Abstract
Objectives:
To estimate the effect of skin-to-skin contact (SSC) on biobehavioral measures of stress (anxiety and salivary cortisol) and attachment (attachment scores and salivary oxytocin) of mothers before and after their infants’ neonatal cardiac surgery.
Design:
A prospective interventional, baseline response–paired, pilot study.
Setting:
Cardiac center of a large, metropolitan, free-standing, children’s hospital.
Participants:
Thirty women whose infants were hospitalized for neonatal cardiac surgery.
Methods:
Participants acted as their own controls before, during, and after SSC at two time points: once before and once after surgery. We measured the stress response of mothers, as indicated by self-reported scores of anxiety and maternal salivary cortisol, and maternal-infant attachment, as indicated by self-reported scores and maternal salivary oxytocin.
Conclusions:
Our findings provide initial evidence of the benefits of SSC as a nurse-led intervention to support maternal attachment and reduce physiologic and psychological stress responses in mothers of infants with critical congenital heart disease before and after neonatal cardiac surgery.
Keywords: infant, mothers, anxiety, stress, psychological, heart diseases, cardiac surgical procedures, mental health, oxytocin
Precis
Skin-to-skin care supported maternal attachment and reduced the stress response in mothers of infants before and after neonatal cardiac surgery.
Stress and the mental health of parents of infants with critical congenital heart disease (cCHD) are growing concerns for the international community of pediatric cardiac health care providers (Lisanti, 2018; Verrall et al., 2019; Wernovsky & Licht, 2016; Woolf-King et al., 2017). Infants born with cCHD require surgery in the neonatal period to survive, which is an overwhelmingly stressful experience for their parents. An ever-growing body of research has demonstrated that parents experience extreme distress, anxiety, and depression during their infants’ hospitalizations for cardiac surgery (Woolf-King et al., 2017), and symptoms of acute stress disorder and post-traumatic stress persist after discharge (Cantwell-Bartl & Tibballs, 2013; Franich-Ray et al., 2013). An important link has been demonstrated between maternal mental health and child developmental outcomes in infants with cCHD (McCusker et al., 2010), which highlights the critical role of mothers and the need to support the mother-infant dyad. However, early attachment in mother-infant dyads is threatened by physical separation of mothers from their infants (Rempel et al., 2013). Maternal-infant separation in cases of cCHD often occurs immediately after birth because of the newborn’s admission to a pediatric cardiac intensive care unit (PCICU) and frequently continues throughout the newborn’s hospitalization (Lisanti et al., 2017; Sood et al., 2018).
Holding the infant is an intervention that can influence stress and attachment for mothers of infants with cCHD (Cong et al., 2015; Li et al., 2014). Skin-to-skin contact (SSC) is a specific form of holding in which the mother holds the unclothed, diapered infant directly to her bare chest. Although researchers during the past decade have called for an increase in developmentally supportive care in PCICUs, including interventions such as SSC (Butler et al., 2017; Harrison, 2019; Lisanti et al., 2016; Lisanti, Vittner, et al., 2019; Peterson, 2018; Peterson & Evangelista, 2017; Torowicz et al., 2012), considerable variation in practice exists across PCICUs (Miller et al., 2020; Sood et al., 2016). Skin-to-skin care and holding in general have not been integrated into the standard of care across PCICUs. This may partly be related to concerns regarding the fragility and hemodynamic instability of infants with cCHD and concerns regarding the safety of moving infants onto their mothers in the presence of required tubes and catheters, such as transthoracic intracardiac lines (Lisanti, Fitzgerald, et al., 2019; Lisanti, Vittner, et al., 2019). Furthermore, mothers of infants with life-threatening anomalies such as cCHD experience extreme and acute stress in the PCICU that may be distinct from mothers of prematurely born infants who receive neonatal intensive care that is primarily focused on physiologic support related to prematurity, growth, and development (Lisanti, Vittner, et al., 2019). Although there may be similarities, full-term infants with cCHD in the PCICU follow a recovery trajectory, with unstable physiology and hemodynamics, in addition to growth and development issues, which presents unique parental challenges (Lisanti, Vittner, et al., 2019).
To date, researchers of three published pilot studies found that after cardiac surgery, SSC was a safe and feasible intervention to support physiologic stability (Gazzolo et al., 2000), autonomic nervous system function (Harrison & Brown, 2017), and cognitive learning (Harrison et al., 2019). However, few researchers have examined the effects of SSC for mothers of infants with cCHD and few have investigated the effect of SSC using maternal biological markers. Although the mental health benefits are well-documented for mothers of premature and healthy full-term infants (Bigelow et al., 2012; Holditch-Davis et al., 2014; Ludington-Hoe, 2011; Moore et al., 2016), research is critically needed to determine whether SSC mitigates stress and supports attachment and mental health of mothers during their infants’ hospitalizations for neonatal cardiac surgery.
Theoretical Framework
The stress experience of mothers with infants in the PCICU was described as a relationship between the stressors mothers perceive and their stress responses in the PCICU Parental Stress Model, which was developed by Lisanti and colleagues (Figure 1; Lisanti, Allen, et al., 2017; Lisanti, Golfenshtein et al., 2017). The PCICU Parental Stress Model includes three categories of stressors, infant, environment, and parent, that combine to produce a maternal stress response. Stressors in the infant category include severity of infant illness (actual and perceived), seeing the infant in pain, and the appearance and behavior of the critically ill infant. Environmental stressors consist of a variety of stimuli from the PCICU such as the sights and sounds of medical equipment, alarms, staff, constant activity, and the other sick patients being cared for in the unit. The environment also creates a lack of privacy and a perceived feeling of never leaving the hospital that can be sources of stress for mothers. Finally, stressors can arise from within mothers themselves. Perhaps the most significant internal stressor for mothers is parental role alteration, defined as a disruption in the instinctual maternal role to hold, feed, care for, and protect the infant Lisanti, Allen, et al., 2017; Lisanti, Golfenshtein et al., 2017). In qualitative studies of mothers of infants with cCHD, researchers found that the parental role alteration that arises from the inability to hold their infants creates substantial stress and may last for weeks or even months in some mothers (Lisanti, Golfenshtein, et al., 2017; Rempel et al., 2013; Sood et al., 2018).
Figure 1.
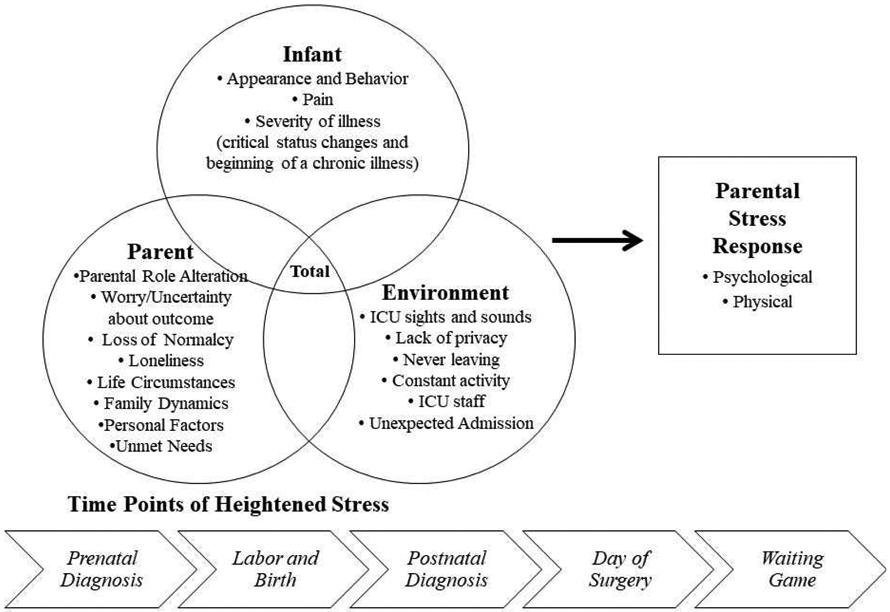
The Pediatric Cardiac Intensive Care Unit Parental Stress Model. From Lisanti, A. J., Golfenshtein, N., & Medoff-Cooper, B. (2017). The Pediatric Cardiac Intensive Care Unit Parental Stress Model: Refinement using directed content analysis. Advances in Nursing Science, 40(4), 319-336. https://doi.org/10.1097/ANS.0000000000000184. Reprinted with permission from Wolters Kluwer Health, Inc.
According to the PCICU Parental Stress Model, maternal responses to stressors in the PCICU are often physical and psychological (Figure 1). One of the body’s primary physiologic responses to stress is through the hypothalamic-pituitary adrenocortical (HPA) axis, which stimulates glucocorticoid production, primarily cortisol (Garfield et al., 2018). Psychological responses can manifest as mental health symptoms of anxiety (Lisanti, Allen, et al., 2017), depression (Solberg et al., 2011; Solberg et al., 2012), and/or post-traumatic stress (Cantwell-Bartl & Tibballs, 2013), which have been documented in mothers of infants with cCHD (Woolf-King et al., 2017). Researchers found a relationship between the stressor of parental role alteration and the stress response of anxiety in mothers after infant cardiac surgery (Lisanti, Allen, et al., 2017). Mothers reported that not being able to hold their infants during hospitalization for cardiac surgery was one of the most stressful experiences (Lisanti, Golfenshtein, et al., 2017). Therefore, nursing interventions that target the enhancement of maternal role may reduce the stress response during the infant’s hospitalization for cardiac surgery. For this study, we hypothesized that mothers who held their infants in SSC would experience fewer symptoms of stress response.
Maternal mental health symptoms have also been shown to negatively influence the developing mother-infant relationship and attachment when infants have cCHD (Jordan et al., 2014). Maternal attachment has been minimally studied in mothers and infants with CHD; however, the few studies that have been published revealed that mothers of infants who undergo neonatal cardiac surgery had difficulty forming attachment feelings towards their infants (Boztepe et al., 2016; Jordan et al., 2014; Ylmaz et al., 2011). Oxytocin is an important hormone that is released by the hypothalamus in response to breastfeeding, holding, and touch. It mediates stress and promotes the psychobiological process of maternal-infant attachment (Amanullah et al., 2016; Naguib et al., 2013). Produced in the supraoptic and paraventricular nuclei of the hypothalamus (Cong et al., 2015; Gordon et al., 2010), oxytocin is naturally present in human saliva, and researchers demonstrated its role in attachment and parenting (Feldman et al., 2011; Gordon et al., 2010; Vittner et al., 2018, 2019) and in the mediation of stress and anxiety (Feldman et al., 2011). Studies conducted in the NICU setting showed that SSC activates the release of endogenous oxytocin in mothers and infants, reduces stress, and facilitates the synchronicity within the dyad (Cong et al., 2015; Vittner et al., 2018). The overall aim of this pilot study was to determine the effect of SSC on biobehavioral measures of stress (anxiety and salivary cortisol) and attachment (attachment scores and salivary oxytocin) of mothers before and after their infants’ neonatal cardiac surgery.
Methods
Design
We conducted a pilot study between January 2018 and January 2019 to estimate the effect of SSC on maternal stress and attachment in women who were mothers of infants with cCHD. Skin-to-skin care was performed twice, once in the preoperative period (T1) and once in the postoperative period (T2). We used a baseline response–paired design with mother-infant pairs acting as their own controls before, during, and after SSC at T1 and T2. Infant outcomes are reported separately (Lisanti et al., 2020). Our study was approved by the institutional review board at the Children’s Hospital of Philadelphia. All hospital policies and procedures for holding and for SSC were followed to ensure safety of infants during the SSC interventions.
Setting
Children’s Hospital of Philadelphia is a free-standing pediatric hospital. The PCICU at Children’s Hospital of Philadelphia is a 32-bed unit that admits more than 180 infants each year for neonatal cardiac surgery. The PCICU has open-bay style and single rooms, and the nurse-patient ratio is one nurse per one to two infants depending on acuity.
Participants
We recruited a convenience sample of 30 women and their infants preoperatively after the infant’s admission to the PCICU. We did not complete a power analysis because a sample size of 30 is generally regarded as adequate for pilot studies (Hertzog, 2008). Participants included women who were biological mothers of infants admitted to the PCICU within one week of birth. Women 18 years of age or older who were able to read and speak English were approached for participation if their infants met the following inclusion criteria: less than 30 days of age, greater than 36 weeks gestational age at birth, and birth weight greater than 2500 grams. Infants were excluded if they were diagnosed with other congenital syndromes or anomalies, were placed on the list for cardiac transplant, or were receiving end of life care.
Measures
We collected data from participants immediately before SSC to obtain baseline measures; 30 minutes after the start of SSC to obtain measures during the intervention; and 30 minutes after the end of SSC, as has been reported by other investigators (Cong et al., 2011; Vittner et al., 2018). Cortisol and oxytocin are released in response to a stimulation in a pulsatile fashion, reaching their peak responses at least 20 minutes after the stressor is initiated (Alley et al., 2019). Therefore, we chose 30 minutes to ensure saliva samples accurately reflected changes in hormone levels as a result of SSC initiation or after SSC ended. We used a combination of questionnaires, self-report measures, and saliva samples to assess the effects of SSC on stress response and attachment. We obtained additional baseline measures of mental health (trait anxiety, symptoms of depression), perceived stressors, and perceived infant severity of illness to address potential confounders on the effect of SSC. We also obtained demographic data on the mothers and infants at T1 and T2 to include these variables as covariates on the outcome variables. We classified infant cCHD using the Society of Thoracic Surgery–European Association for CardioThoracic Surgery (STAT) Congenital Heart Surgery Mortality scoring system (Cavalcanti et al., 2015; O'Brien et al., 2009). The STAT categories range from 1 to 5; surgical procedures in category 5 have the greatest complexity and are associated with the highest risk of mortality.
Salivary cortisol.
Salivary cortisol is unbound or free cortisol and reflects approximately 5% of the circulating levels in the blood (Turpeinen & Hamalainen, 2013). Cortisol has a diurnal pattern with the highest levels in the morning upon awakening and a slow decline until bedtime. We only initiated SSC between the hours of 11 am to 3 pm to account for the diurnal variation of cortisol as reported in other studies on SSC using salivary cortisol (Cong et al., 2015; Neu et al., 2014; Vittner et al., 2018). We asked participants to abstain from smoking, eating, or drinking anything but water for 1 hour before saliva collections to minimize sample contamination. Participants collected their saliva samples using a saliva collection aid (SalivaBio Passive Drool, Salimetrics). We measured salivary cortisol by competitive cortisol immunoassay (Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit, Salimetrics) according to the manufacturer’s directions. Samples were assayed in duplicate, relative to a standard curve. Intra-assay coefficient of variability was calculated to be 4.2%; inter-assay coefficient of variability was calculated to be 9.3% for high (1.0 mg/dl) and low (0.1 mg/dl) controls.
Salivary oxytocin.
We measured salivary oxytocin from the same samples of saliva as used to measure cortisol. In the laboratory, samples underwent an initial spin to pellet debris and cells, remove supernatant, and aliquot into separate vials for cortisol and oxytocin analysis. We extracted salivary oxytocin and tested it using the DetectX OXYTOCIN Enzyme Immunoassay Kit (Arbor Assays, Ann Arbor, Michigan, USA). Extraction was recommended to prevent falsely elevated values (McCullough et al., 2013). For the low control (50 pg/mL), the intra-assay coefficient of variability was 13.98% on average and inter-assay coefficient of variability was 15.74%. For the high control (500 pg/mL), the intra-assay coefficient of variability was 2.35% on average, and the inter-assay coefficient of variability was 3.78%.
Maternal perception of stressors.
The Parental Stressor Scale: Infant Hospitalization (PSS:IH) is a 22-item instrument that is used to measure participants’ perceived stressors, including subscales of infant’s appearance and behavior (eight items), parental role alteration (eight items), and the sights and sounds of the hospital environment (six items; Miles, 2003). Lisanti, Allen, et al (2017) reported an internal consistency reliability coefficient for the PSS:IH of 0.91 when used by mothers of infants with cCHD. In our study, Cronbach’s alpha coefficients were 0.96 for the total instrument and 0.94 for infant appearance and behavior, 0.92 for parental role alteration, and 0.91 for the sights and sounds of the hospital environment. Participants rated each item on a 5-point Likert scale indicating how stressful each item was perceived from not at all stressful to extremely stressful. Items not experienced by participants could be marked not applicable. Means for each subscale were calculated as well as a total mean score. Items scored as not applicable were not included in the calculation of mean scores for the total instrument or the subscales. Mean scores could range from 1 to 5.
Maternal anxiety.
Maternal anxiety was measured with the State Trait Anxiety Inventory (STAI) instrument and a visual analogue scale (VAS). The STAI is a well-established research instrument that has been used by researchers for more than 30 years (Spielberger, 1983). The tool includes two, 20-item subscales: the State-Anxiety scale (STAI Form Y-1) and the Trait-Anxiety scale (STAI Form Y-2; Spielberger, 1983). Internal consistency reliability has been reported for the State-Anxiety scale (Cronbach alpha = 0.95) and the Trait-Anxiety scale (Cronbach alpha = 0.91) in mothers of infants with cCHD (Lisanti, Allen, et al., 2017). In our study, Cronbach’s alpha coefficient was 0.97 for the State-Anxiety scale and 0.91 for the Trait-Anxiety scale. The State-Anxiety scale is used to measure how an individual feels at the current moment and has been frequently used to measure maternal stress response (Fang & Hung, 2012; Lisanti, Allen, et al., 2017; Rychik et al., 2013). The Trait-Anxiety scale is used to measure an individual’s tendency towards anxiety by asking how she or he generally feel outside of the present circumstances. Participants were instructed to rank each item on a four-point Likert scale from 1 (not at all) to 4 (very much so). The total scores of each of the State-Anxiety and Trait-Anxiety subscales were calculated as a total sum that could range from 20 to 80. The State-Anxiety subscale was used to measure maternal stress response.
For the VAS, participants were asked to answer the question: “Many mothers with hospitalized infants report feeling stressed, tense, or anxious. How stressed, tense, or anxious do you feel right now?” To respond, participants were asked to mark a VAS that ranged from not at all on the far left to very much so on the far right. Responses on the VAS were converted to a 100-point scale, where a value of 0 was given to the start of the VAS on the left and a value of 100 was given to the far right of the VAS. A VAS has been used in SSC studies to measure subjective feelings of stress (Cong et al., 2015; Vittner et al., 2018).
Maternal symptoms of depression.
Because baseline symptoms of depression scores have been identified as a confounding variable in SSC studies (Neu et al., 2014), we collected data on symptoms of depression as a covariate. The Center for Epidemiological Studies-Depression (CES-D) is a 20-item instrument that asks participants to rate on a four-point Likert scale how often they experienced symptoms associated with depression in the past week (Radloff, 1977). Total scores are summed and can range from 0 to 60. The CES-D also has been used across a range of populations with acceptable indicators of validity and reliability (Howland et al., 2011). The internal consistency reliability (Cronbach’s alpha) for this study was 0.86.
Perceived maternal-infant attachment.
Participants’ perceptions of attachment to their infants was measured with the Maternal Attachment Inventory (MAI) and a VAS. The MAI is a 26-item, self-report instrument that has been used with women who have given birth to infants with CHD (Boztepe et al., 2016; Ylmaz et al., 2011). Each item is scored on a four-point Likert scale, and higher scores indicate higher maternal-infant attachment; total scores can range from 26 to 104. Although originally reported as .85, Cronbach’s alpha estimate of internal consistency reliability ranged from .92 to .96 in recent studies (Cho et al., 2016; Fang & Hung, 2012).
For the maternal attachment VAS, participants were asked the question: “Many mothers with hospitalized infants report difficulties bonding to their babies. How close or bonded do your feel with your baby right now?” To respond, participants were asked to mark a VAS that ranged from not at all on the far left to very much so on the far right. Participants’ responses on the VAS were converted to a 100-point scale, where a value of zero was given to the start of the VAS on the left and a value of 100 was given to the far right of the VAS.
Perceived severity of infant illness.
According to the PCICU Parental Stress Model, perceived severity of infant illness may contribute to the stress experience for mothers (Figure 1). Therefore, before each SSC intervention, participants were asked to rate on a VAS “How sick or fragile do you believe your baby is today?” from not at all on the far left to very much so on the far right. Participants’ responses were converted to a score from 0 to 100 based on their marking on the VAS, where a value of zero was given to the start of the VAS on the left and a value of 100 was given to the far right of the VAS.
Procedures
Women were approached in person by a member of the study team after their infants’ admissions to the PCICU. If women were willing to consider the research study, the informed consent process was initiated to ensure they comprehended the purpose of the study, the study procedures, and the risk-benefit profile. Time was provided for questions and decision making. After written informed consent was obtained, the preoperative SSC intervention was scheduled.
For the preoperative and postoperative SSC, the SSC was scheduled at a convenient time for participants and their infants between 11 am and 3 pm. SSC was scheduled one hour after breastfeeding or pumping to ensure that these did not affect OT levels during SSC. Participants were instructed to sit quietly for 30 minutes prior to SSC and obtaining the first saliva sample. After the participant’s saliva was obtained, she completed the PSS:IH, STAI, MAI, CES-D, and the VASs on stress and attachment on an electronic tablet. She changed into a hospital gown open in the front and stood at the foot of the infant’s bed with a chair placed directly behind her. With the assistance of the researcher, the participant lifted her undressed infant to her chest in an upright position and then slowly moved to a seated position. If a participant expressed discomfort or soreness from childbirth in moving from a standing to a seated position, she remained seated in a chair and the researcher lifted and positioned the infant for her. Layered blankets covered the infant and participant to ensure comfort for both. After 30 minutes of SSC, participant provided an additional saliva sample and completed the state anxiety subscale of the STAI, MAI, and VASs on an electronic tablet held up for the participant by the researcher. Most participants were able to use one hand to indicate their responses to the surveys while holding their infants in SSC. For participants who preferred, the researcher was able to hold the tablet and provide responses as indicated by the participant. After one hour of SSC holding, the infant was gently placed back into the crib. Thirty minutes after the end of SSC, participants provided a final saliva sample and completed the state anxiety subscale of the STAI, MAI, and VASs.
Analysis and Sample Size
We used the mixed procedure in the statistical software package SAS version 9.4 to compute repeated measures linear mixed-effects models for continuous outcomes at both T1 and T2. Adjustment for clustering of repeated measures within participants was completed using an unstructured covariance matrix. A backward elimination approach was used for variable selection and only those covariates associated with each outcome variable at the p ≤ 0.2 level were considered in the model selection. Covariates that remained significant at p ≤ 0.05 were retained to produce the final analytical models.
Results
One hundred-ninety-five infant PCICU admissions were consecutively screened for eligibility and 158 did not meet criteria for this study (Lisanti et al., 2020). Of the 37 eligible infants during the study period, four women declined participation and two infants went to surgery before their mothers could be approached for participation. A total of 31 women consented to participate in this study; however, the surgical plan was cancelled for one participant’s infant before the T1 SSC intervention and she was withdrawn from the study. The 30 participants had a mean age of 29.5 (Table 1) and had an average of two children, including the infant in the PCICU. The infants were 67% male (n=20) and 33% female (n=10). Fifty-three percent of infants were classified with two-ventricle physiology (n=16) and 47% had single-ventricle physiology (n=14). At the preoperative SSC intervention (T1), infants were within the first few days of life (M=2 days, SD=1.43 days) and recently hospitalized (M=2 days, SD=1.13 days). A majority (90%, n=27) of participants held their infants in open bay-style rooms with the remainder in private rooms.
Table 1:
Demographic Characteristics of Participants (N = 30)
| Variable | Category | n | % |
|---|---|---|---|
| Marital status | Single | 7 | 23 |
| Married | 23 | 77 | |
| Racea | Black or African American | 4 | 13 |
| White | 21 | 70 | |
| Asian | 1 | 3 | |
| Unknown/other | 4 | 13 | |
| Ethnicitya | Hispanic or Latino | 8 | 27 |
| Not Hispanic or Latino | 21 | 72 | |
| Level of education | Graduated from high school | 5 | 17 |
| Technical/vocational school | 3 | 10 | |
| Partial college | 6 | 20 | |
| College graduate | 10 | 33 | |
| Master's degree | 5 | 17 | |
| Doctoral degree | 1 | 3 | |
| Timing of infant diagnosis | Prenatally | 24 | 80 |
| Postnatally | 6 | 20 | |
| Type of birth | Vaginal | 15 | 50 |
| Cesarean | 15 | 50 |
Does not equal 100% because of missing data.
At T2, six mother-infant dyads were no longer eligible for participation because of changes in the plans for surgical intervention (n=3), transition to end of life care (n=2), or a significant neurological injury (n=1). A total of 24 mother-infant dyads were eligible for ongoing participation with a retention rate of 96% (n=23). One participant withdrew after the start of SSC at T2 because the infant began showing feeding cues and the participant wanted to breastfeed. The remaining 23 infants who participated in SSC at T2 were approximately 2 weeks after their cardiac surgeries (M=16 days, SD=19.53) and an average of 10 days after extubation (SD=9.82). Infant surgeries ranged from STAT categories 1 and 2 (21%), 3 and 4 (35%), and 5 (44%). Average deep hypothermic circulatory arrest time was 18 minutes (SD=20.52) during the infants’ surgeries. At T2, 61% (n=14) of participants held their infants in open bay-style rooms, 22% (n=5) in shared rooms, and 17% (n=4) in private rooms.
Baseline mental health measures for participants at T1 and T2 are presented in Table 2. At T1 and T2, participants’ state anxiety scores were significantly reduced during (p < .0001) and after (p < .0001) SSC when compared to baseline (Tables 3 and 4). Models were adjusted for baseline depression symptoms at T1 and trait anxiety at T2. Participants also reported significantly decreased feelings of stress by VAS during (p < .0001) and after (T1, p = .005; T2, p = .0002) SSC at both time points, adjusting for perceived severity of illness at T1 as well as baseline depression symptoms and deep hypothermic circulatory arrest times at T2. Maternal physiologic stress response, as measured by cortisol, also decreased significantly at both time points. At T1, significant reductions in cortisol were demonstrated from baseline to during SSC (p = .0009), from baseline to after SSC (p = .0003), and when comparing during to after SSC (p = .0068). At T1, significant reductions in cortisol were demonstrated from baseline to after SSC (p < .0001), and when comparing during and after SSC (p = .0009). Patterns of physiologic and psychologic stress responses at both time points demonstrated sustained reductions in stress responses during and after SSC at both time points (Figure 2).
Table 2:
Baseline Mental Health Measures of Participants
| Tool | Time 1: Preoperative M (SD) |
Time 2: Postoperative M (SD) |
|---|---|---|
| Center for Epidemiological Stodies–Depression | 15.70 (8.53) | 15.96 (9.74) |
| State–Trait Anxiety Inventory–Trait Anxiety | 36.40 (8.78) | 37.13(9.22) |
| Parental Stressor Scale Infant Hospitalization–Total | 3.30 (0.95) | 3.71 (0.77) |
| Parental Stressor Scale: Infant Hospitalisation, Infant Appearance and Behavior | 3.71 (1.08) | 4.06 (0.79) |
| Parental Stressor Scale: Infant Hospitalization Parental Rote Alteration | 3.29 (0.93) | 3.59 (0.74) |
| Parental Stressor Scale: Infant Hospitalization Sights and Sounds | 2.50 (0.95) | 3.11 (1.21) |
| Perceived severity of infant illness | 42.13(27.75) | 36.80 (27.55) |
Table 3.
Preoperative Outcomes Related to Skin-to-Skin Care (SSC)
| Time 1: Preoperative Period (n=30) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before SSC | Change from Before to During SSC |
During SSC | Change from During to After SSC |
After SSC | Change Before to After SSC |
||||
| M (SD) | M (SD) | M (SD) | |||||||
| p-value | Effect size | p-value | Effect size | p-value | Effect size | ||||
| State-Trait Anxiety Inventory (STAI) State Scorea | 49.90 (12.62) | <.0001 | 2.264 | 25.50 (7.74) | .002 | 0.459 | 30.45 (11.76) | <.0001 | 1.805 |
| Stress Response Visual Analog Scaleb | 59.33 (26.96) | <.0001 | 1.703 | 18.94 (17.73) | .035 | 0.437 | 23.00 (20.74) | .005 | 1.266 |
| Maternal Attachment Inventory State Score | 101.73 (2.29) | .007 | 0.515 | 102.53 (1.94) | .046 | 0.225 | 102.90 (1.78) | <.001 | 0.739 |
| Attachment Visual Analog Scalea,c | 62.69 (20.93) | <.0001 | 4.374 | 91.19 (20.50) | .767 | 0.362 | 89.81 (11.02) | <.0001 | 4.013 |
| Cortisol | 0.21 (0.12) | <.001 | 0.496 | 0.18 (0.09) | .007 | 0.411 | 0.15 (0.07) | <.001 | 0.907 |
| Oxytocin | 90.68 (72.84) | .787 | 0.060 | 98.55 (78.15) | .853 | 0.094 | 98.16 (78.40) | .690 | 0.034 |
Note. Units of measure for each variable: cortisol=ug/dL, oxytocin= pg/mL. Effect sizes reported as Cohen’s D.
Covariate Center for Epidemiological Studies-Depression score (p < .01)
Covariate perceived severity of illness (p = .007)
Covariate room location in preoperative period (p = 0.01)
Table 4.
Postoperative Outcomes Related to Skin-to-Skin Care (SSC)
| Time 2: Preoperative Period (n=23) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before SSC | Change from Before to During SSC |
During SSC | Change from During to After SSC |
After SSC | Change Before to After SSC |
||||
| M (SD) | M (SD) | M (SD) | |||||||
| p-value | Effect size | p-value | Effect size | p-value | Effect size | ||||
| State-Trait Anxiety Inventory (STAI) State Scorea | 42.70 (14.84) | <.0001 | 1.679 | 24.91 (6.15) | .042 | 0.202 | 27.09 (9.55) | <.0001 | 1.477 |
| Stress Response Visual Analog Scale b,c | 54.82 (24.71) | <.0001 | 2.166 | 17.00 (20.58) | .205 | 0.182 | 20.18 (24.58) | <.001 | 1.984 |
| Maternal Attachment | 102.91 (1.90) | .129 | 0.301 | 103.22 (1.35) | .333 | 0.129 | 103.36 (1.18) | .068 | 0.430 |
| Inventory State Score | |||||||||
| Attachment Visual Analog Scale b,d | 56.36 (38.15) | .019 | 0.915 | 85.91 (18.85) | .316 | 0.053 | 87.64 (16.73) | .012 | 0.968 |
| Cortisol | 0.15 (0.07) | .107 | 0.353 | 0.13 (0.06) | <.001 | 0.668 | 0.10 (0.05) | <.0001 | 1.021 |
| Oxytocin | 96.27 (67.35) | .075 | 0.150 | 94.42 (69.06) | .240 | 0.347 | 99.88 (82.12) | .963 | 0.497 |
Note. Units of measure for each variable: cortisol=ug/dL, oxytocin=pg/mL. Effect sizes reported as Cohen’s D.
Covariate trait anxiety, STAI subscale (p = 0.03),
Covariate Center for Epidemiological Studies-Depression score (p < 0.04)
Covariate deep hypothermic circulatory arrest time (p = 0.02)
Covariate days since extubation (p = 0.05)
Figure 2.

Maternal Stress Outcomes
Participants’ MAI scores were significantly increased during (p = .0065) and after (p = .0006) SSC when compared to baseline at T1. Participants also reported at T1 increased feelings of attachment by VAS during (p < .0001) and after SSC (p < .0001) compared to baseline and adjusted for baseline depression symptoms and room location in preoperative period. At T2, no differences were found in MAI scores when comparing before, during, and after SSC. Participants reported significantly increased feelings of attachment by VAS at T2 during (p = .0185) and after SSC (p = .0118) compared to baseline, adjusting for baseline symptoms of depression and days since extubation. Patterns of maternal attachment by VAS and MAI at T1 and T2 demonstrated increased attachment during and after SSC (Figure 3). Oxytocin values ranged from 17.30 to 1191.13 pg/mL across all samples. Because of the wide variability, the top 5% of extreme values were removed from the analysis. These outliers had values greater than 350 pg/mL and were presumed to be biologically implausible. No significant differences were found in oxytocin when comparing before, during, and after SSC at either time point.
Figure 3.

Maternal Attachment Outcomes
Discussion
Our study provides valuable insights regarding the biobehavioral outcomes of SSC for mothers of infants with cCHD. As hypothesized, our results provide preliminary support for SSC as a nurse-driven intervention to mitigate the negative effects of parental role alteration by reducing maternal physiological and psychological stress responses. Additionally, our results provide preliminary evidence that the enhancement of maternal role through SSC can decrease symptoms of anxiety during and immediately after SSC in both preoperative and postoperative phases of their infants’ hospitalization for neonatal cardiac surgery. These findings provide support for the PCICU Parental Stress Model and the interaction of stressors and maternal stress response in the PCICU setting. Our study also presents one of few nurse-led interventional studies conducted in the PCICU directly addressing maternal stress and attachment of infants with cCHD.
Consistent with results of previous studies of SSC with preterm hospitalized infants (Cong et al., 2015; Neu et al., 2014), maternal salivary cortisol levels continuously decreased during and post SSC when compared to baseline levels in our study. Participants reported decreased feelings of stress and decreased state anxiety during SSC. Similar to research in mothers of premature infants (Vittner et al., 2018), maternal anxiety levels post-SSC remained significantly reduced when compared to baseline anxiety levels, suggesting a prolonged effect of the intervention after the completion of SSC. Our findings differ from those reported by Cong and colleagues (2015) who found that mother’s self-reported anxiety by VAS returned to baseline after SSC had ended. SSC may have particular benefit for mothers in the PCICU setting who have described not being able to hold their infants as an extremely significant stressor (Lisanti, Golfenshtein, et al., 2017; Sood et al., 2018). Additionally, we measured stress response by VAS and by the state anxiety subscale of the STAI, which both provided similar results. Since these findings differ from previous research (Cong et al., 2015), future research on SSC may benefit from the inclusion of both measures until studies confirm the pattern and direction of these relationships. The use of the STAI also allowed us to account for trait anxiety as a potential covariate in the statistical modeling. Future research on mothers performing SSC may be strengthened by the inclusion of a developed instrument such as the STAI. Furthermore, symptoms of depression (CES-D) entered into several of the models as a significant covariate influencing the outcome variables, as well as perceived severity of illness, proxy measures of severity of illness (days since extubation, deep hypothermic circulatory arrest times), and room location. These findings provide further support for the PCICU Parental Stress Model, which proposes that parental, infant, and environmental factors influence the stress experience for mothers of infants hospitalized for CHD and should be considered when measuring interventions to reduce parents’ stress response.
Our participants reported increased feelings of attachment to their infants during and after SSC in the preoperative period by VAS and by the MAI. In the postoperative period, significant increases were found by VAS, but not by the MAI, most likely due to a ceiling effect of the instrument, as means were very close to the instrument’s highest possible score. Mean MAI scores pre-SSC were relatively high when compared to other studies of mothers of infants with cCHD in outpatient settings, with reported means ranging in the mid-90’s (Boztepe et al., 2016; Ylmaz et al., 2011). The significant improvements in attachment found in our study provide objective support to the findings of Harrison and colleagues (2017) who performed a daily SSC intervention for 14-days on postoperative infants with cCHD in the hospital and at home. While they did not obtain objective measures from participants, themes of exit interviews with their participants demonstrated that participants felt SSC assisted with attachment despite the difficulties resulting from the infant surgery and postoperative care. They described SSC as a relaxing experience that assisted with stress reduction. One participant stated that SSC “allowed time to make me feel and remind me that I’m a mother” (p. E6), highlighting the important aspect of SSC in enhancing feelings of parental role (Harrison & Brown, 2017).
There were no significant differences in oxytocin levels during or after SSC, despite demonstrated changes in maternal salivary oxytocin found in other SSC studies (Cong et al., 2015; Vittner et al., 2018). We found a wide range of oxytocin values for participants at each time point, despite attempts made to minimize variability (samples were obtained at least one hour after lactation or pumping; samples were extracted as per assay guidelines). We used the same ELISA immunoassay kit reported in other studies measuring oxytocin (Cong et al., 2015; Feldman et al., 2014; Feldman et al., 2011; Vittner et al., 2018). However, these researchers also reported a wide range of oxytocin values in pg/mL. For example, Vitter and colleagues (2018) found mean maternal salivary oxytocin levels pre-SSC were 162 (SD=105), during SSC were 275 (SD=174), and post were 202 (SD=105). In comparison, Cong and colleagues (2015) reported much lower values, pre- (M=50.49, SD=20.05), during (M=57.95, SD=25.07), and post-SSC (M=51.50, SD=24.13). In further contrast, baseline maternal oxytocin values have been reported by other scientists in the single digits (M=6.16, SEM=.37) (Feldman et al., 2011). Our findings confirm doubts of researchers on the lack of specificity of the EIA assays for the measurement of oxytocin (Szeto et al., 2011). As McCullough and colleagues (2013) noted in their review of peripheral oxytocin measurement, assays may tag additional molecules other than oxytocin, which result in discrepant findings between studies. They purported that a standard for the accurate measurement of oxytocin is urgently needed in order to advance the science of oxytocin research (McCullough et al., 2013). In their review of oxytocin research, they demonstrated a 100 to 1000-fold discrepancy in levels, raising a concern regarding the accuracy of oxytocin measurement, particularly with unextracted samples. Samples in our study were extracted as per assay guidelines in an attempt to improve accuracy, but we still found an extremely wide range of values in the samples.
Implications
The significant reductions in stress response and improvements in attachment for participants in our study provide support for the use of SSC as a nurse-driven intervention in PCICUs. Nurses can collaborate with interdisciplinary health care providers in PCICUs to establish specific holding guidelines to standardize when and how infants can be held SSC, as one PCICU nursing team has documented (Torowicz et al., 2012) and others have advocated (Harrison, 2019; Peterson, 2018; Sood et al., 2016). A recent international benchmarking study found that 50% of PCICUs allowed infants to be held out of bed with transthoracic intracardiac catheters, an intravenous catheter used specifically after cardiac surgery (Lisanti, Fitzgerald, et al., 2019). Additional research describing the processes and guidelines required to support the safe holding of infants in PCICUs would add valuable information to the literature that could inform nursing practice.
Nurses can provide education to mothers about the reality of parental role alteration in the PCICU and the potential for disruption in the mother-infant attachment process. Additionally, nurses can recommend SSC as an essential strategy for mitigating parental role alteration, supporting attachment, and decreasing maternal anxiety symptoms. Sood and colleagues (2018) found that mothers felt supported by nurses who encouraged parent participation in care. Nurses working in PCICUs are uniquely positioned to support mothers in this way by advocating for SSC as an essential developmental care strategy to enhance maternal engagement in care and decrease parental role alteration.
Limitations
As with any pilot study, the results reported here should be interpreted with caution. Generalizability is limited by our small sample of mothers with homogeneous characteristics from one hospital setting. Future researchers should include participants with increased diversity in race, ethnicity, socio-economic status, and location. In addition, future studies would be strengthened by the inclusion of a control group or a comparison group receiving a similar intervention such as blanket holding rather than within-subject comparisons. Effect sizes generated from our study can be used for power calculations in future studies, although we only examined the effect of SSC at two distinct time points. Longitudinal studies assessing the overall “dose” required for long-term effects on maternal mental health and attachment are needed.
Conclusion
Our findings provide foundational evidence of the benefits of SSC for mothers of infants with cCHD before and after neonatal cardiac surgery. Our results supported the relationships of parental role alteration and maternal stress response as posited by the PCICU Parental Stress Model. Nurses can integrate SSC into the care of infants admitted preoperatively for neonatal cardiac surgery and continue to support its use as soon as the infant is safe to be held postoperatively. More research is needed to determine whether early and regular SSC between mothers and infants with cCHD results in long-term mental health benefits and increased attachment throughout infancy and into childhood.
Acknowledgement
Dr. Lisanti is a scholar of the Association of PeriOperative Registered Nurses and Stryker of the American Nurses Foundation. Dr. Lisanti was supported by Grant T32NR007100 from the NINR.
Footnotes
Disclosure The authors report no conflicts of interest or relevant financial relationships.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amy J. Lisanti, Cardiac Nursing and the Center for Pediatric Nursing Research and Evidence-based Practice, Children’s Hospital of Philadelphia, Philadelphia, PA; University of Pennsylvania School of Nursing, Philadelphia, PA..
Abigail C. Demianczyk, Department of Child and Adolescent Psychiatry and Behavioral Sciences, Children’s Hospital of Philadelphia, Philadelphia, PA..
Andrew Costarino, Division Chief of Cardiac Critical Care Medicine, Children's Hospital of Philadelphia, Philadelphia, PA; Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA..
Maria G. Vogiatzi, Division of Endocrinology and Diabetes, Children's Hospital of Philadelphia, Philadelphia, PA; Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA..
Rebecca Hoffman, Laboratory of Innovative & Translational Nursing Research, University of Pennsylvania School of Nursing, Philadelphia, PA..
Ryan Quinn, Office of Nursing Research, University of Pennsylvania School of Nursing, Philadelphia, PA..
Jesse L. Chittams, Biostatistics Analysis Core, Office of Nursing Research, University of Pennsylvania School of Nursing, Philadelphia, PA..
Barbara Medoff-Cooper, University of Pennsylvania School of Nursing, Philadelphia, PA..
References
- Alley J, Diamond LM, Lipschitz DL, & Grewen K (2019). Associations between oxytocin and cortisol reactivity and recovery in response to psychological stress and sexual arousal. Psychoneuroendocrinology, 106, 47–56. 10.1016/j.psyneuen.2019.03.031 [DOI] [PubMed] [Google Scholar]
- Amanullah MM, Hamid M, Hanif HM, Muzaffar M, Siddiqui MT, Adhi F, Ahmad K, Khan S, & Hasan Z (2016). Effect of steroids on inflammatory markers and clinical parameters in congenital open heart surgery: A randomised controlled trial. Cardiology in the Young, 26(3), 506–515. 10.1017/S1047951115000566 [DOI] [PubMed] [Google Scholar]
- Bigelow A, Power M, MacLellan-Peters J, Alex M, & McDonald C (2012). Effect of mother/infant skin-to-skin contact on postpartum depression symptoms and maternal physiological stress. Journal of Obstetric, Gynecologic, & Neonatal Nursing, 41(3), 369–382. 10.1111/j.1552-6909.2012.01350.x [DOI] [PubMed] [Google Scholar]
- Boztepe H, Ay A, Kerimoglu Yildiz G, & Cinar S (2016). Does the visibility of a congenital anomaly affect maternal-infant attachment levels? Journal for Specialists in Pediatric Nursing, 21(4), 200–211. 10.1111/jspn.12157 [DOI] [PubMed] [Google Scholar]
- Butler SC, Huyler K, Kaza A, & Rachwal C (2017). Filling a significant gap in the cardiac ICU: Implementation of individualised developmental care. Cardiology in the Young, 27(9), 1797–1806. 10.1017/S1047951117001469 [DOI] [PubMed] [Google Scholar]
- Cantwell-Bartl AM, & Tibballs J (2013). Psychosocial experiences of parents of infants with hypoplastic left heart syndrome in the PICU. Pediatric Critical Care Medicine, 14(9), 869–875. 10.1097/PCC.0b013e31829b1a88 [DOI] [PubMed] [Google Scholar]
- Cavalcanti PE, Sa MP, Santos CA, Esmeraldo IM, Chaves ML, Lins RF, & Lima Rde C (2015). Stratification of complexity in congenital heart surgery: comparative study of the Risk Adjustment for Congenital Heart Surgery (RACHS-1) method, Aristotle basic score and Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery (STS-EACTS) mortality score. Revista Brasileira de Cirurgia Cardiovascular, 30(2), 148–158. 10.5935/1678-9741.20150001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ES, Kim SJ, Kwon MS, Cho H, Kim EH, Jun EM, & Lee S (2016). The Effects of Kangaroo Care in the Neonatal Intensive Care Unit on the Physiological Functions of Preterm Infants, Maternal-Infant Attachment, and Maternal Stress. Journal of Pediatric Nursing, 31(4), 430–438. 10.1016/j.pedn.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Cong X, Ludington-Hoe SM, Hussain N, Cusson RM, Walsh S, Vazquez V, Briere CE, & Vittner D (2015). Parental oxytocin responses during skin-to-skin contact in preterm infants. Early Human Development, 91(7), 401–406. 10.1016/j.earlhumdev.2015.04.012 [DOI] [PubMed] [Google Scholar]
- Cong X, Ludington-Hoe SM, & Walsh S (2011). Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biological Research for Nursing, 13(2), 204–216. 10.1177/1099800410385839 [DOI] [PubMed] [Google Scholar]
- Fang L, & Hung CH (2012). Couples' postpartum health status. Journal of Clinical Nursing, 21(17-18), 2538–2544. 10.1111/j.1365-2702.2012.04104.x [DOI] [PubMed] [Google Scholar]
- Feldman R, Golan O, Hirschler-Guttenberg Y, Ostfeld-Etzion S, & Zagoory-Sharon O (2014). Parent-child interaction and oxytocin production in pre-schoolers with autism spectrum disorder. British Journal of Psychiatry, 205(2), 107–112. 10.1192/bjp.bp.113.137513 [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, & Zagoory-Sharon O (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Developmental Science, 14(4), 752–761. 10.1111/j.1467-7687.2010.01021.x [DOI] [PubMed] [Google Scholar]
- Franich-Ray C, Bright MA, Anderson V, Northam E, Cochrane A, Menahem S, & Jordan B (2013). Trauma reactions in mothers and fathers after their infant's cardiac surgery. Journal of Pediatric Psychology, 38(5), 494–505. 10.1093/jpepsy/jst015 [DOI] [PubMed] [Google Scholar]
- Garfield CF, Simon CD, Rutsohn J, & Lee YS (2018). Stress from the neonatal intensive care unit to home: Paternal and maternal cortisol rhythms in parents of premature infants. Journal of Perinatal and Neonatal Nursing, 32(3), 257–265. 10.1097/JPN.0000000000000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzolo D, Masetti P, & Meli M (2000). Kangaroo care improves post-extubation cardiorespiratory parameters in infants after open heart surgery. Acta Paediatrica, 89(6), 728–729. 10.1080/080352500750044098 [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, & Feldman R (2010). Oxytocin and the development of parenting in humans. Biological Psychiatry, 68(4), 377–382. 10.1016/j.biopsych.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM (2019). Improving neurodevelopment in infants with complex congenital heart disease. Birth Defects Research, 111(15), 1128–1140. 10.1002/bdr2.1517 [DOI] [PubMed] [Google Scholar]
- Harrison TM, & Brown R (2017). Autonomic nervous system function after a skin-to-skin contact intervention in infants with congenital heart disease. Journal of Cardiovascular Nursing, 32 (5), E1–E13. 10.1097/JCN.0000000000000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Chen CY, Stein P, Brown R, & Heathcock JC (2019). Neonatal skin-to-skin contact: Implications for learning and autonomic nervous system function in infants with congenital heart disease. Biological Research in Nursing, 21(3), 296–306. 10.1177/1099800419827599 [DOI] [PubMed] [Google Scholar]
- Hertzog MA (2008). Considerations in determining sample size for pilot studies. Research in Nursing & Health, 31(2), 180–191. 10.1002/nur.20247 [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, White-Traut RC, Levy JA, O'Shea TM, Geraldo V, & David RJ (2014). Maternally administered interventions for preterm infants in the NICU: Effects on maternal psychological distress and mother-infant relationship. Infant Behavior & Development, 37(4), 695–710. 10.1016/j.infbeh.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland LC, Pickler RH, McCain NL, Glaser D, & Lewis M (2011). Exploring biobehavioral outcomes in mothers of preterm infants. American Journal of Maternal Child Nursing, 36(2), 91–97. 10.1097/NMC.0b013e318205587e [DOI] [PubMed] [Google Scholar]
- Jordan B, Franich-Ray C, Albert N, Anderson V, Northam E, Cochrane A, & Menahem S (2014). Early mother-infant relationships after cardiac surgery in infancy. Archives of Disease in Childhood, 99(7), 641–645. 10.1136/archdischild-2012-303488 [DOI] [PubMed] [Google Scholar]
- Li YP, Huang J, Huang SG, Xu YG, Xu YY, Liao JY, Feng X, Zhang XG, Wang JH, & Wang J (2014). The compromised inflammatory response to bacterial components after pediatric cardiac surgery is associated with cardiopulmonary bypass-suppressed toll-like receptor signal transduction pathways. Journal of Critical Care, 29(2), 312 e317–313. 10.1016/j.jcrc.2013.10.008 [DOI] [PubMed] [Google Scholar]
- Lisanti AJ (2018). Parental stress and resilience in CHD: A new frontier for health disparities research. Cardiology in the Young, 28(9), 1142–1150. 10.1017/S1047951118000963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti AJ, Allen LR, Kelly L, & Medoff-Cooper B (2017). Maternal stress and anxiety in the pediatric cardiac intensive care unit. American Journal of Critical Care, 26(2), 118–125. 10.4037/ajcc2017266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti AJ, Cribben J, Connock EM, Lessen R, & Medoff-Cooper B (2016). Developmental care rounds: An interdisciplinary approach to support developmentally appropriate care of infants born with complex congenital heart disease. Clinics in Perinatology, 43(1), 147–156. 10.1016/j.clp.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Lisanti AJ, Demianczyk AC, Costarino A, Vogiatzi MG, Hoffman R, Quinn R, Chittams JL, & Medoff-Cooper B (2020). Skin-to-skin care is a safe and effective comfort measure for infants before and after neonatal cardiac surgery. Pediatric Critical Care Medicine, 21(9), e834–e841. 10.1097/PCC.0000000000002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti AJ, Fitzgerald J, Helman S, Dean S, Sorbello A, & Griffis H (2019). Nursing practice with transthoracic intracardiac catheters in children: International benchmarking study. American Journal of Critical Care, 28(3), 174–181. 10.4037/ajcc2019350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti AJ, Golfenshtein N, & Medoff-Cooper B (2017). The Pediatric Cardiac Intensive Care Unit Parental Stress Model: Refinement using directed content analysis. Advances in Nursing Science, 40(4), 319–336. 10.1097/ANS.0000000000000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti AJ, Vittner D, Medoff-Cooper B, Fogel J, Wernovsky G, & Butler S (2019). Individualized family-centered developmental care: An essential model to address the unique needs of infants with congenital heart disease. Journal of Cardiovascular Nursing, 34(1), 85–93. 10.1097/JCN.0000000000000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludington-Hoe SM (2011). Thirty years of kangaroo care science and practice. Neonatal Network, 30(5), 357–362. 10.1891/0730-0832.30.5.357 [DOI] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, & Mendez AJ (2013). Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neuroscience & Biobehavioral Reviews, 37(8), 1485–1492. 10.1016/j.neubiorev.2013.04.018 [DOI] [PubMed] [Google Scholar]
- McCusker CG, Doherty NN, Molloy B, Casey F, Rooney N, Mulholland C, Sands A, Craig B, & Stewart M (2007). Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Archives of Disease in Childhood, 92(2), 137–141. 10.1136/adc.2005.092320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, Craig B, Stewart M, & Casey F (2010). A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child: Care, Health, and Development, 36(1), 110–117. 10.1111/j.1365-2214.2009.01026.x [DOI] [PubMed] [Google Scholar]
- Miles MS, & Brunssen SH (2003). Psychometric properties of the parental stressor scale: Infant hospitalization. Advances in Neonatal Care, 3, 189–196. 10.1016/S1536-0903(03)00138-3 [DOI] [PubMed] [Google Scholar]
- Miller TA, Lisanti AJ, Witte MK, Elhoff JJ, Mahle WT, Uzark KC, Alexander N, & Butler SC (2020). A collaborative learning assessment of developmental care practices for infants in the cardiac intensive care unit. Journal of Pediatrics, 220, 93–100. 10.1016/j.jpeds.2020.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ER, Bergman N, Anderson GC, & Medley N (2016). Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews, 11, CD003519 10.1002/14651858.CD003519.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib AN, Tobias JD, Hall MW, Cismowski MJ, Miao Y, Barry N, Preston T, Galantowicz M, & Hoffman TM (2013). The role of different anesthetic techniques in altering the stress response during cardiac surgery in children: a prospective, double-blinded, and randomized study. Pediatr Critical Care Medicine, 14(5), 481–490. 10.1097/PCC.0b013e31828a742c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu M, Hazel NA, Robinson J, Schmiege SJ, & Laudenslager M (2014). Effect of holding on co-regulation in preterm infants: a randomized controlled trial. Early Human Development, 90(3), 141–147. 10.1016/j.earlhumdev.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, Hamilton L, Peterson ED, Mavroudis C, & Edwards FH (2009). An empirically based tool for analyzing mortality associated with congenital heart surgery. Journal of Thoracic and Cardiovascular Surgery, 138(5), 1139–1153. 10.1016/j.jtcvs.2009.03.071 [DOI] [PubMed] [Google Scholar]
- Peterson JK (2018). Supporting optimal neurodevelopmental outcomes in infants and children with congenital heart disease. Critical Care Nurse, 38(3), 68–74. 10.4037/ccn2018514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JK, & Evangelista LS (2017). Developmentally supportive care in congenital heart disease: A concept analysis. Journal of Pediatric Nursing, 36, 241–247. 10.1016/j.pedn.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurements, 1, 385–401. [Google Scholar]
- Rempel GR, Ravindran V, Rogers LG, & Magill-Evans J (2013). Parenting under pressure: a grounded theory of parenting young children with life-threatening congenital heart disease. Journal of Advanced Nursing, 69(3), 619–630. 10.1111/j.1365-2648.2012.06044.x [DOI] [PubMed] [Google Scholar]
- Rychik J, Donaghue DD, Levy S, Fajardo C, Combs J, Zhang X, Szwast A, & Diamond GS (2013). Maternal psychological stress after prenatal diagnosis of congenital heart disease. Journal of Pediatrics, 162(2), 302–307 e301. 10.1016/j.jpeds.2012.07.023 [DOI] [PubMed] [Google Scholar]
- Solberg O, Dale MT, Holmstrom H, Eskedal LT, Landolt MA, & Vollrath ME (2011). Emotional reactivity in infants with congenital heart defects and maternal symptoms of postnatal depression. Archives of Women's Mental Health, 14(6), 487–492. 10.1007/s00737-011-0243-1 [DOI] [PubMed] [Google Scholar]
- Solberg O, Gronning Dale MT, Holmstrom H, Eskedal LT, Landolt MA, & Vollrath ME (2012). Trajectories of maternal mental health: a prospective study of mothers of infants with congenital heart defects from pregnancy to 36 months postpartum. Journal of Pediatric Psychology, 37(6), 687–696. 10.1093/jpepsy/jss044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood E, Berends WM, Butcher JL, Lisanti AJ, Medoff-Cooper B, Singer J, Willen E, & Butler S (2016). Developmental care in north american pediatric cardiac intensive care units: Survey of current practices. Advances in Neonatal Care, 16(3), 211–219. 10.1097/ANC.0000000000000264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood E, Karpyn A, Demianczyk AC, Ryan J, Delaplane EA, Neely T, Frazier AH, & Kazak AE (2018). Mothers and fathers experience stress of congenital heart disease differently: Recommendations for pediatric critical care. Pediatric Critical Care Medicine, 19(7), 626–634. 10.1097/PCC.0000000000001528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press. [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, & Mendez AJ (2011). Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine, 73(5), 393–400. 10.1097/PSY.0b013e31821df0c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torowicz D, Lisanti AJ, Rim JS, & Medoff-Cooper B (2012). A developmental care framework for a cardiac intensive care unit: a paradigm shift. Advances in Neonatal Care, 12 Suppl 5(5S), S28–32. 10.1097/ANC.0b013e318265aeef [DOI] [PubMed] [Google Scholar]
- Turpeinen U, & Hamalainen E (2013). Determination of cortisol in serum, saliva and urine. Best Practice & Research: Clinical Endocrinology & Metabolism, 27(6), 795–801. 10.1016/j.beem.2013.10.008 [DOI] [PubMed] [Google Scholar]
- Verrall CE, Blue GM, Loughran-Fowlds A, Kasparian N, Gecz J, Walker K, Dunwoodie SL, Cordina R, Sholler G, Badawi N, & Winlaw D (2019). 'Big issues' in neurodevelopment for children and adults with congenital heart disease. Open Heart, 6(2), e000998 10.1136/openhrt-2018-000998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittner D, Butler S, Smith K, Makris N, Brownell E, Samra H, & McGrath J (2019). Parent engagement correlates with parent and preterm infant oxytocin release during skin-to-skin contact. Advances in Neonatal Care, 19(1), 73–79. 10.1097/ANC.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Vittner D, McGrath J, Robinson J, Lawhon G, Cusson R, Eisenfeld L, Walsh S, Young E, & Cong X (2018). Increase in oxytocin from skin-to-skin contact enhances development of parent-infant relationship. Biological Research for Nursing, 20(1), 54–62. 10.1177/1099800417735633 [DOI] [PubMed] [Google Scholar]
- Wernovsky G, & Licht DJ (2016). Neurodevelopmental outcomes in children with congenital heart disease-What can we impact? Pediatric Critical Care Medicine, 17(8 Suppl 1), S232–242. 10.1097/PCC.0000000000000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf-King SE, Anger A, Arnold EA, Weiss SJ, & Teitel D (2017). Mental health among parents of children with critical congenital heart defects: A systematic review. Journal of the American Heart Association, 6(2), e004862 10.1161/JAHA.116.004862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylmaz HB, Kavlak O, Isler A, Liman T, & Van Sell SL (2011). A study of maternal attachment among mothers of infants with congenital anomalies in Turkey. Infants & Young Children, 24(3), 259–266. 10.1097/IYC.0b013e31821b465b [DOI] [Google Scholar]


