STRUCTURED ABSTRACT:
Aims:
We sought to characterize the direction and associated factors of eGFR change following diagnosis of youth-onset type 1 and type 2 diabetes.
Methods:
We assessed the direction of eGFR change at two visits (mean 6.6 years apart) in SEARCH, a longitudinal cohort study of youth-onset type 1 and type 2 diabetes. We used the CKiDCr-CysC equation to estimate GFR and categorized ‘rising’ and ‘declining’ eGFR as an annual change of ≥3ml/min/1.73m2 in either direction. Multivariable logistic regression evaluated factors associated with directional change in eGFR.
Results:
Estimated GFR declined in 23.8% and rose in 2.8% of participants with type 1 diabetes (N=1225; baseline age 11.4 years), and declined in 18.1% and rose in 15.6% of participants with type 2 diabetes (N=160; baseline age 15.0 years). Factors associated with rising and declining eGFR (versus stable) in both type 1 and type 2 diabetes included sex, age at diagnosis, baseline eGFR and difference in fasting glucose between study visits. Additional factors in type 1 diabetes included time from baseline visit, HbA1c and body mass index.
Conclusions:
Over the first decade of diabetes, eGFR decline is more common in type 1 diabetes whereas eGFR rise is more common in type 2 diabetes.
Keywords: Diabetes, eGFR, Kidney, Longitudinal, Youth, Trajectory
1.1. INTRODUCTION
Diabetes is the leading cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) in the United States and worldwide.[1, 2]. The economic burden of diabetes increases with progression from one CKD stage to the next, with the greatest cost incurred from diabetic ESKD, totaling more than 14 trillion U.S. dollars in 2014.[1, 3] Diabetic Kidney Disease (DKD) is responsible for much of the increased mortality risk in diabetes, mainly attributable to cardiovascular events, the risk of which, increases linearly with decreasing estimated glomerular filtration rate (eGFR).[4]
The rising prevalence of youth-onset diabetes (<20 years of age) is particularly worrisome from a public health viewpoint, as it increases population-level lifetime exposure to the diabetic milieu, thus increasing the opportunity for development of diabetic complications. From 2001 to 2009, the prevalence of youth onset type 1 diabetes increased by 21% and type 2 diabetes by 30%.[5] Kidney-related complications in youth onset type 2 diabetes take a particularly aggressive course. After only 5 years of diabetes, 17–42% of youth with type 2 diabetes exhibit microalbuminuria, as opposed to 11% in youth with type 1 diabetes.[6, 7]. Moreover, albuminuria is more likely to regress in type 1 diabetes and more likely to progress in type 2 diabetes.[8] The risk for ESKD and death after 20 years of diabetes is at least two-fold higher in youth-onset type 2 diabetes versus type 1 diabetes.[9, 10] Based on epidemiologic trends, we anticipate a substantial increase in the human and financial costs from DKD.
While current diabetes management strategies slow the progression of DKD, efforts have focused on reductions in albuminuria, yet the prevalence of decreased eGFR (<60ml/min/1.73m2) has risen.[11] Decreased eGFR with normoalbuminuria occurs in a substantial proportion of people with diabetes and is more common in women and in type 2 diabetes.[12, 13] While historically albuminuria was considered the universal first step of progression to DKD, followed by GFR decline, recent evidence suggests that distinct pathophysiologic and molecular mechanisms are responsible for the two.[14] Biopsy studies show variability in structural characteristics, some of which associate with the magnitude of albuminuria and others with measured GFR.[15, 16] Improved understanding of the early natural history of eGFR changes and associated clinical, structural and molecular factors is imperative for identifying novel biomarkers and therapeutic drug targets for DKD.
Numerous studies have documented the natural history of eGFR decline in DKD, mostly in diabetes of long duration, after GFR has dropped below 90ml/min/1.73m2. Studies of the earliest changes in eGFR are necessary to provide insight into DKD at a stage when interruption of the disease process is most feasible and may have the greatest impact. In early DKD, eGFR may rise toward hyperfiltration (>130ml/min) or fall toward subnormal (<90ml/min) levels. Both phenomena carry increased risk for progression to advanced kidney disease, but the precise time course and clinical factors associated with these changes have not been studied.[17–20]
We sought to: (1) characterize and compare the direction of eGFR changes over the initial years after diagnosis of youth-onset type 1 and type 2 diabetes; and (2) determine the sociodemographic and clinical factors associated with direction of eGFR change.
1.2. SUBJECTS, MATERIALS AND METHODS
1.2.1. Population
Youth with diabetes diagnosed at < 20 years of age were identified from a population-based incidence registry network at five U.S. sites (South Carolina, Ohio, Colorado, Washington, and California) by the SEARCH for Diabetes in Youth Registry Study [21]. Individuals with a new diagnosis of type 1 diabetes or type 2 diabetes in 2002–2006 or 2008 were invited for a baseline visit a mean of 9.4 ± 6.2 months after diagnosis, to measure risk factors for diabetes complications. In 2011–2015, those with ≥ 5 years of diabetes duration and a previously completed baseline visit were invited to participate in a follow-up visit, at which time diabetes risk factors and early diabetes-related complications were assessed (Figure 1). The follow-up visit was completed by 2,777 participants at a mean age of 17.9 + 4.8, and mean diabetes duration of 8.0 ± 2.0 years. A flow chart depicting exclusion criteria is shown in Figure 2. The distribution of demographic, metabolic, and socioeconomic characteristics of participants completing the follow-up visit were similar to that of the larger SEARCH registry.[22] The study was approved by Institutional Review Boards with jurisdiction, and the parent, participant or both provided consent or assent.
Figure 1:
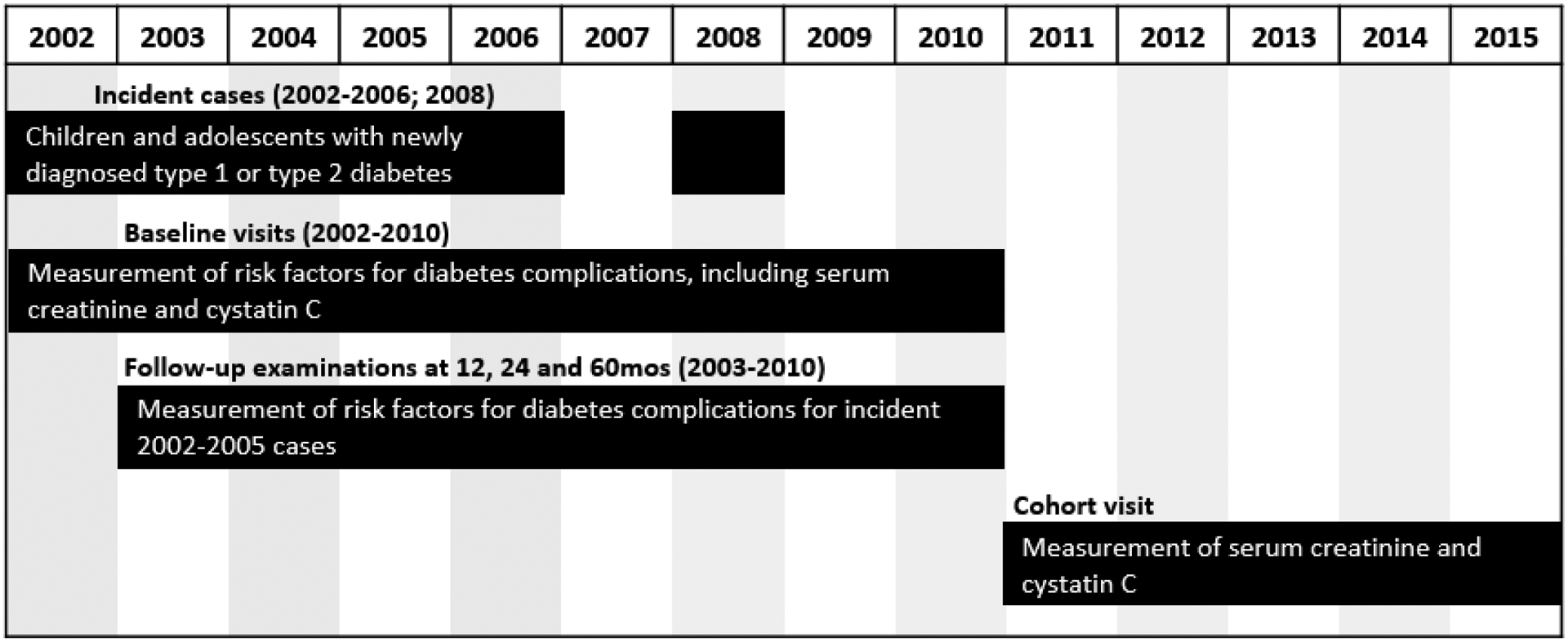
Study design of the SEARCH for Diabetes in Youth Study.
Figure 2.
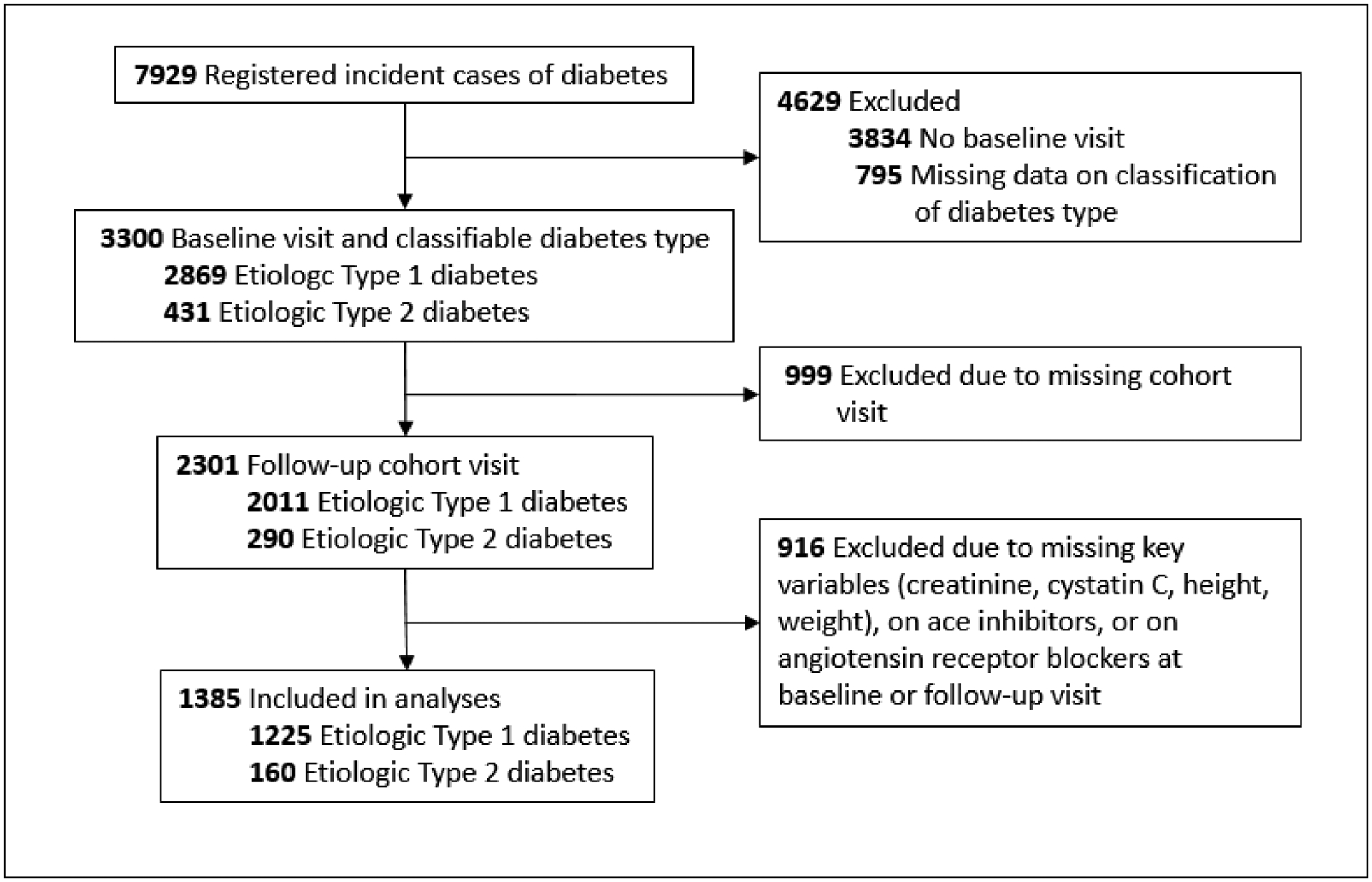
Flow chart of participants of the SEARCH for Diabetes in Youth Study included in the current analyses.
1.2.2. Data Collection and Laboratory Analyses
Race and ethnicity were self-identified using U.S. census methods [23]. Body mass index (BMI) was defined as weight (kilograms) divided by the square of the height (meters2) and converted to a Z score [24]. Waist circumference was measured using the natural waist location. Three systolic and diastolic blood pressures measured with an aneroid manometer after 5 minutes of rest were averaged.
A blood draw occurred after an overnight fast, and was analyzed for hemoglobin A1c (HbA1c), glucose, lipids, creatinine (Jaffe), cystatin-C, and diabetes autoantibodies (GAD-65 antibodies (GAD65) and insulinoma-associated-2 antibodies (IA-2A) at the study’s central laboratory (Northwest Lipid Metabolism and Diabetes Research, Seattle, WA)) [25]. Zinc-T8 autoantibody was analyzed at the Eisenbarth laboratory (University of Colorado, Aurora, CO) [26]. Urine albumin-to-creatinine ratio (UACR) was measured from a randomly collected urine sample. Cystatin C was measured using Siemens reagent on a BNII nephelometer (Siemens Healthcare Diagnostics). As noted in other studies, we identified an assay drift in cystatin C [27]. Calibration equations were utilized to make results traceable to the International Federation of Clinical Chemistry Standard.[28]
1.2.3. Assignment of Diabetes Type
Diabetes type was defined using an etiological classification [29, 30] based on one or more positive diabetes autoantibodies and estimated insulin sensitivity score (validated equation including waist circumference, A1c and triglyceride levels) at the baseline visit.[29] Type 1 diabetes was defined as at least one positive antibody or insulin sensitivity (score ≥8.15). Type 2 diabetes was defined as negative antibodies and insulin insensitivity (score <8.15).
1.2.4. Change in eGFR
We estimated GFR and its change using the Chronic Kidney Disease in Children (CKiDCr-CysC) equation, developed and validated in children with chronic kidney disease and used broadly in clinical and research settings.[31]
As a sensitivity analysis, we estimated GFR and its change using the BouvetCr-CysC equation [32] due to the following features: inclusion of creatinine and cystatin c, which allows greater accuracy and precision in GFR estimation in both children and adults [31, 33]; validity up to 23 years of age [32]; and excellent performance at normal GFR and in hyperfiltration states (100% accuracy within 30% of measured GFRs between 90 and 200ml/min/1.73m2) [34].
Annual change in eGFR was determined by the difference in eGFR between visits, divided by the number of years between visits. Studies have shown that measured GFR remains very stable throughout childhood and into early adulthood [35, 36]. The rate of decline of GFR among people with DKD is highly variable, but typically ranges 3–5ml/min/1.73m2 per year.[37, 38] This led us to define any aberrant annual change in eGFR as ≥ 3mL/min/1.73m2 per year in either direction, as this is well beyond what would be expected for youth and young adults without diabetes, and is not out of range of what would be seen in people with DKD. Over the course of a minimum 5 year interval between the two visits, a change of > 3mL/min/1.73m2 per year translates to a conservative definition of aberrant GFR change (+/− 15ml/min/1.73m2), even considering day-to-day variation and the error inherent in the estimating equations [39].
1.2.5. Statistical Analyses
We performed statistical analyses using SAS, version 9.4 (SAS Institute, Cary, NC).[40] Within each diabetes type, means and percentages of demographic and clinical characteristics were compared using chi square and t-tests. We selected the sample based on key information necessary for analyses (exposure/outcome variables) and assumed missing values in the selected sample were missing at random (Table 1). Transformations were done for continuous characteristics with skewed distributions. We used bivariate and multivariable linear analyses to determine whether baseline and time-varying characteristics (where appropriate) were predictive of the direction of eGFR change. There were 19 participants with type 1 diabetes who were missing one or more covariates and hence were excluded from multivariable analyses (N=1206). Multivariable logistic regression with a generalized logit link was used to evaluate the association between characteristics and categorical eGFR outcome variables (decline, stable, rise). Variables were selected based on existing data and clinical plausibility and included: age at diagnosis, sex (male/female), black race, time from baseline visit, baseline eGFR, glucose change from baseline to follow-up (to account for transient hyperfiltration due to acute hyperglycemia), and time varying HbA1c, BMI-Z score and (systolic blood pressure) SBP-Z score. The AUC variables are longitudinal cumulative exposure variables calculated as the weighted average of the measures of interest during the participant’s time in the study (which varied between 2 and 5 visits) and weighted for the interval between each measurement. Statistical significance when comparing baseline demographics was set at an alpha of 0.0001 to account for multiple comparisons.
Table 1.
Baseline sociodemographic and clinical characteristics of 1,385 participants from the SEARCH for Diabetes in Youth Cohort Study.
| Demographic and Clinical Characteristics | Type 1 DM | Type 2 DM | p-value | ||
|---|---|---|---|---|---|
| missing | n=1225 | missing | n=160 | ||
| Female sex, N (%) | 0 | 598 (48.8) | 0 | 104 (65.0) | 0.0001 |
| Race/Ethnicity, N (%) | |||||
| Age at diagnosis, years, mean (SD) | 0 | 10.6 (3.7) | 0 | 14.0 (2.6) | <0.0001 |
| Age at baseline visit, years, mean (SD) | 0 | 11.4 (3.7) | 0 | 15.0 (2.7) | <0.0001 |
| Duration at baseline visit, months, mean (SD) | 0 | 9.2 (6.2) | 0 | 11.2 (6.3) | 0.0002 |
| Insulin Sensitivity Score (AUC) | 11 | 8.4 (2.6) | 0 | 3.9 (1.6) | <0.0001 |
| HbA1c (AUC), %, mean (SD) | 5 | 8.5 (1.3) | 0 | 8.3 (2.3) | 0.2 |
| HbA1c (AUC), mmol/mol, mean (SD) | 5 | 69 (15) | 0 | 67(25) | 0.2 |
| BMI z-score (AUC) | 0 | 0.6 (0.9) | 0 | 1.9 (0.7) | <0.0001 |
| SBP z-score (AUC) | 9 | −0.5 (0.9) | 0 | 0.8 (1.1) | <0.0001 |
| Total Cholesterol, mmol/L (AUC)* | 42 | 4.2 (0.9) | 8 | 4.2 (1.3) | 0.03 |
| Triglycerides, mmol/L (AUC)* | 42 | 0.8 (0.4) | 8 | 1.3 (2.0) | <0.0001 |
| HDL, mmol/L (AUC) | 43 | 1.4 (0.3) | 8 | 1.1 (0.3) | <0.0001 |
| eGFR at baseline, ml/min/1.73m2 | 0 | 128.4 (22.0) | 0 | 117.4 (22.7) | <0.0001 |
| Albumin/creatinine ratio, mg/mmol (AUC)* | 250 | 0.87 (0.92) | 50 | 1.02 (2.55) | 0.002 |
presented as median (IQR) with tests done on transformed variables due to skewed distributions
1.3. RESULTS
Participants meeting inclusion criteria were 1,225 youth and young adults with type 1 diabetes and 160 with type 2 diabetes. Stratifying by diabetes type, baseline sociodemographic and clinical characteristics of participants are shown in Table 1. With the exception of HbA1c, there were statistically significant differences between diabetes types in all demographic and clinical characteristics (see Table 1).
1.3.1. eGFR Trajectories
Using the CKiDCr-CysC equation and a priori specified criteria to define change (decline versus stable versus rise), the majority of youth and young adults with type 1 and type 2 diabetes maintained a stable eGFR between the baseline and follow-up visit. Decline in eGFR occurred in 23.8% of participants with type 1 diabetes and 18.1% with type 2 diabetes. An increase in eGFR occurred in 2.8% of participants with type 1 diabetes and in 15.6% with type 2 diabetes. The classification of eGFR change as estimated by the CKiDCr-CysC and BouvetCr-CysC equations were in agreement for 67% of the type 1 diabetes and 65% of the type 2 diabetes participants (Tables 2A and 2B), with the BouvetCr-CysC equation classifying more participants, as having a rise in eGFR, regardless of diabetes type.
Table 2A.
Agreement between directional change of eGFR estimated by the CKiDCr-CysC versus BouvetCr-CysC equations from baseline to follow-up visit occurring a mean of 6.6 years later among 1225 SEARCH Cohort Study participants with type 1 diabetes.
| eGFR BouvetCr-CysC | ||||
|---|---|---|---|---|
| eGFR BouvetCr-CysC | Decline | Stable | Rise | Row totals, N (%) |
| Decline | 214 | 78 | 0 | 292 (23.8) |
| Stable | 199 | 579 | 121 | 899 (73.4) |
| Rise | 0 | 5 | 29 | 34 (2.8) |
| Column totals, N (%) | 413 (33.7) | 662 (54.0) | 150 (12.2) | 1225 |
Table 2B.
Agreement between directional change of eGFR estimated by the CKiDCr-CysC versus BouvetCr-CysC equations from baseline to follow-up visit occurring a mean of 6.6 years later among 160 SEARCH Cohort Study participants with type 2 diabetes.
| eGFR BouvetCr-CysC | ||||
|---|---|---|---|---|
| eGFR BouvetCr-CysC | Decline | Stable | Rise | Row totals, N (%) |
| Decline | 13 | 15 | 1 | 29 (18.1%) |
| Stable | 2 | 66 | 38 | 106 (66.3%) |
| Rise | 0 | 0 | 25 | 25 (15.6%) |
| Column totals, N (%) | 15 (9.4) | 81 (50.6) | 64 (40) | 160 |
1.3.2. Factors Associated with eGFR Trajectories
Results of multivariable logistic regression analyses for the outcome of direction of change in eGFR are depicted among those with type 1 diabetes in Table 3A. Characteristics associated with eGFR decline versus stable eGFR included male sex, younger age at diagnosis, shorter time between study visits, higher baseline eGFR, lower glucose level at the follow up compared to baseline visit and lower time-averaged HbA1c. Associations comparing eGFR rise versus stable eGFR were essentially the opposite, with the exception of the time between study visits.
Table 3A.
Multivariable logistic regression (with generalized logic link) analyses of factors associated with annual change in estimated glomerular filtration rate as calculated by the CKiD Cr-CysC equation with ‘Stable’ as the reference group (n=1206) among SEARCH participants with type 1 diabetes.
| Type 1 DM | Overall p-value | Decline (vs Stable) | Rise (vs Stable) | ||||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | ||
| Female sex | <0.0001 | 0.3 | 0.2, 0.4 | <0.0001 | 5.5 | 2.0, 14.7 | 0.0007 |
| NH Black race | 0.7 | 1.0 | 0.6, 1.8 | 1.0 | 0.6 | 0.2, 2.1 | 0.4 |
| Age at diagnosis (years) | 0.0001 | 1.1 | 1.0, 1.1 | 0.003 | 1.2 | 1.1, 1.4 | 0.001 |
| Time from baseline visit (years) | <0.0001 | 0.8 | 0.7, 0.8 | <0.0001 | 0.5 | 0.3, 0.6 | <0.0001 |
| Baseline eGFR (ml/min/1.73m2) (10 unit increase) | <0.0001 | 1.7 | 1.6, 1.9 | <0.0001 | 0.9 | 0.8, 1.2 | 0.6 |
| Glucose change from baseline (mg/dl) (50 unit increase) | <0.0001 | 0.8 | 0.8, 0.9 | <0.0001 | 1.3 | 1.1, 1.5 | 0.002 |
| HbA1c (%) (AUC) | <0.0001 | 0.7 | 0.6, 0.8 | <0.0001 | 2.0 | 1.5, 2.7 | <0.0001 |
| Body mass index Z-score (AUC) | <0.0001 | 1.5 | 1.2, 1.8 | <0.0001 | 1.0 | 0.6, 1.6 | 1.0 |
| Systolic Blood pressure Z-score (AUC) | 0.002 | 0.7 | 0.6, 0.8 | 0.0004 | 0.9 | 0.6, 1.5 | 0.7 |
Multivariable model also adjusts for geographic site
In multivariate analyses of the type 2 diabetes cohort (Table 3B), characteristics associated with decline in eGFR compared to stable eGFR included male sex, younger age at diagnosis, higher baseline eGFR and lower glucose level at the follow up compared to baseline. For rising versus stable eGFR, female sex, lower baseline eGFR, shorter duration between visits, higher glucose level at the follow-up compared to baseline visit and higher time-averaged HbA1c were significant. In sensitivity analysis, we found that our results were largely robust to choice of GFR estimating equation (Supplemental Tables 1A and 1B).
Table 3B.
Multivariable logistic regression (with generalized logic link) analyses of factors associated with annual change in estimated glomerular filtration rate as calculated by the CKiD Cr-CysC equation with ‘Stable’ as the reference group (n=160) among SEARCH participants with type 2 diabetes.
| Type 2 DM | Overall p-value | Decline (vs Stable) | Rise (vs Stable) | ||||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | ||
| Female sex | 0.002 | 0.2 | 0.1, 0.7 | 0.01 | 7.5 | 1.2, 46.1 | 0.03 |
| NH Black race | 0.9 | 0.9 | 0.3, 3.5 | 0.9 | 1.3 | 0.3, 5.9 | 0.8 |
| Age at diagnosis (years) | 0.009 | 0.7 | 0.5, 0.9 | 0.002 | 0.9 | 0.7, 1.2 | 0.5 |
| Time from baseline visit (years) | 0.08 | 0.7 | 0.5, 1.0 | 0.08 | 0.7 | 0.5, 1.1 | 0.1 |
| Baseline eGFR (ml/min/1.73m2) (10 unit increase) | 0.0004 | 1.8 | 1.3, 2.4 | 0.0002 | 0.9 | 0.6, 1.3 | 0.5 |
| Glucose change from baseline (mg/dl) (50 unit increase) | <0.0001 | 0.8 | 0.6, 1.1 | 0.1 | 2.4 | 1.6, 3.5 | <0.0001 |
| HbA1c (%) (AUC) | 0.2 | 0.8 | 0.6, 1.1 | 0.1 | 1.1 | 0.8, 1.5 | 0.8 |
| Body mass index Z-score (AUC) | 0.5 | 1.5 | 0.6, 4.1 | 0.4 | 0.6 | 0.2, 2.5 | 0.5 |
| Systolic Blood pressure Z-score (AUC) | 0.3 | 1.6 | 0.9, 2.7 | 0.1 | 1.1 | 0.6, 2.2 | 0.8 |
Multivariable model also adjusts for geographic site
1.4. DISCUSSION
The rising incidence of type 1 and type 2 diabetes in youth [5] portends a surge in CKD and ESKD in the coming years. In contrast to the known stability of GFR over the course of childhood and early adulthood in the healthy state [35, 36], we have provided the first documentation that aberrant changes in eGFR (≥3mL/min/1.73m2 per year) can occur early in the early years following diabetes diagnosis. Moreover, we have shown that the direction of change in eGFR differs by diabetes type, wherein, youth and young adults with type 1 diabetes have a greater risk for eGFR decline than rise, while those with type 2 diabetes are at greater risk of rise than decline.
1.4.1. Effect of Diabetes Type
The type 2 diabetes group has a much higher prevalence of obesity than those with type 1 diabetes, which may account for the greater proportion with rising eGFR, as obesity can result in a hyperfiltration state even in the absence of diabetes.[41] Additionally, there has been speculation that insulin resistance, rather than obesity, plays a causal role in the development of hyperfiltration in type 2 diabetes.[42] The opposing direction of eGFR change between the type 1 and type 2 diabetes groups could also be related to the older age of type 2 diabetes onset as opposed to type 1 diabetes. In support of this potential explanation is the finding that age of diabetes onset was older within the type 1 diabetes group with rising (13.6 yrs) versus declining (8.6 yrs) eGFR (data not shown). The pathophysiologic basis for this is unclear, as GFR is constant between ages 2 and about 35–40 in the healthy, physiologic state.[35, 43]
1.4.2. Effect of Baseline eGFR
Baseline eGFR was a highly significant predictor of direction of eGFR trajectory in both type 1 and type 2 diabetes, even after accounting for differences in acute glycemia at the time of eGFR measurement. It is possible that the observed changes may be partially due to regression to the mean [44], however, this seems unlikely given our conservative definition of eGFR change (≥3mL/min/1.73m2 per year) and since adjustment for baseline eGFR did not entirely explain the differences between the three groups. The baseline visit occurred soon after diabetes diagnosis, and there was no statistical difference in diabetes duration at the baseline visit between those with rising versus declining eGFR. It may be that some individuals are at higher risk of developing hyperfiltration within the first year of diagnosis, which predicts GFR decline over the ensuing several years. Our data suggests this may be the case in those who are younger at diagnosis, among females and particularly among youth and young adults with type 2 diabetes. Hyperfiltration has been shown to be a predictor of rapid (>3ml/min/1.73m2/year) GFR decline among adults with type 1 diabetes.[18]
1.4.3. Effect of Glycemia
Both acute and chronic glycemic status influenced direction and magnitude of eGFR change. Physiology studies using 24-hour euglycemic clamps and measured GFR have demonstrated that an acute high glucose level over a number of hours reliably causes an increase in GFR.[45–47] We observed this pattern as well; within both diabetes types, those with eGFR rise had the highest gain in glucose between visits, and those with eGFR decline between visits had the least gain or a decline in glucose between visits (type 1 diabetes) or had a lower serum glucose level at follow up than at baseline (type 2 diabetes) (data not shown). To account for this effect, the change in fasting glucose between the two visits was included in the multivariate model. The reason for this physiologic effect may lie in the sodium-glucose cotransporter of the proximal tubule, which, under circumstances of hyperglycemia, will also reabsorb sodium, resulting in decreased sodium delivery to the macula densa and resultant vasodilatation of the afferent arteriole.[48, 49]
1.4.4. Study Limitations
This study has limitations, the most notable being lack of measured GFR. Choice of the appropriate GFR estimating equation proved quite difficult, given that obesity, hyperfiltration, hyperglycemia, and the transition from childhood to adulthood all affect accuracy and validity of GFR estimates. We utilized the CKiDCr-CysC equation because it is widely used in clinical pediatrics and hence provides the most clinical relevance. One caveat to this is that it was derived and validated within a cohort of children with CKD and hence performs best within a much lower range of GFR than that seen in our study population. In contrast, the BouvetCr-CysC equation was derived in a cohort of children and young adults up to age 23 with normal or supranormal GFR, leading to excellent performance in this range of GFR [32]. The BouvetCr-CysC was therefore the most appropriate equation to verify our results and also provided the greatest test to their validity, given its strengths lie in opposition to those of the CKiDCr-CysC equation. Notably, results of our analyses were mostly in agreement using the two equations, lending confidence to our findings.
The Bouvet equation could be considered problematic, particularly in our type 2 DM population because it includes weight, raising the question of whether observed eGFR changes could be due to change in weight rather than change in GFR. In order to address this, we performed post hoc analyses of changes in weight in both type 1 and type 2 diabetes. Weight change was much greater in those who had an eGFR decline than in those with rise in eGFR opposite of what would have been observed had weight been responsible for direction of eGFR change (data available upon request).
We also acknowledge that it is less than ideal to draw longitudinal conclusions from eGFR estimated at only two timepoints. Attempts to overcome these deficiencies included a broad definition of stable eGFR, defining change as much greater than observed physiologic change from year to year in this age group, and more than the error known to be inherent in GFR estimating equations. Over the mean follow up time of 6 years between visits, the defined threshold change of 3mL/min/1.73m2 would translate to a mean change of 18mL/min/1.73m2, which most nephrologists would deem clinically significant.
1.4.5. Study Strengths
Our study also has several strengths. The SEARCH for Diabetes in Youth Study is a large, multicenter longitudinal study with significant representation from ethnic minorities and includes youth with type 1 and type 2 diabetes. Its rigor is underscored by baseline data captured close to diabetes diagnosis. Very few studies have had the opportunity to document and contrast the early natural history of eGFR according to diabetes type, particularly without the added confounding of vast differences in age of onset between diabetes types.
1.4.6. Conclusion
We have identified distinct trajectories of eGFR over the early course of diabetes. Regardless of diabetes type, factors associated with the rising or declining trajectories (versus stable) included sex, age at diagnosis, baseline eGFR and baseline glucose levels. A greater preponderance of youth and young adults with type 2 diabetes had a rise in eGFR to hyperfiltration levels, and a greater proportion of individuals with type 1 diabetes had an early decline in eGFR. Whether these two phenotypes predict differing risks for progression to advanced kidney disease will be the focus of future study.
Supplementary Material
HIGHLIGHTS.
Estimated glomerular filtration rate (eGFR) generally remains stable over the first 5–10 years of youth onset type 1 and type 2 diabetes.
Approximately 20% of individuals with youth onset type 1 and type 2 diabetes will have a significant decline in eGFR, though still remaining within the normal range.
Very few individuals with youth onset type 1 diabetes will have a rise in eGFR over the first 5–10 years, however, roughly 15% of youth onset type 2 diabetes experiences a significant rise in eGFR.
Predictors of change in eGFR shared in youth onset type 1 and type 2 diabetes include age at diagnosis, sex, baseline eGFR and fasting glucose level.
ACKNOWLEDGEMENTS:
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
The SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), University of Washington School of Medicine (U48/CCU019235-4, U01 DP000244, and U18DP002710-01), Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171).
The authors wish to acknowledge the involvement of the Kaiser Permanente Southern California’s Clinical Research Center (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group), the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR000062; Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflict of interest related to this manuscript.
REFERENCES
- 1.United States Renal Data System. USRDS 2017 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States In: National Institutes of Health, editor. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2017. [Google Scholar]
- 2.Atkins RC and Zimmet P. Diabetic kidney disease: act now or pay later. Kidney Int 2010;77(5):375–7. [DOI] [PubMed] [Google Scholar]
- 3.Vupputuri S, Kimes TM, Calloway MO, et al. The economic burden of progressive chronic kidney disease among patients with type 2 diabetes. J Diabetes Complications 2014;28(1):10–6. [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20(8):1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med 2017;376(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013;36(6):1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 2007;30(10):2593–8. [DOI] [PubMed] [Google Scholar]
- 8.Kahkoska AR, Isom S, Divers J, et al. The early natural history of albuminuria in young adults with youth-onset type 1 and type 2 diabetes. J Diabetes Complications 2018;32(12):1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dart AB, Sellers EA, Martens PJ, et al. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care 2012;35(6):1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013;36(12):3863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afkarian M, Zelnick LR, Hall YN, et al. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988–2014. JAMA 2016;316(6):602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mottl AK, Kwon KS, Mauer M, et al. Normoalbuminuric diabetic kidney disease in the U.S. population. J Diabetes Complications 2013;27(2):123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macisaac RJ and Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 2011;20(3):246–57. [DOI] [PubMed] [Google Scholar]
- 14.Porrini E, Ruggenenti P, Mogensen CE, et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol 2015;3(5):382–91. [DOI] [PubMed] [Google Scholar]
- 15.Mauer M, Caramori ML, Fioretto P and Najafian B. Glomerular structural-functional relationship models of diabetic nephropathy are robust in type 1 diabetic patients. Nephrol Dial Transplant 2015;30(6):918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fufaa GD, Weil EJ, Lemley KV, et al. Structural Predictors of Loss of Renal Function in American Indians with Type 2 Diabetes. Clin J Am Soc Nephrol 2016;11(2):254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sochett EB, Cherney DZ, Curtis JR, et al. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 2006;17(6):1703–9. [DOI] [PubMed] [Google Scholar]
- 18.Bjornstad P, Cherney DZ, Snell-Bergeon JK, et al. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant 2015;30(10):1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Porrini EL, Gaspari F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012;35(10):2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson HJ, Ekinci EI, Radcliffe NJ, et al. Elevated baseline glomerular filtration rate (GFR) is independently associated with a more rapid decline in renal function of patients with type 1 diabetes. J Diabetes Complications 2016;30(2):256–61. [DOI] [PubMed] [Google Scholar]
- 21.Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 2014;37(12):3336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA 2017;317(8):825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2 2003(135):1–55. [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000(314):1–27. [PubMed] [Google Scholar]
- 25.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95(7):3360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lampasona V, Schlosser M, Mueller PW, et al. Diabetes antibody standardization program: first proficiency evaluation of assays for autoantibodies to zinc transporter 8. Clin Chem 2011;57(12):1693–702. [DOI] [PubMed] [Google Scholar]
- 27.Larsson A, Hansson LO, Flodin M, et al. Calibration of the Siemens cystatin C immunoassay has changed over time. Clin Chem 2011;57(5):777–8. [DOI] [PubMed] [Google Scholar]
- 28.Kanakatti Shankar R, Dolan LM, Isom S, et al. Serum cystatin C in youth with diabetes: The SEARCH for diabetes in youth study. Diabetes Res Clin Pract 2017;130:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabelea D, D’Agostino RB Jr., Mason CC, et al. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia 2011;54(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dabelea D, Pihoker C, Talton JW, et al. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care 2011;34(7):1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20(3):629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouvet Y, Bouissou F, Coulais Y, et al. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol 2006;21(9):1299–306. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Inker LA and Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis 2014;63(5):820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma AP, Yasin A, Garg AX and Filler G. Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clin J Am Soc Nephrol 2011;6(7):1599–608. [DOI] [PubMed] [Google Scholar]
- 35.Pottel H, Mottaghy FM, Zaman Z and Martens F. On the relationship between glomerular filtration rate and serum creatinine in children. Pediatr Nephrol 2010;25(5):927–34. [DOI] [PubMed] [Google Scholar]
- 36.Grewal GS and Blake GM. Reference data for 51Cr-EDTA measurements of the glomerular filtration rate derived from live kidney donors. Nucl Med Commun 2005;26(1):61–5. [DOI] [PubMed] [Google Scholar]
- 37.de Boer IH, Sun W, Cleary PA, et al. Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol 2014;25(4):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- 39.Waikar SS, Rebholz CM, Zheng Z, et al. Biological Variability of Estimated GFR and Albuminuria in CKD. Am J Kidney Dis 2018;72(4):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2013. [Google Scholar]
- 41.Stefansson VT, Schei J, Jenssen TG, et al. Central obesity associates with renal hyperfiltration in the non-diabetic general population: a cross-sectional study. BMC Nephrol 2016;17(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naderpoor N, Lyons JG, Mousa A, et al. Higher glomerular filtration rate is related to insulin resistance but not to obesity in a predominantly obese non-diabetic cohort. Sci Rep 2017;7:45522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz GJ and Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009;4(11):1832–43. [DOI] [PubMed] [Google Scholar]
- 44.Tu YK and Gilthorpe MS. Revisiting the relation between change and initial value: a review and evaluation. Stat Med 2007;26(2):443–57. [DOI] [PubMed] [Google Scholar]
- 45.Skott P, Vaag A, Hother-Nielsen O, et al. Effects of hyperglycaemia on kidney function, atrial natriuretic factor and plasma renin in patients with insulin-dependent diabetes mellitus. Scand J Clin Lab Invest 1991;51(8):715–27. [DOI] [PubMed] [Google Scholar]
- 46.Cherney DZ, Sochett EB, Dekker MG and Perkins BA. Ability of cystatin C to detect acute changes in glomerular filtration rate provoked by hyperglycaemia in uncomplicated Type 1 diabetes. Diabet Med 2010;27(12):1358–65. [DOI] [PubMed] [Google Scholar]
- 47.Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol 1999;10(8):1778–85. [DOI] [PubMed] [Google Scholar]
- 48.Skrtic M and Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens 2015;24(1):96–103. [DOI] [PubMed] [Google Scholar]
- 49.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129(5):587–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


