Abstract
Background:
Esophageal adenocarcinoma (EAC) is a lethal cancer with rising incidence. There are limited data in younger (< 50 years) patients with EAC. We aimed to assess time trends in the incidence and outcomes of “young onset” EAC using a population-based database.
Methods:
We queried the Surveillance, Epidemiology, and End Results (SEER) 9 database to identify EAC patients between 1975–2015. Patients were stratified into three age strata: < 50, 50–69 and ≥ 70 years. Staging was stratified as localized, regional and distant. Trends in incidence, disease stage, and survival were assessed in three periods (1975–89, 1990–99 and 2000–2015). Univariate and multivariate models were created to identify predictors of mortality.
Results:
EAC incidence has increased in patients < 50 years, with an annual percentage change of 2.9% (95% CI: 1.4%−4.4%) from 1975–2015. Young onset EAC presented at more advanced stages (regional + distant) compared to older patients (84.9% vs 67.3%; p<0.01) with increasing proportion of advanced stages over the study period. These patients also experienced poorer 5-year EAC free survival compared to older patients (22.9%% vs 29.6%; p<0.01), though this finding was attenuated on stage-stratified analysis.
Conclusion:
Young onset EAC, while uncommon, is rising in incidence. Concerningly, the proportion of advanced disease continues to increase. Young onset EAC also presents at more advanced stages resulting in poorer EAC-free survival.
Impact:
EAC patients younger than 50 present at more advanced stages with higher EAC-specific mortality compared to older peers. Current diagnostic and management strategies for young-onset EAC may need to be reevaluated.
Keywords: Esophageal Adenocarcinoma, Epidemiology, Mortality, Young Onset Cancer, trends, Barrett’s Esophagus
Introduction
The incidence of esophageal adenocarcinoma (EAC) has been rising rapidly over the last four decades, and approximately 17,650 esophageal cancer cases were expected to be diagnosed in the US in 2019.1, 2 Barrett’s esophagus (BE), a form of intestinal metaplasia in which the normal esophageal squamous epithelium is replaced by columnar epithelium, is the only known precursor lesion for the development of EAC.3 The risk of malignant transformation for dysplastic BE is dramatically increased compared to the general population, and can be as high as 7% per year for patients who have BE with high grade dysplasia.4
Gastroenterological societies have advocated for endoscopic screening for BE in those with chronic gastroesophageal reflux and other risk factors.5, 6 Age greater than 50 is one of the risk factors for BE and is based on studies showing an increase in the incidence of BE diagnosed on endoscopy done in the 5th and 6th decade.7 The median age of EAC diagnosis is the 6th decade. Hence EAC is relatively uncommon before the age of 50, and data on the incidence, stage distribution and outcomes of this segment of patients with EAC are relatively limited.
A recent report of patients at a single tertiary care center demonstrated that younger patients (less than age 50) presented with advanced cancers (stages III and IV) at higher rates than patients aged 50–69 (77.1% compared to 61.4%; p <0.001).8 Further, survival outcomes were also poorer in this younger cohort of patients. Other studies have also reported that a small proportion of EAC cases are diagnosed in patients younger than 50.9–11 However, most were from tertiary care centers, making them subject to referral bias, and therefore their conclusions may not be generalizable to the entire population.
In this study, we aimed to further understand the epidemiology, including trends in incidence (using annual percent change), stage at presentation and survival outcomes (cancer free and overall), of young onset EAC (patients < 50 years old at diagnosis) over the last 4 decades, in comparison to older patients using a population-based national cancer database.
Materials and Methods
The Surveillance, Epidemiology, and End Results (SEER) database is a comprehensive, national database which is maintained by the National Institutes of Health (NIH), and contains de-identified data for the incidence and outcomes for a number of cancers, including EAC. We utilized the SEER 9 release, which incorporates data from 18 geographic regions in the United States with a catchment area of approximately 35% of the United States population.12 Data were obtained through SEER*Stat version 8.3.6 software.13 Data were included on patients diagnosed over 40 years (1975–2015) except for survival outcomes where data were included from 1975 to 2011 to allow at least 5 years of follow up after diagnosis. Additionally, a subset analysis of 1-year survival outcomes was done for patients diagnosed from 2011–2015.
Data Abstraction and Patient Classification
SEER9 cases were limited to histologically confirmed cases of esophageal adenocarcinoma utilizing International Classification of Diseases for Oncology (ICD-O-3)/World Health Organization (WHO) 2008 site recodes for the esophagus, with confirmation of the diagnosis with ICD-O-3 codes 8140–8389 (adenomas and adenocarcinomas) with exclusion of non-malignant cases. Cases with an unknown stage at diagnosis, unknown age at diagnosis, diagnosis based only on autopsy report at death, and without histologic confirmation of EAC were excluded. Demographic variables, including age at diagnosis, gender, race (as white, black, or other), and year of diagnosis were retrieved. Stage was recoded based on SEER historic stages (as localized, regional, and distant). Localized disease was disease confined to the boundaries of the esophagus, regional disease included either direct extension of tumor to adjacent structures or regional lymph nodes, and distant disease included all metastatic disease.14 A sensitivity analysis utilizing the American Joint Commission on Cancer (AJCC) staging system (version 6 for 2004–2009 and version 7 for 2010–2015) was also used to stratify outcomes for localized T1 disease (T1N0M0), disease with positive regional nodes (TXNXM0), and metastatic disease (TXNXM1). From the time period of 2010–2015, AJCC version 7 data were available and used to compare outcomes stratified by T1a status. Survival time and cause of death were recorded, with appropriate censoring at last follow up. The SEER9 database does not record cancer recurrence, but does provide data on whether death was due to EAC. As such, the outcome of EAC-free survival was defined as either not dying by the end of follow up or dying from a cause other than EAC. First line surgical or endoscopic treatment modalities are available after 1998 and collected utilizing SEER site specific therapy codes (localized therapies: 10–29; surgery, including partial and total esophagectomy: 30–98; no surgery: 0). Age at diagnosis was stratified into the following categories in order to effectively compare across age groups: 1. Age less than 50, 2. Age 50–69, 3. Age greater than or equal to 70.
Statistical Analysis
Age-standardized incidence, standardized to 2000 US census data, was calculated utilizing SEER*Stat software. Annual percent change (APC) in incidence, presented as an average of the percent change over the time period of interest, was calculated utilizing the weighted least squares method, with the Tiwari modification for confidence intervals (CI).15, 16 Significance testing for the APC was tested against the null hypothesis of no change (APC = 0). Analysis of variance was used to assess for changes in continuous values (means), followed by multiple stepwise comparisons to identify significant differences. For categorical variables, we utilized χ2 analysis. Univariate and multivariate Cox-proportional hazard models were created in order to identify predictors of survival. As the variables incorporated into our study were predetermined, we used a two-sided alpha level of 0.05 as our threshold for statistical significance. This alpha level has been used in other epidemiologic studies of EAC utilizing the SEER database.1, 17 JMP (SAS Software, Cary, NC) was used for the analysis.
This study was exempt from review by the Mayo Clinic Institutional Review Board given the use of de-identified data in this analysis.
Results
Incidence Based Outcomes
We identified 34,443 cases of EAC diagnosed from 1975–2015 that were included in the incidence analysis, and basic demographics are displayed in Table 1. Young onset EAC has a strong male predominance: 90.0% of all EAC cases in those < 50 years were men in 1975 and this proportion remained high at 86.4% in 2015.
Table 1:
Basic Demographics of Esophageal Adenocarcinoma Cases, 1975–2015 (N=34,443)
| <50 | 50–69 | ≥70 | Total | ||
|---|---|---|---|---|---|
| Gender | Male | 2,442 (88.3%) | 15,750 (89.2%) | 11,503 (82.0%) | 29,695 (86.2%) |
| Female | 323 (11.7%) | 1,900 (10.8%) | 2,525 (18.0%) | 4,748 (13.8%) | |
| Race | White | 2,578 (93.2%) | 16,699 (94.6%) | 13,468 (96.0%) | 32,745 (95.1%) |
| Black | 84 (3.0%) | 532 (3.0%) | 247 (1.8%) | 863 (2.5%) | |
| Other/Unknown | 103 (3.7%) | 419 (2.4%) | 313 (2.2%) | 835 (2.3%) | |
| Stage | Localized | 460 (16.6%) | 4,047 (22.9%) | 4,590 (3.3%) | 9,098 (26.4%) |
| Regional/Distant | 2,305 (83.4%) | 13,603 (77.1%) | 9,438 (67.2%) | 25,345 (73.6%) | |
| Year of Diagnosis | 1975–1989 | 168 (10.4%) | 895 (55.4%) | 551 (34.2%) | 1,614 (4.7%) |
| 1990–1999 | 371 (9.1%) | 1,986 (48.8%) | 1,715 (42.1%) | 4,072 (11.8%) | |
| 2000–2015 | 2,226 (7.7%) | 14,769 (51.4%) | 11,762 (40.9%) | 28,757 (83.5%) | |
| Receipt of Chemotherapy* | 1,331 (74.0%) | 8,634 (67.4%) | 5,056 (47.0%) | 15,021 (59.2%) | |
| Receipt of Radiation Therapy* | 963 (53.5%) | 6,948 (54.2%) | 4,974 (46.3%) | 12,885 (50.8%) | |
| Receipt of Surgical or Endoscopic Therapy* | 607 (33.7%) | 4,742 (37.0%) | 2,448 (22.8%) | 7,797 (30.7%) | |
| Total | 2,765 (8.0%) | 17,650 (51.2%) | 14,028 (40.8%) | 34,442 | |
Data presented only for patients diagnosed from 2004–2015
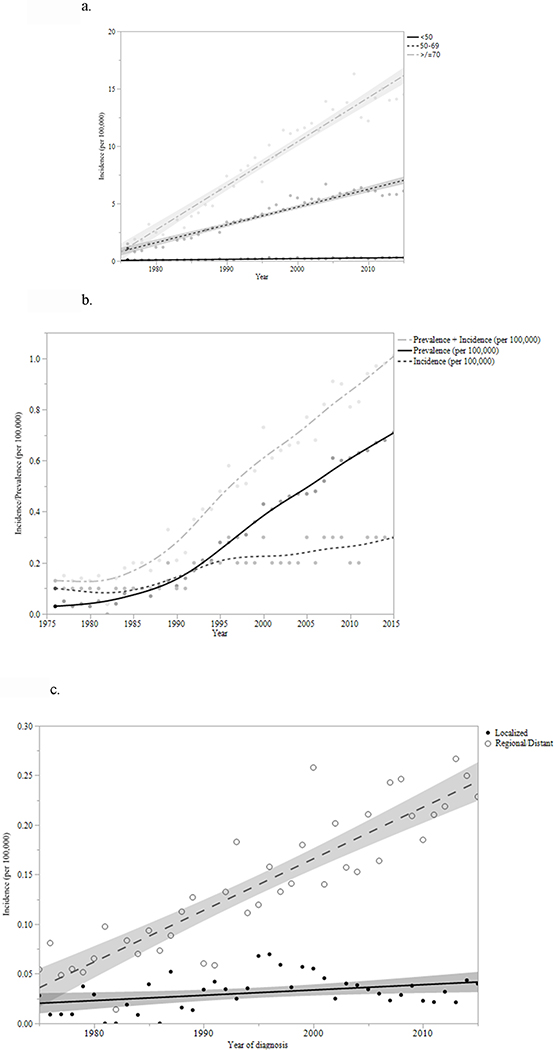
When comparing changes in standardized incidence from 1975–2015, there has been an increase in all three age groups (Figure 1a). The largest increase has occurred in patients over the age of 70, (APC = 5.4, 95% CI 3.9–6.9; p < 0.01). The annual incidence of EAC in those < 50 has also increased by more than threefold from 0.08/100,000 in 1975 to 0.27/100,000 in 2015 (Figure 1b and Supplementary table 1). Similarly, an increase in the incidence of regional and distant disease affecting those younger than 50 during the same time period accounted for most of the overall increase in EAC within that age group (APC = 3.6, 95% CI 2.0–5.2; p < 0.01; Figure 1c and supplementary figure 2a).
Figure 1.
a: Incidence of EAC by age groups, from 1975 to 2015 (per 100,000) Figure 1b: Prevalence and Incidence of Young-Onset EAC, 1975–2015 (per 100,000) Figure 1c: Incidence by Stage at Diagnosis in young onset EAC patients (age <50), 1975–2015
The proportion of EAC diagnosed in patients < 50 (as a fraction of all incident EAC) remains small overall (< 10%). It also appears to have decreased over time (from 10.7% in 1975 to 1989 to 7.7% in 2000–2015, p<0.0001; Supplementary Figure 1), likely due to the rather steep rise in the incidence of EAC in those ≥ 70 years old. There was a corresponding rise in the proportion of EAC diagnosed in patients older than 70 (from 33.9% in 1975–1989 to 40.9% in 2000–2015; p<0.01). The proportion of disease burden in patients aged 50–69 has remained relatively stable (55.4% in 1975 to 1989, 55.6% in 2000 to 2015; p > 0.05).
Notably, young onset EAC presented at more advanced stages when compared to both groups of older patients (Table 2a). In the time period from 2000–2015, regional/distant disease made up 84.9% of young onset EAC, compared to 77.6% of EAC in patients aged 50–69 and 67.8% of EAC in those over age 70 (p<0.01). Somewhat concerning was the observation that the proportion of young onset EAC patients presenting with regional/distant disease has also increased over time (1975–1989: 81.8%; 1990–1999: 75.5%; 2000–2015: 84.9%; p<0.01; Table 2a) at a rate faster than the older age groups. Ordinal logistic regression analysis confirmed this observation (supplementary figure 2a, 2b, and 2c; F-test for difference among regression lines <0.01).
Table 2a:
Incidence of Localized and Advanced Stage EAC by Age and Year of Diagnosis (%)
| < 50 years | 50–69 years | ≥ 70 years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1975–1989 | 1990–1999 | 2000–2015 | 1975–1989 | 1990–1999 | 2000–2015 | 1975–1989 | 1990–1999 | 2000–2015 | |
| N | 168 | 371 | 2226 | 895 | 1986 | 14769 | 551 | 1715 | 11762 |
| % Localized | 18.2 | 24.5 | 15.1 | 21.3 | 27.5 | 22.4 | 39.9 | 36.8 | 32.2 |
| % Regional/Distant | 81.8 | 75.5 | 84.9 | 78.7 | 72.5 | 77.6 | 67.1 | 63.3 | 67.8 |
Denominator: Total EAC cases in age group.
A sensitivity analysis utilizing the AJCC staging system versions 6 (2004–2009) and 7 (2010–2015) was also congruent with the above findings, and demonstrated that in the time period from 2004–2015, patients less than 50 were significantly more likely to present with metastatic disease, and less likely to present with localized T1 disease than their older counterparts (Supplementary table 2; p<0.01). From 2010–2015, AJCC version 7 staging was available to stratify outcomes by T1a status. During this time period, fewer (9.2%) patients younger than 50 were diagnosed with T1a disease, compared to 12.3% of patients aged 50–59 (p < 0.01) and 13.3% of patients older than 70 (p<0.01).
Survival Outcomes
We identified 25,813 cases of EAC that were included in the final survival analysis (1975–2011). Across all age groups, 5-year EAC free survival has improved from 1975 to 2011 (Table 2b). However, patients younger than 50 had the lowest rates of 5-year EAC free survival compared to older cohorts in the time period from 2000 to 2011 (age < 50: 22.9%; age 50–69: 29.6%; age ≥ 70: 29.6%; p<0.01; table 2b and figure 2). This trend toward lower 5-year EAC free survival was mirrored in earlier time periods as well. As shown in table 2b, when analyzing survival stratified by stage at diagnosis, results appear similar, with younger patients having poorer 5-year EAC free survival compared to patients over age 50 (apart from those diagnosed with localized disease, in which 5-year EAC free survival in those <50 was worse compared to those aged 50–69).
Table 2b:
EAC Survival Outcomes by Age and Year of Diagnosis
| < 50 years | 50–69 years | ≥ 70 years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1975–1989 | 1990–1999 | 2000–2011 | 1975–1989 | 1990–1999 | 2000–2011 | 1975–1989 | 1990–1999 | 2000–2011 | |
| 5-Year EAC-Free Survival (%) | |||||||||
| N | 168 | 371 | 1644 | 895 | 1986 | 10206 | 551 | 1715 | 8276 |
| Total | 10.3 | 20.5 | 22.9 | 13.8 | 21.8 | 29.6 | 13.0 | 21.6 | 29.6 |
| Localized | 35.3 | 53.5 | 63.2 | 37.1 | 47.9 | 66.2 | 22.1 | 38.8 | 50.9 |
| Regional/Distant | 4.3 | 9.7 | 14.7 | 7.0 | 11.1 | 17.6 | 8.2 | 9.6 | 17.2 |
A cutoff of 2011 was used to allow for a potential of 5 years of follow-up. Denominator is total cases within each age group.
Figure 2:
Kaplan-Meier Survival Curve for 5-year EAC-Free Survival (<50 vs Older Cohorts: Log-rank <0.0001)
In contrast, overall 5-year survival was significantly better in the those < 50 years old compared to patients over 70, but was significantly worse compared to patients aged 50–69 (for time period 2000 to 2011, age < 50: 19.7%; aged 50–69: 21.4%; age ≥ 70: 12.3%; p<0.01). This trend of superior overall survival in patients aged 50–69 compared to other age cohorts has persisted since 1975 (Supplementary Table 3). As shown in supplementary table 3, when analyzing survival by stage of diagnosis, results remained consistent, with superior 5-year overall survival in patients aged 50–69 compared to the other age groups over the time periods studied.
Univariate and multivariate predictors of 5-year EAC-specific mortality are shown in table 3. Univariate predictors of higher EAC-related mortality included age less than 50, diagnosis of regional or distant disease, and diagnosis prior to 1990. However, on multivariate analysis adjusted by gender, age, race, stage at diagnosis, and year of diagnosis, EAC-specific mortality was higher in patients older than 50 (p=0.03). The determinant of this effect appears to be stage at diagnosis, as construction of a model excluding stage (but incorporating the other aforementioned characteristics) demonstrated poorer mortality outcomes in the younger cohorts compared to those older than 50 (supplementary table 4). Multivariate analysis also demonstrated poorer outcomes for females, patients with regional/distant disease, and patients diagnosed prior to 1990. There was a trend toward poorer survival in African-Americans compared to whites, though this did not meet our a priori level of significance. EAC-related mortality has continued to improve for each time period compared to the preceding time period.
Table 3:
Predictors of EAC Specific Mortality (HR > 1 = increased mortality)
| Hazard Ratio (95% CI Range) | ||||
|---|---|---|---|---|
| Univariate | P-Value | Multivariate* | P-value | |
| Gender | ||||
| Male | REF | REF | ||
| Female | 1.05 (1.01–1.10) | 0.02 | 1.08 (1.04–1.13) | <0.01 |
| Age | ||||
| Age < 50 | REF | REF | ||
| Age ≥ 50 | 0.88 (0.83–0.93) | <0.01 | 1.06 (1.00–1.11) | 0.03 |
| Race | ||||
| White | REF | REF | ||
| Black | 1.17 (1.06–1.30) | <0.01 | 1.09 (0.99–1.21) | 0.08 |
| Other | 1.12 (1.01–1.24) | 0.03 | 1.05 (0.95–1.17) | 0.31 |
| Stage | ||||
| Localized | REF | REF | ||
| Regional | 1.99 (1.90–2.08) | <0.01 | 2.00 (1.91–2.10) | <0.01 |
| Distant | 4.89 (4.68–5.10) | <0.01 | 5.02 (4.81–5.25) | <0.01 |
| Year of Diagnosis | ||||
| 1975–1989 | REF | REF | ||
| 1990–1999+ | 0.75 (0.70–0.80) | <0.01 | 0.83 (0.78–0.89) | <0.01 |
| 2000–2011+ | 0.61 (0.58–0.65) | <0.01 | 0.60 (0.56–0.63) | <0.01 |
Model Adjusted for Variables in Table
Univariate and multivariate predictors of 5-year overall mortality are shown in supplementary table 5. Females appear to have poorer survival compared to males on both univariate and multivariate analyses. Other predictors of poor 5-year overall mortality included age >50, African-Americans race, regional/advanced disease, and diagnosis prior to 1990.
Supplementary table 6 shows 1-year survival data for patients diagnosed from 2011–2015. One-year EAC free survival was significantly lower in patients younger than 50 (51.9%) compared to patients aged 50–69 (57.9%; p<0.01) and ≥70 (54.2%; p<0.01). Overall 1-year survival was similar between patients aged <50 (48.7%) and those aged 50–69 (50.6%; p>0.05), but significantly higher compared to patients ≥70 (37.9%; p<0.01).
Surgical and Endoscopic Treatment Modalities
Data for endoscopic therapy are available from 1998. During the time period from 1998–2015, of patients with localized disease, only 7.4% of those <50 were treated with endoscopic therapies compared to 11.2% (p=0.05) of those aged 50–60 and 14.0% of patients ≥70 (p<0.01; supplementary table 7). In a sensitivity analysis of T1a lesions, for which data were available from 2010–2015, receipt of endoscopic eradication therapy as first line treatment modality appeared to be highest in the oldest age group (age <50: 27.4%; age 50–69: 37.5%; age ≥70: 43.3%; <0.01). Rates of surgical resection for localized disease were similar between patients <50 (44.8%) compared to those aged 50–69 (45.2%; p=0.25) but were significantly higher compared to patients ≥70 (20.5%; p<0.01; supplementary table 7). This trend persisted in a sensitivity analysis of patients with T1a lesions diagnosed from 2010–2015, with patients older than 70 significantly less likely to undergo surgery (13.3%) compared to the younger cohorts (age < 50: 35.5%; age 50–69: 32.9%; p<0.0001).
Data are available from 1975–2015 in regards to receipt of chemotherapy or radiation therapy as primary treatment modality. However, well over half of patients have “unknown” status for these outcomes and presentation of the remaining numbers may not present informative trends. However, acknowledging the importance of treatment options in patients with EAC, we did a multivariate analysis of patients diagnosed from 2004–2015, in whom treatment information is reliably available, and found poorer EAC-free survival in those without treatment (supplementary table 8).
Discussion
In this large population-based study on the epidemiology and outcomes of “young onset” EAC over the last 4 decades, we make the following observations. While this subset continues to constitute a small proportion of all EACs (< 10%), its incidence has increased by over 200% over the last few decades. Young onset EAC also presents at more advanced stages, along with a worrying trend of increasing proportion of advanced stage presentation over time, compared to older age groups. Lastly, while young onset EAC was associated with lower EAC-free survival, this appeared to be a reflection of the advanced stage at presentation.
Several findings in our study mirror trends in young onset colorectal cancer (CRC). Patients younger than 50 years also make up approximately 5–10% of all CRC. Over the last 30 years, the incidence of young onset CRC has increased by 2% per year.2, 18–20 These patients are also more likely to present with more advanced stage disease.21–23 Cancer-specific mortality appears to be increased in young onset CRC in a number of studies, even in stage-stratified analyses.24–31
Some investigators have reported similar findings. Sawas et al, in a single center study from a quaternary referral center susceptible to referral bias, reported a higher proportion of advanced stages (stage III and IV disease) and poorer EAC free survival in younger (<50 years) compared to older patients.8 In another recent study utilizing the National Cancer Database (NCDB), the authors found that EAC patients aged 18–57 had the highest proportion of metastatic disease at presentation (34%).32 EAC free survival was unfortunately not reported in this cohort. 33 This is an inpatient database, not population-based, and hence may have been biased by sicker hospitalized patients. Our study demonstrates that the increased burden of advanced disease in young patients is not due to referral bias but a true population-based phenomenon with adverse survival outcomes.
There is no clear explanation for the higher proportion of advanced disease in younger patients, and further study is required to identify biologic, genetic, and environmental factors that may underlie this observation. A potential hypothesis is that “young onset EAC” may involve rapid transition from intestinal metaplasia to EAC, driven by an increase in signaling molecules that are active in the intestine, such as Wnt/β-catenin, Notch, and TGF-β,34 or this may be EAC that is potentially driven by a process independent of intestinal metaplasia. Poorer survival in patients without BE or intestinal metaplasia on EAC specimens has been reported recently.35 Unfortunately the SEER9 database does not contain histology data regarding the presence of intestinal metaplasia. While a genetic sequencing analysis of EAC in patients younger than 40 compared to patients older than 68 found no difference in mutational load, the two most commonly mutated genes were noted to be TP53 and P16 in both age cohorts.36 As such, the similar survival noted on multivariate and stage-stratified analysis suggests the EAC affecting younger populations is no different from that seen in older age groups, and may reflect the inability to identify early stage disease in this population. The delay in diagnosis of EAC may be due to several factors, including lack of clinical suspicion (for malignancy) in young patients presenting with dysphagia, lack of screening and surveillance recommendations for BE in those younger than 50, as well as the fact that younger individuals, in general, are less likely to seek care compared to older individuals.37
Some insight into the pathogenesis of this subset of EAC may be garnered by evaluating traditional risk factors for BE. Central obesity has been strongly linked to the pathogenesis of BE, as visceral fat produces pro-inflammatory cytokines which can contribute to carcinogenesis.38–40 It is well known that obesity rates have dramatically increased in the United States over the last several decades.41–43 Further, rates of current obesity appear to be higher in those aged 40–59 compared to over those 60.43 Mirroring these trends in obesity, the prevalence of another BE risk factor, gastroesophageal reflux disease, has also increased over the last decade, and may be partly explained by increased intra-abdominal pressure as a result of obesity.44, 45 As such, rising rates of obesity and gastroesophageal reflux disease, affecting all age groups, but particularly those aged 40–59,46 may contribute to the burden of disease found in this age cohort.47
On stage-stratified analysis, EAC free survival for localized disease in patients younger than 50 was superior to those over 70 but were poorer compared to patients aged 50–69. This may be due to differing therapeutic options, as younger patients were more likely to undergo esophagectomy (curative for T1 disease and likely also for T1b and T2 disease which is also part of “localized disease”), whereas patients aged 50–69 were more likely to undergo EET (which may not be curative for T1b or T2 disease). Further, as mentioned previously, prior work has demonstrated the existence of a phenotype of EAC without associated BE, which resulted in poorer EAC free survival outcomes.35 It is possible that younger patients may be more likely to carry this phenotype of EAC, but unfortunately such information is not available in the SEER9 database.
Our study has several strengths. We utilized the SEER9 database, which is drawn from a significant sample of the United States population, therefore avoiding the introduction of referral bias. This database regularly undergoes quality checks to ensure accuracy of included patient level data, and has been the basis for a number of high impact articles in many medical journals.48 Additionally we were also able to assess informative time trends. Hence, the use of the SEER9 database strengthens the validity of our findings, and accurately assesses patient characteristics that would be encountered in clinical practice throughout the United States. Other strengths include the strict definition of EAC, requiring histologic confirmation, the length of follow up (dating back to 1975), and exclusion of patients of unknown age and stage at diagnosis. Furthermore, we have constructed a multivariate model that takes into account several factors that could affect EAC-free survival.
Limitations of this study include the lack of ability to thoroughly review individual case health records to confirm the stage of disease (as well as other demographic variables) at diagnosis. Though a trend toward poorer survival was found in African Americans in our multivariate analysis, the disproportionate ratio of white cases to other races makes it difficult to truly compare outcomes across races. Comorbidity information, which could be valuable in identifying a high risk population, is unfortunately not available in the SEER9 database. Notably, there is a SEER-Medicare linked database that includes comorbidity data, but unfortunately does not have information on patients younger than 65, which excludes the target cohort of our present study. Information on chemotherapy and radiation therapy is clinically relevant, but given that therapy data were unknown for a large subset of the database, we did not include it in the primary analysis. However, in a subset analysis of patients in which this data is available, a lack of receipt of therapy was associated with poorer EAC free survival.
In conclusion, while young onset EAC remains uncommon, it presents disproportionately with advanced disease (with a worrisome trend of increase over the last four decades) and is associated with poorer 5 year EAC free survival compared to older cohorts. Concerningly, the proportion of advanced disease in this age group is steadily increasing. While it is unclear at this time what biologic, genetic, or environmental factors may influence these findings, until such factors are elucidated, reevaluation of our diagnostic and treatment strategies in this age group might need to be considered.
Supplementary Material
Acknowledgments
Grant Support: Funded in part by NCI R01 CA241164 (to PGI); This project was made possible by the CTSA Grant UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosures:
Don C. Codipilly: None
Tarek Sawas: None
Lovekirat Dhaliwal: None
Michele Johnson: None
Ramona Lansing: None
Kenneth K. Wang: None
Cadman Leggett: Research support but no direct monetary compensation from Nine Point Medical
David A. Katzka: Honorarium from Celgene and Education Advisory Board of Takeda
Prasad G. Iyer: Research funding from Exact Sciences, Medtronic, Pentax Medical, Nine Point Medical, Consulting: Pentax Medical, CSA Medical, Medtronic
Abbreviations:
- AJCC
American Joint Commission on Cancer
- APC
Annual Percent Change
- BE
Barrett’s Esophagus
- CI
Confidence Interval
- CRC
Colorectal Cancer
- EAC
Esophageal Adenocarcinoma
- ICD
International Classification of Diseases for Oncology
- NCDB
National Cancer Database
- NIH
National Institutes of Health
- WHO
World Health Organization
- SEER
Surveillance, Epidemiology, and End Results
References:
- 1.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013;119:1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 3.Spechler SJ, Souza RF. Barrett’s Esophagus. New England Journal of Medicine 2014;371:836–845. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointestinal Endoscopy 2008;67:394–8. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. American Journal of Gastroenterology 2016;111:30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.di Pietro M, Fitzgerald RC. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett's oesophagus with low-grade dysplasia. Gut 2018;67–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010;71:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawas T, Manrique GC, Iyer PG, et al. Young Adults With Esophageal Adenocarcinoma Present With More Advanced Stage Tumors and Have Shorter Survival Times. Clin Gastroenterol Hepatol 2019;17:1756–1762. [DOI] [PubMed] [Google Scholar]
- 9.Iwaya Y, Shimamura Y, Goda K, et al. Clinical characteristics of young patients with early Barrett’s neoplasia. World J Gastroenterol 2019;25:3069–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashemi N, Loren D, Dimarino AJ, et al. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Digestive Diseases and Sciences 2009;54:1708–1712. [DOI] [PubMed] [Google Scholar]
- 11.Portale G, Peters JH, Hsieh CC, et al. Esophageal adenocarcinoma in patients < or = 50 years old: delayed diagnosis and advanced disease at presentation. Am Surg 2004;70:954–8. [PubMed] [Google Scholar]
- 12.Wang S, Zhan M, Yin J, et al. Transcriptional profiling suggests that Barrett’s metaplasia is an early intermediate stage in esophageal adenocarcinogenesis. Oncogene 2006;25:3346–56. [DOI] [PubMed] [Google Scholar]
- 13.Aviles A, Reymunde A, Santiago N. Balloon-based electrode for the ablation of non-dysplastic Barrett’s esophagus: ablation of intestinal metaplasia (AIM II Trial). Boletin - Asociacion Medica de Puerto Rico 2006;98:270–5. [PubMed] [Google Scholar]
- 14.Dubecz A, Solymosi N, Stadlhuber RJ, et al. Does the Incidence of Adenocarcinoma of the Esophagus and Gastric Cardia Continue to Rise in the Twenty-First Century?—a SEER Database Analysis. Journal of Gastrointestinal Surgery 2014;18:124–129. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15:547–69. [DOI] [PubMed] [Google Scholar]
- 16.Walters KA, Li Y, Tiwari RC, et al. A Weighted-Least-Squares Estimation Approach to Comparing Trends in Age-Adjusted Cancer Rates Across Overlapping Regions. Journal of data science : JDS 2011;8:631–644. [PMC free article] [PubMed] [Google Scholar]
- 17.Tramontano AC, Nipp R, Mercaldo ND, et al. Survival Disparities by Race and Ethnicity in Early Esophageal Cancer. Digestive diseases and sciences 2018;63:2880–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey CE, Hu C-Y, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA surgery 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silla IO, Rueda D, Rodríguez Y, et al. Early-onset colorectal cancer: a separate subset of colorectal cancer. World journal of gastroenterology 2014;20:17288–17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy CC, Singal AG, Baron JA, et al. Decrease in Incidence of Young-Onset Colorectal Cancer Before Recent Increase. Gastroenterology 2018;155:1716–1719.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kneuertz PJ, Chang GJ, Hu C-Y, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA surgery 2015;150:402–409. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari A, Rognone A, Casanova M, et al. Colorectal carcinoma in children and adolescents: the experience of the Istituto Nazionale Tumori of Milan, Italy. Pediatric blood & cancer 2008;50:588–593. [DOI] [PubMed] [Google Scholar]
- 23.Khan SA, Morris M, Idrees K, et al. Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients. Journal of pediatric surgery 2016;51:1812–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen L, Mo M, Jia L, et al. Poorer prognosis in young female patients with non-metastatic colorectal cancer: a hospital-based analysis of 5,047 patients in China. Cancer management and research 2018;10:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sultan I, Rodriguez-Galindo C, El-Taani H, et al. Distinct features of colorectal cancer in children and adolescents: a population-based study of 159 cases. Cancer 2010;116:758–765. [DOI] [PubMed] [Google Scholar]
- 26.Shida D, Ahiko Y, Tanabe T, et al. Shorter survival in adolescent and young adult patients, compared to adult patients, with stage IV colorectal cancer in Japan. BMC cancer 2018;18:334–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou C-L, Tseng C-J, Shiue Y-L. The impact of young age on the prognosis for colorectal cancer: a population-based study in Taiwan. Japanese journal of clinical oncology 2017;47:1010–1018. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Bao F, Yan J, et al. Poor prognosis of young patients with colorectal cancer: a retrospective study. International journal of colorectal disease 2017;32:1147–1156. [DOI] [PubMed] [Google Scholar]
- 29.Fu J, Yang J, Tan Y, et al. Young patients (≤ 35 years old) with colorectal cancer have worse outcomes due to more advanced disease: a 30-year retrospective review. Medicine 2014;93:e135–e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou C-L, Chang S-C, Lin T-C, et al. Differences in clinicopathological characteristics of colorectal cancer between younger and elderly patients: an analysis of 322 patients from a single institution. American journal of surgery 2011;202:574–582. [DOI] [PubMed] [Google Scholar]
- 31.Lieu CH, Renfro LA, de Gramont A, et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD Clinical Trials Program. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobel TB, Curry M, Gennarelli R, et al. Higher clinical suspicion is needed for prompt diagnosis of esophageal adenocarcinoma in young patients. The Journal of Thoracic and Cardiovascular Surgery 2020;159:317–326.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feig B Comprehensive Databases: A Cautionary Note. Annals of Surgical Oncology 2013;20:1756–1758. [DOI] [PubMed] [Google Scholar]
- 34.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol 2004;20:695–723. [DOI] [PubMed] [Google Scholar]
- 35.Sawas T, Killcoyne S, Iyer PG, et al. Identification of Prognostic Phenotypes of Esophageal Adenocarcinoma in 2 Independent Cohorts. Gastroenterology 2018;155:1720–1728.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Nistelrooij AM, van Marion R, Biermann K, et al. Early onset esophageal adenocarcinoma: a distinct molecular entity? Oncoscience 2016;3:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim MT, Lim YMF, Tong SF, et al. Age, sex and primary care setting differences in patients’ perception of community healthcare seeking behaviour towards health services. PloS one 2019;14:e0224260–e0224260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Booth A, Magnuson A, Foster M. Detrimental and protective fat: body fat distribution and its relation to metabolic disease. Horm Mol Biol Clin Investig 2014;17:13–27. [DOI] [PubMed] [Google Scholar]
- 39.Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. New England Journal of Medicine 2017;376:254–266. [DOI] [PubMed] [Google Scholar]
- 40.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:872–8. [DOI] [PubMed] [Google Scholar]
- 41.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obesity Trends in the United States. J Natl Cancer Inst 2016;108. [DOI] [PubMed] [Google Scholar]
- 43.Stokes A, Ni Y, Preston SH. Prevalence and Trends in Lifetime Obesity in the U.S., 1988–2014. Am J Prev Med 2017;53:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki T, Hemond C, Eisa M, et al. The Changing Epidemiology of Gastroesophageal Reflux Disease: Are Patients Getting Younger? Journal of neurogastroenterology and motility 2018;24:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017:1–8. [PubMed] [Google Scholar]
- 47.Murphy CC, Yang YC, Shaheen NJ, et al. An age-period-cohort analysis of obesity and incident esophageal adenocarcinoma among white males. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus 2017;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duggan MA, Anderson WF, Altekruse S, et al. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. The American journal of surgical pathology 2016;40:e94–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.