Abstract
Background
Despite the superiority of mitral valve repair (MVr) over replacement for degenerative disease, repair rates vary widely across centers. Traveling to a Mitral Reference Center (MRC) is one way to increase the odds of MVr. This study assessed the economic value (quality/cost) and long-term outcomes of distant referral to a MRC.
Methods
Among 746 mitral surgery patients between January 2011–June 2013, low-risk patients with ejection fraction>40% undergoing isolated degenerative MVr were identified and included 26 out-of-state (DISTANT) and 104 in-state patients (LOCAL). Short- and long-term outcomes and institutional financial data (including travel expenses) were used to compare groups. National average and MRC-specific MVr rates, clinical outcomes, and marginal value of quality-adjusted life years collected from STS database and Medicare estimates were used to perform a nationally-representative cost-benefit analysis for distant referral.
Results
Age, ejection fraction, operative time, blood transfusions, and annuloplasty ring size did not differ between groups. Median charges were $76,022 for LOCAL and $74,171 for DISTANT (p=0.35), while median payments (including travel expenses) were $57,795 for LOCAL and $58,477 for DISTANT (p=0.70). Short- and long-term outcomes were similar between groups and median follow-up was 7.1 years. Estimated 5-year survival was 97% (96% for LOCAL and 100% for DISTANT; p=0.24). Cost-benefit analysis showed a net benefit through distant referral to a MRC ranging from $436–$6,078 to the payor and $22,163–$30,067 to the patient, combining for an estimated $22,599–$32,528 societal benefit.
Conclusions
These data suggest that distant referral to a MRC is achievable and reasonable.
Mitral valve repair (MVr) is the standard of care and a quality marker for treatment of degenerative mitral valve (MV) disease. Numerous studies have reported superior survival and outcomes for patients undergoing repair compared to replacement for degenerative disease.1–7 While the American Heart Association/American College of Cardiology (AHA/ACC) Valvular Guidelines include recommendations that complex MVr be performed at a Heart Valve Center of Excellence,8 this designation is loosely defined and not used by referring physicians or patients for mitral valve surgery.9 To address this gap, the Mitral Foundation and AHA have created the Mitral Valve Repair Reference Center Award pilot program, defining a Mitral Reference Center as performing ≥50 degenerative repairs per year, <1% operative mortality for all MVr, and degenerative repair rate >95%, in addition to heart team, process, and best practice standards.10 Despite various recommendations for referral to experienced centers and surgeons8–13 and near universal consensus on the benefit of repair for degenerative disease, the national repair rate remains only 75%.14
While existing evidence supports improved clinical outcomes associated with MVr, less economic evidence exists to support distant referral to a reference center. Whether the possible increased costs of distant referral are offset by higher MVr rates has not been established. As healthcare expenditures rise to almost 18% of the U.S. gross domestic product,15 identifying opportunities to improve outcomes while controlling costs is important, especially in the context of emerging bundled payments and value-based reimbursement models.
To determine cost-effectiveness for patients, payors, and society, this study assessed the economic value (quality/cost) and long-term outcomes of distant referral to a reference center for degenerative MVr.
PATIENTS AND METHODS
This study was approved by the University of Michigan Institutional Review Board (HUM#00081443).
Data Sources
Our primary data source included the institutional component of the Society of Thoracic Surgeons (STS). Additional short- and long-term clinical, echocardiographic, and reoperative data were obtained during clinical in-person or telephone follow-up with patients, shared regional electronic medical records (EMR), and/or telephone calls with primary care physicians or cardiologists. Post-procedure echocardiographic data were obtained from intraoperative transesophageal echocardiogram and operative reports, while long-term echocardiographic data were collected from transcribed reports read by cardiologists at either in-state or out-of-state institutions. Financial data, including charges, professional fees, and payments from hospital accounting systems were used in conjunction with national Medicare estimates.
Clinical follow-up was obtained in 100%(130/130) of patients. A long-term follow-up telephone survey was attempted up to five times per patient for each living study patient (n=124/130, 95%; Supplemental Figure 1). In addition to 109/124(88%) patients providing direct long-term clinical follow-up by telephone, these data were supplemented with institutional and regional EMR review and/or telephone calls with each patient’s primary care physician or cardiologist to complete 100% clinical follow-up.
Patient Population
Patients undergoing MV surgery at our center between January 1, 2011–June 30, 2013 were identified (n=746). From these, those with preoperative ejection fraction ≤40%, functional MR, endocarditis, preoperative dialysis, history of cardiac surgery, preoperative arrhythmia, immunosuppression and/or cancer, and those undergoing concomitant aortic valve replacement, coronary artery bypass grafting, and/or anti-arrhythmia surgery were excluded. From the remaining population, patients undergoing isolated degenerative MVr by a single surgeon were identified and divided into out-of-state (DISTANT, n=26) and in-state (LOCAL, n=104) groups based on state of residence (Supplemental Figure 2). Mitral techniques were performed through standard approaches which have been described in prior series.16
To estimate patient-related travel costs, the DISTANT cohort was prospectively sent a questionnaire (Supplemental Figure 3). Using standard methodology, travel costs were estimated by multiplying distance by the transport method’s average total cost per mile and rental car, hotel, and meal costs were estimated using number of days in Ann Arbor and published per-diem rates (Supplemental Table 1).
Cost-Benefit Analysis
A cost-benefit analysis (Figure 1) was performed to quantify the impact of distant referral from the patient, payor, and societal perspectives, similar in methodology to prior work.17
Figure 1.
Cost-benefit analysis equation.
From the patient perspective, benefit was the expected increase in quality-adjusted life years (QALY) following surgery. To determine this, the probability of repair versus biological or mechanical replacement was multiplied by each expected survival rate, respectively.5 For the purposes of this model, we estimated a 99% chance of repair at a reference center based on multiple single-center series reporting >99% degenerative repair rates.7,18,19 Repair rate at a local hospital was estimated at 75%, which is the national STS average among patients contemporary to this study.14 Notably, this figure by definition overestimates the expected repair rate at a non-reference center, since this national average also includes the repair rates at reference centers. For replacement, the probability of using a biological valve (over mechanical) was estimated at 10% for patients under age 50, 20% for patients 50–59, 36% for patients 60–69, 71% for patients 70–79, and 89% for patients ≥80.20 The expected increases in QALY at a reference center versus local hospital was multiplied by the marginal value per QALY to arrive at a dollar figure.21 We estimated a loss of 2 weeks in QALYs for the patient, with an additional 1 week loss of QALYs for distant referral patients due to patient and family travel. The net benefit from distant referral to a reference center was calculated by taking the reference center benefit (minus $2000 to approximate travel expenses) and subtracting the benefit of undergoing surgery at a local hospital.
From the payor perspective, benefit was calculated by determining cost savings from undergoing MV surgery at a reference center versus local hospital. The expected costs of MVr, bioprosthetic replacement, and mechanical replacement were multiplied by the probabilities of undergoing each at a reference center versus local hospital. Medicare charge and payment data were used to estimate surgery cost (non-reference center: $48,000 and reference center: $54,000). Total cost to the payor was calculated by determining surgery costs at a reference center and local hospital and adding complication costs separately for repair, bioprosthetic replacement, or mechanical replacement.22–26 Operative costs for replacement were assumed to be the same as repair, with $7000 added to account for the replacement valve, based on manufacturer coding sheets. Stroke rate for mechanical replacement was assumed to be 1.6 times greater than that of bioprosthetic replacement, which was assumed to be 1.4 times greater than repair.27 The costs of reoperation for recurrent MR following repair was also included, at a rate of 1% per year.28 For bioprosthetic replacement, the costs of explantation and need for valve replacement were included at a rate of 2.5% per valve-year.29 Finally, for mechanical replacement, the costs of anticoagulation and associated bleeding complications were included.23–25,30,31 Net benefit of distant referral was calculated by subtracting the total cost at a reference center from total cost at a local hospital.
Benefit to society was estimated by summing the net benefit to patients and payors.
Statistical Analysis
Primary outcomes included MVr rate, charges (including travel costs), payments, and 30-day mortality. Secondary outcomes included short-term outcomes and long-term rate of reoperation, residual/recurrent MR, and all-cause mortality. Death was confirmed by ≥1 of 4 methods: institutional and regional EMR, STS database file, the National Death Index (utilized to confirm deceased but not to confirm alive), or through telephone calls to patients, primary care physicians, and/or cardiologists.
Analyses were performed using Stata 16.0 (StataCorp LLC, College Station, TX). Continuous variables were tested for normality using the Shapiro-Wilk test. Normally-distributed continuous variables are reported as mean±standard deviation and were analyzed using the Student’s t-test with Levene’s test for equality of variances. Non-normally distributed continuous variables are reported as median (interquartile range, IQR) and analyzed using the Mann-Whitney U test. Categorical variables are reported as percentages (number) and were analyzed using two-sided Fisher’s exact test. A P≤0.05 was used for statistical significance.
RESULTS
Patient Characteristics and Operative Data
There was no difference in age between LOCAL and DISTANT patients (57.3±13.3 vs. 58.9±14.2 years, P=0.59), though a higher proportion of DISTANT patients were female (15/26[58%] vs. 35/104[34%], P=0.04). Other clinical characteristics and demographics (including payor type) did not statistically differ between groups (Table 1).
Table 1.
Preoperative patient characteristics and payor demographics for in-state (LOCAL, n=104) and out-of-state (DISTANT, n=26) groups.
| Variable | Overall (n=130) | In-State (LOCAL, n=104) | Out-of-State (DISTANT, n=26) | P-value |
|---|---|---|---|---|
| Preoperative Patient Characteristics | ||||
| Age, mean±standard deviation | 57.6±13.4 | 57.3±13.3 | 58.9±14.2 | 0.59 |
| Female gender | 50(38.5) | 35(33.7) | 15(57.7) | 0.04 |
| Renal failure requiring dialysis | 2(1.5) | 2(1.9) | 0 | 1.00 |
| Preoperative creatinine, median mg/dL (IQR) | 0.90(0.8–1.1) | 0.90(0.8–1.1) | 0.85(0.8–1.0) | 0.37 |
| Chronic lung disease | 4(3.1) | 2(1.9) | 2(7.7) | 0.18 |
| Diabetes | 3(2.3) | 3(2.9) | 0 | 1.00 |
| Cerebrovascular disease | 1(0.8) | 1(1.0) | 0 | 1.00 |
| CHF | 3(2.3) | 2(1.9) | 1(3.8) | 0.49 |
| Ejection fraction, median %(IQR) | 60(60–65) | 60(60–65) | 60(60–65) | 0.27 |
| Previous myocardial infarction | 0 | 0 | 0 | |
| Previous cardiovascular intervention | 6.2(8) | 7.7(8) | 0 | 0.36 |
| Previous CABG | 0 | 0 | 0 | |
| Previous valve surgery | 0 | 0 | 0 | |
| Previous congenital heart surgery | 0.8(1) | 1.0(1) | 0 | 1.00 |
| Previous PCI | 3.1(4) | 3.8(4) | 0 | 0.58 |
| Previous AICD/pacemaker | 1.5(2) | 1.9(2) | 0 | 1.00 |
| Previous other CV intervention | 1.5(2) | 1.9(2) | 0 | 1.00 |
| Payor Demographics | ||||
| Medicare | 33(25.4) | 24(18.5) | 9(34.6) | 0.31 |
| Medicaid | 5(3.8) | 4(3.1) | 1(3.8) | 1.00 |
| State-specific health plan | 1(0.8) | 1(1.0) | 0 | 1.00 |
| Commercial health insurance | 105(80.8) | 83(79.8) | 22(84.6) | 0.78 |
| Health maintenance organization | 12(9.2) | 11(10.6) | 1(3.8) | 0.46 |
| None/self-pay | 1(0.8) | 1(1.0) | 0 | 1.00 |
AICD, automated implantable cardioverter defibrillator; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CV, cardiovascular; IQR, interquartile range; PCI, percutaneous coronary intervention.
The proportion of patients undergoing concomitant tricuspid repair, ASD/PFO repair, and both did not statistically differ between groups (Table 2). Median operative duration was similar between LOCAL and DISTANT groups (271[IQR 253–290] vs. 265[IQR 255–285] minutes, P=0.68), although cross-clamp (52[IQR 44–63] vs. 47[IQR 43–51] minutes, P=0.03) and cardiopulmonary bypass times (71[IQR 64–83] vs. 65[60–71] minutes, P=0.01) were statistically shorter in the DISTANT cohort. Overall 95%(123/130) of patients had no post-procedure residual MR and MR grade did not differ between groups.
Table 2.
Operative characteristics and short- and long-term outcomes for in-state (LOCAL, n=104) and out-of-state (DISTANT, n=26) groups.
| Outcome | Overall, n=130 (%) | In-State, n=104 (%) | Out-of-State, n=26 (%) | P-value |
|---|---|---|---|---|
| Operative Data | ||||
| Concomitant procedures | ||||
| Tricuspid valve repair | 16(12.3) | 15(14.4) | 1(3.8) | 0.19 |
| ASD closure | 24(18.5) | 21(20.2) | 3(11.5) | 0.40 |
| Tricuspid valve repair & ASD closure | 3(2.3) | 3(11.5) | 0 | 1.00 |
| Surgery duration, median min (IQR) | 270(254–289) | 271(253–290) | 265(255–285) | 0.68 |
| CPB time, median min (IQR) | 69(63–81) | 71(64–83) | 65(60–71) | 0.012 |
| Cross-clamp time, median min (IQR) | 50(44–61) | 52(44–63) | 47(43–51) | 0.031 |
| Intraoperative blood product use | 10(7.7) | 9(8.7) | 1(3.8) | 0.69 |
| Mitral implant size (n=129) | 32(28–34) | 32(30–34) | 32(28–34) | 0.58 |
| Short-Term Outcomes | ||||
| MR grade on post-procedure echocardiogram, n (%) | ||||
| None | 123(96) | 99(95) | 24(92) | |
| Mild | 7(5) | 5(5) | 2(8) | 0.56 |
| Moderate | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | |
| Missing | 0 | 0 | 0 | |
| Any complication, n (%) | 41(31.5) | 21(30.8) | 9(34.6) | 0.81 |
| Reoperation | 1(0.8) | 0 | 1(3.8) | 0.20 |
| Transient ischemic attack | 1(0.8) | 1(1.0) | 0 | 1.00 |
| Atrial fibrillation | 30(23) | 26(25.0) | 4(15.4) | 0.44 |
| Renal Failure | 0 | 0 | 0 | |
| Superficial sternal wound infection | 2(1.5) | 2(1.9) | 0 | 1.00 |
| Postoperative blood products used | 2(1.5) | 2(1.9) | 0 | 1.00 |
| Gastrointestinal event | 2(1.5) | 2(1.9) | 0 | 1.00 |
| Prolonged ventilation | 1(0.8) | 0 | 1(3.8) | 0.20 |
| Reintubation | 1(0.8) | 0 | 1(3.8) | 0.20 |
| Other complication | 15(11.5) | 9(8.7) | 6(23.1) | 0.08 |
| Readmission to intensive care unit | 3(2.3) | 2(1.9) | 1(3.8) | 0.20 |
| Length of stay, median days (IQR) | 4(3–5) | 4(3–5) | 4(3–5) | 0.50 |
| 30-day mortality | 0 | 0 | 0 | |
| 30-day readmissions | 3(2.3) | 3(2.9) | 0 | 1.00 |
| Long-Term Outcomes | ||||
| All-cause mortality | 6(4.6) | 6(5.8) | 0 | 0.60 |
| Post-discharge mitral reoperation | 1(0.8) | 0 | 1(3.8) | 0.20 |
| MR grade on latest echocardiographic follow-up, n (%) | ||||
| None/trace/trivial | 33(25) | 27(26) | 6(23) | |
| Mild | 17(13) | 15(14) | 2(8) | 0.76 |
| Moderate | 6(5) | 4(4) | 2(8) | |
| Severe | 1(1) | 1(1) | 0 | |
| Missing | 73(56) | 57(55) | 16(62) | |
| Echocardiographic follow-up time, median (IQR) years | 5.8(4.8–6.8) | 6.0(4.7–6.8) | 5.6(5.3–6.9) | 0.77 |
| Clinical follow-up time, median(IQR) years | 7.1(6.1–7.7) | 7.1(6.0–7.8) | 7.1(6.4–7.6) | 0.93 |
ASD, atrial septal defect; CPB, cardiopulmonary bypass; IQR, interquartile range; MR, mitral regurgitation; SD, standard deviation.
Postoperative Outcomes and Follow-Up
Overall complication rates were similar between LOCAL and DISTANT (21/104[31%] vs. 9/26[35%], P=0.81) and specific complications also did not statistically differ [Table 2]. There were two major complications. One early reoperation (postoperative day 5) in the DISTANT cohort was required due to Gore-Tex neochord failure and one LOCAL patient experienced a transient ischemic neurologic deficit. Neither patient had residual sequelae at follow-up.
Median length of stay was not different between LOCAL and DISTANT cohorts (4[IQR 3–5] days vs 4[IQR 3–5] days, P=0.50), nor was intensive care unit readmission (2/104[2%] vs. 1/26[4%], P=0.49). Thirty-day mortality was 0% in both groups and 30-day readmissions did not differ (LOCAL: 3/104[3%] vs. DISTANT: 0/26[0%], P=0.38).
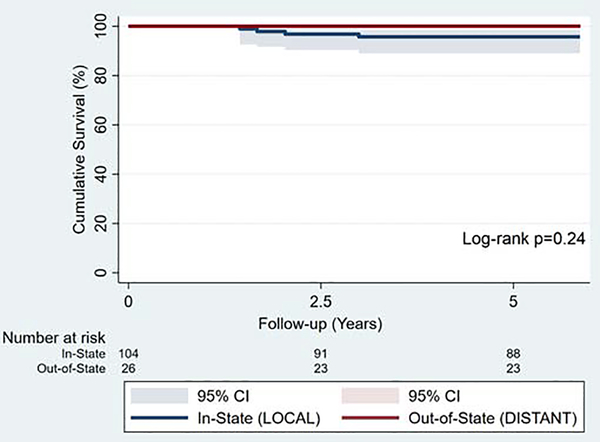
Median follow-up was 7.1(IQR 6.1–7.7) years and did not differ between groups (LOCAL: 7.1[IQR 6.0–7.8] vs. DISTANT: 7.1[IQR 6.4–7.6] years, p=0.93). Long-term reoperation occurred in one patient from the DISTANT group, who underwent re-repair at our center approximately 3 years after primary operation. Overall estimated 5-year survival was 97% (LOCAL: 96±5% vs. DISTANT: 100%, p=0.24) [Figure 2]. MR grade on latest long-term echocardiogram did not differ between groups at median 5.8[IQR 4.9–6.8] years.
Figure 2.
Kaplan-Meier survival estimates for LOCAL(n=104) and DISTANT(n=26) groups.
Economic Analyses
Among 23 (89%) travel expense survey respondents from the DISTANT group, the mean and median round-trip travel distances were 1520±1457 miles and 1020(IQR 110–2520) miles, respectively. Median travel expenses were $1688(IQR $530-$2351). Including professional fees, median charges for LOCAL and DISTANT groups were $76,022 and $74,171, respectively (P=0.35), and median payments (including travel expenses for DISTANT) were $57,795 and $58,477, respectively (P=0.70, Table 3).
Table 3.
Financial outcomes.
| Overall (n=130) | In-State (LOCAL, n=104) | Out-of-State (DISTANT, n=26) | P-value | |
|---|---|---|---|---|
| Facilities charges | $57,452 ($52,652–$64,622) | $57,519 ($52,626–$65,320) | $57,386 ($53,391–$62,883) | 0.64 |
| Professional fee charges* | $18,472 ($16,570–$23,433) | $18,574 ($16,573–$23,610) | $18,140 ($ 16,478–$20,087) | 0.30 |
| Total Charges | $75,258 ($70,916–$86,358) | $76,022 ($70,573–$87,227) | $74,171 ($72,173–$79,622) | 0.35 |
| Facilities payments | $50,827 ($43,656–$58,717) | $50,927 ($42,988–$66,791) | $49,987 ($43,950–$54,930) | 0.44 |
| Professional fee payments* | $6,367 ($4,578–$8,828) | $6,273 ($4,601–$8,732) | $6,456 ($4,545–$11,219) | 0.65 |
| Travel expenses | N/A | N/A | $1,688 ($530–$2,351) | - |
| Total Payments | $57,984 ($51,553–$69,749) | $57,795 ($50,837–$73,383) | $58,477 ($53,142–$61,506) | 0.70 |
Data are presented in median (interquartile range) dollars.
For professional fee charges and payments, total n=127, in-state n=102, and out-of-state n=25.
For travel expenses, total n=23 (all out-of-state by study design). Travel expenses calculated based on mean round-trip travel distance: 1520±1457 miles; median round-trip travel distance: 1020(IQR 110–2520) miles(n=23).
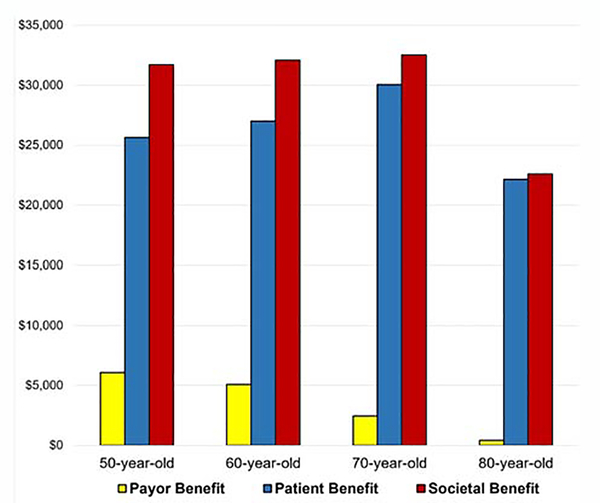
Payor benefit for patients aged 50, 60, 70, and 80 was $6078, $5082, $2461, and $436; patient benefit was $25,644, $27,011, $30,067 and $22,163; And societal benefit was $31,722, $32,093, $32,528, and $22,599, respectively (Figure 3).
Figure 3.
Net benefit of distant referral to a reference center for mitral valve repair from the payor, patient, and societal perspectives by patient age group.
COMMENT
In this analysis comparing in-state and out-of-state patients undergoing degenerative MVr at a single center, we demonstrate comparable short- and long-term clinical and financial outcomes between groups. Additionally, a nationally-representative cost-benefit analysis found a net benefit to the patient, payor, and society for distant referral to a reference center for all age groups, driven by differences in repair rates. Collectively, these data reinforce the importance of degenerative MVr as a quality marker and suggest that the benefits of undergoing repair may outweigh the burden incurred by traveling to a reference center.
MVr confers numerous advantages over replacement for degenerative disease, including lower mortality, avoidance of anticoagulation, and preserving left ventricular function.1–7 MVr is potentially “curative,” in that the resultant survival for patients after degenerative MVr mirrors that of an aged-matched general population.4,6,7,32–34 Given these benefits, it remains concerning that the nationwide repair rate widely varies by hospital and surgeon and overall remains only 75%,14 despite multiple series at single centers of excellence repairing >99% of degenerative MVs.7,18,19 Although there is now increased awareness that repair is superior to replacement, cardiologists and surgeons may have difficulty determining which patients are likely to be repaired versus those needing replacement.
To address this practice variation, some have suggested that low-risk patients with degenerative MR be referred to a mitral specialist and reference center.9,11 In Europe, Bridgewater and colleagues have proposed MVr best practice standards, which set annual volume thresholds of 25 mitral repairs per surgeon and 50 repairs to be a reference center.12 In the U.S., Adams and colleagues have suggested referral to a “mitral subspecialist” with an anticipated mitral repair rate of 95% or higher when managing degenerative disease,13 while a broader analysis of cardiac surgery found traveling past the nearest surgical center to be associated with lower operative mortality and failure to rescue.35 The concept of out-of-state referral to cost-effectively optimize outcomes is not limited to MVr, as private corporations such as Walmart have established a robust Centers of Excellence Program to send employees out-of-state for specialist care.36 The current study shows that even if traveling to a reference center in a different state to undergo a durable degenerative MVr, the clinical and financial benefits of a higher chance at repair outweigh the potential cost, most notably from the patient perspective. These data not only indicate the potential for profound benefit to the patient by undergoing repair over replacement, but also inform value-based reimbursement models, since distant referral was also beneficial to the payor.
Using dialysis and left ventricular assist device utilization as examples, the American healthcare system appears to support the cost of a validated QALY at approximately $50,000-$60,000 per year.37 Although assumptions and modeling errors may influence final estimates of distant referral to a reference center, the QALY cost in this study appears to be mere fractions of this, in the range of a few hundreds of dollars. This study therefore suggests that distant referral of patients undergoing MVr to a reference center is a cost-effective way to improve both MVr rates and quality of life. Even if payors assumed responsibility for travel costs, distant referral would generate a positive net benefit for nearly all patients. Future economic models for distant referral beyond this simplified model for low-risk patients should be able to take into consideration the effect of common comorbidities, including age, hypertension, heart failure, diabetes, renal disease, atrial fibrillation, and chronic lung disease.
This study has several limitations. First, our analysis was performed at a single center and by a single surgeon. However, as a tertiary referral center, we include patients from around the U.S. and provide granular hospital billing and payment data that may not otherwise be available. Second, multiple financial assumptions and estimations were made to quantify the potential benefit of distant referral to a reference center. However, actual charges and payments are presented for our clinical cohort, while all assumptions and estimates in our cost-benefit model were based on literature or contemporary billing information. Third, we do not have universal long-term echocardiographic follow-up for all patients, since performing routine echocardiograms on asymptomatic patients is not indicated by the AHA/ACC guidelines. However, the frequency of missingness for these data did not differ between in-state and out-of-state patients and we do have post-procedure echocardiographic, long-term survival, and mitral-related reoperation data for 100% of patients. Fourth, we cannot rule out unmeasured confounding in our clinical analysis and are at risk of a type II error due to the small sample size of out-of-state patients. However, we felt it most important to isolate comparable low-risk degenerative groups to perform an appropriate comparison and capture purely elective, low-risk, degenerative MVr.
In conclusion, MVr for degenerative disease is superior to replacement and is a quality marker. In an era of emerging value-based payment models, achieving higher quality at lower costs through distant referral is achievable, reasonable, and appealing, and may inform reimbursement strategies and policy surrounding access to degenerative MVr.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Anyanwu AC, Bridgewater B, Adams DH. The lottery of mitral valve repair surgery. Heart 2010;96:1964–7. [DOI] [PubMed] [Google Scholar]
- 2.Gillinov AM, Blackstone EH, Nowicki ER, et al. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg 2008;135:885–93,893.e1–2. [DOI] [PubMed] [Google Scholar]
- 3.Suri RM, Vanoverschelde J-L, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310:609–16. [DOI] [PubMed] [Google Scholar]
- 4.Badhwar V, Peterson ED, Jacobs JP, et al. Longitudinal outcome of isolated mitral repair in older patients: results from 14,604 procedures performed from 1991 to 2007. Ann Thorac Surg 2012;94:1870–7. [DOI] [PubMed] [Google Scholar]
- 5.Daneshmand MA, Milano CA, Rankin JS, et al. Influence of patient age on procedural selection in mitral valve surgery. Ann Thorac Surg 2010;90:1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David TE, Armstrong S, McCrindle BW, Manlhiot C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation 2013;127:1485–92. [DOI] [PubMed] [Google Scholar]
- 7.Watt TMF, Brescia AA, Murray SL, et al. Degenerative Mitral Valve Repair Restores Life Expectancy. Ann Thorac Surg 2020;109(3):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135(25):e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy PM. When is your surgeon good enough? When do you need a “referent surgeon”? Curr Cardiol Rep 2009;11:107–113. [DOI] [PubMed] [Google Scholar]
- 10.Mitral Valve Repair Reference Center Award. Mitral Foundation. https://www.mitralfoundation.org/mvrrca/mitral-valve-repair-reference-center-award Accessed May 2, 2020.
- 11.Gillinov M, Mick S, Suri RM. The Specialty of Mitral Valve Repair. J Am Coll Cardiol 2017;69:2407–9. [DOI] [PubMed] [Google Scholar]
- 12.Bridgewater B, Hooper T, Munsch C, et al. Mitral repair best practice: proposed standards. Heart 2006;92:939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams DH, Anyanwu AC. Seeking a higher standard for degenerative mitral valve repair: begin with etiology. J Thorac Cardiovasc Surg 2008;136:551–6. [DOI] [PubMed] [Google Scholar]
- 14.Badhwar V, Rankin JS, He X, et al. The Society of Thoracic Surgeons Mitral Repair/Replacement Composite Score: A Report of The Society of Thoracic Surgeons Quality Measurement Task Force. Ann Thorac Surg 2016;101:2265–71. [DOI] [PubMed] [Google Scholar]
- 15.National Health Expenditures 2017 Highlights. National Health Expenditure Accounts, Centers for Medicare and Medicaid Services, 2018. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf.
- 16.Brescia AA, Watt TMF, Rosenbloom LM, et al. Anterior versus Posterior Leaflet Mitral Valve Repair: A Propensity-Matched Analysis. J Thorac Cardiovasc Surg 2020; 10.1016/j.jtcvs.2019.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Li J, Hopp WJ, et al. Using Patient-Specific Quality Information to Unlock Hidden Healthcare Capabilities. M&SOM 2019;21:582–601. [Google Scholar]
- 18.Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg 2012;144:308–12. [DOI] [PubMed] [Google Scholar]
- 19.Gammie JS, Barlett ST, Griffith BP. Small-incision mitral valve repair: safe, durable, and approaching perfection. Ann Surg 2009;250:409–15. [DOI] [PubMed] [Google Scholar]
- 20.Gammie JS, Sheng S, Griffith BP, et al. Trends in Mitral Valve Surgery in the United States: Results From The Society of Thoracic Surgeons Adult Cardiac Database. Ann Thorac Surg 2009;87:1431–9. [DOI] [PubMed] [Google Scholar]
- 21.Mason H, Jones-Lee M, Donaldson C. Modelling the monetary value of a QALY: a new approach based on UK data. Health Econ 2009;18:933–50. [DOI] [PubMed] [Google Scholar]
- 22.LaPar DJ, Hennessy S, Fonner E, Kern JA, Kron IL, Ailawadi G. Does urgent or emergent status influence choice in mitral valve operations? An analysis of outcomes from the Virginia Cardiac Surgery Quality Initiative. Ann Thorac Surg 2010;90:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chikwe J, Goldstone AB, Passage J, et al. A propensity score-adjusted retrospective comparison of early and mid-term results of mitral valve repair versus replacement in octogenarians. Eur Heart J 2011;32:618–26. [DOI] [PubMed] [Google Scholar]
- 24.Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S. Cost analysis of isolated mitral valve surgery in the United States. Ann Thorac Surg 2012;94:1429–36. [DOI] [PubMed] [Google Scholar]
- 25.Iribarne A, Burgener JD, Hong K, et al. Quantifying the incremental cost of complications associated with mitral valve surgery in the United States. J Thorac Cardiovasc Surg 2012;143:864–72. [DOI] [PubMed] [Google Scholar]
- 26.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics—2014 Update: A Report from the American Heart Association. Circulation 2014;129:e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo A, Grigioni F, Avierinos J-F, et al. Thromboembolic complications after surgical correction of mitral regurgitation incidence, predictors, and clinical implications. J Am Coll Cardiol 2008;51:1203–11. [DOI] [PubMed] [Google Scholar]
- 28.Dumont E, Gillinov AM, Blackstone EH, et al. Reoperation after mitral valve repair for degenerative disease. Ann Thorac Surg 2007;84:444–50. [DOI] [PubMed] [Google Scholar]
- 29.Bourguignon T, Bouquiaux-Stablo A-L, Loardi C, et al. Very late outcomes for mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg 2014;148:2004–11.e1. [DOI] [PubMed] [Google Scholar]
- 30.Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol 2000;36:1152–8. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko T, Aranki S, Javed Q, et al. Mechanical versus bioprosthetic mitral valve replacement in patients <65 years old. J Thorac Cardiovasc Surg 2014;147:117–26. [DOI] [PubMed] [Google Scholar]
- 32.David TE, Ivanov J, Armstrong S, Rakowski H. Late outcomes of mitral valve repair for floppy valves: Implications for asymptomatic patients. J Thorac Cardiovasc Surg 2003;125:1143–52. [DOI] [PubMed] [Google Scholar]
- 33.Enriquez-Sarano M, Avierinos J-F, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875–83. [DOI] [PubMed] [Google Scholar]
- 34.Montant P, Chenot F, Robert A, et al. Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: A propensity score–based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg 2009;138:1339–48. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins RB, Byler M, Fonner C, et al. Travel distance and regional access to cardiac valve surgery. J Card Surg 2019;34:1044–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farr C Walmart is so desperate to fix health care, it flies employees to top hospitals in other states for treatment. CNBC 2019. https://www.cnbc.com/2019/03/14/walmart-sends-employees-to-top-hospitals-out-of-state-for-treatment.html Accessed November 22, 2019.
- 37.Cohen DJ, Reynolds MR. Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol 2008;52:2119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.