1. Introduction
Migraine affects up to 28% of children and adolescents [2, 38, 61, 79, 101]. Treatments for migraine have traditionally focused on pharmacological approaches [26], however, a recent meta-analysis found that there is little evidence supporting the superiority of pharmacologic preventive treatments in children and adolescents with migraine compared with placebo (although the overall effect was similar to adult effect for pharmacological intervention) [44]. Cognitive-behavioral therapy (CBT) is a behavioral intervention that involves the development of coping skill strategies to reduce the experience of pain [13, 36, 76]. A recent meta-analysis showed the efficacy of CBT in reducing pain in various chronic pain syndromes [13] including headache and migraine [42] and in children and adolescents with migraine [57]. Although CBT is a promising intervention to reduce headache frequency, the response rate of children and adolescents with headache to CBT ranges between 50–90% [29, 37, 41, 60, 73], suggesting that some individuals may be less responsive than others to CBT [39]. Identifying markers that could predict headache reduction after CBT is a critical step towards personalized medicine and would lead to increased success rates of CBT and improved outcomes in patients with migraine.
The response to CBT can be predicted using brain function at baseline in adult patients with chronic pain [99], and psychiatric disorders [40] [84] [6] [90]. These studies provide preliminary support for the idea that aspects of brain function before CBT could be used to predict CBT response in adolescents with migraine. Since currently, fMRI is relatively costly and may not be accessible for routine use, another measure was examined.
Quantitative sensory testing (QST) measures including the conditioned pain modulation (CPM) paradigm can be used in a clinical setting. CPM responses at baseline can predict the trajectory of postoperative pain [95] and the efficacy of pain medications in adults [14, 97] [20]. CPM is a sensory testing paradigm used to evaluate spatial filtering of nociceptive input that represents a ‘pain inhibits pain’ phenomenon of pain modulation [54, 92]. CPM has been demonstrated to be an effective treatment for the acute relief of a migraine headache [96, 98]. The mechanisms of CPM induced pain inhibition are not fully understood. One suggestion is that a portion of the CPM response relies upon cognitive mechanisms of attention [25, 49]. Similarly, engaging cognitive techniques such as cognitive reappraisal is one component of CBT [76]. Thus, CPM may be related to the effect of CBT on headache frequency via the enhancing cognitive control on pain.
In this study, we examined the relationships between neural and psychophysical parameters at baseline and reduction in headache days after CBT in adolescents with migraine. We hypothesized that brain function and CPM response would predict changes in headache frequency after 8 weeks of CBT in adolescents with migraine. For brain function, we focused on resting-state functional connectivity of the amygdala. The amygdala was chosen based on a study that found alterations in amygdalar connectivity after an interdisciplinary treatment, which included CBT, in adolescents with complex regional pain syndrome [78].
2. Methods
A detailed description of the methods can be found in a previous publication from this data set in which the brain mechanisms engaged following CBT were examined [55]. A short description of the study methods is presented below.
2.1. Participants
Participants were recruited from the Cincinnati Children’s Headache Center. Participants were between the ages of 10 - 17, diagnosed with migraine by a headache neurologist, and reported between 8 - 28 headache days/month with disability scores between 10-140 (based on the Pediatric Migraine Disability Assessment Scale (PedMIDAS) [28]). Exclusion criteria were based on incompatibility with MRI scanner (claustrophobia, orthodontic braces or other metallic implants, weight/size restrictions), presence of other chronic pain, neurological or psychiatric disorders, and medication usage (current use of preventive medication for migraine or pain). The rationale for exclusion of medication overuse and exclusion of concomitant use of preventive medication was that frequent usage of acute and active usage of preventive medications may alter neural function and pain perception [12, 14, 23, 50] and confound the results. The exclusion criteria are similar to those used for several of our previous studies [27, 66].
2.2. Study design overview
The study was approved by the Cincinnati Children’s Hospital Institutional Review Board (IRB). Informed parental consent and participant assent were obtained before study procedures began. Participants first completed a 28-day headache diary. The instructions for completing the diaries are in Supplementary Section 1. Participants were instructed to complete a paper-format diary and, if required, could ask for parental assistance. During each study visit, the diaries were collected and reviewed by the study coordinator or psychology fellow for data completeness. In the event missing data fields were identified, the study coordinator (or psychology fellow) interviewed the participant (and parent/legal guardian if applicable), and the diary was updated with information queried and collected during the study visit.
After the 28-day baseline period, participants completed a baseline visit in which they completed questionnaires, an MRI scan and, psychophysical testing. After the baseline visit, participants completed 8 weekly CBT sessions. During the 8 weeks of CBT, participants filled out a daily diary. Participants were allowed to use medications for the acute treatment of an attack during the 8 weeks of CBT. At the end of the CBT sessions, participants completed a post-treatment visit that was identical to the baseline visit. Analyses of the changes in brain function before vs. after 8 weeks of CBT are reported elsewhere [55]. Data from this study will be shared at the request of other investigators for purposes of replicating procedures and results.
2.3. CBT sessions
Sessions were about 45 minutes in duration. The CBT protocol that was used in this study was shown to reduce migraine severity (frequency and disability) [66]. CBT included discussion of gate control theory of pain, relaxation training and, cognitive restructuring.
2.4. Outcome measures
Based on recent guidelines of the International Headache Society for controlled trials of preventive treatment of migraine in children and adolescents, there are two possible efficacy endpoints to assess intervention efficacy. These endpoints are the change in headache frequency and % responder rate as measured by migraine days (traditionally been set at 50% or greater) with the recommendation of absolute reduction in headache frequency preferred [1]. Additionally, it is recommended that the responder rate should not be used to determine clinically meaningful treatment effects in individuals [1]. The absolute reduction in headaches days is also less confounded by the baseline number of days compared to percentage reduction. Thus, our outcome measure was a continuous variable of change in absolute headache days from 28-day baseline to the last 28 days of the study.
Headache days were defined as the number of days with any headache in a 24 hour period starting and ending at midnight. If headache symptoms crossed over midnight, this was considered a new and distinct headache day, which would result in a frequency count of two headache days. If painful symptoms resolved and then recurred within the same midnight to midnight period, this is considered a single headache day. Change in headache frequency was determined by examining the rate of the absolute number of headache days, per 28-day period, at baseline and during the last 28 days of the study (the last four weeks of CBT sessions). Percent headache reduction, which was the percent change in headache between baseline and during the last 28 days of the study was included as an exploratory variable in order to ensure that the interpretation of effects related to change in headache frequency (absolute days reduction) was not confounded by baseline differences in headache frequency.
2.5. Pain ratings during MRI scanning and QST
Pain intensity and unpleasantness were defined using a radio analogy [68]. Participants were asked to indicate the magnitude of pain sensation along the VAS [67].
2.6. Quantitative sensory testing
In the CPM paradigm, one noxious stimulus (e.g., test stimulus) is delivered during another noxious stimulus (e.g., conditioning stimulus), which is delivered elsewhere in the body [4]. The CPM response is defined as a reduction in the perceived magnitude of pain from the test stimulus when it is delivered alone vs. when it is delivered concurrently with the conditioning stimulus. In the current study, the test stimulus was delivered alone and then, after an 8-minute break, delivered concurrently for the last 30 seconds of the conditioning stimulus. Based on our previous meta-analysis, which indicated alterations in pain sensitivity based on stimulus location [94], we tested two CPM paradigms on two different test stimulus locations (trapezius and leg). The two test stimuli were delivered first in random order with a 2-minute break between them. An 8-minute break was kept between the application of the two conditioning stimuli.
2.6.1. Test stimuli.
Pressure pain thresholds (PPT) were used as the test stimuli in the study. Pressure was delivered with a Pressure Algometer (1cm diameter probe, Medoc, Israel), which was delivered to the lower dominant leg (anterior tibialis muscle) or the trapezius in random order. The increase rate was 60 kilopascals (kPa)/second. The pressure was gradually increased until the participant felt a sensation of pain. Once the sensation of pain was experienced, the participants were instructed to press a button and the force at pain threshold (kPa) was recorded. The PPT value was an average of two PPT tests.
2.6.2. Conditioning stimulus.
The conditioning stimulus was an immersion of the non-dominant foot into a cold-water bath (8°C) for 60 seconds. Participants rated pain intensity from this stimulus after 20 seconds of immersion using a separate mechanical VAS. During the last 30 seconds of the conditioning stimulus, the test stimulus was also delivered.
2.7. Statistical analysis of behavioral data
CPM response was calculated by the difference (Δ) between PPT obtained before vs. during the conditioning stimulus. A negative value indicates a more efficient pain modulation response (i.e. increased in PPT when the conditioning stimulus was applied). Spearman correlations were used to determine if CPM responses before CBT predict the change in headache days after CBT. Paired T-tests were used to compare the difference in headache days before and after CBT and in PPT before and during the conditioning stimulus.
Exploratory analyses were conducted in which associations between the other QST measures that were collected as part of the CPM paradigm (PPT and pain ratings to cold water immersion) and headache reduction after CBT were examined.
Another analysis was performed in order to examine if changes in headache frequency after CBT relates to the change in pain modulation. Correlations (Spearman) were calculated between the change in CPM after CBT and the change in headache days after CBT. The change in CPM response was calculated as CPM response at visit 2 minus CPM response at visit 1. A larger negative value indicates an improvement in pain modulation response. The results of these analyses are presented in Supplementary sections 2 & 3.).
Data are presented as mean ± SD. A p-value of < 0.05 was considered significant.
2.8. Neuroimaging data
2.8.1. Imaging acquisition
Participants were scanned on a 3T Philips Ingenia scanner with a 32 Channel head coil using the 5.09 software. The total scan time was up to 1 hour. A T1 anatomical scan was always completed first following two resting-state pseudo-continuous arterial spin labeling (pCASL), and two resting-state blood oxygen level-dependent (BOLD) scans in a random order. Participants were directed to close their eyes during all scans. The methods and results for pCASL are presented in Supplementary section 4 due to findings that are outside of the brain. A radiologist (R.R.) reviewed the MRI scans in order to detect incidental findings.
High-resolution T1 was performed using a multiecho weighted images. Scan parameters were TR = 10ms, TE = 1.8, 3.8, 5.8, 7.8 ms, field of view = 256 x 224 x 200mm, voxel size = 1 x 1 x 1mm, number of slices = 200, flip angle = 8, slice orientation = sagittal, total scan time = 4:42 minutes.
Scan parameters of the BOLD fMRI were TR = 2000ms, TE = 35ms, field of view = 240 x 240mm, voxel size = 3 x 3mm, slice thickness = 4mm, 34 slices, flip angle = 90 degrees, number of volumes = 193, slice orientation = transverse, slice order = ascending, dummy scans = 2 (data not saved during export from scanner), total scan duration = 6:34 minutes.
2.8.2. Image processing and statistical analysis
FSL (FMRIB’s Software Library, Oxford, UK, version 5.0.8) and scripts were used for the analyses. T1-weighted images were bias-corrected, brain extracted, segmented into the different tissue types, and masked with a probability threshold of 0.95 for white matter and CSF [31, 32]. EPI images were slice timing corrected using slicetimer and outlier detection using FSL motion outliers routines (using the default setting in which the threshold used to define an outlier is the 75th percentile + 1.5 times the InterQuartile Range). Time points of corrupted volumes were regressed out of the analysis. The mean corrupted volumes per participant was 7.9 (range of 2-27). Images were then co-registered to the high resolution T1 structural image and normalized using FLIRT. A component-based noise correction method (aCompCor) was used for noise reduction [5], and included adding the top five principal components from the white matter and CSF and 24 motion regressors. The collinearity of these nuisance regressors was not assessed since the GLM removes the shared variability from all regressors [51]. Additional image processing included data smoothing (5 mm), high-pass temporal filtering (> 0.01 hz), intensity normalization, and registration to the high-resolution structural image [19] and to the standard space (MNI152) with warp resolution of 10 mm.
Time courses of activity were extracted from the right and left amygdala. The decision to focus only on the amygdala as a seed region was based on a previous study that found involvement of the amygdala in behavioral therapy in adolescents with chronic pain [78]. The Juelich atlas was used to create a mask of the seed regions of the right and left amygdala using a 50% probability (combining amygdala sub-structures into one mask).
The time courses of each seed were separately analyzed. First-level, fixed-effects analyses were run for each BOLD series to identify voxels that had time courses that were significantly positively and negatively correlated with that of the amygdala seed. Second-level fixed effects analyses were used to examine within participants’ effects across imaging series. In the third-level random-effects analyses, demeaned values of absolute headache reduction after CBT were included in the model as a covariate of interest. Clusters of connectivity were identified using a threshold of Z > 2.3, and corrected cluster significance of p < 0.05 were estimated according to Gaussian random field theory [91].
In order to better describe the relationship between amygdalar connectivity and headache reduction, values within each cluster were extracted using Featquery from each subject. A Spearman correlation was then performed to determine the relationship between values within each cluster and headache reduction.
An additional analysis was performed to determine if the combination of neuroimaging (functional connectivity of the right amygdala) and CPM response could produce better prediction than either factor independently. Correlations between amygdalar connectivity and CPM response were examined. In addition, prediction of headache reduction was estimated using a block-wise regression model which included headache reduction as the dependent variable and 1) parameter estimate of right amygdala functional connectivity, 2) CPM data at the trapezius and, 3) both parameter estimate of right amygdala functional connectivity and CPM as the independent variables. A p-value of < 0.05 was considered significant.
3. Results
Twenty adolescents (4 Males, 16 Females; Mean age: 14.8 ± 2.2 years old, Table 1) were recruited with 19 participants completing the baseline imaging session. One participant had high anxiety about the MRI scanner and did not complete the imaging session. All 20 patients completed the QST sessions. Participants self-reported having headaches for 56.2±37.1 months prior to enrollment with average duration of untreated headaches of 7.4±6.9 hours. The average pain intensity and unpleasantness ratings during the MRI scans were 1.2±1.8 and 1.2±1.9, respectively.
Table 1-.
Characteristics of Patients
| Patient number | Age | Sex | Race | Ethnicity | Headache days at baseline | Headache days after CBT | ≥ 50% response rate |
|---|---|---|---|---|---|---|---|
| 1 | 10 | F | Caucasian | Not Hispanic or Latino | 10 | 7 | |
| 2 | 11 | F | Caucasian | Not Hispanic or Latino | 8 | 4 | + |
| 3 | 14 | F | Caucasian | Not Hispanic or Latino | 28 | 28 | |
| 4 | 15 | F | Caucasian | Not Hispanic or Latino | 24 | 13 | |
| 5 | 11 | F | Caucasian | Not Hispanic or Latino | 11 | 6 | |
| 6 | 16 | F | Caucasian | Not Hispanic or Latino | 15 | 11 | |
| 7 | 12 | M | Caucasian | Not Hispanic or Latino | 9 | 9 | |
| 8 | 17 | F | Caucasian | Not Hispanic or Latino | 9 | 2 | + |
| 9 | 15 | M | Caucasian | Not Hispanic or Latino | 9 | 8 | |
| 10 | 17 | F | African American | Not Hispanic or Latino | 8 | 2 | + |
| 11 | 17 | F | Caucasian | Not Hispanic or Latino | 27 | 11 | + |
| 12 | 16 | F | Caucasian | Not Hispanic or Latino | 16 | 10 | |
| 13 | 15 | F | Caucasian | Not Hispanic or Latino | 18 | 5 | + |
| 14 | 14 | F | African American | Not Hispanic or Latino | 16 | 13 | |
| 15 | 16 | F | Caucasian | Not Hispanic or Latino | 9 | 6 | |
| 16 | 16 | M | Caucasian | Not Hispanic or Latino | 27 | 28 | |
| 17 | 16 | F | African American | Not Hispanic or Latino | 8 | 7 | |
| 18 | 17 | F | Caucasian | Not Hispanic or Latino | 8 | 4 | + |
| 19 | 11 | M | African American | Not Hispanic or Latino | 8 | 10 | |
| 20 | 17 | F | Caucasian | Not Hispanic or Latino | 23 | 15 |
3.1. Headache frequency at baseline and after CBT
The frequency of headaches was reduced after CBT from 14.6 ± 7.4 days per 28-day assessment before CBT (range 8-28) to 9.9 ± 7.2 days per 28-day assessment after CBT (range 2-28) (p < 0.001, Table 1). Interestingly, six patients had substantial alleviation of their headaches (responder rate of 50 % or greater, patients # 2, 8, 10, 11, 13, 18) while others had no improvement, and even increase in headache frequency (for example patients # 3,7,16).
There was no correlation between headache days at baseline and the reduction in headache days after CBT such that greater headache reduction after CBT was not due to greater headaches days at baseline (r= −0.375, p=0.103). Absolute headache reduction was significantly correlated with percent headache reduction (r= −0.774, p<0.001).
3.2. BOLD functional connectivity at baseline relates to headache frequency reduction after CBT
Baseline functional connectivity of the right amygdala was related to post CBT reduction in headache days in multiple brain regions. These brain regions encompassed three separate clusters identified in the whole brain search: (i) Precentral cluster, which included the left precentral and left postcentral gyrus (Spearman r = 0.684, p = 0.001, Fig. 1A, Supplementary section 5), (ii) Anterior cingulate cortex (ACC) cluster, which included the right ACC, bilateral supplementary motor area and the right precentral gyrus (Spearman r = 0.756, p < 0.001, Fig. 1B), (iii) Frontal cluster, which includes bilateral frontal pole, bilateral paracingulate gyrus, bilateral superior frontal gyrus and the right middle frontal gyrus (Spearman r = 0.716, p = 0.001, Fig. 1C). Patients with greater baseline connectivity between the right amygdala and these clusters had greater reduction in headache days after CBT.
Figure 1. Amygdalar connectivity relates to headache frequency reduction after cognitive behavioral therapy (CBT).
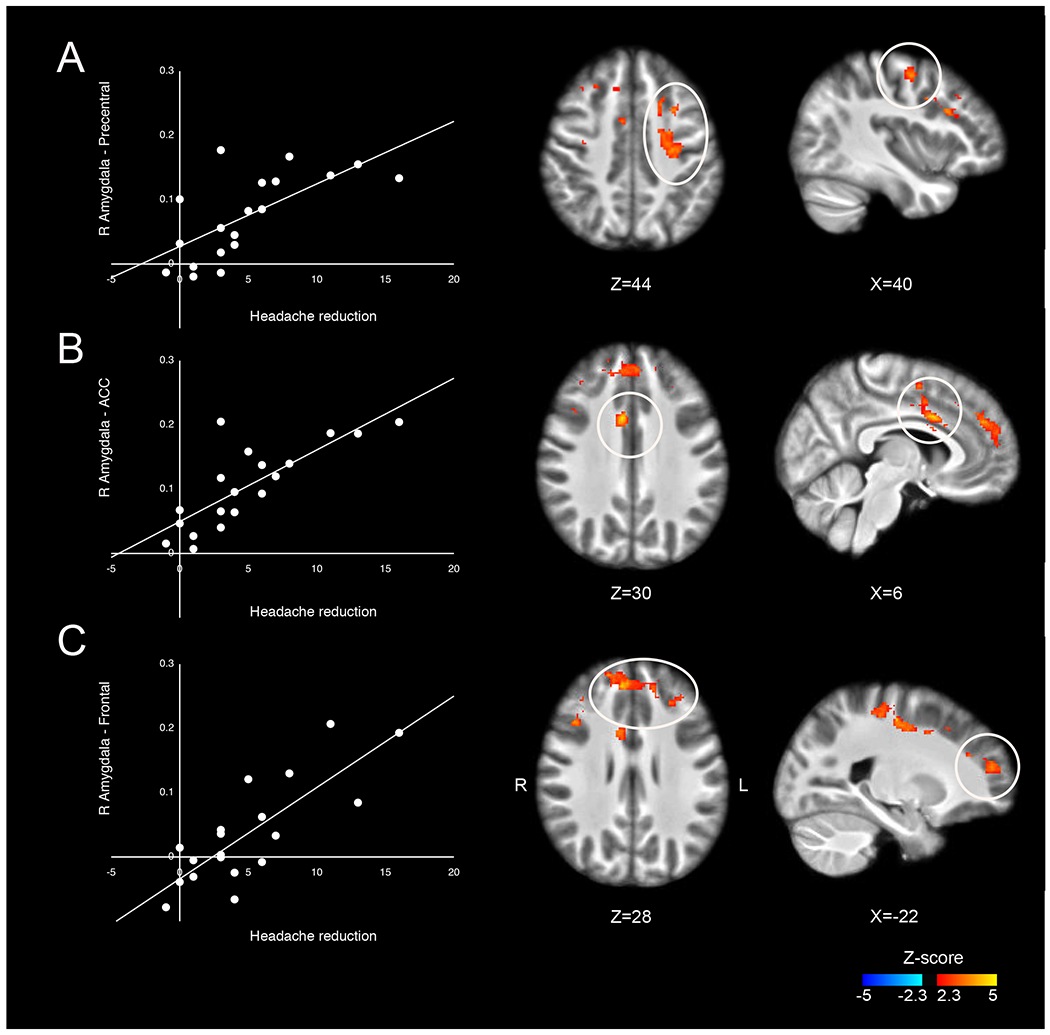
Positive correlations between headache frequency reduction after CBT and connectivity between the right amygdala and the following clusters: (A) Precentral cluster, which included the left precentral and the left postcentral gyrus; (B) ACC cluster, which included the right ACC, bilateral SMA and the right precentral gyrus; and, (C) Frontal cluster, which includes bilateral frontal pole, bilateral paracingulate gyrus, bilateral superior frontal gyrus and the right middle frontal gyrus.
For the left amygdala, no brain regions exhibited functional connectivity at baseline that was related with reductions in headache days following CBT.
3.3. CPM at baseline relates to headache frequency reduction after CBT
A significant CPM response was observed in adolescents with migraine before CBT. During the conditioning stimulus of cold immersion, a significant increase in PPT at the trapezius (t(19)=−2.36, p = 0.029) and leg (t(19)=−2.50, p = 0.022) was found for the group as a whole (Table 2). This response varied substantially across individuals. Baseline CPM responses were related to headache frequency reduction after CBT. Lower CPM response at the trapezius was related with greater reduction in headache days (r = 0.492, p = 0.028, n=20, Fig. 2). No correlation was found between CPM responses at the leg and headache frequency reduction (r = 0.240, p = 0.309, n=20, Fig. 2).
Table 2. CPM response in adolescents with migraines before Cognitive Behavioral Therapy (n=20).
Pressure pain thresholds (PPT, kPa) at the trapezius and leg were collected before (Baseline PPT) and after cold immersion of the hand (CPM PPT). CPM response reflects the magnitude of pain inhibition in which negative values indicate greater inhibition (e.g., greater increase in PPT during cold immersion).
| Testing Site | Baseline PPT (kPa) | CPM PPT (kPa) | CPM Response |
|---|---|---|---|
| Trapezius | 142.7±77.2 | 161.5±82.5 | −18.9±35.7 |
| Leg | 280.6± 124.4 | 324.4±136.8 | −43.8±78.3 |
Figure 2. Pain modulation response at baseline relates to headache frequency reduction after cognitive behavioral therapy (CBT).
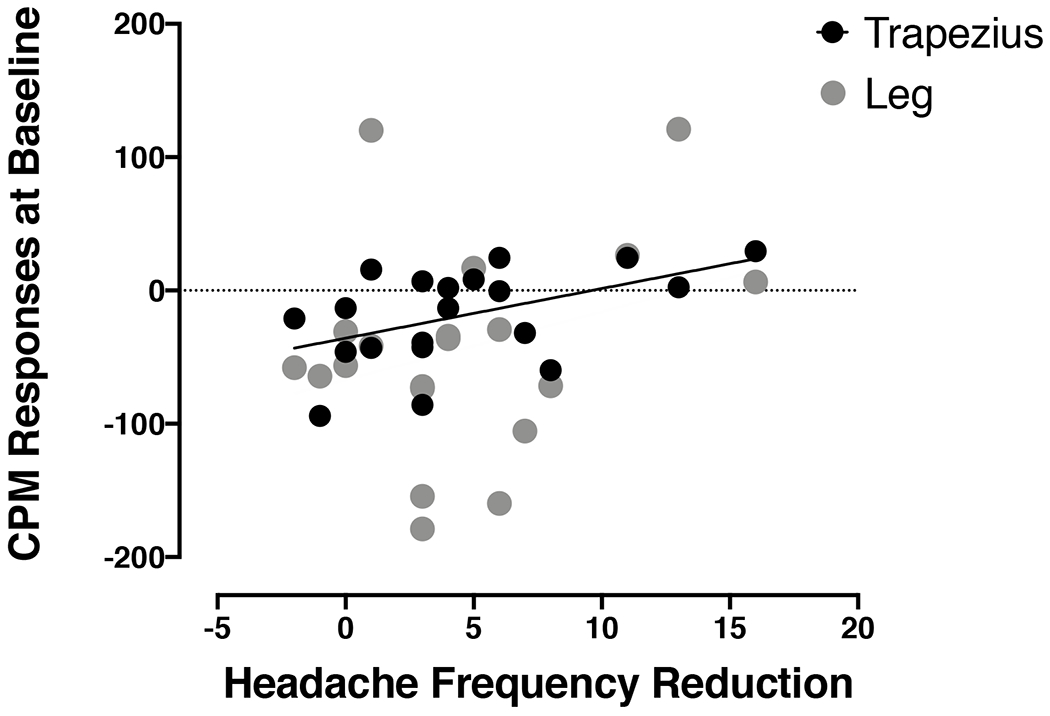
Greater reduction in headache frequency was related to lower conditioned pain modulation (CPM) response before CBT at the trapezius (r =0.492, p = 0.028, n=20) but not at the leg (r = 0.240, p = 0.309, n=20).
Additional exploratory analyses were conducted to examine the relationships between other QST measures and headache reduction after CBT. No correlations were found between headache reduction after CBT and PPT at the trapezius (r= 0.279, p=0.234), PPT at the leg (r= 0.063, p= 0.793), pain intensity of cold water immersion (r= −0.400, p= 0.080) and pain unpleasantness of cold water immersion (r= −0.355, p= 0.124).
3.4. Prediction model including both functional connectivity and CPM at baseline
Both the connectivity of the right amygdala with frontal, ACC and sensorimotor regions at baseline and CPM responses at the trapezius at baseline were each significantly correlated with headache reduction after CBT. Thus, we included both of them in a block-wise regression model in order to see if the combination would increase the explained variance (Table 3). The explained variance from the combined model for absolute headache reduction (Table 3A) was R2 = 0.712, which was similar to the explained variance from the right amygdalar connectivity alone (R2 = 0.709, n=19) but much higher than the explained variance from the CPM alone (R2 = 0.262, n=19). In the combined model, the right amygdalar connectivity was a significant predictor for headache reduction after CBT (p <0.001), but not CPM (p = 0.703, n=19). Similar results were found with percent headache change (Table 3B). The explained variance from the combined model was R2 = 0.397 which was similar to the explained variance from the right amygdalar connectivity alone (R2 = 0.377) but higher than the explained variance from the CPM alone (R2 = 0.213). In the combined model, the right amygdalar connectivity was a significant predictor for percent headache reduction after CBT (p=0.042) but not CPM (p=0.469).
Table 3.
Regression models predicting headache reduction after CBT (n=19)
| Unstandardized B | Coefficients Std. Error | Standardized Coefficient Beta | t | P value | |
|---|---|---|---|---|---|
| A. Dependent variable: absolute headache reduction | |||||
| Model 1 | |||||
| Constant | −2.223 | 0.712 | −3.122 | 0.006 | |
| Parameter estimate right amygdala | 28.877 | 4.486 | 0.842 | −6.437 | 0.000 |
| Model 2 | |||||
| Constant | −6.082 | 1.041 | −5.845 | <0.001 | |
| CPM at the trapezius | 0.63 | 0.26 | 0.512 | 2.457 | 0.025 |
| Model 3 | |||||
| Constant | −2.480 | 0.984 | −2.519 | 0.023 | |
| Parameter estimate right amygdala | 27.678 | 5.540 | 0.807 | −4.996 | <0.001 |
| CPM at the trapezius | 0.008 | 0.020 | 0.063 | 0.389 | 0.703 |
| B. Dependent variable: percent headache reduction | |||||
| Model 1 | |||||
| Constant | 24.830 | 5.784 | 4.293 | <0.001 | |
| Parameter estimate right amygdala | 116.765 | 36.440 | 0.614 | 3.204 | 0.005 |
| Model 2 | |||||
| Constant | 41.565 | 5.965 | 6.968 | <0.001 | |
| CPM at the trapezius | 0.317 | 0.148 | 0.461 | −2.142 | 0.047 |
| Model 3 | |||||
| Constant | 28.755 | 7.900 | 3.640 | 0.002 | |
| Parameter estimate right amygdala | 98.432 | 44.451 | 0.517 | 2.214 | 0.042 |
| CPM at the trapezius | 0.119 | 0.160 | 0.173 | −0.741 | 0.469 |
In order to further understand the results of the regression analysis, we sought to determine if amygdalar connectivity was also related to the CPM response.
Significant correlation was found between amygdalar connectivity and the CPM response at the trapezius (r= 0.556, p= 0.013) but not at the leg (r= 0.069, p= 0.779).
4. Discussion
CBT is a highly effective non-pharmacological intervention for patients with migraine [57], however, some patients do not respond to CBT. This study found that baseline amygdalar connectivity with frontal, ACC and sensorimotor regions predicts headache frequency reduction after CBT in adolescents with migraine. Pain modulation capacity also predicted headache frequency reduction after CBT. In a combined model, only the right amygdalar connectivity remained a significant predictor. The results of the present study conducted in adolescents, support previous literature regarding the ability of neural and psychophysical measures to predict clinical outcomes in adults [14, 22, 74, 83, 97, 100].
4.1. Baseline amygdalar connectivity relates to changes in headache frequency after CBT
This study focused on resting-state functional connectivity of the amygdala. Resting-state represents spontaneous brain activation, which is not dependent on a specific task. Interestingly, similar patterns of connectivity for the amygdala sub-regions were found during both task and resting-state [34]. This may indicate that changes that are observed during tasks would also be observed during resting-state (such in the present study).
In the present study adolescents with greater resting-state functional connectivity between the right amygdala and the ACC before CBT had greater headache frequency reduction after CBT. The amygdala is involved in sensory processing, emotional-affective and cognitive aspects of pain and is connected to other brain regions important in pain [43, 65, 77, 80, 85]. Alterations in amygdalar connectivity and structure have been observed in patients with migraine [15, 21, 45]. The ACC is involved in pain processing and plays a role in sensory, attentional and affective components of pain [8, 11, 16, 59, 75]. It interacts with multiple brain regions including regions that are involved in emotional regulation (e.g., amygdala) and cognitive regulation of pain (e.g prefrontal cortex) [9, 75, 81, 86–89]. Patients with migraine have greater activation in the ACC in response to trigeminal noxious stimuli compared to healthy controls [71].
In the present study CBT has a stronger effect in patients with pre-existing high connectivity between the right amygdala and ACC. CBT then reduces this amygdala-ACC connectivity, which was found in additional analyses performed in this data set [55]. Thus, if CBT acts via reducing the amygdala–ACC connectivity, it may have a stronger effect in patients that have greater amygdala–ACC connectivity at baseline. CBT intervention may have a small or no effect in patients that already have a reduced amygdala – ACC connectivity.
Adolescents with greater connectivity between the amygdala and PFC at baseline had a greater reduction in headache days after CBT. One component of CBT is cognitive reappraisal, in which maladaptive negative thoughts are identified and modified [76]. A meta-analysis indicates that the PFC is involved in cognitive reappraisal [7]. The amygdala and PFC are connected both structurally and functionally [17, 18, 30, 46, 47, 85, 86] with projections from the PFC to the amygdala [47, 64] and from the amygdala to PFC [3]. The amygdala has an inhibitory effect on the PFC [17, 33, 85]. Thus, the greater amygdala-PFC connectivity at baseline may suggest greater influence of the amygdala on functions within the PFC. CBT may decrease the inhibitory influence of the amygdala on the PFC. Patients with preexisting weaker amygdala-PFC connectivity may already have a less inhibitory effect of the amygdala on the PFC. Thus, in these patients, the clinical benefit from CBT may be limited.
Headache reduction after CBT was also associated with the connectivity between the amygdala and the sensorimotor cortex. Evidence from primates about connections between the amygdala and primary sensory areas suggest that the amygdala is also involved in modulating somatosensory processing [69]. Certain aspects of coping strategies in CBT are focused on muscle relaxation and controlled breathing [76], and are associated with activation in the sensory and motor cortex [24, 48, 58]. Thus, in order for the muscle relaxation and controlled breathing components of CBT to exert an effect on pain, greater connectivity between the sensorimotor cortex and the right amygdala is needed. This greater connectivity possibly allows a greater influence of sensorimotor regions on the amygdala and on the initiation of headaches. Thus, adolescents with stronger amygdala-SI resting state functional connectivity might benefit from CBT particularly related to muscle relaxation and controlled breathing.
4.2. Pain modulation and changes in headache frequency after CBT
The present study assessed pain modulation capacity using the CPM paradigm. In patients with migraine, impaired CPM capacity has been found in some studies [10, 52, 72] but not in others [35, 53, 63, 82] (for a review see [56]). Nevertheless, the CPM response can predict clinical outcomes such as chronic pain development and responses to medications in adults [95] [20] [14] [97]. Recently, a remote electrical neuromodulation device, which evokes CPM, was developed. This device is effective for the acute relief of a migraine headache [96, 98]. Even though the device can affect acute headache via the CPM mechanism, examining the CPM response should not have an effect on the study’s results. Patients were examined during a headache-free period and preventing headaches rather than treating acute headaches were examined. In addition, the CPM response was tested at the end of the QST session, which was after the neuroimaging session.
In the present study, two CPM paradigms were used based on recommendation on CPM methodology [94]. The CPM paradigms differed in the location of the test stimulus (trapezius vs. leg). CPM response at the trapezius but not at the leg predicted headache reduction in adolescents with migraine after CBT, indicating that stimulus location may be a critical factor affecting the CPM response in this population. Since the specific mechanisms underlying CPM are not known yet and cannot be discerned [93], it is difficult to explain how CPM can predict responsiveness to CBT, particularly when the primary outcome variable is the frequency of headaches vs. magnitude of pain from headaches. Moreover, when the right amygdalar connectivity was included with CPM in a regression model, CPM did not remain a significant predictor for headache reduction after CBT. Thus, the explained variance of amygdalar connectivity and CPM may overlap. However, CPM can still be used as a predictor for headache reduction after CBT when functional connectivity cannot be obtained.
4.3. Study strengths and limitations
A strength of this study is in the homogenous study sample (adolescents without preventive pain medications or other neurological or psychiatric disorders). Even though migraine is highly prevalent in children and adolescents, they remain an understudied population [62]. A limitation of this study is the somewhat small sample size. The sample size was based on a previous study that examined changes in functional connectivity in children and adolescents with chronic pain after a behavioral intervention [78]. Due to the small sample size, an analysis comparing participants based on their response rate (responders vs. non-responders) was not possible. Future studies with a larger n are needed to explore the sensitivity of our predictors to discriminate between CBT responders vs. non-responders. In addition, functional connectivity analysis does not inform about the direction of a relationship between two brain regions. Identifying the direction of the relationship is more complex and may involve brain stimulation methods or examining patients with brain lesions. Another limitation is the lack of a control group. Previous studies [29, 37, 41, 57, 60, 73] have demonstrated the efficacy of CBT in adolescents with migraine, thus the intent of this study was to determine the underlying mechanism, not to address the efficacy of CBT. It is possible that the reduction in headache frequency is not solely in response to CBT and might be also due to other factors such as an evaluation by a headache specialist, being diagnosed with migraine, being introduced to a healthy habits routine, or expecting positive treatment outcome [70]. In real-life situations, there is no option to differentiate between these factors. Thus, this study identified predictors for headache reduction in a normal clinical environment.
5. Conclusions
Amygdala connectivity with the ACC, sensorimotor, and frontal regions, and pain modulation capacity, predicts headache reduction after CBT in adolescents with migraine. This study advances the understanding of the role of the amygdala and pain modulation in preventing headaches in adolescents with migraine. The clinical relevance of this basic mechanistic study is that it is the first to identify potential predictors of CBT efficacy. Now that such predictors have been identified, a larger clinical trial that is powered to assess sensitivity and specificity of these predictors is feasible. This can lead to a personalized medicine approach in which patients with the greatest likelihood of a positive response are directed towards CBT, yielding both cost savings and greater acceptance of CBT as a treatment for migraine among patients, physicians, and insurance companies. Whether the findings of the present study are generalized to other chronic pain syndromes is yet to be determined. Future studies should examine if using a different outcome measure (e.g. reduction in pain intensity) affects the results. In addition, replicating this study in adult patients with migraine will clarify whether our findings are specific to adolescents or not.
Supplementary Material
Supplementary section 3. Changes in pain modulation response after cognitive behavioral therapy (CBT). CPM responses at the trapezius (A, p = 0.140) or leg (B, p = 0.202) did not show any significant changes before vs. after CBT. (C) greater reduction in headache days after CBT was related to greater change in CPM response at the trapezius (e.g. improved pain modulation response after CBT, r = −0.486, p = 0.030) but not CPM at the leg (r = −0.225, p = 0.340).
Acknowledgments:
We thank Dr. Marielle Kabbouche Samaha, Dr. Hope L. O’Brien, Dr. Joanne Kacperski, Jessica L. Weberding, Susan L. LeCates, Mimi N. Miller and Shannon Kathleen K. White. We thank Thomas Maloney (Cincinnati Children’s Hospital) for his aCompCor scripts and Maria Ashton (Department of Anesthesiology, Cincinnati Children’s Hospital) for her copyediting of the manuscript. This project was funded using 2016 internal funding from a Research Innovation and Pilot Program on behalf of the Basic Science Research (BSR) and the Clinical Translational, Outcomes and Health Services (CTOHS) Committee at Cincinnati Children’s.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- [1].Abu-Arafeh I, Hershey AD, Diener HC and Tassorelli C. Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine in children and adolescents, 1st edition. Cephalalgia : an international journal of headache 2019;39(7):803–816. [DOI] [PubMed] [Google Scholar]
- [2].Abu-Arefeh I and Russell G. Prevalence of headache and migraine in schoolchildren. BMJ (Clinical research ed) 1994;309(6957):765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amaral DG and Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis). The Journal of comparative neurology 1984;230(4):465–496. [DOI] [PubMed] [Google Scholar]
- [4].Arendt-Nielsen L and Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. The journal of pain : official journal of the American Pain Society 2009;10(6):556–572. [DOI] [PubMed] [Google Scholar]
- [5].Behzadi Y, Restom K, Liau J and Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 2007;37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A and Williams L. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychological medicine 2008;38(4):555–561. [DOI] [PubMed] [Google Scholar]
- [7].Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J and Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral cortex (New York, NY : 1991) 2014;24(11):2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coghill RC, Sang CN, Maisog JM and Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. Journal of neurophysiology 1999;82(4):1934–1943. [DOI] [PubMed] [Google Scholar]
- [9].Dahlke LA, Sable JJ and Andrasik F. Behavioral therapy: emotion and pain, a common anatomical background. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 2017;38(Suppl 1):157–161. [DOI] [PubMed] [Google Scholar]
- [10].de Tommaso M, Losito L, Difruscolo O, Sardaro M, Libro G, Guido M, Lamberti P and Livrea P. Capsaicin failed in suppressing cortical processing of CO2 laser pain in migraine patients. Neuroscience letters 2005;384(1–2):150–155. [DOI] [PubMed] [Google Scholar]
- [11].Devinsky O, Morrell MJ and Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain : a journal of neurology 1995;118 (Pt 1):279–306. [DOI] [PubMed] [Google Scholar]
- [12].Duale C, Daveau J, Cardot JM, Boyer-Grand A, Schoeffler P and Dubray C. Cutaneous amitriptyline in human volunteers: differential effects on the components of sensory information. Anesthesiology 2008;108(4):714–721. [DOI] [PubMed] [Google Scholar]
- [13].Eccleston C, Palermo TM, Williams AC, Lewandowski Holley A, Morley S, Fisher E and Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. The Cochrane database of systematic reviews 2014(5):Cd003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Edwards RR, Dolman AJ, Martel MO, Finan PH, Lazaridou A, Cornelius M and Wasan AD. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC musculoskeletal disorders 2016;17:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Faria V, Erpelding N, Lebel A, Johnson A, Wolff R, Fair D, Burstein R, Becerra L and Borsook D. The migraine brain in transition: girls vs boys. Pain 2015;156(11):2212–2221. [DOI] [PubMed] [Google Scholar]
- [16].Fuchs PN, Peng YB, Boyette-Davis JA and Uhelski ML. The anterior cingulate cortex and pain processing. Frontiers in integrative neuroscience 2014;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Giustino TF and Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Frontiers in behavioral neuroscience 2015;9:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goetschius LG, Hein TC, Mattson WI, Lopez-Duran N, Dotterer HL, Welsh RC, Mitchell C, Hyde LW and Monk CS. Amygdala-prefrontal cortex white matter tracts are widespread, variable and implicated in amygdala modulation in adolescents. NeuroImage 2019;191:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Greve DN and Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage 2009;48(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grosen K, Vase L, Pilegaard HK, Pfeiffer-Jensen M and Drewes AM. Conditioned pain modulation and situational pain catastrophizing as preoperative predictors of pain following chest wall surgery: a prospective observational cohort study. PloS one 2014;9(2):e90185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, Caramia F, Tinelli E and Mainero C. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia : an international journal of headache 2013;33(15):1264–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ and Apkarian AV. Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain 2012;153(12):2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hebestreit JM and May A. Topiramate modulates trigeminal pain processing in thalamo-cortical networks in humans after single dose administration. PloS one 2017;12(10):e0184406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Rezaie R, Freeman WJ, Wheless JW, Papanicolaou AC, Ruszinko M, Sokolov Y and Kozma R. Breathing as a Fundamental Rhythm of Brain Function. Frontiers in neural circuits 2016;10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hermans L, Van Oosterwijck J, Goubert D, Goudman L, Crombez G, Calders P and Meeus M. Inventory of Personal Factors Influencing Conditioned Pain Modulation in Healthy People: A Systematic Literature Review. Pain practice : the official journal of World Institute of Pain 2016;16(6):758–769. [DOI] [PubMed] [Google Scholar]
- [26].Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. The Lancet Neurology 2010;9(2):190–204. [DOI] [PubMed] [Google Scholar]
- [27].Hershey AD, Powers SW, Coffey CS, Eklund DD, Chamberlin LA and Korbee LL. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache 2013;53(5):799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA and Maynard MK. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology 2001;57(11):2034–2039. [DOI] [PubMed] [Google Scholar]
- [29].Hicks CL, von Baeyer CL and McGrath PJ. Online psychological treatment for pediatric recurrent pain: a randomized evaluation. Journal of pediatric psychology 2006;31(7):724–736. [DOI] [PubMed] [Google Scholar]
- [30].Janak PH and Tye KM. From circuits to behaviour in the amygdala. Nature 2015;517(7534):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jenkinson M, Bannister P, Brady M and Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- [32].Jenkinson M and Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis 2001;5(2):143–156. [DOI] [PubMed] [Google Scholar]
- [33].Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V and Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. The Journal of neuroscience : the official journal of the Society for Neuroscience 2010;30(15):5451–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kerestes R, Chase HW, Phillips ML, Ladouceur CD and Eickhoff SB. Multimodal evaluation of the amygdala’s functional connectivity. NeuroImage 2017;148:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kisler LB, Granovsky Y, Coghill RC, Sprecher E, Manor D, Yarnitsky D and Weissman-Fogel I. Do patients with interictal migraine modulate pain differently from healthy controls? A psychophysical and brain imaging study. Pain 2018. [DOI] [PubMed] [Google Scholar]
- [36].Knoerl R, Lavoie Smith EM and Weisberg J. Chronic Pain and Cognitive Behavioral Therapy: An Integrative Review. Western journal of nursing research 2016;38(5):596–628. [DOI] [PubMed] [Google Scholar]
- [37].Kroener-Herwig B and Denecke H. Cognitive-behavioral therapy of pediatric headache: are there differences in efficacy between a therapist-administered group training and a self-help format? Journal of psychosomatic research 2002;53(6):1107–1114. [DOI] [PubMed] [Google Scholar]
- [38].Krogh AB, Larsson B and Linde M. Prevalence and disability of headache among Norwegian adolescents: A cross-sectional school-based study. Cephalalgia 2015;35(13):1181–1191. [DOI] [PubMed] [Google Scholar]
- [39].Kroon Van Diest AM and Powers SW. Cognitive Behavioral Therapy for Pediatric Headache and Migraine: Why to Prescribe and What New Research Is Critical for Advancing Integrated Biobehavioral Care. Headache 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kumari V, Peters ER, Fannon D, Antonova E, Premkumar P, Anilkumar AP, Williams SC and Kuipers E. Dorsolateral prefrontal cortex activity predicts responsiveness to cognitive-behavioral therapy in schizophrenia. Biological psychiatry 2009;66(6):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Labbe EL and Williamson DA. Treatment of childhood migraine using autogenic feedback training. Journal of consulting and clinical psychology 1984;52(6):968–976. [DOI] [PubMed] [Google Scholar]
- [42].Lee HJ, Lee JH, Cho EY, Kim SM and Yoon S. Efficacy of psychological treatment for headache disorder: a systematic review and meta-analysis. The journal of headache and pain 2019;20(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Linnman C, Moulton EA, Barmettler G, Becerra L and Borsook D. Neuroimaging of the periaqueductal gray: state of the field. NeuroImage 2012;60(1):505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Locher C, Kossowsky J, Koechlin H, Lam TL, Barthel J, Berde CB, Gaab J, Schwarzer G, Linde K and Meissner K. Efficacy, Safety, and Acceptability of Pharmacologic Treatments for Pediatric Migraine Prophylaxis: A Systematic Review and Network Meta-analysis. JAMA pediatrics 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Maleki N, Bernstein C, Napadow V and Field A. Migraine and Puberty: Potential Susceptible Brain Sites. Seminars in pediatric neurology 2016;23(1):53–59. [DOI] [PubMed] [Google Scholar]
- [46].McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in neurobiology 1998;55(3):257–332. [DOI] [PubMed] [Google Scholar]
- [47].McDonald AJ, Mascagni F and Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 1996;71(1):55–75. [DOI] [PubMed] [Google Scholar]
- [48].McKay LC, Evans KC, Frackowiak RS and Corfield DR. Neural correlates of voluntary breathing in humans. Journal of applied physiology (Bethesda, Md : 1985) 2003;95(3):1170–1178. [DOI] [PubMed] [Google Scholar]
- [49].Moont R, Pud D, Sprecher E, Sharvit G and Yarnitsky D. ‘Pain inhibits pain’ mechanisms: Is pain modulation simply due to distraction? Pain 2010;150(1):113–120. [DOI] [PubMed] [Google Scholar]
- [50].Morgan V, Pickens D, Gautam S, Kessler R and Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005;54(5):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mumford JA, Poline JB and Poldrack RA. Orthogonalization of regressors in FMRI models. PloS one 2015;10(4):e0126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nahman-Averbuch H, Granovsky Y, Coghill RC, Yarnitsky D, Sprecher E and Weissman-Fogel I. Waning of “conditioned pain modulation”: a novel expression of subtle pronociception in migraine. Headache 2013;53(7):1104–1115. [DOI] [PubMed] [Google Scholar]
- [53].Nahman-Averbuch H, Leon E, Hunter BM, Ding L, Hershey AD, Powers SW, King CD and Coghill RC. Increased pain sensitivity but normal pain modulation in adolescents with migraine. Pain 2019. [DOI] [PubMed] [Google Scholar]
- [54].Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D and Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain 2014;155(12):2491–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nahman-Averbuch H, Schneider VJ 2nd, Chamberlin LA, Kroon Van Diest AM, Peugh JL, Lee GR, Radhakrishnan R, Hershey AD, King CD, Coghill RC and Powers SW. Alterations in Brain Function After Cognitive Behavioral Therapy for Migraine in Children and Adolescents. Headache 2020. [DOI] [PubMed] [Google Scholar]
- [56].Nahman-Averbuch H, Shefi T, Schneider VJ 2nd, Li D, Ding L, King CD and Coghill RC. Quantitative sensory testing in patients with migraine: a systematic review and meta-analysis. Pain 2018;159(7):1202–1223. [DOI] [PubMed] [Google Scholar]
- [57].Ng QX, Venkatanarayanan N and Kumar L. A Systematic Review and Meta-Analysis of the Efficacy of Cognitive Behavioral Therapy for the Management of Pediatric Migraine. Headache 2017;57(3):349–362. [DOI] [PubMed] [Google Scholar]
- [58].Oga T, Honda M, Toma K, Murase N, Okada T, Hanakawa T, Sawamoto N, Nagamine T, Konishi J, Fukuyama H, Kaji R and Shibasaki H. Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer’s cramp: an fMRI study. Brain : a journal of neurology 2002;125(Pt 4):895–903. [DOI] [PubMed] [Google Scholar]
- [59].Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA and Coghill RC. Brain mechanisms supporting discrimination of sensory features of pain: a new model. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29(47):14924–14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Osterhaus SO, Lange A, Linssen WH and Passchier J. A behavioral treatment of young migrainous and nonmigrainous headache patients: prediction of treatment success. International journal of behavioral medicine 1997;4(4):378–396. [DOI] [PubMed] [Google Scholar]
- [61].Ozge A, Sasmaz T, Bugdayci R, Cakmak SE, Kurt AO, Kaleagasi SH and Siva A. The prevalence of chronic and episodic migraine in children and adolescents. European journal of neurology 2013;20(1):95–101. [DOI] [PubMed] [Google Scholar]
- [62].Ozge A and Yalin OO. Chronic Migraine in Children and Adolescents. Current pain and headache reports 2016;20(2):14. [DOI] [PubMed] [Google Scholar]
- [63].Perrotta A, Serrao M, Sandrini G, Burstein R, Sances G, Rossi P, Bartolo M, Pierelli F and Nappi G. Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia 2010;30(3):272–284. [DOI] [PubMed] [Google Scholar]
- [64].Pinard CR, Mascagni F and McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 2012;205:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Porrino LJ, Crane AM and Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. The Journal of comparative neurology 1981;198(1):121–136. [DOI] [PubMed] [Google Scholar]
- [66].Powers SW, Kashikar-Zuck SM, Allen JR, LeCates SL, Slater SK, Zafar M, Kabbouche MA, O’Brien HL, Shenk CE, Rausch JR and Hershey AD. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. Jama 2013;310(24):2622–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Price DD, Bush FM, Long S and Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 1994;56(2):217–226. [DOI] [PubMed] [Google Scholar]
- [68].Price DD, McGrath PA, Rafii A and Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17(1):45–56. [DOI] [PubMed] [Google Scholar]
- [69].Price JL. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences 2003;985:50–58. [DOI] [PubMed] [Google Scholar]
- [70].Rastogi RG, Borrero-Mejias C, Hickman C, Lewis KS and Little R. Management of Episodic Migraine in Children and Adolescents: a Practical Approach. Current neurology and neuroscience reports 2018;18(12):103. [DOI] [PubMed] [Google Scholar]
- [71].Russo A, Tessitore A, Esposito F, Di Nardo F, Silvestro M, Trojsi F, De Micco R, Marcuccio L, Schoenen J and Tedeschi G. Functional Changes of the Perigenual Part of the Anterior Cingulate Cortex after External Trigeminal Neurostimulation in Migraine Patients. Frontiers in neurology 2017;8:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sandrini G, Rossi P, Milanov I, Serrao M, Cecchini AP and Nappi G. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia : an international journal of headache 2006;26(7):782–789. [DOI] [PubMed] [Google Scholar]
- [73].Scharff L, Marcus DA and Masek BJ. A controlled study of minimal-contact thermal biofeedback treatment in children with migraine. Journal of pediatric psychology 2002;27(2):109–119. [DOI] [PubMed] [Google Scholar]
- [74].Schmidt-Wilcke T, Ichesco E, Hampson JP, Kairys A, Peltier S, Harte S, Clauw DJ and Harris RE. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. NeuroImage Clinical 2014;6:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ and Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature reviews Neuroscience 2011;12(3):154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Simons LE and Basch MC. State of the art in biobehavioral approaches to the management of chronic pain in childhood. Pain management 2016;6(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L and Borsook D. The human amygdala and pain: evidence from neuroimaging. Human brain mapping 2014;35(2):527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Simons LE, Pielech M, Erpelding N, Linnman C, Moulton E, Sava S, Lebel A, Serrano P, Sethna N, Berde C, Becerra L and Borsook D. The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain 2014;155(9):1727–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Split W and Neuman W. Epidemiology of migraine among students from randomly selected secondary schools in Lodz. Headache 1999;39(7):494–501. [DOI] [PubMed] [Google Scholar]
- [80].Stefanacci L and Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. The Journal of comparative neurology 2000;421(1):52–79. [DOI] [PubMed] [Google Scholar]
- [81].Stevens FL, Hurley RA and Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. The Journal of neuropsychiatry and clinical neurosciences 2011;23(2):121–125. [DOI] [PubMed] [Google Scholar]
- [82].Teepker M, Kunz M, Peters M, Kundermann B, Schepelmann K and Lautenbacher S. Endogenous pain inhibition during menstrual cycle in migraine. European journal of pain (London, England) 2014;18(7):989–998. [DOI] [PubMed] [Google Scholar]
- [83].Tetreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV and Baliki MN. Brain Connectivity Predicts Placebo Response across Chronic Pain Clinical Trials. PLoS biology 2016;14(10):e1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Thompson DG, Kesler SR, Sudheimer K, Mehta KM, Thompson LW, Marquett RM, Holland JM, Reiser R, Rasgon N, Schatzberg A and O’Hara RM. FMRI activation during executive function predicts response to cognitive behavioral therapy in older, depressed adults. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2015;23(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Thompson JM and Neugebauer V. Amygdala Plasticity and Pain. Pain research & management 2017;2017:8296501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Thompson JM and Neugebauer V. Cortico-limbic pain mechanisms. Neuroscience letters 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Vogt BA. Midcingulate cortex: Structure, connections, homologies, functions and diseases. Journal of chemical neuroanatomy 2016;74:28–46. [DOI] [PubMed] [Google Scholar]
- [88].Vogt BA and Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. The Journal of comparative neurology 1987;262(2):271–289. [DOI] [PubMed] [Google Scholar]
- [89].Vogt BA, Rosene DL and Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science (New York, NY) 1979;204(4389):205–207. [DOI] [PubMed] [Google Scholar]
- [90].Whitfield-Gabrieli S, Ghosh SS, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai XJ, Reynolds GO, Hofmann SG, Pollack MH and Gabrieli JD. Brain connectomics predict response to treatment in social anxiety disorder. Molecular psychiatry 2016;21(5):680–685. [DOI] [PubMed] [Google Scholar]
- [91].Worsley KJ, Evans AC, Marrett S and Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. JCerebBlood Flow Metab 1992;12:900–918. [DOI] [PubMed] [Google Scholar]
- [92].Yarnitsky D Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015;156 Suppl 1:S24–31. [DOI] [PubMed] [Google Scholar]
- [93].Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S and Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. European journal of pain (London, England) 2010;14(4):339. [DOI] [PubMed] [Google Scholar]
- [94].Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen KB, Stubhaug A, Treede RD and Wilder-Smith OH. Recommendations on practice of conditioned pain modulation (CPM) testing. European journal of pain (London, England) 2015;19(6):805–806. [DOI] [PubMed] [Google Scholar]
- [95].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA and Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138(1):22–28. [DOI] [PubMed] [Google Scholar]
- [96].Yarnitsky D, Dodick DW, Grosberg BM, Burstein R, Ironi A, Harris D, Lin T and Silberstein SD. Remote Electrical Neuromodulation (REN) Relieves Acute Migraine: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Headache 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M and Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012;153(6):1193–1198. [DOI] [PubMed] [Google Scholar]
- [98].Yarnitsky D, Volokh L, Ironi A, Weller B, Shor M, Shifrin A and Granovsky Y. Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology 2017;88(13):1250–1255. [DOI] [PubMed] [Google Scholar]
- [99].Yoshino A, Okamoto Y, Okada G, Takamura M, Ichikawa N, Shibasaki C, Yokoyama S, Doi M, Jinnin R, Yamashita H, Horikoshi M and Yamawaki S. Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychological medicine 2018;48(7):1148–1156. [DOI] [PubMed] [Google Scholar]
- [100].Zeng P, Huang J, Wu S, Qian C, Chen F, Sun W, Tao W, Liao Y, Zhang J, Yang Z, Zhong S, Zhang Z, Xiao L and Huang B. Characterizing the Structural Pattern Predicting Medication Response in Herpes Zoster Patients Using Multivoxel Pattern Analysis. Frontiers in neuroscience 2019;13:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zwart JA, Dyb G, Holmen TL, Stovner LJ and Sand T. The prevalence of migraine and tension-type headaches among adolescents in Norway. The Nord-Trondelag Health Study (Head-HUNT-Youth), a large population-based epidemiological study. Cephalalgia 2004;24(5):373–379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary section 3. Changes in pain modulation response after cognitive behavioral therapy (CBT). CPM responses at the trapezius (A, p = 0.140) or leg (B, p = 0.202) did not show any significant changes before vs. after CBT. (C) greater reduction in headache days after CBT was related to greater change in CPM response at the trapezius (e.g. improved pain modulation response after CBT, r = −0.486, p = 0.030) but not CPM at the leg (r = −0.225, p = 0.340).


