washed twice with 1% PBS-serum. Livers were perfused with 1% PBS-serum prior to harvesting, dissociated using the GentleMACS program E0.1 (Miltenyi Biotech), and filtered; samples were washed three times in 1% PBS-serum, overlayed onto a two-step discontinuous Percoll gradient (GE Healthcare Bio-Sciences), and centrifuged at room temperature for 20 minutes at 2500 RPM. Thymi were dissociated in 1% PBS-serum and filtered. Live cell counts were obtained using trypan blue to exclude dead cells with a hemocytometer or propidium iodide to exclude dead cells on a MACSQuant (Miltenyi).
Adoptive transfer.
Splenic iNKT cells from MyD88 control or cKO animals were enriched using magnetic depletion on an AutoMACS or OctoMACS (Miltenyi) with CD19 and CD8 MicroBeads (Miltenyi). Samples were then i.v. injected into B6.SJL recipients and 3 hours post-injection recipients were infected with MCMV. At 36 hours post-infection, recipients were sacrificed and splenic iNKT cells were enriched using magnetic depletion on an AutoMACS or OctoMACS (Miltenyi) with CD19 and CD8 MicroBeads (Miltenyi). The entire splenic negative fraction and liver samples were then stained to determine IFN-γ production.
Antibodies and flow cytometry.
Staining was performed in 1% PBS-serum containing CD1d-, M45-, or M57-loaded tetramer (NIH Tetramer Facility), extracellular antibodies, and Fc block (2.4G2) in the dark for 15 minutes at room temperature followed by 15 minutes on ice. Intracellular staining was performed by fixing samples for 30 minutes with Cytofix/Cytoperm (BD Biosciences), followed by staining in Perm/Wash Buffer (BD Biosciences) for 30 minutes. Intranuclear staining was performed by fixing samples for 30 minutes with Fixation/Permeabilization Solution (eBioscience), followed by staining in Permeabilization Buffer (eBioscience) for 30 minutes. To maintain eYFP expression during intranuclear staining, samples were prefixed with fresh 4% PFA (Electron Microscopy Science) for 15 minutes, fixed using Fixation/Permeabilization Solution 2 (Miltenyi) for 40 minutes, incubated with 1% Triton X-100 for 15 minutes, and then stained for 30 minutes in PBS. Samples were run on a FACSAria III (BD Biosciences) or MACSQuant (Miltenyi) and analyzed using FlowJo (Tree Star Inc.). The antibodies listed below were used for flow cytometry and purchased from BD, BioLegend, eBioscience, or Thermo Fisher Scientific: CD4-APC, CD4-BV570, CD8α-BV605, CD19-BV785, CD19-PE, CD45-APC-eF780, CD45-BV570, CD45.1-APC, CD45.2-PerCP-Cy5.5, CD69-FITC, CD69-PE, CD69-PerCP-Cy5.5, CD127-PerCP-eF710, IFN-γ-APC, IFN-γ-PE, IL-4-PE-Cy7, KLRG1-PE-Cy7, NK1.1-BV785, NK1.1-PE-Cy7, PLZF-PE, RORγt-PerCP-eF710, RORγt-APC, T-bet-PE-Cy7, TCRβ-BV510, TCRβ-FITC, TCRβ-PerCP-Cy5.5.
Western blot.
Protein lysate was run on a 4–20% Mini-Protean TGX gel (BioRad) and transferred onto a nitrocellulose membrane (BioRad). Membranes were incubated overnight at 4 °C with purified anti-MyD88 antibody (ProSci) followed by peroxidase-conjugated donkey anti-rabbit (Jackson ImmunoReseach) and Precision Protein StrepTactin-HRP Conjugate (BioRad). SuperSignal West Pico Chemiluminescent Substrate was used for imaging (Thermo Scientific). Membranes were stripped and incubated overnight at 4 °C with purified anti-β-actin (Invitrogen) followed by peroxidase-conjugated donkey anti-mouse (Jackson ImmunoResearch) and Precision Protein StrepTactin-HRP Conjugate (BioRad).
Statistical analysis.
Statistical analyses were performed with Prism 7.0 or 8.0 (Graph-Pad Software, Inc.). Unpaired two-tailed Student’s t-tests were used to compare two individual groups. Error bars indicate SEM and *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
RESULTS
IL-12Rβ2 and MyD88 signaling are dispensable for iNKT cell development and peripheral localization
To characterize the roles of IL-12, IL-18, and TLRs on iNKT cell activation during MCMV infection, we generated two conditionally deficient mouse lines. We utilized MyD88 as a means to broadly begin looking at the importance of IL-18 and TLRs in tandem, because it is an adaptor protein that signals downstream of all TLRs (except TLR3) and the IL-1R/IL-18R superfamily (23). MyD88 conditional knockouts (cKOs) were generated by crossing MyD88fl/fl mice with CD4Cre recombinase expressing animals and a line expressing enhanced yellow fluorescent protein (eYFP) in the Rosa locus. Cre recombinase driven by the CD4 promoter allows for deletion at the DN4/DP stage of T cell development (24, 25), and Rosa-eYFP is surrounded by loxP-flanked stop sequences, acting as a reporter of Cre recombinase activity (26). Western blot confirms T cell-specific loss of MyD88 protein expression from MyD88fl/fl eYFP+/− CD4cre+/− mice (referred to as MyD88 cKO), compared to littermate controls (MyD88fl/+ eYFP+/− CD4cre+/−, referred to as MyD88 control) (Fig. S1A). eYFP expression also effectively labels almost the entirety of cells in the T cell lineage, including iNKT cells (Fig. S1B). To investigate the role of IL-12, IL-12Rβ2 cKO were made by crossing IL-12Rβ2fl/fl mice our lab recently generated (21) with CD4cre expressing mice; however, IL12rb2 and Rosa-eYFP are both on Chromosome 6, which prohibited the use of this tool.
We first wanted to establish that iNKT cells from naïve animals were not affected by loss of IL-12Rβ2 or MyD88. IL-12Rβ2fl/fl CD4cre+/− mice (referred to as IL-12Rβ2 cKO) have comparable T cell development and peripheral iNKT cell populations to littermate controls (IL-12Rβ2fl/+ CD4cre+/−, referred to as IL-12Rβ2 control) (Fig. 1A, B). IL-15 and IL-7 are known to be important for differentiation into NKT1 and NKT17, respectively (27–30), thus we also investigated the ratio of NKT1, NKT2, and NKT17 populations in the absence of IL-12; using PLZF, RORγt, and T-bet expression allows for the differentiation of these three subsets. The majority of thymic iNKT cells from mice on the C57BL/6 background are NKT1 cells (20). Since NKT2 and NKT17 cells are scarce, a BALB/c control was used to establish the gating for these experiments, however even in this strain there can be variation (Fig. S1C, D). As expected, IL-12Rβ2 control mice are predominantly populated by NKT1 cells, and loss of IL-12 signaling has no observable effects on the proportion of thymic NKT1, NKT2, and NKT17 cells (Fig. 1C, S1C). Similarly to what we find for IL-12Rβ2 cKO mice, T cell development and iNKT cell populations in the thymus and periphery are similar between MyD88 cKO mice and littermate controls (Fig. 1D–F, S1D). These data illustrate that IL-12, IL-18, and TLRs are dispensable for the development of iNKT cells and their peripheral localization.
Figure 1.
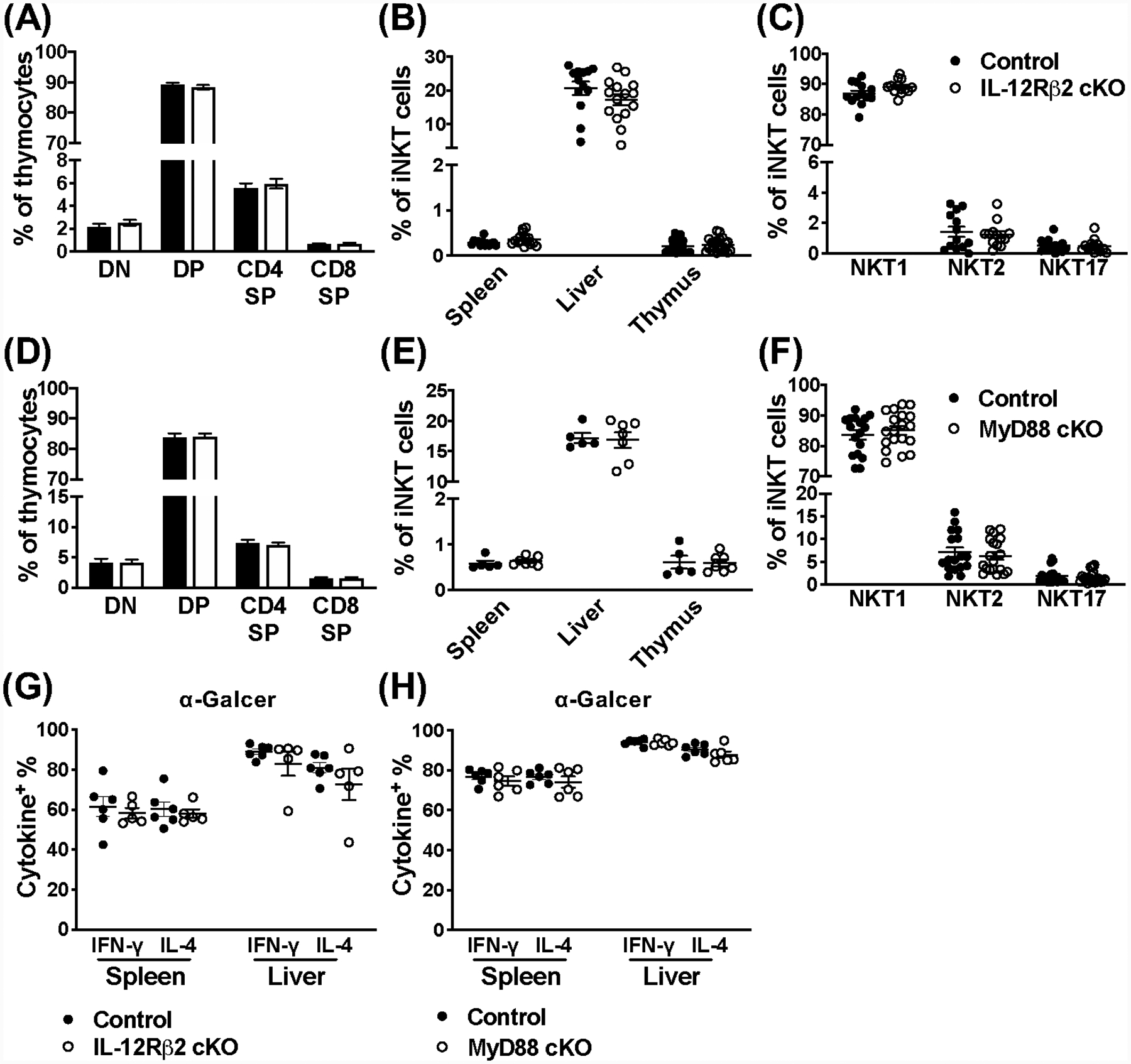
IL-12Rβ2 and MyD88 signaling are dispensable for iNKT cell development, peripheral localization, and their response to a strong agonist. (A) The CD4−CD8− double negative (DN), CD4+CD8+ double positive (DP), and CD4+ or CD8 + single positive (SP) stages of T cell development in the thymus of IL-12Rβ2 control (black) and cKO (open) mice (n=18–20). (B) Frequency of iNKT cells (TCRβ+CD1dtet+) in indicated organs from IL-12Rβ2 control (black) and cKO (open) mice (n=13–20). (C) Frequency of NKT1, NKT2, and NKT17 lineages from the thymus of IL-12Rβ2 control (black) and cKO (open) mice (n=13). NKT cell lineages (TCRβ+CD1dtet+) were differentiated using PLZF and RORγt expression. (D) The DN, DP, and CD4+ or CD8+ SP stages of T cell development in the thymus of MyD88 control (black) and cKO (open) mice (n=17). (E) Frequency of iNKT cells (CD45+TCRβ+CD1dtet+) in indicated organs from MyD88 control (black) and cKO (open) mice (n=5–7). (F) Frequency of NKT1, NKT2, and NKT17 lineages from the thymus of MyD88 control (black) and cKO (open) mice (n=17–19). NKT cell lineages (TCRβ+CD1dtet+) were differentiated using PLZF and RORγt expression. (G) Frequency of IFN-γ+ and IL-4+ iNKT cells (TCRβ+CD1dtet+) from the spleen and liver of IL-12Rβ2 control (black) and IL-12Rβ2 cKO (open) mice 2 hours post-stimulation with α-Galcer (n=5–6). (H) Frequency of IFN-γ+ and IL-4+ iNKT cells (TCRβ+CD1dtet+eYFP+) from the spleen and liver of MyD88 control (black) and MyD88 cKO (open) mice 2 hours post-stimulation with α-Galcer (n=6). Data are pooled from two (E, G, H) or at least three (A-D, F) independent experiments and error bars indicate SEM.
IL-12 and IL-18 are dispensable during agonistic stimulation of iNKT cells
To determine whether IL-12 or IL-18 have any roles during iNKT cell TCR-mediated activation, we utilized α-GalCer (KRN7000). α-GalCer is a potent synthetic iNKT cell agonist, and results in both rapid and robust IFN-γ and IL-4 production (2, 3). Mice were stimulated with α-GalCer and iNKT cells were assessed 2 hours post-stimulation, and vehicle alone was used as a control. We found that neither IL-12Rβ2 (Fig. 1G) or MyD88 (Fig. 1H) affect the IFN-γ and IL-4 response of iNKT cells to α-GalCer treatment. Previous studies have suggested a role for co-stimulation and IL-12 produced by activated dendritic cells (6, 7), however the data presented here indisputably demonstrate that when iNKT cells are activated via their TCR using a strong agonist, other signals from IL-12, IL-18 or TLRs are dispensable.
Loss of IL-12Rβ2 signaling has major effects on iNKT cell activation following MCMV infection, and MyD88 signaling has organ-specific effects
We next wanted to investigate the iNKT cell-specific response to MCMV, where they are activated independently of TCR engagement, in the absence of the IL-12R and MyD88. Our lab, and others, have previously shown that IL-12 in particular, but also IFN-α/β and IL-18, are important for an optimal iNKT cell response (16, 17). The peak of the iNKT cell response is 36 hours post-infection, and is characterized by robust IFN-γ production in the spleen and liver, but not IL-4 (16). At day 1.5 post-infection, IL-12Rβ2 cKO mice and littermate controls have comparable frequencies and numbers of iNKT cells in the spleen and liver (Fig. 2A, B), however the IFN-γ response is completely abolished in the absence of IL-12 signaling (Fig. 2C, D). In contrast, the NK cell response is unaffected, since they retain their ability to respond to IL-12 (Fig. 2E & Fig. S2A).
Figure 2.
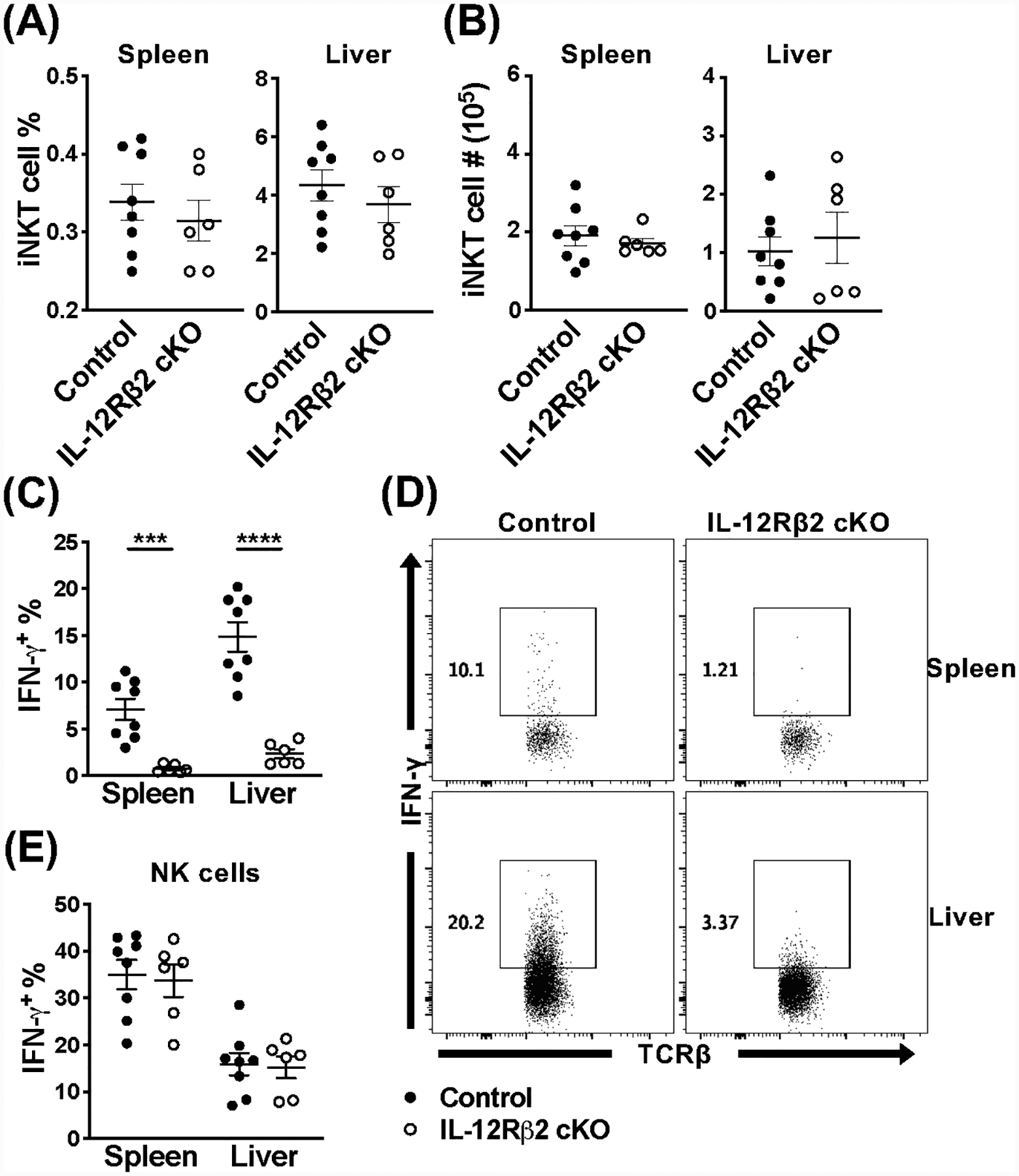
Loss of IL-12Rβ2 signaling has major effects on iNKT cell activation during acute MCMV infection. (A) Frequency and (B) absolute number of iNKT cells (TCRβ+CD1dtet+) from the spleen and liver of IL-12Rβ2 control (black) and IL-12Rβ2 cKO (open) mice at 36 hours post-infection with MCMV. (C) Frequency and (D) representative flow cytometry of IFN-γ+ iNKT cells (TCRβ+CD1dtet+) and (E) frequency of IFN-γ+ NK cells (TCRβ−NK1.1+) from the spleen and liver of indicated mice at 36 hours post-MCMV. Data are pooled from or representative of two (A-E, n=6–8) independent experiments and error bars indicate SEM.
Loss of MyD88 signaling in iNKT cells also does not affect their frequency or number in the spleen and liver at 36 hours post-infection with MCMV (Fig. 3A, B). Surprisingly however, splenic iNKT cells appear to be hyporesponsive compared to littermate controls (Fig. 3C, D), with approximately half the amount of IFN-γ+ iNKT cells. This impairment does not coincide with decreased CD69 expression (Fig. 3E). In contrast, the frequency of iNKT cells producing IFN-γ in the liver is unaffected in the absence of MyD88 (Fig. 3C, D), indicating there may be organ specific requirements. This hyporesponsiveness is not a global defect, as indicated by the normal NK cell response (Fig. 3F & Fig. S2B). Notably, these diminished responses are not the result of an intrinsic defect in these cell’s ability to produce IFN-γ upon stimulation, since they are fully capable of responding to other stimuli, including α-GalCer (Fig. 1H).
Figure 3.
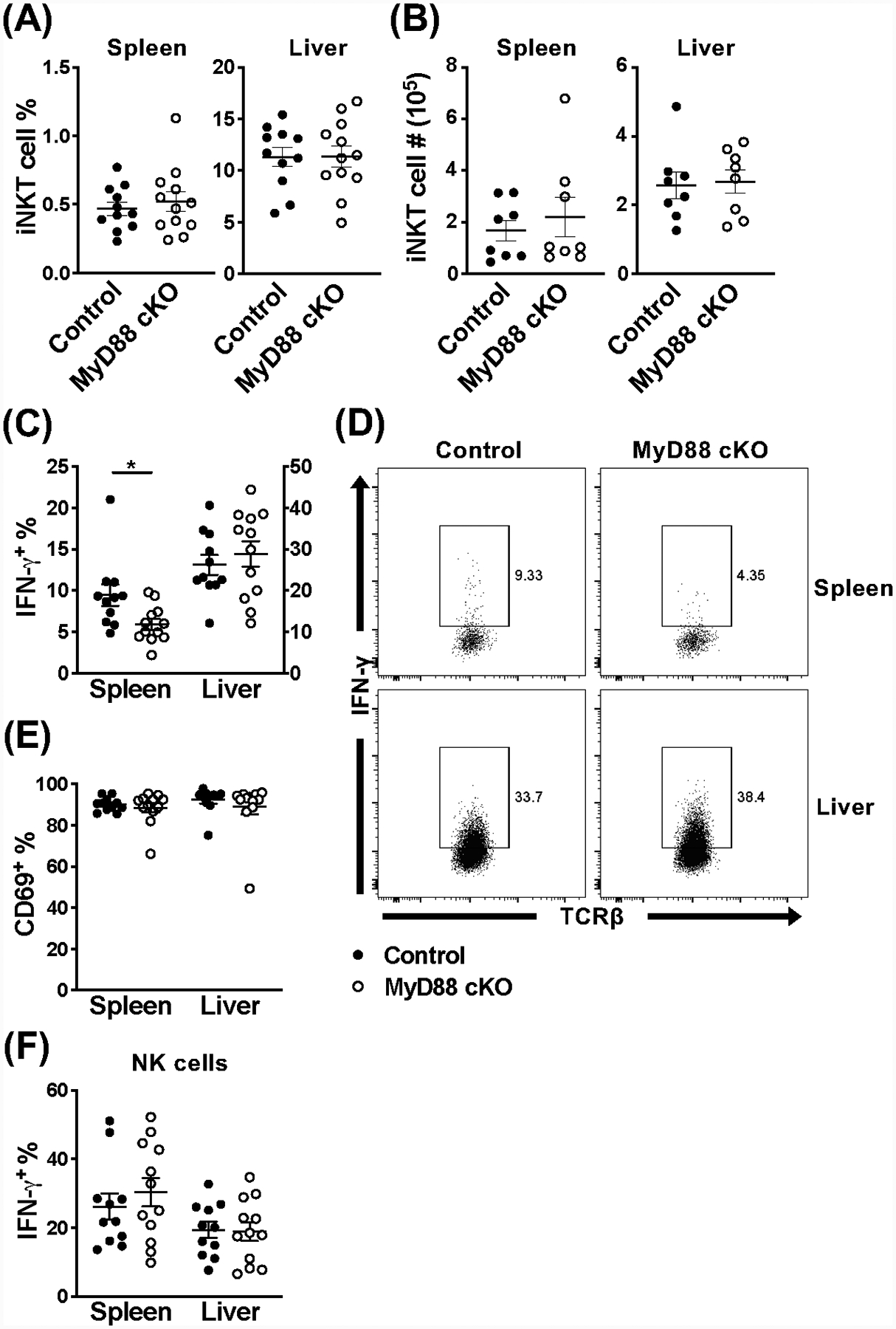
Splenic iNKT cells are hyporesponsive during MCMV infection in the absence of MyD88 signaling. (A) Frequency and (B) absolute number of iNKT cells (TCRβ+CD1dtet+) from the spleen and liver of MyD88 control (black) and MyD88 cKO (open) mice at 36 hours post-infection with MCMV. (C) Frequency and (D) representative flow cytometry of IFN-γ+ iNKT cells (TCRβ+CD1dtet+eYFP+) and (E) frequency of CD69+ iNKT cells (TCRβ+CD1dtet+eYFP+) from the spleen and liver of indicated mice at 36 hours post-MCMV. (F) Frequency of IFN-γ+ NK cells (TCRβ−NK1.1+) from the spleen and liver of indicated mice at 36 hours post-MCMV. Data are pooled from two (B, n=8) or three (A, C-F, n=11–12) independent experiments and error bars indicate SEM.
The CD8+ T cell response does not require MyD88 during MCMV infection
To determine whether the hyporesponsive phenotype of MyD88-deficient iNKT cells was unique, or a potential issue for other T cells during MCMV infection as well, we next considered the adaptive response. It is well known that there is a robust non-inflationary CD8+ T cell response, whose expansion and activation both peak on day 7 post-infection; in C57BL/6 mice, this population recognizes a number of MCMV-derived antigens, including M45 and M57 (31, 32). Following MCMV infection, we find a comparable frequency and number of CD8+ T cells from MyD88 cKO and littermate controls in the spleen and liver on day 7 (Fig. 4A, B). We then examined the quality of the CD8+ T cell response by staining with MCMV-specific MHC class I tetramers loaded with either the M45 or M57 epitopes. In the absence of MyD88 signaling, these antigen-specific CD8+ T cells are also unaffected in MyD88 cKO mice, compared to littermate controls (Fig. 4C, D). Finally, we looked at the proportion of responding cells that differentiated into T effector cells (TEFF, KLRG1+CD127−) on day 7 post-infection. However, loss of MyD88 also had no effect on the formation of this effector population (Fig. 4E). Together, these data indicate that MyD88 signaling does not influence all T cell populations during MCMV infection, rather that splenic iNKT cells are uniquely sensitive to its absence.
Figure 4.
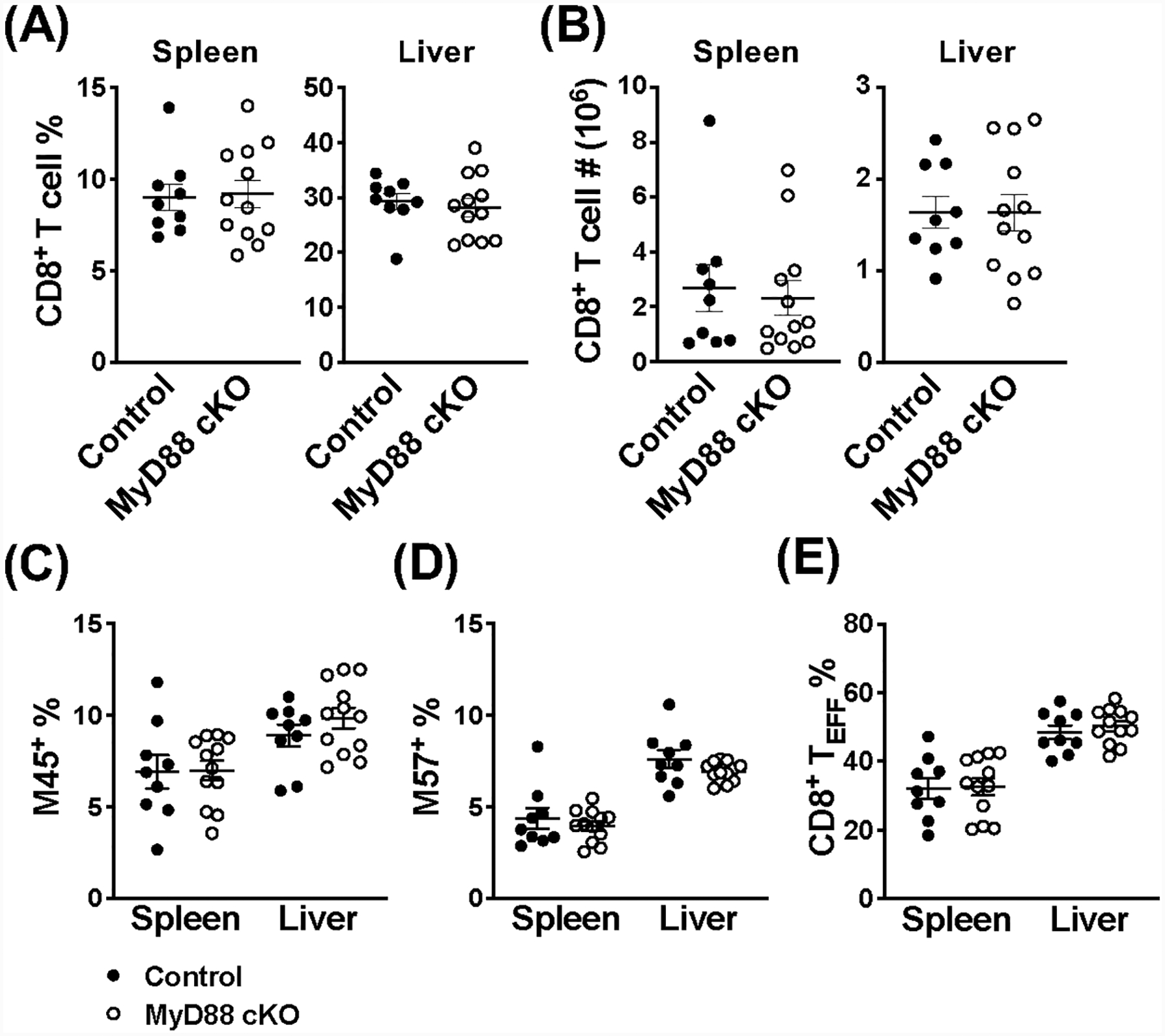
CD8+ T cells do not require MyD88 signaling during MCMV infection. (A) Frequency and (B) absolute number of CD8+ T cells (TCRβ+CD8+) from the spleen and liver of MyD88 control (black) and MyD88 cKO (open) mice on day 7 post-infection with MCMV. Frequency of antigen-specific CD8+ T cells (TCRβ+CD8+eYFP+) in the spleen and liver of indicated mice on day 7 post-MCMV using (C) M45-loaded and (D) M57-loaded MHC class I tetramers. (E) Frequency of CD8+ TEFF cells (TCRβ+CD8+eYFP+KLRG1+CD127−) from the spleen and liver of indicated mice on day 7 post-MCMV. Data are pooled from three independent experiments (n=9–12) and error bars indicate SEM.
NKT1 cells are not detrimentally affected by loss of MyD88 signaling
We next wanted to establish whether splenic iNKT cell hyporesponsiveness was due to a difference in the subsets of iNKT cells during acute MCMV infection. To answer this question, we assessed whether the proportion of NKT1, NKT2, and NKT17 cells in the spleen was altered in the absence of MyD88 by looking at PLZF, RORγt, and T-bet expression; similarly to CD4+ T helper subsets, NKT1 cells predominantly produce IFN-γ. While MyD88 cKO mice have a slight increase in the frequency of NKT1 cells in the spleen and liver at 36 hours post-infection, there is no difference in absolute number (Fig. 5A, B & Fig. S3A). Conversely, there is also a modest decrease in NKT17 cell frequency (spleen and liver) and absolute number (liver), compared to littermate controls (Fig. 5A, B & Fig. S3A). These minor alterations are not observed in naïve animals (data not shown). While it is difficult to make conclusions about NKT2 and NKT17 cells given their scarcity, the hyporesponsiveness of MyD88-deficient splenic iNKT cells is likely not due to a loss of IFN-γ-producing cells during MCMV infection.
Figure 5.
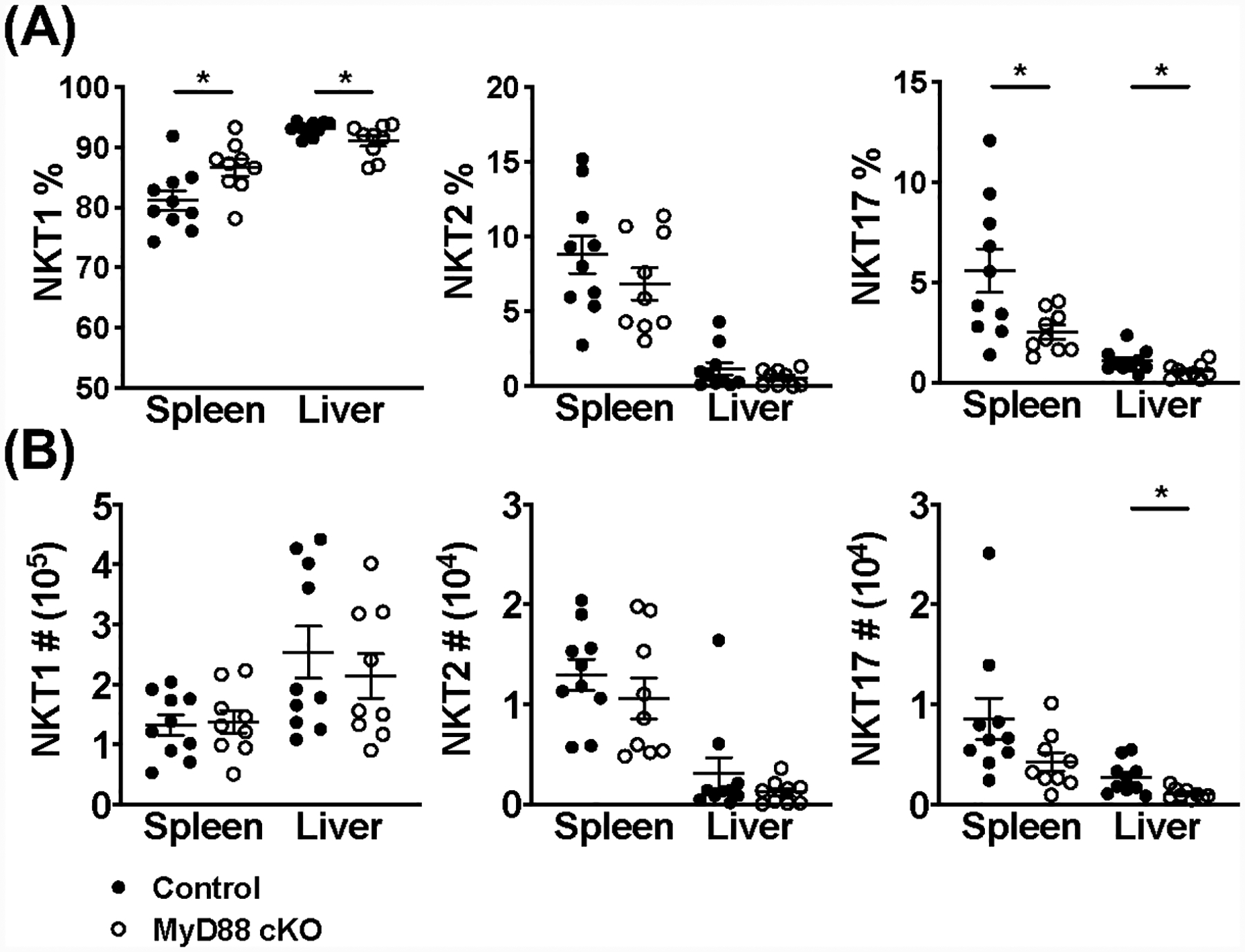
NKT1 cells are not detrimentally affected by loss of MyD88 signaling during acute MCMV infection. (A) Frequency and (B) absolute number of NKT1, NKT2, and NKT17 cells (CD45+CD1dtet+TCRβ+) in the spleen and liver of MyD88 control (black) and MyD88 cKO (open) animals at 36 hours post-infection with MCMV. NKT cell lineages were differentiated using PLZF, RORγt, and T-bet expression. Data are pooled from two independent experiments (n=9–10) and error bars indicate SEM.
Loss of IL-18 signaling phenocopies the response of iNKT cells in MyD88 cKO mice
Since MyD88 is an adaptor protein that is downstream of a number of important signaling pathways, we wanted to determine which of its functions are responsible for the phenotype we are observing. IL-18 has been shown to be critical for iNKT cell activation in some studies (33), but dispensable in others (17). We therefore began by comparing the iNKT cell response in IL-18−/− mice and C57BL/6 wild-type controls. At 36 hours post-MCMV infection, there is no difference in the frequency or absolute number of splenic or hepatic iNKT cells (Fig. 6A, B). Surprisingly however, when we looked at the frequency of cells producing IFN-γ, the iNKT cells in the spleen of IL-18−/− mice were hyporesponsive, while unaffected in the liver (Fig. 6C, D). Similarly to our findings in MyD88 cKO animals, there was no difference in CD69 expression accompanying the defect in IFN-γ+ splenic iNKT cells (Fig. 6E). As previously observed, NK cells are also affected by the absence of IL-18 signaling in the spleen and have a decreased frequency of IFN-γ producing cells, compared to wild-type animals (Fig. 6F). Together, these data illustrate that loss of IL-18 signaling by iNKT cells phenocopies the results we observed in MyD88 cKO animals. In addition, it demonstrates that the effects observed are likely exclusively mediated by IL-18, with minimal direct roles for TLR ligands on iNKT cells, if any.
Figure 6.
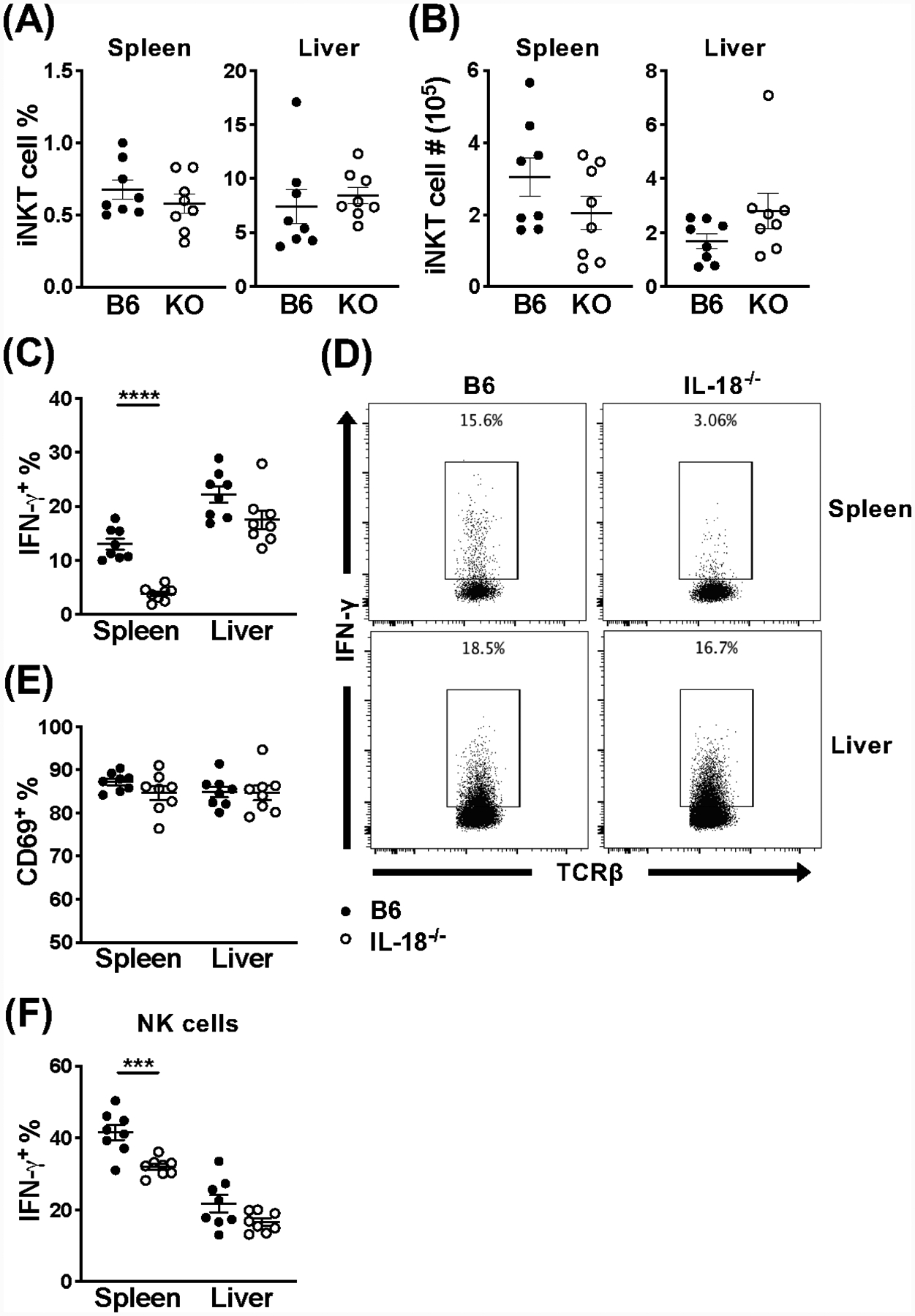
Loss of IL-18 signaling results in hyporesponsive splenic iNKT cells, however hepatic iNKT cells respond normally. (A) Frequency and (B) absolute number of iNKT cells (CD45+TCRβ+CD1dtet+) from the spleen and liver of C57BL/6 (black) or IL-18−/− (open) mice at 36 hours post-infection with MCMV. (C) Frequency and (D) representative flow cytometry of IFN-γ+ iNKT cells (CD45+TCRβ+CD1dtet+) and (E) frequency of CD69+ iNKT cells (CD45+TCRβ+CD1dtet+) from the spleen and liver of indicated mice at 36 hours post-MCMV. (F) Frequency of IFN-γ+ NK cells (CD45+TCRβ−NK1.1+) from the spleen and liver of indicated mice at 36 hours post-MCMV. Data are pooled from or representative of two independent experiments (n=8) and error bars indicate SEM.
Splenic iNKT cells are intrinsically affected by loss of MyD88 signaling
We next wanted to determine whether the defect in MyD88-deficient splenic iNKT cells was due to an extrinsic result of their environment, or an intrinsic consequence on the cells themselves. To tease these two circumstances apart, we took an adoptive transfer approach. Bulk splenocytes from MyD88 cKO mice or littermate controls (CD45.2+) were enriched for iNKT cells and i.v. injected into B6.SJL congenic recipients (CD45.1+). Three hours post-transfer, recipients were infected with MCMV, which allowed us to examine the donor cell response in a wild-type environment. When we look at the donor cells that trafficked back to the spleen, surprisingly MyD88 cKO iNKT cells remain hyporesponsive compared to MyD88 control cells (Fig. 7A, C). We also see a similar trend for the donor cells that trafficked to the liver, however it was not statistically significant (Fig. 7A, C). To illustrate that we are not detrimentally affecting the cells during adoptive transfer, we also examined the IFN-γ production of donor NK cells from both groups, and found a comparable response in the spleen and liver (Fig. 7B). Thus, splenic iNKT cell IFN-γ production is intrinsically affected in the absence of MyD88 signaling under these conditions. Since these cells were only given three hours to acclimate to their new environment, it remains possible that if they were allowed to reconstitute for a longer time period before infection, MyD88 signaling may have different requirements.
Figure 7.
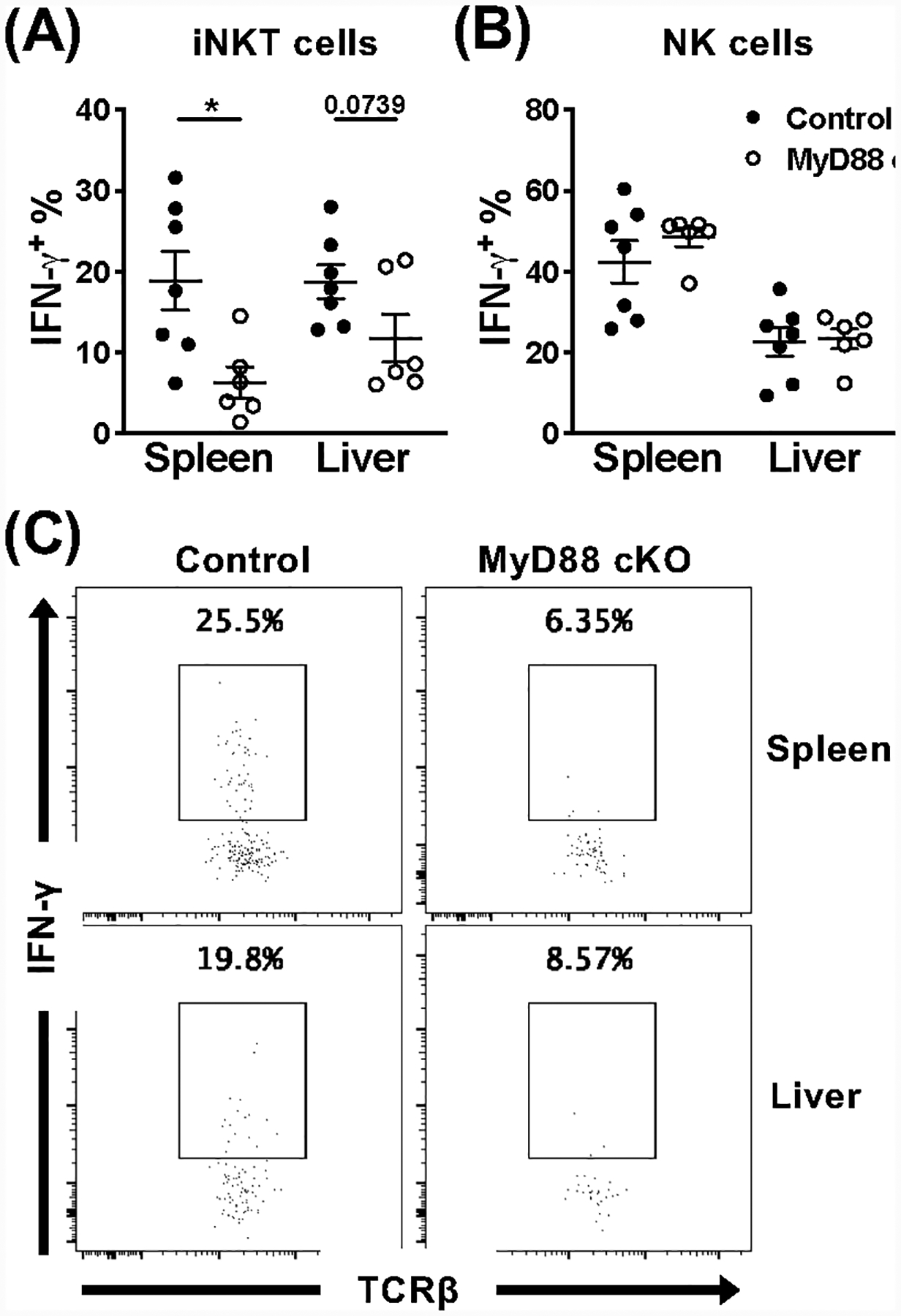
Splenic iNKT cells are intrinsically affected by loss of MyD88 signaling during acute MCMV infection. Frequency of IFN-γ+ donor (A) iNKT cells (CD45+TCRβ+CD1dtet+CD45.1−CD45.2+eYFP+) and (B) NK cells (CD45+TCRβ−NK1.1+CD45.1−CD45.2+) from the spleen and liver of B6.SJL recipients at 36 hours post-infection with MCMV. Donor cells were isolated from either MyD88 control (black) or MyD88 cKO (open) animals and enriched for iNKT cells prior to adoptive transfer. (C) Representative flow cytometry of IFN-γ production by indicated donor iNKT cells from the spleen and liver of B6.SJL recipients. Data are pooled from or representative of two independent experiments (n=6–7) and error bars indicate SEM.
DISCUSSION
The goal of these studies was to determine the importance of different signaling pathways on the iNKT cell response. We expanded upon earlier studies using a genetic approach in which iNKT cells were impaired in their ability to respond to inflammatory cytokines and TLR ligands. iNKT cells can be activated directly via their TCR, a mix of TCR signaling and cytokine receptor signaling, and through inflammatory cytokines only (8, 9). Brigl and colleagues initially postulated that IL-12 and CD1d recognition were the major pathways responsible for the rapid activation of NKT cells during different microbial infections (34). However, upon revisiting these findings they proposed that cytokine-driven signals overwhelmingly dictate iNKT cell activation during microbial infections, rather than microbial antigens (35). These conclusions, supported by data previously obtained by us and others using viral infections, demonstrate that iNKT cells can be exclusively activated via inflammatory cytokines (12, 16, 17, 36). In this study, we therefore focused on the two main stimulation pathways that have been ascribed to iNKT cells, and used α-GalCer and MCMV infection for direct and indirect stimulation, respectively.
Regarding direct TCR stimulation, it has been proposed that blockade of CD28-CD80/CD86 and CD40-CD154 pathways inhibit the α-GalCer-induced IFN-γ production by splenic iNKT cells due to reduced IL-12 production by DC cells (7). Our results demonstrate that IL-12, IL-18, and TLRs are completely dispensable when a strong agonist is used, which is supported by the presence of preformed mRNA for IL-4 and IFN-γ in resting iNKT cells (5). However, although compounds likely closely related to α-GalCer have been reported as endogenous and physiological ligand (37), it is nevertheless an extremely strong stimulus, which does raise the question of how comparable the response we observe is to a more relevant infection model. For example, iNKT cells are known to become activated in a CD1d-dependent mechanism during Streptococcus pneumoniae infection in mice (38, 39). Regarding cytokine receptor signaling, we and others have previously shown an essential requirement of IL-12 for IFN-γ production by iNKT cells during MCMV infection, however these were performed using global knockout mice (16, 17). Our results using the new IL-12Rβ2 cKO mice presented here are congruent with these previous findings (Fig. 2), that IL-12 signaling is essential for iNKT cell activation during MCMV infection.
Importantly, our data also clarify the role of TLR engagement (with the exception of TLR3) directly on iNKT cells, particularly when the infection is known to involve several TLR ligands. MCMV is primarily recognized in a TLR9-dependent manner by conventional and plasmacytoid DCs, but also through TLR7 and TLR3, resulting in the production of inflammatory cytokines, e.g. IL-12, IL-15, IL-18, IL-33, and IFN-α/β (40, 41). As a result, global loss of MyD88 signaling results in the severe impairment of both NK and iNKT cell activation following MCMV infection (40, 41). Whether or not iNKT cells actually express TLRs themselves, or are directly stimulated by their ligands, remains controversial. Human iNKT cells express all TLRs, except TLR8, but are not activated in the presence of TLR ligands (42). Others find that murine iNKT cells express TLR2 and TLR4, and are capable of activation through their ligands (13, 14). The expression of TLRs by murine iNKT cells is also upregulated following TCR-activation, which enhances stimulation (15). Using MyD88 cKO mice allowed us to avoid affecting the inflammatory cytokine response from other innate immune cells. Since the data obtained with IL-18−/− mice phenocopies MyD88 cKO mice, this rules out a role for MyD88-dependent TLRs expressed by iNKT cells during MCMV infection. Rather, these results imply that TLR engagement on non-iNKT cells leads to iNKT cell activation indirectly.
Somewhat unexpectedly, we find that iNKT cell IFN-γ production is differentially affected in MyD88 cKO mice, compared to control animals – splenic iNKT cells are hyporesponsive, while hepatic iNKT cells have a normal response. These data illustrate that hepatic iNKT cells do not require TLR engagement, but also do not require IL-18R engagement for appropriate levels of IFN-γ. In contrast, a component of the MyD88 signaling pathway is necessary for a robust cytokine response in the spleen during MCMV infection. Even though splenic iNKT cells in MyD88 cKO mice maintain their ability to respond to IL-12, in conjunction with other pro-inflammatory cytokines, this is not sufficient (Fig. 3C, D). We determined this hyporesponsive phenotype is due to an absence of IL-18 (Fig. 6), illustrating a previously unappreciated role on the splenic iNKT cell response during MCMV infection.
Whether IL-18 is playing a role during co-stimulation or in the priming of splenic iNKT cells during MCMV infection is unclear. NK cells are hyporesponsive in IL-18−/− animals when stimulated with IL-12 ex vivo, however NK cells with previous access to IL-18 can later adequately respond to IL-12 in the absence of IL-18 co-stimulation (43). Thus, it is possible that loss of IL-18 priming and loss of IL-18 co-stimulation will have different effects on the iNKT cell response during MCMV infection. However unlike NK cells, when wild-type iNKT cells are stimulated ex vivo with either IL-12, IL-18, or IFN-β alone, no single cytokine successfully provokes iNKT cells to produce IFN-γ (17). A coordinated requirement for IL-12 and either IL-18 or IFN-β was necessary for activation in vitro, whereas IL-18 and IFN-β together were insufficient (17). Why access to IL-12 and IL-18 by splenic iNKT cells is required for optimal IFN-γ production, versus IL-12 and co-stimulation with another pro-inflammatory cytokine (such as type I IFNs) is sufficient in the liver remains unclear. It could also be that in the absence of IL-18, other cytokines are present in an adequate concentration for co-stimulation within the liver, but this threshold is not reached in the spleen. Whether this is the case, and which cytokine(s) are involved, requires further elucidation. Nevertheless, because splenic iNKT cells are intrinsically affected by loss of MyD88 signaling, these data suggest that there may be diversity even within the NKT1 population.
There also remains the possibility that the loss of MyD88 signaling pathways in iNKT cells could have a multifactorial effect, and not necessarily an additive defect. It will be important to continue investigating the roles of additional MyD88-dependent cytokines such as IL-33 (44, 45), especially since our data clearly show that the iNKT cell response during MCMV infection has different requirements based on anatomic location. Furthermore, our work is consistent with findings demonstrating that the localization of iNKT cells governs their cytokine response, both at steady state and upon activation (46).
We also looked at whether loss of IL-12 or IL-18 signaling affected the proportion of iNKT cell subsets during thymic development and acute MCMV infection. In both instances, we observed no major differences between cKO animals and littermate controls, and that the majority of the iNKT cells present were NKT1 cells. However, given the rarity of NKT2 and NKT17 cells in mice on the C57BL/6 background, it would be interesting to examine how T cell-specific loss of IL-12 and IL-18 signaling on the Balb/c background would influence the ratio of these subsets.
To summarize, the findings presented here greatly improve our understanding of the signaling requirements for iNKT cell activation. For a strong agonist like α-GalCer, that directly engages with the iNKT cell TCR, no secondary signal from IL-12, IL-18, or TLRs are required. In contrast, during viral infections like MCMV, which lack glycolipid antigens, external signals are essential for an appropriate iNKT cell response. We discovered a unique requirement for IL-18 by splenic iNKT cells during MCMV infection. This intrinsic requirement of MyD88 by splenic iNKT cells begs the question of what distinguishes iNKT cells in different localizations to have distinct responses and signaling requirements, when they are characterized as the same lineage.
Supplementary Material
Key Points.
IL-12, IL-18, and TLRs are dispensable for TCR-dependent activation of iNKT cells
During TCR-independent activation, IL-12 is necessary, but not sufficient
Splenic, but not hepatic, iNKT cells need IL-18 during TCR-independent activation
ACKNOWLEDGEMENTS
We thank Céline Fugère for i.v. injections, Kevin Carlson for cell sorting, and the NIH tetramer facility for providing CD1d- and MHC class I-loaded tetramers. We also thank Mimi Le for initial technical support. In addition, we would like to acknowledge Dr. Christian Vosshenrich (The Pasteur Institute) for sharing his protocol to preserve fluorescent protein expression during intranuclear staining.
FINANCIAL SUPPORT
This work was supported by an American Association of Immunologists Career in Immunology Fellowship (L.B.) and the following NIH research grants: R01 AI46709 (L.B.), R01 AI122217 (L.B.) and F31 AI124556 (C.K.A.).
REFERENCES
- 1.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, and Brenner MB. 1994. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 372: 691–694. [DOI] [PubMed] [Google Scholar]
- 2.Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, Keating R, Kronenberg M, Smyth MJ, and Godfrey DI. 2003. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. Journal of immunology (Baltimore, Md. : 1950) 171: 4020–4027. [DOI] [PubMed] [Google Scholar]
- 3.Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, and Van Kaer L. 2003. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proceedings of the National Academy of Sciences of the United States of America 100: 10913–10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, and Kronenberg M. 2003. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proceedings of the National Academy of Sciences of the United States of America 100: 8395–8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, and Locksley RM. 2003. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. The Journal of experimental medicine 198: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, and Nishimura T. 1999. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. The Journal of experimental medicine 189: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, and Okumura K. 2001. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. Journal of immunology (Baltimore, Md. : 1950) 166: 6012–6018. [DOI] [PubMed] [Google Scholar]
- 8.Reilly EC, Wands JR, and Brossay L. 2010. Cytokine dependent and independent iNKT cell activation. Cytokine 51: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Suryadevara N, Hill TM, Bezbradica JS, Van Kaer L, and Joyce S. 2017. Natural Killer T Cells: An Ecological Evolutionary Developmental Biology Perspective. Frontiers in immunology 8: 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, and Trottein F. 2007. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27: 597–609. [DOI] [PubMed] [Google Scholar]
- 11.Cohen NR, Tatituri RV, Rivera A, Watts GF, Kim EY, Chiba A, Fuchs BB, Mylonakis E, Besra GS, Levitz SM, Brigl M, and Brenner MB. 2011. Innate recognition of cell wall beta-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe 10: 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, and Kronenberg M. 2008. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. Journal of immunology (Baltimore, Md. : 1950) 181: 4452–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Kim HS, Kim HY, Oh SJ, and Chung DH. 2012. Direct engagement of TLR4 in invariant NKT cells regulates immune diseases by differential IL-4 and IFN-gamma production in mice. PloS one 7: e45348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Askenase PW, Itakura A, Leite-de-Moraes MC, Lisbonne M, Roongapinun S, Goldstein DR, and Szczepanik M. 2005. TLR-dependent IL-4 production by invariant Valpha14+Jalpha18+ NKT cells to initiate contact sensitivity in vivo. Journal of immunology (Baltimore, Md. : 1950) 175: 6390–6401. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni RR, Villanueva AI, Elawadli I, Jayanth P, Read LR, Haeryfar SM, and Sharif S. 2012. Costimulatory activation of murine invariant natural killer T cells by toll-like receptor agonists. Cell Immunol 277: 33–43. [DOI] [PubMed] [Google Scholar]
- 16.Wesley JD, Tessmer MS, Chaukos D, and Brossay L. 2008. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS pathogens 4: e1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyznik AJ, Verma S, Wang Q, Kronenberg M, and Benedict CA. 2014. Distinct requirements for activation of NKT and NK cells during viral infection. Journal of immunology (Baltimore, Md. : 1950) 192: 3676–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orange JS, Wang B, Terhorst C, and Biron CA. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. The Journal of experimental medicine 182: 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loh J, Chu DT, O’Guin AK, Yokoyama WM, and Virgin H. W. t.. 2005. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. Journal of virology 79: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, and Hogquist KA. 2013. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nature immunology 14: 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodard G, Tata A, Erick TK, Jaime D, Miah SMS, Quatrini L, Escaliere B, Ugolini S, Vivier E, and Brossay L. 2020. Inflammation-Induced Lactate Leads to Rapid Loss of Hepatic Tissue-Resident NK Cells. Cell Rep 32: 107855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry SC, Schmader K, Brown TT, Miller SE, Howell DN, Daley GG, and Hamilton JD. 2000. Enhanced green fluorescent protein as a marker for localizing murine cytomegalovirus in acute and latent infection. J Virol Methods 89: 61–73. [DOI] [PubMed] [Google Scholar]
- 23.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, and Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9: 143–150. [DOI] [PubMed] [Google Scholar]
- 24.Sawada S, Scarborough JD, Killeen N, and Littman DR. 1994. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell 77: 917–929. [DOI] [PubMed] [Google Scholar]
- 25.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, and Wilson CB. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15: 763–774. [DOI] [PubMed] [Google Scholar]
- 26.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, and Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo EF, Acero LF, Stonier SW, Zhou D, and Schluns KS. 2010. Thymic and peripheral microenvironments differentially mediate development and maturation of iNKT cells by IL-15 transpresentation. Blood 116: 2494–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, Stanic AK, Boothby MR, He YW, Zhao Z, Van Kaer L, and Joyce S. 2011. IL-15 regulates homeostasis and terminal maturation of NKT cells. Journal of immunology (Baltimore, Md. : 1950) 187: 6335–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, and Taniguchi M. 2012. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol 10: e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster KE, Kim HO, Kyparissoudis K, Corpuz TM, Pinget GV, Uldrich AP, Brink R, Belz GT, Cho JH, Godfrey DI, and Sprent J. 2014. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol 7: 1058–1067. [DOI] [PubMed] [Google Scholar]
- 31.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, and Hill AB. 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. Journal of immunology (Baltimore, Md. : 1950) 177: 450–458. [DOI] [PubMed] [Google Scholar]
- 32.Schlub TE, Sun JC, Walton SM, Robbins SH, Pinto AK, Munks MW, Hill AB, Brossay L, Oxenius A, and Davenport MP. 2011. Comparing the kinetics of NK cells, CD4, and CD8 T cells in murine cytomegalovirus infection. Journal of immunology (Baltimore, Md. : 1950) 187: 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leite-De-Moraes MC, Hameg A, Arnould A, Machavoine F, Koezuka Y, Schneider E, Herbelin A, and Dy M. 1999. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. Journal of immunology (Baltimore, Md. : 1950) 163: 5871–5876. [PubMed] [Google Scholar]
- 34.Brigl M, Bry L, Kent SC, Gumperz JE, and Brenner MB. 2003. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nature immunology 4: 1230–1237. [DOI] [PubMed] [Google Scholar]
- 35.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, and Brenner MB. 2011. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. The Journal of experimental medicine 208: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holzapfel KL, Tyznik AJ, Kronenberg M, and Hogquist KA. 2014. Antigen-dependent versus -independent activation of invariant NKT cells during infection. Journal of immunology (Baltimore, Md. : 1950) 192: 5490–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kain L, Webb B, Anderson BL, Deng S, Holt M, Costanzo A, Zhao M, Self K, Teyton A, Everett C, Kronenberg M, Zajonc DM, Bendelac A, Savage PB, and Teyton L. 2014. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity 41: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, Li Y, Imamura M, Kaneko Y, Okawara A, Miyazaki Y, Gomez-Velasco A, Rogers P, Dahesh S, Uchiyama S, Khurana A, Kawahara K, Yesilkaya H, Andrew PW, Wong CH, Kawakami K, Nizet V, Besra GS, Tsuji M, Zajonc DM, and Kronenberg M. 2011. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nature immunology 12: 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crosby RA, Mena L, and Arnold T. 2017. Disclosure of newly diagnosed HIV infection and condom use at first sex after diagnosis: a study of young Black men who have sex with men. Sex Health 14: 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, and Beutler B. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America 101: 3516–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puttur F, Francozo M, Solmaz G, Bueno C, Lindenberg M, Gohmert M, Swallow M, Tufa D, Jacobs R, Lienenklaus S, Kuhl AA, Borkner L, Cicin-Sain L, Holzmann B, Wagner H, Berod L, and Sparwasser T. 2016. Conventional Dendritic Cells Confer Protection against Mouse Cytomegalovirus Infection via TLR9 and MyD88 Signaling. Cell Rep 17: 1113–1127. [DOI] [PubMed] [Google Scholar]
- 42.Moreno M, Mol BM, von Mensdorff-Pouilly S, Verheijen RH, de Jong EC, von Blomberg BM, van den Eertwegh AJ, Scheper RJ, and Bontkes HJ. 2009. Differential indirect activation of human invariant natural killer T cells by Toll-like receptor agonists. Immunotherapy 1: 557–570. [DOI] [PubMed] [Google Scholar]
- 43.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, and Walzer T. 2008. Cutting edge: Priming of NK cells by IL-18. Journal of immunology (Baltimore, Md. : 1950) 181: 1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, and Herbelin A. 2009. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. European journal of immunology 39: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 45.Nabekura T, Girard JP, and Lanier LL. 2015. IL-33 receptor ST2 amplifies the expansion of NK cells and enhances host defense during mouse cytomegalovirus infection. Journal of immunology (Baltimore, Md. : 1950) 194: 5948–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, and Hogquist KA. 2015. Tissue-Specific Distribution of iNKT Cells Impacts Their Cytokine Response. Immunity 43: 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


