Abstract
Heavy metal exposure in humans and animals commonly occurs through the consumption of metal-contaminated drinking water and food. Although many studies have focused on the remediation of metals by purification of water using sorbents, limited therapeutic sorbent strategies have been developed to minimize human and animal exposures to contaminated water and food. To address this need, a medical grade activated carbon (MAC) and an acid processed montmorillonite clay (APM) were characterized for their ability to bind heavy metals and mixtures. Results of screening and adsorption/desorption isotherms showed that binding plots for arsenic, cadmium and mercury sorption on surfaces of MAC (and lead on APM), fit the Langmuir model. The highest binding percentage, capacity and affinity were shown in a simulated stomach model, and the lowest percentage desorption (<18%) was shown in a simulated intestine model. The safety and protective ability of MAC and APM were confirmed in a living organism (Hydra vulgaris) where 0.1% MAC significantly protected the hydra against As, Cd, Hg, and a mixture of metals by 30-70%. In other studies, APM showed significant reduction (75%) of Pd toxicity, compared to MAC and heat-collapsed APM, suggesting that the interlayer of APM was important for Pb sorption. This is the first report showing that edible sorbents can bind mixtures of heavy metals in a simulated gastrointestinal tract and prevent their toxicity in a living organism.
Keywords: Heavy metals, activated carbon, adsorption/desorption isotherms, hydra, montmorillonite clay, acid activation
Graphical Abstract
Introduction
Heavy metal contaminants cause important problems due to their mobility in the aqueous ecosystem, their accumulation in the environment and food chain, their hazards to higher life forms, and their non-biodegradable nature (Lasheen et al. 2012; Mishara 2014). Diet is the main route of exposure to metals and significant health risks to humans and animals have been strongly associated with metal exposure. Arsenic, cadmium, lead and mercury (FDA 2019, 2020) are at the top of the list and have been prioritized as the most hazardous metals by the Agency for Toxic Substances and Disease Registry (ATSDR 2019).
Arsenic (As) is commonly found in groundwater from natural and industrial processes. It is the most common cause of acute heavy metal poisoning in adults. Chronic exposure to As from groundwater can cause skin lesions, cancer, neurological effects, hypertension, cardiovascular problems, and pulmonary disease (Smith et al. 2000). Inorganic As, the most common form of As in groundwater, is usually found in two forms, arsenite and arsenate, referred to as As (III) and As (V), respectively.
Cadmium (Cd) is commonly present in the diet, and the background exposure of Cd for the general North American population is approximately 30 μg per day (Satarug et al. 2003). The sources that are potentially the highest in Cd are mushrooms, shellfish, freshwater fish, dried algae, and potable water, among others. Cd exerts damaging effects on the lungs, kidneys, and bones of humans (Meneguin et al. 2017; Van et al. 2019). WHO and EPA established a maximum acceptable concentration for Cd in drinking water of 0.003 mg/L (Siegel et al. 2002).
Dietary intake of Hg is an important source of exposure. Mercury has three forms, including elemental (liquid) Hg, inorganic Hg, and organic Hg. Methylmercury (MeHg), which is known to be the most toxic form, is created when inorganic Hg is dissolved in water in the environment. It is known to accumulate through the ecological food chain, resulting in human and animal exposures and adverse impacts on health. Between 80% to 90% of organic mercury in humans is derived from fish and shellfish intake, and 75% to 90% of organic mercury in fish and shellfish is in the MeHg form (Hong et al. 2012).
Lead (Pb) is especially hazardous for children since they can absorb a higher quantity than adults (Cruz-Olivares et al. 2016). The principal exposure route for Pb in the general population is also through the diet (e.g., vegetables, meat, fruits, seafood, and wine). Low concentrations of Pb in water can be toxic, therefore, the permissible level for Pb in drinking water is 0.05 mg/L according to the EPA (Bhattacharjee et al. 2003).
Importantly, heavy metals such as As, Cd, Hg and Pb can frequently occur as mixtures since they are widely distributed in the environment (Anyanwu et al. 2018; Fairbrother et al. 2007). Previous studies have reported that co-exposure to metal mixtures of As, Cd and Pb has produced severe effects at both high and low dose levels (Wang and Fowler 2008). Also, studies in cells have suggested that exposure to mixtures of Pb, Cd, As and Hg may evoke a synergistic effect, even if individual metals are below NOAEL levels (Zhou et al. 2018). Thus, this present study was designed to investigate the ability of edible carbon and clay sorbents (and combinations) to bind mixtures of As, Cd, Hg and Pb and prevent their toxicity in a living organism.
Many treatment techniques are available to reduce heavy metal contamination in water, such as chemical precipitation, electrolysis, ion exchange, liquid extraction, and membrane separation. Among these, sorbent filtration and water purification have proven to be the simplest and most effective techniques (Mckay et al. 1999; Wang et al. 2010; Yang et al. 2013). However, food is also a major source of metal contamination, and very limited work has been done to remediate metal-contaminated food and feed. Also, no edible sorbent therapy has been reported that can decrease exposure to mixtures of metals in foods and drinking water.
The idea of including broad-acting sorbents in the diet to reduce metal exposure is a logical spinoff of our earlier work in the US and Africa. That work demonstrated that montmorillonite clay was effective against aflatoxins when ingested in capsules, food or water and was safe for consumption in humans and animals for up to 3 months of treatment time. The mechanism involved tight binding of aflatoxins by the sorbent in the stomach and intestines which resulted in decreased toxin bioavailability and toxicity (Phillips 1999; Phillips et al. 2019). To develop broad-acting sorbents for environmental toxins and metals, parent montmorillonite clays were activated with sulfuric acid to enhance porosity and surface area. An important physicochemical property of acid processed montmorillonite (APM) clay was its heterogeneity and structural diversity, including delaminated clay layers with silica chains and amorphous silica formed by cross-linked SiO4 (Tyagi et al. 2006). APM, like carbon, was shown to be broad-acting and has been reported to bind diverse mixtures of hazardous mycotoxins, pesticides and polychlorinated biphenyls (PCBs) (Hearon et al. 2020; Wang and Phillips 2019; Wang et al. 2019a, b). Additionally, activated carbon, which has been commonly used to filter metals from contaminated water streams, is broad-acting and has been shown to remove inorganic heavy metals in aqueous solution. Medical grade activated carbon (MAC) used in this study was derived from selected grades of coconut shell and has been shown to be suitable for drinking water and food applications (Generalcarbon.com).
This study focused on the application of MAC and APM for the binding of heavy metals and metal mixtures (As, Cd, Hg and Pb). We have characterized and optimized metal-sorbent interactions and binding parameters using methods to: 1) prescreen for optimal sorbent/metal interactions and metal dosimetry, 2) determine equilibrium adsorption and desorption with isothermal analysis, and 3) investigate optimal sorbent/metal combinations in a simulated gastrointestinal tract model. Additionally, the hydra assay was used to predict the safety of sorbent treatment and sorbent ability to prevent the adverse effects of individual metals and metal mixtures.
Materials and Methods
Reagents and materials
Certified ACS plus grade hydrochloric acid and trace metal grade nitric acid were purchased from Fisher Scientific (Waltham, MA). ACS reagent grade cadmium nitrate tetrahydrate, methylmercury chloride, lead nitrate, sodium metarsenite, sodium arsenate, potassium permanganate, potassium persulphate, hydroxylamine hydrochloride, stannous chloride, and Omnitrace Ultra nitric acid (NX0408) were purchased from Sigma Aldrich (Saint Louis, MO). ICPMS trace metal standard mixture and internal standards were purchased from Absolute Standards Inc (Hamden, CT). ICPMS spike solution was purchased from Perkin Elmer (Waltham, MA) and mercury stock standards were purchased from Ricca (Arlington, TX). Medical grade activated carbon (MAC), purity > 99%, was obtained from General Carbon Corporation (Paterson, NJ). MAC is a virgin powdered activated carbon derived from a selected grade of coconut shell with 1100 m2/g surface area, 5% moisture, and pH 8-10 (Generalcarbon.com). APM was synthesized based on a previously described method (Wang and Phillips 2019) with a surface area equal to 1213 m2/g and a pH of 3. To investigate the importance of intact interlayers as binding sites for Pb, APM was heated at 200°C for 30 min and 800°C for 1 hr to dehydroxylate the siloxane surface and collapse the interlayer (Wang et al. 2017). Clays and carbon were sieved at 100 mesh to achieve uniform particle size equal to, or less than 149 microns (Grant and Phillips 1998; Wang et al. 2019c).
Screening
A multi-metal stock solution containing 40 ppm As (III, V), 18 ppm Cd (II), 9 ppm Hg (II), and 31 ppm Pd (II) in water was prepared from pure crystals of sodium metarsenite, sodium arsenate, cadmium nitrate tetrahydrate, methylmercury chloride, and lead nitrate, respectively. Serial dilutions of the stock solution at pH 2 resulted in 3 concentrations per metal. These concentrations were based on previous studies that showed distinguishable binding (slight, intermediate, and nearly maximum) for the various sorbents. Into 15 mL of acid-cleaned polypropylene tubes, 5 mL of metal solution was added along with 5 mg sorbent (1 mg/mL). The tubes were mixed at 1000 rpm and 37°C for 2 hr, centrifuged at 2000 g for 20 min, and 3.5 mL of the supernatant was collected for metal analysis.
Metal analysis
A 1:100 dilution of the resulting supernatants was performed by mixing 100 μL of the supernatant and 9.9 mL of 1% Omnitrace Ultra nitric acid (stored in Teflon) for Cd, As, and Pb. To adjust for any matrix effects, calibration standards (7 points) ranging from 0 to 200 μg/L were prepared daily from certified standards in 1% nitric acid. For QA/QC, blanks containing acid were run with each experiment, and calibration standards were checked after every 10 samples followed by a blank analysis. Precision of the metal measurement was determined by analyzing the metal concentration in triplicate for all samples. Samples and calibration standards were detected by an inductively coupled Plasma-Mass Spectrophotometer (ICP-MS) NexIon 300 (PerkinElmer). The interferences of oxide and doubly-charged species were minimized by means of the optimization procedure, primarily by the nebulizer gas flow setting. The nebulizer gas flow rate was set at the highest value that keeps the oxide ratio below 3%. If the other settings are also optimal, then sufficient intensity (ion count rates) can be obtained at a lower nebulizer gas flow. The optimized setting should provide the lowest oxide/doubly-charged ratios as possible. To avoid any carryover, a 2% nitric acid was injected as wash solution between each sample. Concentrations were determined using isotopes (Cd111, Cd114, As75, Pb207 and Pb208) for the best sensitivity according to linear calibration curves (r2 greater than 0.99) using indium, terbium or rhodium as the internal standards for Cd, Pb, or As, respectively. The possible isobaric interferences were monitored and edited using correction equations including the most significant empirical set by PerkinElmer. Sample concentrations were then adjusted to account for the sample dilution factor.
The total Hg concentrations in samples were determined by Cold Vapor Atomic Absorption Spectrometry (CV-AAS, PerkinElmer) following digestion. In summary, 0.1 mL of the water sample was added in a 50 mL digestion vessel. The samples in 2 mL of concentrated nitric acid were heated on a hot block (90-95°C) for 30 min. After removing from the hot block, the samples were swirled and cooled. Then 10 mL of milli-Q water, 5% potassium permanganate and 5% potassium persulphate were added. The digestion vessels were tightly capped and swirled before they were put on a hot block for another 30 min. The vessels were then cooled before 5 mL of hydroxylamine hydrochloride was added. Finally, volumes were brought to 40 mL by adding milli-Q water and samples were ready for the analysis. For QA/QC, blank and laboratory blank spike (LBS) were analyzed with each batch and QC was checked every 10 samples. Calibration standards were prepared in 5% nitric acid and four drops of potassium permanganate were added to reduce the evaporation of mercury.
Adsorption isotherms
A metal solution consisted of an equal final concentration of 20 ppm (μg/mL) As, Cd and Pb, and 8 ppm Hg that was prepared from pure crystals in pH 2 distilled water, which is the average stomach pH. The concentrations were set based on the screening results to ensure the complete saturation of metal binding sites on sorbents, to confirm sorption efficacy, and to select the best sorbents for metal binding. 1 mg/mL of each sorbent was then added to a concentration gradient from 5% to 100% of 5 mL metal solution. Controls included 5 mL of blank solution (pH 2 water), metal solution, and sorbent suspension. All samples were vibrated at 37°C and 1000 rpm using an IKA® electric shaker (VIBRAX VXR basic, Werke, Germany) for 2 hr, as 2 hr is the average digestion time in a human stomach. The sorbent/metal complex was then separated from solution by centrifugation at 2000 g for 20 min and analyzed by ICP-OES for As, Cd and Pb, and AAS for Hg.
Desorption isotherms
The desorption of metals from the surface of sorbents was tested in a simulated intestine model. At the end of adsorption experiments, metal-loaded sorbents were separated from the aqueous solutions and rinsed once with pH 7 water to washout unbound metals and traces of acid (Yardim et al. 2003; Vacca et al. 2005). The tubes were then filled with 5 mL of pH 7 water solution and agitated at 500 rpm and 37°C for 48 hr. The 48 hr equilibration time was chosen based on the maximum duration of digestion in human intestines. Then the suspensions were centrifuged at 2000 g for 20 min, and an aliquot of supernatant was extracted for analysis. Desorbed metal concentrations were detected at the end of the desorption experiment and the amount of metals that remained bound on sorbents was determined as the difference between the initial adsorbed and the desorbed amount. For both adsorption and desorption, the dry weight of sorbents, before and after, the experiments were determined for selected concentrations, and no significant change was observed.
Data calculations and curve fitting
Metals detected by ICP-MS and CV-AAS were calculated to determine the free metal concentrations in solution. In the adsorption study, the quantity of bound metal was calculated by the concentration difference between control and test groups and expressed as g/kg on the isotherm plots. The amount of metal that remained bound in the desorption study was derived from the difference between the initial amount bound in the adsorption and the dissociated metal in the solution. Table-Curve 2D was used to plot these data and derive values for the variable parameters. The adsorption and desorption isotherms were plotted by Langmuir or Freundlich models using mean values from triplicate analyses. The Langmuir isotherm describes monolayer adsorption onto a surface with a finite number of identical sites and uniform energies of adsorption. The Langmuir equation was entered as user-defined functions:
| (1) |
q = the amount of metal adsorbed (g/kg), Qmax = maximum binding capacity (g/kg), Kd = Langmuir distribution constant, Cw = equilibrium concentration of metal (g/L) (Grant and Phillips 1998).
The Freundlich isotherm is used to describe the adsorption characteristics for a heterogenous surface. The Freundlich model is represented by the following equation:
| (2) |
Kf = Freundlich distribution constant, 1/n = degree of heterogenicity (Ji et al. 2010). The percentage of metal ions desorbed was calculated by the following equation:
Qmax = maximum capacity in the adsorption study (g/kg), qmax = remaining capacity in the desorption study (g/kg) (Akpomie et al. 2015).
Hydra assay
Hydra vulgaris were obtained from Environment Canada (Montreal, Qc) and maintained at 18°C. Using a hydra classification method, morphology of the hydra was rated over time as an indicator of solution toxicity. The morphological scoring of hydra was classified using a dissecting microscope based on a 10-0 point scale, where scores were assigned based on changes that occurred in hydra morphology following exposure to metals. A score of 10 represented normal, healthy hydra and a score of 0 represented disintegrated (dead) hydra (Brown et al. 2014). Scores between 10 and 8 were classified as minor toxicity. Scores between 8 and 6 were classified as moderate toxicity, and scores between 6 and 0 were classified as severe toxicity. The hydra assay has been shown to be objective and repeatable (Dash et al. 2012). The hydra response was scored after exposure to metal solutions, with and without, sorbent inclusion. The assay included closely monitoring mature and non-budding hydra at 0, 4, 20, 28, 44, 68, and 92 hr, without changing solutions during testing. Hydra media was included as a control. Metal control groups consisted of individual metal solutions at 8 ppm As, 8 ppm Cd, 0.5 ppm Hg, 15 ppm Pb, and a metal mixture based on 0.2 ppm of each of the above metals dissolved in hydra media. These concentrations of metals were equivalent to minimum effective doses that resulted in 100% mortality of hydra in 92 hr. Sorbents including MAC, APM, collapsed APM, and an equal mixture of MAC and APM were individually added to the metal solutions at 0.1% inclusion rate. All solutions were mixed at 1000 rpm for 2 hr and centrifuged at 2000 g for 20 min before exposure of hydra in Pyrex dishes. Three hydra colonies in each group were exposed to 4 mL of test media at 18°C. The average score for each group was used to determine the toxicity rating at each time point.
Statistical analysis
A two-way t-test was used to determine statistical significance. Each experiment was independently conducted in triplicate to derive means and standard deviations for the average binding percentages from the screening experiments and the toxicity scores from the hydra assay. These were then used to calculate t-value using a Tukey test and p-value. Results were considered significant at p ≤ 0.05.
Results
Standard curve and percent recovery
A multipoint standard curve for each metal was constructed using concentrations ranging from the limit of detection (LOD) to 200 ppb for As, Cd and Pb, and 10 ppb for Hg. LOD was determined by intensity being one standard deviation higher than ultra-pure water as a blank. The calibration solutions were prepared daily and verified before running test samples. The linearity (r2) in all cases was 1 > r2> 0.99 as shown in Table 1. One of the calibration standards was run as continuing calibration verification standard (CCV) with every 10 samples and the recoveries were calculated (Table 1). As mercury samples required digestion, LBS was run with each batch of samples and the recovery for mercury was also calculated (Table 1).
Table 1.
Linear regression for the standard curve of each metal
| Metal | Linearity, r2 | Intensity of blank (ppb) | LOD (ppb) | Percent recovery |
|---|---|---|---|---|
| As | 0.9998 | 4.89E-2 | 2 | 100.6% |
| Cd | 0.9997 | 7.46E-4 | 2 | 103.73% |
| Hg | >0.995 | 0.02 | 0.04 | 102.8% |
| Pb | 0.9995 | 3.03E-3 | 2 | 103.1% |
Screening of sorbents for efficacy of metal binding
The mean binding percentages from triplicate analyses of the data were calculated for 3 concentrations of As, Cd, Hg, and Pb onto surfaces of APM and MAC (shown in Figure 1). Based on the screening result, MAC proved to be the most effective sorbent for As and Cd with > 10% binding (Figure 1a, b), and Hg with > 90% binding in solution (Figure 1c). Although APM showed moderate binding for Cd at approximately 10%, it adsorbed more than 20% Pb, which was higher than MAC (Figure 1d).
Fig. 1.
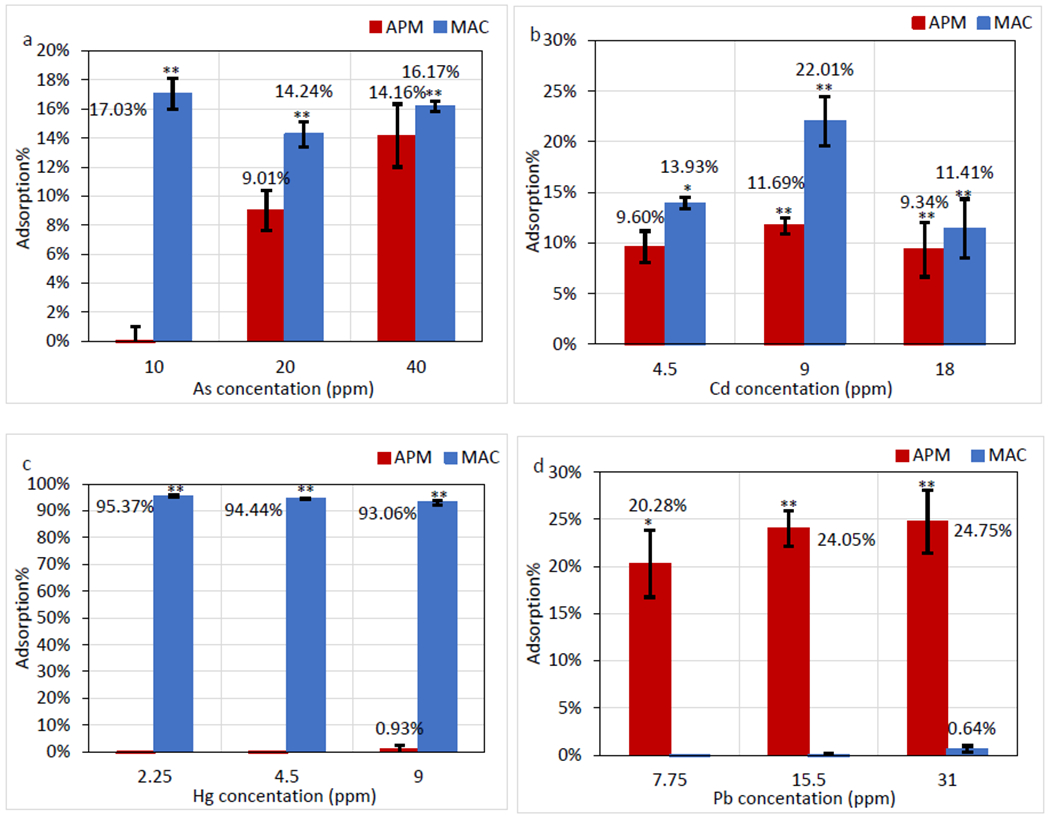
Adsorption percentage at different concentrations of As (III, V) (A), Cd (II) (B), Hg (II) (C), and Pb (II) (D) onto 1 mg/mL APM and MAC Data represent the mean percent adsorption at each concentration, run in triplicate (* p ≤ 0.05; ** p ≤ 0.01)
Adsorption analyses
Isothermal data reflecting the sorption of As, Cd, Hg and Pb onto APM and MAC surfaces were plotted in a Langmuir or Freundlich model based on their highest r2 values (Figure 2). The adsorption plots of As, Cd and Hg binding onto MAC are similar in terms of good fit to the Langmuir model as indicated by good correlation coefficients, curved shape and a plateau, suggesting the presence of saturable active sites. Specifically, MAC showed the highest binding capacity and affinity (Qmax = 7.97 g/kg and Kd = 7.28E4) for Hg compared to other metals, which was consistent with the screening result that MAC delivered the highest binding percentage for Hg (> 90%) at the same inclusion rate. The binding of As, Cd and Hg onto APM fit the Freundlich model the best, indicating heterogeneous binding sites and a partitioning activity onto the APM surfaces. Specifically, the smaller 1/n in the Freundlich model, the greater the expected heterogeneity (Puttamat and Pavarajarn 2016). Since 1/n for As, Cd and Hg binding onto APM was smaller than 1 (Table 2), this indicates the existence of more than one preferred site of binding on APM surfaces. Possible sites include delaminated clay structure, silica chains on the edge of the clay, cross-linked amorphous silica and combinations of these. Interestingly, binding of Pb showed a different activity compared to other metals. The adsorption of Pb to APM fit the Langmuir model with a Qmax equal to 5.98 g/kg, indicating homogeneous sites and saturable binding. Pb binding onto MAC showed a linear correlation between bound Pb and free Pb concentration in solution indicating a lack of a saturable site. From the adsorption parameters described by the Langmuir and Freundlich models summarized in Table 2, the value of 1/n for Pb binding to MAC was equal to 1.08, indicating relatively low heterogenicity on MAC surfaces for Pb (Butnariu et al. 2015; Dada et al. 2012; Goldberg 2005). This adsorption isotherm for Pb agrees with the screening result, where MAC only adsorbed 0-0.64% of Pb from solution.
Fig. 2.
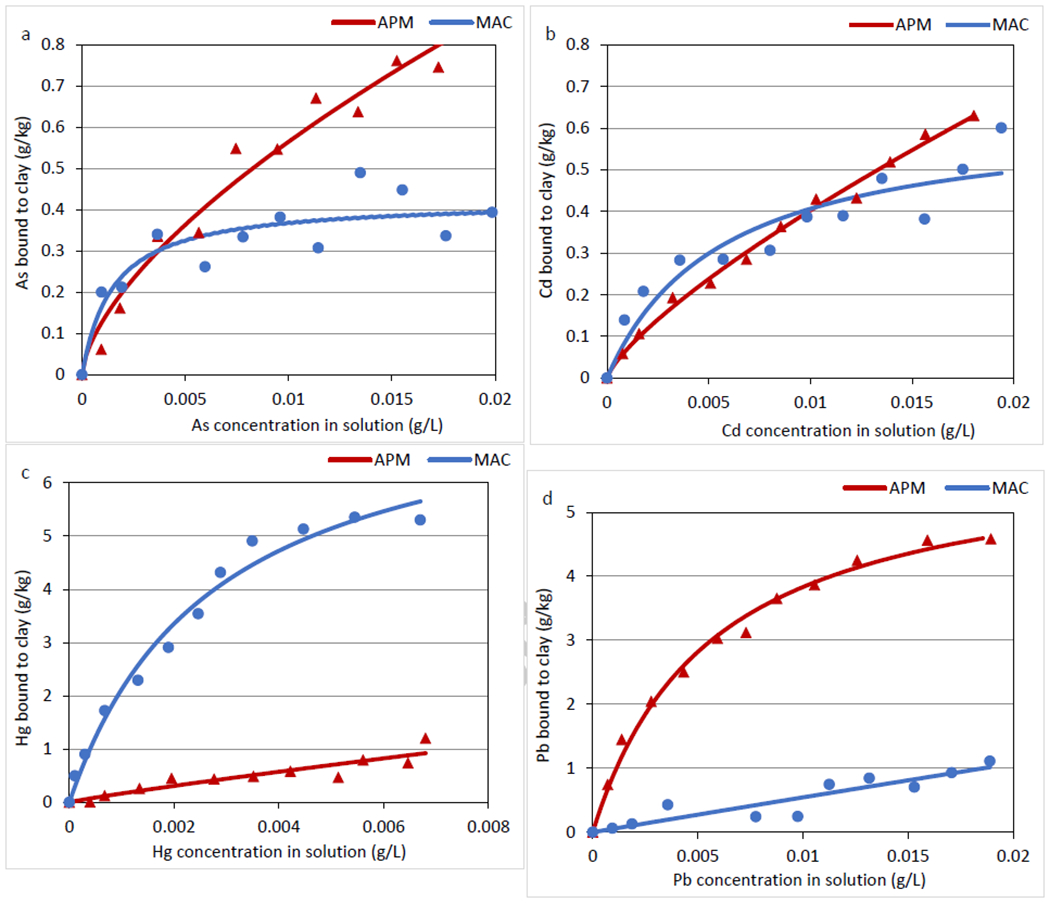
Adsorption isotherms of As (III, V) (A), Cd (II) (B), Hg (II) (C), and Pb (II) (D) onto APM and MAC surfaces, plotted by their best fit model Data represent the mean adsorption (g/kg) at each concentration, run in triplicate
Table 2.
Parameters and correlation coefficients of adsorption isotherms according to Langmuir and Freundlich
| Metal | Sorbents | Langmuir |
Sorbents | Freundlich |
||||
|---|---|---|---|---|---|---|---|---|
| Qmax (g/kg) | Kd | r2 | 1/n | Kf | r2 | |||
| As | MAC | 0.425 | 4.96E4 | 0.86 | APM | 0.64 | 2.22 | 0.97 |
| Cd | MAC | 0.63 | 2.0E4 | 0.88 | APM | 0.76 | 4.24 | 0.98 |
| Hg | MAC | 7.97 | 7.28E4 | 0.91 | APM | 0.9 | 46.76 | 0.86 |
| Pb | APM | 5.98 | 3.69E4 | 0.95 | MAC | 1.08 | 119.9 | 0.85 |
Qmax, binding capacity; Kd, binding affinity; r2, correlation coefficients; 1/n, degree of heterogenicity; Kf, Freundlich distribution constant
Desorption analysis
To calculate the percentage of metal desorption and identify optimal binding agents, only those sorbents that fit the Langmuir model with quantifiable Qmax values were tested. Figure 3 presents the desorption plots of As, Cd, Hg onto MAC, and Pb onto APM. Desorption parameters were summarized in Table 3, and showed that desorption isotherms for As, Cd, and Hg on MAC and for Pb on APM, fit the Langmuir model (r2 > 0.85) with lower qmax values, compared to Qmax from their respective adsorption isotherms. This was due to the desorption of bound metals from sorbent surfaces resulting in less metal bound and more metal released in solution. Percent desorption was calculated from the ratio of Qmax for the adsorption process versus qmax after the desorption process, and in all cases small desorption ratios were observed, indicating that the majority of metals adsorbed to surfaces remained bound in a simulated intestine model. The highest desorption percentage occurred for the case of Hg binding onto surfaces of MAC, where 17.94% of bound Hg was dissociated and released in solution. These low desorption percentages confirmed that the interactions of As, Cd, and Hg with MAC, and Pb with APM, were strong at saturable sites.
Fig. 3.
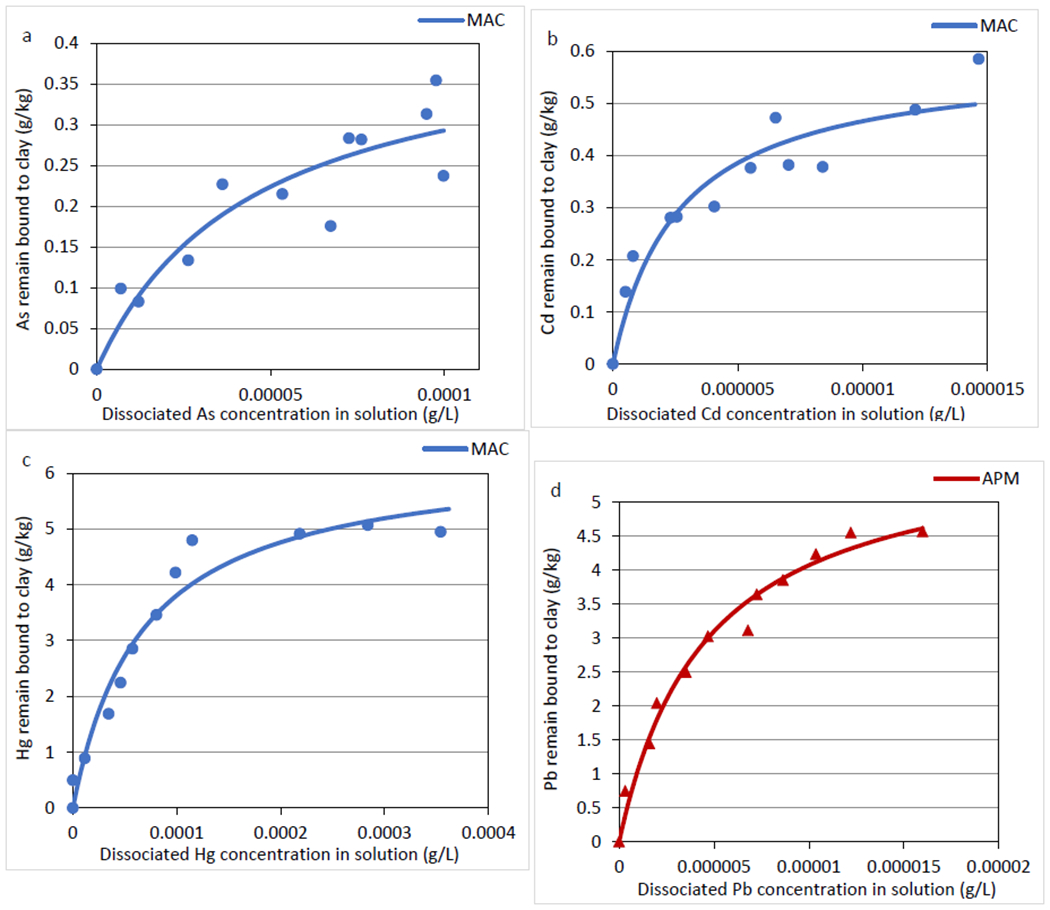
Desorption isotherms of As (III, V) (A), Cd (II) (B), Hg (II) (C) onto MAC, and Pb (II) (D) onto APM surfaces, plotted by the Langmuir model Data represent the mean adsorption (g/kg) at each concentration, run in triplicate
Table 3.
Parameters and correlation coefficients for desorption isotherms
| Metal | Sorbents | Langmuir | |||
|---|---|---|---|---|---|
| qmax (g/kg) | Kd | r2 | Desorption% | ||
| As | MAC | 0.423 | 1.68E6 | 0.85 | 0.47% |
| Cd | MAC | 0.59 | 4.28E7 | 0.9 | 6.35% |
| Hg | MAC | 6.54 | 3.02E6 | 0.94 | 17.94% |
| Pb | APM | 5.94 | 4.53E7 | 0.96 | 0.67% |
qmax, binding capacity of sorbent following desorption; Kd, binding affinity; r2, correlation coefficient
Hydra assay
The toxicity of individual metals and a design mixture of metals was determined by hydra vulgaris (Figure 4). The minimum effective doses (MEDs) that caused complete mortality of hydra following 92 hr exposure were equal to 8 ppm As, 8 ppm Cd, 0.5 ppm Hg, 15 ppm Pb, and 0.2 ppm of a mixture of these metals. The inclusion of only 0.1% MAC showed significant protection of hydra at 30%, 33.3%±4.7%, and 70% against As, Cd, and Hg toxicity, respectively (p ≤ 0.01). APM at the same inclusion showed 75%±5% protection of hydra against Pb toxicity, whereas collapsed APM showed less protection, 65%±5% (Figure 4d). Additionally, to test sorption efficacy for metal mixtures, individual sorbents and a mixture of 50% MAC and 50% APM were administered at 0.1%. Results in Figure 4E showed that the protection with APM was negligible, however, MAC and the sorbent mixture showed significant protection of 76.7%±4.7% against the metal mixture.
Fig. 4.
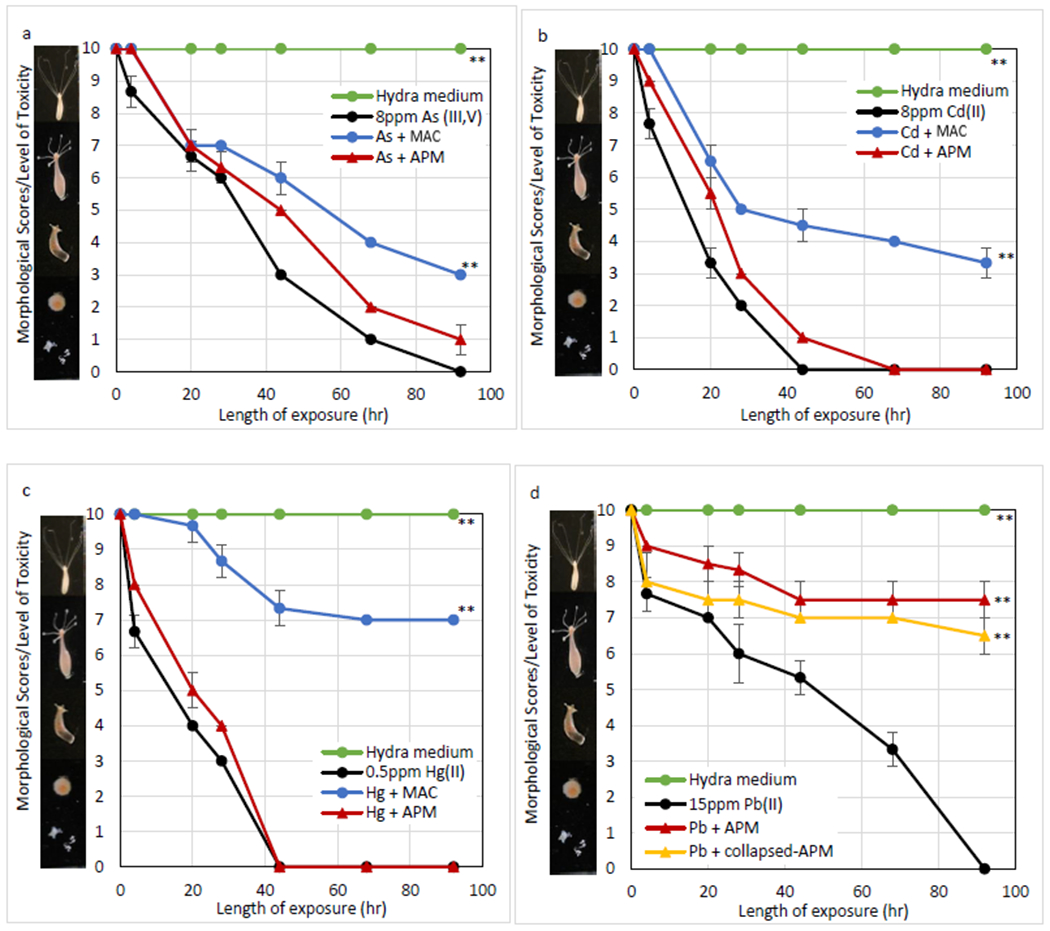
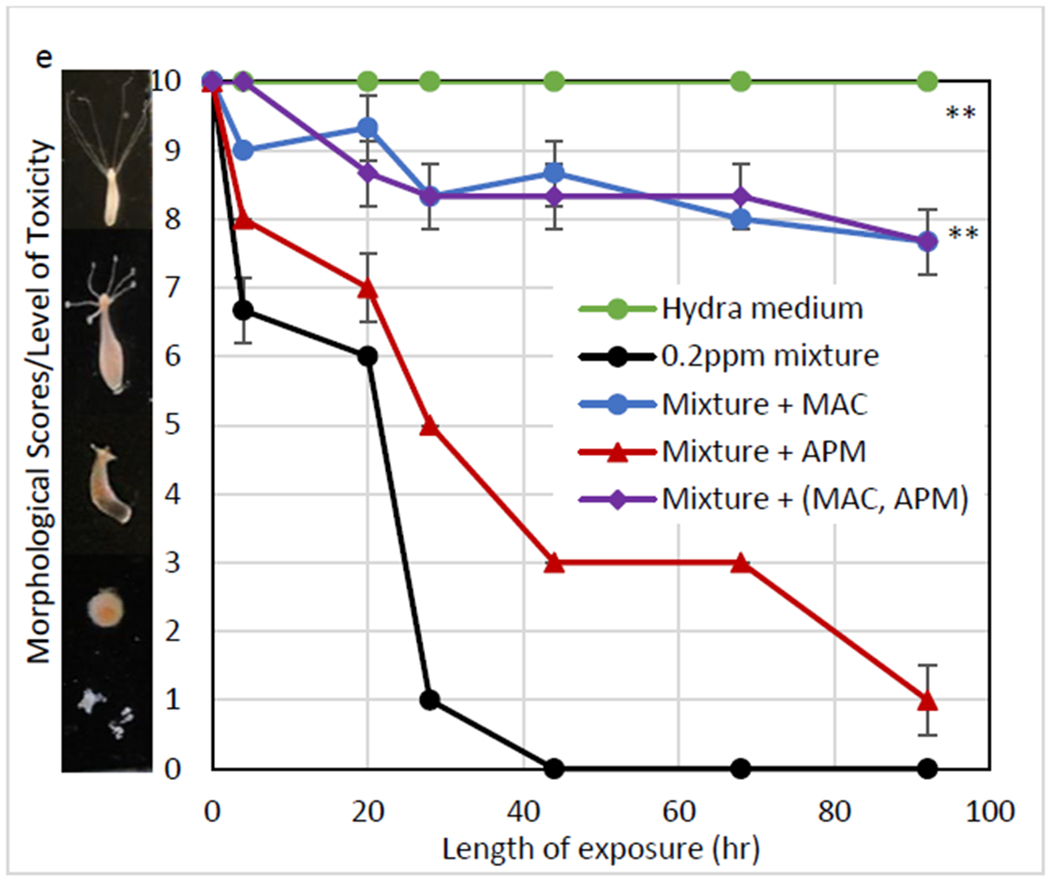
Hydra toxicity from As (III, V) (A), Cd (II) (B), Hg (C), Pb (II) (D) and a metal mixture (0.2 ppm/metal) (E)and protection by sorbents at an inclusion rate of 0.1% Hydra media and metal controls are included for comparison Data represent the mean morphological score at each time point, run in triplicate (* p ≤ 0.05, ** p ≤ 0.01)
Discussion
Carbonaceous materials and dioctahedral smectite clays (including montmorillonite) have been widely used to remove heavy metals from wastewater and water streams. The unique properties of carbons, such as large specific surface area, hollow and layered structures, and high chemical and thermal stabilities, have also facilitated their applications as broad-acting binding agents for toxins.
Like carbon, clays have been used as ancient medicine for diarrhea, cholera, bacterial infections, and mitigation of poisonings. For centuries, eating clay has been a global practice that exists among humans as well as numerous animal species, including non-human primates, birds, butterflies, elephants, bats, dogs, cats, various isopods, etc. In previous intervention studies and clinical trials in the US and Africa, calcium montmorillonite clay was shown to be safe for human and animal consumption. However, the clay had a preference for binding aflatoxins and similar chemicals (Phillips et al 2019). To enhance its ability to bind diverse environmental chemicals, we processed this clay with sulfuric acid to develop novel, acid processed montmorillonites (APM) with increased surface area, porosity and structural diversity similar to carbon. The safety of APM and its increased binding efficacy for a wide variety of mycotoxins, pesticides, and PCBs have been previously described (Wang et al. 2019a, b; Wang and Phillips 2019).
This study was designed to investigate the binding efficacy and safety of MAC and APM for commonly detected, and highly toxic, heavy metals in solution. Preliminary binding studies to screen for the best sorbents for As, Cd, Hg and Pb were run using 1 mg/mL of different sorbents including MAC, APM, calcium and sodium montmorillonite clays, and montmorillonite amended with ferrihydrite, thiamine, carnitine or choline (data not shown). Among all sorbents tested, MAC and APM consistently delivered the highest binding percentage for all four metals (Figure 1), and therefore, these sorbents were further tested for efficacy by equilibrium isothermal analysis.
Adsorption isotherms were conducted in a simulated stomach model, using metal mixtures in aqueous solution at pH 2 and 37°C mixed for 2 hr, to simulate the average duration of the digestion process in the stomach. From isothermal analyses in Figure 2, a Langmuir plot showed that MAC was an effective binder of As (III, V), Cd (II), and Hg (II). All adsorption sites had equal adsorbate affinity and that adsorption at one site did not affect adsorption at an adjacent site, indicating saturable and homogenous metal binding sites on the surface of MAC. The binding capacities for MAC were 0.43 g/kg for As, 0.63 g/kg for Cd, and 7.97 g/kg for Hg (Table 2). While the measured capacities were lower than some reported in the literature, these adsorption isotherms were conducted in simulated stomach conditions using high concentrations of metal mixtures (to simulate high level exposures). Also, isothermal results are consistent with our preliminary screening experiment, showing that MAC: 1) was the most effective sorbent, 2) significantly reduced high concentrations of As and Cd (p ≤ 0.05), and 3) adsorbed nearly 100% of Hg (p ≤ 0.01) at the same inclusion rate (Figure 1). According to the maximum adsorption capacities in Table 2, the preference for binding was Hg > Cd > As for MAC. The dominant binding mechanism for As and Cd onto MAC surfaces has been shown to involve ion-exchange interactions, which is compatible with tight binding and Langmuir chemisorption isotherms (Mohan et al. 2007; Kadirvelu et al. 2000). The binding plots of As, Cd and Hg onto APM clay were best described by a Freundlich model, indicating the presence of partitioning mechanisms, weak binding, and heterogeneous binding sites. On the contrary, the screening results for Pb showed that APM delivered a higher binding % (p ≤ 0.05) than that of MAC. Consistently, the adsorption of Pb onto APM fit a Langmuir model with a high Qmax that was equal to 5.98 g/kg, which indicated that Pb adsorption onto APM is site selective and site specific. This is in alignment with the literature that Pb adsorption is preferred by a montmorillonite-rich clay (bentonite) and fits well with the Langmuir model (Alexander et al. 2018; Meneguin et al. 2017). Interestingly, Pb adsorption onto MAC was defined best by a Freundlich, instead of a Langmuir model, and Pb ions had a preferential uptake onto APM compared to the other metals. This difference in Pb binding is consistent with the literature that showed Pb adsorption, in contrast to other metals, was not competitive with Cd or Hg uptake. This indicates a different site of binding and a separate mechanism for Pb, possibly involving cation exchange and inner sphere complex formations (Rowley et al. 1984; Inglezakis et al. 2002; Saha et al. 2002; Uddin 2017).
All of the correlation coefficient values for the Langmuir and Freundlich models were greater than 0.85, the standard deviations were all lower than 4%, and all points had small deviations from the plots supporting the validity of these models for describing the metal adsorption process. Blank tests of each metal without sorbent were performed and confirmed that metal precipitation did not occur and was not associated with metal sorption.
Besides the high capacity and high affinity for binding in stomach conditions, stability and tightness of the adsorption complex are important in the evaluation of the potential for metal dissociation from the complex in intestine conditions. Therefore, desorption studies were run using metal-loaded sorbents following the adsorption study and were suspended in pH 7 water and incubated at 37°C for 48 hr to simulate the intestine conditions. The amount of metal remaining bound after dissociation (qmax) was calculated from the difference between the maximum adsorption value Qmax and the dissociated metal concentration in the solution. From the results in Figure 3, desorption isotherms of As, Cd and Fig from MAC and Pb from APM maintained a Langmuir shape as suggested by r2 ≥ 0.85 (Table 3), indicating the persistence of saturable binding sites during the dissociation. The desorption percentages are less than 1% for As and Pb, 6.53% for Cd, and 17.94% for Hg. All these suggest that only a small amount of bound metals was dissociated from the binding complex in the intestine model. This result further indicates that As, Cd and Hg binding to MAC and Pb binding to APM involve tight interactions, the binding was not easily dissociated, and the complex is expected to be stable in the intestine.
To investigate the potential safety and the binding efficacy of MAC and APM in vivo, individual metals and a mixture of these metals (0.2 ppm/metal) were tested in the hydra assay. The hydra assay has been used to accurately predict the safety and efficacy of toxin-binding sorbents prior to animal studies (Afriyie-Gyawu et al. 2005; Marroquin-Cardona et al. 2009), and has been utilized along with an in vitro gastrointestinal model (Lemke et al. 2001) and in silico multi-dynamic simulations (Wang et al. 2019) for screening purposes. Hydra vulgaris is very sensitive to environmental toxins and has been widely used as an indicator of toxicity. The minimal effective concentrations from As, Cd, Hg, Pb, and a metal mixture consisting of the four metals were equal to 8 ppm, 8 ppm, 0.5 ppm, 15 ppm, and 0.2 ppm after 92 hr, respectively (Figure 4). MAC, at 0.1% w/v inclusion, displayed significant protection that reduced toxicity by 30% for As, 33.3%±4.7% for Cd, and 70% for Hg (p ≤ 0.01), compared to less protection from APM. However, APM delivered 75%±5% protection against Pb toxicity (p ≤ 0.01). The hydra assay results agree with the in vitro study, where As, Cd and Hg sorption onto surfaces of MAC and Pb sorption onto surfaces of APM are well-described by a Langmuir model. To investigate the binding site and mechanism for Pb, heat-collapsed APM at the same level, showed less protection compared to intact APM. This indicated that intact interlayer surfaces on APM are important for Pb binding. This result is consistent with a previous XAFS (X-ray absorption fine structure spectroscopy) study showing that Pb adsorption mostly occurs in the inner sphere (Heidmann et al. 2005). Additionally, to mitigate mixtures of metals, sorbents including MAC, APM and a mixture of MAC and APM were tested in the hydra assay. Results showed negligible protection with APM, possibly due to the high toxicity of Hg (MEC = 0.5 ppm) and the limited binding of APM. Importantly, MAC and the sorbent mixture were able to equally reduce the toxicity of the metal mixture by 76.7%±4.7% (p ≤ 0.01). It is possible that higher doses of sorbents will contribute to even better protection against toxicity, based on previous toxin dosimetry studies (Maki et al. 2017; Mitchell et al. 2014; Phillips et al. 2008).
Mitigation strategies for heavy metals have focused mainly on the purification of contaminated water, with limited emphasis on the development of therapeutic strategies to protect humans and animals from metal contaminated food and drinking water. For example, disodium and calcium EDTA are intensive chelators that can successfully bind to heavy metals, but they require strict supervision by doctors due to their adverse effects. Penicillamine and dimercaprol have been shown to bind lead and mercury, which then are excreted in the urine (Sears 2013). Natural foods high in vitamins and minerals, such as cilantro and garlic, have protective effects against low level exposures with heavy metals (Flora 2002). Activated carbon has been used as a general detoxification treatment due to its high porosity and surface area (Gerhardsson and Aaseth 2016). Furthermore, thiol-functionalized silica nanomaterials removed metals from blood and urine (better than carbon), and reduced Fig accumulation in rat tissues when orally administered (Sangvanich et al. 2014; Yantasee et al. 2010). In our laboratory, ferrihydrite was shown to decrease arsenic levels in urine in a dose-dependent manner (Taylor et al. 2009). These studies have suggested that the inclusion of edible sorbents with optimal affinity and capacity for metals could be used to decrease the bioavailability of mixtures of metals from the diet.
Conclusion
In this study, we characterized the binding ability of MAC and APM for important metals (and a mixture). Using screening and adoption/desorption isotherms, we validated the stability of complex formation of As, Cd, and Hg on MAC and Pb on APM based on: 1) the Langmuir model, 2) the highest binding percentage, capacity and affinity in a simulated stomach model, and 3) the lowest percentage desorption in a simulated intestine model. The safety and efficacy of the interaction were confirmed in a living organism where 0.1% MAC significantly reduced toxicity of As, Cd, and Hg, while APM significantly reduced Pb toxicity. Our results show that these edible sorbents have a notable potential for the detoxification of heavy metals and mixtures from the diet. Further work is warranted to confirm the ability of MAC, APM (and optimal mixtures of these sorbents) to decrease unintended metal exposures from contaminated food and water in animals and humans.
Acknowledgements
Not applicable
Funding
This work was supported by the Superfund Hazardous Substance Research and Training Program (National Institutes of Health) [P42 ES027704]; and the United States Department of Agriculture [Hatch 6215].
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
References
- Afriyie-Gyawu E, Wiles MC, Huebner HJ, Richardson MB, Fickey C, Phillips TD (2005) Prevention of zearalenone-induced hyperestrogenism in prepubertal mice. Toxicol Environ Health A 68(5):353–368 [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Sbstances and Disease Registry (2019) ATSDR’s Substance Priority List. https://www.atsdr.cdc.gov/spl/index.html#2019spl. Accessed 9 May 2020
- Akpomie KG, Dawodu FA, Adebowale KO (2015) Mechanism on the sorption of heavy metals from binary-solution by a low cost montmorillonite and its desorption potential. Alex Eng J 54(3):757–767 [Google Scholar]
- Alexander JA, Zaini MA, Surajudeen A, Aliyu EU, Omeiza AU (2018) Insight into kinetics and thermodynamics properties of multicomponent lead(II), cadmium(II) and manganese(II) adsorption onto Dijah-Monkin bentonite clay. Particul Sci Technol 36(5):569–577 [Google Scholar]
- Anyanwu BO, Ezejiofor AN, Igweze ZN, Orisakwe OE (2018) Heavy metal mixture exposure and effects in developing nations: An update. Toxics 6(4):65 10.3390/toxics6040065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S, Chakrabarty S, Maity S, Kar S, Thakur P, Bhattacharyya G (2003) Removal of lead from contaminated water bodies using sea nodule as an adsorbent. Water Res 37(16):3954–3966 [DOI] [PubMed] [Google Scholar]
- Brown KA, Mays T, Romoser A, Marroquin-Cardona A, Mitchell NJ, Elmore SE, Phillips TD (2014) Modified hydra bioassay to evaluate the toxicity of multiple mycotoxins and predict the detoxification efficacy of a clay-based sorbent. J Appl Toxicol 34(1):40–48. 10.1002/jat.2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butnariu M, Negrea P, Lupa L, Ciopec M, Negrea A, Pentea M, Sarac I, Samfira I (2015) Remediation of rare earth element pollutants by sorption process using organic natural sorbents. Int J Environ Res Public Health 12(9):11278–11287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Olivares J, Marinez-Barrera G, Perez-Alonso C, Barrera-Diaz CE, Chaparro-Mercado MC, Urena-Nunez F (2016) Adsorption of lead Ions from aqueous solutions using gamma irradiated minerals. Water Remediation. 10.1155/2016/8782469 [DOI] [Google Scholar]
- Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. ISORJ Appl Chem 3(1):38–45. 10.9790/5736-0313845 [DOI] [Google Scholar]
- Dash B, Phillips TD (2012) Molecular Characterization of a Catalase from Hydra vulgaris. Gene 501(2):144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother A, Wenstel R, Sappington K, Wood W (2007) Framework for metals risk assessment. Ecotoxicol Environ Saf 68:145–227. https://doi.Org/10.1016/j.ecoenv.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Flora SJS (2002) Nutritional components modify metal absorption, toxic response and chelation therapy. J Nutr Environ Med 12(1):53–67 [Google Scholar]
- General Carbon Corporation. GC powdered S. https://generalcarbon.com/wp-content/uploads/2020/03/Powdered-S-Specs-030920-2.pdf. Accessed 9 May 2020
- Gerhardsson L, Aaseth J (2016) Guidance for clinical treatment of metal poisonings-sse and misuse of chelating agents In: Chelation Therapy in the Treatment of Metal Intoxication. ELSEVIER, London, UK [Google Scholar]
- Goldberg S (2005) Equations and models describing adsorption processes in soils In: Soil Science Society of America Chemical Processes in Soils, SSSA Book Series, Madison, WI, USA [Google Scholar]
- Grant PG, Phillips TD (1998) Isothermal adsorption of aflatoxin B(l) on HSCAS clay. J Agric Food Chem 46:599–605 [DOI] [PubMed] [Google Scholar]
- Hearon SE, Wang M, Phillips TD (2020) Strong adsorption of dieldrin by parent and processed montmorillonite clays. Environ Toxi Chem 39(3):517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann I, Christl I, Leu C, Kretzchmar R (2005) Competitive sorption of protons and metal cations onto kaolinite: experiments and modeling. J Colloid and Interf Sci 282(2):270–282 [DOI] [PubMed] [Google Scholar]
- Hong Y, Kim Y, Lee K (2012) Methylmercury exposure and health effects. J Prev Med Public Health 45(6):353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglezakis VJ, Loizidou MD, Grigoropoulou HP (2002) Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res 36:2784–2792 [DOI] [PubMed] [Google Scholar]
- Ji L, Shao Y, Xu Z, Zheng S, Zhu D (2010) Adsorption of monoaromatic compounds and pharmaceutical antibiotics on carbon nanotubes activated by KOH etching. Environ Sci Technol 44:6429–6436 [DOI] [PubMed] [Google Scholar]
- Kadirvelu K, Faur-Brasquet C, Le Cloirec P (2000) Removal of Cu(II), Pb(II), and Ni(II) by adsorption onto activated carbon cloths. Langmuir 16(22):8404–8409. 10.1021/la0004810 [DOI] [Google Scholar]
- Lasheen M, Ammar NS, Ibrahim HS (2012) Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci 14(2):202–210. 10.1016/j.solidstatesciences.2011.11.029 [DOI] [Google Scholar]
- Lemke SL, Ottinger SE, Mayura K, Ake CL, Pimpukdee K, Wang N, Phillips TD (2001) Development of a multi-tiered approach to the in vitro prescreening of clay-based enterosorbents. Animal Feed Sci Technol 93(1–2):17–29. 10.1016/S0377-8401(01)00272-3 [DOI] [Google Scholar]
- Maki CR, Haney S, Wang M, Ward SH, Rude BJ, Bailey HR, Harvey RB, Phillips TD (2017) Calcium montmorillonite clay for the reduction of aflatoxin residues in milk and dairy products. Journal of Dairy & Veterinary Sciences 2(3):1–8. 10.19080/JDVS.2017.02.555587 [DOI] [Google Scholar]
- Marroquin-Cardona A, Deng Y, Taylor JF, Hallmark CT, Johnson NM, Phillips TD (2009) In vitro and in vivo characterization of mycotoxin binding agents used for animal feeds in Mexico. Food Addit Contam Part A 26(5):733–743 [DOI] [PubMed] [Google Scholar]
- Mckay G, Porter JF, Prasad GR (1999) The removal of dye colours from aqueous solutions by adsorption on low-cost materials. Water, Air, Soil Pollut 144:423–438. 10.1023/A:1005197308228 [DOI] [Google Scholar]
- Meneguin JG, Moisés MP, Karchiyappan T, Faria SHB, Gimenes ML, Barros MA, Venkatachalam S (2017) Preparation and characterization of calcium treated bentonite clay and its application for the removal of lead and cadmium ions: Adsorption and thermodynamic modeling. Process Saf Environmen 111:244–252 [Google Scholar]
- Mishara SP (2014) Adsorption-desorption of heavy metal ions. Curr Sci 107(4):601–612 [Google Scholar]
- Mitchell NJ, Xue KS, Lin S, Marroquin-Cardona A, Brown KA, Elmore SE, Tang L, Romoser A, Gelderblom WC, Wang JS, Phillips TD (2014) Calcium montmorillonite clay reduces AFB1 and FB1 biomarkers in rats exposed to single and co-exposures of aflatoxin and fumonisin. J Appl Toxicol 34(7):795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan D, Pittman CU Jr, Bricka M, Smith F, Yancey B, Mohammad J, Steele PH, Alexandre-Franco MF, Gómez-Serrano V, Gong H (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interf Sci 310:57–73 [DOI] [PubMed] [Google Scholar]
- Phillips TD (1999) Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicol Sci 52(2 Suppl):118–126 [DOI] [PubMed] [Google Scholar]
- Phillips TD, Afriyie-Gyawu E, Williams J, Huebner H, Ankrah NA, Ofori-Adjei D, Jolly P, Johnson N, Taylor J, Marroquin-Cardona A, Xu L, Tang L, Wang JS (2008) Reducing human exposure to aflatoxin through the use of clay: A review. Food Addit and Contam Part A 25(2):134–145 [DOI] [PubMed] [Google Scholar]
- Phillips TD, Wang M, Elmore SE, Hearon S, Wang JS (2019) NovaSil clay for the protection of humans and animals from aflatoxins and other contaminants. Clays Clay Miner 67:99–110. 10.1007/s42860-019-0008-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttamat S, Pavarajarn V (2016) Adsorption study for removal of Mn (II) ion in aqueous solution by hydrated ferric (III) oxides. Int J Chem Eng Appl 7(4):239–243 [Google Scholar]
- Rowley AG, Husband FM, Cunningham AB (1984) Mechanisms of metal adsorption from aqueous solutions by waste tyre rubber. Water Res 18(8):981–984. 10.1016/0043-1354(84)90248-3 [DOI] [Google Scholar]
- Saha UK, Taniguchi S, Sakurai K (2002) Simultaneous adsorption of cadmium, zinc, and lead on hydroxyalumium- and hydoroxyaluminosilicate-montmorillinite complexes. Soil Sci Soc Am J 66(1):117–128. 10.2136/sssaj2002.1170 [DOI] [Google Scholar]
- Sangvanich T, Morry J, Fox C, Ngamcherdtrakul W, Goodyear S, Castro D, Fryxell GE, Addleman RS, Summers AO, Yantasee W (2014) Novel oral detoxification of mercury, cadmium, and lead with thiol-modified nanoporous silica. ACS Appl Mater Interfaces 6(8):5483–5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ et al. (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137(1–2):65–83 [DOI] [PubMed] [Google Scholar]
- Sears ME (2013) Chelation: Harnessing and enhancing heavy metal detoxification-a review. Sci World J 219840. 10.1155/2013/219840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel FR (2002) Environmental geochemistry of potentially toxic metals. Springer; 77–101 [Google Scholar]
- Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organization 78(9):1093–1103 [PMC free article] [PubMed] [Google Scholar]
- Taylor JF, Robinson A, Johnson N, Marroquin-Cardona A, Brattin B, Taylor R, Phillips TD (2009) In vitro evaluation of ferrihydrite as an enterosorbent for arsenic from contaminated drinking water. Environ Sci Technol 43(14):5501–5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi B, Chudasama CD, Jasra RV (2006) Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 64:273–278 [DOI] [PubMed] [Google Scholar]
- U.S Food and Drug Administration (2019) CVM CY15-17 report on heavy metals in animal food. https://www.fda.gov/media/132046/download. Accessed 9 May 2020
- U.S Food and Drug Administration (2020) Metals and your food. https://www.fda.gov/food/chemicals-metals-pesticides-food/metals-and-your-food. Accessed 9 May 2020
- Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462 [Google Scholar]
- Vacca DJ, Bleam WF, Hickey WJ (2005) Isolation of soil bacteria adapted to degrade humic acid-sorbed phenanthrene. Appl Environ Microbiol 71(7):3797–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van HT, Nguyen LH, Nguyen VD, Nguyen XH, Nguyen TH, Nguyen TV, Vigneswaran S, Rinklebe J, Tran HN (2019) Characteristics and mechanisms of cadmium adsorption onto biogenic aragonite shells-derived biosorbent: Batch and column studies. J Environ Manage 241:535–548 [DOI] [PubMed] [Google Scholar]
- Wang G, Fowler BA (2008) Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol Appl Pharmacol 233(1):92–99 [DOI] [PubMed] [Google Scholar]
- Wang M, Hearon SE, Johnson NM, Phillips TD (2019c) Development of broad-acting clays for the tight adsorption of benzo [a] pyrene and aldicarb. Appl Clay Sci 168:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hearon SE, Phillips TD (2019a) Development of enterosorbents that can be added to food and water to reduce toxin exposures during disasters. J Environ Sci Health B 54(6):514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Maki CR, Deng Y, Tian Y, Phillips TD (2017) Development of high capacity enterosorbents for aflatoxin B1 and other hazardous chemicals. Chem Res Toxicol 30(9):1694–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Orr AA, He S, Dalaijamts C, Chiu WA, Tamamis P, Phillips TD (2019) Montmorillonites can tightly bind glyphosate and paraquat reducing toxin exposures and toxicity. ACS Omega 4(18):17702–17713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Phillips TD (2019) Potential applications of clay-based therapy for the reduction of pesticide exposures in humans and animals. Appl Sci 9(24):5325 10.3390/app9245325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Safe S, Hearon SE, Phillips TD (2019b) Strong adsorption of polychlorinated biphenyls by processed montmorillonite clays: potential applications as toxin enterosorbents during disasters and floods Environ Pollut 255(1):113210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang J, Zhao R, Li Y, Li C, Zhang C (2010) Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour Technol 101(5):5808–5814 [DOI] [PubMed] [Google Scholar]
- Yang W, Ding P, Zhou L, Yu J, Chen X, Jiao F (2013) Preparation of diamine modified mesoporous silica on multi-walled carbon nanotubes for the adsorption of heavy metals in aqueous solution. Appl Surf Sci 282:38–45 [Google Scholar]
- Yantasee W, Rutledge RD, Chouyyok W, Sukwarotwat V, Orr G, Warner CL, Warner MG, Fryxell GE, Wiacek RJ, Ctimchalk C, Addleman RS (2010) Functionalized nanoporous silica for the removal of heavy metals from biological systems: adsorption and application. ACS Appl Mater Interfaces 2(10):2749–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardim MF, Budinova T, Ekinci E, Petrov N, Razvigorov M, minkova V (2003) Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural. Chemosphere 52:835–841 [DOI] [PubMed] [Google Scholar]
- Zhou F, Xie J, Zhang S, Yin G, Gao Y, Zhang Y, Bo D, Li Z, Liu S, Feng C, Fan G (2018) Lead, cadmium, arsenic, and mercury combined exposure disrupted synaptic homeostasis through activating the Snk-SPAR pathway. Ecotoxicol Environ Saf 163:674–684 [DOI] [PubMed] [Google Scholar]


