Abstract
Chemical modifications on RNA can regulate fundamental biological processes. Recent efforts have illuminated the chemical diversity of post-transcriptional (“epitranscriptomic”) modifications on eukaryotic mRNA, and have begun to elucidate their biological roles. In this review, we discuss our current molecular understanding of epitranscriptomic RNA modifications and their effects on gene expression. In particular, we highlight the role of modifications in mediating RNA-protein interactions, RNA structure, and RNA-RNA base pairing, and how these macromolecular interactions control biological processes in the cell.
1. Introduction
Chemical modifications on macromolecules play an important role in biological processes. These modifications not only diversify the chemical functionality afforded to proteins and nucleic acids by the basic building blocks of life (i.e. amino acids and nucleotides), but also provide a mechanism for regulating molecular function in a dynamic and reversible manner through enzyme-mediated transformations. This is perhaps no more evident than in the >150 structurally distinct post-transcriptional modifications that have been described on cellular RNA1. These modifications, which range from simple base and ribose methylation installed by a single enzyme to more exotic modifications requiring dedicated biosynthetic pathways2, serve to expand the somewhat limited chemical diversity of building blocks (i.e. A, C, G, and U) available to canonical RNA polymers, and play important roles in regulating RNA folding and structure, base pairing, and RNA-protein interactions3.
RNA modifications are most prevalent on structured, non-coding RNA3. The most heavily modified RNA is transfer RNA (tRNA), with on average 13 modifications per ~76mer RNA molecule4. Due to tRNA abundance, high modification stoichiometry, and ease of biochemical reconstitution and structural analysis, tRNA modifications are among the best understood and most extensively studied RNA post-transcriptional modifications5. Indeed, we now know most of the tRNA modification enzymes in bacteria and simple eukaryotes2, and understand the involvement of many modifications in processes such as decoding, tRNA folding and structure, and recognition by tRNA-aminoacyl-synthetase enzymes4,6,7. In contrast, less is known about the extent of chemical modification on messenger RNA (mRNA), and how modifications on internal mRNA sequences (in contrast to the better studied modifiations at the 5’ and 3’ ends of the transcript) may function in the absence of well-defined RNA secondary and tertiary structure. Spurred by advances in next-generation RNA sequencing approaches, numerous chemical modifications on mRNA have been now identified and mapped across transcriptomes8, including N6-methyladenosine (m6A), pseudouridine (Ψ), N6,2′-O-dimethyladenosine (m6Am), N1-methyladenosine (m1A), 5-methylcytidine (m5C), 5-hydroxymethylcytidine (hm5C), N4-acetylcytidine (ac4C), N7-methylguanosine (m7G), inosine (I), and 2′-O-methylated nucleotides (Fig. 1). The study of the role that these modifications play in mRNA behavior, gene expression and higher-order biological processes has led to the emergence of the field of RNA epigenetics or “epitranscriptomics”8,9.
Figure 1.
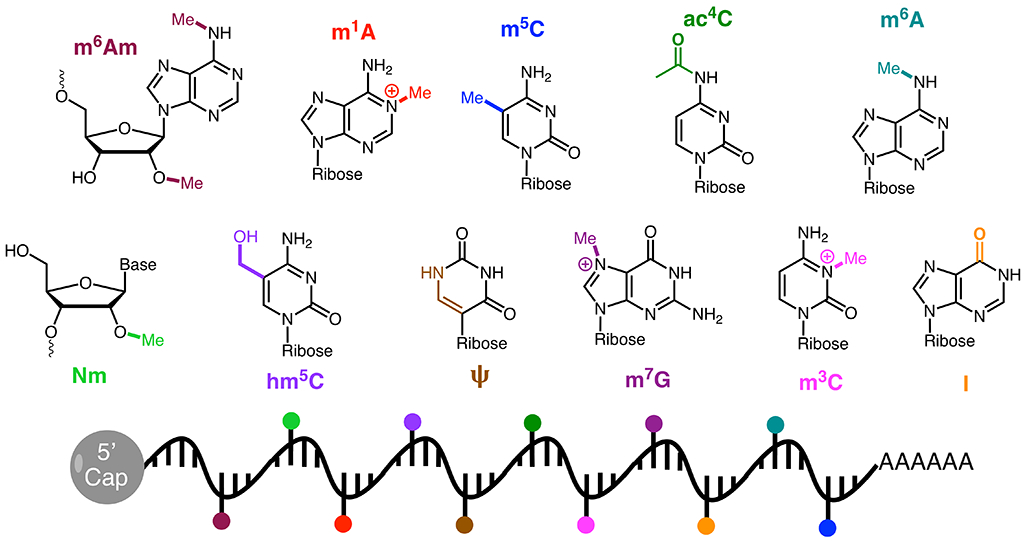
Chemical structures of known epitranscriptomic mRNA modifications.
Several mechanisms have been proposed by which modifications may affect mRNA properties. A number of modification sites have been mapped to the coding region of mRNA transcripts where they could function to alter tRNA selection rules and/or modulate the efficiency of ribosomal translation10. Such a mechanism has been well established for inosine, which base pairs preferentially with C and can efficiently recode translation11. This property has also enabled mapping of inosine sites by RNA mutational analysis12. In contrast, dramatic effects on base pairing have not been observed with other epitranscriptomic modifications, such as m6A and m5C, which both behave similarly to the corresponding unmodified base with regards to their capacity to form canonical Watson-Crick pairs13. Further, modification sites can be found in intronic regions14 or within the 5’-UTR15, or 3’-UTR16,17. Therefore, additional functional mechanisms such as perturbation of local RNA structure or recruitment of modification-specific RNA-binding proteins (“readers”) are likely to be important. In this review, we describe the mechanisms underlying the function of the best-characterized epitranscriptomic mRNA modifications, focusing on their role in modulating RNA-protein and RNA-RNA interactions.
2. Protein readers of epitranscriptomic modifications
2.1. N6-methyladenosine
N6-methyladenosine (m6A) was one of the first epitranscriptomic modifications to be mapped transcriptome-wide16,17. It is also the most abundant internal modification that has so far been identified in mammalian mRNA, with ~ 10,000 annotated sites in humans, and estimated to consitute 0.5% of all adenosine bases18-21. Most m6A sites reside within the consensus motif RRACH (R = A, G; H = A, C, U)22 and are installed by a methyltransferase complex containing the methyltransferase like 3 (METTL3), methyltransferase like 14 (METTL14), and other protein adaptors including WTAP, KIAA1429/VIRMA, RBM15/15B, CBLL1/HAKAI, and Zc3h13.21,23-29 N6-methyladenosine can also be reverted to adenosine via oxidative demethylation by fat mass and obesity-associated protein (FTO) and alkylation repair homolog protein 5 (ALKBH5), which are Fe(II)-dependent and 2-oxoglutarate-dependent dioxygenases19,20. The action of these enzymes indicates that m6A is reversible and dynamic in nature, however the physiologically relevant substrates of these proteins have been debated. FTO and ALKBH5 are primarily nuclear, although the localization of FTO does vary in different cell lines30, suggesting that demethylation of m6A mRNA sites in the cytosol may be a rare phenomenon31. Interestingly, FTO has been shown to demethylate the related modification m6Am32 and to act upon snRNA33.
Studies of m6A have shown that this modification can regulate many different aspects of RNA biology including mRNA stability, splicing, nuclear export and translation8. Most of these roles are thought to be mediated by recognition of m6A-modified transcripts by m6A-specific RNA-binding proteins, or reader proteins (Fig. 2). The first m6A readers to be identified were the YT521-B homology (YTH) proteins YTHDF2 and YTHDF317. The defining feature of these proteins is the YTH domain, which binds specifically to m6A-modified RNA sequences, and which is also found in 3 other human proteins – YTHDF1, YTHDC1, and YTHDC2. Structural and biochemical studies of YTH domains together with m6A-modified substrates have indicated that discrimination of the methylated base is facilitated by an aromatic pocket lined with 3 Trp residues34-37. Interestingly, despite making contacts with residues surrounding the m6A site, YTH domains (particularly those from YTHDF1-3) do not show strong sequence bias and appear to function as general m6A-binding modules, although studies have shown a preference for the predominant m6A-containing GGACU consensus sequence38-41. In addition to YTH domain proteins, several diverse RNA-binding proteins have been identified as m6A readers through affinity proteomics and crosslinking and immunoprecipitation (CLIP) approaches. These include eukaryotic translation initiation factor 3 (eIF3), ELAV-like protein 1 (ELAVL1), leucine-rich PPR motif-containing protein (LRPPRC), fragile X mental retardation protein 1 (FMR1), insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs), hnRNP proteins and proline rich coiled-coil 2A (Prrc2a) (Fig. 2)15,42-45. While most of these proteins possess established RNA-binding domains (e.g. KH domain, RRM domain), in the absence of detailed structural and biochemical data the specificity of m6A binding and the mechanism by which these proteins recognize the methylated base are largely unknown. Nevertheless, the existence of m6A readers containing multiple distinct RNA binding motifs suggests multiple possible solutions to m6A recognition.
Figure 2.
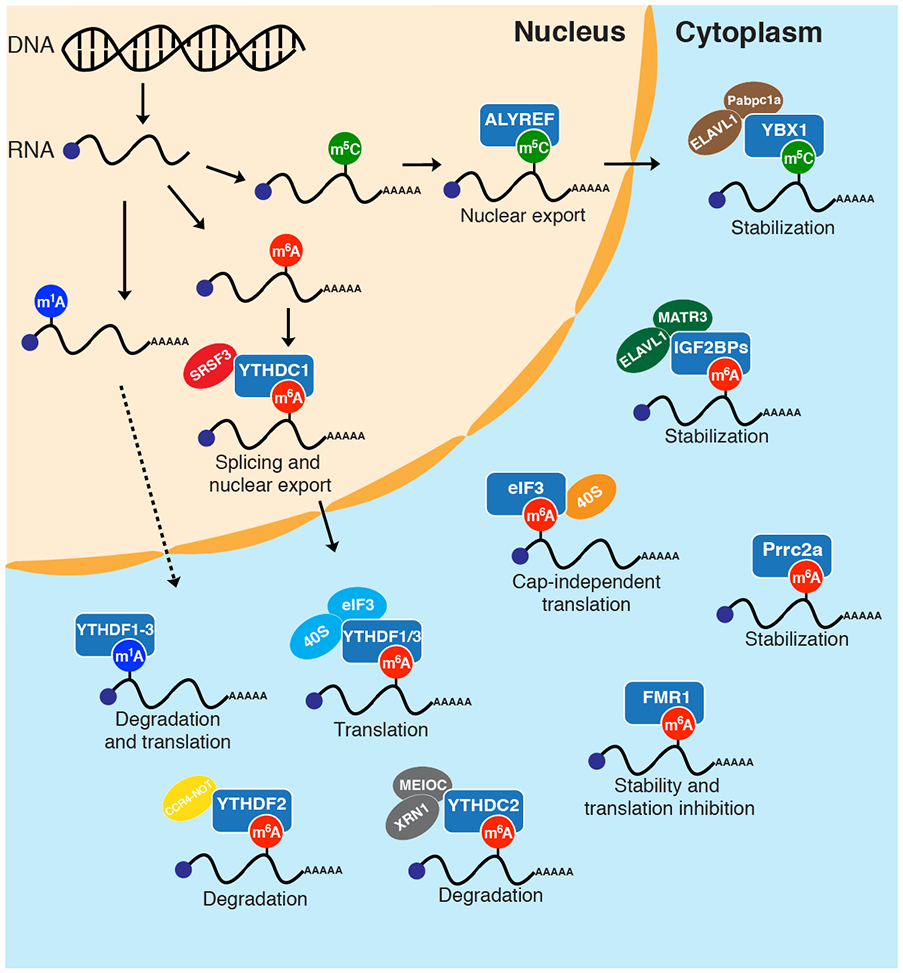
Protein readers of epitranscriptomic modifications. Different reader proteins bind to m6A, m5C, and m1A and regulate cellular fate of modified RNAs.
The identification and characterization of m6A readers has paved the way for understanding the role of m6A modifications in diverse biological processes. A number of studies have established that m6A-modified mRNAs show accelerated turnover and enhanced translation38-41. These effects, which have been shown to play important roles in modulating gene expression during early development, differentiation, immune response, stress response, and disease progression46-58, appear to be primarily mediated by the YTH-domain proteins. YTHDF2 was the first YTH-domain protein to be characterized in a biological context, and promotes RNA decay through multiple mechanisms including localization of m6A-modified mRNAs to processing bodies (sites of RNA decay in the cytoplasm)41 and recruitment of deadenylase59 and endonuclease enzymes60. YTHDF2 has a modular architecture whereby its N-terminal domain is responsible for interactions with effector proteins and its C-terminal YTH domain selectively recognizes m6A-modified mRNAs. YTHDF1, which shares 67% sequence similarity with YTHDF2, does not appear to play a role in RNA degradation but instead enhances translation by interacting with translation initiation factors40. Interestingly, while YTHDF1 and YTHDF2 appear to affect different molecular processes, they share many target transcripts in common, as revealed by CLIP analysis,39 and possess similar in vitro affinity and specificity for m6A-modified sequences61. YTHDF3, which has been less well studied than YTHDF1 and YTHDF2, appears to have dual roles in promoting degradation and translation in concert with YTHDF1 and YTHDF2.38,39 While the aforementioned work has established a model whereby YTHDF proteins have distinct biological roles, 2 recent studies from Jaffrey62 and Hanna63 have called this model into question by proposing that YTHDF1/2/3 proteins are functionally redundant and all mediate mRNA decay but have no role in mRNA translation. The recent work, which includes both independent experiments and re-analysis of previously published data, stands in sharp contrast to previous studies, particularly regarding the finding that YTHDF1/3 do not promote mRNA translation. Notably, YTHDF1 has been shown to promote translation in multiple different biological contexts40,49,64 and its N-terminal domain has been used as a translational activator in artificial tethering assays40,65, suggesting that it can regulate translation in certain systems. Further investigation will be needed in order to fully eludicate the role of YTHDF proteins in diverse biological processes.
The biological effects of m6A are not limited to mature mRNAs in the cytoplasm and can also regulate pre-mRNA processes occurring in the nucleus. YTHDC1 is a nuclear YTH-domain protein that reads m6A and regulates the splicing of modified transcripts through interactions with the splicing factor SRSF3.66 This m6A-YTHDC1-SRSF3 interaction promotes exon inclusion; without m6A or YTHDC1, other splicing factors cause differentially spliced transcripts. In Drosophila, YT521-B (the homolog of YTHDC1) recognizes m6A in the intron of Sxl and mediates the female specific alternative splicing of Sxl.46-48 In mouse, YTHDC1 is essential for embryo viability and germline development; the loss of YTHDC1 in oocytes leads to extensive altered splicing, polyadenylation, and 3′ UTR length of mRNA.67 YTHDC1 can also promote nuclear export of m6A-methylated mRNAs by recruiting the binding of nuclear export adapter protein SRSF3 and export receptor NXF1.68 Similarly, FMRP can promote the nuclear export of m6A-modified transcripts69, together with its roles in translation and stability regulation in the cytoplasm. Finally, m6A reading events in the nucleus can extend to non-coding RNAs including lncRNA25 and miRNA70, as well as the recent demonstration that chromosome-associated regulatory RNAs (carRNA) are controlled by m6A modification and YTHDC1 recognition71.
Increasing evidence suggests that other m6A readers can compete with YTH-domain proteins to regulate RNA transcript behavior. FMRP was identified as a selective m6A reader that stabilizes its target transcripts and inhibits translation42,44,72, acting in an opposing manner to YTHDF1-344,72. While FMRP does bind to m6A-modified RNAs in vitro42,44, its specificity for methylated transcripts is modest, and its cellular effects may be due to overlap of FMRP binding sites with m6A sites, rather than direct m6A recognition72. Additionally, IGF2BPs bind to GG(m6A)C motifs in mRNAs and increase stability and translation of modified transcripts.45 In mouse neural cells, Prcc2a was identified to bind m6A-modified mRNAs and stabilize its target transcripts43. These findings indicate that distinct m6A readers may compete with one another for RNA binding and that the biological consequences of m6A modification may consist of the cumulative sum of such protein-RNA interactions distributed over a set of transcripts. Alternatively, particular m6A-protein interactions may dominate in certain biological contexts based on the expression of individual protein factors or other regulatory features. Further studies are needed to elucidate the network of m6A-reader interactions and how these individual binding events specify the ultimate fate of m6A-containing mRNA.
2.2. N1-methyladenosine (m1A)
N1-methyladenosinse (m1A), which is methylated at the N1 position of adenine instead of the N6 position, is a well-established and abundant post-transcriptional modification on tRNA and rRNA73. While the vast majority of cellular m1A is found on tRNA, it has recently also been detected in mRNA74,75. In contrast to most known epitranscriptomic modifications, m1A is positively charged and is incompatible with canonical Watson-Crick pairing74,75. Further, it is chemically labile and can undergo Dimroth rearrangement to m6A under alkaline conditions76. The occurrence of m1A on mRNA has been highly controversial to date, with estimates from different studies ranging from thousands to only a handful of sites74,75,77,78. There are likely many reasons for this, including the chemical lability of the modification, its low abundance on mRNA, and the differences in mapping strategies and bioinformatic analysis platforms utilized by different groups74,75,77-79. Further, m1A could show context-dependent distribution on mRNA. It is unlikely that m1A modifications can be tolerated within the coding sequence given the dramatic effect of this modification on Watson-Crick pairing. Indeed, studies have mapped most mRNA m1A modifications to the 5’-UTR of transcripts74,75,77,78. Writers and erasers of m1A on mRNA appear to be the same enzymes that modulate modification levels on tRNA, including the TRMT6/61A methyltransferase complex77,78 and the AlkB family demethylases ALKBH1 and ALKBH330,74,75,80. Further studies will be necessary to comprehensively map m1A modifications in diverse biological contexts and characterize relevant writer and eraser proteins acting upon mRNA.
While m1A sequence maps have generated considerable controversy in the field77,78, multiple studies of m1A readers have converged on the finding that this mark is read by YTH-domain proteins, particularly the YTHDF1-3 family (Fig. 2)81-83. While this is somewhat surprising, given the preference of these proteins for m6A-modified sequences, it is conceivable that the hydrophobic m6A-binding pocket can also accommodate the proximal N1-methyl group in a similar fashion. Recognition of m1A by YTHDF proteins appears to have similar functional consequences as binding of these proteins to m6A. A study from our group showed that in living cells, YTHDF2 knockdown increased the abundance of m1A-modified mRNAs, whereas the knockdown of the m1A eraser ALKBH3 resulted in global destabilization of m1A-containing transcripts83. Further building the case for YTH-protein reading of m1A, a recent report showed that YTHDF3 recognizes m1A-modified IGF1R mRNA to promote its degradation, inhibiting migration, invasion, and proliferation of trophoblast.82 Taken together, these findings demonstrate a role for m1A in transcript turnover, likely in part through recruitment of YTHDF proteins81-83. Whether there are functional differences between m1A and m6A-mediated recruitment of YTH proteins remains to be seen, as well as whether unique m1A reader proteins exist.
2.3. 5-methylcytidine (m5C)
5-methylcytidine (m5C) is an abundant epitranscriptomic modification on eukaryotic mRNA. While less prevalent than m6A, thousands of m5C mRNA sites have been mapped and show enrichment in coding sequences and near the translation initiation site84-87. NSUN2, a tRNA m5C methyltransferase, appears to be the major mRNA m5C methyltransferase, but it is likely that other m5C mRNA methyltransferases exist84-88. In contrast to the adenosine modifications discussed above, which occur on the Watson-Crick face, m5C involves methylation on the opposite edge of the nucleobase at the C5 position, and therefore might be expected to have a less dramatic effect on RNA structure or on processes involving base pairing (i.e. decoding). This makes recognition by RNA-binding reader proteins a particularly appealing model for understanding its functional role, particularly, in the larger context of well-characterized reader proteins that bind to 5-methylcytosine bases on DNA such as methyl-binding domain (MBD) proteins89. Currently, our understanding of the effect of m5C modification on mRNA transcripts is poorly developed, but recent studies identifying m5C reader proteins have helped the field coalesce. One challenge in the study of m5C and associated readers has been the lack of a clear consensus sequence for m5C deposition sites, although recent work has begun to implicate sequence motifs within mRNA that resemble the well-established m5C sites on tRNA84,85. Nevertheless, researchers seeking to identity reader proteins have pushed forward with m5C-modified oligonucleotides mimicking individual high-confidence m5C sites within the transcriptome. In 2017, Yang and co-workers identified Aly/REF export factor (ALYREF) as a direct reader of m5C (Fig. 2)87. Using a combination of genetic knockdown and RNA imaging, they proposed that ALYREF could promote the nuclear export of m5C-modified mRNA. HNRPNK has also been implicated as a nuclear m5C reader that can regulate chromatin state through its interactions with chromatin-associated RNAs and various transcriptional regulators90. While ALYREF and HNRPNK may function as nuclear m5C readers, several groups have characterized the transcriptional and translational regulator YBX1 as a cytoplasmic m5C reader protein through its cold shock domain (CSD) (Fig. 2). Similar to other epigenetic and epitranscriptomic readers that bind to methylated epitopes, YBX1 recognizes the C5-methyl group through a hydrophobic pocket containing aromatic residues91,92. YBX1 stabilizes its mRNA clients by interacting with accessory proteins (ELAVL1,HUR in humans, Pabpc1a in zebrafish)91,92, supporting a role for m5C in mRNA stability.
5-methylcytosine on RNA can also be oxidized to 5-hydroxymethylcytosine and 5-formylcytosine93-95, although the prevalence of these oxidative modifications on mRNA has not been firmly established. These modifications may serve as an added layer of regulation on interactions between m5C and its reader proteins, which may be unable to bind in their presence. Just as well, dedicated readers for these 5-methylcytidine derivative may exist, as has been shown for the analogous modifications on DNA96.
3. Anti-readers of epitranscriptomic modifications
While most studies of epigenetic and epitranscriptomic readers have focused on proteins that bind specifically to the modified epitope, an alternative mechanism by which RNA modifications can affect biological function is by abrogating interactions between RNA binding proteins and their preferred unmodified sequence motifs, We refer to such proteins that bind preferentially to unmodified sequences over their modified counterparts as “anti-readers”. In principle, many RNA-binding proteins that bind sequence specifically could exhibit this property since modified bases may be incompatible with recognition in the same way that a sequence mutation can modulate the interaction affinity97. Further, since modified bases constitute a small fraction of the transcriptome, it is likely that the majority of RNA-binding proteins have evolved to recognize unmodified sequence motifs. In practice, however, only a small number of proteins have been identified as anti-readers of methylated bases, which may speak to the promiscuity of their RNA binding sites with regards to nucleobase structure.
Using affinity proteomics and biochemical characterization with recombinant protein, our group and Vermeulen, Carrell, He and co-workers identified the stress granule protein G3BP1 as an anti-reader of m6A (Fig. 3)42,44. G3BP1 binds 10-fold worse to an oligonucleotide containing the major m6A motif GGm6ACU, as compared to the corresponding unmodified sequence lacking m6A methylation42. Cellular studies of G3BP1-mRNA interactions showed that a substantial amount of G3BP1 RNA binding sites overlap with known m6A sites, and demonstrated that G3BP1 promotes mRNA stability44. Together, these findings suggest that m6A could act as a molecular switch that could regulate the recruitment of proteins with opposing functions, such as YTHDF2 to promote RNA decay or G3BP1 to maintain RNA stability. Additionally, G3BP1 is a critical stress granule protein, and other stress granule proteins USP10 and CAPRIN1 were identified as potential anti-readers of m6A.42,44 This hints that m6A could be involved in the trafficking of mRNAs to stress granule by abrogating interactions with G3BP1 – which would be predicted to exclude certain mRNAs from recrtuiment to these structures. Interestingly, a number of studies have suggested that m6A-modified mRNAs and m6A readers are enriched in stress granules.98,99 Therefore, more comprehensive investigation is needed to dissect the potential role of m6A in stress granule assembly.
Figure 3.
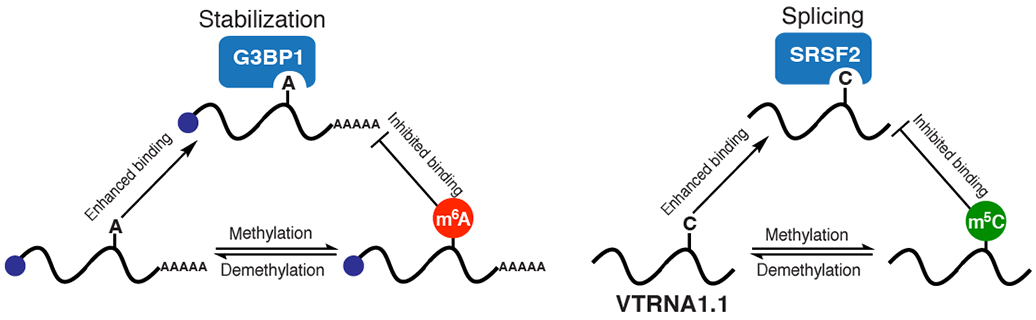
Anti-readers of m6A and m5C. RNA-binding of G3BP1 and SRSF2 is significantly reduced with m6A- and m5C-modified RNAs, respectively.
Similar to m6A, m5C can also repel certain RNA-binding proteins. Frye and co-workers demonstrated that NSUN2-mediated m5C methylation at cytosine 69 of vault RNA 1.1 (VTRNA1.1) inhibits binding of the splicing factor SRSF2, leading to different splicing patterns in the presence or absence of m5C (Fig. 3)100. Together, NSUN2 and SRSF2 orchestrates the maturation of the vault RNAs and produce distinct small-vault RNAs100. Further, m5C methylation has been shown to abrogate binding of the chromatin-modifying PRC2 complex to the XIST and HOTAIR lncRNAs, which both contain multiple m5C sites101. Now that m5C sites on mRNA are becoming better characterized, it is likely that such anti-reader mechanisms function in this context as well. It is also plausible that other RNA modifications could act in a similar way to inhibit RNA recognition by RNA-binding proteins. For instance, m1A introduces a positive charge and alters the hydrogen bonding pattern of the Watson-Crick face. This could serve to repel RNA-binding proteins via charge-charge repulsion and/or altered hydrogen bonding. The identification and characterization of anti-readers of RNA modifications will be important to further our understanding of epitranscriptomic RNA regulation.
4. Effects on RNA structure and ribosomal translation
In addition to the ability of epitranscriptomic modifications to modulate interactions between mRNAs and RNA-binding proteins, several complementary mechanisms have been proposed to provide a biochemical framework underlying the role of these modifications in gene expression regulation. These include effects on RNA secondary structure, which may directly or indirectly (through modulation of RNA-binding protein affinity) affect downstream processes as well as modulation of ribosomal decoding and translational elongation efficiency. While the effects of m6A on mRNA have been attributed primarily to its recruitment of reader proteins, model studies of m6A-modified oligonucleotides102 as well as transcriptome-wide structure mapping103 have shown that m6A can also change local RNA structure. These effects appear to be mediated primarily by disrupted Watson-Crick base pairing as well as through enhanced stacking interactions that serve to stabilize unpaired, single-stranded regions102. While such structural modulation can have widespread effects on mRNA behavior, it has been specifically implicated in the regulation of protein binding through what has been termed an “m6A switch” (Fig. 4A)14. Pan et al. proposed that these switches are ubiquitous throughout the transcriptome and can affect the binding of HNRNPC/G proteins on pre-mRNAs to control splicing and mRNA abundance14,104. In contrast to the YTH-domain proteins, HNRNPC does not appear to directly recognize the m6A-modified base. Instead, methylation in the stem of an RNA hairpin structure can weaken the stem-loop structure and expose the single-stranded HNRNPC binding site, thereby promoting the recruitment of this protein in the presence of m6A (Fig 4A)14. Further, a similar m6A-switch like mechanism has been proposed for the binding of the nuclear m6A reader HNRNPA2B1, which mediates methylation-dependent primary microRNA processing and affects alternative splicing70,105.
Figure 4.
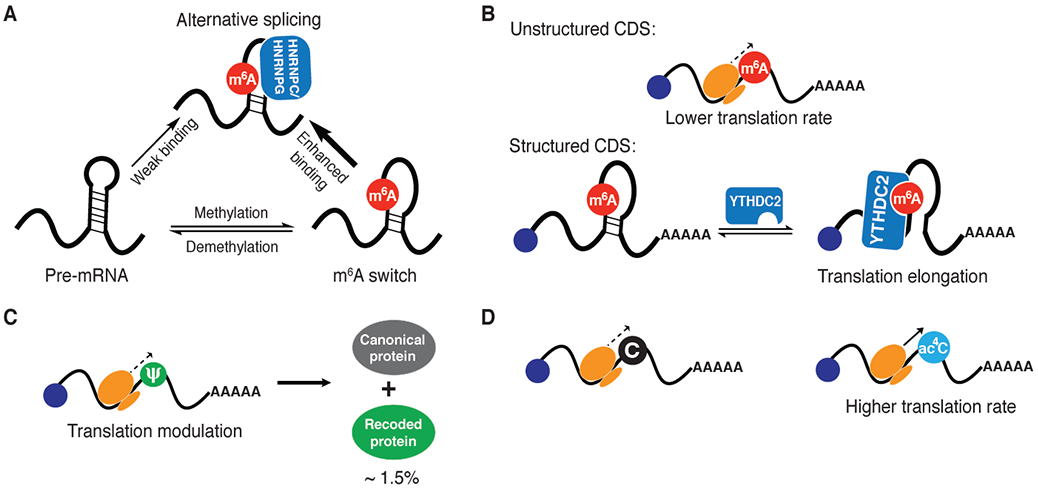
Structural and translational effects of RNA modifications. A) m6A-switch: m6A destabilizes RNA duplex and exposes the opposite strand that is a binding target for HNRNPC/G. B) m6A in CDS can impede translation but can recruit YTHDC2 to increase translational rate in structured regions. C) Pseudouridine can recode mRNAs during translation. D) N4-acetylcytidine in CDS increases translation elongation.
While m6A is an epitranscriptomic modification primarily found on mRNA, other epitranscriptomic modifications that are being actively investigated are predominantly found on structured RNAs such as tRNA and rRNA, in addition to their presence on mRNA. Pseudouridine (Ψ) has long been studied in non-coding RNA where it is known as the “fifth ribonucleotide”106 and has been shown to stabilize RNA structure through the formation of a water-bridged hydrogen bond between the ΨNH1 proton and adjacent phosphate oxygen atoms as well as through increased stacking interactions107,108. High-throughput chemical mapping of Ψ sites, taking advantage of its unique reactivity with carbodiimide reagents, has revealed widespread pseudouridylation on mRNA109-111. Interestingly, a number of these sites are found within sequences predicted to adopt a characteristic “bulged stem loop” structural motif112. Currently, it is not known whether Ψ is involved in the stabilization and regulation of such structures in mRNA or whether this motif is simply a requirement for deposition of Ψ by pseudouridine synthase (PUS) enzymes. Similarly, 5-methylcytosine (m5C), an epitranscriptomic mRNA modification, is primarily found in the variable loop (VL) of tRNA where it protects these molecules from stress-induced endonucleolytic cleavage113. Recent work suggests that m5C modifications are predominantly found in stem-loop forming structures84,85. Studies have indicated that m5C does not have a large effect on base pairing interactions but can induce structural distortions in the helical backbone114.
Another mechanism by which mRNA modifications can affect gene expression is through the regulation of ribosomal translation115. In most cases, the mechanism underlying this process appears to involve alteration in the efficiency or specificity of tRNA selection, either resulting in modulation of the rate of ribosomal elongation or the incorporation of non-cognate amino acids opposite modified codons. This is analogous to the well-established role in decoding of different RNA modifications in the anticodon of tRNA (particularly at the “wobble position”)5. The most striking example is the recoding of translation that occurs upon A:I editing, resulting in high efficiency incorporation of non-cognate amino acids opposite inosine-containing codons due to its propensity to base pair with C instead of T11. While RNA A:I editing has been studied extensively, our understanding of the effects of more recently discovered epitranscriptomic modifications on translational fidelity and efficiency is still in its infancy. Several studies relying on both in vitro and cellular experimentation have begun to reveal insights into this process, as we describe below.
Early studies by Yu and Ramakrishnan into the effects of artificial pseudouridylation on translation led to the remarkable finding that pseudouridine could convert stop codons into sense codons 116,117. Structural analysis of the 30S ribosomal subunit in complex with the anticodon stem-loop of tRNA-Ser bound to the ΨAG codon indicated that the Ψ modification at the 1st position made possible normally forbidden purine-purine base pairs at the 2nd and 3rd codon positions, in which the purine bases in the codon adopt a syn conformation, rather than the more commonly observed anti conformation. Interestingly, while Ψ induced non-canonical pairing at adjacent base pairs, the Ψ residue itself is found in a standard Ψ-A base pair, and the underlying role of Ψ in inducing non-canonical pairing and recoding is not fully understood. While this work put forth an attractive hypothesis for the biological function of Ψ on mRNA, in the past decade since the initial report of Ψ recoding, few studies have been conducted to further expand on this finding. Further, epitranscriptomic Ψ maps have not shown enrichment of Ψ at stop codons, suggesting that such a mechanism may not be relevant to the majority of endogenous Ψ sites. More recently, using a reconstituted bacterial translation system and studies in human cells, Koutmou and co-workers have explored the effect of Ψ within coding sequences118. Their study demonstrates that Ψ increases the rate of amino acid substitutions (i.e. miscoding) and reduces the rate of translation elongation (Fig 4C). While these findings do connect with the prior work on Ψ recoding116,117, the observed effects are more modest than previously claimed. In part, this may be due to sequence or structural-context dependent effects of Ψ in mRNA. Further work will be needed to comprehensively elucidate the role of this abundant epitranscriptomic mark in diverse biological systems.
The effects of m6A on ribosomal translation elongation demonstrate how modifications can function in different manners depending upon sequence and structural context. In reconstituted prokaryotic in vitro translation systems, m6A impedes translational elongation by perturbing tRNA selection (Fig. 4B)119. Consistent with this, Qian and co-workers found that m6A modifications in vivo correlate with ribosomal pausing120. Somewhat paradoxically, however, removal of these modifications by methyltransferase depletion results in further decreased translational efficiency, suggesting a positive effect of m6A in promoting translation. To reconcile these somewhat contradictory findings, the authors suggest a model whereby m6A functions to resolve RNA secondary structures (which would otherwise impede ribosomal progression) through the action of the YTHDC2, a YTH-domain protein containing an RNA helicase domain (Fig. 4B)120. In contrast, m6A residues found in unstructured regions of mRNA coding sequences likely have a net negative effect on translation120. As a further context-dependent function, m6A in the 5’-UTR has been proposed to promote translation by mediating cap-independent translation through the direct recruitment of eIF315, although detailed structural and biochemical information on this interaction is lacking.
In addition to the modifications mentioned above, several other epitranscriptomic marks have been associated with translational regulation, including m5C and N4-acetylcytidine (ac4C). Since manipulation of individual endogenous RNA modification sites in vivo is still an unsolved challenge, insights into the effects of these modifications have been primarily generated through in vitro translation assays using site-specifically modified synthetic mRNAs121, or through cellular transfection of fully modified mRNA templates generated through in vitro transcription with modified NTP building blocks. Measurements using these artificial mRNAs can then be integrated with cellular/organismal assays that correlate translational phenotypes with annotated modification sites. Using these approaches, the presence of m5C in the CDS has been associated with lower translation rate, though m5C modifications on 5’ and 3’ UTRs had negligible correlation with translation.84,122 The mechanistic basis of these effects are unclear, although it may be related to direct interactions of the modified base with the ribosome or modulation of RNA structure114,115. In contrast, ac4C, which is one of the most recent epitranscriptomic modifications identified on mRNA, has been found to promote translation (Fig. 4D)123. It has been proposed that ac4C modification found at the wobble sites in the CDS stabilize anticodon-codon interactions enabling efficient tRNA discrimination and decoding efficiency123. Such a mechanism is analogous to the known role of ac4C in bacteria, where it is present in the anticodon of tRNA, and promotes proper pairing124.
5. CONCLUSION
Epitranscriptomic modifications on mRNA have emerged as a new modality for gene expression regulation. Led by studies of m6A, modifications have been shown to regulate diverse aspects of mRNA biology including splicing, nuclear export, stability, translation, and more14,15,38-42,44,45,66,68,69,81-83,87,91,92,104,105,118-120,123. Importantly, in several contexts, RNA modification-associated effects on gene expression have been shown to have functional consequences in higher order biological processes including development, cancer, immune activation, and learning/memory46-57,91,92. In this review, we have described our current understanding of the major molecular mechanisms underlying the role of epitranscriptomic RNA modifications including the recruitment of reader proteins, effects on intramolecular RNA structure, and modulation of codon-anticodon pairing interactions during ribosomal decoding. While these studies have focused on only a small number of known mRNA modifications, they have laid a conceptual framework for understanding the function of nucleobase modifications in the context of mRNA.
Moving forward, as additional modifications are identified, and we delve deeper into the biology of known modifications, there are several gaps that the field should work towards addressing. First and foremost, we lack a general strategy for mapping modification sites transcriptome wide. Current approaches rely heavily on antibody-based detection, which is known to have inherent limitations with regards to specificity and generality, and can only be applied to one modification per experiment. Further, information regarding modification stoichiometry is difficult to ascertain using antibody-based enrichment approaches. While RNA bisulfite sequencing can provide stoichiometry information it is plagued by false positives due to RNA structure-mediated non-conversion, and is primarily used for m5C mapping. Promising developments in single-molecule direct RNA sequencing125 may ultimately lead to improvement in this area and have already been applied to m6A with some success126,127, although it is still too early to conclude whether such approaches will be applicable to the majority of RNA modifications. Given the importance of reliable modification maps for generating biological hypotheses concerning the function of modifications, this is an important step towards a comprehensive understanding of RNA modification biology. Second, as additional proteins are characterized as RNA modification readers, generating a systems level understanding of how different modification-reader interactions are regulated and interplay with one another in diverse biological contexts will be critical to understand functional consequences. Work towards this end has already provided insight into YTH-domain protein interactions with m6A128, but similar analyses need to be performed for other RNA modification reader proteins. Finally, as we make progress in uncovering transcriptome-wide RNA modification maps for diverse modifications, identifying the functional consequence of any individual site is still a major undertaking. Robust approaches for reconstituting and manipulating endogenous modifications sites in a specific fashion would be enabling for such studies.
ACKNOWLEDGEMENTS
Research in the Kleiner lab is supported by the NIH (R01GM132189) and the NSF (MCB-1942565).
Footnotes
CONFLICT OF INTEREST
No competing interests have been declared.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Boccaletto P et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res 46, D303–D307, doi: 10.1093/nar/gkx1030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonkhout N et al. The RNA modification landscape in human disease. Rna 23, 1754–1769, doi: 10.1261/rna.063503.117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ontiveros RJ, Stoute J & Liu KF The chemical diversity of RNA modifications. Biochem J 476, 1227–1245, doi: 10.1042/bcj20180445 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan T Modifications and functional genomics of human transfer RNA. Cell Res 28, 395–404, doi: 10.1038/s41422-018-0013-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenz C, Lünse CE & Mörl M tRNA Modifications: Impact on Structure and Thermal Adaptation. Biomolecules 7, doi: 10.3390/biom7020035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato N, Hoshino H & Harada F Minor serine tRNA containing anticodon NCA (C4 RNA) from human and mouse cells. Biochem Int 7, 635–645 (1983). [PubMed] [Google Scholar]
- 7.van Tol H, Stange N, Gross HJ & Beier H A human and a plant intron-containing tRNATyr gene are both transcribed in a HeLa cell extract but spliced along different pathways. Embo j 6, 35–41 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J & Yi C Reading Chemical Modifications in the Transcriptome. J Mol Biol, doi: 10.1016/j.jmb.2019.10.006 (2019). [DOI] [PubMed] [Google Scholar]
- 9.He C Grand challenge commentary: RNA epigenetics? Nat Chem Biol 6, 863–865, doi: 10.1038/nchembio.482 (2010). [DOI] [PubMed] [Google Scholar]
- 10.McCown PJ et al. Naturally occurring modified ribonucleosides. Wiley Interdiscip Rev RNA, e1595, doi: 10.1002/wrna.1595 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walkley CR & Li JB Rewriting the transcriptome: adenosine-to-inosine RNA editing by ADARs. Genome Biol 18, 205, doi: 10.1186/s13059-017-1347-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levanon EY et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol 22, 1001–1005, doi: 10.1038/nbt996 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Harcourt EM, Kietrys AM & Kool ET Chemical and structural effects of base modifications in messenger RNA. Nature 541, 339–346, doi: 10.1038/nature21351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu N et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564, doi: 10.1038/nature14234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer KD et al. 5' UTR m(6)A Promotes Cap-Independent Translation. Cell 163, 999–1010, doi: 10.1016/j.cell.2015.10.012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer KD et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell 149, 1635–1646, doi: 10.1016/j.cell.2012.05.003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominissini D et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206, doi: 10.1038/nature11112 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Desrosiers R, Friderici K & Rottman F Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A 71, 3971–3975, doi: 10.1073/pnas.71.10.3971 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng G et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Molecular cell 49, 18–29, doi: 10.1016/j.molcel.2012.10.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia G et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7, 885–887, doi: 10.1038/nchembio.687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10, 93–95, doi: 10.1038/nchembio.1432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper JE, Miceli SM, Roberts RJ & Manley JL Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Res 18, 5735–5741, doi: 10.1093/nar/18.19.5735 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol 16, 191–198, doi: 10.1038/ncb2902 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P & Rottman F Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem 269, 17697–17704 (1994). [PubMed] [Google Scholar]
- 25.Patil DP et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537, 369–373, doi: 10.1038/nature19342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen J et al. Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell 69, 1028–1038.e1026, doi: 10.1016/j.molcel.2018.02.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue Y et al. VIRMA mediates preferential m(6)A mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell Discov 4, 10, doi: 10.1038/s41421-018-0019-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz S et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep 8, 284–296, doi: 10.1016/j.celrep.2014.05.048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ping XL et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24, 177–189, doi: 10.1038/cr.2014.3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei J et al. Differential m(6)A, m(6)Am, and m(1)A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Molecular cell 71, 973–985 e975, doi: 10.1016/j.molcel.2018.08.011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ke S et al. m(6)A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev 31, 990–1006, doi: 10.1101/gad.301036.117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauer J et al. Reversible methylation of m(6)Am in the 5' cap controls mRNA stability. Nature 541, 371–375, doi: 10.1038/nature21022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mauer J et al. FTO controls reversible m(6)Am RNA methylation during snRNA biogenesis. Nat Chem Biol 15, 340–347, doi: 10.1038/s41589-019-0231-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma C, Liao S & Zhu Z Crystal structure of human YTHDC2 YTH domain. Biochem Biophys Res Commun 518, 678–684, doi: 10.1016/j.bbrc.2019.08.107 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Xu C et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol 10, 927–929, doi: 10.1038/nchembio.1654 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Li F, Zhao D, Wu J & Shi Y Structure of the YTH domain of human YTHDF2 in complex with an m(6)A mononucleotide reveals an aromatic cage for m(6)A recognition. Cell Res 24, 1490–1492, doi: 10.1038/cr.2014.153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu T et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res 24, 1493–1496, doi: 10.1038/cr.2014.152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li A et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res 27, 444–447, doi: 10.1038/cr.2017.10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi H et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res 27, 315–328, doi: 10.1038/cr.2017.15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X et al. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161, 1388–1399, doi: 10.1016/j.cell.2015.05.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120, doi: 10.1038/nature12730 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arguello AE, DeLiberto AN & Kleiner RE RNA Chemical Proteomics Reveals the N(6)-Methyladenosine (m(6)A)-Regulated Protein-RNA Interactome. J Am Chem Soc 139, 17249–17252, doi: 10.1021/jacs.7b09213 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Wu R et al. A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res 29, 23–41, doi: 10.1038/s41422-018-0113-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edupuganti RR et al. N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 24, 870–878, doi: 10.1038/nsmb.3462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20, 285–295, doi: 10.1038/s41556-018-0045-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kan L et al. The m(6)A pathway facilitates sex determination in Drosophila. Nat Commun 8, 15737, doi: 10.1038/ncomms15737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haussmann IU et al. m(6)A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature 540, 301–304, doi: 10.1038/nature20577 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Lence T et al. m(6)A modulates neuronal functions and sex determination in Drosophila. Nature 540, 242–247, doi: 10.1038/nature20568 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Han D et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274, doi: 10.1038/s41586-019-0916-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z et al. METTL3-mediated N(6)-methyladenosine mRNA modification enhances long-term memory consolidation. Cell Res 28, 1050–1061, doi: 10.1038/s41422-018-0092-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi H et al. m(6)A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 563, 249–253, doi: 10.1038/s41586-018-0666-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J et al. YTH domain family 2 orchestrates epithelial-mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle 16, 2259–2271, doi: 10.1080/15384101.2017.1380125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen M et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology 67, 2254–2270, doi: 10.1002/hep.29683 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Li Z et al. Suppression of m(6)A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res 28, 904–917, doi: 10.1038/s41422-018-0072-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivanova I et al. The RNA m(6)A Reader YTHDF2 Is Essential for the Post-transcriptional Regulation of the Maternal Transcriptome and Oocyte Competence. Mol Cell 67, 1059–1067.e1054, doi: 10.1016/j.molcel.2017.08.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C et al. m(6)A modulates haematopoietic stem and progenitor cell specification. Nature 549, 273–276, doi: 10.1038/nature23883 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Zhao BS et al. m(6)A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 542, 475–478, doi: 10.1038/nature21355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu PJ et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res 27, 1115–1127, doi: 10.1038/cr.2017.99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du H et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun 7, 12626, doi: 10.1038/ncomms12626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park OH et al. Endoribonucleolytic Cleavage of m(6)A-Containing RNAs by RNase P/MRP Complex. Mol Cell 74, 494–507.e498, doi: 10.1016/j.molcel.2019.02.034 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Arguello AE, Leach RW & Kleiner RE In Vitro Selection with a Site-Specifically Modified RNA Library Reveals the Binding Preferences of N(6)-Methyladenosine Reader Proteins. Biochemistry, doi: 10.1021/acs.biochem.9b00485 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaccara S & Jaffrey SR A Unified Model for the Function of YTHDF Proteins in Regulating m(6)A-Modified mRNA. Cell 181, 1582–1595 e1518, doi: 10.1016/j.cell.2020.05.012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lasman L et al. Context-dependent functional compensation between Ythdf m6A readers. bioRxiv, doi: 10.1101/2020.06.03.131441 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu T et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res 48, 3816–3831, doi: 10.1093/nar/gkaa048 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rauch S, He C & Dickinson BC Targeted m(6)A Reader Proteins To Study Epitranscriptomic Regulation of Single RNAs. J Am Chem Soc 140, 11974–11981, doi: 10.1021/jacs.8b05012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao W et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Molecular cell 61, 507–519, doi: 10.1016/j.molcel.2016.01.012 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Kasowitz SD et al. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet 14, e1007412, doi: 10.1371/journal.pgen.1007412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roundtree IA et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife 6, doi: 10.7554/eLife.31311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu PJ et al. The RNA-binding protein FMRP facilitates the nuclear export of N (6)-methyladenosine-containing mRNAs. J Biol Chem 294, 19889–19895, doi: 10.1074/jbc.AC119.010078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alarcon CR, Lee H, Goodarzi H, Halberg N & Tavazoie SF N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485, doi: 10.1038/nature14281 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J et al. N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367, 580–586, doi: 10.1126/science.aay6018 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang F et al. Fragile X mental retardation protein modulates the stability of its m6A-marked messenger RNA targets. Hum Mol Genet 27, 3936–3950, doi: 10.1093/hmg/ddy292 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunn DB The occurrence of 1-methyladenine in ribonucleic acid. Biochim Biophys Acta 46, 198–200, doi: 10.1016/0006-3002(61)90668-0 (1961). [DOI] [PubMed] [Google Scholar]
- 74.Dominissini D et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature 530, 441–446, doi: 10.1038/nature16998 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X et al. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol 12, 311–316, doi: 10.1038/nchembio.2040 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Macon JB & Wolfenden R 1-Methyladenosine. Dimroth rearrangement and reversible reduction. Biochemistry 7, 3453–3458, doi: 10.1021/bi00850a021 (1968). [DOI] [PubMed] [Google Scholar]
- 77.Safra M et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551, 251–255, doi: 10.1038/nature24456 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Li X et al. Base-Resolution Mapping Reveals Distinct m(1)A Methylome in Nuclear- and Mitochondrial-Encoded Transcripts. Molecular cell 68, 993–1005 e1009, doi: 10.1016/j.molcel.2017.10.019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou H et al. Evolution of a reverse transcriptase to map N(1)-methyladenosine in human messenger RNA. Nat Methods, doi: 10.1038/s41592-019-0550-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu F et al. ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell 167, 816–828 e816, doi: 10.1016/j.cell.2016.09.038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai X, Wang T, Gonzalez G & Wang Y Identification of YTH Domain-Containing Proteins as the Readers for N1-Methyladenosine in RNA. Anal Chem 90, 6380–6384, doi: 10.1021/acs.analchem.8b01703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng Q et al. Cytoplasmic m(1)A reader YTHDF3 inhibits trophoblast invasion by downregulation of m(1)A-methylated IGF1R. Cell Discov 6, 12, doi: 10.1038/s41421-020-0144-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seo KW & Kleiner RE YTHDF2 Recognition of N(1)-Methyladenosine (m(1)A)-Modified RNA Is Associated with Transcript Destabilization. ACS Chem Biol 15, 132–139, doi: 10.1021/acschembio.9b00655 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schumann U et al. Multiple links between 5-methylcytosine content of mRNA and translation. BMC Biol 18, 40, doi: 10.1186/s12915-020-00769-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang T, Chen W, Liu J, Gu N & Zhang R Genome-wide identification of mRNA 5-methylcytosine in mammals. Nat Struct Mol Biol 26, 380–388, doi: 10.1038/s41594-019-0218-x (2019). [DOI] [PubMed] [Google Scholar]
- 86.Squires JE et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res 40, 5023–5033, doi: 10.1093/nar/gks144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang X et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res 27, 606–625, doi: 10.1038/cr.2017.55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khoddami V & Cairns BR Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat Biotechnol 31, 458–464, doi: 10.1038/nbt.2566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du Q, Luu PL, Stirzaker C & Clark SJ Methyl-CpG-binding domain proteins: readers of the epigenome. Epigenomics 7, 1051–1073, doi: 10.2217/epi.15.39 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Cheng JX et al. RNA cytosine methylation and methyltransferases mediate chromatin organization and 5-azacytidine response and resistance in leukaemia. Nat Commun 9, 1163, doi: 10.1038/s41467-018-03513-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Y et al. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal mRNA Decay. Mol Cell 75, 1188–1202.e1111, doi: 10.1016/j.molcel.2019.06.033 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Chen X et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol 21, 978–990, doi: 10.1038/s41556-019-0361-y (2019). [DOI] [PubMed] [Google Scholar]
- 93.Zhang HY, Xiong J, Qi BL, Feng YQ & Yuan BF The existence of 5-hydroxymethylcytosine and 5-formylcytosine in both DNA and RNA in mammals. Chem Commun (Camb) 52, 737–740, doi: 10.1039/c5cc07354e (2016). [DOI] [PubMed] [Google Scholar]
- 94.Fu L et al. Tet-mediated formation of 5-hydroxymethylcytosine in RNA. J Am Chem Soc 136, 11582–11585, doi: 10.1021/ja505305z (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delatte B et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science 351, 282–285, doi: 10.1126/science.aac5253 (2016). [DOI] [PubMed] [Google Scholar]
- 96.Muñoz-López Á & Summerer D Recognition of Oxidized 5-Methylcytosine Derivatives in DNA by Natural and Engineered Protein Scaffolds. Chem Rec 18, 105–116, doi: 10.1002/tcr.201700088 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Dimitrova-Paternoga L, Jagtap PKA, Chen PC & Hennig J Integrative Structural Biology of Protein-RNA Complexes. Structure 28, 6–28, doi: 10.1016/j.str.2019.11.017 (2020). [DOI] [PubMed] [Google Scholar]
- 98.Ries RJ et al. m(6)A enhances the phase separation potential of mRNA. Nature 571, 424–428, doi: 10.1038/s41586-019-1374-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Markmiller S et al. Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell 172, 590–604 e513, doi: 10.1016/j.cell.2017.12.032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sajini AA et al. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat Commun 10, 2550, doi: 10.1038/s41467-019-10020-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amort T et al. Long non-coding RNAs as targets for cytosine methylation. RNA Biol 10, 1003–1008, doi: 10.4161/rna.24454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roost C et al. Structure and thermodynamics of N6-methyladenosine in RNA: a spring-loaded base modification. J Am Chem Soc 137, 2107–2115, doi: 10.1021/ja513080v (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spitale RC et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature 519, 486–490, doi: 10.1038/nature14263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu N et al. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res 45, 6051–6063, doi: 10.1093/nar/gkx141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alarcon CR et al. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308, doi: 10.1016/j.cell.2015.08.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohn WE & Volkin E Nucleoside-5′-Phosphates from Ribonucleic Acid. Nature 167, 483–484, doi: 10.1038/167483a0 (1951). [DOI] [Google Scholar]
- 107.Deb I et al. Computational and NMR studies of RNA duplexes with an internal pseudouridine-adenosine base pair. Sci Rep 9, 16278, doi: 10.1038/s41598-019-52637-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kierzek E et al. The contribution of pseudouridine to stabilities and structure of RNAs. Nucleic Acids Res 42, 3492–3501, doi: 10.1093/nar/gkt1330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartz S et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159, 148–162, doi: 10.1016/j.cell.2014.08.028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li X et al. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol 11, 592–597, doi: 10.1038/nchembio.1836 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Carlile TM et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515, 143–146, doi: 10.1038/nature13802 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carlile TM et al. mRNA structure determines modification by pseudouridine synthase 1. Nat Chem Biol 15, 966–974, doi: 10.1038/s41589-019-0353-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blanco S et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. Embo j 33, 2020–2039, doi: 10.15252/embj.201489282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang T, Low JJA & Woon ECY A general strategy exploiting m5C duplex-remodelling effect for selective detection of RNA and DNA m5C methyltransferase activity in cells. Nucleic Acids Res 48, e5, doi: 10.1093/nar/gkz1047 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ranjan N & Leidel SA The epitranscriptome in translation regulation: mRNA and tRNA modifications as the two sides of the same coin? FEBS Lett 593, 1483–1493, doi: 10.1002/1873-3468.13491 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Fernandez IS et al. Unusual base pairing during the decoding of a stop codon by the ribosome. Nature 500, 107–110, doi: 10.1038/nature12302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karijolich J & Yu YT Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 474, 395–398, doi: 10.1038/nature10165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eyler DE et al. Pseudouridinylation of mRNA coding sequences alters translation. Proc Natl Acad Sci U S A 116, 23068–23074, doi: 10.1073/pnas.1821754116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choi J et al. N(6)-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat Struct Mol Biol 23, 110–115, doi: 10.1038/nsmb.3148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mao Y et al. m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat Commun 10, 5332, doi: 10.1038/s41467-019-13317-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoernes TP et al. Nucleotide modifications within bacterial messenger RNAs regulate their translation and are able to rewire the genetic code. Nucleic Acids Res 44, 852–862, doi: 10.1093/nar/gkv1182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang T, Chen W, Liu J, Gu N & Zhang R Genome-wide identification of mRNA 5-methylcytosine in mammals. Nature Structural & Molecular Biology 26, 380–388, doi: 10.1038/s41594-019-0218-x (2019). [DOI] [PubMed] [Google Scholar]
- 123.Arango D et al. Acetylation of Cytidine in mRNA Promotes Translation Efficiency. Cell 175, 1872–1886 e1824, doi: 10.1016/j.cell.2018.10.030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taniguchi T et al. Acetate-dependent tRNA acetylation required for decoding fidelity in protein synthesis. Nat Chem Biol 14, 1010–1020, doi: 10.1038/s41589-018-0119-z (2018). [DOI] [PubMed] [Google Scholar]
- 125.Garalde DR et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat Methods 15, 201–206, doi: 10.1038/nmeth.4577 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Liu H et al. Accurate detection of m(6)A RNA modifications in native RNA sequences. Nat Commun 10, 4079, doi: 10.1038/s41467-019-11713-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Workman RE et al. Nanopore native RNA sequencing of a human poly(A) transcriptome. Nat Methods 16, 1297–1305, doi: 10.1038/s41592-019-0617-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zaccara S & Jaffrey SR A Unified Model for the Function of YTHDF Proteins in Regulating m(6)A-Modified mRNA. Cell, doi: 10.1016/j.cell.2020.05.012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


