Abstract
Historically, murine models of inflammation in biomedical research have been shown to minimally correlate with genomic expression patterns from blood leukocytes in humans. In 2019, our laboratory reported an improved surgical sepsis model of cecal ligation and puncture (CLP) that provides additional daily chronic stress (DCS), as well as adhering to the Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS) guidelines. This model phenotypically recapitulates the persistent inflammation, immunosuppression, and catabolism syndrome observed in adult human surgical sepsis survivors. Whether these phenotypic similarities between septic humans and mice are replicated at the circulating blood leukocyte transcriptome has not been demonstrated. Our analysis, in contrast with previous findings, demonstrated that genome-wide expression in our new murine model more closely approximated human surgical sepsis patients, particularly in the more chronic phases of sepsis. Importantly, our new model of murine surgical sepsis with chronic stress did not reflect well gene expression patterns from humans with community-acquired sepsis. Our work indicates that improved preclinical murine sepsis modeling can better replicate both the phenotypic and transcriptomic responses to surgical sepsis, but cannot be extrapolated to other sepsis etiologies. Importantly, these improved models can be a useful adjunct to human-focused and artificial intelligence-based forms of research in order to improve septic patients’ morbidity and mortality.
Keywords: inflammation, sepsis, transcriptome, translational medicine
1 |. INTRODUCTION
In 2013, Seok et al published the manuscript “Genomic responses in mouse models poorly mimic human inflammatory diseases” in the Proceedings of the National Academy of Science (PNAS).1 This article highlighted the lack of correlation between host murine and human genomic responses in circulating leukocytes after trauma, burn or lipopolysaccharide (LPS) injection. This work has been cited at least 2000 times between 2013 and 2019 (Google Scholar) and has been the source of many subsequent debates regarding the appropriateness of murine studies to investigate human inflammatory or infectious conditions.1–4 Of note, a secondary analysis of the same data was subsequently published, also in PNAS, which concluded murine models could replicate human inflammation well.2 However, the lack of progress over the last several decades regarding immunomodulative therapies for human sepsis, despite positive murine model results,5 has appropriately tempered dogmatic enthusiasm for murine research in these fields.6–8
Although the importance and accuracy of the Seok et al paper is not in question, some have questioned the subsequent reaction to its results.9 “Strive for progress, not perfection” has been stated by some regarding animal research for inflammation and infection.9 Our laboratory has been particularly active in trying to decipher if murine sepsis and trauma research can be clinically relevant, thus providing insights in underlying mechanisms and allowing the testing therapeutic interventions.9–12 Recently, the scientific community has published the “Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): “an international expert consensus initiative for improvement of animal modeling in sepsis”.13 Using “reverse translation” and following the recommendations of MQTiPPS, we created a murine model of surgical sepsis that was a significant improvement over pre-existing rodent sepsis models, especially in how it replicated the human disease.14 This was accomplished by following current definitions of human sepsis, applying surviving sepsis guidelines to the host and simulating the chronic stress of the intensive care environment.
We hypothesized that improvements in the murine surgical sepsis model would produce a more comparable circulating blood leukocyte transcriptomic response between mice and humans after sepsis. In addition, we explored how specific variables such as sex, age and type of sepsis, all vital for future personalized medicine,9,10 affected these correlations.
2 |. MATERIALS AND METHODS
2.1 |. Study site and patients
Over the 4 year period during which the study was conducted, surgical intensive care unit (ICU) patients were enrolled who were either admitted with, or who subsequently developed sepsis during their hospitalization.1 Screening for sepsis was carried out using the Modified Early Warning Signs-Sepsis Recognition System (MEWS-SRS), which quantifies derangements in vital signs, white blood cell count, and mental status.2 All patients with sepsis were managed using a standardized, evidence-based protocol that emphasizes early goal-directed fluid resuscitation as well as other time-appropriate interventions such as administration of broad-spectrum antibiotics. Empiric antibiotics were chosen based on current hospital antibiograms in conjunction with the suspected source of infection.3 Antimicrobial therapy was then narrowed based on culture and sensitivity data.
2.2 |. Inclusion and exclusion criteria
Patients eligible for participation in the study met the following inclusion criteria: (1) admission to the surgical or trauma ICU; (2) age ≥ 18 years; (3) clinical diagnosis of sepsis, severe sepsis or septic shock with this being the patient’s first septic episode; and (4) entrance into our sepsis clinical management protocol.1
Patients were excluded if any of the following were present: (1) refractory shock (ie, patients expected to die within the first 24 hours); (2) an inability to achieve source control (ie, irreversible disease states such as unresectable dead bowel); (3) pre-sepsis expected lifespan < 3 months; (4) patient/family not committed to aggressive management; (5) severe CHF (NYHA Class IV); (6) Child-Pugh Class C liver disease or preliver transplant; (7) known HIV with CD4+ count <200 cells/mm3; (8) organ transplant recipient or use of chronic corticosteroids or immunosuppressive agents; (9) pregnancy; (10) institutionalized patients; (11) chemotherapy or radiotherapy within 30 days; (12) severe traumatic brain injury (ie, evidence of neurological injury on CT scan and a GCS < 8); (13) spinal cord injury resulting in permanent sensory and/or motor deficits; or, (14) inability to obtain informed consent.
2.3 |. Patient classification
Patients were diagnosed with sepsis, severe sepsis, or septic shock using the definitions established by the Society of Critical Care Medicine, the European Society of Intensive Care Medicine, the American College of Chest Physicians, the American Thoracic Society, and the Surgical Infection Society (SCCM/ESICM/ACCP/ATS/SIS) 2001 International Sepsis Definitions Conference.4 This was utilized rather than “Sepsis-3” due to the fact that the study began prior to the publication and universal international acceptance of Sepsis-3.5 Chronic critical illness (CCI) was defined as an ICU length of stay (LOS) greater than or equal to 14 days with evidence of persistent organ dysfunction, measured using components of the Sequential Organ Failure Assessment (SOFA) score at 14 days (ie, cardiovascular SOFA ≥ 1, or score in any other organ system ≥ 2).6 Patients with an ICU LOS less than 14 days would also qualify for CCI if they were discharged to another hospital, a long-term acute care facility, or to hospice and demonstrated evidence of organ dysfunction at the time of discharge. Those patients experiencing death within 14 days of sepsis onset were excluded from the clinical and biomarker analyses. Any patient who did not meet criteria for CCI or early death was classified as rapid recovery.
2.4 |. Human blood collection
EDTA human whole blood was collected by venipuncture on days 1 (n = 70), 4 (n = 66) and 14 (n = 121). Samples were stored on ice and processed within 6 hours after blood drawing. An additional cohort of de-identified patient blood samples7 from previous published data, was analyzed for total leukocyte genome-wide expression analysis. This study included 257 samples from patients meeting Sepsis-2 severe sepsis or septic shock criteria, as well as 41 sex and age matched control subjects.1,8
2.5 |. Animals
Young (3-5 month) and old (18-22 month) C57BL/6J female and male (~50%) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were cared for by the University of Florida Animal Care Services (Gainesville, FL) and housed in transparent cages (three to four animals of the same sex/age per cage) within specific pathogen free facilities at ambient room temperatures (23°C). The animals were provided standard rodent chow and water ad libitum for the duration of the study. Prior to initiation of the experiment, mice were acclimated to the humidity-controlled housing room programmed for a 12-hour light-dark cycle for a minimum of 1 week. This also allowed the animals’ microbiomes to become similar. The animals were cared for and used according to the Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the University of Florida Institutional Animal Care and Use Committee (protocols: #201608141, #201807157, #201708454).
2.6 |. Intra-abdominal sepsis and daily chronic stress model
This model was conducted as previously described.9 Briefly, CLP was conducted under isoflurane anesthesia. The cecum was ligated 1 cm from its tip and a 25-gauge needle was used to puncture the cecum. Buprenorphine analgesia was provided for 48 hours post-surgery. Antibiotics (imipenem monohydrate; 25 mg/kg diluted in 1mL of 0.9% sodium chloride [NaCl]) were administered 2 hours post-CLP then continued twice daily for 72 hours. Chronic restraint was conducted by placing mice in weighted plexiglass animal restraint holders (Kent Scientific, Torrington, CT) for 2 hours daily starting the day after CLP. Mice were euthanized at specified time intervals on days 1 (n = 12), 4 (n = 12), 7 (n = 12) and 14 (n = 13) post sepsis.
2.7 |. Murine blood sample collection
Mice were euthanized following isoflurane inhalation, after which mixed arteriovenous blood was collected in a Becton Dickinson sodium heparin vacutainer tube (Franklin Lakes, NJ) from the neck vasculature.
2.8 |. Gene expression profiling
Total blood leukocytes were isolated from whole blood of human subjects and mouse subjects. Very briefly, blood was centrifuged at 500 g for 10 minutes at 4°C and plasma removed.
The red and white cell pellet was lysed with 10 volumes of Qiagen red cell lysis buffer and the process was repeated three times. Remaining white blood cells were pelleted and lysed with RLT buffer. Human total RNA was extracted and hybridized onto GeneChip Human Transcriptome Array 2.0 (Affymetrix, Santa Clara, CA) and processed following manufacturer’s instructions. Mouse total RNA was isolated using RNeasy kit (Qiagen, Valencia, CA) following the manufacturer’s instructions and was hybridized to mouse genome 430 2.0 arrays (Affymetrix, Santa Clara, CA). BRBArray Tools® was used to identify significant microarray gene expression differences.
2.9 |. Datasets for previously used human sepsis and mouse model
The additional datasets analyzed in the study were the same as those used by Seok et al and Takao and Miyakawa10,11 and are available in from Gene Expression Omnibus (GEO)12 under the accession numbers GSE28750 for human community-acquired sepsis and GSE5663 for the traditional CLP murine model.
2.10 |. Comparison of gene responses
Fold expression changes of the significant genes were calculated over age/sex-matched controls. Means, standard deviations and coefficients of variation were calculated from biological replicates at each time point. Significant genes were selected using fold change and false discovery rate adjusted p-value. Significant genes were also selected with the analytic methods discussed in Seok et al.10 Takao and Miyakawa11 for comparison, adjusting the fold change cutoff to account for differences in microarray chips (HU133 Plus 2.0 vs HTA 2.0). Between two datasets, the correlation of the gene expression fold changes (Pearson and Spearman) and % of genes that changed in the same direction was compared.
In the comparison between our data and datasets accessed from GEO, all four Affymetrix datasets were robust multi-array average (RMA) renormalized from original cell intensity files (CEL) using Transcriptome Analysis Console Software. A total of 13 429 orthologs were matched between the four datasets. The microarray datasets were then co-normalized by COCONUT version 1.02. In brief, COCONUT normalizes datasets by assuming that healthy controls are derived from the same distributions.
2.11 |. IPA and GO analyses
Functional pathway analysis was performed using Ingenuity Pathway Analysis (IPA, Redwood City, CA, USA), which allows for the discovery of signaling pathways associated with the dataset of interest. A Gene Ontology (GO) enrichment analysis13–15 was also performed to find significant representative pathways/biogroups shared between human post-sepsis day 14 and the novel murine model at day 14. Only genes that changed significantly with a FDR Q < 0.001 and had greater than 1.25-fold change from control subjects were subjected to analysis. IPA and GO identify those pathways that are overrepresented, indicating that their expression is affected by the intervention. Significance was determined using a Z-score in IPA, with values of 2 < Z<–2 are considered significant and correspond to a 95% confidence interval. Additionally, the IPA canonical pathway analysis displays pathways that are most associated with the genes in our dataset and significance is calculated at a P < .05 level of significance. Within the canonical pathway analysis, the Molecule Activity Predictor tool was used to determine whether the overall effects on the pathway were activated or inhibited.
3 |. RESULTS
3.1 |. Correlation of gene changes between human surgical sepsis and our novel murine preclinical surgical sepsis
Total white blood cell transcriptomics were determined in human and murine surgical sepsis at fixed intervals. Table 1 presents demographics of the septic patients. Fold changes in blood leukocyte gene expression were calculated at each time point studied using a linear scale for septic and healthy patients and mice. Murine and human orthologues were identified, and the correlations in fold change, as well as the directionality of the changes (percentages of genes that changed in the same direction between the two datasets) were compared. The Kolmogorov-Smirnov test revealed that none of our datasets were normally distributed (P < .0001). Thus, we relied on the Spearman’s correlations for these datasets since normal distribution or linearity of datasets are not required for this test.
TABLE 1.
Demographics of human and mouse cohorts
| Cohort | |||
|---|---|---|---|
| Human | n | Mean, median age | % Male |
| Control | 41 | 58.4, 60 | 48.8 |
| 12 hours | 70 | 59.3, 61 | 52.9 |
| Day 1 | 66 | 59.2, 62 | 56.1 |
| Day 14 | 121 | 60.3, 63 | 57.4 |
| Mice | n | # old (18-22 mo) | # Male |
| Control | 12 | 6 | 6 |
| Day 1 | 12 | 6 | 6 |
| Day 4 | 12 | 6 | 6 |
| Day 7 | 12 | 7 | 5 |
| Day 14 | 13 | 6 | 7 |
Seok et al compared genome-wide expression including those genes whose expression was invariant due to sepsis.10 We repeated Seok et al’s initial analysis and compared between mice and humans the fold change for all human-mouse matched orthologs (17 368 genes) at each time point, regardless of their change in expression and significance. We found weak correlations among genes expressed in the human and murine datasets when comparing early times points of 1 or 4 days after surgical sepsis (Table 2; ρ = 0.03-0.17; P < .0001 for all comparisons; 52%-58% common directionality). Surprisingly, correlations and percentages improved when the datasets where compared at the later sepsis time points of 7 and 14 days in both humans and mice. For all matched orthologs assayed, sepsis-induced changes in gene expression at 14 days between mice and humans were significantly correlated (ρ = 0.30, P < .0001; 60% directionality) across the entire genome.
TABLE 2.
Correlations of gene expression changes from all orthologs among human surgical sepsis and the CLP + DCS mouse model at each time point
| Human 12 hours | R = 0.0894 | R = 0.1428 | R = 0.2308 | R = 0.2431 |
|---|---|---|---|---|
| ρ = 0.0675 | ρ = 0.1178 | ρ = 0.2315 | ρ = 0.2553 | |
| P < .0001 | P < .0001 | P < .0001 | P < .0001 | |
| 53.0% | 54.6% | 58.1% | 59.1% | |
| Human Day 1 | R = 0.0924 | R = 0.1925 | R = 0.2888 | R = 0.2938 |
| ρ = 0.0731 | ρ = 0.1720 | ρ = 0.2889 | ρ = 0.3004 | |
| P < .0001 | P < .0001 | P < .0001 | P < .0001 | |
| 53.6% | 58% | 60.7% | 62.2% | |
| Human Day 14 | R = 0.0580 | R = 0.1332 | R = 0.2357 | R = 0.2745 |
| ρ = 0.0368 | ρ = 0.1291 | ρ = 0.2561 | ρ = 0.3031 | |
| P < .0001 | P < .0001 | P < .0001 | P < .0001 | |
| 52% | 54.4% | 58.3% | 60.2% | |
| All orthologs: 17 368 genes | Mouse Day 1 | Mouse Day 4 | Mouse Day 7 | Mouse Day 14 |
Note: Pearson’s (R) and Spearman’s rank correlations (ρ) of the gene expression fold changes to both conditions for each pair of interest in all 17 368 orthologs. Percent represents the percentages of genes changed to the same direction between the two datasets. Murine models had weak correlations with humans that improved to moderate correlation with the longer models (P < .0001 for all comparisons).
3.2 |. Correlation of gene changes between human surgical sepsis and our murine preclinical surgical sepsis model, focusing on significant gene expression changes in sepsis
We subsequently focused only on the human genes whose expression changed in response to sepsis using a false discovery rate (FDR) adjusted probability of Q < 0.001, and fold change ≥1.25. This approach identified genes that show a significant sepsis response in humans and how they correlate with their murine orthologues. We used a fold change of ≥1.25 for our analysis due to the compressed response associated with the newer microarray chip, specifically the HTA 2.0, instead of the HU133 Plus 2.0 used in Seok et al.10 Gene selection with this method (Q < 0.001 and FC ≥ 1.25) revealed that the human expression of 3494 genes changed 12 hours after sepsis, 3357 genes 1 day after sepsis, and 2615 genes 14 days after sepsis. Expression and directionality of those changes among human genes and their murine orthologs were improved at later time points (Figure 1; Supporting Information Dataset 1). For example, the greatest correlation and directionality of the changes in gene expression was from comparison of 14-day human data to 14-day sepsis mouse data (ρ = 0.4215, P < .0001; 71.4%).
FIGURE 1.
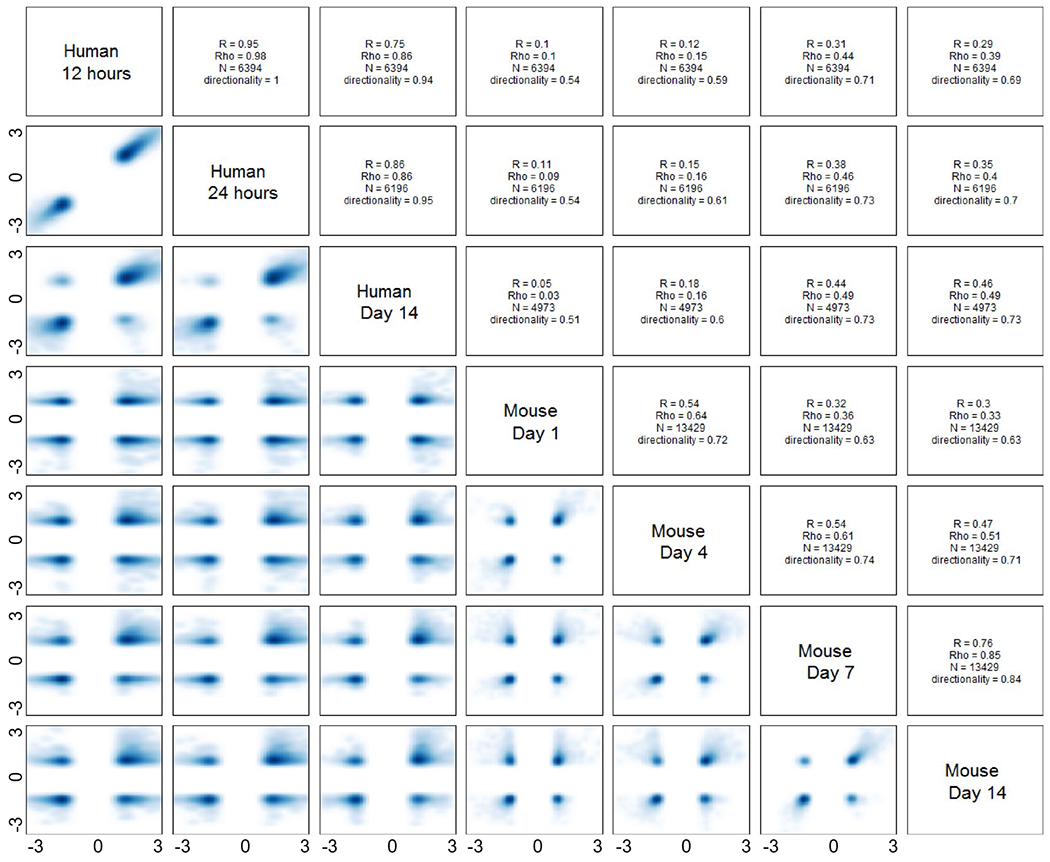
Correlations of genes changes among our human surgical sepsis and novel CLP + DCS mouse model at each time point. Scatter plots, Pearson’s (R) and Spearman’s rank correlations (ρ) of the fold changes of the gene expression to both conditions for each pair of interest (FDR < 0.001 and |FC| > 1.25 in humans, N = number of significant genes by this criteria). Directionality represents the percentages of genes changed to the same direction between the two datasets. Day 1 and Day 4 of the murine models had weak correlations with human conditions (P < .0001) at all compared time points. However, the correlation between humans and mice significantly improved for mouse Day 7 and day 14
3.3 |. Correlation of gene changes between human surgical sepsis and our novel murine preclinical surgical sepsis model using the Takao and Miyakawa selection criteria
We also calculated correlations and percentages of gene directionality based on the method used in Takao and Miyakawa in their 2015 PNAS publication. These authors used a selection criteria for genes of interest at P < .05 and absolute fold change ≥ 1.25 for both humans and mice.11 Their analysis restricts the results to only those genes significantly changed in both humans and mice. Not surprisingly, correlations of the gene expression changes at all time points were considerably better than the previous analysis using the Seok et al approach. Again, correlations improved when later murine sepsis time points were compared to the human datasets (Supporting Information Dataset 1). We found 872 orthologs with significant correlation (ρ = 0.4438, P < .0001), with 91.4% of the genes changing in the same direction, when comparing changes in gene expression 14 days after sepsis in humans with mice 14 days after sepsis and restraint.
3.4 |. Effect of sex and age on human and murine leukocyte genomic expression after surgical sepsis
We also explored the effect of age and sex on the gene expression patterns of our murine sepsis model to human sepsis. For the analysis of age, patients were classified as being either young adults (≤45 years old) or older adults (≥65 years old), as commonly defined by the US Centers for Disease Control and Prevention and National Institute on Aging (NIA). Middle aged adults (46-64 years old) were excluded from the age analysis as middle age mice were not tested. Young adult (3-5 months) and old adult (18-22 months) C57BL/6 mice were also subjected to sepsis and chronic restraint stress, as these are the relative equivalents of the young and older adult human patients.16 No marked improvement in correlations was observed from analysis of the variable of sex between mice and humans (Supporting Information Dataset 1). Although the correlations for genome-wide expression human between males and females were very strong at all time points (ρ = 0.92, 0.94, 0.91; P < .0001 at all time points; directionality 88%, 90%, and 88%; at 12 hours, 1 day and 14 days respectively), they were weak within the mouse cohort (ρ = 0.04, 0.34, 0.24, 0.24; P < .0001 at all time points; directionality 52%, 64%, 62%, 59%; at day 1, 4, 7, and 14, respectively) suggesting that sex differences are considerably more important for mice than humans.
In the analysis of age as a variable, changes in gene expression after sepsis in the humans were strong but still less well correlated between young and old sepsis patients (ρ = 0.66, 0.74, 0.76; P < .0001 at all time points; directionality 70%, 78%, 79%; at 12 hours, 1 day and 14 days respectively) than they were for sex. Within the mouse cohort, correlation of the gene expression patterns between the different age groups was nonexistent at Day 1 (ρ = −0.0187, P = .01; 49%) but improved at the later time points with the best correlation at Day 7 (ρ = 0.38, 0.48, 0.40; P < .0001; 65%, 70%, 65%; at day 4, 7, and 14, respectively). Therefore, it was not surprising that the gene expression of young mice did not correlate well with old human sepsis patients over time (young mice at Day 1 vs old humans at 12 hours ρ = −0.04, P < .0001, 46%; young mice at Day 14 vs old humans at Day 14 ρ = 0.22, P < .0001, 57%). The correlation of gene expression in old human patients to old mice and young human patients to young mice was slight (Supporting Information Dataset 1). The best correlation was identified between young human sepsis patients and young septic mice at Day 14 (ρ = 0.28, P < .0001, 60%).
3.5 |. Comparison to previous datasets
Using the GEO public accession database, we accessed human sepsis (GSE28750—community-acquired sepsis) and murine surgical sepsis (GSE5663—traditional/historical model of CLP) datasets used in previous papers for comparison to our novel model. In the GEO dataset GSE5663, mice were subjected to either administration of LPS or a cecal ligation and puncture to emulate sepsis. The previous PNAS papers10,11 appear to have combined all of these mice into one cohort, which could account for the noted poor correlations. In this current study, we selected only mice that underwent CLP to provide an appropriate comparison of our surgical sepsis model to that of traditional models. Mice in those studies underwent CLP with a 25 or 27-gauge needle followed by intraperitoneal antibiotics at 1, 12, and 24 hours after their initial surgery.17 Since the data from that work was from different platforms, COCONUT was used to co-normalize the datasets for comparison yielding a total of 13 429 common orthologs after matching the four datasets. Between two datasets, the Spearman rank correlations as well as the directionality of the changes were compared as above (Figure 2; Supporting Information Dataset 2). Moderate to high correlation of gene response between our human surgical sepsis patients and the community-acquired sepsis patients (ρ = 0.6432, P < .0001, 73%) was identified. Interestingly, a poor correlation between the traditional CLP mouse model (GSE5663) and our novel surgical sepsis model was noted (Figure 2; ρ = 0.13, P < .0001, 54% at day 1; ρ = 0.19, P < .0001, 57% all time points combined).
FIGURE 2.

Correlations of gene changes among our human surgical sepsis patients, human community-acquired sepsis (GSE28750), our novel murine model for surgical sepsis and the traditional model of murine surgical sepsis (GSE5663). Scatter plots, Spearman’s rank (rho) and Pearson (R) correlations of the absolute fold changes of all orthologs (no selection criteria). Vertical and horizontal bar represent fold change. Correlations of the gene expression changes at all time points in human surgical sepsis was better with the novel murine model than with the traditional method, with improvement noted with time in the novel murine model. There was very poor correlation between our novel surgical sepsis model compared to human community-acquired sepsis
In a comparison between our surgical sepsis patients at any time point and the original cecal ligation and puncture mouse model (GSE5663), correlation of all orthologs was marginal (Figure 2, P < .0001 at all time points). Stronger correlation was detected between the novel murine sepsis and restraint model and surgical sepsis patients at the later time points (ρ = 0.0476-0.3942, P < .0001, 51%-65%). Comparing our mouse model to the community-acquired human sepsis cohort revealed poor to no significant correlation in the analysis of all orthologs (Figure 2; Supporting Information Dataset 2). This is not surprising given that our murine model is one of abdominal surgical sepsis compared to a non-surgical sepsis cohort, primarily pneumonia, and urinary tract infections.
3.6 |. Pathway analysis of significant genes at post-sepsis day 14
Ingenuity Pathway Analysis (IPA) was conducted on gene expression post-sepsis Day 14 patients and murine sepsis and restraint at Day 14 that was significantly different (Q < 0.001 and FC ≥ 1.25 for humans). Remarkedly, expression of toll-like receptors and inflammatory interleukins were changed in the same direction in both humans and mice. All of the significantly expressed cell-differentiation (CD) molecules were also changed in the same direction except three: CD163, CD38 and CD274 (Supporting Information Dataset 3). These three molecules were significantly upregulated in late sepsis patients but significantly downregulated in the novel murine model. The gene CD274 encodes the transmembrane protein programmed death-ligand 1 (PD-L1) and is associated with decreased T-cell activation and cytokine production in cancer.18,19 Additional IPA software analysis of hematological system development and functional pathways demonstrated similar alterations in quantity, activation, and differentiation in post-sepsis Day 14 patients compared to our novel murine model as visualized in Supporting Information Figure S1. Further analysis in Gene Ontology (GO) of functional pathways revealed significant overlap between gene expression involved in the innate immune response and lymphocyte differentiation from post-sepsis Day 14 patients and sepsis plus restraint mice (Supporting Information Figure S2).
4 |. DISCUSSION
Current dogma argues that genomic responses in mouse models poorly mimic human inflammatory diseases.10,20,21 We believe that this is due in part to the inadequacy of earlier surgical sepsis models. Our current work has revealed an improved correlation of the genomic response between human surgical sepsis patients and this novel preclinical model of murine sepsis (ρ = 0.38-0.71, 64%-86%),10,11 especially at later times points in both humans and mice. Importantly, this model also displayed an improved correlation to our human surgical sepsis cohort when compared to the more traditional murine abdominal sepsis models used in previous studies.
Our laboratory’s previous work revealed that our novel sepsis and restraint model produces a murine phenotype that better recapitulates the human septic response.9 Our results in this study indicate that this model also produces a transcriptomic profile that better correlates with that of human sepsis. In particular, the correlation of genomic responses is greatly improved at later time points providing a promising model for chronic critical illness in sepsis survivors.
In part, the improved correlations of the genomic response may be due to adherence to the recommendations of MQTiPPS and the inclusion of both sexes of mice. Importantly, this murine model induces abdominal infection with acute organ insufficiency (SEPSIS-3 definition) and then follows the Surviving Sepsis Guidelines by delivering antibiotics and fluid resuscitation after the infection, as well analgesia, which humans receive in the ICU.22 In addition, the mice receive daily restraint, recapitulating the medications and stress of residing in a surgical intensive care unit. In addition, early mortality (<14 days) is low in young adult animals in this new surgical sepsis murine model, as is now the case for human surgical sepsis.1,23–25 Finally, the contained ischemic cecum acts as a constant source of low grade inflammation as well as the release of both pathogen and danger associated molecular patterns (PAMPs, DAMPs), imitating the long-term status of the human patient who may develop secondary infections or require ventilation and renal replacement therapy.26–29
Advancements in the detection and treatment of surgical sepsis have led to a decrease in acute in-hospital mortality.23,30 Currently, two common clinical trajectories exist in the surgical sepsis population that survive the immediate “cytokine storm”: those who rapidly recover and those who develop chronic critical illness (CCI).1,23,25,31 CCI is characterized by a prolonged hospital course and ongoing organ dysfunction, and up to 40% of sepsis survivors develop CCI. These patients can enter a state of persistent inflammation, immunosuppression, and cachexia/catabolism and have significantly worse 1 year outcomes, with higher mortality and persistent impaired performance status 1 year of their initial infection.23 Older adults are more likely to develop CCI after sepsis.23,32,33 Additionally, older adults have increased susceptibility and mortality to sepsis as compared to their younger counterparts before and after developing CCI.34 Therefore, it is important to develop a preclinical model that can best imitate the human response in late sepsis for interventional studies that can alter this potentially devastating post-septic clinical trajectory.
We found that many of the positively correlated genes between human and mice in late sepsis are related to immune cell function, including BCL2, MMP8/9, CCR7, CD274, LCN2, and TLR2. These specific genes are also important to the function of myeloid derived suppressor cells, which have been shown to be expanded and able to suppress T-cell function at Day 14 in septic patients.35 Therefore, the gene signature seen in the both humans and mice at Day 14 appears to represent the continued expansion of immune cells secondary to a state of persistent low grade inflammation and immunosuppression after sepsis, especially in elderly patients whom are more likely to follow a clinical trajectory to CCI.9,36 Interestingly, although this murine model of sepsis better recapitulates late human sepsis in mixed age cohorts, we found that the aged mice transcriptomic response to CLP + DCS still does not correlate better with the older adult human transcriptome after sepsis. Thus, although CLP + DCS in older adult mice better imitate (versus young adult mice) adult human persistent inflammation, immunosuppression and catabolism after surgical sepsis,9 our data indicate this may not be true of the leukocyte transcriptome. Previous research has indicated the correlation between mRNA and protein levels has been shown to vary, that is, depending on tissue type.37
Mice will never perfectly imitate the human condition, but this may not be required in order to have useful and appropriate preclinical sepsis and/or trauma murine models.38,39 For example, among the common genes in both old human and old mice cohorts were LCN2 and LGALS3 which are both implicated in immune cell responses to sepsis and could be used as potential targets for immunomodulation therapy.40–44 Importantly, our collective data illustrate the importance of further evaluating preclinical models of sepsis and emphasizes that with modifications more relevant rodent models can be engendered to achieve better and more appropriate comparisons (as all sepsis is not equivalent).45,46
There are many possible explanations as to why there have been no interventional therapies, proven successful in murine models of sepsis, translated in clinical trials. First, biomedical research has traditionally used young male mice despite human sepsis population being predominantly older adults with a significant proportion being female.16 This was the case in both of the seminal papers by Seok et al10 and Takao and Miyakawa.11 In fact, older adults are known to have worse outcomes and different immune response to sepsis and other inflammatory insults.24,47–49 Second, these mouse models have depended on the reproducibility facilitated by the genetic stability of inbred mice.50 However, the heterogeneity of the human sepsis population and the sepsis should be replicated by the genetic heterogeneity in any animal model that seeks to understand the disease. Finally, we should consider that not all sepsis is the same—that is, community- versus hospital-acquired, medical versus surgical sepsis or pneumonia versus abdominal sepsis.45,46 We compared an animal model of surgical sepsis to both human surgical sepsis and community-acquired sepsis in our work. Further studies to compare the genomic response of different murine models of sepsis to specific corresponding types of human sepsis are warranted.
There are limitations to our study. We noted that the coefficient of variation for some genes within the septic and healthy mouse cohorts were elevated, but not localized to any particular GeneChip or individual mouse. This is surprising given that we expected the genetic variation to be small between inbred mice under the same conditions. However, this variability within the mouse model is not completely unwanted in that the genetic response in the human sepsis population has been found to be highly variable. The addition of this variation in genetic expression with the inclusion of females and aged mice to this novel model improves upon the goal to match the heterogeneity of human sepsis.51 In addition, there is work to suggest that “outbred” mice have an immune system more similar to humans52,53; thus, comparing the transcriptomic response of these mice after sepsis to humans may also improve correlations. Furthermore, we have only studied one model of abdomen sepsis in mice, which is the most common form of sepsis in surgical ICUs.23 In medical ICUs, pneumonia is the most common source for sepsis54,55 and future research should evaluate the correlations of a murine sepsis pneumonia model that follows MQTIPPS guidelines56,57 to medical ICU sepsis patients.
Finally, future studies should investigate the correlation of transcription levels with protein translation levels, as well as, transcriptomics of other organ systems beside hematologic. This is especially important with new definition of sepsis-3 which requires organ insufficiency/failure.5
Our results illustrate the potential utility of murine sepsis models contingent on investigators working to create mouse models for disease that can also mirror the heterogeneity of the human sepsis population. Mice do not imitate human genomic responses to severe inflammation perfectly, but with continued improvement in murine modeling, mouse research can be a powerful adjunct to human translational and clinical research as well as computer modeling.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported, in part, by the following National Institutes of Health grants: R01 GM-040586 and R01 GM-104481 (LLM), R01 GM-113945 (PAE), P50 GM-111152 (FAM, SCB, LLM, PAE, BAB, AMM, and AB) awarded by the National Institute of General Medical Sciences (NIGMS). In addition, this work was supported, in part, by a postgraduate training grant T32 GM-008721 (DD, RH, and MCC) in burns, trauma, and perioperative injury by NIGMS.
Funding information: National Institutes of Health, Grant/Award Number: R01 GM-040586, R01 GM-104481, R01 GM-113945 and P50 GM-111152; National Institute of General Medical Sciences, Grant/Award Number: T32 GM-008721
Abbreviations:
- CEL
cell intensity files
- CLP
cecal ligation and puncture
- CCI
chronic critical illness
- DAMPs
danger associated molecular patterns
- DCS
daily chronic stress
- FDR
false discovery rate
- GEO
Gene Expression Omnibus
- GO
Gene Ontology™
- IPA
Ingenuity Pathway Analysis™
- LPS
lipopolysaccharide
- LOS
length of stay
- MEWS-SRS
Modified Early Warning Signs-Sepsis Recognition System
- MQTiPSS
Minimum Quality Threshold in Pre-Clinical Sepsis Studies
- PNAS
Proceedings of the National Academy of Science
- RMA
robust multi-array average
- SOFA
Sequential Organ Failure Assessment
- PAMPs
pathogen associated molecular patterns
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interests.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- 1.Stortz JA, Murphy TJ, Raymond SL, et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock. 2018;49(3):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croft CA, Moore FA, Efron PA, et al. Computer versus paper system for recognition and management of sepsis in surgical intensive care. J Trauma Acute Care Surg. 2014;76(2):311–317; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 3.Fitousis K, Moore LJ, Hall J, Moore FA, Pass S. Evaluation of empiric antibiotic use in surgical sepsis. Am J Surg. 2010;200(6):776–782; discussion 82. [DOI] [PubMed] [Google Scholar]
- 4.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. [DOI] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. [DOI] [PubMed] [Google Scholar]
- 7.Mathias B, Delmas AL, Ozrazgat-Baslanti T, et al. Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg. 2017;265(4):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca AG, Moldawer LL. Myeloid-derived suppressor cells in sepsis: friend or foe? Intensive Care Med. 2012;38(6):928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stortz JA, Hollen MK, Nacionales DC, et al. Old mice demonstrate organ dysfunction as well as prolonged inflammation, immunosuppression, and weight loss in a modified surgical sepsis model. Crit Care Med. 2019;47(11):e919–e929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2015;112(4):1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47(D1):D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Gene Ontology C The gene ontology resource: 20 years and still Going strong. Nucleic Acids Res. 2019;47(D1):D330–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. [DOI] [PubMed] [Google Scholar]
- 17.Chung TP, Laramie JM, Meyer DJ, et al. Molecular diagnostics in sepsis: from bedside to bench. J Am Coll Surg. 2006;203(5):585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian S, Xie R, Ye Y, et al. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBioMedicine. 2019;42:281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norde WJ, Maas F, Hobo W, et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011;71(15):5111–5122. [DOI] [PubMed] [Google Scholar]
- 20.Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA. Murine models of sepsis and trauma: can we bridge the gap? ILAR J. 2017;58(1):90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentile LF, Nacionales DC, Lopez MC, et al. A better understanding of why murine models of trauma do not recapitulate the human syndrome. Crit Care Med. 2014;42(6):1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43(1):3–12. [DOI] [PubMed] [Google Scholar]
- 23.Brakenridge SC, Efron PA, Cox MC, et al. Current epidemiology of surgical sepsis: Discordance between inpatient mortality and 1-year outcomes. Ann Surg. 2019;270(3):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brakenridge SC, Efron PA, Stortz JA, et al. The impact of age on the innate immune response and outcomes after severe sepsis/septic shock in trauma and surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;85(2):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner AK, Ghita GL, Wang Z, et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit Care Med. 2019;47(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efron PA, Mohr AM, Bihorac A, et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery. 2018;164(2):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenck EJ, Ma KC, Murthy SB, Choi AMK. Danger signals in the ICU. Crit Care Med. 2018;46(5):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raymond SL, Holden DC, Mira JC, et al. Microbial recognition and danger signals in sepsis and trauma. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2564–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mankowski RT, Anton SD, Ghita GL, et al. Older sepsis survivors suffer persistent disability burden and poor long-term survival. J Am Geriatr Soc. 2020;68(9): 1962–1969. 10.1111/jgs.16435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn JM, Le T, Angus DC, et al. and ProVent Study Group I. The epidemiology of chronic critical illness in the United States. Crit Care Med. 2015;43(2):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. [DOI] [PubMed] [Google Scholar]
- 35.Hollen MK, Stortz JA, Darden D, et al. Myeloid-derived suppressor cell function and epigenetic expression evolves over time after surgical sepsis. Crit Care. 2019;23(1):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horiguchi H, Loftus TJ, Hawkins RB, et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Frontiers in Immunology. 2018;9 10.3389/fimmu.2018.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edfors F, Danielsson F, Hallstrom BM, et al. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol Syst Biol. 2016;12(10):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osuchowski MF, Ayala A, Bahrami S, et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Infection. 2018;46(5):687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Efron PA, Mohr AM, Moore FA, Moldawer LL. The future of murine sepsis and trauma research models. J Leukoc Biol. 2015;98(6):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu F, Inoue K, Kato J, Minamishima S, Morisaki H. Functions and regulation of lipocalin-2 in gut-origin sepsis: a narrative review. Crit Care. 2019;23(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreira RG, Rodrigues LC, Nascimento DC, et al. Galectin-3 aggravates experimental polymicrobial sepsis by impairing neutrophil recruitment to the infectious focus. J Infect. 2018;77(5):391–397. [DOI] [PubMed] [Google Scholar]
- 42.Yorulmaz H, Ozkok E, Kaptan E, Ates G, Tamer S. Therapeutic effects of simvastatin on Galectin-3 and oxidative stress parameters in endotoxemic lung tissue. Biosci Rep. 2018;38(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasioudis D, Witkin SS. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med Microbiol Immunol. 2015;204(4):471–479. [DOI] [PubMed] [Google Scholar]
- 44.Mishra BB, Li Q, Steichen AL, et al. Galectin-3 functions as an alarmin: pathogenic role for sepsis development in murine respiratory tularemia. PLoS One. 2013;8(3):e59616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox MC, Brakenridge SC, Stortz JA, et al. Abdominal sepsis patients have a high incidence of chronic critical illness with dismal long-term outcomes. Am J Surg 2020. 10.1016/j.amjsurg.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stortz JA, Cox MC, Hawkins RB, et al. Phenotypic heterogeneity by site of infection in surgical sepsis: a prospective longitudinal study. Crit Care 2020;24(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanzant EL, Hilton RE, Lopez CM, et al. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care. 2015;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turnbull IR, Clark AT, Stromberg PE, et al. Effects of aging on the immunopathologic response to sepsis. Crit Care Med. 2009;37(3):1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raymond SL, Lopez MC, Baker HV, et al. Unique transcriptomic response to sepsis is observed among patients of different age groups. PLoS One. 2017;12(9):e0184159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remick DG, Ayala A, Chaudry IH, et al. Premise for standardized sepsis models. Shock. 2019;51(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93(3):1247–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masopust D, Sivula CP, Jameson SC. Of mice, dirty mice, and men: using mice to understand human immunology. J Immunol. 2017;199(2):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beura LK, Hamilton SE, Bi K, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson K, Venkatesh B, Finfer S. Sepsis and septic shock: current approaches to management. Intern Med J. 2019;49(2):160–170. [DOI] [PubMed] [Google Scholar]
- 55.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. [DOI] [PubMed] [Google Scholar]
- 56.Osuchowski MF, Ayala A, Bahrami S, et al. Correction to: Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Infection. 2018;46(5):745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osuchowski MF, Ayala A, Bahrami S, et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Shock. 2018;50(4):377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


