Abstract
Background:
To date, the impact of the human papillomavirus (HPV) vaccine on invasive cervical cancers in the United States has not been documented due, in part, to the time needed for cancer to develop, and to recent changes to cervical cancer screening guidelines and recommendations which complicate data interpretation.
Methods:
We examined incidence rates of cervical squamous cell carcinoma (SCC) and adenocarcinoma (AC) among women aged 15–29 years diagnosed during 1999–2017 using population-based cancer registry data covering 97.8% of the US population. Trends were stratified by age and histology. The annual percent change in cervical cancer incidence per year was calculated using Joinpoint regression.
Results:
During 1999–2017, SCC rates decreased 7.9% per year among women aged 15–20 years, 5.5% among women aged 21–24 years, and 2.3% among women aged 25–29 years. The declines in SCC rates were largest among women aged 15–20 years from 2011 to 2017, with a decrease of 22.5% per year. Overall, AC rates decreased 4.1% per year among women aged 15–20 years, 3.6% per year among women aged 21–24 years, and 1.6% per year among women 25–29 years. AC rates declined the most among women aged 15–20 years during 2005 to 2017, decreasing 11.2% per year.
Conclusions:
Since HPV vaccine introduction, both SCC and AC incidence rates declined among women aged 15–20 years, a group not typically screened for cervical cancer, which may suggest HPV vaccine impact.
Impact:
Timely vaccination and improved screening and follow-up among recommended age groups could result in further reductions in invasive cervical cancer.
Keywords: cervical cancer, human papillomavirus, screening, National Program of Cancer Registries (NPCR)
Introduction
In the United States, cervical cancer incidence rates have declined since the introduction of cervical cancer screening with the Papanicolaou (Pap) test in the 1950s. This reduction has mainly been observed among squamous cell carcinomas (SCCs), which account for 75% of cervical cancers. Similar declines in the incidence of adenocarcinomas (ACs) have not been observed because of the lower detection of glandular cancers with the Pap test, and the relatively short time since HPV vaccine introduction. Despite the decline in incidence, cervical cancer continues to be a burden in the United States. In 2017, 12,831 new cases of invasive cervical cancer were reported (8 per 100,000 women); approximately 5% of these occurred among women younger than age 30(1).
Routine vaccination with the HPV vaccine for females aged 11 – 12 years was recommended in 2006. Most vaccinations given through 2014 were the quadrivalent vaccine, which targets oncogenic HPV types 6, 11, 16, and 18 (2). The 9-valent HPV vaccine became available in 2015 which targets the same types as the quadrivalent vaccine, plus five additional oncogenic types (31, 33, 45, 52, and 58) (3) and in 2016 became the only vaccine distributed in the United States. Current recommendations for routine HPV vaccination include all persons aged 11–12 years, and catch-up vaccination for those who have not been adequately vaccinated through age 26 (4). Although the HPV vaccine is approved for use in adults up to age 45, vaccination is not routinely recommended for persons older than age 26; instead, shared clinical decision-making is recommended for persons in this age group to determine if vaccination is likely to be beneficial (4). HPV vaccination coverage in the United States has gradually increased since its introduction; coverage of ≥ 1 dose of HPV vaccine among female adolescents aged 13 to 17 years increased from 37% in 2008 to 73% in 2019 (5,6).
Despite suboptimal coverage when the vaccine was first introduced, significant reductions in HPV infection (7), anogenital warts (8), cervical precancers (9,10), and vaccine-type HPV prevalence among cervical precancers (11) has been observed in the United States. Modeling studies in high income countries indicate that observing the full impact of HPV vaccination on invasive cervical cancer incidence may take decades, but the earliest impact would be seen in the youngest age groups of women (12), with substantial herd effects even with less optimal HPV vaccine coverage (13). Rapidly aggressive cancers associated with HPV types 16 and 18 that are found more frequently in younger women are also more likely to be impacted earlier by the HPV vaccine. Since it has been over a decade since initial implementation of the HPV vaccine in the United States, we can now begin to examine the possible early impact of vaccination on cervical cancer incidence in young US women.
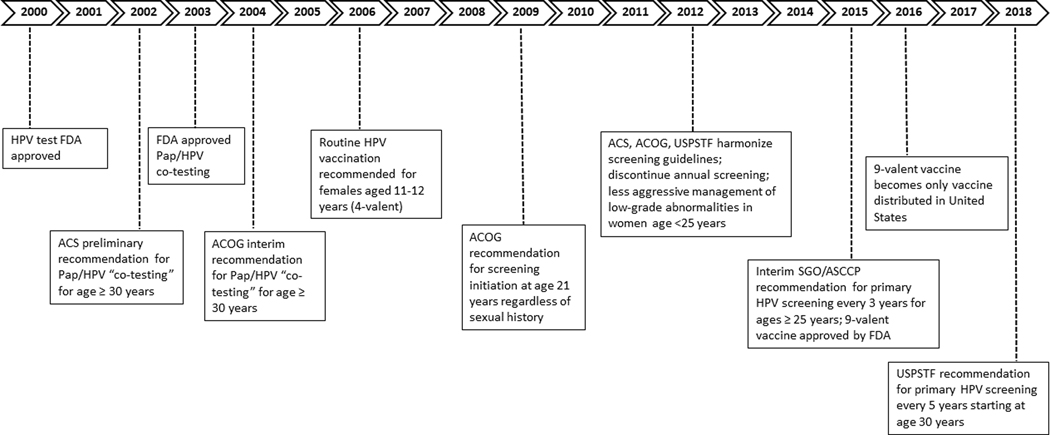
Assessing the impact of HPV vaccination on cervical cancer incidence rates is complicated by changes in screening and follow-up care over time (Figure 1). Prior to 2009, women were advised to start screening at age of sexual initiation or at age 21 years, whichever came first. In 2009, the American College of Obstetricians and Gynecologists (ACOG) recommended to starting screening at age 21 years regardless of sexual initiation (14); in 2012 the American Cancer Society (ACS) and United States Preventive Services Tasks Force made similar recommendations. In 2012 all 3 organizations recommended Pap screening intervals be extended to 3 years among women aged 21–65 years and to stop annual testing. Additionally in 2012, US consensus management guidelines recommended less aggressive management and more observation of women under 25 with low-grade abnormalities (15).
Figure 1. Timeline of relevant recommendations and guidelines in the United States for cervical cancer testing, HPV vaccination, screening, and management.
Abbreviations: HPV = human papillomavirus, FDA = Food and Drug Administration, ACS = American Cancer Society, ACOG = American College of Obstetrics and Gynecology, USPSTF = United States Preventive Services Task Force. This timeline depicts relevant events in the type of testing (Pap/HPV), HPV vaccination, age and frequency of testing, and management of cervical cancers and precancers.
In this study, we examined population-based national cancer registry data in the United States to describe trends in cervical cancer incidence among women aged 15–29 years by age group, histology, and tumor stage. We also estimated cervical cancer incidence rates based on trends during the pre-HPV vaccine period and compared these with observed rates to provide further insight on the potential impact of the HPV vaccine.
Materials and Methods
Study Population and Design
We analyzed cancer incidence data from the United States Cancer Statistics (USCS) database, which combines data from population-based cancer registry data from the Centers for Disease Control and Prevention’s (CDC’s) National Program of Cancer Registries (NPCR) dataset and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program dataset (16). Cancer registries demonstrate that data were of high quality by meeting U.S. Cancer Statistics publication criteria (16); during 1999–2017, data from 48 cancer registries met these criteria, covering 97.8% of the United States population. Invasive cervical cancers among women aged 15–29 years were identified using the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) site codes C53.0-C53.9 (17) diagnosed during 1999–2017; excluding histology codes 9050–9055, 9140, and 9590–9992. We examined microscopically confirmed cases separately for squamous cell carcinoma (SCC; 8050–8084 and 8120–8131) and adenocarcinoma (AC; 8140–8575, adenosquamous included).
Statistical Analysis
We calculated age-adjusted incidence rates for women aged 15–29 years per 100,000 women standardized to the 2000 US population. Data were suppressed for groups with fewer than 6 cases. Analyses were stratified into 3 age groups based on cutoffs used for current cervical cancer screening guidelines and recommendations: 15–20, 21–24, and 25–29 years. Because of sparse data in the women aged 15–20 years, particularly at the end of the study period, years of diagnosis were aggregated into 2-year intervals from 1999–2014 and one 3-year interval from 2015–2017. Rates were calculated using SEER*Stat software version 8.3.6 (18).
To describe trends in cervical cancer incidence rates, we used Joinpoint regression, which fits a series of joined straight lines on a logarithmic scale to the trends in the age-adjusted rates (19). Year of diagnosis was included as a continuous independent variable using the midpoint of each interval. The number of observations allowed us to fit a maximum of 1 joinpoint (2 trend segments) for women aged 15–20 years and a maximum of 2 joinpoints (3 trend segments) for women aged 21–24 and 25–29 years. Statistics derived from these models included the annual percent change (APC) between two time points and the average annual percent change (AAPC), which is a weighted average of the APCs over the entire time period under study. We used Bayesian Information Criteria (BIC) for model selection and the empirical quantile method (method two) to calculate confidence intervals for APCs and AAPCs (20). Rates were considered to increase if the APC or AAPC was greater than zero (P < 0.05) and to decrease if the APC/AAPC was less than zero (P < 0.05). Otherwise, rates were considered stable.
To descriptively visualize impact, we calculated predicted rates of cervical cancer incidence if trends during the pre-vaccine period continued. First, we used weighted least squares (WLS) regression models to calculate the slope of the linear trend of the incidence rate for each age and histology group during 1999–2008. This time period was used to calculate predicted trends based on studies that show HPV impact in cervical precancers in 3 to 4 years after vaccination. We then calculated the predicted values from the WLS regression models for each year during 2009–2017 and transformed those predicted values back to incidence rates for 2009–2017. All predicted trends were calculated using SAS 9.4 (SAS Institute, Cary NC).
To examine whether cervical cancer screening guideline changes may have resulted in increased diagnosis of late-stage cancers over time, we categorized tumor stage at diagnosis into early (localized), late (regional and distant), or unstaged using SEER Summary Stage guidelines (21–23). Data were grouped into 2-year or 3-year intervals and we calculated rate ratios (RR) and 95% confidence intervals to compare incidence rates in each age group using 1999–2000 as the referent time period. Confidence intervals were calculated using the Tiwari method (24).
Results
From 1999 to 2017, a total of 13,231 invasive cervical cancer cases (an average of approximately 700 each year) among women aged 15–29 years were reported (Table 1). Of these, 67.5% (n = 8,930) were SCCs and 23.1% (n = 3,057) were ACs. The total number of cervical cancer cases in all age groups decreased from the beginning of the study period to the most recent time period. In women aged 15–20 years, the total number of cervical cancer cases decreased from 79 (1999/2000) to 32 (2015–2017); among women aged 21–24 years the number of cases decreased from 151 (1999) to 69 (2017), and in women aged 25–29 years, the number of cases decreased from 685 (1999) to 492 (2017). Among women aged 15–20, the total proportion of SCC decreased from 1999/2000 to 2015–2017 (54.4% to 18.8%), while the proportion of AC increased (17.7% to 28.1%). Among women aged 21–24 years, the proportion of SCC decreased during 1999 to 2017 from 66.2% to 56.5%, and the proportion of AC increased from 20.5% to 27.5%. There was very little change in the proportion of SCC and AC in women ages 25–29 during 1999 to 2017 (72.1% to 70.5% among SCC, and no change in AC).
Table 1.
Cervical cancer incidence rates1 among women aged 15–29 years by histology2 and year of diagnosis, United States3, 1999–2017
| Age 15–20 years | Age 21–24 years | Age 25–29 years | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SCC | AC | Total | SCC | AC | Total | SCC | AC | ||||||||||||||||
| Year4 | n | Rate | n | (%) | Rate | n | (%) | Rate | n | Rate | n | (%) | Rate | n | (%) | Rate | n | Rate | n | (%) | Rate | n | (%) | Rate |
| 1999–2017 | 485 | 0.21 | 193 | 39.8 | 0.08 | 122 | 25.2 | 0.05 | 2,208 | 1.41 | 1,491 | 67.5 | 0.95 | 417 | 18.9 | 0.27 | 10,538 | 5.65 | 7246 | 68.8 | 3.89 | 2,518 | 23.9 | 1.35 |
| 1999 | 79 | 0.35 | 43 | 54.4 | 0.19 | 14 | 17.7 | 0.06 | 151 | 2.13 | 100 | 66.2 | 1.41 | 31 | 20.5 | 0.44 | 685 | 7.21 | 494 | 72.1 | 5.21 | 142 | 20.7 | 1.49 |
| 2000 | 136 | 1.89 | 89 | 65.4 | 1.23 | 23 | 16.9 | 0.32 | 676 | 7.27 | 474 | 70.1 | 5.10 | 153 | 22.6 | 1.64 | ||||||||
| 2001 | 61 | 0.26 | 24 | 39.3 | 0.10 | 14 | 23.0 | 0.06 | 120 | 1.61 | 86 | 71.7 | 1.15 | 17 | 14.2 | 0.23 | 618 | 6.85 | 430 | 69.6 | 4.77 | 147 | 23.8 | 1.62 |
| 2002 | 147 | 1.91 | 112 | 76.2 | 1.45 | 23 | 15.6 | 0.30 | 580 | 6.52 | 397 | 68.4 | 4.45 | 128 | 22.1 | 1.44 | ||||||||
| 2003 | 53 | 0.23 | 21 | 39.6 | 0.09 | 14 | 26.4 | 0.06 | 134 | 1.68 | 88 | 65.7 | 1.10 | 27 | 20.1 | 0.34 | 550 | 6.12 | 395 | 71.8 | 4.39 | 115 | 20.9 | 1.29 |
| 2004 | 131 | 1.63 | 87 | 66.4 | 1.08 | 27 | 20.6 | 0.33 | 509 | 5.62 | 354 | 69.5 | 3.91 | 122 | 24.0 | 1.35 | ||||||||
| 2005 | 72 | 0.30 | 31 | 43.1 | 0.13 | 20 | 27.8 | 0.08 | 132 | 1.62 | 87 | 65.9 | 1.07 | 29 | 22.0 | 0.35 | 507 | 5.56 | 334 | 65.9 | 3.69 | 132 | 26.0 | 1.44 |
| 2006 | 125 | 1.55 | 90 | 72.0 | 1.12 | 21 | 16.8 | 0.26 | 543 | 5.73 | 355 | 65.4 | 3.75 | 146 | 26.9 | 1.55 | ||||||||
| 2007 | 59 | 0.24 | 22 | 37.3 | 0.09 | 17 | 28.8 | 0.07 | 119 | 1.47 | 74 | 62.2 | 0.91 | 26 | 21.8 | 0.32 | 524 | 5.39 | 366 | 69.8 | 3.77 | 118 | 22.5 | 1.23 |
| 2008 | 109 | 1.34 | 74 | 67.9 | 0.91 | 21 | 19.3 | 0.26 | 551 | 5.53 | 367 | 66.6 | 3.68 | 135 | 24.5 | 1.38 | ||||||||
| 2009 | 54 | 0.21 | 24 | 44.4 | 0.09 | 12 | 22.2 | 0.05 | 135 | 1.64 | 80 | 59.3 | 0.97 | 24 | 17.8 | 0.29 | 574 | 5.71 | 382 | 66.6 | 3.80 | 153 | 26.7 | 1.52 |
| 2010 | 113 | 1.36 | 82 | 72.6 | 0.98 | 18 | 15.9 | 0.22 | 514 | 5.10 | 351 | 68.3 | 3.47 | 134 | 26.1 | 1.35 | ||||||||
| 2011 | 48 | 0.19 | 15 | 31.3 | 0.06 | 13 | 27.1 | 0.05 | 116 | 1.37 | 79 | 68.1 | 0.94 | 20 | 17.2 | 0.24 | 499 | 4.89 | 324 | 64.9 | 3.18 | 139 | 27.9 | 1.37 |
| 2012 | 121 | 1.39 | 85 | 70.2 | 0.98 | 22 | 18.2 | 0.25 | 462 | 4.52 | 322 | 69.7 | 3.17 | 111 | 24.0 | 1.07 | ||||||||
| 2013 | 27 | 0.11 | 7 | 25.9 | 0.03 | 9 | 33.3 | 0.04 | 93 | 1.04 | 63 | 67.7 | 0.70 | 21 | 22.6 | 0.24 | 553 | 5.38 | 349 | 63.1 | 3.40 | 154 | 27.8 | 1.51 |
| 2014 | 97 | 1.07 | 67 | 69.1 | 0.73 | 20 | 20.6 | 0.22 | 562 | 5.40 | 407 | 72.4 | 3.92 | 119 | 21.2 | 1.14 | ||||||||
| 2015 | 32 | 0.09 | 6 | 18.8 | 0.02 | 9 | 28.1 | 0.02 | 77 | 0.84 | 54 | 70.1 | 0.59 | 13 | 16.9 | 0.14 | 556 | 5.30 | 378 | 68.0 | 3.61 | 134 | 24.1 | 1.28 |
| 2016 | 83 | 0.93 | 55 | 66.3 | 0.61 | 15 | 18.1 | 0.17 | 583 | 5.45 | 420 | 72.0 | 3.94 | 134 | 23.0 | 1.24 | ||||||||
| 2017 | 69 | 0.79 | 39 | 56.5 | 0.45 | 19 | 27.5 | 0.22 | 492 | 4.50 | 347 | 70.5 | 3.16 | 102 | 20.7 | 0.95 | ||||||||
Data Sources: Center for Disease Control and Prevention’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program.
Abbreviations: SCC = squamous cell carcinoma, AC = adenocarcinoma
Per 100,000 women, age adjusted to the US standard population
Cervical cancers (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] site codes C53.0–C53.9) are limited to microscopically confirmed cases and exclude (ICD-O-3 histology codes 9050–9055, 9140, and 9590–9992); SCC includes ICD-O-3 codes 8050–8084,8120–8131; AC includes ICD-O-3 codes 8140–8575
Cancer incidence compiled from cancer registries that meet the data quality criteria for all invasive cancer sites combined for each year during the period 1999–2017 (covering 97.8% of the U.S. population).
Year of diagnosis in women ages 15–20 is grouped into 2-year intervals during the years 1999–2014, and one 3-year interval (2015–2017) due to sparse data
Squamous cell carcinoma
SCC incidence rates decreased from 1999 to 2017 in all age groups (Table 2). Among women aged 15–20 years, SCC rates decreased 12.7% per year on average, with the largest decline occurring from 2011 to 2017, with a decrease of 22.5% per year. Among women aged 21–24 years, SCC rates from 1999 to 2017 declined on average 5.5% per year, with the largest decline occurring from 2012 to 2017 at 11.2% per year. Among women aged 25–29 years, SCC decreased on average 2.3% per year from 1999 to 2017, showing nonsignificant increase of 7.9% per year (2012–2014) followed by a nonsignificant decrease of 8.4% from 2015–2017
Table 2.
| Trends 1999–2017 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Trend 1 |
Trend 2 |
Trend 3 |
||||||
| Age (years) | Histology1 | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | AAPC (95% CI) 1999–2017 |
| Total | 1999–2012 | −4.2 (−5.9, −0.4) | 2012–2017 | −17.4† (−30.6, −13.2) | -- | -- | −8.0† (−12.0, −4.7) | |
| 15–203 | SCC | 1999–2010 | −5.6 (−10.3, 6.3) | 2010–2017 | −22.5† (−45.2, −13.2) | -- | -- | −12.7† (−19.5, −8.8) |
| AC | 1999–2006 | 6.0 (−1.1, 25.2) | 2006–2017 | −9.4† (−19.7, −6.7) | -- | -- | −4.1† (−7.8, −7.8) | |
| Total | 1999–2012 | −3.1 (−4.2, 1.4) | 2012–2017 | −9.9† (−25.4, −5.2) | -- | -- | −5.0† (−6.8, −3.7) | |
| 21–24 | SCC | 1999–2012 | −3.1 (−4.3, 1.6) | 2012–2017 | −11.2† (−29.2, −5.7) | -- | -- | −5.5† (−7.7, −3.9) |
| AC | 1999–2017 | −3.6† (−5.9, −1.5) | -- | -- | -- | -- | −3.6† (−5.9, −1.5) | |
| Total | 1999–2012 | −3.0 (−6.6, 4.1) | 2012–2015 | 5.7 (−7.8, 9.4) | 2015–2017 | −9.1 (−18.0, 2.2) | −2.3† (−3.3, −1.0) | |
| 25–29 | SCC | 1999–2012 | −3.6 (−7.2, 4.8) | 2012–2015 | 7.9 (−9.5, 12.3) | 2015–2017 | −8.4 (−18.5, 4.3) | −2.3† (−3.4, −1.0) |
| AC | 1999–2017 | −1.6† (−2.8, −0.4) | -- | -- | -- | -- | −1.6† (−2.8, −0.4) | |
Data Sources: Center for Disease Control and Prevention’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program.
Abbreviations: SCC = squamous cell carcinoma, AC = adenocarcinoma, APC = annual percent change, AAPC = average annual percent change
Cervical cancers (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] site codes C53.0–C53.9) are limited to carcinomas (ICD-O-3 histology codes 8010–8671, 8940–8941); SCC includes ICD-O-3 codes 8050–8084,8120–8131; AC includes ICD-O-3 codes 8140–8575
Cancer incidence compiled from cancer registries that meet the data quality criteria for all invasive cancer sites combined for each year during the period 1999–2017 (covering 97.8% of the U.S. population).
Year of diagnosis in women ages 15–20 is grouped into 2-year intervals during the years 1999–2014, and one 3-year interval (2015–2017) due to sparse data
Significant at p<0.05. Trends were measured with AAPC in rates and were considered to increase or decrease if p<0.05; otherwise rates were considered stable.
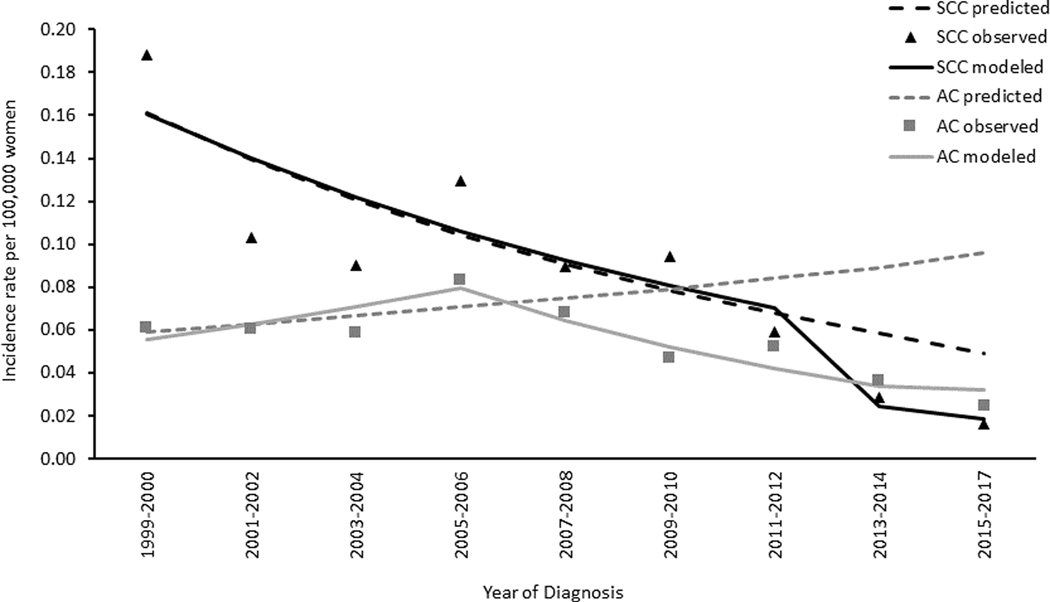
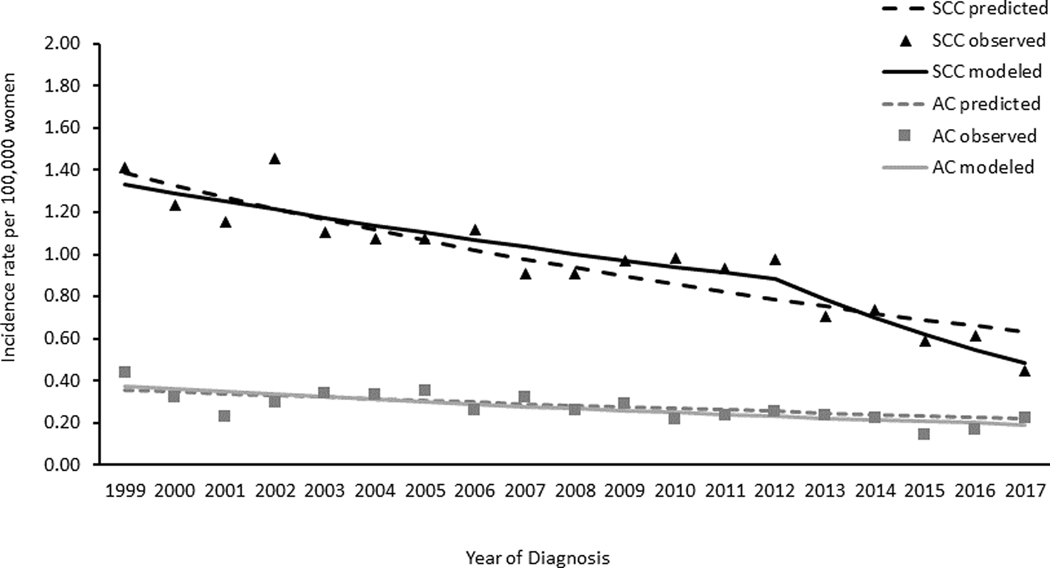
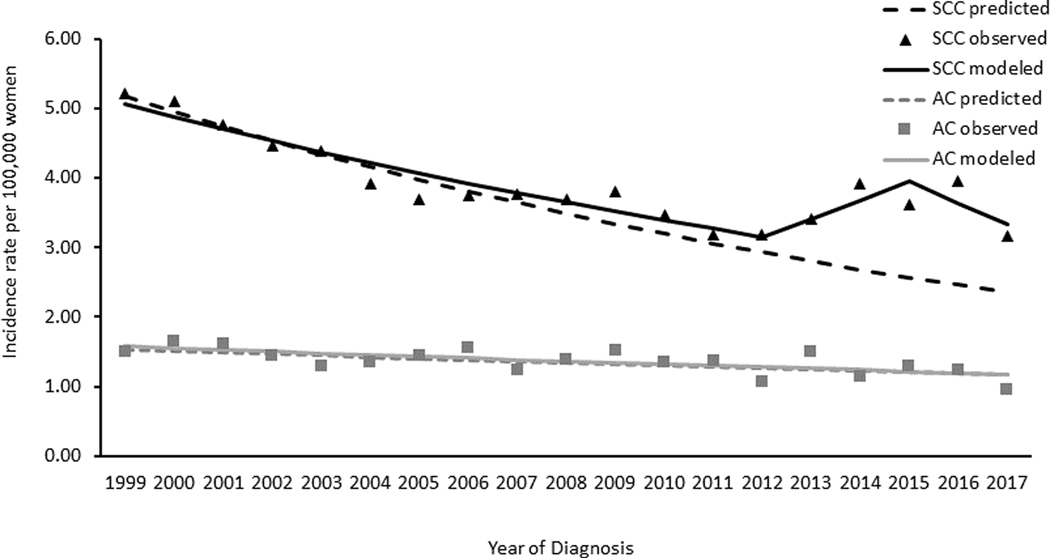
Descriptively comparing observed and predicted incidence rates of SCC, we found that the observed rates declined faster than predicted rates beginning at 2011 among women aged 15–20 years (Figure 2). In women aged 21–24 years and 25–29 years, trends in observed and predicted rates were similar (Figure 3 and Figure 4, respectively).
Figure 2. Cervical Cancer Observed and Predicted Incidence Trends among Women Aged 15–20 Years by Histology, United States, 1999–2017.
Data Sources: Center for Disease Control and Prevention’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program. Incidence data compiled from cancer registries that met data quality standards from 1999 to 2017, covering 97.8% of the US population. AC refers to adenocarcinomas defined by ICD-O-3 histology codes 8140–8575; SCC refers to squamous cell carcinomas defined by ICD-O-3 histology codes 8050–8084 and 8120–8131. Black triangles represent observed incidence rates of SCC; black solid lines are the SCC modelled trends; black dotted lines are the SCC predicted trends; gray squares represent observed incidence rates of AC; gray dotted lines are the AC modelled trends; gray dotted lines are the AC predicted trends. Predicted rates were estimated based on the trends in 1999 to 2008 continuing to 2017 (see methods section for further information on methodology).
Figure 3. Cervical Cancer Observed and Predicted Incidence Trends among Women Aged 21–24 Years by Histology, United States, 1999–2017.
Data Sources: Center for Disease Control and Prevention’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program. Incidence data compiled from cancer registries that met data quality standards from 1999 to 2017, covering 97.8% of the US population. AC refers to adenocarcinomas defined by ICD-O-3 histology codes 8140–8575; SCC refers to squamous cell carcinomas defined by ICD-O-3 histology codes 8050–8084 and 8120–8131. Black triangles represent observed incidence rates of SCC, black solid lines are the SCC modelled trends, black dotted lines are the SCC predicted trends; gray squares represent observed incidence rates of AC, gray dotted lines are the AC modelled trends, gray dotted lines are the AC predicted trends. Predicted rates were estimated based on the trends in 1999 to 2008 continuing to 2017 (see methods section for further information on methodology).
Figure 4. Cervical Cancer Observed and Predicted Incidence Trends among Women Aged 21–24 Years by Histology, United States, 1999–2017.
Data Sources: Center for Disease Control and Prevention’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program. Incidence data compiled from cancer registries that met data quality standards from 1999 to 2017, 97.8% of the US population. AC refers to adenocarcinomas defined by ICD-O-3 histology codes 8140–8575; SCC refers to squamous cell carcinomas defined by ICD-O-3 histology codes 8050–8084 and 8120–8131. Black triangles represent observed incidence rates of SCC, black solid lines are the SCC modelled trends, black dotted lines are the SCC predicted trends; gray squares represent observed incidence rates of AC, gray dotted lines are the AC modelled trends, gray dotted lines are the AC predicted trends. Predicted rates were estimated based on the trends in 1999 to 2008 continuing to 2017 (see methods section for further information on methodology).
Adenocarcinoma
On average, trends in AC incidence rates decreased from 1999 to 2017 in all age groups. Among women aged 15–20 years, rates decreased 4.1% per year on average, with the largest decline occurring from 2005/2006 to 2015–2017 of 9.4% per year. Among women aged 21–24 years, rates decreased on average 3.6% per year; in women aged 25–29 rates decreased 1.6% per year. Descriptively comparing observed and predicted incidence rates of AC, observed rates decreased starting at 2005 (Figure 2). This was in contrast to predicted rates, which increased from 1999 to 2017. No differences in the observed or predicted rates of AC were observed in women aged 21–24 or 25–29 years (Figure 3 and Figure 4, respectively).
Cancer Stage
Compared to the referent period, the rate of early stage cancer decreased in all age groups over time, where rates of late-stage cancers did not change over time (Supplementary Table). Unstaged cancers appeared to decrease in all age groups, but not all rate ratios were significant.
Discussion
In this analysis of population-based cancer registry data in the United States, we found that during 1999–2017, cervical SCC declined in young women aged 15–29 years. We found the largest reduction occurred in women aged 15–20 years during 2011–2017, just 3–4 years after the introduction of the HPV vaccine. In addition, observed incidence rates of SCC and AC in women aged 15–20 years were lower than rates that would be expected if trends in the pre-vaccine period had continued.
Early evidence of the impact of HPV vaccination on the incidence of cervical precancers has been demonstrated in high income countries with established HPV vaccination programs, such as Australia (25) and Scotland(26) and in the United States. For example, a statewide registry in New Mexico collecting both cervical precancer and screening data observed significant reductions in cervical intraepithelial neoplasia grade 2 (CIN2), and grade 3 (CIN3) incidence from 2007–2014 among women aged 15–19 years and a decrease in CIN2 incidence among women aged 20–24 years even after adjusting for changes in cervical screening across the study period (9). Another US study using population-based surveillance of high-grade cervical lesions in 5 catchment areas in the US reported significant reductions in CIN2, CIN3 and adenocarcinoma in situ (CIN2+) incidence from 2008 to 2015 among women aged 18–24 years (also adjusted for screening) just four to five years after vaccine implementation (10,27). Further, a decreasing trend in the proportion of HPV types 16 and 18 in CIN2+ lesions was observed among vaccinated women (11). Taken altogether, these US studies show a population-level impact of HPV vaccination on high-grade cervical precancers, a proximal outcome to invasive cervical cancer.
On the other hand, few studies have examined the impact on invasive cervical cancer. Recently, results from long-term passive follow-up of two HPV vaccination trial cohorts (65,656 women years) and unvaccinated control cohorts (124,245 women years) conducted in Finland from 2007 to 2015 showed 100% vaccine efficacy among invasive cervical cancer cases (28). A limiting factor for examining this association in most countries is a small population size in the youngest cohorts of women, as a large population size is needed to examine trends in cervical cancer incidence by characteristics such as age or histology. In the United States, an older study compared cervical cancers 4-year periods before and after HPV vaccination introduction (2003–2006 and 2010–2014) and noted a 29% reduction in cervical cancer in women aged 15–24 years and a 13% reduction in those aged 25–34 years (29). However, the analysis was limited by not being able to examine AC specifically or to examine age groups that aligned with screening guidelines. In the current study of the U.S. population over 19 years, we were able to examine incidence trends for both SCC and AC among women aged younger than 30 years, which allowed us to explore the potential impact of HPV vaccination alongside changes in screening among women aged 15–20 years.
Although the natural history of HPV infection and cervical cancer development can take decades, there is evidence that suggests that HPV impact can be observed earlier in younger women. First, rapidly progressing cervical cancers most frequently occur in women younger than age 35 years (30), can occur as early as 3 years from HPV infection(30), and are frequently associated with HPV18(31). Our finding of decreasing incidence rates of SCC and AC among women aged 15–20 years may be a result HPV impact in the number of rapidly progressing cancers. Also, as HPV16 represents a larger attributable fraction of cervical cancers among younger women (32), and HPV18 is more frequent in ACs (10,33), this may allow better observation of cancer reduction due to HPV vaccine in this age group (31,34).
An alternative explanation of declining cervical cancer incidence in women aged 15–20 years is that screening guidelines which recommended delaying screening to age 21 has resulted in less cancers being diagnosed in this age group. However, we might then expect to find increasing incidence rates of cancers, or cancers diagnosed at a later stage among women aged 21–24 years and 25–29 years, which we did not observe. In fact, after 2012, the annual percent decline in SCC among women 21–24 years was 11% per year and the fluctuations seen in women 25–29 years (first up and down) were non-significant.
In addition, we found that incidence rates of AC started to decline among women aged 15–20 years around the time HPV vaccine was introduced. ACs are not as easily detected by Pap testing because they are generally located higher in the endocervix (35), which suggest that the decline could be partly a result of HPV vaccination. In contrast, we observed a steady decline in AC incidence rates among women aged 21–24 years and 25–29 years throughout the study period, however the decline was greater in women aged 21–24 than in the older age group, consistent with another US study that found declines in AIS in the 21–24 age group after HPV vaccination introduction (10). The observed reduction in ACs among women aged 15–20 years around the time of HPV vaccine introduction lends support that HPV vaccination may also have contributed to the overall reduction in incidence observed in this age group. We did not observe as large of a decline among women aged 21–29 years, possibly explained in part by vaccine uptake being low in the years immediately following implementation and distribution of age at immunization varied over time (6) with most vaccinated women in this study being vaccinated at catch-up ages.
Some limitations to this study warrant consideration. First, cancers are relatively rare in younger women and as a result there are a relatively small number of cervical cancer cases that occurred among women aged 15–20 years. In order to increase statistical stability in this age group, we aggregated data into multi-year intervals, which hampered our ability to observe more detailed trends. However, given the large population of young women and the comprehensive cancer registry, we still have a relatively large number of cervical cancers in the United States in this age group, where European countries with HPV vaccination programs are much smaller and don’t have such a volume of cancers. Our data show an absolute reduction in the number of invasive cervical cancer cases and the rates over time in young women, which reflects progress in light of the World Health Organization’s initiative for cervical cancer elimination (36). Second, this study is ecological and we were not able to assess vaccination or screening status of invasive cancer cases as linkage to vaccine registries or screening registries is not routine nor robust at a national level in the United States, although it holds promise in select settings for precancers (9,37). Routine genotyping of all cervical cancers, particularly in younger women, could support these ecologic data in assessing HPV vaccination impact going forward. Third, our estimates of predicted cervical cancer rates are limited because they do not account for population changes in HPV vaccination uptake or cervical cancer screening after 2008.
The major strength of our study is the use of population-based cervical cancer data for nearly the entire US population, allowing us to examine rates and trends by age, especially ages under 20, and histology. These data could be used in the coming years to continue to monitor the impact of HPV vaccination. Comparisons of cervical cancer trends in younger women from countries that have HPV vaccination, cervical cancer screening programs, and high-quality cancer registries could help identify contributing factors to the decreasing incidence rates.
In this population-based study of US cancer registry data from 1999–2017, both SCC and AC cervical cancer incidence rates declined, with the largest declines among women aged 15–20 years, an age group most likely to be impacted by the introduction of HPV. Continued monitoring and surveillance can determine the impact of HPV vaccine on invasive cervical cancer incidence. Timely vaccination and improved screening and follow-up among recommended age groups could result in further reductions in invasive cervical cancer.
Supplementary Material
Acknowledgements:
Funding support for the primary author was received from Oak Ridge Institute for Science and Education, an asset of the United States Department of Energy.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Centers for Disease Control and Prevention. 2016. October 17. United States Cancer Statistics: Data Visualizations. <https://gis.cdc.gov/Cancer/USCS/DataViz.html>. Accessed 2019 October 17.
- 2.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports 2007;56(Rr-2):1–24. [PubMed] [Google Scholar]
- 3.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. Journal of the National Cancer Institute 2015;107(6):djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and mortality weekly report 2019;68(32):698–702 doi 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2008. January 8 2008 Adolescent Human Papillomavirus (HPV) Vaccination Coverage Report. <https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/hpv/reports/2008.html>. Accessed 2020 January 8. [Google Scholar]
- 6.Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years - United States, 2019. MMWR Morbidity and mortality weekly report 2020;69(33):1109–16 doi 10.15585/mmwr.mm6933a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver SE, Unger ER, Lewis R, McDaniel D, Gargano JW, Steinau M, et al. Prevalence of human papillomavirus among females after vaccine introduction-National Health and Nutrition Examination Survey, United States, 2003–2014. The Journal of infectious diseases 2017;216(5):594–603 doi 10.1093/infdis/jix244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health 2013;103(8):1428–35 doi 10.2105/AJPH.2012.301182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benard VB, Castle PE, Jenison SA, Hunt WC, Kim JJ, Cuzick J, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017;3(6):833–7 doi 10.1001/jamaoncol.2016.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleveland AA, Gargano JW, Park IU, Griffin MR, Niccolai LM, Powell M, et al. Cervical adenocarcinoma in situ: human papillomavirus types and incidence trends in five states, 2008–2015 [published online ahead of print April 16, 2019]. Int J Cancer 2019. doi: 10.1002/ijc.32340. doi 10.1002/ijc.32340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClung NM, Gargano JW, Bennett NM, Niccolai LM, Abdullah N, Griffin MR, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008–2014. Cancer Epidemiol Biomarkers Prev 2019;28(3):602–9 doi 10.1158/1055–9965.EPI-18–0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MT, Simms KT, Lew JB, Smith MA, Brotherton JM, Saville M, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health 2019;4(1):e19–e27 doi 10.1016/S2468-2667(18)30183-X. [DOI] [PubMed] [Google Scholar]
- 13.Brisson M, Benard E, Drolet M, Bogaards JA, Baussano I, Vanska S, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health 2016;1(1):e8–e17 doi 10.1016/S2468-2667(16)30001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saraiya M, Steben M, Watson M, Markowitz L. Evolution of cervical cancer screening and prevention in United States and Canada: implications for public health practitioners and clinicians. Prev Med 2013;57(5):426–33 doi 10.1016/j.ypmed.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013;17(5 Suppl 1):S1–S27 doi 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Cancer Statistics Working Group. 2020 United States Cancer Statistics: 1999–2017 Incidence and mortality Web-based report. Atlanta, GA: U.S. Department of Health and Human Services, CDC, National Cancer Institute; 2017. Available at www.cdc.gov/uscs/. [Google Scholar]
- 17.Fritz A PC, Jack A, Shanmugarathnam K, Sobin L, Parkin D, et al. , editors. International Classification of Diseases for Oncology. 3rd edition Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 18.National Cancer Institute; Surveillance E, and End Results Program,. August 25. SEER*Stat Software. <https://seer.cancer.gov/seerstat/>. Accessed 2020 August 25. [Google Scholar]
- 19.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19(3):335–51. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Luo J, Chen HS, Green D, Buckman D, Byrne J, et al. Improved confidence interval for average annual percent change in trend analysis. Stat Med 2017;36(19):3059–74 doi 10.1002/sim.7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Summary staging guide for the Cancer Surveillance, Epidemiology, and End Results (SEER) Program. Bethesda, MD: National Cancer Institute, National Institutes of Health; 1977. [Google Scholar]
- 22.National Cancer Institute. Summary staging guide for the Cancer Surveillance, Epidemiology, and End Results (SEER) Program Bethesda, MD: National Cancer Institute, National Institutes of Health; 2000. [Google Scholar]
- 23.Young JL RS Jr, Ries LAG, Fritz AG, Hurlbut AA(eds) SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. [Google Scholar]
- 24.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15(6):547–69 doi 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 25.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet (London, England) 2011;377(9783):2085–92 doi 10.1016/s0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 26.Cameron RL, Kavanagh K, Cameron Watt D, Robertson C, Cuschieri K, Ahmed S, et al. The impact of bivalent HPV vaccine on cervical intraepithelial neoplasia by deprivation in Scotland: reducing the gap. Journal of epidemiology and community health 2017;71(10):954–60 doi 10.1136/jech-2017-209113. [DOI] [PubMed] [Google Scholar]
- 27.Gargano JW, Park IU, Griffin MR, Niccolai LM, Powell M, Bennett NM, et al. Trends in high-grade cervical lesions and cervical cancer screening in 5 states, 2008–2015. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2019;68(8):1282–91 doi 10.1093/cid/ciy707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luostarinen T, Apter D, Dillner J, Eriksson T, Harjula K, Natunen K, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer 2018;142(10):2186–7 doi 10.1002/ijc.31231. [DOI] [PubMed] [Google Scholar]
- 29.Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018;55(2):197–204 doi 10.1016/j.amepre.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildesheim A, Hadjimichael O, Schwartz PE, Wheeler CM, Barnes W, Lowell DM, et al. Risk factors for rapid-onset cervical cancer. American journal of obstetrics and gynecology 1999;180(3 Pt 1):571–7 doi 10.1016/s0002-9378(99)70256-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang KL, Yang YC, Wang TY, Chen JR, Chen TC, Chen HS, et al. Neuroendocrine carcinoma of the uterine cervix: a clinicopathologic retrospective study of 31 cases with prognostic implications. Journal of chemotherapy (Florence, Italy) 2006;18(2):209–16 doi 10.1179/joc.2006.18.2.209. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WG, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. Journal of the National Cancer Institute 2009;101(7):475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007;121(3):621–32 doi 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 34.Castle PE, Pierz A, Stoler MH. A systematic review and meta-analysis on the attribution of human papillomavirus (HPV) in neuroendocrine cancers of the cervix. Gynecologic oncology 2018;148(2):422–9 doi 10.1016/j.ygyno.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Niu S, Molberg K, Thibodeaux J, Rivera-Colon G, Hinson S, Zheng W, et al. Challenges in the Pap diagnosis of endocervical adenocarcinoma in situ. Journal of the American Society of Cytopathology 2019;8(3):141–8 doi 10.1016/j.jasc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Canfell K. Towards the global elimination of cervical cancer. Papillomavirus research (Amsterdam, Netherlands) 2019;8:100170 doi 10.1016/j.pvr.2019.100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potter RC, Flagg EW, Datta SD, Saraiya M, Copeland G. Monitoring the impact of human papillomavirus vaccines on high-grade pre-invasive cervical lesions: designing a framework of linked immunization information system and cancer registry data in Michigan. Vaccine 2015;33(11):1400–5 doi 10.1016/j.vaccine.2014.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.