Abstract
Background:
Prenatal alcohol exposure (PAE) affects early brain development and has been associated with hippocampal damage. Animal models of PAE have suggested that some subfields of the hippocampus may be more susceptible to damage than others. Recent advances in structural MRI processing now allow us to examine the morphology of hippocampal subfields in humans with PAE.
Method:
Structural MRI scans were collected from 40 children with PAE and 39 typically developing children (ages 8–16). The images were processed using the Human Conneetome Project Minimal Preprocessing Pipeline (v4.0.1) and the Hippocampal Subfields package (v21) from FreeSurfer. Using a large dataset of typically developing children enrolled in the Human Conneetome Project in Development (HCP-D) for normative standards, we computed age-speeifie volumetric z-seores for our two samples. Using these norm-adjusted hippocampal subfield volumes, comparisons were performed between children with PAE and typically developing children, controlling for total intracranial volume. Lastly, we investigated whether subfield volumes correlated with episodic memory (i.e., Picture Sequence Memory test of the NIH toolbox).
Results:
Five subfields had significantly smaller adjusted volumes in children with PAE than in typically developing controls: CA1, CA4, subiculum, presubiculum, and the hippocampal tail. Subfield volumes were not significantly correlated with episodic memory.
Conclusions:
The results suggest that several regions of the hippocampus may be particularly affected by PAE. The finding of smaller CA1 volumes parallels previous reports in rodent models. The novel findings of decreased volume in the subicular cortex, CA4 and the hippocampal tail suggest avenues for future research.
Keywords: Prenatal alcohol exposure, Brain, Hippocampus, MRI, Memory
1. Introduction
Alcohol is a potent teratogen associated with a range of neurodevelopmental and behavioral sequelae. Prenatal alcohol exposure (PAE) has a global effect on brain development, marked by a wide range of outcomes. These include reduced cerebral and cerebellar volumes, agenesis of the corpus callosum, and abnormalities in white matter microstructure among others (Lebel et al. 2011, Sowell et al., 2008, Wozniak and Muetzel, 2011).
Hippocampal damage has long been a suspected consequence of PAE due to the memory and learning impairments common in children with Fetal Alcohol Spectrum Disorders (FASD) and was among the early targets of PAE research (Lewis et al., 2015, Rasmussen et al., 2013). Since the time of the first descriptions of Fetal Alcohol Syndrome in the clinical literature (Jones et al., 1973, Lemoine, 1968), alcohol has been known to pass the placental barrier and ultimately accumulate in the hippocampi (along with several other subcortical structures) of fetal hamsters and primates (Ho et al., 1972).
The nature and extent of hippocampal injury following PAE has been investigated primarily through animal models. One unique aspect of hippocampal injury from PAE is the long-term impact on neurogenesis that carries into adolescence and adulthood (Boschen and Klintsova, 2017). Following an early finding of lower counts of pyramidal neurons in the dorsal hippocampus in adult rats after PAE (Barnes and Walker, 1981), many studies have investigated relative damage in substructures of the hippocampus. Decreased cell counts and/or lower dendritic density in CA1 of the rodent hippocampus after gestational exposure to alcohol have been frequently reported (Gibson et al., 2000, Livy et al., 2003, Mcgoey et al., 2003, Tran and Kelly, 2003, Wigal et al., 1990, Miller 1995). Evidence of cell loss in other hippocampal subfields has also been described, though less reliably. Livy et al. (2003) reported decreased cell counts in CA3 after third-trimester equivalent alcohol exposure. Several other studies failed to show any significant decreases in this region (Byrnes et al., 2004, Maier and West, 2001, West et al., 1986). A reduction of granule cells in the dentate gyrus was also found in some studies (Wigal et al., 1990, Livy et al., 2003) but not others (Bonthius and West, 1988, Miki et al., 2003, Tran and Kelly, 2003). Methodological differences (i.e. strain of animal chosen, route of administration, and amount/duration of exposure) may explain the variability in results from rodent studies. Thus far, the extent to which any of these findings might be paralleled in humans is not fully understood (Gil-Mohapel et al., 2010).
Recently, magnetic resonance imaging (MRI) and automated cortical and subcortical parcellation tools such as FreeSurfer have given researchers a non-invasive method to study abnormalities in brain morphology in humans following PAE (Fischl et al., 2002). Using such methods, the hippocampus as a whole has been shown to be smaller in children with PAE (Nardelli et al., 2011, Willoughby et al., 2008), though this difference is significant only prior to correction for total brain volume in some reports (Astley et al., 2009, Coles et al., 2011, Roussotte et al., 2012, Treit et al., 2013).
While volume reductions in the whole hippocampus have been reported in humans following PAE, limitations in MRI resolution and contrast have made it difficult to reliably subdivide the hippocampus into its constituent subfields. As a result, subfield-level descriptions of volume differences have not been feasible outside of animal research. This has changed in recent years as higher field magnets, advances in MRI acquisition and preprocessing, and the development of novel automated hippocampal segmentation methods have been introduced. Hippocampal subfield morphometry has now been used to investigate subfield-level alterations in a variety of pediatric conditions, including prenatal exposure to maternal obesity (Alves et al., 2020), childhood mood disorders (Tannous et al., 2018), and the effects of preterm birth (Aanes et al., 2019).
Here we apply an automated approach to hippocampal subfield morphometry (Iglesias et al., 2015) to derive volumes for ten hippocampal subfields. We created an age-adjusted normative dataset from the Human Connectome Project in Development (HCP-D) (Harms et al., 2017) and applied the normative corrections to hippocampal subfield volumes in our samples (those with PAE and controls). We then compared the adjusted volumes across groups.
A further aim of this study was to explore the relationship between hippocampal subfield volumes and a behavioral measure of hippocampal function. Episodic memory, which involves discrete learning, retention, and recall of information (as opposed to motor learning or personal memory), is especially dependent on the hippocampus (Winocur et al., 2010). Episodic memory has been repeatedly shown to be impaired in children with PAE (du Plooy et al., 2016). In those with PAE, verbal declarative memory is known to be impaired in terms of initial learning, discrimination, and recall (Crocker et al. 2011). Nonverbal memory is also impaired following PAE, especially on measures that involve a delay (Uecker and Nadel, 1996). To measure episodic memory performance, the Picture Sequence Memory Test (PSMT) from NIH Toolbox (Weintraub et al. 2013) was chosen due to its appropriateness over a wide age range and previous use as a measure of hippocampal-dependent episodic memory. We hypothesized that PAE would be associated with lower PSMT scores and that smaller hippocampal subfields would be associated with poorer PSMT performance in the group with PAE.
2. Materials and methods
2.1. Participants – CIFASD
Children with PAE (n = 40) and controls (n = 39) were part of a Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) study. Information about the CIFASD project is available at www.cifasd.org and in previous CIFASD publications (Mattson et al., 2010). Participants were recruited between 2017 and 2019, primarily by referral from the University of Minnesota Fetal Alcohol Spectrum Disorder Clinic. Other recruitment sources included self-referral, community flyers, and referral by other local clinics.
Clinical interviews in combination with a review of records, including retrospective maternal report, social service, legal, and/or medical records, were used to determine PAE history. Participants were included in the group with PAE if there was evidence of heavy PAE, defined as 13+ drinks per week or 4+ drinks per occasion at least once per week during pregnancy. When a detailed history of the alcohol exposure was unobtainable, decisions to include or exclude were made by consensus of the clinical team and the investigators based on available evidence. If, for example, the mother was known to have had an alcohol use disorder and had contact with law enforcement or social services during pregnancy, PAE was categorized as “suspected.” Finally, in some cases detailed PAE information was not available but participants were included following a diagnosis of Fetal Alcohol Syndrome (FAS) or Partial Fetal Alcohol Syndrome based on dysmorphology (see Classification section below).
Exclusion criteria for participants were comorbid neurological or developmental disorders (e.g. epilepsy or Autism), severe psychiatric disabilities that would prevent participation (e.g. psychosis or mania), drug or alcohol use by the participant, very low birthweight (<1500 g), and contraindications to MRI scanning (e.g. non-MR-safe medical devices, braces, or claustrophobia). Use of drugs other than alcohol by the mother during pregnancy was not considered a cause for exclusion, so long as alcohol was the primary and most common substance used. For control participants, maternal drug or alcohol use during pregnancy (excluding tobacco and caffeine) was also an exclusion criterion.
Participants were 8–16 years old at enrollment. A total of 84 participants were enrolled in the study. Table 1 contains demographic information for the 79 participants who were included in the analyses after eliminating five participants with excessive movement during the MRI scan and/or aberrant image processing (see MRI Acquisition and Processing). These five participants were enrolled in the group with PAE.
Table 1.
Characteristics of participants included in final analyses.
| N(%) or mean (SD) | PAE (n = 40) | Control (n = 39) | Statistical test (PAE vs Control) | HCP-D (n = 514) |
|---|---|---|---|---|
| Age (years) | 12.00 (2.49) | 12.21 (2.67) |
t(77) = −0.353 p = 0.725 |
12.18 (2.44) |
| Gender | ||||
| Male | 18 (45.0%) | 21 (53.8%) | x2(1) = 0.618 p = 0.432 |
258 (50.2%) |
| Female | 22 (55.0%) | 18 (46.2%) | x2(1) = 0.618 p = 0.432 |
256 (49.8%) |
| Racial Categories | ||||
| White | 22 (55.0%) | 30 (76.9%) | x2(6) = 10.579 p = 0.102 |
342 (66.5%) |
| Black or African American | 6 (15.0%) | 0 (0.0%) | 49 (9.5%) | |
| American Indian/Alaska Native | 1 (2.5%) | 2 (5.1%) | 2 (0.3%) | |
| Asian | 2 (5.0%) | 1 (2.6%) | 26 (5.0%) | |
| Native Hawaiian or Other Pacific Islander | 0 (0.0%) | 1 (2.6%) | 0 (0.0%) | |
| Other or Not Reported | 1 (2.5%) | 0 (0.0%) | 3 (0.5%) | |
| More than One Race | 8 (20.0%) | 5 (12.8%) | 92 (17.9%) | |
| Alcohol Exposure | ||||
| Alcohol Confirmed | 34 (85.0%) | |||
| Alcohol Suspected | 6 (15.0%) | |||
| Other Drug Exposure | ||||
| None | 8 (20.0%) | |||
| Drug Exposure Suspected | 10 (25.0%) | |||
| Drug Exposure Confirmed | 22 (55.0%) | |||
| Dysmorphic Facial Features | ||||
| Lip (score 4 or 5) | 11 (27.5%) | 3 (7.7%) | x2(1) = 3.207 p = 0.073 |
|
| Philtrum (score 4 or 5) | 15 (37.5%) | 3 (7.7%) | x2(1) = 6.723 p = 0.010 |
|
| Palpebral Fissure (≤10th percentile) | 1 (2.5%) | 3 (7.7%) | x2(1) = 1.850 p = 0.174 |
|
| ≥ 2 Facial Features Present | 11 (27.5%) | 1 (2.6%) | x2(1) = 6.978 p = 0.008 |
|
| Growth Deficiency (≤ 10th percentile) | ||||
| Height | 4 (10.0%) | 0 (0.0%) | x2(1) = 3.138 p = 0.077 |
|
| Weight | 1 (2.5%) | 3 (7.7%) | x2(1) = 1.850 p = 0.174 |
|
| Deficient Brain Growth (≤10th percentile) a | ||||
| Occipital-Frontal Circumference (OFC) | 4 (10.0%) | 0 (0.0%) | x2(1) = 3.138 p = 0.077 |
|
| IOM Diagnostic Category | ||||
| FAS | 1 (2.50%) | |||
| Partial FAS | 11 (27.50%) | |||
| ARND | 26 (65.00%) | |||
Note. 2 participants in the group with PAE and 11 participants in the control group did not have available physical exam information for analysis. 3 of the 6 participants in the group with PAE classified under “suspected alcohol exposure” were included because they had characteristics of pFAS. The remaining 3 participants in this group received this classification for the following reasons: 1) information in adoption documents indicating chronic maternal alcohol use, 2) information from the biological father indicating maternal alcohol use and 2 siblings with pFAS diagnoses, 3) thin Vermillion border at a previous point in development, a biological sibling with known FASD diagnosis, and documentation of multiple siblings removed from the biological mother’s home due to alcohol use.
2.2. Human connectome project in development as normative reference group
In order to maximize the precision of our volumetric analyses, we referenced our sample against a large, independent reference group of typically developing children enrolled in the Lifespan Human Connectome Project in Development (HCP-D; Somerville et al., 2018). HCP-D is a large-scale, multi-center project with data collection sites at the University of Minnesota, Washington University, Harvard University, and the University of California, Los Angeles. Exclusion criteria for HCP-D included learning disabilities, insufficient English fluency, health problems, psychiatric disorders, and MRI contraindications.
Because the age range of the HCP-D participants (i.e., 5–21 years) was broader than that of our CIFASD sample, we excluded HCP-D subjects with ages outside of the CIFASD age range. Of the 655 subjects included in the HCP-Development 1.0 release, 514 were included after limiting the age range to 8–16 years. Demographic data for these 514 subjects is included in Table 1. Chi-square tests revealed no significant demographic differences between the HCP-D reference group and the study groups in race, age, or sex.
2.3. Procedures – CIFASD and controls
After participants assented to study procedures and parents provided informed consent, CIFASD participants completed an MRI scan, a cognitive testing battery including the NIH Toolkit (Bauer et al., 2013), and a brief physical exam to assess for FASD-related characteristics. All study procedures were reviewed and approved by the University of Minnesota Institutional Review Board (IRB). Participants were compensated per IRB guidelines for their time and provided travel reimbursement.
2.4. Classification
For all CIFASD participants, dysmorphology information was collected by a trained dysmorphologist (KLJ) who was blinded to group status. The physical assessment included rating the Vermillion border of the upper lip and philtrum, measuring palpebral fissure length (PFL), measuring occipital-frontal circumference (OFC), and measuring the height and weight of the participant. Norms for these measures were the University of Washington’s 4-Digit Diagnostic System for the Vermillion and philtrum (Astley, 2011), Stromland et al. (1999), Nellhaus data for head circumference percentiles (Nellhaus, 1968), and CDC growth charts for height and weight (Kuczmarski et al., 2000).
Multiple FASD diagnostic classification systems exist and, it is worth acknowledging that there are differences in how these systems characterize individuals diagnostically (FAS, partial FAS, ARND) (Coles et al., 2016). Examples of existing diagnostic systems include the 4-Digit Diagnostic Code system (Astley, 2004, Astley, 2013), the Canadian diagnostic criteria (Chudley et al., 2005), the Centers for Disease Control & Prevention diagnostic criteria (Bertrand et al., 2004), and the Emory Fetal Alcohol Center Clinical Criteria (Coles et al., 1997). For the current study, Modified Institute of Medicine criteria were used to determine FASD classifications (Hoyme et al., 2016). A combination of standardized neurocognitive testing and parent reports were used to characterize neurobehavioral functioning: global intellectual ability (IQ or IQ index score), behavioral and self-regulation, other cognition (memory, executive functioning, specific, or visual spatial processing), and adaptive functioning. Per Hoyme et al. (2016), a participant was considered “impaired” in a category if the standardized score was 1.5 standard deviations or more below the mean. All participants in the PAE category had impairment in at least one domain of neurobehavioral functioning and all but two participants had impairment in two or more domains.
2.5. MRI acquisition and processing
Structural MRI data for the CIFASD group and the local controls were acquired at the University of Minnesota’s Center for Magnetic Resonance Research on two 3 T Siemens Prisma scanners (Siemens, Erlangen, Germany) equipped with standard 32-channel head coils. For each participant, a Tl-weighted and T2-weighted scan were acquired using custom pulse sequences which included automatic real-time motion detection and k-space line rejection and replacement software. Pulse sequence parameters were chosen to match those used in the HCP-D project (Harms et al., 2018). These parameters are described in Table 2.
Table 2.
MRI scan parameters.
| Sequence | Imaging parameters |
|---|---|
| T1-weighted | Multi-echo MP-RAGE sequence with TR = 2500 ms, TE = 1.8/3.6/5.4/7.2 ms, TI = 1000 ms, voxel size = 0.8 mm isotropic, flip angle = 8 degrees |
| T2-weighted | SPACE sequence with TR = 3200 ms, TE = 564 ms, voxel size = 0.8 mm isotropic, variable flip angle |
Note. TR = repetition time, TE = echo time; ms = milliseconds.
MRI data for the HCP-D reference group were acquired at four collection sites, also using the Siemens Prisma platform. We obtained these data, unprocessed, through the National Data Archive as made available in HCP-Development Release 1.0.
The HCP Minimal Preprocessing Pipeline (v4.0.1) was used to pre-process the structural data for all groups (Glasser et al., 2013). In this pipeline, the initial processing includes an alignment between the Tlw and T2w images, bias field and gradient distortion corrections, and registration of the data to MNI space. FreeSurfer (v6.0.0) was then used for cortical parcellation and subcortical segmentation of the Tlw volume, including the estimation of total intracranial volume used in correction for head size (surfer.nmr.mgh.harvard.edu) (Dale et al., 1999, Fischl et al., 2002). FreeSurfer processing included removal of non-brain tissue, automated Talairach transformation, segmentation, intensity normalization, tessellation of the grey matter / white matter boundary, topology correction, and surface deformation. The T2w volume was included in the FreeSurfer processing stream to better refine the pial surface by excluding dura and vasculature. To maximize reproducibility, we have encapsulated the HCP preprocessing pipeline into a Singularity container (Kurtzer et al., 2017). This container is available upon request.
After preprocessing, the right and left hippocampi were parcellated into constituent subfields using the hippocampal subfield segmentation tool (v21) included with development releases of FreeSurfer 6.0.0 (Iglesias et al., 2015). Both Tlw and T2w volumes were used to inform the segmentation. This hippocampal parcellation method utilizes statistical maps created from high resolution ex vivo imaging to inform a segmentation of each hippocampus into 19 preliminary subregions which are then combined using one of three merging schemes. The “CA” merging scheme was chosen, resulting in 10 hippocampal subfields per hemisphere in the final segmentation. An example of the resulting segmentation on an individual from the CIFASD group is shown in Fig. 1.
Fig. 1.
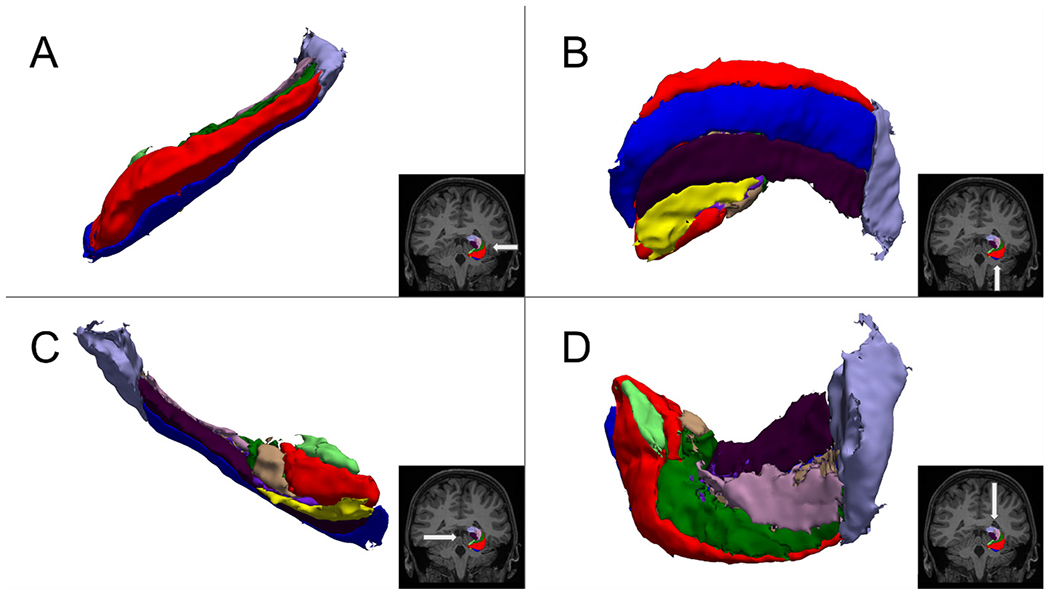
Results of automated hippocampal segmentation.
Note. The segmented left hippocampus of a CIFASD participant rendered as a 3D isosurface. Lateral (A), inferior (B), medial (C), and superior (D) aspects are shown.
All segmented volumes of CIFASD participants were visually inspected by a trained operator (DJR). Hippocampal subfield volumes were plotted and outliers were flagged for a secondary inspection. Additionally, ENIGMA imaging protocols (http://enigma.ini.usc.edu) were used to assess the quality of the overall FreeSurfer parcellation, including cortical parcellation (Hibar et al., 2015). In cases where subject images had obvious processing errors (e.g. significant cortical boundary misidentification) the data were excluded from this analysis even if the hippocampal segmentation appeared normal.
2.6. Episodic memory
To assess episodic memory, all 84 CIFASD participants completed the Picture Sequence Memory Test (PSMT) from the NIH Toolbox ® for Assessment of Neurological and Behavioral Function (NIH Toolbox) (Weintraub et al., 2013) on an iPad, though only those participants with usable MRI data were ultimately included in the subsequent analyses (n = 79). In this task, participants were asked to recall a series of illustrated objects and activities that were presented in a particular order on the screen, while audio-recorded phrases describing the objects and activities were played. Participants were asked to recall the sequence of pictures presented over two learning trials. Fifteen pictures were shown during the first trial and 18 pictures were shown during the second trial. We used fully corrected T-scores (i.e., corrected for age, gender, education and race/ethnicity) in the analyses. T-scores have a mean of 50 and standard deviation of 10; higher T-scores indicate better episodic memory.
3. Data analysis
SPSS version 22 was used for all analyses described below.
3.1. Z-scores using HCP-D participants as a normative reference group
The means and standard deviations of the 10 hippocampal subfields within HCP-D participants were computed for each one-year age group (i.e., 8-year-olds, 9-year-olds, 10-year-olds, etc.) separately. These values were used to compute z-scores for each subfield for each CIFASD participant (those with PAE and controls). These z-scores thus reflect age-corrected volumes using HCP-D participants as a reference group, and they were used in all analyses reported below. Z-scores were computed separately for the left and right hemispheres of each subfield, and the mean of the z-scores for both hemispheres was subsequently computed and used in all analyses, since we had no a priori hypotheses about lateralized effects and to limit the number of comparisons.
3.2. Group comparison: CIFASD group with PAE vs. controls
To account for the correlations among different subfield volumes (r’s up to 0.91) and to reduce the number of univariate comparisons, group differences in subfield volumes were evaluated by performing a repeated-measures ANCOVA with the 10 hippocampal subfields as within-subjects variables, group (those with PAE vs. Controls) as the between-subjects factor and total intracranial volume as a covariate. Total intracranial volume (z-score) was included as a covariate in order to account for the group difference (Controls > those with PAE) in intracranial volume (t = −2.09, p = 0.04), and the significant correlations between intracranial volume and subfield volumes (r’s 0.31–0.69; p’s < 0.01). Because there were no sex differences in either the group with PAE or the control group after controlling for intracranial volume, sex was not included as a covariate in any of the subsequent analyses.
3.3. Associations of hippocampal subfield volumes with spatial memory
We performed multiple regression analyses with each of the subfields that showed a significant group difference as a dependent variable, and fully corrected T-score for the PSMT and total intracranial volume (z-score) as independent variables. Regression analyses were performed collapsed across both groups, and, subsequently, within children with PAE and controls separately.
4. Results
4.1. Group differences in hippocampal subfield volumes
The repeated-measures ANCOVA revealed a significant effect of group on adjusted hippocampal subfield volumes (F(1,76) = 9.58; p = 0.003; partial η 2 = 0.112). Parameter estimates showed that children with PAE had significantly smaller volumes of the CA1, CA4, presubiculum, subiculum, and hippocampal tail (see Table 3 and Fig. 2). The other five subfields did not differ significantly between the groups (see Table 3 and Fig. 2).
Table 3.
Comparison of hippocampal subfield volumes between participants with prenatal alcohol exposure (PAE; n = 40) and control participants (n = 39).
| Subfield | Mean | SD | t | p | ||
|---|---|---|---|---|---|---|
| PAE | Control | PAE | Control | |||
| Parasubiculum | 132 | 138 | 26 | 29 | 0.14 | 0.890 |
| Presubiculum | 693 | 782 | 101 | 94 | −3.09 | 0.003 |
| Subiculum | 968 | 1089 | 136 | 114 | −3.62 | 0.001 |
| CA1 | 1599 | 1760 | 226 | 184 | −2.48 | 0.015 |
| CA3 | 498 | 523 | 65 | 65 | −0.72 | 0.471 |
| CA4 | 1201 | 1301 | 156 | 135 | −2.16 | 0.034 |
| HATA | 96 | 101 | 17 | 14 | −0.64 | 0.523 |
| Fimbria | 172 | 194 | 37 | 39 | −1.35 | 0.182 |
| Fissure | 197 | 212 | 50 | 41 | −0.56 | 0.579 |
| Tail | 1071 | 1189 | 163 | 146 | −2.56 | 0.012 |
Note. SD = standard deviation. Untransformed volumes (in mm3) are presented here to allow inclusion of these data in future meta-analyses, but t-scores and p-values were obtained from analyses using transformed volumes (i.e., z-scores computed using HCP-D data). Reported p-values were corrected for total intracranial volume.
Fig. 2.
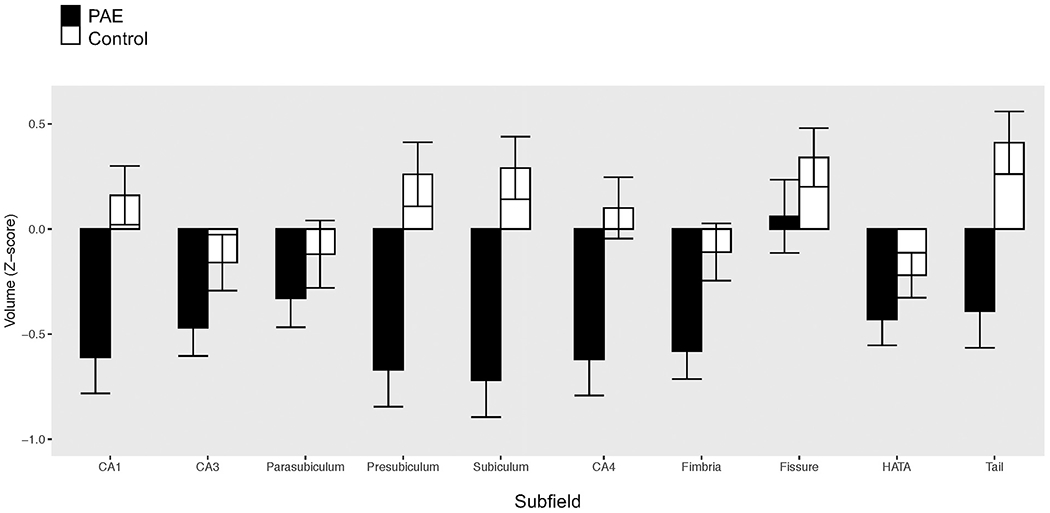
Subfield volume Z-scores for those with PAE and controls.
4.2. Associations between hippocampal subfield volumes and episodic memory
Participants with PAE had significantly lower performance on the PSMT than control participants (t = −3.38, p < 0.001). However, volumes of the five subfields that showed significant group differences were not found to be significantly associated with episodic memory, neither in the full sample, nor when examined separately in children with PAE and in controls (all p’s > 0.07). The only association that approached significance was with the presubiculum in the full sample (t = 1.78, p = 0.079). This association was in the expected direction; i.e., a larger volume was associated with better episodic memory.
5. Discussion
To our knowledge, this report is the first application of automated hippocampal segmentation to a clinical population of children with PAE. This approach allows a more detailed mapping of the hippocampus than has previously been feasible with MRI. Of the ten subfields examined, five were significantly smaller in children with PAE after correction for head size.
5.1. Observed differences in adjusted hippocampal subfield volumes
Our observation of decreased volume in CA1 is in agreement with similar findings in animal models (Livy et al., 2003, Mcgoey et al., 2003, Miller 1995, Tran and Kelly, 2003, Wigal et al., 1990, Gibson et al., 2000). In humans, CA1 is thought to be involved in long term episodic memory, especially that of an autobiographical nature (Bartsch et al., 2011). Decreased volume in CA4 is a novel finding. This region is comprised of mostly mossy cells at the junction of the dentate gyrus and CA3 of the hippocampus. CA4 has been proposed to play a role in pattern separation, ensuring that memories are encoded separately and thus can be retrieved without the contamination of information from other memories (Senzai 2019). Encoding of episodic memories has been shown to be impaired in FASD, independently of global memory impairment (du Plooy et al., 2016). PAE’s impact on CA4 volume may play a role in these impairments.
In the subicular cortex, two subfields were found to be smaller in children with PAE compared to controls. The subiculum has not been a specific target of PAE research in past studies. However, as it is one of the primary gateways from the hippocampus to the entorhinal cortex and neocortex, the subiculum is thought to play an important role in memory recall and consolidation (Ledergerber and Moser, 2017).
Another novel finding was decreased volume in the hippocampal tail among our group with PAE. While the hippocampal tail has not been explored in prior literature concerning PAE, the volume of this region has recently been associated with Major Depressive Disorder and used as a biomarker to predict antidepressant treatment outcome (with smaller volume associated with a lessened likelihood of remission) (Nogovitsyn et al., 2020). Depression in children with FASD is a common comorbidity, and suicide risk is markedly increased in this population (O’connor and Kasari, 2000, O’connor et al., 2019, Famy et al., 1998).
No significant differences were detected in the remaining subfields (CA3, fimbria, HATA, hippocampal fissure, and parasubiculum). A post-hoc power analysis was performed to estimate the sample size that would be required to detect significant group differences in these subfields. The results suggest that a sample size greater than 336 would be needed to detect group differences in adjusted fimbria volumes, while the remaining subfields would require samples in excess of 1000 participants. Notably, the five subfields in which no significant differences were found are also the smallest of the 10 subfields (by mean absolute volume across all subjects; see Table 3). Volumes of these smaller subfields may be more difficult to reliably measure using this automated approach at the current resolution. More reliable segmentations of these smaller structures may reveal group differences that were obscured in our data.
5.2. Picture sequence memory task and hippocampal subfield volumes
While impairment in episodic memory as measured by the PSMT was apparent in our group with PAE compared to controls, we did not observe any of the predicted associations between hippocampal subfield volumes and PSMT performance in either group. The PSMT was chosen for this analysis from a limited battery of cognitive measures that had been obtained by the parent study (CIFASD) due to its face validity and appropriateness for the age range of participants in our study. Impaired performance on visual-spatial episodic memory tasks such as the PSMT has been linked to smaller hippocampal volumes in individuals with PAE (DU Plooy et al., 2016). However, impaired performance on these tasks may also be associated with volumes of other brain areas (i.e., the caudate nucleus) (Fryer et al., 2012), and with deficits in other aspects of cognitive processing known to be impaired following PAE including attention and executive functioning (Mattson et al., 2019). Future investigations would benefit from a broader battery of tasks specifically chosen for their sensitivity to hippocampal-dependent functions, possibly including verbal memory measures such as the California Verbal Learning Test (Delis et al., 2000), or multi-modal memory tasks like those from the NEPSY (i.e., Memory for Names) (Korkman et al., 2007). Other memory domains such as spatial memory (as could be assessed with a virtual water maze task; Hamilton et al. (2003)) may show greater specificity to hippocampal function (Dodge et al., 2020), whereas episodic memory is supported by a broader network of brain regions in children, including the hippocampus, parahippocampal gyrus, perirhinal cortex, lateral prefrontal cortex and parietal cortex (Ghetti and Bunge, 2012). Additionally, the choice of memory measures for future investigations may benefit from targeting domains related to the subfields that were most affected in this analysis: CA1 (long term autobiographical memory), CA4 (pattern separation during encoding), hippocampal tail (emotional memory), and subicular regions (long term memory recall and consolidation).
A recent meta-analysis from DU Plooy et al. (2016) revealed that episodic memory encoding is impacted by PAE while delayed recall performance is spared in many tests after controlling for encoding deficits. The PSMT does not measure delayed recall so we were unable to investigate this phenomenon. Future investigations should employ tests of episodic memory that measure both immediate and delayed recall, ideally including tasks with longer delays that allow for memory consolidation before recall. Several of the hippocampal subfields which exhibited reduced volume in PAE (CA1 and subicular regions) are thought to be functionally related to recall rather than encoding, but these subfields are likely involved in the recall of remote consolidated memories, largely of an autobiographical nature.
5.3. Other limitations and future directions
Several limitations in the present study are related to the recruitment of children with PAE. Imperfect demographic matching between the group with PAE and controls is a limitation of the present study. Some of this variance was reduced by applying adjustments from a large normative dataset (HCP-D) based on age. The inclusion of six children with suspected, but not confirmed, alcohol exposure in the group with PAE may have diluted our sample. However, the evidence supporting inclusion was convincing (see note, Table 1) and our ultimate results did not differ if these six participants were excluded from the analysis (except that the observed difference in adjusted CA4 volumes would fail to meet our significance threshold; p = 0.065). Prenatal exposure to drugs other than alcohol is common is this population (Astley 2010) as well as in our clinical sample. By not excluding children with polydrug exposure, we benefit from a sample that is more representative of the clinical population. However, polydrug exposure has the potential to drive changes in regional brain volumes and may be a confound (Rivkin et al., 2008). We strived to mitigate this effect by ensuring that alcohol was the primary and most common substance of exposure. Additionally, a post hoc analysis determined that hippocampal subfield volumes in participants with PAE and confirmed polydrug exposure (n = 22) did not differ significantly from rest of the sample with PAE (F(1,37) = 1.36, p = 0.252). Future studies may be able to better address this confound if thorough maternal substance use histories are available and sufficiently large samples of children with confirmed alcohol-only exposure can be recruited.
The relatively small sample size did not provide enough power to examine different diagnostic subgroups. A post hoc analysis was run to compare subjects with ARND diagnosis (n = 26) to those with either FAS or pFAS diagnoses (n = 12). There were no significant differences in adjusted volumes between these two groups in any of the ten subfields. Compared to controls, both groups had significantly smaller adjusted volumes only in the subiculum, likely a result of decreased power after dividing the sample.
Imaging and image processing limitations are also a factor due to the relatively small size of the structures involved. Because the hippocampal subfield segmentation tool relies in part on predefined statistical maps, the segmentations may not be completely reliable and quality control beyond exclusion of obvious errors is difficult due to insufficient contrast and resolution (Iglesias et al., 2015). As noted previously, our ability to detect to group differences in the five largest structures but not the five smallest structures suggests that reliability of volume measurements in the smallest subfields may be a significant limitation. Nonetheless, the ability to perform in vivo segmentations of a structure that has otherwise been largely treated as monolithic is valuable. Continued improvements in image resolution with higher field strength MRI will likely lead to more reliable hippocampal segmentation methods.
Finally, the hippocampal volume-memory relationship may be complex and dependent on both age and neuropsychiatric diagnosis, with some populations exhibiting a “bigger is better” relationship while others may exhibit a negative correlation between volume and memory (Van Petten, 2004). Volume as a measure may be too coarse and insensitive to the variations in cell type, density, and connectivity that may drive cognitive and behavioral impairments.
In conclusion, the present study may be the first to report hippocampal subfield measurements in children with PAE. We observed that several subfields (CA1, CA4, presubiculum, subiculum, and hippocampal tail) are smaller in children exposed to alcohol in utero, while the remaining subfields are relatively spared. These findings parallel earlier reports in rodent models which suggested a variation in the extent to which different regions of the hippocampus are affected by PAE. Further research using this approach may provide a more detailed description of the teratological effects of alcohol on the hippocampus, allow for cross-species comparisons, and inform future clinical interventions.
Acknowledgments
Sources of support
This work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. Support for this project was provided by the NIAAA (5U01AA026102, 5U01AA014834, 5U24AA014815, 5U24AA014811), the National Institute of Biomedical Imaging and Bioengineering (NIBIB P41 EB027061), the Biotechnology Research Center (P41 EB015 894), the NINDS Institutional Center Core Grants to Support Neuroscience Research (P30 NS076408), and the High Performance Connectome Upgrade for Human 3T MR Scanner (1S10OD017974-01).
Footnotes
Declaration of Competing Interest
None to report.
References
- Aanes S, Bjuland KJ, Sripada K, Solsnes AE, Grunewaldt KH, Haberg A, Lohaugen GC, Skranes J, 2019. Reduced hippocampal subfield volumes and memory function in school-aged children born preterm with very low birthweight (VLBW). Neuroimage Clin. 23, 101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves JM, Luo S, Chow T, Herting M, Xiang AH, Page KA, 2020. Sex differences in the association between prenatal exposure to maternal obesity and hippocampal volume in children. Brain Behav. 10, e01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, 2004. Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code. University of Washington, Seattle, WA. [Google Scholar]
- Astley SJ, 2010. Profile of the first 1,400 patients receiving diagnostic evaluations for fetal alcohol spectrum disorder at the Washington State Fetal Alcohol Syndrome Diagnostic & Prevention Network. Can. J. Clin. Pharmacol 17, e132–e164. [PubMed] [Google Scholar]
- Astley SJ, 2011. Canadian palpebral fissure length growth charts reflect a good fit for two school and FASD clinic-based U.S. populations. J. Popul. Ther. Clin. Pharmacol 18, e231–e241. [PubMed] [Google Scholar]
- Astley SJ, 2013. Validation of the fetal alcohol spectrum disorder (FASD) 4-digit diagnostic code. J. Popul. Ther. Clin. Pharmacol 20, e416–e467. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, 2009. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res 33, 1671–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Walker DW, 1981. Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Dev. Brain Res 1, 333–340. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Dohring J, Rohr A, Jansen O, Deuschl G, 2011. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc. Natl. Acad. Sci. U. S. A 108, 17562–17567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Dikmen SS, Heaton RK, Mungas D, Slotkin J, Beaumont JL, 2013. III. NIH toolbox cognition battery (CB): measuring episodic memory. Monogr. Soc. Res. Child Dev 78, 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK, O’connor MJ, Riley EP, Johnson KA, Cohen DE, 2004. Fetal alcohol syndrome: guidelines for referral and diagnosis. In: FAS/FAE NTFO (ed.). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Bonthius DJ, West JR, 1988. Blood alcohol concentration and microencephaly: A dose-response study in the neonatal rat. Teratology 37, 223–231. [DOI] [PubMed] [Google Scholar]
- Boschen KE, Klintsova AY, 2017. Neurotrophins in the brain: interaction with alcohol exposure during development. Vitam. Horm 104, 197–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes M, Richardson D, Brien J, Reynolds J, Dringenberg H, 2004. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult Guinea pig following brain growth spurt-prenatal ethanol exposure. Neurotoxicol. Teratol 26, 543–551. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Conry J, Cook JL, Loock C, Rosales T, Leblanc N, Public Health Agency of Canada’s National Advisory Committee On Fetal Alcohol Spectrum, D, 2005. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. CMAJ 172, S1–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE, 1997. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol. Clin. Exp. Res 21, 150–161. [PubMed] [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, Hu X, 2011. Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn. 75, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Gailey AR, Mulle JG, Kable JA, Lynch ME, Jones KL, 2016. A comparison among 5 methods for the clinical diagnosis of fetal alcohol Spectrum disorders. Alcohol. Clin. Exp. Res 40, 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN, 2011. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/ hyperactivity disorder. Alcohol. Clin. Exp. Res 35, 1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA, 2000. California Verbal Learning Test - 2nd Edition (CVLT-II). The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Dodge NC, Thomas KG, Meintjes EM, Molteno CD, Jacobson JL, Jacobson SW, 2020. Reduced Hippocampal Volumes Partially Mediate Effects of Prenatal Alcohol Exposure on Spatial Navigation on a Virtual Water Maze Task in Children. Clinical and Experimental Research, Alcoholism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU Plooy CP, Malcolm-Smith S, Adnams CM, Stein DJ, Donald KA, 2016. The effects of prenatal alcohol exposure on episodic memory functioning: A systematic review. Arch. Clin. Neuropsychol 31, 710–726. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS, 1998. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am. J. Psychiatry 155, 552–554. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, VAN DER Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Mattson SN, Jernigan TL, Archibald SL, Jones KL, Riley EP, 2012. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcohol. Clin. Exp. Res 36, 1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Bunge SA, 2012. Neural changes underlying the development of episodic memory during middle childhood. Dev. Cognit. Neurosci 2, 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M, Butters N, Reynolds J, Brien J, 2000. Effects of chronic prenatal ethanol exposure on locomotor activity, and hippocampal weight, neurons, and nitric oxide synthase activity of the young postnatal Guinea pig. Neurotoxicol. Teratol 22, 183–192. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR, 2010. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res. Rev 64, 283–303. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M & Polimeni JR, 2013. The minimal preprocessing pipelines for the human Connectome project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD, 2003. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav. Brain Res 143, 85–94. [DOI] [PubMed] [Google Scholar]
- Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Bookheimer SY, Brown TB, Buckner RL, Burgess GC, 2018. Extending the human Connectome project across ages: imaging protocols for the lifespan development and aging projects. Neuroimage 183, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivieres S, Jahanshad N, Toro R, Wittfeld K, Abramovic L, Andersson M, 2015. Common genetic variants influence human subcortical brain structures. Nature 520, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B, Bt H, Ge F, Je IH, 1972. Placental Transfer and Tissue Distribution of Ethanol-1-14C. A Radioautographic Study in Monkeys and Hamsters. [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA, 2016. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, Roy N, Frosch MP, Mckee AC, Wald LL, 2015. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115, 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P, 1973. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1, 1267–1271. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S, 2007. NEPSY-II: Second Edition PsychCorp, San Antonio, TX. [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL, 2000. CDC growth charts: United States. Adv. Data 1–27. [PubMed] [Google Scholar]
- Kurtzer GM, Sochat V, Bauer MW, 2017. Singularity: scientific containers for mobility of compute. PLoS One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER, 2011. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsyehol. Rev 21, 102–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledergerber D, Moser EI, 2017. Memory retrieval: taking the route via Subiculum. Curr. Biol 27, R1225–R1227. [DOI] [PubMed] [Google Scholar]
- Lemoine D, 1968. Les enfants de parents alcoholiques Anomalies, observees de 127 cas. Quest. Med 25, 477–482. [Google Scholar]
- Lewis CE, Thomas KGF, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW, 2015. Verbal learning and memory impairment in children with fetal alcohol Spectrum disorders. Alcohol. Clin. Exp. Res 39, 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy D, Miller EK, Maier SE, West JR, 2003. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol. Teratol 25, 447–458. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR, 2001. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol 23, 49–57. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Ramo I, May PA, Adnams CM, Konovalova V, 2010. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol 44, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA, Doyle LR, 2019. Fetal alcohol Spectrum disorders: A review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol. Clin. Exp. Res 43, 1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgoey TN, Reynolds JN, Brien JF, 2003. Chronic prenatal ethanol exposure-induced decrease of Guinea pig hippocampal CA1 pyramidal cell and cerebellar Purkinje cell density. Can. J. Physiol. Pharmacol 81, 476–484. [DOI] [PubMed] [Google Scholar]
- Miki T, Harris SJ, Wilce PA, Takeuchi Y, Bedi KS, 2003. Effects of alcohol exposure during early life on neuron numbers in the rat hippocampus. I. Hilus neurons and granule cells. Hippocampus 13, 388–398. [DOI] [PubMed] [Google Scholar]
- Miller MW, 1995. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcohol. Clin. Exp. Res 19, 1500–1509. [DOI] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C, 2011. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res 35, 1404–1417. [DOI] [PubMed] [Google Scholar]
- Nellhaus G, 1968. Head circumference from birth to eighteen years: practical composite international and interracial graphs. Pediatrics 41, 106–114. [PubMed] [Google Scholar]
- Nogovitsyn N, Muller M, Souza R, Hassel S, Arnott SR, Davis AD, Hall GB, Harris JK, Zamyadi M, Metzak PD, Ismail Z, Downar J, Parikh SV, Soares CN, Addington JM, Milev R, Harkness KL, Frey BN, Lam RW, Strother SC, Rotzinger S, Kennedy SH, Macqueen GM, 2020. Hippocampal tail volume as a predictive biomarker of antidepressant treatment outcomes in patients with major depressive disorder: a CAN-BIND report. Neuropsychopharmacology 45, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’connor MJ, Kasari C, 2000. Prenatal alcohol exposure and depressive features in children. Alcohol. Clin. Exp. Res 24, 1084–1092. [PubMed] [Google Scholar]
- O’connor MJ, Portnoff LC, Lebsack-Coleman M, Dipple KM, 2019. Suicide risk in adolescents with fetal alcohol spectrum disorders. Birth Defects Res. 111, 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C, Tamana S, Baugh L, Andrew G, Tough S, Zwaigenbaum L, 2013. Neuropsychological impairments on the NEPSY-II among children with FASD. Child Neuropsychol. 19, 337–349. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, Robson CD, Rose-Jacobs R, Frank DA, 2008. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics 121, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’connor MJ, Narr KL, Sowell ER, 2012. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum. Brain Mapp 33, 920–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzai Y, 2019. Function of local circuits in the hippocampal dentate gyrus-CA3 system. Neurosci. Res 140, 43–52. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, Elam JS, Gaffrey MS, Harms MP, Hodge C, Kandala S, Kastman EK, Nichols TE, Schlaggar BL, Smith SM, Thomas KM, Yacoub E, VAN Essen DC, Barch DM, 2018. The lifespan human Connectome project in development: A large-scale study of brain connectivity development in 5–21 year olds. Neuroimage 183, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O’connor MJ, Bookheimer SY, 2008. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J. Neurosci 28, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromland K, Chen Y, Norberg T, Wennerstrom K, Michael G, 1999. Reference values of facial features in Scandinavian children measured with a range-camera technique. Scand. J. Plast. Reconstr. Surg. Hand Surg 33, 59–65. [DOI] [PubMed] [Google Scholar]
- Tannous J, Amaral-Silva H, Cao B, Wu MJ, Zunta-Soares GB, Kazimi I, Zeni C, Mwangi B, Soares JC, 2018. Hippocampal subfield volumes in children and adolescents with mood disorders. J. Psychiatr. Res 101, 57–62. [DOI] [PubMed] [Google Scholar]
- Tran TD, Kelly SJ, 2003. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol. Teratol 25, 519–528. [DOI] [PubMed] [Google Scholar]
- Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C, 2013. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J. Neurosci 33, 10098–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uecker A, Nadel L, 1996. Spatial locations gone awry: object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsyehologia 34, 209–223. [DOI] [PubMed] [Google Scholar]
- Van Petten C, 2004. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia 42, 1394–1413. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC, 2013. Cognition assessment using the NIH toolbox. Neurology 80, S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Hamre KM, Cassell MD, 1986. Effects of ethanol exposure during the third trimester equivalent on neuron number in rat hippocampus and dentate gyrus. Alcohol. Clin. Exp. Res 10, 190–197. [DOI] [PubMed] [Google Scholar]
- Wigal S, Amsel A, Wilcox RE, 1990. Fetal ethanol exposure diminishes hippocampal β-adrenergic receptor density while sparing muscarinic receptors during development. Dev. Brain Res 55, 161–169. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J, 2008. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J. Int. Neuropsyehol. Soc 14, 1022–1033. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B, 2010. Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia 48, 2339–2356. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, 2011. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol. Rev 21, 133–147. [DOI] [PubMed] [Google Scholar]


