To the Editor,
Chronic granulomatous disease (CGD) is a rare immunodeficiency caused by defects in the phagocytic nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex.[1] Defective NADPH oxidase results in impaired production of microbicidal reactive oxygen species. CGD is therefore typically characterized by recurrent life-threatening bacterial and fungal infections, and the most common pathogens include Staphylococcus aureus, Serratia marsescens, Burkholderia cepacia, Nocardia species, and Aspergillus species.[1] Patients with CGD may also experience a spectrum of inflammatory complications and autoimmune disorders.[1]
CGD is caused by mutations in the genes CYBB, CYBA, CYBC1, NCF1, NCF2, and NCF4, which encode phagocyte oxidase (phox) subunits of the NADPH oxidase complex.[1] X-linked CGD is caused by mutations in CYBB, encoding for gp91phox, and accounts for 65% of cases in western countries.[1-3] Female CYBB mutation carriers have dual phagocyte populations with one set expressing the wild-type X chromosome and the other expressing the mutant X chromosome. Female carriers of CYBB mutations are usually unaffected in terms of infectious disease susceptibility because the number of cells expressing wild-type CYBB and having superoxide production is adequate.[4] There are an increasing number of reports, however, of X-linked CGD carriers being affected by various inflammatory and autoimmune manifestations. Manifestations previously reported include, but are not limited to, discoid lupus erythematosus and other features of systemic lupus erythematous, oral ulcers, arthritis, Raynaud’s phenomenon, alopecia, inflammatory bowel disease, and other gastrointestinal manifestations like abdominal pain and diarrhea.[4]
We present an adolescent female X-linked CGD carrier with a de novo mutation in CYBB and skewed X chromosome inactivation. She originally presented with severe neutrophilic dermatoses manifestations and no significant infectious history, which initially mislead the clinical diagnosis. A 16-year-old female presented with an 8-year history of various mucocutaneous manifestations, including pustular scalp psoriasis, pyoderma gangrenosum (PG), hidradenitis suppurativa, scarring acne, oral ulcers, recurrent hordeolum and chalazion, and pustules of the nose and fingertips. Prior infection history was unremarkable, and prior lesion cultures were negative. Her family history was negative for immune deficiency. Previous immune evaluation, including quantitative immunoglobulins, lymphocyte markers, lymphocyte proliferation to mitogens and antigens, and complement levels, was also normal. Evaluation of NADPH oxidase activity with either dihydrorhodomine flow cytometric assay or nitroblue tetrazolium test had not been performed.
A punch biopsy of a cutaneous lesion demonstrated an abundance of neutrophils filling the interstitial spaces and forming confluent sheets characteristic of a neutrophilic dermatosis (Online Resource 1). Her lesions were typically steroid responsive, and she was often on cycles of systemic steroids for several weeks at a time. Previous steroid-sparing therapies included methotrexate and the tumor necrosis factor (TNF) inhibitor etanercept with no response and also no adverse effects. The patient also previously tried the TNF inhibitor adalimumab, but this was discontinued after 3 doses due to lack of insurance coverage. She ultimately restarted adalimumab 40 mg subcutaneously every 2 weeks after insurance coverage was obtained.
After 2 months of adalimumab therapy, the patient developed a leg ulcer with malodorous drainage. A culture isolated Serratia marascens. She then developed fevers, and blood cultures isolated Serratia marascens. She was receiving care at an outside, local facility during this time. Despite appropriate antibiotic therapy, she continued with fevers and was hospitalized locally for septicemia due to Providencia stuartii. Her hospital course was complicated by macrophage activation syndrome (MAS) with pancytopenia, elevated transaminases, hypofibrinogenemia, hypertriglyceridemia, coagulopathy, hyperferritinemia, and an elevated soluble IL-2 receptor level. A bone marrow biopsy also demonstrated multiple hemophagocytic histiocytes. For the MAS, the patient received high-dose intravenous (IV) methylprednisolone, the IL-1 receptor antagonist anakinra IV, and IVIg. She unfortunately did not respond to therapy and within 10 days of admission died of complications from the MAS and multi-organ failure.
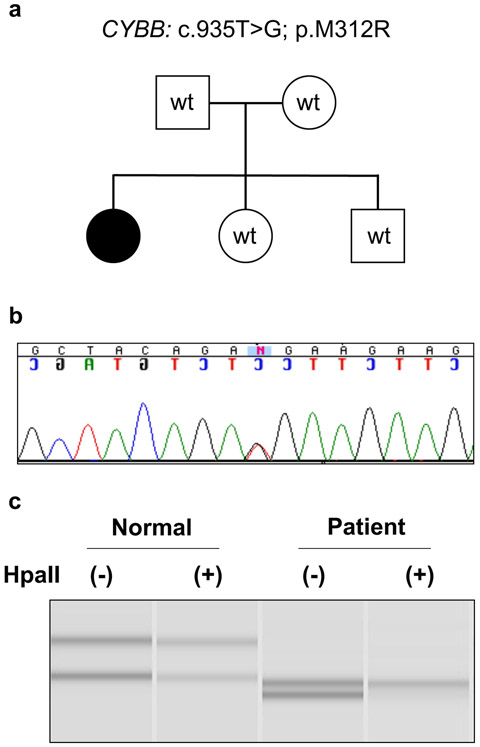
Given her clinical history of predominantly neutrophilic dermatoses, the patient was initially evaluated for an autoinflammatory syndrome. PSTPIP1 sequencing for pyogenic arthritis, PG, and acne (PAPA) syndrome was negative. Whole exome sequencing was then performed postmortem and identified a missense variant in CYBB NM_000397.3: c.935T>G, p.Met312Arg (M312R). The missense variant has been previously reported in a patient with CGD, but no clinical information provided.[5] Her mother, father, and two other siblings did not carry this variant when peripheral blood genomic DNA was tested (Fig. 1a,b). This variant is not reported in large public databases gnomAD and dbSNP and is predicted to be deleterious by in silico analysis. A HUMARA assay using patient’s DNA sample confirmed a fully skewed X chromosome inactivation pattern in our patient when compared to a healthy control (Fig. 1c). Since the missense variant was identified post-mortem, we did not have the patient’s RNA or primary cells to confirm skewing towards mutant cells and/or a functional defect in the NADPH oxidase activity.
Fig. 1.
(a) Family pedigree with filled circle representing our affected patient. Squares represent males and circles represent females. (b) Sequencing of patient genomic DNA confirming CYBB mutation (c.935T>G, p.M312R). (c) Determining the patient’s X chromosome inactivation using HUMARA assay. One X allele is active and completely digested by the methylation-sensitive restriction enzyme HpaII (bottom allele), while the second allele is inactive, due to methylated chromosome that resists digestion (top band). The assay confirms a fully skewed X chromosome inactivation pattern in the patient (0:100) when compared to a healthy control (42:58).
In summary, CGD is a rare immunodeficiency that typically presents with recurrent life-threatening bacterial and fungal infections due to impaired production of reactive oxygen species. X-linked CGD carriers have an increased susceptibility to inflammatory and autoimmune manifestations. We identified a female patient with a de novo X-linked CYBB mutation who originally presented with various mucocutaneous symptoms and no significant infectious history that unfortunately passed away from MAS triggered by Providencia stuartii septicemia. The mutation is presumed pathogenic since it occurs in a domain where all previously identified pathogenic mutations reside and has been previously reported in a CGD patient.[5] In addition, the patient had a confirmed immunodeficiency clinically consistent with CGD. Our case highlights that female carriers of X-linked CGD are at risk for CGD-type infections like Serratia marascens. Our case is also significant in that it expands the spectrum of symptoms associated with CGD to include severe neutrophilic dermatoses. Multiple, chronic inflammatory skin conditions including severe neutrophilic dermatoses may be an indication to screen for CGD. Our case also adds to prior reports that MAS may be a rare complication of CGD, including in X-linked CGD carriers.[6] Lastly, our case also supports prior observations that TNF inhibition may have untoward effects in CGD.[2]
Supplementary Material
Online Resource 1 Skin punch biopsy (hematoxylin and eosin stain). Neutrophils fill interstitial spaces and form confluent sheets characteristic of neutrophilic dermatosis. Arrows designate more concentrated areas of neutrophils and debris.
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.Holland SM. Chronic granulomatous disease. Hematol Oncol Clin North Am. 2013;27(1):89–99, viii. doi: 10.1016/j.hoc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uzel G, Orange JS, Poliak N, Marciano BE, Heller T, Holland SM. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin Infect Dis. 2010;51(12):1429–34. doi: 10.1086/657308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnadottir GA, Norddahl GL, Gudmundsdottir S, Agustsdottir AB, Sigurdsson S, Jensson BO et al. A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease. Nat Commun. 2018;9(1):4447. doi: 10.1038/s41467-018-06964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marciano BE, Zerbe CS, Falcone EL, Ding L, DeRavin SS, Daub J et al. X-linked carriers of chronic granulomatous disease: Illness, lyonization, and stability. The Journal of allergy and clinical immunology. 2018;141(1):365–71. doi: 10.1016/j.jaci.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Roos D, de Boer M, Kuribayashi F, Meischl C, Weening RS, Segal AW et al. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87(5):1663–81. [PubMed] [Google Scholar]
- 6.Urriola N, Williams A, Keat K. Macrophage activation syndrome/haemophagocytic lymphohistiocytosis secondary to Burkholderia cepacia complex septicaemia in an elderly female carrier of X-linked chronic granulomatous disease with extreme lyonisation: 'cepacia syndrome' revisited. BMJ Case Rep. 2019;12(8). doi: 10.1136/bcr-2019-230434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1 Skin punch biopsy (hematoxylin and eosin stain). Neutrophils fill interstitial spaces and form confluent sheets characteristic of neutrophilic dermatosis. Arrows designate more concentrated areas of neutrophils and debris.