Abstract
Background.
Weight-bearing jump tests that measure lower-extremity muscle power may be more strongly related to physical performance measures vs. non-weight-bearing leg press power, leg press strength and grip strength. We investigated if multiple muscle function measures differentially related to standard physical performance measures.
Materials/Methods.
In the Developmental Epidemiologic Cohort Study (DECOS; N=68; age 78.5±5.5 years; 57% women; 7% minorities), muscle function measures included power in Watts/kg (functional, weight-bearing: jump; mechanical: Nottingham power rig; Keiser pneumatic leg press) and strength in kg/kg body weight (Keiser pneumatic leg press; hand-held dynamometry). Physical performance outcomes included 6m usual gait speed (m/s), usual-paced 400m walk time (seconds), and 5-repeated chair stands speed (stands/s).
Results.
Women (N=31; 79.8±5.0 years) had lower muscle function and slower gait speed compared to men (N=25; 78.7±6.6 years), though similar 400m walk time and chair stands speed. In partial Pearson correlations adjusted for age, sex, race and height, muscle function measures were moderately to strongly correlated with each other (all p<0.05), though the individual correlations varied. In multiple regression analyses, each muscle function measure was statistically associated with all physical performance outcomes in models adjusted for age, sex, race, height, self-reported diabetes, self-reported peripheral vascular disease and self-reported pain in legs/feet (all p<0.05). Jump power (β=0.75) and grip strength (β=0.71) had higher magnitudes of association with faster gait speed than lower-extremity power and strength measures (β range: 0.32 to 0.7158). Jump power (β=0.56) had a slightly lower magnitude of association with faster 400m walk time vs. Keiser power70% 1-RM (β=0.61), and a higher magnitude of association vs. Nottingham power, Keiser strength and grip strength (β range: 0.41 to 0.47). Jump power (β=0.38) had a lower magnitude of association with chair stands speed than any other power or strength measures (β range: 0.50 to 0.65).
Conclusions.
Jump power/kg and grip strength/kg may be more strongly related to faster gait speed, a standard measure of physical function and vital sign related to disability and mortality in older adults, compared to leg press power/strength. However, jump power/kg had a similar magnitude of association with 400m walk time as Keiser power70% 1-RM/kg and a lower magnitude of association with faster chair stands speed than the other muscle function measures. Importantly, choice of muscle function measures should carefully reflect the study focus and methodologic considerations, including population.
Keywords: Epidemiology, muscle, countermovement, power, physical function
1. Introduction
Both muscle power (force*velocity)1,2 and strength3-9 have been extensively used as measures of muscle mechanical function in aging studies. However, inconsistent relationships of lower-extremity muscle power and strength have been found with physical function including short (e.g. 6-meter) and long distance gait speed (e.g. 400m walk, 6 minute walk), chair stand speed, the Short Physical Performance Battery (SPPB) score and stair climb time.10-22 These inconsistent findings may be due to the different intrinsic properties of power vs. strength (e.g. eccentric vs. concentric muscle actions, velocity of movement included, dynamic vs. static) and the diversity of the protocols used.23 Most studies have measured lower-extremity muscle function using dynamic or isometric movements in the seated position with power rigs10-12,17 or leg press,13,14 and static upper-extremity muscle function using grip strength.10,16,17,22 Although both muscle power and strength are lower at older ages compared to younger ages, the magnitudes are greater in muscle power compared with muscle strength,24-29 suggesting that power may be an earlier indicator of age-related muscle function loss compared to strength.
Weight-bearing tests that assess muscle power may be more strongly related to the ability to perform objective measures of physical performance that are also weight-bearing (e.g., walking or rising from a chair) vs. non-weight-bearing tests. Few studies have examined the relationship between weight-bearing tests (i.e. countermovemnt jump tests) and physical performance.16,19,20,30 Importantly, not all have included both men and women in the same study and none of these past studies included and/or compared other muscle mechanical function tests (e.g., grip strength, Keiser leg press power). Additionally, critical biomechanical differences exist between weight-bearing and non-weight-bearing tests. Countermovement jump tests include both eccentric and concentric muscle action, as well as velocity of movement and other neuromuscular factors (e.g., postural control)31 that are required to complete physical performance measures. These countermovement jumps performed under unrestricted conditions may be more similar to physical performance measures than tests with other postural set-ups, such as the Nottingham leg press power and Keiser leg strength/power testing completed in the seated position. Identifying if certain muscle function measures are more strongly related to physical performance vs. others may help when selecting the most appropriate tests for future studies examining these relationships in older adults.
We examined associations of multiple muscle function measures, including lower-extremity muscle mechanical power and strength, and upper-extremity muscle strength with physical performance (6m usual gait speed, 400m walk time and 5-repeated chair stands speed) in older adults. We hypothesized that weight-bearing power would be more strongly related to physical performance than other non-weight-bearing lower-extremity muscle function measures and upper-extremity grip strength.
2. Materials and methods
2.1. Participants
The Developmental Epidemiologic Cohort Study (DECOS) was conducted at the University of Pittsburgh in community-dwelling older adults (age 70+ years) recruited using the Pittsburgh Claude D. Pepper Older Americans Independence Center Research Registry.32 Exclusion criteria included any self-reported health contraindication to physical testing and the inability to perform basic mobility tasks (e.g. severe pain, aching, or stiffness while walking). A total of 68 participants enrolled in the study. Participants had two clinic visits scheduled 8 to 14 days apart, and 64 completed both visits. Of those 64 participants, 87.5% (N=56) completed all muscle function measures (jump power, Nottingham power, Keiser power and strength, and grip strength) and all physical performance measures (6m usual gait, 400m usual walk, and chair stands); 12.5% (N=8) were excluded for inability to complete to 400m usual walk test. The tests were completed in the same order across the two test days. The University of Pittsburgh Institutional Review Board approved this study and all participants provided written informed consent prior to participation. Participants were excluded if they had Modified Mini-Mental State Exam33 score of <80, which was administered at the beginning of the first visit.
2.2. Muscle strength
2.2.1. Grip strength
Grip strength was measured using Jamar dynamometers (Sammons Preston Rolyan, Bolingbrook, IL, USA)34 for two trials of both hands. Exclusions included recent worsening of pain/arthritis in hands or hand surgery in the past three months. Maximum grip strength was normalized to body weight (kg/kg body weight). In community-dwelling older adults ≥65years, the Jamar dynamometer had excellent reliability (Intraclass Correlation Coefficients (ICC); range: 0.91 to 0.95).35
2.2.2. Lower limb dynamic muscle strength
The Reiser pneumatic resistance device (A420 model; Reiser Sports Health Equipment, Fresno, CA) was used to assess dynamic single leg press strength as 1 repetition maximum (1-RM). Exclusions were for brain aneurysm or cerebral hemorrhage in the past six months; bilateral knee and/or hip replacement; significant difficulty bending or straightening both knees due to pain, arthritis, injury or some other condition; resting systolic blood pressure (SBP) >180 mmHg or <90 mmHg, or diastolic blood pressure (DBP) >110 mmHg. Participants seated with the leg at a 90° angle were instructed to press their leg as fast as possible through a full range of motion. Two repetitions were performed as a warm-up to leg press strength/power testing. 1-RM was assessed with a starting resistance of 40 pounds of force (the lowest setting). The Borg Rating of Perceived Exertion (RPE; scale: 6 = “no exertion at all” to 20 = “maximal exertion”) was reported with rest for 30 seconds between each repetition for which the RPE ≥ 15 “hard/heavy”. Resistance was gradually increased until the participant reported an RPE=18 (between “very hard” and “extremely hard”). 1-RM testing ended when participants reported that they could not continue with higher resistance. Absolute muscle strength from raw data was standardized by body weight (kg/kg body weight).
2.3. Muscle power
2.3.1. Lower limb weight-bearing muscle power: countermovement jump test
Advanced Mechanical Technology Inc. (AMTI) AccuPower force plates (Netforce Acquisition Software Version 3.05.01 with Accugait RS-232 setting and Biosoft Analysis software version 2.3.0) collected force signals from jump trials at a 1000 Hz sampling rate. The jump test protocol has been previously described.24 Exclusions were for inability to walk with or without an aid, inability to perform the test without orthotics, or endoprosthesis in knee/hip. Briefly, three countermovement jumps (4-5 maximum if ≥1/3 had data quality or technical problems) on the force plate were performed. Jump instructions were to jump as quickly and as high as possible without pausing between bending the knees and jumping, land smoothly, and then stand up straight and remain still. Participants were not instructed on use of arms during jumps for a more free-living movement. Pain (scale of 0-10, “0”=none to “10”=severe pain) and location of pain were reported after jump tests; no participants stopped further testing due to pain. No serious adverse safety events occurred. Peak power from the trial with the highest jump height was selected for analysis. All participants had at least ≥1 trial without any technical and data processing exclusions. The analytic variable from the selected trial was jump peak power standardized by body weight (Watts/kg body weight). The intra-person jump reproducibility was assessed in the Osteoporotic Fractures in Men (MrOS) Study at two sites that completed testing twice on the same day with two separate examiners per site; jump peak power/kg were significantly and highly correlated (ICC=0.85) with low Coefficients of Variation (CV=7.2%).24
2.3.2. Lower limb non-weight-bearing muscle power: single leg press (Nottingham power rig and Keiser pneumatic resistance device)
Single seated leg press power was measured using the Nottingham power rig (Nottingham University, Nottingham, England)36 and Keiser pneumatic resistance device (A420 model; Keiser Sports Health Equipment, Fresno, CA). Nottingham single leg press exclusions were for bilateral hip replacement in the past six months. Participants were instructed to push the pedal as hard and as fast as possible through a full range of motion. Testing was performed until power plateaued, or participants completed 5-10 trials to obtain peak power (Watts). The analytic variable from the selected trial was Nottingham peak power standardized by body weight (Watts/kg body weight). In older adults, power measures from initial and repeat measures separated by approximately 1 week were significantly correlated (r=0.97) and had low CVs (range: 3.5% to 9.4%), indicating high reliability.36,37,38
Keiser single less press exclusions were the same as exclusions for the Keiser 1-RM testing (outlined in section 2.2.2). Peak power was assessed by participants pushing at 40%, 50%, 60%, and 70% of the 1-RM dynamic strength assessment. After approximately 30 minutes of no physical activity following the 1-RM assessment, power testing was started. Participants completed 2 trials for each intensity (40%, 50%, 60%, and 70% 1-RM) with 30 seconds of rest between each trial at the same level of resistance and 1 minute of rest between each increase in resistance. Raw data exported from the Keiser to the computer through a chip system was analyzed. The analytic variable was Keiser peak power from raw data standardized by body weight (Watts/kg body weight), which was obtained at intensity = 70% 1-RM for all participants. If participants had difficulty bending or straightening either knee fully due to pain, arthritis, injury, or some other condition, or ever had an injury that made one leg weaker than the other, that leg was not tested. The same leg tested during the Nottingham power assessment was tested during Keiser strength and power assessments. Keiser strength and power were validated against the 1-RM achieved on the double leg press, the vertical jump test and maximal power in older women (R2 range: 0.30 to 0.58)39 and average power and resistance were highly reliable in older men and women (inter- and intra-tester reliability, r=0.97 and 0.99, respectively).40
2.4. Lower-extremity physical performance measures
2.4.1. 6m usual gait speed
Time to walk 6m at the participant’s usual pace was recorded with a stop watch.41 Timing started when the participant began walking from a parallel foot position and ended when the first foot crossed the 6m mark. Exclusion was for inability to attempt the test. Gait speed was calculated from the fastest time of two trials (m/s).
2.4.2. Usual-paced 400m walk time
Time to walk 400m (10 laps on 20m course) at the participant’s usual pace without overexertion32 was recorded at the second visit. Exclusions were for inability to attempt the test due to use of a walking aid other than a single straight cane; SBP >199 mmHg, DBP >109 mmHg, heart rate <40 or <110 beats/minute, or heart rhythm abnormalities; shortened clinic visit; or course obstruction/unavailability. Participants could rest for ≤60 seconds at any time during testing (without leaning on any surface or sitting down).
2.4.3. Chair stands speed
Ability to rise once from a standard chair was recorded. Exclusion was for inability to attempt the test. Time to complete 5-repeated chair stands without using the arms was recorded for all study participants. Chair stands speed was calculated as five stands divided by the time to complete the test (stands/second).
2.5. Covariates
Information on age (years), sex (women/men), race (white/other), smoking status (current/former/never),42 and education (college or higher/ less than college) was obtained from self-administered questionnaires. Body mass index (BMI) was calculated from weight (balance beam or digital scales) and height (Harpenden stadiometers; Dyved UK). Self-reported physical activity was obtained from the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire.43 CHAMPS score was the sum of total time (minutes/day) spent in all physical activities ≥2 METs (high-light and moderate to vigorous physical activity)44 Multimorbidity included self-reported health from the 12-Item Short-Form Health Survey (SF-12),45 self-reported difficulty walking ¼ mile (yes/no), self-reported very easy to walk ¼ mile (yes/no), self-reported diabetes (yes/no), and self-reported peripheral vascular disease (PVD; yes/no). Sensory nerve function was assessed using standard 10-g and light touch 1.4-g monofilaments at the dorsum of the great toe by trained examiners after warming the participant’s right foot (unless contraindicated, in which case testing was performed on the left side) to 30°C.46 Insensitivity (yes/no) was defined as the inability to detect at least 3 of 4 touches (combined as one variable in analyses). Peripheral neuropathy symptoms included self-reported pain (yes/no) or numbness (yes/no) in the feet or legs in the past 12 months.
2.6. Statistical analyses
Descriptive statistics included two-sided t-tests and Chi-square tests to compare baseline characteristics between women and men. Muscle function measures were normalized by body weight to account for total body mass. Partial Pearson correlations were calculated between jump power/kg, Nottingham power/kg, Keiser power70% 1-RM/kg, Keiser strength/kg, grip strength/kg, gait speed, 400m walk time and chair stands speed, and were adjusted for age, sex, race and height. BMI was not entered as the outcomes were adjusted for weight. Correlations were classified as weak (r < 0.4), moderate (0.4 ≤ r ≤ 0.6), or strong (r > 0.6).47 Separate stepwise multivariable-adjusted linear regression models were built to evaluate the associations per standard deviation (SD) of jump power/kg, Nottingham power/kg, Keiser power70% 1-RM/kg, Keiser strength/kg and grip strength/kg with physical performance. Covariates with p<0.10 in any model were retained in all final models. As a final step, muscle function measure*sex interaction was entered into the fully adjusted models and retained if p<0.05. Standardized β coefficients and 95% confidence intervals were reported to compare results across all models. We also calculated percent difference as [(β1-β2)/β2]*100, where β2=jump power and β1=other power/strength measure, with negative values indicating lower β for power/strength measure vs. jump power. As a sensitivity analysis, we replaced the normalized muscle function variable with absolute muscle function variable and adjusted for body weight; any attenuation in β coefficients or change in significance level was assessed. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
The study sample included 31 women (age, mean ± standard deviation = 79.8±5.0 years) and 25 men (age, mean ± standard deviation = 78.7±6.6 years). Compared to men, women had 31% lower jump power, 42% lower Nottingham power, 31% lower Keiser power70% 1-RM, 26% lower Keiser strength and 33% lower grip strength (Table 1; all p<0.05). Women also had 9% slower usual gait speed compared to men (p<0.05), though similar usual-paced 400m walk time and chair stands speed (Table 1). No sex differences were found in age, race, body mass index, education, smoking status, self-reported physical activity, self-reported health, self-reported difficulty walking ¼ mile, self-reported diabetes, self-reported PVD, or monofilament sensitivity (Table 1). However, women were more likely to report pain and numbness in legs/feet vs. men (Table 1; all p<0.05).
Table 1.
Baseline characteristics by muscle function and physical performance completion, N=56
| Total (N=56) |
Women (N=31) |
Men (N=25) |
|
|---|---|---|---|
| Demographics | |||
| Age, years | 79.3 ± 5.9 | 79.8 ± 5.0 | 78.7 ± 6.6 |
| Women | 55.4 (31) | ||
| White race | 92.9 (52) | 87.1 (27) | 100.0 (25) |
| Anthropometry | |||
| Height, cm | 165.5 ± 7.7 | 160.2 ± 4.8* | 171.9 ± 5.3* |
| Weight, kg | 73.8 ± 12.4 | 69.4 ± 12.9* | 79.3 ± 9.5* |
| Body Mass Index, kg/m2 | 26.9 ± 4.1 | 27.0 ± 4.9 | 26.8 ± 3.0 |
| Lifestyle characteristics | |||
| Education ≥ college | 83.9 (47) | 83.9 (26) | 84.0 (21) |
| Former smoker | 51.8 (29) | 54.8 (17) | 48.0 (12) |
| Physical activity, CHAMPS score mins/day | 117.1 ± 75.6 | 121.9 ± 81.7 | 111.2 ± 68.3 |
| Comorbidity | |||
| Self-report excellent health | 33.9 (19) | 35.5 (11) | 32.0 (8) |
| Self-report no difficulty walking ¼ mile | 92.9 (52) | 90.3 (28) | 96.0 (24) |
| Self-report very easy to walk ¼ mile | 65.4 (34) | 60.7 (17) | 70.8 (17) |
| Self-reported diabetes | 3.6 (2) | 3.2 (1) | 4.0 (1) |
| Self-reported PVD | 5.4 (3) | 6.5 (2) | 4.0 (1) |
| Sensory nerve function | |||
| Monofilament sensitivity | |||
| Light monofilament insensitivity | 41.1 (23) | 41.9 (13) | 40.0 (10) |
| Standard monofilament insensitivity | 17.9 (10) | 16.1 (5) | 20.0 (5) |
| Pain in legs/feet | 17.9 (10) | 29.0 (9)* | 4.0 (1) |
| Numbness in legs/feet | 32.1 (18) | 45.2 (14) | 16.0 (4) |
| Muscle function | |||
| Jump power, W/kg body wt | 20.4 ± 6.7 | 17.9 ± 5.9* | 23.6 ± 6.3 |
| Nottingham power, W/kg body wt | 1.4 ± 0.5 | 1.2 ± 0.4* | 1.7 ± 0.6 |
| Keiser power70% 1-RM, W/kg body wt | 5.1 ± 1.8 | 4.5 ± 1.3* | 5.9 ± 2.0 |
| Keiser strength, kg/kg body wt | 4.8 ± 1.5 | 4.3 ± 1.2* | 5.4 ± 1.7 |
| Grip strength, kg/kg body wt | 0.3 ± 0.1 | 0.3 ± 0.1* | 0.4 ± 0.1 |
| Physical performance | |||
| 6m usual gait speed, m/s | 1.2 ± 0.2 | 1.1 ± 0.1* | 1.2 ± 0.2 |
| Usual-paced 400m walk time, seconds | 377.4 ± 59.9 | 376.7 ± 58.5 | 378.3 ± 62.9 |
| Chair stands speed, stands/second | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
p<0.05 for women vs. men; values in table are mean ± standard deviation or % (n)
In partial Pearson correlations adjusted for age, sex, race and height, muscle function measures were moderately to strongly correlated with each other (Table 2; all p<0.05), though the strength of individual correlations varied. For correlations with performance measures, jump power/kg was moderately correlated with 400m walk time (r=0.54; p<0.05) but weakly correlated with gait speed and chair stands speed (r=0.31 and 0.27, respectively; all p<0.05). Nottingham power/kg and Keiser power70% 1-RM/kg were moderately correlated with all physical performance measures (range: r=0.40 to 0.55; all p<0.05). Keiser leg strength was moderately correlated with 400m walk time and chair stands speed (both r=0.49; all p<0.05), but weakly correlated with gait speed (r=0.32; p<0.05). Grip strength was weakly correlated with all physical performance measures (range: r=0.29 to 0.35; all p<0.05).
Table 2.
Correlations between jump measures, grip strength and physical performance, N=56
| Nottingham power, W/kg body wt |
Keiser power70% 1-RM, W/kg body wt |
Keiser strength, kg/kg body wt |
Grip strength, kg/kg body wt |
6m usual gait speed, m/s |
Usual-paced 400m walk time, seconds |
Chair stands speed, stands/second |
|
|---|---|---|---|---|---|---|---|
| Jump power, W/kg body wt | 0.50* | 0.63* | 0.50* | 0.43* | 0.31* | −0.54* | 0.27 |
| Nottingham power, W/kg body wt | 0.58* | 0.39* | 0.34* | 0.42* | −0.41* | 0.40* | |
| Keiser power70% 1-RM, W/kg body wt | 0.72* | 0.52* | 0.46* | −0.50* | 0.55* | ||
| Keiser strength, kg/kg body wt | 0.50* | 0.32* | −0.49* | 0.49* | |||
| Grip strength, kg/kg body wt | 0.29* | −0.35* | 0.35* |
p<0.05 for correlations adjusted for age, sex, race, and height; r<0.4 weak, 0.4≤r≤0.6 moderate, r>0.6 strong; W=Watts, wt=weight
In multiple regression analyses, each muscle function measure was statistically associated with all physical performance outcomes in models adjusted for age, sex, race, height, self-reported diabetes, self-reported PVD and self-reported pain in legs/feet (Figure 1; all p<0.05). Muscle function became more strongly associated with physical performance outcomes with the addition of diabetes, PVD and pain symptoms in the legs/feet. Jump power (β=0.75; additionally adjusted for jump power/kg*sex interaction) had a higher magnitude of association with faster usual gait speed than any other power or strength measure (Figure 2): 32% higher than Nottingham power/kg (β=0.51), 23% higher than Keiser power70% 1-RM/kg (β=0.58), 57% higher than Keiser strength/kg (β=0.32), and 5% higher than grip strength/kg (β=0.71; additionally adjusted for grip strength/kg*sex interaction. Jump power (β=0.56) had a 9% lower magnitude of association with longer 400m walk time than Keiser power70% 1-RM (β=0.61), though was 18% higher than Nottingham power (β=0.46), 16% higher than Keiser strength (β=0.47) and 27% higher than grip strength (β=0.41) (Figure 2). Jump power (β=0.38) had a lower magnitude of association with faster chair stands speed than any other power or strength measure: 32% lower than Nottingham power (β=0.50), 71% lower than Keiser power70% 1-RM (β=0.65), 32% lower than Keiser strength (β=0.50) and 37% lower than grip strength/kg. In sensitivity analyses, β coefficients were not attenuated when replacing the normalized muscle function variable with the absolute variable and adjusting for body weight (data not shown).
Figure 1.
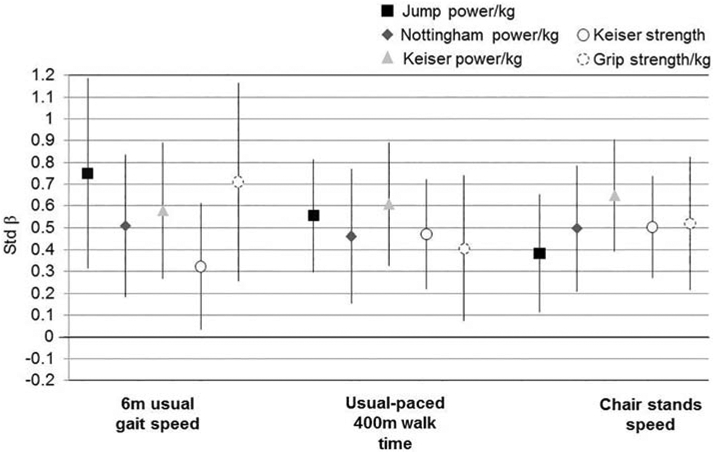
Associations (standardized β and 95% confidence intervals) of muscle power and strength measures with physical performance*, N=56
*all p<0.05; models for jump power/kg with 6m usual gait speed and grip strength/kg with 6m usual gait speed include the measure*sex interaction term; all models adjusted for age, sex, race, height, self-reported diabetes, self-reported PVD and self-reported pain in legs/feet
Figure 2.
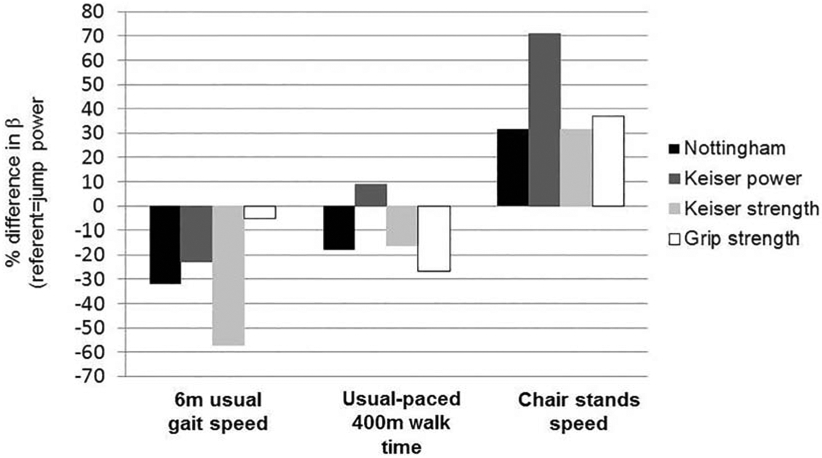
Percent difference in βs between continuous jump power and other power and strength variables*, N=56
*% difference calculated as [(β1-β2)/β2]*100, where β2=jump power and β1=other power/strength measure; negative values indicate lower beta for power/strength measure vs. jump power (e.g., for 6m usual gait speed, Nottingham power β is 32% lower vs. jump power β)
4. Discussion
The magnitude of association of jump power and grip strength with faster gait speed was approximately 1.5 to 2-fold higher than each of the other lower-extremity muscle function measures, even after adjusting for potentially confounding factors. Gait speed is commonly considered a standard measure of physical function and vital sign related to functional outcomes of sarcopenia and frailty,48 and higher risk of disability49,50 and mortality49,51 in large epidemiologic studies of older adults. The magnitude of associations of both jump power and Keiser power with longer 400m walk time was higher than other muscle function tests. Peak power from the Keiser leg press was obtained at 70% 1-RM for all participants in our study. The relatively high external loading (70% 1-RM) may be more similar to the forces generated during task-based power measures (e.g. maximum peak force in the jump tests) in our study. Power at lower percentages of the 1-RM (e.g. 40%) may be more similar to power from other seated methods (e.g. Nottingham leg press). The magnitude of association of jump power with faster chair stands speed was lower than any other power and strength measure. Diabetes, PVD and pain symptoms in the legs/feet may impact both muscle function and physical performance in older adults, as muscle function became more strongly associated with physical performance outcomes with the addition of these covariates. To our knowledge, this is the first study in older adults to compare magnitudes of association with multiple physical performance measures for weight-bearing and non-weight-bearing lower-extremity muscle mechanical function, as well as upper-extremity grip strength. The muscle function measures selected for future studies should consider not only the study outcomes, but also methodologic considerations including functional ability of the population.
In contrast with the unilateral, non-weight-bearing leg press measures in our study that include concentric but not eccentric muscle action, the jump test is a bilateral weight-bearing muscle function measure that may more closely replicate the weight-bearing conditions of physical performance measures. Countermovement jumping includes both eccentric and concentric muscle contractions (stretch-shortening cycle, SCC). This potentially translates into large force at the lowest position of the center of mass (transition between eccentric and concentric phases)1 which are dependent on the downward acceleration and deceleration and the subject’s body weight. The SSC allows for generation of greater impulse and upward acceleration of the body, and potentially higher velocity compared to shortening-only tests (e.g. Nottingham power rig). In addition, the countermovement jump is performed under unrestricted conditions requiring other neuromuscular factors (e.g., postural control)31 which may be more similar to physical performance measures than other postural set-ups (e.g. seated tests, Nottingham power rig, Keiser pneumatic resistance machine). In previous studies of associations between both lower-extremity muscle power or strength and physical performance,10,11,13,14,17,19,20,30 most lower-extremity power measures were non-weight-bearing tests in the seated position.10,11,13,14,17 Past studies that included weight-bearing muscle function tests, found associations with physical performance that were lower than in our study,19,20,30 possibly since we included healthier older adults who were able to complete all muscle function and physical performance measures. Hannam et al.20 (N=463; 71-87 years; age 77±4 years) included only women and adjusted standardized estimates were stronger for jump power (gait speed β=0.44; chair time β =0.42) than lower-extremity strength from jump tests (gait speed β=0.13; chair stands time β=0.23). Winger et al.30 (N=1,242, 71-101 years, age 77±4 years) found stronger associations for jump power (gait speed β=0.42; 400m walk time β=0.47; chair stands speed β=0.43) than lower-extremity strength from jump tests (gait speed β=0.18; 400m walk time β=0.24; chair stands speed β=0.23) in older men. None of these past studies included non-weight-bearing muscle function tests; therefore, they could not compare lower-extremity jump power and strength to leg press power or leg press strength or determine whether specific muscle function measures are more related to certain performance outcomes than others. Our results suggest that weight-bearing jump test measures and grip strength may be more robust predictors of gait speed compared to seated power and strength measures and therefore should be considered in studies of muscle function and physical performance in aging populations, especially those with difficulty performing seated tests.
Dynamic lower-extremity muscle mechanical function tests assess muscle groups involved in weight-bearing tasks, whereas upper-extremity grip strength is a static muscle action measure that does not include weight-bearing muscle groups. We showed that all lower-extremity muscle function measures were more strongly associated with the 400m walk test, an assessment of mobility that requires endurance, fatigue resistance and aerobic capacity,52 than upper-extremity grip strength.. Only one previous study compared associations of lower-extremity power, lower-extremity strength, and upper-extremity strength with physical performance. Winger et al.30 found that jump power in older men had a stronger relationships with 400m walk time, gait speed and chair stands speed (β range: 0.42 to β=0.47) than both lower-extremity strength from jump tests (β range 0.18 to 0.24) and grip strength (β range: 0.23 to 0.28), and relationships of lower-extremity strength from jump tests and grip with physical performance were similar. Compared to our study population of men (age=79.8±5.0 years; 400m walktime=378.3±62.9 seconds; gait speed=1.2±0.2 m/s; chair stands speed=0.4±0.1 stands/second), this past study included older men (age=84±4 years) with similar physical performance (400m walk=369±85 seconds; gait speed=1.18±0.22 m/s; chair stands speed=0.42±0.14 stands/second). This suggests that lower-extremity weight-bearing tests may be more appropriate than upper-extremity non-weight bearing tests in older, less functional populations of older adults than well-functioning older adults. Jump power may be able to differentiate within poor functioning individuals, likely due to values of jump power with wider ranges in the oldest adults and those with functional limitation.24 Additionally, correlations with 400m walk time were higher for lower-extremity power measures (r range: −0.41 to −0.54) compared to grip strength (r=−0.35), indicating that weight-bearing and non-weight bearing power may be more direct measures of the muscle groups needed to complete the 400m walk test, whereas grip strength may be a proxy measure of lower-extremity muscle function. Assessments that measure lower-extremity muscle mechanical function should be considered in studies of older adults because the same muscle groups are also required to complete the performance measures.
Both the weight-bearing and traditional seated lower-extremity muscle power and strength measures in our study have varied methodological considerations. The Nottingham power rig test protocol requires 5-10 total leg presses per leg, which may be more timeconsuming than other measures. The operator effort is also substantial since the seat and heavy flywheel are low to the ground and must be manually adjusted prior to each test. The Keiser pneumatic resistance protocol has a total test time of approximately 1 hour and may have participant burden as well since it requires the 1-RM to be established first, and then up to 9 total leg extensions for the power assessment and may result in participant fatigue. Frail older adults or those with chronic health conditions, may have difficulty sitting in the required positions for both Nottingham and Keiser testing and/or pushing against the leg press through the full range of motion, such those with knee osteoarthritis, which affects approximately 40% of older adults.53 Therefore, these seated measures may not be practical due to testing time for a large epidemiologic study of older adults who must be able to complete multiple study measures. Jump testing may be more appropriate in studies of older adults who may not be able to perform the seated tests. However, data processing of jump test trials in our study was time intensive due to custom-designed algorithms to evaluate data post-processing for participants with issues (e.g., unstable baseline that affected onset of movement and calculation of several parameters, such as mean power).24 This allowed us to capture a wider range of results in older adults with poor performance that are typically excluded with an automatized approach only. For those with poor balance who may not be able to perform the jump test safely, future studies may consider modifications to the jump test methodology to reduce exclusions while still ensuring participant safety, such as use of a harness. In larger epidemiological studies, tests with high reliability that require less time to complete may be more appropriate than tests that take more time. The factors regarding burden of testing and data processing must be considered in specific studies of older adults.
Our study had several strengths and limitations. Our cohort included men and women over age 70 years, with half of the sample ≥80 years. The study evaluated multiple power, strength and physical performance tests that are rarely considered in one study. The cross-sectional design did not allow examination of muscle function as predictors of future physical performance decline. While magnitudes of association were compared with each outcome, statistical tests of differences between standardized estimates of muscle function measures were not completed. Separate associations for women and men were not examined due to the small sample size. The community-dwelling, largely white population who were able to attend a clinic exam limits generalizability. Finally, other methods for measuring lower-extremity muscle function, such as the stair climb and knee extension strength tests, were not included.
In conclusion, functional, task-based power methods (e.g., jump tests) and grip strength may be more strongly related to faster usual gait speed, a standard measure of physical function related to both mobility-related disability and mortality in older adults, compared to traditional measures of leg press power and strength in older adults. However, jump power had similar magnitude of associations with longer 400m walk time as Keiser power/kg and a lower magnitude of association with faster chair stands speed than the other muscle function measures. The choice of muscle function measure should carefully reflect the study focus and methodologic considerations, including the specific population of older adults.
Highlights.
Jump power/grip strength more strongly related to gait speed vs. leg press measures
Jump/leg press power more strongly related to 400m walk vs. other muscle function
Jump power less strongly related to chair stands vs. other muscle function
5.
Funding
The Developmental Epidemiologic Cohort Study (DECOS) was supported by Pittsburgh Claude D. Pepper Older Americans Independence Center, Research Registry, and Developmental Pilot Grant (PI: Glynn) NIH P30 AG024827; National Institute on Aging Professional Services Contract HHSN271201100605P; University of Pittsburgh Department of Epidemiology Small Grants Program (PI: Ward); SEA Pilot – RC2AG036594; Department of Sports Science and Clinical Biomechanics, University of Southern Denmark (Caserotti P with engineer programming by Cuno Ramussen); NIA Aging Training Grant (PI: AB Newman) T32 AG 000181-29. The project was also supported, in part, by the Intramural Research Program of the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Caserotti P, Aagaard P, Simonsen EB, Puggaard L. Contraction-specific differences in maximal muscle power during stretch-shortening cycle movements in elderly males and females. European journal of applied physiology. 2001;84(3):206–212. [DOI] [PubMed] [Google Scholar]
- 2.Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50(3):461–467. [DOI] [PubMed] [Google Scholar]
- 3.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal Muscle Strength as a Predictor of All-Cause Mortality in Healthy Men. The Journals of Gerontology: Series A. 2002;57(10):B359–B365. [DOI] [PubMed] [Google Scholar]
- 4.Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age and ageing. 2003;32(6):650–656. [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. The journals of gerontology. Series A, Biological sciences and medical sciences 2005;60(3):324–333. [DOI] [PubMed] [Google Scholar]
- 6.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age and ageing. 1994;23(5):371–377. [DOI] [PubMed] [Google Scholar]
- 7.Guadalupe-Grau A, Carnicero JA, Gómez-Cabello A, et al. Association of regional muscle strength with mortality and hospitalisation in older people. Age and ageing. 2015;44(5):790–795. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Kupelian V, Visser M, et al. Strength, But Not Muscle Mass, Is Associated With Mortality in the Health, Aging and Body Composition Study Cohort. The Journals of Gerontology: Series A. 2006;61(1):72–77. [DOI] [PubMed] [Google Scholar]
- 9.Byrne C, Faure C, Keene DJ, Lamb SE. Ageing, Muscle Power and Physical Function: A Systematic Review and Implications for Pragmatic Training Interventions. Sports Medicine. 2016;46(9):1311–1332. [DOI] [PubMed] [Google Scholar]
- 10.Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. The journals of gerontology. Series A, Biological sciences and medical sciences 2012;67(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? The journals of gerontology. Series A, Biological sciences and medical sciences 2003;58(8):728–733. [DOI] [PubMed] [Google Scholar]
- 12.Marsh AP, Miller ME, Saikin AM, et al. Lower extremity strength and power are associated with 400-meter walk time in older adults: The InCHIANTI study. The journals of gerontology. Series A, Biological sciences and medical sciences 2006;61(11):1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. The journals of gerontology. Series A, Biological sciences and medical sciences 2004;59(11):1200–1206. [DOI] [PubMed] [Google Scholar]
- 14.Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Physical therapy. 2007;87(10):1334–1347. [DOI] [PubMed] [Google Scholar]
- 15.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Archives of physical medicine and rehabilitation. 2007;88(5):604–609. [DOI] [PubMed] [Google Scholar]
- 16.Siglinsky E, Krueger D, Ward RE, et al. Effect of age and sex on jumping mechanography and other measures of muscle mass and function. Journal of musculoskeletal & neuronal interactions. 2015;15(4):301–308. [PMC free article] [PubMed] [Google Scholar]
- 17.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. Journal of applied physiology (Bethesda, Md. : 1985). 2003;95(5):1851–1860. [DOI] [PubMed] [Google Scholar]
- 18.Hong N, Kim CO, Youm Y, Kim HC, Rhee Y. Low peak jump power is associated with elevated odds of dysmobility syndrome in community-dwelling elderly individuals: the Korean Urban Rural Elderly (KURE) study. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Thompson BJ, Whitson M, Sobolewski EJ, Stock MS. Effects of Age, Joint Angle, and Test Modality on Strength Production and Functional Outcomes. International journal of sports medicine. 2018;39(2):124–132. [DOI] [PubMed] [Google Scholar]
- 20.Hannam K, Hartley A, Clark EM, Aihie Sayer A, Tobias JH, Gregson CL. Feasibility and acceptability of using jumping mechanography to detect early components of sarcopenia in community-dwelling older women. Journal of musculoskeletal & neuronal interactions. 2017;17(3):246–257. [PMC free article] [PubMed] [Google Scholar]
- 21.Maden-Wilkinson TM, McPhee JS, Jones DA, Degens H. Age-Related Loss of Muscle Mass, Strength, and Power and Their Association With Mobility in Recreationally-Active Older Adults in the United Kingdom. Journal of aging and physical activity. 2015;23(3):352–360. [DOI] [PubMed] [Google Scholar]
- 22.Fragala MS, Alley DE, Shardell MD, et al. Comparison of Handgrip and Leg Extension Strength in Predicting Slow Gait Speed in Older Adults. Journal of the American Geriatrics Society. 2016;64(1):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alcazar J, Guadalupe-Grau A, Garcia-Garcia FJ, Ara I, Alegre LM. Skeletal Muscle Power Measurement in Older People: A Systematic Review of Testing Protocols and Adverse Events. The journals of gerontology. Series A, Biological sciences and medical sciences 2017. [DOI] [PubMed] [Google Scholar]
- 24.Strotmeyer ES, Winger ME, Cauley JA, et al. Normative Values of Muscle Power using Force Plate Jump Tests in Men Aged 77-101 Years: The Osteoporotic Fractures in Men (MrOS) Study. The journal of nutrition, health & aging. 2018;22(10):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietzel R, Gast U, Heine T, Felsenberg D, Armbrecht G. Cross-sectional assessment of neuromuscular function using mechanography in women and men aged 20-85 years. Journal of musculoskeletal & neuronal interactions. 2013;13(3):312–319. [PubMed] [Google Scholar]
- 26.Stephenson ML, Smith DT, Heinbaugh EM, et al. Total and Lower Extremity Lean Mass Percentage Positively Correlates With Jump Performance. Journal of strength and conditioning research / National Strength & Conditioning Association. 2015;29(8):2167–2175. [DOI] [PubMed] [Google Scholar]
- 27.Zengin A, Pye SR, Cook MJ, et al. Associations of muscle force, power, cross-sectional muscle area and bone geometry in older UK men. Journal of cachexia, sarcopenia and muscle. 2017;8(4):598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dionyssiotis Y, Galanos A, Michas G, Trovas G, Lyritis GP. Assessment of musculoskeletal system in women with jumping mechanography. International journal of women's health. 2010;1:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scandinavian journal of medicine & science in sports. 2008;18(6):773–782. [DOI] [PubMed] [Google Scholar]
- 30.Winger ME, Caserotti P, Cauley JA, et al. Associations between novel jump test measures, grip strength, and physical performance: the Osteoporotic Fractures in Men (MrOS) Study. Aging clinical and experimental research. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards MH, Buehring B. Novel Approaches to the Diagnosis of Sarcopenia. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2015;18(4):472–477. [DOI] [PubMed] [Google Scholar]
- 32.Lange-Maia BS, Newman AB, Strotmeyer ES, Harris TB, Caserotti P, Glynn NW. Performance on fast- and usual-paced 400-m walk tests in older adults: are they comparable? Aging clinical and experimental research. 2015;27(3):309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 34.Harkonen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. Journal of hand therapy : official journal of the American Society of Hand Therapists. 1993;6(4):259–262. [DOI] [PubMed] [Google Scholar]
- 35.Bohannon RW. Test-retest reliability of the five-repetition sit-to-stand test: a systematic review of the literature involving adults. Journal of strength and conditioning research / National Strength & Conditioning Association. 2011;25(11):3205–3207. [DOI] [PubMed] [Google Scholar]
- 36.Blackwell T, Cawthon PM, Marshall LM, Brand R. Consistency of leg extension power assessments in older men: the Osteoporotic Fractures in Men (MrOS) Study. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2009;88(11):934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. European journal of applied physiology and occupational physiology. 1990;60(5):385–390. [DOI] [PubMed] [Google Scholar]
- 38.Hurst C, Batterham AM, Weston KL, Weston M. Short- and long-term reliability of leg extensor power measurement in middle-aged and older adults. Journal of Sports Sciences. 2018;36(9):970–977. [DOI] [PubMed] [Google Scholar]
- 39.Thomas M, Fiatarone MA, Fielding RA. Leg power in young women: relationship to body composition, strength, and function. Medicine and science in sports and exercise. 1996;28(10):1321–1326. [DOI] [PubMed] [Google Scholar]
- 40.Earles DR, Judge JO, Gunnarsson OT. Velocity training induces power-specific adaptations in highly functioning older adults. Archives of physical medicine and rehabilitation. 2001;82(7):872–878. [DOI] [PubMed] [Google Scholar]
- 41.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26(5):569–585. [DOI] [PubMed] [Google Scholar]
- 42.Ewing JA. Detecting alcoholism. The CAGE questionnaire. Jama. 1984;252(14):1905–1907. [DOI] [PubMed] [Google Scholar]
- 43.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS Physical Activity Questionnaire for Older Adults: outcomes for interventions. Medicine & Science in Sports & Exercise. 2001;33(7). [DOI] [PubMed] [Google Scholar]
- 44.Glynn NW, Meinhardt AJ, LaSorda KR, et al. An Optimal Self-Report Physical Activity Measure for Older Adults: Does Physical Function Matter? Journal of aging and physical activity. 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 46.Ward RE, Boudreau RM, Caserotti P, et al. Sensory and motor peripheral nerve function and longitudinal changes in quadriceps strength. The journals of gerontology. Series A, Biological sciences and medical sciences 2015;70(4):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans JD. Straightforward statistics for the behavioral sciences. Belmont, CA, US: Thomson Brooks/Cole Publishing Co; 1996. [Google Scholar]
- 48.Perera S, Patel KV, Rosano C, et al. Gait Speed Predicts Incident Disability: A Pooled Analysis. The journals of gerontology. Series A, Biological sciences and medical sciences 2016;71(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005;53(10):1675–1680. [DOI] [PubMed] [Google Scholar]
- 50.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. The journals of gerontology. Series A, Biological sciences and medical sciences 2000;55(4):M221–231. [DOI] [PubMed] [Google Scholar]
- 51.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011;305(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. The journals of gerontology. Series A, Biological sciences and medical sciences 2011;66(11):1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allen KD, Golightly YM. State of the evidence. Curr Opin Rheumatol. 2015;27(3):276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


