Abstract
Background:
There is limited information on perinatal outcomes in HIV-HBV coinfection.
Methods:
HPTN 046 was a randomized double-blind placebo-controlled trial of perinatal transmission that evaluated 6 months of infant nevirapine vs placebo among breast-fed infants. Women living with HIV and their infants enrolled in sub-Saharan Africa from 2007-2010; 78% received antiretroviral therapy (ART). Maternal samples were tested for hepatitis B surface antigen (HBsAg). High and low HBV VL was defined as ≥106 IU/ml and <106 IU/ml. The association between HIV-HBV coinfection and maternal and infant outcomes was assessed using multivariate (MV) logistic and Cox regression.
Results:
Among 2025 women, 88 (4.3%) had HBV. HIV-HBV women with high HBV VL had lower median CD4, versus HIV alone or HIV-HBV women with low HBV VL (320, 490, and 434 cells/mm3, respectively (p<0.007)). In MV analysis, adjusted for maternal CD4, age, and maternal ART, infants born to women with high HBV VL were more likely to be low birth weight (LBW), versus HIV+/HBV− and low HBV VL women: [30% (3/10) vs 10% (194/1953) vs 6% (5/78), respectively, p=0.03). High HBV VL was associated with HIV perinatal transmission [(Hazard Ratio 6.75 (95% CI 1.86 – 24.50)]. There was no impact on infant mortality or maternal outcomes at 18 months.
Conclusions:
In HIV-HBV women, high HBV viral loads increase the risk of LBW and potentially HIV perinatal transmission. Reduction of antepartum HBV viremia may have beneficial effects beyond the prevention of HBV perinatal transmission.
Keywords: Pregnancy, low birth weight, HBV Viral Load, HIV/HBV coinfection, Sub-saharan Africa
Introductory Statement:
Although HBV coinfection occurs in 3-9% [1–4] of pregnant women living with HIV in sub-Saharan Africa, there is little information on perinatal and infant outcomes in this population, including HIV transmission. In an HIV perinatal transmission study in India where women received single dose nevirapine but no other antepartum ART, researchers found similar rates of HIV transmission at one year in infants born to HIV monoinfected mothers when compared to HIV/HBV coinfected mothers (13% vs 9%, p=0.79) but a non-statistically significant trend towards increased infant mortality.[5]
With regards to non-HIV outcomes, some HBV monoinfection studies have shown a correlation between maternal HBV infection and adverse perinatal outcomes, including low birth weight, preterm labor, and miscarriages, while others have not.[6–10] One large study from China, however, did describe an association with an elevated risk of preterm birth in HBeAg positive, compared to HBeAg-negative women [6]. This study did not include maternal HBV viral loads. Indeed, few studies have examined the impact of HBV viremia on maternal and infant outcomes despite the fact that HBV viremia is associated with long term adverse outcomes, including cirrhosis and hepatocellular carcinoma (HCC).[11, 12] Given the dearth of information on the influence of maternal HBV infection on maternal perinatal and infant outcomes, our objectives were to characterize the impact of HBV infection, stratified by maternal HBV viral loads, on infant HIV infection, mortality, and other adverse events from a sample and data repository of an HIV perinatal transmission study conducted in Sub-Saharan Africa, the HPTN 046 trial.
Experimental Procedures
HPTN 046 was a phase 3, randomized, double-blind, placebo-controlled trial that assessed the efficacy and safety of once-daily nevirapine to 6 months of age or until cessation of breastfeeding for the prevention of HIV perinatal transmission and mortality in HIV-exposed breastfeeding infants who were followed through 18 months of age. The methods and results of the parent study were published in 2012.[13] Briefly, the study had two protocol versions, v2.0 and v3.0. In protocol version 2.0, infants born to HIV-infected women were randomized at birth to receive once-daily nevirapine or placebo for six months. In response to new information from the Six-Week Extended Nevirapine (SWEN) trial[14], the protocol was modified to version 3.0 where infant randomization to nevirapine or placebo occurred at six weeks of age. Data and samples were available from both protocols and this analysis of HBV infected mothers includes both cohorts. All infants, regardless of their randomization, were included with the exception that infants with a birth HIV positive PCR were excluded as there was no additional data collected on these maternal-infant pairs. Pregnant women living with HIV from South Africa, Tanzania, Uganda, and Zimbabwe were enrolled within 7 days of delivery. Infant study visits were undertaken within 7 days postpartum, at 2, 5, 6, and 8 weeks, and at 3, 6, 9, 12, and 18 months. Infants who developed HIV-1 infection were taken off study drug but followed on study.
Sample Testing and Data Extraction
Maternal study entry and labor and delivery samples were stored regardless of randomization status. Archived maternal samples were retrospectively tested for HBsAg. Those that were identified as HBsAg positive were also assessed for HBeAg and HBV viral load at study entry and labor and delivery, respectively. High and low HBV VL was defined as ≥106 IU/ml and <106 IU/ml, respectively. These thresholds were selected given their association with adverse liver disease outcomes.[15] Data abstracted from the parent study database included: maternal age, CD4 T-cell count at delivery, antiretroviral receipt ever during pregnancy (yes/no), infant nevirapine randomization (none, 6 weeks of NVP, 6 months of NVP), and country of participation. Maternal HIV VL were not performed as part of the parent study and thus were unavailable for this analysis.
All specimens were stored at −80C. Serological testing for HBsAg was performed using Siemens ADVIA Centaur HBsAg assay (Siemens Healthineers, Los Angeles, CA, USA) at UCLA and GS HBsAg EIA 3.0 (ref 32591, Bio-Rad, Hercules, CA, USA) in Zimbabwe and Uganda. HBeAg testing was performed using ETI-EBK PLUS (DiaSorin, Stillwater, Minnesota, USA) at UCLA. HBV DNA testing was done with the Abbott DNA assay with lower limit of detection of 50 IU/ml at UCLA.
Statistical Analysis
Primary infant outcomes included the cumulative incidence of HIV transmission assessed as infant HIV PCR positivity through 12 months of life, infant mortality through 12 months of life, LBW (defined as <2500 g at birth), congenital malformations, and a composite endpoint of HIV infection or LBW. Primary maternal outcomes were Caesarian-section, episiotomy or primary tears, and prolonged rupture of membranes. Univariate analyses were performed, with the outcomes of interest as defined above. To compare continuous variables between maternal HBV status and its sub categories, Kruskal-Wallis or Wilcoxon rank sum test was used, and to compare categorical variables, chi-square or Fisher’s exact test was utilized. For the outcomes of infant HIV infection and death, multivariable model covariates included maternal age, maternal CD4 count at delivery, maternal receipt of ART during pregnancy (yes/no) and infant nevirapine treatment assignment defined with 3 levels (no NVP, 6 weeks of NVP, and 6 months of NVP). For infant outcomes that occurred before nevirapine initiation (infant birth weight, congenital malformations, maternal Caesarian section, episiotomy, and prolonged rupture of membranes) covariates included maternal age, maternal CD4 at delivery and maternal receipt of ART during pregnancy.
For HIV infection, we also conducted a time-to-event analysis to evaluate the relative risk of HIV infection between infants of HIV monoinfected and HIV/HBV coinfected mothers and to assess whether HBV viral load influenced the time to HIV infection. A Cox proportional hazard regression analysis was used to examine the impact of maternal HBV infection on HIV infection while adjusting for maternal age, maternal CD4 count, maternal receipt of ART and infant treatment group (no NVP vs 6 weeks of NVP vs 6 months of NVP). For many outcomes of interest, such as HIV infection and death, the events were rare and Firth’s penalized likelihood approach[16] was used to address issues of data separation, rare events, and bias of the parameter estimates in the logistic regression and Cox regression analysis.
The protocol was approved by the UCLA IRB 12-000123 and by institutional review boards/ medical ethics councils in Zimbabwe, Tanzania, South Africa, and Uganda. The parent study protocol IRB approval is referenced here.[13]
Results
The analysis cohort included 2025 mothers and 2050 infants (347 and 1678 mother-infant pairs from v2.0 and v3.0, respectively). 9 mothers and 9 infants were excluded due to infant HIV infection at birth, resulting in the inclusion of 2016 mothers with 2041 infants. (Figure 1) Infant nevirapine vs placebo randomization assignments were distributed similarly among all subgroups. Nonrandomized participants represent those mother-infant pairs who were enrolled but not randomized due to exclusion criteria, i.e. missed randomization window, maternal comorbid illness that precluded enrollment etc.
Figure 1: Flow diagram of HIV and HIV/HBV Women and Infants in HBV Analysis of HPTN 046.
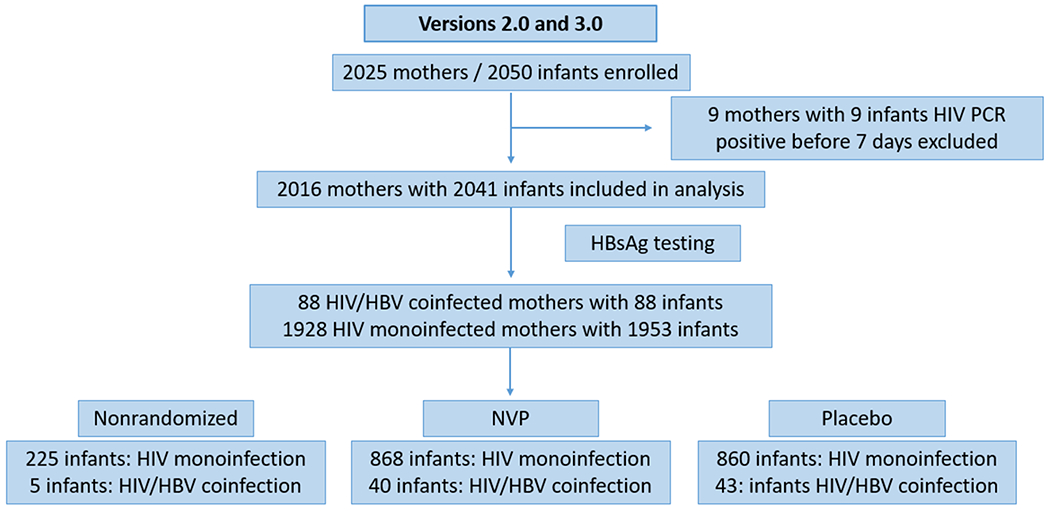
Abbreviations: HIV (Human Immunodifficiency Virus), HBV (Hepatitis B Virus), NVP (Nevirapine), HBsAg (Hepatitis B Surface Antigen), PCR (Polymerase Chain Reaction), HPTN (HIV Prevention Trials Network).
Baseline Demographics: (Table 1)
Table 1.
Baseline Characteristics of HIV and HIV/HBV Coinfected Participants in HPTN 046
| Variable | HIV | HBV VL <106 IU/ml | HBV VL ≥106 IU/ml | All Participants | *p Value | |
|---|---|---|---|---|---|---|
| Mother | 1928 | 78 | 10 | 2016 | ||
| Median Age (years) | 27 (23-31) | 27 (23-32) | 28.5 (27-32) | 27 (23-31) | 0.2413 | |
| Median CD4 (cells/mm3)1 | 490 (329-698) | 433.5 (263-609) | 319.5 (210-454) | 488 (326-695) | 0.0068 | |
| ART During Pregnancy2 | Yes | 1502(78) | 69(88) | 8(80) | 1579 | 0.0645 |
| Median Duration of ART2 (days) | 62 (41-85, N=1414) | 69 (43-99, N=65) | 93.5 (71.5-1236.5, N=8) | 63 (42-86, N=1487) | 0.0092 |
ART= Antiretroviral therapy
At maternal delivery.
Ever Receipt of ART during pregnancy, women could have been off of ART at time of delivery
p value represents a three-way comparison between the groups
After testing for HBsAg, eighty-eight (88/2016) women were HBsAg (+), representing 4.4% (95% CI 3.5% - 5.3%) of the cohort (Table 1). Among HIV/HBV women, 25% (22/88) were HBeAg-positive. Eighty-six per cent (19/22) of HBeAg-positive women had a detectable HBV VL (median 4.54 x 105 IU/ml). All three HBeAg-positive women with undetectable HBV DNA were on HBV-active ART, defined as receipt of lamivudine or tenofovir in the seven days prior to delivery. Seventy-five per cent (66/88) were HBeAg-negative; the median HBV VL for the 10 (15%) HBeAg-negative women with detectable viremia was 3225 IU/ml. Thirty-nine per cent (26/66) of HBeAg-negative women were receiving HBV-active ART.
Women with HBV VL of ≥106 IU/ml (high HBV VL) had lower CD4 counts, when compared to HIV alone or HBV VL < 106 IU/ml (low HBV VL) , 320, 490, and 434 cells/mm3 respectively (p<0.007). More HIV/HBV women (77/88, 88%) were receiving ART than those without HBV (1502/1928, 78%) (p=0.03) (data not shown in table). HIV/HBV women with high HBV VL also had longer ART duration, compared to mothers with low HBV VL (median time 93.5 vs 69 days, p=0.07). Similar results were seen when assessing by HBeAg status and a threshold of HBV VL of 105 IU/ml (data not shown).
In those women who were receiving ART up to seven days before delivery, the majority were on AZT alone (48% 736/1529) followed by 34% (517/1529) who were on triple ART regimens. Of these triple ART regimens, 98% (505/517) were on NNRTI-containing regimens.
Infant Outcomes (Table 2)
Table 2.
Infant Outcomes by Maternal HBV VL Status
| HBV VL <106 IU/ml vs HIV | HBV VL ≥106 IU/ml vs HIV | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome N=2041 | HIV N=1953 | HBV VL <106 IU/ml N=78 | p | Adj P | HBV VL ≥106 IU/ml N=10 | p | Adj P | Odds Ratio (OR) | |
| Low Birth Weight (grams) | >2500 | 1759(90) | 73(94) | 0.43 | 0.38 | 7(70) | 0.07 | 0.04 | 3.99(1.04-15.3) |
| ≤2500 | 194(10) | 5(6) | 3(30) | ||||||
| Median Birth Weight (kilograms) | 3.1 (2.8-3.4) | 3.2 (3-3.5) | 0.03 | 3.1 (2.4-3.5) | 0.94 | ||||
| 3.1 +/− 0.43 | 3.2 +/− 0.42 | 3.06 +/− 0.51 | |||||||
| Congenital Malformation | Yes | 83(4) | 2(3) | 0.77 | 0.68 | 0 | 1.00 | 0.96 | 1.07(0.05-21.0) |
| HIV Infection at Any Time | Yes | 71(4) | 0 | 0.11 | 0.23 | 2(20) | 0.05 | 0.01 | 6.89(1.48-32.1) |
| Death at Any Time | Yes | 75(4) | 0 | 0.11 | 0.18 | 0 | 1.00 | 1.00 | 1.00(0.05-19.5) |
| HIV Infection at Any Time/Low Birth Weight | Yes | 254(13) | 5(6) | 0.12 | 0.13 | 4(40) | 0.03 | 0.02 | 4.33(1.21-15.5) |
Table 2 represents the results of multivariable analysis (adjusted p value) and odds ratio where covariates for infant low birth weight and congenital malformations were maternal age, maternal CD4 at delivery, and maternal receipt of ART during pregnancy (yes/no). Odds ratio represents the comparison between women with HBV VL ≥106 IU/ml vs women with HIV alone. Covariates for infant HIV infection and death and the composite endpoint of HIV infection at any time and low birth weight were maternal age, maternal CD4 count at delivery, maternal receipt of ART during pregnancy (yes/no), and infant nevirapine treatment assignment (no NVP, 6 weeks of NVP, and 6 months of NVP).
In multivariable analysis, the incidence of infant LBW defined as <2500 g at birth was greatest in those women with high HBV VL, compared to those with low HBV VL and HIV infection alone, representing 30% (3/10), 6% (5/77), and 10% (194/1953), respectively. Women with high HBV VL, when compared to HIV infection alone, were more likely to have infants with low birth weight (OR 3.99 (1.04-15.3)).
The incidence of HIV infection at any time and composite endpoint of HIV Infection/LBW was similarly increased in high HBV VL women, compared to low HBV VL women and HIV infection alone; 20% (2/10) and 40% (4/10); 0% (0/78) and 6% (5/78); 4% (71/2041) and 13% (254/2041) respectively.
Women with high HBV VL, when compared to HIV infection alone, were more likely to have perinatal HIV transmission (OR 6.89 (1.48-32.1)). There was no statistically significant difference in congenital malformations or infant death amongst groups. No infants with LBW born to women with high HBV VL were exposed to maternal protease inhibitor containing ART.
Infant HIV Infection
Seventy-one infants (3.6%, 95% CI: 2.8%-4.5%) born to HIV monoinfected mothers were infected with HIV and two (2.3%, 95% CI: 0.3%-8.0%) infants born to HIV/HBV women were infected with HIV during study follow-up. The median age of infant HIV diagnosis was 126.5 days for all infants born to HIV monoinfected and HIV/HBV coinfected women. Infant HIV infection occurred at 7 and 27 days for infants who were born to HIV/HBV women. Of the two incident infant HIV infections, one occurred in a women on ART and one occurred in a women not on ART. The incidence of infant HIV infection was 3.6 % (71/1953), 20% (2/10), 0% (0/78) in HIV monoinfection, high HBV VL, and low HBV VL, respectively. Similar results were seen when assessing by HBeAg status and a threshold of HBV VL of 105 IU/ml (data not shown). Women with high HBV VL had a significantly increased risk of infant HIV infection compared to infants of HIV monoinfected mothers (OR 6.89 (1.48-32.1)). Similarly, in Cox regression analyses, the HR for HIV perinatal transmission in HIV/HBV with high HBV VL was 6.7 (95% CI 1.9-24.9). (not shown) No statistically significant difference was found for the risk of HIV infection between infants of HIV monoinfected mothers and those of HIV/HBV mothers with low HBV VL. In all models, maternal ART receipt during pregnancy, higher maternal CD4 count at baseline and infant receipt of NVP treatment for 6 weeks or 6 months was protective against HIV infection (p<0.05). Figure 2 represents the KM curve for infant time to HIV infection by high HBV VL thresholds. A sensitivity analysis was also performed such that HIV transmission event time and follow-up time were censored at the time of breastfeeding cessation; this yielded similar results.
Figure 2: Kaplan Meier curve for time to infant HIV infection by maternal HBV and HBV VL status.
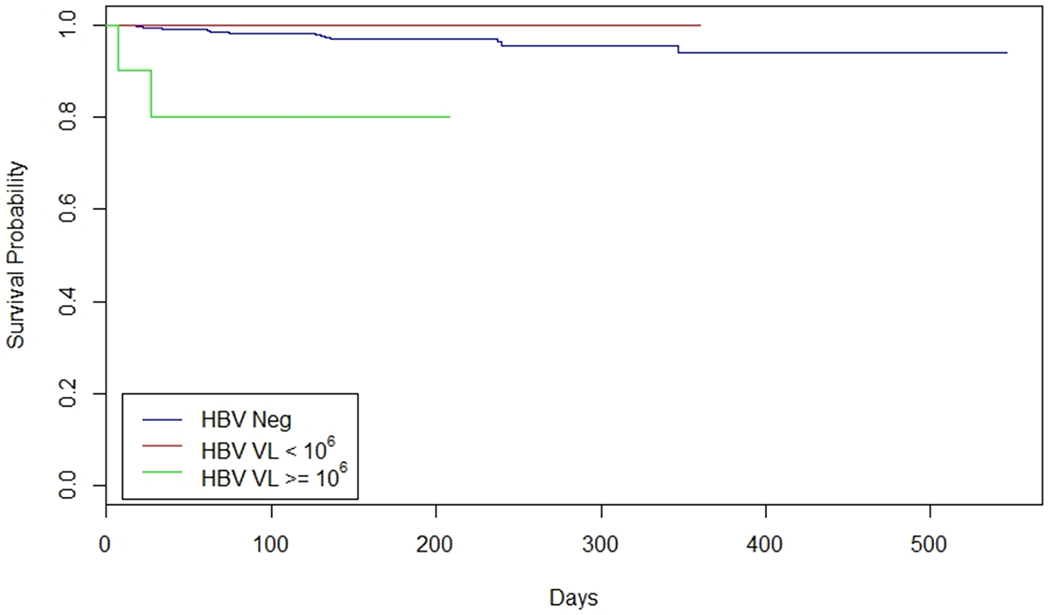
HIV events and follow-ups were censored at the end of mother’s breast feeding. Abbreviations: HBV (Hepatitis B Virus), HIV (Human Immunodifficiency Virus), VL (Viral Load).
Maternal Outcomes (Table 3)
Table 3.
Maternal Outcomes by Maternal HBV VL Status
| HBV VL <106 IU/ml vs HIV | HBV VL ≥106 IU/ml vs HIV | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HIV | HBV VL <106 IU/ml | p | Adj P | HBV VL ≥106 IU/ml | p | Adj P | |
| Duration of Ruptured Membranes (hrs) | ≤4 | 1493(80) | 68(92) | 0.01 | 0.02 | 6(60) | 0.12 | 0.11 |
| >4 | 365(20) | 6(8) | 4(40) | |||||
| Birth Type | Vaginal | 1613(84) | 61(78) | 0.21 | 0.52 | 8(80) | 0.67 | 0.83 |
| Caesarian | 314(16) | 17(22) | 2(20) | |||||
| Episiotomy or Primary Tears | Yes | 682(35) | 28(37) | 0.81 | 0.37 | 5(50) | 0.34 | 0.25 |
There was no difference in incidence of prolonged rupture of membranes (PROM), Caesarian section or the proportion with episiotomies when comparing high HBV VL and HIV monoinfection. More women living with HIV alone had PROM (20% vs 8%), compared to women living with HIV and low HBV VL but this is of unclear clinical significance.
We performed separate analyses with a maternal HBV viral load threshold of 105 IU/ml and HBeAg status for maternal and infant outcomes and found similar results (results not shown).
Discussion
In summary, we found that high maternal HBV viral loads at delivery were associated with infant LBW and incident HIV infection, although event numbers were small. Our findings of increased incidence of LBW are supported by other studies. In a large Thai study of 1446 HBV infected women, HBeAg positive women had a higher incidence of low birth weight, compared to HBeAg negative women, 22% vs 14% p=0.005, respectively.[17] Preterm birth is associated with low birth weight [18] and a large Chinese study of 489,965 pregnant women, 20,827 of whom had HBV infection, demonstrated that maternal HBV infection was associated with a higher risk of preterm birth.[6] Indeed, in an accompanying editorial[19], the authors postulated that HBV viremia may be the etiologic agent of these adverse perinatal events. Our findings are one of the first to support this hypothesis that maternal HBV viremia itself is associated with adverse perinatal outcomes.
The etiologies of LBW are multifactorial, including preterm birth (birth before 37 weeks), intrauterine growth restriction (IUGR), and congenital malformations or genetic factors.[20] HIV itself is associated with LBW as is the use of protease inhibitor-containing ART during pregnancy, at least in some studies.[21] Placental insufficiency is thought to be a common pathogenetic pathway. Hypotheses include the possibilities that HBV can cause chorion angiopathy which in turn decreases placental function[22] and that HBV may accumulate in the placenta initiating a placental inflammatory response.[17]
The finding that incident HIV infection was increased in HIV/HBV exposed infants was notable. HIV viral load, the primary predictor for HIV perinatal transmission, was not available to us and we cannot exclude the possibility that HIV viremia contributed to these incident HIV infections and low birth weight. However, one of the two incident HIV infections occurred in a woman on ART and our multivariable analyses included both CD4 count and maternal receipt of ART, both of which are proxies for HIV viral load.[23, 24] Also, the findings of increased LBW and incident HIV infection amongst women with high HBV viral loads are supported by large studies in HBV monoinfection which reported similar associations with HBeAg-emia, a proxy for high HBV viral loads, and other adverse events, including low birth weight and pre-term labor.[17, 18] The fact that these two infant HIV infections occurred early suggests that there was a relationship to maternal factors at delivery, including HBV viral load. Other potential etiologies may include accelerated placental inflammation (as described above) and enhanced immunosuppression from HBV. Authors of one study who found a lower incidence of pre-eclampsia in HBV monoinfected pregnant women hypothesized that the increase in circulating regulatory T cells, which induces immunotolerance in chronic HBV, may also be responsible for generalized immunosuppression.[25]
Limitations of our study included the age of the parent study which enrolled between 2007-2010 prior to current treatment guidelines whereby pregnant women are treated with triple drug therapy, generalizability to non-African populations in low and middle income countries (LMIC), the lack of HIV viral load information as detailed above, and small numbers of events which led to the conclusions (the 95% CI for HIV infection was (1.86, 24.5). If HIV viremia had also been associated with preterm birth and incident HIV, we would still need to explore the possibility that concomitant HBV viremia also contributed to these events, given the results of other supporting studies and pathophysiologic mechanisms detailed here. Finally, although this study occurred in a time before widespread HBV-active ART for pregnant women living with HIV where antepartum therapy can lower maternal HBV viral loads to below 106 IU/ml [26], the combination of low antepartum HBV screening rates in LMIC [27] and the advent of new ART regimens without HBV activity make the study of uncontrolled HBV viremia in HIV infection still relevant. Generalizability of study results to other populations is always a concern although it is notable that similar findings of adverse perinatal outcomes in HBV-monoinfected women were seen in a large recent Chinese study.[6]
Our finding that maternal HBV replication may increase adverse perinatal outcomes has implications beyond HIV/HBV coinfection. The current US guidance for the management and timing of antiviral therapy for antepartum monoinfection with HBV maternal viremia is based on HBV viral loads that are predictive of HBV perinatal transmission.[28] If our findings are corroborated by larger studies in HBV monoinfection, this may suggest that antepartum antiviral therapy for HBV be initiated earlier in pregnancy in order to decrease HBV viral loads prior to the third trimester to avoid adverse infant outcomes, independent of HBV perinatal transmission. Future research should investigate comparisons between HBV monoinfected and HIV/HBV coinfected cohorts and the mechanisms behind increased low birth weight and incident HIV infection.
Acknowledgments
Conflicts of Interest and Source of Funding:
Dr. Bhattacharya: Dr. Bhattacharya was on the protocol teams for studies within the AIDS Clinical Trials Group (ACTG) for which Abbvie and Regeneron donated or will donate study drug. For the remaining authors none were declared.
Funding: This work was supported by the following sources of support. HIV Prevention Trials Network (HPTN) 046 (ClinicalTrials.gov Identifier: NCT00074412) was funded by the US National Institutes of Health (NIH), initially through the HPTN and later through the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group. The HPTN (U01AI46749) has been funded by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Drug Abuse (NIDA), and National Institute of Mental Health (NIMH). The IMPAACT Group (U01AI068632) has been funded by NIAID, NICHD, and NIMH. Additional funding from NIAID R01 01AI100748-01 and NICHD R01 5R01HD085862-02 to DB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1).Fomulu NJ, Morfaw FL, Torimiro JN, Nana P, Koh MV, William T. Prevalence, correlates and pattern of Hepatitis B among antenatal clinic attenders in Yaounde-Cameroon: is perinatal transmission of HBV neglected in Cameroon? BMC Pregnancy and Childbirth 2013; 13:158. doi: 10.1186/1471-2393-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Rouet F, Chaix ML, Inwoley A, et al. HBV and HCV prevalence and viraemia in HIV-positive and HIV-negative pregnant women in Abidjan, Cote d’Ivoire: the ANRS 1236 study. Journal of medical virology 2004; 74:34–40. doi: 10.1002/jmv.20143. [DOI] [PubMed] [Google Scholar]
- 3).Chasela CS, Kourtis AP, Wall P, et al. Hepatitis B virus infection among HIV-infected pregnant women in Malawi and transmission to infants. Journal of hepatology 2014; 60:508–14. doi: 10.1016/j.jhep.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Andersson MI, Maponga TG, Ijaz S, et al. The epidemiology of hepatitis B virus infection in HIV-infected and HIV-uninfected pregnant women in the Western Cape, South Africa. Vaccine 2013; 31:5579–84. doi: 10.1016/j.vaccine.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Mave V, Kadam D, Kinikar A, et al. Impact of maternal hepatitis B virus coinfection on mother-to-child transmission of HIV. HIV medicine 2014; 15:347–54. doi: 10.1111/hiv.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Liu J, Zhang S, Liu M, Wang Q, Shen H, Zhang Y. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: a population-based cohort study. The Lancet Global Health 2017; 5:e624–e32. doi: 10.1016/S2214-109X(17)30142-0. [DOI] [PubMed] [Google Scholar]
- 7).Chen S, Yang Y, Qu Y, et al. Both maternal and paternal risk factors for term singleton low birthweight infants in rural Chinese population: a population-based, retrospective cohort study. Scientific reports 2018; 8:12539-. doi: 10.1038/s41598-018-30036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Cui A-M, Cheng X-Y, Shao J-G, et al. Maternal hepatitis B virus carrier status and pregnancy outcomes: a prospective cohort study. BMC pregnancy and childbirth 2016; 16:87. doi: 10.1186/s12884-016-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Lao TT, Chan BC, Leung WC, Ho LF, Tse KY. Maternal hepatitis B infection and gestational diabetes mellitus. Journal of hepatology 2007; 47:46–50. doi: 10.1016/j.jhep.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 10).Bajema KL, Stankiewicz Karita HC, Tenforde MW, Hawes SE, Heffron R. Maternal Hepatitis B Infection and Pregnancy Outcomes in the United States: A Population-Based Cohort Study. Open forum infectious diseases 2018; 5:ofy134. doi: 10.1093/ofid/ofy134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–86. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 12).Chen C-J, Yang H-I, Su J, et al. Risk of Hepatocellular Carcinoma Across a Biological Gradient of Serum Hepatitis B Virus DNA Level. Jama 2006; 295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 13).Coovadia HM, Brown ER, Fowler MG, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2012; 379:221–8. PMCID: PMC3539769.doi: 10.1016/s0140-6736(11)61653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Bedri A, Gudetta B, Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet (London, England) 2008; 372:300–13. doi: 10.1016/s0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 15).Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clinics in liver disease 2007; 11:797–816, viii. doi: 10.1016/j.cld.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16).Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Statistics in medicine 2002; 21:2409–19. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 17).Sirilert S, Traisrisilp K, Sirivatanapa P, Tongsong T. Pregnancy outcomes among chronic carriers of hepatitis B virus. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2014; 126:106–10. doi: 10.1016/j.ijgo.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 18).Nobile CG, Raffaele G, Altomare C, Pavia M. Influence of maternal and social factors as predictors of low birth weight in Italy. BMC public health 2007; 7:192. doi: 10.1186/1471-2458-7-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Huang Q-T, Zhong M. Maternal hepatitis B virus infection and risk of preterm birth in China. The Lancet Global Health 2017; 5:e563–e4. doi: 10.1016/S2214-109X(17)30175-4. [DOI] [PubMed] [Google Scholar]
- 20).WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization; Copyright (c) World Health Organization 2013. [PubMed] [Google Scholar]
- 21).Saleska JLTA, Maierhofer C, Clark J, Kwiek JJ. Use of Antiretroviral Therapy During Pregnancy and Adverse Birth Outcomes Among Women Living With HIV-1 in Low- and Middle-Income Countries: A Systematic Review. Journal of acquired immune deficiency syndromes (1999) 2018; 79:1–9. doi: 10.1097/QAI.0000000000001770. [DOI] [PubMed] [Google Scholar]
- 22).Yang H, Chen R, Li Z, et al. Analysis of fetal distress in pregnancy with hepatitis B virus infection. Zhonghua fu chan ke za zhi 2002; 37:211–3. [PubMed] [Google Scholar]
- 23).Njom Nlend AE, Same Ekobo C, Moyo ST, et al. Virological profile of pregnant HIV positive women with high levels of CD4 count in low income settings: can viral load help as eligibility criteria for maternal triple ARV prophylaxis (WHO 2010 option B)? Pan Afr Med J 2011; 10:27. doi: 10.4314/pamj.v10i0.72239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Venet A, Lu W, Beldjord K, Andrieu JM. Correlation between CD4 cell counts and cellular and plasma viral load in HIV-1-seropositive individuals. AIDS (London, England) 1991; 5:283–8. doi: 10.1097/00002030-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 25).Lao TT, Sahota DS, Cheng YKY, Law LW, Leung TY. Maternal hepatitis B surface antigen status and incidence of pre-eclampsia. Journal of viral hepatitis 2013; 20:343–9. doi: 10.1111/jvh.12037. [DOI] [PubMed] [Google Scholar]
- 26).Wang L, Wiener J, Bulterys M, et al. Hepatitis B Virus (HBV) Load Response to 2 Antiviral Regimens, Tenofovir/Lamivudine and Lamivudine, in HIV/ HBV-Coinfected Pregnant Women in Guangxi, China: The Tenofovir in Pregnancy (TiP) Study. The Journal of Infectious Diseases 2016; 214:1695–9. doi: 10.1093/infdis/jiw439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Chotun N, Preiser W, van Rensburg CJ, et al. Point-of-care screening for hepatitis B virus infection in pregnant women at an antenatal clinic: A South African experience. PloS one 2017; 12:e0181267. doi: 10.1371/journal.pone.0181267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology (Baltimore, Md) 2018; 67:1560–99. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]


