Abstract
Background:
An enduring question from cross-sectional clinical studies is whether the structural and functional differences often observed between cocaine users and healthy controls result from a history of drug use, or instead, reflect preexisting differences. What is needed to assess causality from drug exposure are true pre-drug baseline imaging and neurocognitive assessments.
Methods:
In the present study, we address this fundamental question of causality using longitudinal anatomical MR imaging and neurocognitive assessments in rhesus macaques. Cognitive tasks employed were stimulus reversal learning as a measure of cognitive flexibility/inhibitory control, and delayed match to sample as a measure of visual working memory. Timepoints examined were prior to and following 12 months of chronic cocaine (n=8) or water (n=6) self-administration. An MR only timepoint was also obtained following 2 years forced abstinence.
Results:
We identified localized patterns of gray matter density (GMD) changes that were largely concordant with cross-sectional clinical studies. These included decreases in orbitofrontal cortex, insula, amygdala, and temporal cortex. There was also a prominent increase in GMD in the caudate putamen. In only select cortical regions were GMD decreases significantly correlated with cognitive impairments across individuals. Following abstinence, changes in GMD in some regions, including the orbitofrontal cortex, insula, and amygdala were persistent, and thus may play an important role in risk of relapse following extended abstinence.
Conclusions:
Cocaine use is causal in producing regional changes in GMD, and those changes appear to drive cognitive impairments.
Keywords: cocaine, cognition, structural imaging, macaque, impairment, addiction
Introduction
Structural brain alterations and cognitive deficits have been associated with stimulant use disorders(1–5). Specifically, clinical observations indicate reduced gray matter volume or density in frontal, temporal, and insular cortices as well as increases in the caudate/putamen. The structural changes in these areas have been hypothesized to underlie the differences in cognitive assessments that are frequently observed between stimulant dependent and healthy control individuals(6).
However, difficult to address in cross-sectional clinical studies is whether these differences in structure and/or cognition are a consequence of prolonged stimulant use, or instead, reflect pre-existing traits that confer greater vulnerability to abuse and dependence. An additional confound for determining causality is that of variable poly-drug abuse in clinical populations. Several studies have observed that the magnitude of structural differences correlated with duration of abuse, suggesting the magnitude of stimulant exposure drove the gray matter alterations. Specifically, decreased gray matter was related to duration of use in orbitofrontal, insular, parahippocampal and anterior cingulate cortices as well as the cerebellum(1, 7–10). Supporting the hypothesis that differences in structure could drive cognitive deficits are observations of significant correlations between gray matter density (GMD) and measures of cognitive performance among stimulant using populations(11–13). Critical elements missing in these clinical studies are true baseline measures of GMD and cognitive performance prior to drug exposure.
Upon extended abstinence, the extent of structural and functional recovery, or lack thereof, is also a fundamentally important question because it directly relates to what might be contributing to the enduring risk of relapse. It appears that some changes in gray matter exhibit at least a partial recovery with abstinence(10, 11, 14). While recovery is obviously a good thing, it also complicates many cross-sectional comparisons between stimulant-dependent individuals with differing abstinence durations and control subjects.
Herein, we address each of these critically important questions: 1) Does chronic cocaine intake produce GMD structural changes and if so, in which brain regions? 2) Do those GMD changes relate to cognitive consequences? 3) Following extended abstinence, what structural consequences remain? We do so using neurocognitive performance-matched groups of rhesus monkeys coupled with longitudinal imaging acquisition at baseline prior to any drug exposure, following 12 months of drug (or water) self-administration, and finally after 24 months of imposed drug abstinence. Using the resulting structural gray matter interaction across time, we next determined if GMD changes from baseline were linked to the magnitude of any cognitive impairment observed in the same subjects(15). The cognitive domains assessed previously were associative learning, measured by stimulus discrimination, cognitive flexibility/inhibitory control, measured in a reversal learning (RevL) task, and visual working memory, measured by a delayed match to sample (DMS) task. The rationale for choosing those three cognitive domains in our earlier study was that cross-sectional clinical studies have observed significant differences in reversal learning, but not in stimulus discrimination(16). The literature on working memory is mixed, as discussed previously(15). We did not determine any relationship between structural changes and stimulus discrimination because cocaine exposure had no effect on it(15). Scans acquired following the prolonged abstinence in a subset of the original cohort were contrasted with those obtained at the end of self-administration to determine GMD changes following abstinence. We also contrasted the post-abstinence scan with pre-exposure baseline scans to determine what differences between groups remained following self-administration and extended abstinence.
This within subject design offers greater sensitivity to detect small but systematic changes within a relatively small number of subjects. Importantly, non-human primate (NHP) models of chronic drug exposure can offer critical insight to clinical observations due to similarities in brain structure, circuitry, and neuroanatomical assignment of function(17). Moreover, compared to rodent studies, NHP models correspond more closely to the human condition in acute neurometabolic response(18), and progressive neurochemical and neurometabolic adaptations upon drug challenge after a prolonged duration of exposure(19–21). Finally, cognitive performance of NHPs can be assessed using tasks very similar to those used in human studies(15, 16, 21)
Materials & Methods
All procedures were in accordance with the USPHS Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. A cohort of 14 drug-naive, adult male rhesus macaques (4-5 years old) was trained to perform several cognitive tasks on a touch screen monitor for water reward(15, 22). Subjects received sufficient monkey chow (Labdiet 5038, PMI Richmond, IN) to maintain a healthy body weight supplemented with daily fruit treats. All subjects were water regulated (25 ml/kg/day).
A vascular access port was implanted midscapulae in all subjects prior to baseline cognitive assessments(15). Subsequently, animals were divided into performance-matched groups and the experimental group of 8 subjects proceeded to self-administer cocaine intravenously via their vascular access port. Subjects typically would self-administer the maximum daily allotment of 6 infusions (0.5 mg cocaine/kg/infusion; cumulative daily dose 3 mg/kg) during the course of 4 sessions/week (Tue-Fri; max session duration 2 hrs). Over the year of self-administration, the total cumulative dose of cocaine was approximately 600 mg/kg with negligible variation between individuals. Subjects in the control group (n=6) contemporaneously performed a similar operant task (4 days/wk) on the touch screen for oral water reward. All subjects performed cognitive testing reinforced by water reward on Mondays (cocaine subjects were in a drug-free state, approximately 72 hrs after the last cocaine session) (Fig 1). RevL and DMS performance were assessed on alternating Mondays. A more detailed description of the self-administration and cognitive testing procedures has been reported previously(15).
Fig 1.
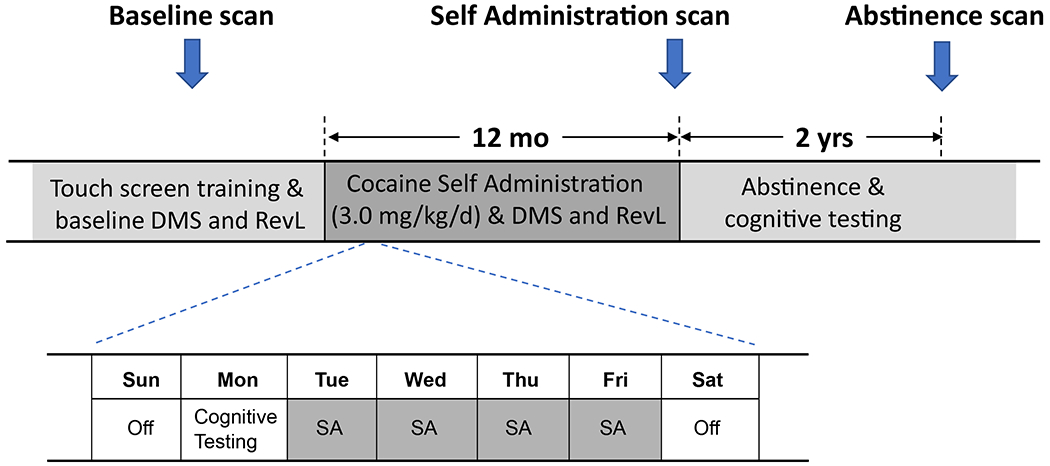
Experimental timeline. Subjects received a structural MRI scan and were trained on a touch screen to perform a Delayed Match to Sample (DMS) task and a stimulus discrimination with reversal learning (RevL) task prior to assignment into one of two groups (cocaine self-administration (N=8), water self-administration (SA) control (N=6)). Cocaine animals self-administered intravenously up to 3.0mg/kg cocaine 4 days a week, while control subjects performed similar tasks for water reward. Cognitive performance on DMS and RevL tasks were assessed on alternating Mondays.
Structural MRI scans were performed using a Siemens 3T Allegra (TR 1.68 sec/ TE 3.04 msec; flip angle 8°; FOV 12/16 cm) at 3 time points: Pre-drug baseline scan (1); Post Self-administration scan (2) after 12 months of self-administration; and Abstinence scan (3) following a 2-year period of abstinence in 11 of the original subjects (cocaine group n=6, control group n=5).
A custom imaging processing pipeline was developed in order to maximize sensitivity for longitudinal comparisons(23). Structural scans were manually skull stripped, N4 bias-corrected(24), and segmented in native space(25) using FSL-FAST. Individual scans were linearly registered to the implicit mean for each subject (the average of baseline, self-administration, and if available, abstinence scan) using Freesurfer mri_robust_template(26) and each implicit mean was subsequently aligned to the INIA-19 rhesus template(27) using linear and non-linear registration (mri_robust_register & FSL-FNIRT)(23). Individual raw GMD images were then aligned to the INIA-19 space using the same (concatenated) alignment parameters and used to build a symmetrical (left/right) GMD template using mri_robust_template. Next the individual raw GMD images were linearly aligned to the subject’s implicit mean, and each subject’s implicit mean GMD image was linearly aligned to the symmetrical GMD template. The concatenated alignment parameters from these 2 linear steps were used to build an updated symmetrical GMD template. Finally, the raw GMD images were linearly aligned to each subject’s implicit mean, and each implicit mean GMD image was non-linearly aligned to the updated symmetrical GMD template and the final normalized GMD estimates were smoothed to 6 voxels (3.6mm) using AFNI 3dBlurToFWHM prior to statistical comparison. Compared to the FSL-VBM pipeline, this process better respects the longitudinal nature of the datasets(26).
A GMD group X time interaction was analyzed with a general linear model using total intracranial volume as a covariate. Note that we focus on the group X time interaction analysis which reflects changes in structure with the passage of time that are not common to both groups. Statistical thresholds were determined using Permutation Analysis of Linear Models (PALM with speed-up)(28, 29) using threshold-free cluster enhancement (TFCE) and multiple comparison correction maintaining FWE at p<0.05(30). Ten iterations of 500 permutations each were averaged. This work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). All maps are presented according to radiologic convention.
The change in DMS performance was quantified as the difference in slope of the accuracy versus delay plot in DMS sessions before (baseline) and after cocaine self-administration as described previously(15). The change in RevL performance was quantified as the difference in trials to criterion (TTC; >70% high value choice over a ten trial sliding window.
Primary structural analyses determined the effect of cocaine self-administration on GMD by contrasting the post-self-administration scan with the baseline scan in a group X time longitudinal design. We then determined correlations between these GMD changes and cooccurring changes in cognitive performance. Specifically, decreases in GMD averaged for each significant cluster were regressed against changes in RevL and DMS performance (only using individuals in the cocaine group in order to avoid artefactual correlations driven by group mean differences), using a directional null-hypothesis with p<0.05. All structural changes combined were held to False Discovery Rate of 0.05, but correlational analyses were not (further) corrected for multiple comparisons, and thus should be considered exploratory.
To determine persistent or compensatory structural changes following 2 years of abstinence, the scan at that timepoint was contrasted with the post-self-administration scan in 11 remaining subjects (6 cocaine, 5 controls) using the same PALM with TFCE procedure as described above. At abstinence, only a subset of the original subjects was available due to an unexpected illness or loss of motivation(31). Lastly, to determine if there were persisting differences between groups following self-administration and extended abstinence, a comparison between the abstinence and baseline scan time points was conducted.
Results
The first question posed in the Introduction was whether chronic cocaine intake produces GMD changes and if so, in which brain regions? The 2-way ANOVA group X time interaction corrected for multiple comparisons (FWE threshold p < 0.05) is shown in Fig 2. From that contrast, sixteen regions greater than 100 voxels (voxel size 0.6 mm, total volume of 22 microliters) were selected for regression of change in GMD with cognitive changes. This threshold was arbitrarily chosen in order to avoid spurious correlations with regions too small to likely be functionally relevant.
Fig 2.
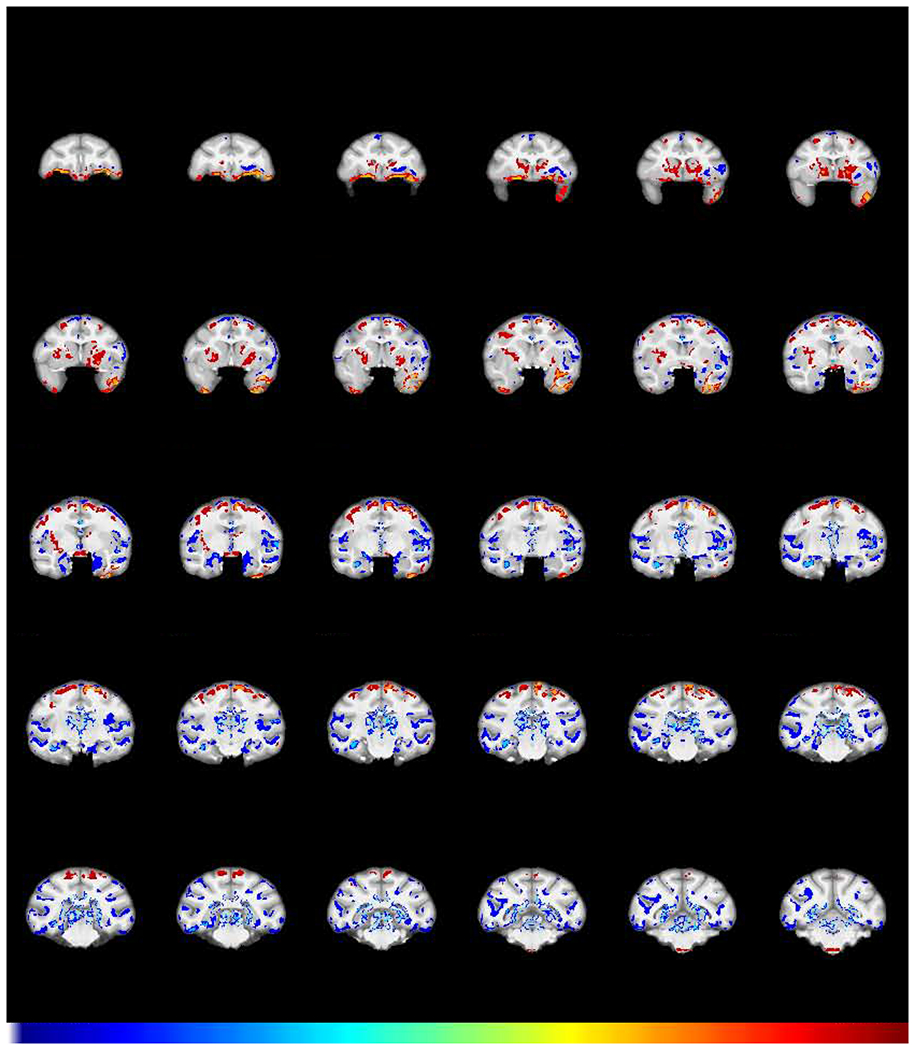
Effect of chronic cocaine on GMD. Coronal sections of structural interaction resulting from cocaine self-administration (FWE-corrected −log(p) TFCE maps showing the difference between groups in GMD change from baseline to post-self-administration scans). Regions in which cocaine subjects (N=8) exhibited a greater relative decline in GMD compared to control subjects (N=6) are colored in blue. Regions in which the cocaine subjects showed relatively greater increases in GMD are colored in red. Numbers above sections represent distance in mm from the crossing of the anterior commissure.
Brain regions with a greater relative decline in GMD in cocaine vs. control group subjects (cold, blue colors) were found in left orbitofrontal cortex (OFC), thalamus, and multiple regions throughout the temporal lobe including the amygdala, hippocampus and adjacent fusiform/entorhinal cortex, parietal cortex, and large extended areas of insular cortex. In contrast, brain regions with relative increases in GMD in the cocaine vs. control group (warm, red colors) were observed in the temporal pole, precentral gyrus, ventral frontal cortex, and caudate putamen (Fig 2). Supplementary Fig S1 is a compendium plot of the individual subject GMD values at baseline and post self-administration for each regional cluster. Although animals were assigned to each group to achieve matched mean task performance, comparison between the two groups at baseline indicated that 2 regions showed GMD differences that survived a Bonferroni-corrected t-test. One was in the left insula (Fig. S1, cluster #3), wherein the cocaine group had a greater GMD at baseline (pcorrected=0.023), a difference opposite to the subsequent effect of cocaine. The other region was an ambiguous region incorporating the optic chiasm (#12) at the rostral extent of the brainstem (see the three sections from −0.3 to −2.7 in Fig 2), where segmentation was extremely difficult, rendering those differences difficult to interpret.
The second question we posed was whether any of the alterations in GMD shown in Fig 2 have functional consequences. Based on reports of associations between measures of gray matter and cognitive performance in stimulant using populations(11–13), we explored whether decreases in GMD from baseline would correlate with decreases in cognitive performance on the RevL and/or DMS task. We identified 3 regions with significant correlations. First, the impairments in RevL significantly correlated with decreased GMD in the OFC (L) (Fig 3; 471 voxels, Pearson ρ = −0.634, p = 0.046). In addition, there was a significant correlation between RevL impairment and decreased GMD in the lateral parietal area (LIP: 187 voxels, Pearson ρ = −0.790, p = 0.0098). For the DMS task, decreases in cognitive performance significantly correlated with decreased GMD in a large region that spanned the insula and the temporal lobe (L) (Fig 4; 7635 voxels, Pearson ρ = 0.650, p = 0.040). That large region encompasses multiple areas with distinct functionality that were connected by single voxels. Consequently, it was separated into subregions by an erosion/dilation procedure (a common image analysis process called opening(32)), producing six regions. Two of those six resulting subregions showed significant correlations that appear to drive the relationship shown by the larger cluster. One is in the insula proper, consisting of 2637 voxels, ρ = 0.647, p = 0.041 and the other is a middle temporal cortex (area TE) consisting of 658 voxels, ρ = 0.914, p = 0.0007 (Fig 4b).
Fig 3.
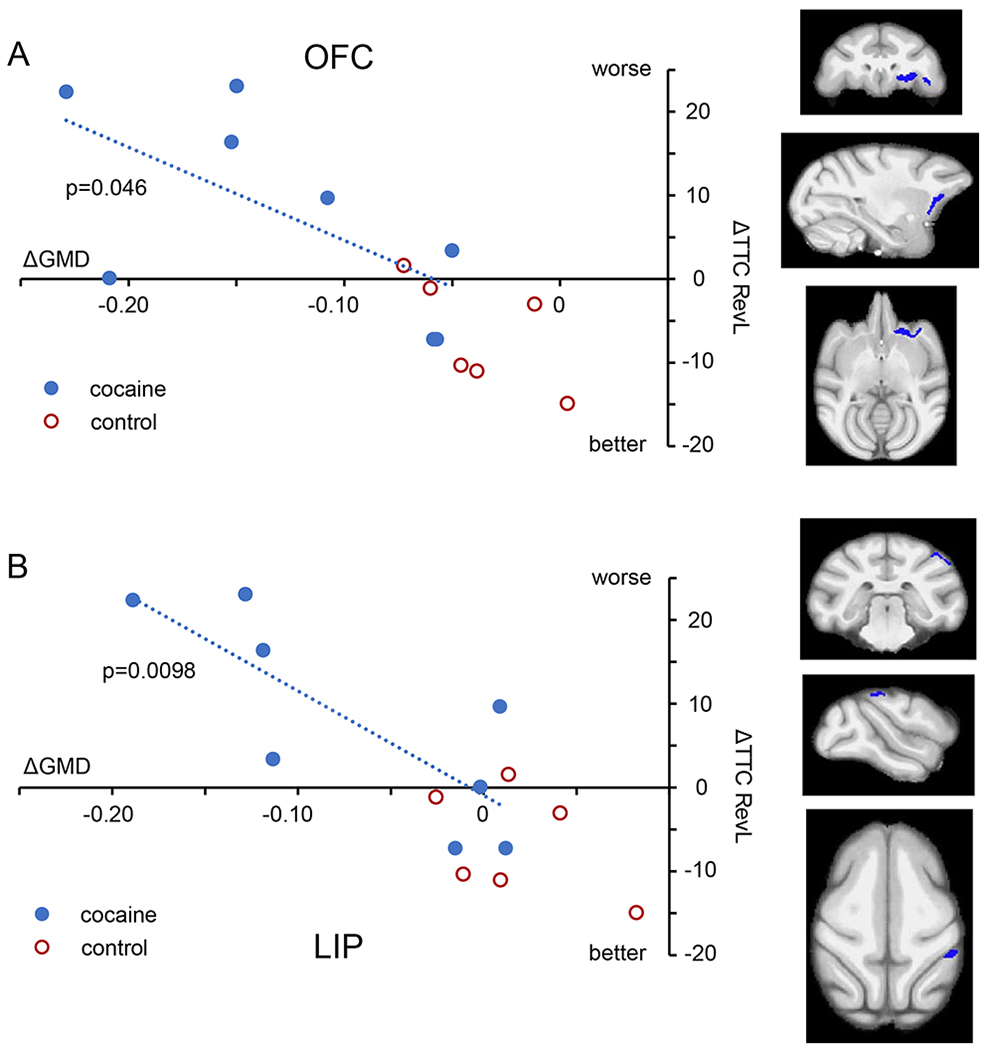
Impact of structural changes on RevL performance. A. Within the cocaine subjects (blue symbols), the impairment in RevL performance (greater number of trials to criterion) correlated with the magnitude of GMD decline in regions of the orbitofrontal cortex (OFC; 471 voxels). B. The lateral parietal area (LIP; 187 voxels) also showed a significant correlation. Control subjects (red) are shown for comparison. Orthogonal views (coronal, parasagittal, and axial) indicate the location of the region of decreased GMD. For all regressions, only cocaine subjects were used.
Fig 4.
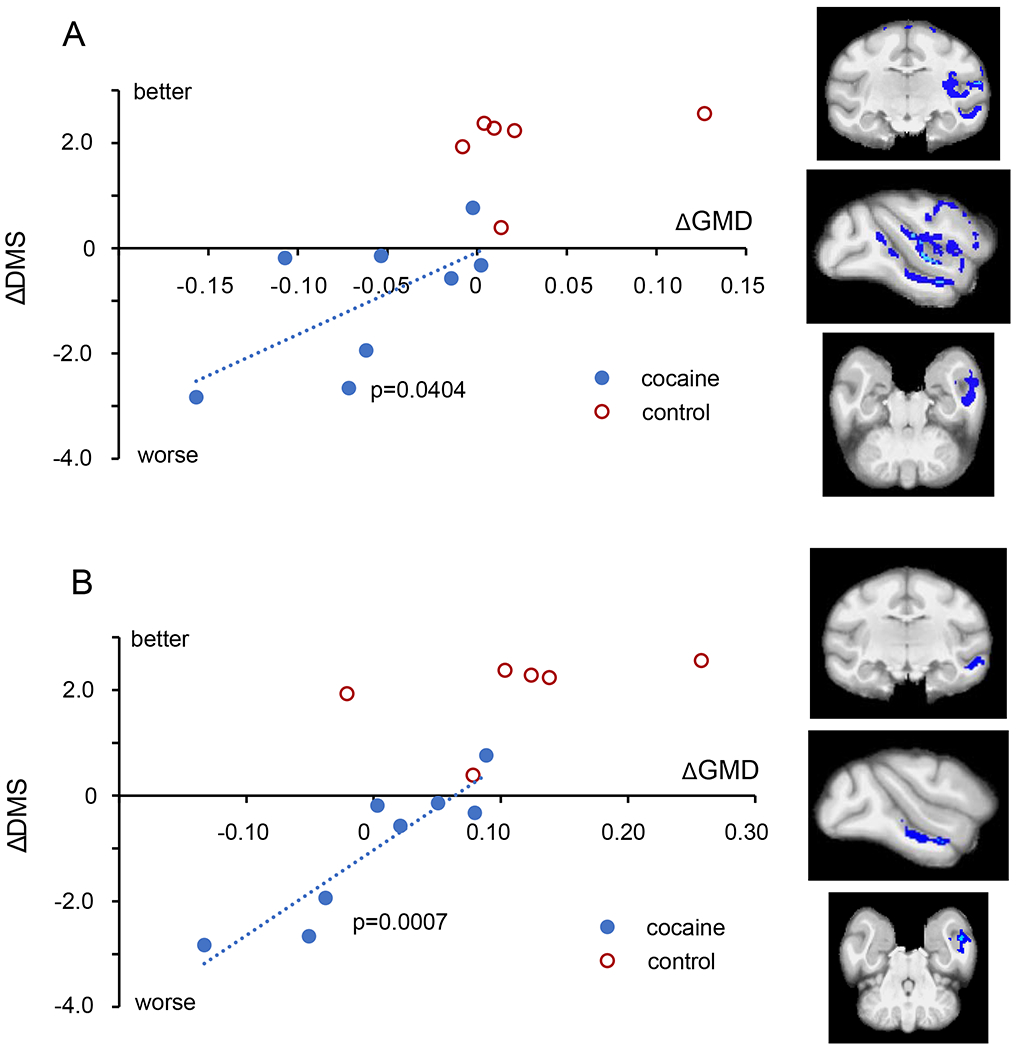
Impact of structural changes on DMS performance. A. Within the cocaine subjects (blue circles), the impairment in DMS performance (steeper decline in accuracy with delay) correlated with the magnitude of GMD decline in a large region spanning the insula and the temporal lobe (7635 voxels). B. Within the larger contiguous region shown in A, a subregion within temporal cortex area TE (658 voxels, lower panel) was also highly correlated.
To address the third question, namely, does extended abstinence reveal either further, or persistent changes, another structural scan (Abstinence scan 3) was acquired in 6 subjects in the cocaine group and 5 subjects in the control group after two years of abstinence. A group X time interaction of Abstinence scan 3 contrasted with Self-administration scan 2 is shown in Fig 5. Between groups differences over the time following abstinence were: a) decreases in GMD in frontal pole regions and b) increases in GMD most prominently in the caudate putamen. Without it being conclusive, we note an absence of statistically significant change in some areas where there were significant decreases in GMD following self-administration (Fig 2), such as the amygdala and insula.
Fig 5.
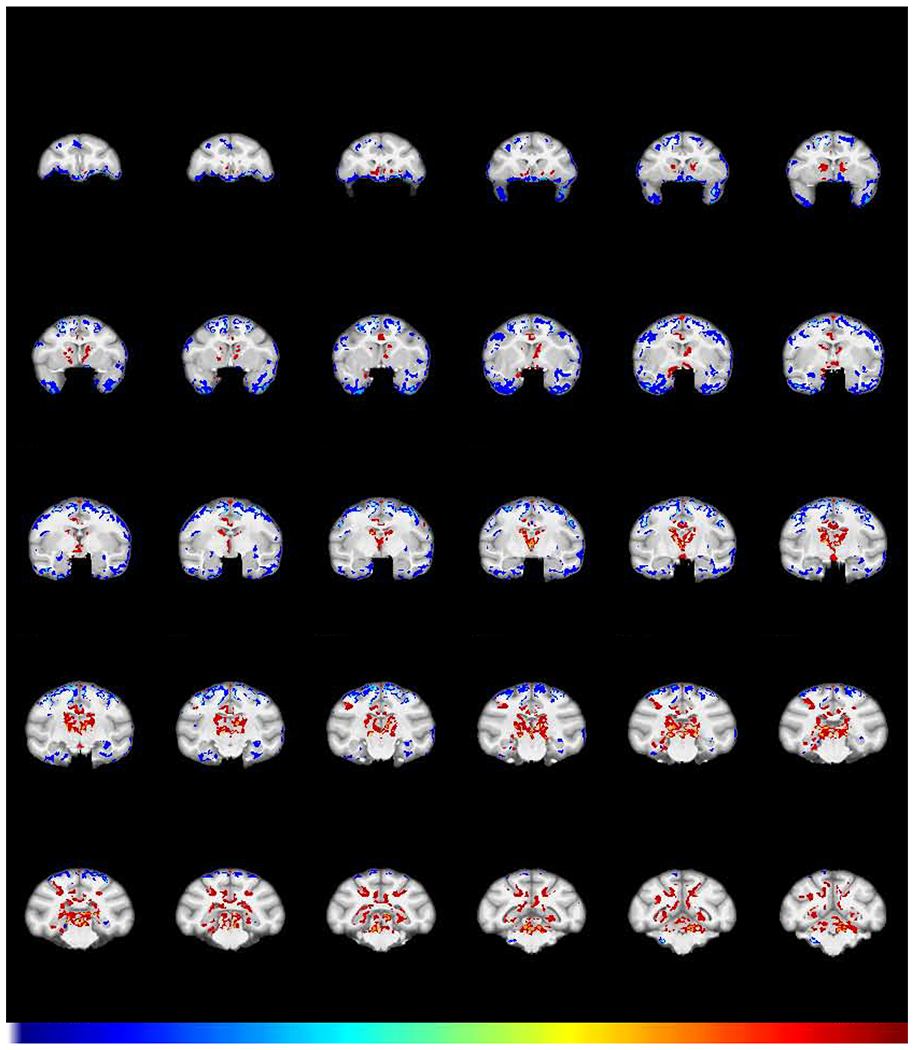
Effect of abstinence on GMD. Shown is the structural interaction after a 2-year period of abstinence from cocaine (FWE-corrected −log(p) TFCE maps showing the difference between groups for GMD change from the post-self-administration scan to the abstinence scan). Regions in which cocaine subjects (N=6) exhibited a greater decline in GMD compared to control subjects (N=5) are colored in blue. Regions in which the cocaine subjects showed a relative increase in GMD compared to control subjects are colored in red.
Given that a lack of effect of abstinence in areas such as the amygdala and insula could be due to a lack of power, we asked a follow-on question: are there areas of significantly different GMD that remain following self-administration and extended abstinence? To answer this question, a group X time interaction contrasted the Abstinence scan (3) with the Baseline scan (1). The results (Fig 6) demonstrate prominently increased GMD in the caudate/putamen, consistent with the significant increase seen in Figs 2 and 5. There is also decreased GMD in the insula, amygdala, and left OFC in the cocaine group, relative to the controls, consistent with a lack of significant change in those regions in Fig. 5
Fig 6.
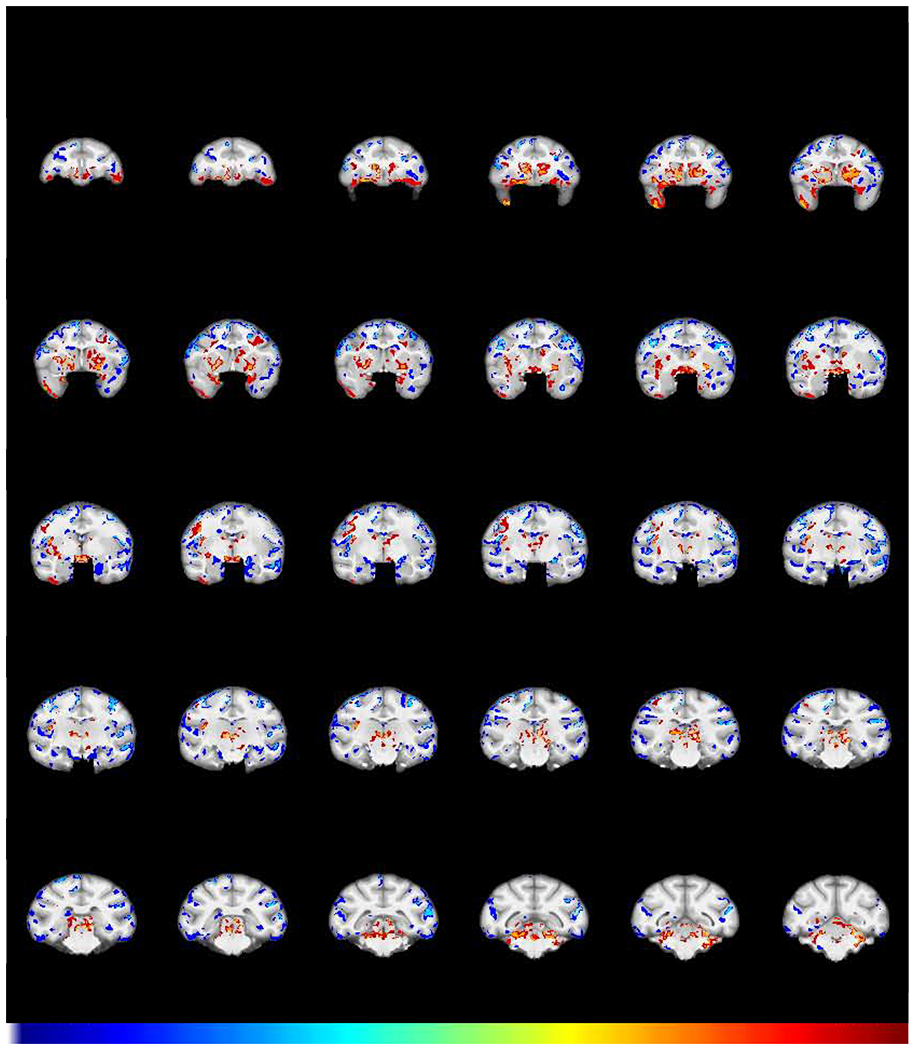
GMD differences remaining after chronic cocaine and abstinence. Shown is the structural interaction (FWE-corrected −log(p) TFCE map) comparing scans obtained after a 2-year period of abstinence with the baseline scans for the cocaine and control group. Regions in which cocaine subjects (N=6) exhibited a greater decline in GMD compared to control subjects (N=5) are colored in blue. Regions in which the cocaine subjects showed a relative increase in GMD compared to control subjects are colored in red. This demonstrates the changes that persisted after one year of chronic self-administration followed by a 2-year period of abstinence from cocaine.
Discussion
In this report, using performance matched groups of non-human primates, we show not only that one year of regular, yet relatively moderate cocaine exposure causes GMD structural differences similar to those observed clinically in human cocaine dependent individuals, but that in a few specific cortical regions, the magnitude of these structural differences correlates with accompanying cognitive deficits. Importantly, our results suggest a causal link between cocaine-induced structural changes and impaired cognition in the two cognitive domains examined. We also observed that despite long-term abstinence, some of the structural changes induced by cocaine remained, and therefore, may be implicated in long-term risk of relapse.
A confound almost always discussed in cross-sectional studies examining structural and/or cognitive differences between drug using and control populations is that of potential preexisting differences. Hence the need to address the question of causation by cocaine posed in the Introduction. Similar to findings from cross-sectional studies in human stimulant users(1, 2, 4, 5, 8, 9, 33, 34), we observed declines in GMD in the cocaine group (relative to controls) in the orbitofrontal, insular, and temporal cortices, as well as in the amygdala and thalamus. We also found several regions where the cocaine group showed increases in GMD relative to the control group following cocaine self-administration. In particular, the increase in caudate putamen GMD is reminiscent of the areas of increased gray matter observed in some cross-sectional studies in humans with stimulant dependence(7, 35–37). Increased gray matter in the temporal pole was previously demonstrated in cocaine dependent individuals as well(33). The parallels between clinical data sets and the present one suggest that the structural differences between stimulant users and control subjects in clinical studies are caused, in large part, by exposure to stimulants. The strengths of this type of non-human primate longitudinal imaging approach has also been exploited to examine the structural impact of chronic ethanol consumption(38).
The second question we posed is that of whether functional consequences accompany the structural effects we observed. We previously reported deficits in RevL and DMS performance in the cocaine exposed animals(15), which were similar to clinical observations of impaired RevL and DMS in cocaine using populations(6, 11, 16, 21). Impairments in cognitive performance subserved by neuroanatomical areas that also showed structural deficits in clinical studies suggests a link between them, but the lack of true baseline measures prior to cocaine exposure has precluded any inferences of causality. Our results fill that gap.
In only a very limited number of anatomical regions do we see a significant correlation between the magnitude of structural change and cognitive impairment across individuals. Reversal performance has classically been associated with impaired OFC function(39, 40), and decreases in gray matter in the OFC as well as impairment on RevL performance have been frequently reported in stimulant abuse subjects(7, 16). Our results suggest the decreased GMD in OFC is a likely contributor to the deficits in RevL observed in the cocaine exposed animals. While not classically associated with RevL, the lateral intraparietal cortex is associated with inhibitory control, an essential part of RevL performance(41), which may explain the correlation with RevL in that region.
DMS performance, as a measure of visual working memory, has traditionally been related to function of the inferior frontal cortex/lateral convexity and the medial temporal lobe(42–44). Visual working memory has also been associated with function of the insula(45) and lesions of temporal cortex area TE impair matching performance(46). We observed a significant correlation between changes in GMD and DMS performance in a large region that incorporated the insula and temporal cortex. Given that this large region encompasses multiple areas with distinct functionality that were connected by single voxels, we employed an erosion/dilation procedure that generated six subregions. Of those six, a large one in the insula proper, and another in area TE showed significant correlations between decreases in GMD and DMS performance.
The third general question we posed related to the effect of extended abstinence on cocaine-induced structural changes. The group X time interaction of scan 3 and scan 2 revealed a general pattern of results in which areas where cocaine had produced changes now showed changes in the opposite direction, suggesting that the GMD alterations resulting from cocaine self-administration might partially reverse. We previously reported a normalization of working memory and reversal performance in these subjects after approximately 3-5 months abstinence(47). Normalization of cognitive function and increases in gray matter have also been observed in abstinent cocaine users(6, 14). After a 6-month period of abstinence, cocaine users exhibited increased gray matter volume in occipital and frontal cortex compared to control subjects over a similar time period(14). Moreover, the improvement in cognitive flexibility and decision making over the abstinence period correlated with increased inferior frontal gyrus GM in cocaine users(14). Gray matter differences between active cocaine users and abstainers largely in temporal and frontal cortex could also be interpreted as signs of recovery in combination with normalization of cognitive performance in former cocaine users (>1 month abstinence)(6, 11). Collectively, these data suggest that some structural and functional changes are transient and seem to ‘recover’ with abstinence. Ideally, we would have also evaluated any relationship between cognitive performance after abstinence and changes in structure. Though we established recovery of cognitive function after approximately 5 months, at which time routine cognitive testing stopped, the lack of cognitive assessments extending to the time of the abstinence scan and the reduced number of subjects precluded a meaningful correlational analysis.
Because we were not looking selectively at the regions defined by the group X time interaction in Fig. 2, we cannot specifically speak to a reversal by abstinence of those effects as depicted in Fig 5. Given the voxel-wise nature of our analyses, we cannot specifically determine if specific regional changes reverse upon abstinence, because the subset of voxels that differ in a particular contrast are not identical to the ones resulting from a different contrast, resulting in what would be a “circular analysis,” from “double dipping”(48). (A future study could use our present results for hypothesis-driven predictions of how anatomically defined regions of interest change upon exposure and abstinence.) However, the third group X time interaction we conducted, scan 3 contrasted with scan 1 does permit us to make definitive statements about what differences remain between the groups following self-administration and abstinence. As seen in Fig. 6, some regions with significant GMD alterations following self-administration, such as the OFC, caudate/putamen, insula, and amygdala/parahippocampal cortex (shown in Fig 2) were present, even following prolonged abstinence. These regions are part of functional networks implicated in impaired response inhibition and salience attribution that are affected in human addiction(49). It is plausible these represent more permanent changes in GMD that may contribute to the long-lasting impact characteristic of drug addiction such as the increased risk of relapse. For example, the lack of ‘recovery’ in insula may contribute to lasting failure to integrate salient interoceptive activity and emotional behavior(50, 51) and functional connectivity in a circuit identified from differences in insula predict risk of relapse(33). Such areas of lasting structural change could only have been identified with a true, pre-drug baseline measure, and further highlights the strength of NHP models of chronic drug use. We note that there may be differences in the effects of a “forced” abstinence as in this study, and “volitional” clinical abstinence outside the context of administrative (judicially enforced) abstinence.
The distribution of the regions of reduced GMD in the cocaine group are more widespread than simply brain regions with a dense monoaminergic innervation, known to be a primary target for stimulants. Although the cellular mechanisms are unclear, GMD reduction has frequently been interpreted as an indication of neuronal (and/or synaptic bouton) loss based on a coinciding reduction of the neural marker N-acetyl-aspartate(52). This interpretation is supported by observations that chronic cocaine administration selectively decreases neural spine counts in some brain regions such as OFC and hippocampus, but not in other regions(53–55). Aside from neural loss, other factors potentially contributing to distributed alterations in GMD are activation of the innate immune response resulting in astrocyte activation, microgliosis, and glial hypertrophy, which all have been reported in response to stimulant drugs including cocaine(56–58). In addition, cocaine-induced angiogenesis(59) and reductions in cerebral blood flow in insula, orbitofrontal cortex, and temporal regions, observed both clinically and in preclinical models(59–62) might contribute to the alterations in GMD observed.
One finding from this study of particular interest is the increase in GMD in the caudate/putamen. Ersche and colleagues(7) report that cocaine users, as a group, show increased GMD in this region, with a significant positive correlation between GMD and an “inatttention” component of impulsivity. However, they also report a negative correlation between duration of cocaine use and GMD, suggesting a potential preexisting difference at baseline, which was a marker of vulnerability that was “normalized” by cocaine use. Our findings would suggest that cocaine use does increase GMD there, and that even after extended abstinence, Fig 6 demonstrates enduring increases relative to the control group. Strikingly, Fig 5 shows that at a regional level, not only was there no suggestion of any reversal upon abstinence, but there were further scattered increases in GMD in caudate/putamen when contrasting scan 3 and scan 2. Why this region of the greatest dopaminergic impact of cocaine (based on orders of magnitude greater innervation density) shows such an opposite effect relative to others is unclear. Ersche and colleagues(7) discuss the literature relating increased regional volume to D2 antagonist effects, and also the well documented effects of cocaine at decreasing D2 levels, but the need for further exploration of mechanism remains.
This study is uniquely informative to the large clinical literature of cross-sectional studies between cocaine users and controls. It conclusively demonstrates that use of cocaine can result in structural changes of both decreased, and increased GMD. Critically, it also strongly suggests that decreases in GMD in select cortical regions is linked to altered cognition. While abstinence appears to reverse some of the changes, there are striking exceptions that may contribute to the enduring risk of relapse commonly seen in addiction.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | none | |||
| Bacterial or Viral Strain | none | |||
| Biological Sample | none | |||
| Cell Line | none | |||
| Chemical Compound or Drug | none | |||
| Commercial Assay Or Kit | none | |||
| Deposited Data; Public Database | none | |||
| Genetic Reagent | none | |||
| Organism/Strain | none | |||
| Peptide, Recombinant Protein | none | |||
| Recombinant DNA | none | |||
| Sequence-Based Reagent | none | |||
| Software; Algorithm | none | |||
| Transfected Construct | none | |||
| Other | none |
Acknowledgements:
This research was supported by the Intramural Research Program of the NIH, NIDA , the Veterans Affairs Medical Research Service and NIH/NIDA grant DA 25636. The authors greatly acknowledge the expert assistance of Carol Ehnerd, Kate Gurnsey, Rebecca MacCloud, and Jessica Nicolazzo and statistical consultation with Dr. Thomas Ross, NIDA intramural program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References:
- 1.Ersche KD, Williams GB, Robbins TW, Bullmore ET (2013): Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Current opinion in neurobiology. 23:615–624. [DOI] [PubMed] [Google Scholar]
- 2.Mackey S, Paulus M (2013): Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehav Rev 37:300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. (2015): Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, et al. (2019): Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am J Psychiatry 176:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. (2002): Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological psychiatry. 51:134–142. [DOI] [PubMed] [Google Scholar]
- 6.Potvin S, Stavro K, Rizkallah E, Pelletier J (2014): Cocaine and cognition: a systematic quantitative review. J Addict Med. 8:368–376. [DOI] [PubMed] [Google Scholar]
- 7.Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET (2011): Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain : a journal of neurology. 134:2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, et al. (2007): Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 32:2229–2237. [DOI] [PubMed] [Google Scholar]
- 9.Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, et al. (2011): Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Archives of general psychiatry. 68:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly CG, Bell RP, Foxe JJ, Garavan H (2013): Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS One. 8:e59645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ (2011): Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl). 218:681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, et al. (2010): Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 50:1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, et al. (2006): Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 9:221–228. [DOI] [PubMed] [Google Scholar]
- 14.Parvaz MA, Moeller SJ, d’Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, et al. (2017): Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 22:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW (2011): Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 31:4926–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ (2008): Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 197:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, et al. (2005): Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 25:8854–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandeville JB, Choi JK, Jarraya B, Rosen BR, Jenkins BG, Vanduffel W (2011): fMRI of cocaine self-administration in macaques reveals functional inhibition of basal ganglia. Neuropsychopharmacology. 36:1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradberry CW (2007): Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology (Berl) 191:705–717. [DOI] [PubMed] [Google Scholar]
- 20.Narendran R, Martinez D (2008): Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 62:851–869. [DOI] [PubMed] [Google Scholar]
- 21.Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ (2008): Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philosophical transactions of the Royal Society of London. 363:3257–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, et al. (2010): Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Molecular psychiatry. 15:512–522, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuter M, Fischl B (2011): Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 57:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. (2010): N4ITK: improved N3 bias correction. IEEE transactions on medical imaging. 29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Brady M, Smith S (2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE transactions on medical imaging. 20:45–57. [DOI] [PubMed] [Google Scholar]
- 26.Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012): Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, et al. (2012): The INIA19 Template and NeuroMaps Atlas for Primate Brain Image Parcellation and Spatial Normalization. Front Neuroinform. 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM (2016): Faster permutation inference in brain imaging. Neuroimage. 141:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage. 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SM, Nichols TE (2009): Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44:83–98. [DOI] [PubMed] [Google Scholar]
- 31.Porter JN, Minhas D, Lopresti BJ, Price JC, Bradberry CW (2014): Altered cerebellar and prefrontal cortex function in rhesus monkeys that previously self-administered cocaine. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez RC, Woods RE (2017): Digital Imaging Processing. Pearson. [Google Scholar]
- 33.Geng X, Hu Y, Gu H, Salmeron BJ, Adinoff B, Stein EA, et al. (2017): Salience and default mode network dysregulation in chronic cocaine users predict treatment outcome. Brain : a journal of neurology. 140:1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, et al. (2000): Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 98:93–102. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH (2001): Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 158:486–489. [DOI] [PubMed] [Google Scholar]
- 36.Mackey S, Stewart JL, Connolly CG, Tapert SF, Paulus MP (2014): A voxel-based morphometry study of young occasional users of amphetamine-type stimulants and cocaine. Drug Alcohol Depend. 135:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaquero L, Camara E, Sampedro F, Perez de Los Cobos J, Batlle F, Fabregas JM, et al. (2017): Cocaine addiction is associated with abnormal prefrontal function, increased striatal connectivity and sensitivity to monetary incentives, and decreased connectivity outside the human reward circuit. Addict Biol. 22:844–856. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke CD, Rohlfing T, Park B, Sullivan EV, Pfefferbaum A, Grant KA (2014): Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology. 39:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fellows LK, Farah MJ (2003): Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain : a journal of neurology. 126:1830–1837. [DOI] [PubMed] [Google Scholar]
- 40.Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A (2017): The neural basis of reversal learning: An updated perspective. Neuroscience. 345:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osada T, Ohta S, Ogawa A, Tanaka M, Suda A, Kamagata K, et al. (2019): An Essential Role of the Intraparietal Sulcus in Response Inhibition Predicted by Parcellation-Based Network. J Neurosci. 39:2509–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JD, et al. (2009): Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J Neurosci. 29:11880–11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schon K, Ross RS, Hasselmo ME, Stern CE (2013): Complementary roles of medial temporal lobes and mid-dorsolateral prefrontal cortex for working memory for novel and familiar trial-unique visual stimuli. Eur J Neurosci. 37:668–678. [DOI] [PubMed] [Google Scholar]
- 44.Wilson FA, Scalaidhe SP, Goldman-Rakic PS (1993): Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 260:1955–1958. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA (2003): Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 15:1028–1038. [DOI] [PubMed] [Google Scholar]
- 46.Buffalo EA, Ramus SJ, Squire LR, Zola SM (2000): Perception and recognition memory in monkeys following lesions of area TE and perirhinal cortex. Learn Mem. 7:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter JN, Gurnsey K, Jedema HP, Bradberry CW (2013): Latent vulnerability in cognitive performance following chronic cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl). 226:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI (2009): Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 12:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ (2018): Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron. 98:886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garavan H (2010): Insula and drug cravings. Brain Struct Funct. 214:593–601. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. (2009): The neurocircuitry of impaired insight in drug addiction. Trends in cognitive sciences. 13:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang L, Ernst T, Strickland T, Mehringer CM (1999): Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry. 156:716–722. [DOI] [PubMed] [Google Scholar]
- 53.DePoy LM, Zimmermann KS, Marvar PJ, Gourley SL (2017): Induction and Blockade of Adolescent Cocaine-Induced Habits. Biological psychiatry. 81:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, et al. (2015): The Contingency of Cocaine Administration Accounts for Structural and Functional Medial Prefrontal Deficits and Increased Adrenocortical Activation. J Neurosci. 35:11897–11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selvas A, Coria SM, Kastanauskaite A, Fernaud-Espinosa I, DeFelipe J, Ambrosio E, et al. (2017): Rat-strain dependent changes of dendritic and spine morphology in the hippocampus after cocaine self-administration. Addiction biology. 22:78–92. [DOI] [PubMed] [Google Scholar]
- 56.Clark KH, Wiley CA, Bradberry CW (2013): Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotoxicity research. 23:174–188. [DOI] [PubMed] [Google Scholar]
- 57.Little KY, Ramssen E, Welchko R, Volberg V, Roland CJ, Cassin B (2009): Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res. 168:173–180. [DOI] [PubMed] [Google Scholar]
- 58.Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, et al. (2016): Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biological psychiatry. 80:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q, You J, Volkow ND, Choi J, Yin W, Wang W, et al. (2016): Chronic cocaine disrupts neurovascular networks and cerebral function: optical imaging studies in rodents. J Biomed Opt. 21:26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Suh J, Duan D, Darnley S, Jing Y, Zhang J, et al. (2017): A hypo-status in drug-dependent brain revealed by multi-modal MRI. Addict Biol. 22:1622–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adinoff B, Braud J, Devous MD, Harris TS (2012): Caudolateral orbitofrontal regional cerebral blood flow is decreased in abstinent cocaine-addicted subjects in two separate cohorts. Addict Biol. 17:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K (1988): Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 152:641–648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


