Abstract
Background:
Determining the extent of Cryptococcal disease (CD) is key to therapeutic management. Treatment with fluconazole is only recommended for localized pulmonary disease. Induction therapy with amphotericin B (AmB) and flucytosine is recommended for disease at other sites, irrespective of central nervous system (CNS) involvement, but this is not often followed in patients without meningitis. In this study, we compared treatment and mortality between patients with CD of the CNS and other extrapulmonary (OE) sites.
Methods:
This is a retrospective, single-center study of all hospitalized patients with non-pulmonary Cryptococcal infection from 2002 to 2015 who underwent lumbar puncture. Demographics, predisposing factors, comorbidities, clinical presentation, laboratory values, antifungal treatment, and mortality data were collected to evaluate 90-day mortality and treatment differences between patients with OE and CNS CD. Survival analysis was performed using multivariable Cox Regression analysis.
Results:
Of 193 patients analyzed, 143 (74%) had CNS CD and 50 (26%) had OE CD. Ninety-day mortality was 23% and similar between the OE and CNS CD groups (22% vs 23%, P=0.9). In the comorbidity adjusted multivariable Cox Regression model, mortality risk was similar in the OE and CNS groups. Fewer patients with OE CD received induction therapy with AmB and flucytosine compared to those with CNS disease (28% vs 71.3%, P<0.001).
Conclusion:
Patients with OE CD had similar 90-day mortality compared to those with CNS disease. Despite current guideline recommendations, patients with OE disease were less likely to receive appropriate induction therapy with AmB and flucytosine compared to patients with CNS disease.
Keywords: Cryptococcus, Mortality, Prognosis, Therapy, HIV, Transplant, Other Extrapulmonary Cryptococcal Disease, Disseminated Disease
Introduction
Cryptococcal Disease (CD) spreads through the whole body but preferentially causes disease in the lungs and central nervous system (CNS). CNS disease is more common in people living with HIV (PLWH) [1,2], however, epidemiology is shifting with an increasing number of cases now occurring in people without HIV, in whom clinical presentation is more heterogeneous, and has a higher rate of other extrapulmonary (OE) sites [2–7].
Current Infectious Diseases Society of America (IDSA) guidelines for the management of CD are based on localization of disease and population at risk, including PLWH, organ transplant recipients and non-HIV nontransplant (NHNT) individuals [8,9]. Localization of CD primarily focuses on identifying meningoencephalitis and localized pulmonary disease. Infection beyond these two sites, including disseminated disease, is categorized as nonmeningeal, nonpulmonary cryptococcosis [8]. Recently, the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) abandoned the term “disseminated cryptococcosis” in favor of reclassifying CD using the terms: pulmonary, CNS and OE sites [10].
Previously defined disseminated disease encompasses both CNS and OE CD, which current guidelines recommend treating with induction therapy with amphotericin B and flucytosine. In clinical practice, under-recognition of disease dissemination outside of the CNS may lead to less aggressive management and worse outcomes [11]. This study aims to compare management strategies and mortality in patients with OE and CNS CD. We hypothesize that patients with OE CD are more likely to be undertreated compared to patients with CNS disease.
Methods
Study Design
We conducted a retrospective cohort analysis of all patients diagnosed with CD at Barnes Jewish Hospital, a 1,368-bed tertiary urban referral center in St. Louis, MO with a significant suburban and rural referral base. The Washington University in St. Louis Human Research Protection Office approved this study with a waiver of informed consent.
Cohort Construction
We reviewed all patients aged 18 and older hospitalized between January 1, 2002 and December 31, 2015 who were diagnosed with CD. CD was defined as 1) a positive cryptococcal antigen (CrAg) in the blood or cerebrospinal fluid (CSF), 2) isolation of Cryptococcus neoformans in any culture, or 3) International Classification of Diseases (ICD) 9th (117.5, 321.0) or 10th revision codes for cryptococcal infection (B45.1-B45.9). Through chart review, we excluded patients with localized pulmonary infection (i.e. microbiologic or histologic identification of C. neoformans from a pulmonary source with no other sites of involvement) and those without a lumbar puncture (LP) to rule out CNS involvement, as those patients could not be adequately classified. Patients were then classified as having CNS disease if they had: 1) a positive culture or CrAg in the CSF or 2) a brain or meningeal biopsy positive for C. neoformans by Mucicarmine stain. Patients without evidence of CNS disease were defined as having OE CD.
We used a combination of electronic query and manual chart review from the BJC Healthcare Medical Informatics database to obtain demographic data, presenting symptoms, predisposing risk factors for Cryptococcal infection, comorbidities, treatment, and mortality data as previously described [2,11–13].
Definitions
Time to diagnosis was defined as time from admission to the day the patient was identified to have a positive Cryptococcal antigen or culture results indicating the presence of C. neoformans or encapsulated yeast, or histologic finding of encapsulated yeast consistent with CD. Duration of symptoms was defined as the time from symptom onset (as recorded in the admission note) to the diagnosis as defined above. Culture site, antigen detection, imaging, and histopathology were used to define the site of infection. Localized pulmonary cryptococcosis was defined as disease localized to the lungs, without radiographic, histopathologic, physical exam or culture evidence of extrapulmonary sites. CNS infection was defined as a positive cerebrospinal fluid Cryptococcal antigen (CrAg) or cerebrospinal fluid or brain biopsy cultures growing C. neoformans. Other extrapulmonary cryptococcosis was defined as patients with cryptococcal infection involving other sites excluding the lungs and the CNS, determined by the presence of Cryptococcus in culture or histology of biopsies taken from other organs or cryptococcemia (formerly defined as disseminated disease) determined by growth of C. neoformans in bacterial or fungal blood cultures.
Patients who did not receive a lumbar puncture (LP) were reviewed and defined as postmortem diagnosis, lost to follow-up, presumed localized infection, LP contraindicated, refusal, transition to comfort care, other and unknown (if no clear reason was given). Patients who did not receive treatment were reviewed and defined as presumed to have a false positive result (determined by the attending physician who was caring for the patient), transition to hospice or comfort care, leaving the hospital against medical advice, and unknown (if no clear reason was given).
The primary outcome was 90-day mortality, as mortality beyond 90 days was considered less likely to be related to CD. Patients were censored at the time of last follow-up or at 90 days. Mortality data were obtained from the hospital’s medical informatics system and supplemented with review of the Social Security Death Index to include deaths outside the hospital network. Secondary outcomes included type and total duration of antifungal treatment received.
Statistical Analysis
Between-group differences were compared using Chi square tests and Fisher exact tests for categorical variables. For continuous variables a t-test was used; Mann-Whitney U test was used when the assumption of normality was not visually satisfied using a histogram. Continuous variables are presented as mean and standard deviation unless they were not normally distributed, and then they are presented as median and interquartile range. All statistical tests were two-tailed and significance was set at α=0.05. We performed univariate analysis to assess the association of demographic factors and underlying diseases with 90-day mortality, and variables found to be plausible (p<0.2) were assessed in the multivariable Cox proportional hazards model to compare 90-day mortality between patients with OE and CNS disease. All variables were assessed in a stepwise manner, with a focus on improving the model performance, while maintaining plausibility for the variables. Presence of CNS disease vs OE was assessed in the model regardless of significance, as it is the central focus of the manuscript. All statistical analysis was performed using SPSS Software V24 (IBM, Armonk, New York).
Results
Cohort
We identified 304 patients with CD. One-hundred eleven patients were excluded, 60 patients with localized pulmonary disease and 51 patients who did not receive a LP (Figure 1). Reasons to not receive a LP included: perimortem diagnosis of CD (22), presumed localized infection (11), contraindication to LP (5), patient refusal (5), transition to hospice/comfort care (2), other (3), and unknown (3).
Figure 1.
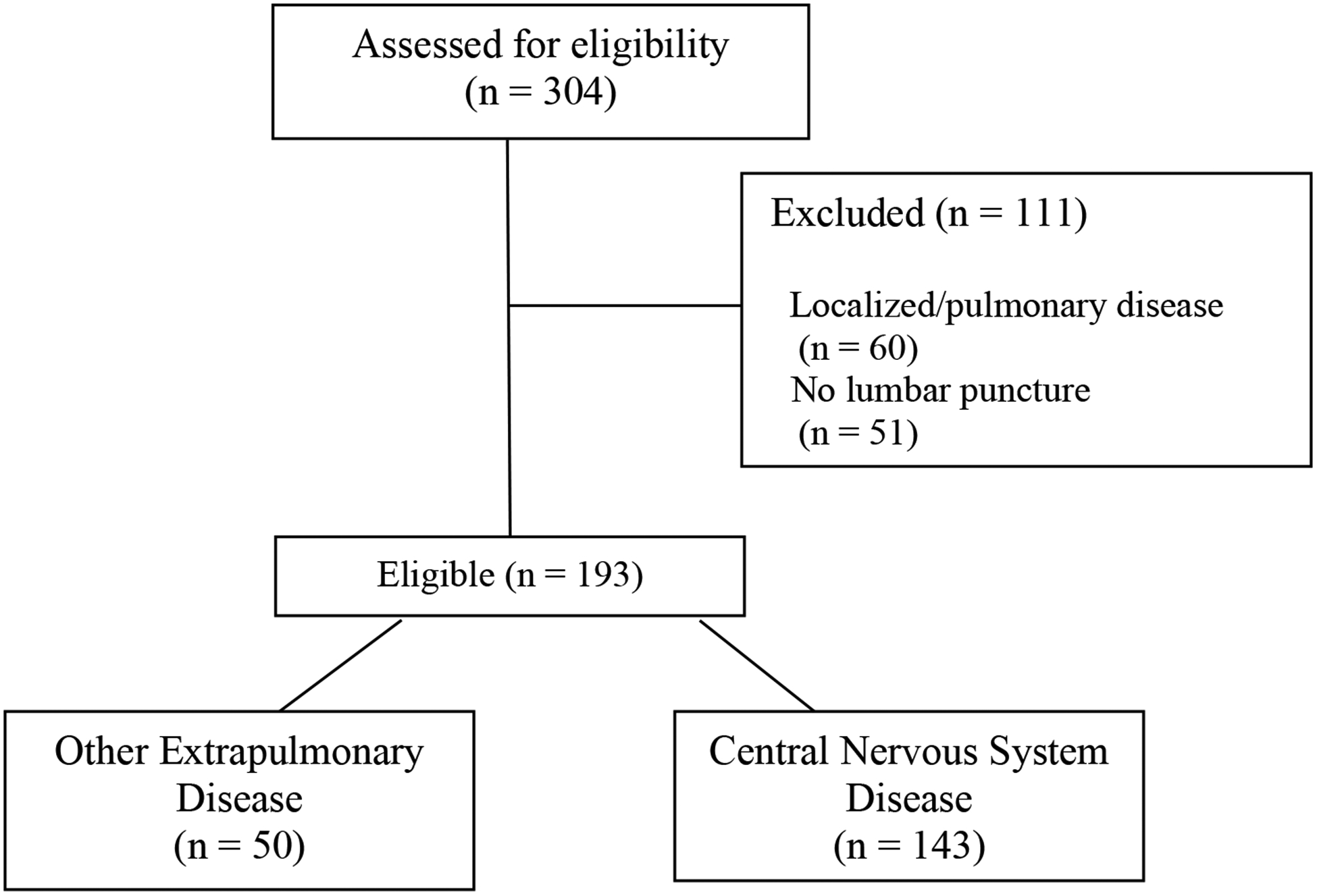
Cohort Construction
Of the 193 patients included in the final analysis, the mean age was 48.9 years (SD 15.7), 134 (69.4%) were men and 93 (48.2%) were white. Fifty patients (26%) had OE CD, and 143 (74%) had CNS disease. Distribution of patients with CNS disease was not significantly different between PLWH, transplant recipients or NHNT (73.4% vs 61.9% vs 78.2%, respectively, p=0.31). The sites of infection of OECD included Cryptococcemia (80%), osteomyelitis (6%), skin and soft tissue infection (10%) and liver and biliary tract infection (4%). In the CNS-CD group, 90 had concomitant Cryptococcemia (62.9%), three had skin and soft tissue infection (2.1%), one ocular (0.7%), one lymphadenitis (0.7%), one urinary tract disease (0.7%) and the rest CNS involvement only (32.9%).
Compared to patients with CNS disease, those with OE disease presented less often with neck stiffness (4% vs 36.4%, p<0.001), headache (36% vs 72%, p<0.001), altered mental status (18% vs 60.8%, p<0.001) or vision changes (4% vs 10.5%, p=0.2). In addition, patients with OE CD presented more often with cough (34% vs 8.4%, p<0.001) and dyspnea (38% vs 7%, p<0.001). Neither the median time to diagnosis from hospitalization (1 vs 2 days; p=0.32) nor the median time to diagnosis from symptom onset (12.5 vs 21 days; p=0.09) was significantly different between the OE and CNS disease groups (Table 1). Patients with OE CD were less likely to have high serum CrAg titers (≥1:1024) compared to those with CNS disease (8.3% vs 42.3%, p<0.001).
Table 1.
Baseline Characteristics of 193 patients with Cryptococcal Disease by site of involvement (Other Extrapulmonary compared with Central Nervous System Disease) 2002–2017.
| Variable | CNS N=143(%) | OECD N=50 (%) | p |
|---|---|---|---|
| Age (±SD) | 48.1(15.1) | 46.1(15.9) | 0.4 |
| Male | 96 (67.1) | 38 (76) | 0.2 |
| White | 69 (48.3) | 24 (48) | 0.9 |
| Presenting Symptoms | |||
| Fever | 98 (68.5) | 29 (58) | 0.2 |
| Fatigue | 118 (82.5) | 37 (74) | 0.2 |
| Neck stiffness | 52 (36.4) | 2 (4) | <0.001 |
| Headache | 103 (72) | 18 (36) | <0.001 |
| AMS | 87 (60.8) | 9 (18) | <0.001 |
| Visual changes | 15 (10.5) | 2 (4) | 0.2 |
| Weight loss | 40 (28) | 10 (20) | 0.3 |
| CN Palsy | 5 (3.5) | 1 (2) | 0.6 |
| Cough | 12 (8.4) | 17 (34) | <0.001 |
| Dyspnea | 10 (7) | 19 (38) | <0.001 |
| Time to Diagnosis from hospitalization | 1 (0–3.25) | 2 (1–4) | 0.32 |
| Time to diagnosis from symptom onset | 12.5 (6–24) | 21 (5.75–41) | 0.09 |
| Underlying condition | |||
| Non-HIV, non- transplant (%) | 61 (42.7) | 17 (34) | 0.3 |
| On immunosuppressive therapy | 39 (27.2) | 11 (22) | 0.42 |
| Immunocompetent | 22 (15.4) | 6 (12) | 0.6 |
| HIV/AIDS | 69 (48.3) | 25 (50) | 0.8 |
| Median CD4 at CD diagnosis (IQR) | 21 (6–42) | 29 (8–57) | 0.02 |
| Any Transplant | 13 (9.1) | 8 (16) | 0.2 |
| Diabetes Mellitus | 15 (11) | 9 (18) | 0.2 |
| ESLD | 18 (12.6) | 4 (8) | 0.4 |
| ESRD | 9 (6.3) | 5 (10) | 0.4 |
| Solid organ CA | 13 (9.1) | 9 (18) | 0.08 |
| Hematological CA | 13 (9.1) | 5 (10) | 0.8 |
| Microbiological Data | |||
| Serum CrAg positivity | 87 (89.7) | 43 (89.6) | 0.9 |
| 0 | 10 (10.3) | 5 (10.4) | <0.001 |
| 1:2 – 1:8 | 6 (6.2) | 8 (9.5) | |
| 1:16–1:512 | 40 (41.2) | 31 (64.6) | |
| ≥1:1024 | 41 (42.3) | 4 (8.3) |
CSN, Central Nervous System; AMS; altered mental status, OECD, Other Extrapulmonary Cryptococcal Disease; ESLD, End stage Liver Disease; ESRD, End stage Renal Disease; CA, Cancer; HIV/AIDS Human Immunodeficiency Virus/ Acquired Immunodeficiency Syndrome; CrAg, Cryptococcal Antigen; CD Cryptococcal Disease.
Management
Only 28% of patients with OE CD received induction therapy with amphotericin B (AmB) and flucytosine, compared to the majority of patients with CNS disease (71.3%) (p<0.001) (Table 2). Median duration of induction therapy was 14 days (IQR 11–17) and not different between the OE and CNS disease groups (13 vs 14 days; p=0.3). Azole monotherapy was more commonly given to patients with OE disease compared to those with CNS CD (48% vs 4.2%; p=<0.001). Overall duration of azole therapy was not significantly different between patients with OE CD and CNS disease (227 days [IQR 112–554] vs 309 days [IQR 61–674]; p=0.9).
Table 2.
Initial treatment and duration in 193 patients with Cryptococcal Disease by localization (Other Extrapulmonary compared with Central Nervous System Disease) 2002–2017.
| Variable | CNS N=143(%) | OECD N=50(%) | p |
|---|---|---|---|
| Induction therapy | |||
| AmB +5 FC | 102 (71.3) | 14 (28) | <0.001 |
| AmB + azole | 22 (15.4) | 6 (12) | 0.55 |
| AmB alone | 6 (4.2) | 1 (2) | 0.47 |
| Azole alone | 6 (4.2) | 24 (48) | <0.001 |
| 5 FC + azole | 1 (0.7) | 0 | |
| No induction therapy | 6 (4.2) | 5 (10) | 0.12 |
| Presumed false positive | 3 (2) | 2 (4) | 0.54 |
| Hospice/comfort care | 2 (1.4) | 2 (4) | |
| Left AMA | 1 (0.7) | 0 | |
| Unknown | 0 | 1 (2) | |
| Median (IQR) duration of therapy in days | |||
| AmB therapy | 14 (12–17) | 13 (10–17) | 0.3 |
| 5 FC therapy | 11 (4–16) | 11.5 (5–13) | 0.8 |
| Azole therapy | 309 (61–674) | 227 (112–554) | 0.9 |
CSN, Central Nervous System; AMS, OECD, Other Extrapulmonary Cryptococcal Disease; AmB liposomal amphotericin B, 5 FC Flucytosine, IQR interquartile range. SNF, Skilled nurse facility; LTCF Long-term Care Facility.
The distribution was not significantly different between CNS and OE disease.
Distribution was significantly different between CNS only and OE disease.
Five (10%) patients in the OE disease group and six (4.2%) in the CNS group did not receive any antifungal treatment (p=0.12). Reasons for lack of therapy included: presumed false positive CrAg (5), transition to hospice/comfort care (4), leaving the hospital against medical advice (1) and unknown (1).
Mortality
Overall, 90-day mortality in the cohort was 23% and there was no significant differences between the OE and CNS disease groups (22% vs 23%; p=0.9). In the univariable analysis, age ≥50 years (HR 3.2 [1.7–5.9]; p<0.001), end-stage liver disease (HR 5.5 [3–10.2]; p<0.001), lack of therapy (HR 4 [1.9–8.7]; p<0.001), altered mental status (HR 2.3 [1.3–4.0]; p=0.006) and receipt of chemotherapy (HR 2.2 [1.1–4.2]; p=0.02), were significantly associated with increased risk of 90-day mortality (p<0.2). White race (HR 1.8 [0.9–3.2]; p=0.05), headache on admission (HR 0.54 [0.3–0.9]; p=0.03), and the immune status of being immunocompetent (HR 0.4 [0.1–1.1]; p=0.08), a transplant recipient (HR 0.25 [0.1–0.6]; p=0.002) or PLWH (HR 0.31 [0.2–0.6]; p<0.001), were associated with decreased 90-day mortality (p<0.2) (Supplementary Table 1).
In the multivariable Cox regression model, we included the most significant variables associated with mortality (p<0.001) to avoid overfitting. In the multivariable model, after adjusting for age ≥50 years (HR 2.36 [1.3–5.3]; p=0.007), end-stage liver disease (HR 5.8 [2.9–11.6]; p<0.001), lack of therapy (HR 3.8 [1.7–8.9]; p=0.002) and immune status (HR 0.08 [0.01–0.6]; p=0.01 for transplant recipients), 90-day mortality between the OE and CNS disease groups was not significantly different (HR 1.23 [0.61, 2.46]; p=0.55) (Figure 2).
Figure 2. Survival curve of 193 patients with Cryptococcal disease by localization (Other Extrapulmonary compared with Central Nervous System Disease), 2002–2017.
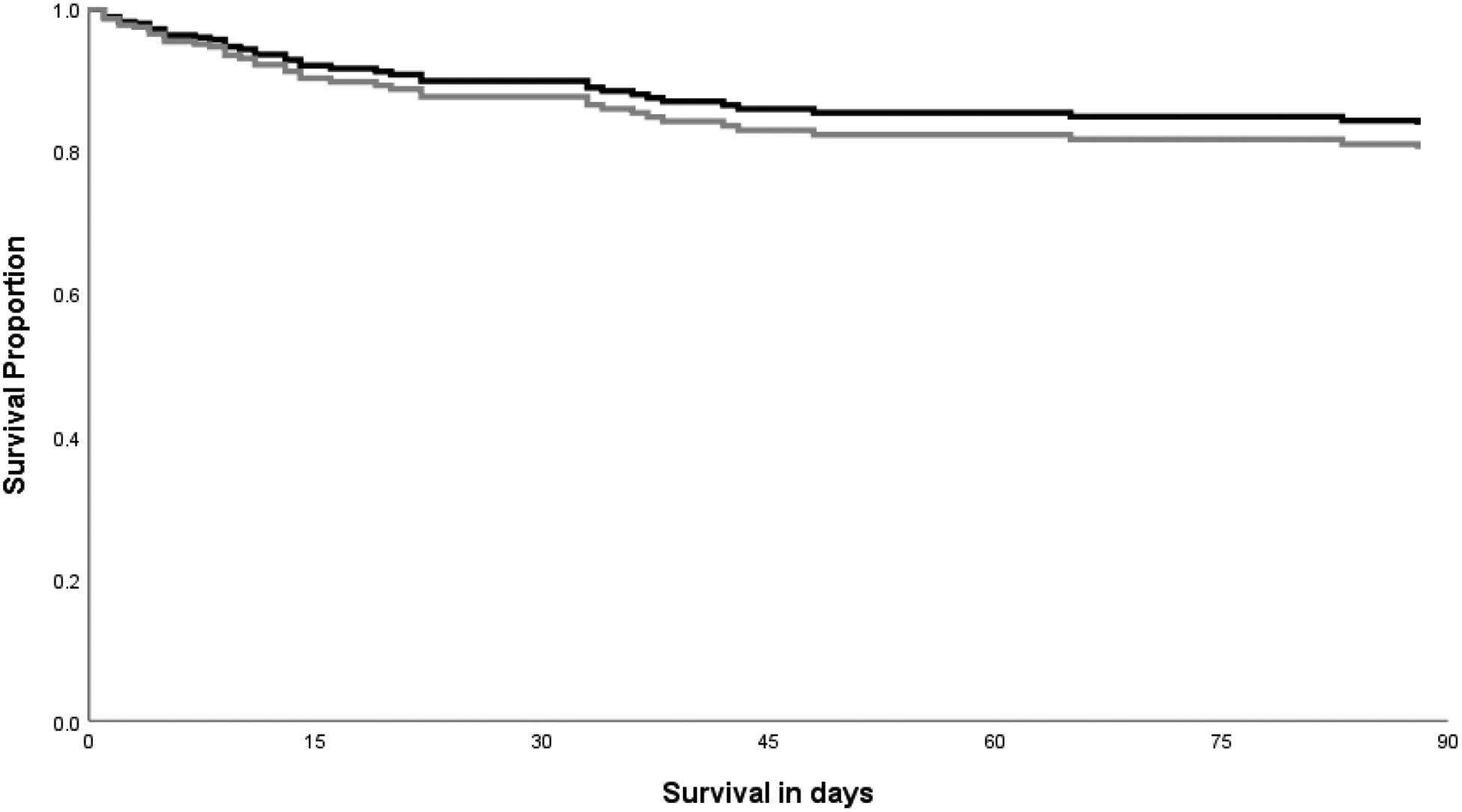
Survival curve of patients infected with cryptococcus by location of dissemination adjusted for age ≥50 years, end-stage liver disease (ESLD), immune status and lack of therapy. Mortality risk was similar in the other extrapulmonary group (OE) compared to the Central Nervous System (CNS) disease group (1.23 [0.61, 2.46]; p=0.55). Mortality was censored after 90 days as it was less likely to be related to cryptococcosis. Dark line indicates CNS disease; gray line indicates no OE disease.
Discussion
This study showed that patients with OE CD had similar 90-day mortality compared to those with CNS disease even after adjusting for factors associated with increased mortality. In the study, more than two thirds of patients in the OE CD group did not receive appropriate induction therapy with AmB and flucytosine as recommended by current treatment guidelines. The overall 90-day mortality rate of 23% seen in our cohort is consistent with previous studies, where mortality rates of 20–25% for disseminated CD have been described [5,14]. Other studies have evaluated risk factors for the progression of dissemination in patients with pulmonary disease, but have not assessed mortality outcomes and treatment in patients with OE CD [14,15]. Although previous studies have observed that the majority of PLWH had CNS disease [2], after removing patients with unproven CNS disease (i.e. no lumbar puncture performed) and those with localized pulmonary CD, we saw a similar distribution of PLWH within the CNS disease and the OE group in our cohort (48.3% vs 50%).
In our cohort, older age and end-stage liver disease were associated with increased mortality, consistent with prior studies [4,12,14,16]. Earlier studies identified immunosuppression to be associated with increased mortality [17–19]. However, organ transplant recipients and PLWH had a 46% and 60% decreased risk of 90-day mortality, respectively, compared to the non-HIV, non-transplant (NHNT) group. Keeping with the recognized change in the epidemiology of CD, with recent publications showing an increase in the incidence of CD in the NHNT population, it is becoming more apparent that NHNT patients have worse outcomes, including increased mortality and neurologic sequelae compared to other groups [4,5,16,20]. This increased incidence is also reflected in our cohort, where NHNT comprised 40% of all patients. Although the underlying cause for this difference in outcomes is unclear, it has been hypothesized that contributing factors could be a delay in diagnosis and undertreatment, other underlying risk factors which were not captured in the patient demographics, or an intact immune response resulting in a more robust inflammatory response [16].
The framework of classifying CD primarily in terms of pulmonary or CNS involvement was established at the time of the HIV epidemic, and given the changing epidemiology of this disease, this may mislead physicians to undertreat a serious infection with high mortality. The new consensus definitions released by EORTC/MSGERC have discarded the term “disseminated cryptococcosis” in favor of describing CD as “pulmonary,” “CNS,” and “other extrapulmonary sites” [10], which may help clarify that CD has multiple clinical presentations and that infection at other sites concurrent with pulmonary disease represents a multi-site infection that should be managed accordingly. To the best of our knowledge this is the first study comparing mortality and management outcomes between with CNS CD and OE CD. Management differences between patients with non-CNS and CNS disease have been compared in other studies, but interpretation of the results is limited due to inclusion of patients with isolated pulmonary infection in the non-CNS group [7,14]. Brizendine and colleagues found that patients with non-CNS disease treated with fluconazole alone or in combination with amphotericin for induction had the lowest mortality. However, they also included patients with localized, pulmonary disease in the non-CNS group [5]. In our cohort, receiving induction therapy with AmB and flucytosine was less common in the OE CD group compared to the CNS disease group (28% vs 71%) and 48% of OE CD patients were only given azole monotherapy, the recommended treatment for pulmonary disease. Similar to our study, in a study by Bratton et al, only 52% of patients with severe disease (defined as CNS disease, cryptococcemia or disseminated disease with serum CrAg≥1:512) received appropriate induction therapy [14].
Lack of recognition of disseminated disease when non-CNS extrapulmonary sites are involved, along with the perception that OE CD may not be as severe as CNS disease could explain some of these management differences. In our cohort where we excluded localized pulmonary disease cases, we suspect that undertreatment of patients with OE CD may likely be contributing to the elevated mortality seen in this group. Although the term “disseminated” was removed from the latest guidance [10], it may still help conceptualize the treatment algorithm of CD as localized (i.e. pulmonary) disease, which may be managed with azole monotherapy, and all other presentations (CNS and OE), which should be treated with induction therapy with AmB and flucytosine followed by azole step-down therapy. The mortality outcomes and management differences seen in the OE group in our study support this approach. Unsurprisingly, regardless of the disease group, lack of antifungal therapy for CD was associated with a four-fold increase in mortality. The recently developed EQUAL Cryptococcus score could aid clinical decision making for the optimal management of cryptococcosis [9].
The main limitation of our study is that it is a single-center, retrospective study, subject to misclassification and referral bias. To decrease misclassification, we excluded patients who did not receive a LP as most of these patients were diagnosed perimortem, which may have resulted in underestimation of mortality. However, it is unclear if this may have affected the distribution of patients preferentially into any of the groups being compared. As a tertiary-care center with a substantial referral base, severe CD may be over represented in our cohort, however, this is likely consistent with other large academic institutions as evidenced by the similar distribution of risk factors and overall mortality seen in previously published studies [5,14,21].
In conclusion, our study showed that patients with OE Cryptococcal disease have similar 90-day mortality compared to those with CNS disease. As our understanding of this serious infection continues to evolve, it is apparent that labeling CD in terms of the presence or absence of pulmonary and/or CNS involvement alone can be misleading and fails to address the wide array of clinical presentations associated with CD. It remains to be seen how the new consensus definitions will be reflected in future treatment guidelines and how it will reshape the management of CD.
Supplementary Material
Clinical Question:
How do outcomes and management differ between patients with Cryptococcal disease at other extrapulmonary sites compared to central nervous system disease?
Funding Statement
Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant ULTR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Footnotes
Disclosure of Potential Conflicts of Interest.
WGP has received research support from Merck & Co. and serves on the advisory board for Merck & Co. and Gilead Sciences. AS has received research support from Astellas, Scynexis, Cidera, MeraVista, and Mayne and consulting fees from Mayne, Scynexis, Astellas, Viamet, and Minnetronix. All other authors report no conflicts of interests relevant to this article.
REFERENCES
- 1.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PloS One. 2013;8(2):e56269. doi: 10.1371/journal.pone.0056269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hevey MA, George IA, Raval K, Powderly WG, Spec A. Presentation and Mortality of Cryptococcal Infection Varies by Predisposing Illness: A Retrospective Cohort Study. Am J Med. 2019;132(8):977–983.e1. doi: 10.1016/j.amjmed.2019.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Halloran JA, Powderly WG, Spec A. Cryptococcosis Today: It Is Not All About HIV Infection. Curr Clin Microbiol Rep. 2017;4(2):88–95. doi: 10.1007/s40588-017-0064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas PG. CRYPTOCOCCAL INFECTIONS IN NON-HIV-INFECTED PATIENTS. Trans Am Clin Climatol Assoc. 2013;124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 5.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PloS One. 2013;8(3):e60431. doi: 10.1371/journal.pone.0060431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George IA, Spec A, Powderly WG, Santos CAQ. Comparative Epidemiology and Outcomes of Human Immunodeficiency virus (HIV), Non-HIV Non-transplant, and Solid Organ Transplant Associated Cryptococcosis: A Population-Based Study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;66(4):608–611. doi: 10.1093/cid/cix867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marr KA, Sun Y, Spec A, et al. A Multicenter, Longitudinal Cohort Study of Cryptococcosis in Human Immunodeficiency Virus-negative People in the United States. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;70(2):252–261. doi: 10.1093/cid/ciz193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perfect JR, Dismukes WE, Dromer F, et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spec A, Mejia-Chew C, Powderly WG, Cornely OA. EQUAL Cryptococcus Score 2018: A European Confederation of Medical Mycology Score Derived From Current Guidelines to Measure QUALity of Clinical Cryptococcosis Management. Open Forum Infect Dis. 2018;5(11):ofy299. doi: 10.1093/ofid/ofy299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis Off Publ Infect Dis Soc Am. Published online December 5, 2019. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spec A, Olsen MA, Raval K, Powderly WG. Impact of Infectious Diseases Consultation on Mortality of Cryptococcal infection in Patients without HIV. Clin Infect Dis. Published online December 7, 2016:ciw786. doi: 10.1093/cid/ciw786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spec A, Raval K, Powderly WG. End-Stage Liver Disease Is a Strong Predictor of Early Mortality in Cryptococcosis. Open Forum Infect Dis. 2016;3(1):ofv197. doi: 10.1093/ofid/ofv197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hevey MA, Presti RM, OʼHalloran JA, et al. Mortality After Cryptococcal Infection in the Modern Antiretroviral Therapy Era: JAIDS J Acquir Immune Defic Syndr. 2019;82(1):81–87. doi: 10.1097/QAI.0000000000002095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bratton EW, El Husseini N, Chastain CA, et al. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PloS One. 2012;7(8):e43582. doi: 10.1371/journal.pone.0043582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baddley JW, Perfect JR, Oster RA, et al. Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2008;27(10):937–943. doi: 10.1007/s10096-008-0529-z [DOI] [PubMed] [Google Scholar]
- 16.Ecevit IZ, Clancy CJ, Schmalfuss IM, Nguyen MH. The poor prognosis of central nervous system cryptococcosis among nonimmunosuppressed patients: a call for better disease recognition and evaluation of adjuncts to antifungal therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2006;42(10):1443–1447. doi: 10.1086/503570 [DOI] [PubMed] [Google Scholar]
- 17.Pappas PG, Alexander BD, Andes DR, et al. Invasive Fungal Infections among Organ Transplant Recipients: Results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50(8):1101–1111. doi: 10.1086/651262 [DOI] [PubMed] [Google Scholar]
- 18.Henao-Martínez AF, Beckham JD. Cryptococcosis in solid organ transplant recipients. Curr Opin Infect Dis. 2015;28(4):300–307. doi: 10.1097/QCO.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 19.Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7(3):375–381. doi: 10.3201/eid0703.010302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day JN, Chau TTH, Wolbers M, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368(14):1291–1302. doi: 10.1056/NEJMoa1110404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58(5):736–745. doi: 10.1093/cid/cit794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


