SUMMARY
Oxytocinergic neurons in the paraventricular nucleus of the hypothalamus that project to extrahypothalamic brain areas and the lumbar spinal cord play an important role in the control of erectile function and male sexual behavior in mammals. The gastrin-releasing peptide (GRP) system in the lumbosacral spinal cord is an important component of the neural circuits that control penile reflexes in rats, circuits that are commonly referred to as the “spinal ejaculation generator (SEG).” We have examined the functional interaction between the SEG neurons and the hypothalamo-spinal oxytocin system in rats. Here, we show that SEG/GRP neurons express oxytocin receptors and are activated by oxytocin during male sexual behavior. Intrathecal injection of oxytocin receptor antagonist not only attenuates ejaculation but also affects pre-ejaculatory behavior during normal sexual activity. Electron microscopy of potassium-stimulated acute slices of the lumbar cord showed that oxytocin-neurophysin-immunoreactivity was detected in large numbers of neurosecretory dense-cored vesicles, many of which are located close to the plasmalemma of axonal varicosities in which no electron-lucent microvesicles or synaptic membrane thickenings were visible. These results suggested that, in rats, release of oxytocin in the lumbar spinal cord is not limited to conventional synapses but occurs by exocytosis of the dense-cored vesicles from axonal varicosities and acts by diffusion—a localized volume transmission—to reach oxytocin receptors on GRP neurons and facilitate male sexual function.
Graphical Abstract
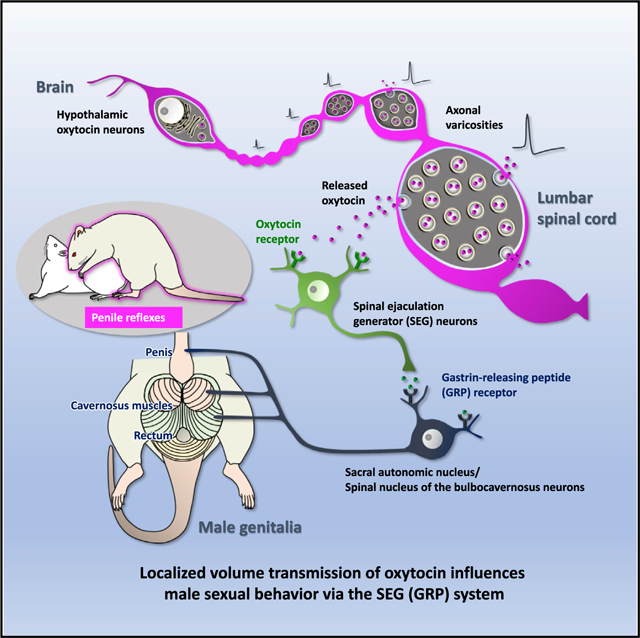
In Brief
Oti et al. show that oxytocin directly activates SEG/GRP neurons via OXTRs and influences male sexual function in the rat lumbar spinal cord. Release of oxytocin in the lumbar cord is not limited to conventional synapses and acts by diffusion—a localized volume transmission—to reach OXTRs on SEG/GRP neurons and facilitate male sexual activity.
INTRODUCTION
There is considerable current interest concerning the role of the neuropeptide oxytocin in the regulation of socio-sexual behaviors, including sexual intercourse, parturition, lactation, maternal attachment, social bonding,1,2 and empathy3 in mammals. However, the precise mechanisms by which sparsely dispersed oxytocin fibers in the central nervous system lead to activation of distributed receptor populations are unclear.4 The neuropeptides oxytocin and vasopressin are mainly synthesized by neurons in the paraventricular (PVN) and supraoptic nuclei of the hypothalamus and stored in ~160-nm dense-cored vesicles; they are well known to be released into the systemic circulation from the posterior pituitary, where the neuropeptide-containing dense-cored vesicles are exocytosed from both the perivascular axonal terminals and also the preterminal axonal varicosities.1,5 They are also released from dendrites of the neurons into the hypothalamus, where they have important roles in social behaviors via non-synaptic volume transmission.4,6–13 Malfunction of these peptide systems has been linked to behavioral disorders, and the peptides have been proposed as treatments for autism and certain psychiatric conditions.11,14,15 Because the most familiar functions of oxytocin are to regulate female reproductive functions, including parturition, milk ejection, and maternal behavior, oxytocin is often thought of as a “female” hormone.5 However, there is evidence that a group of oxytocin neurons located in the posterior PVN project to the lower spinal cord and control penile erection and ejaculation in male rats16–22 (Figure 1). The injection of oxytocin into the PVN induced an increase in the penile erections and yawning episodes in male rats, suggesting a physiological role of hypothalamic oxytocin in the regulation of such responses.23 Although oxytocin has also been reported to enhance a number of components of sexual functions in men, including libido, erection, and orgasm,24–26 the regulatory roles of oxytocin in male sexual functions remain unclear.
Figure 1. Schematic Drawing Summarizing the Brain-Spinal Cord Neural Network that Controls Male Sexual Function.
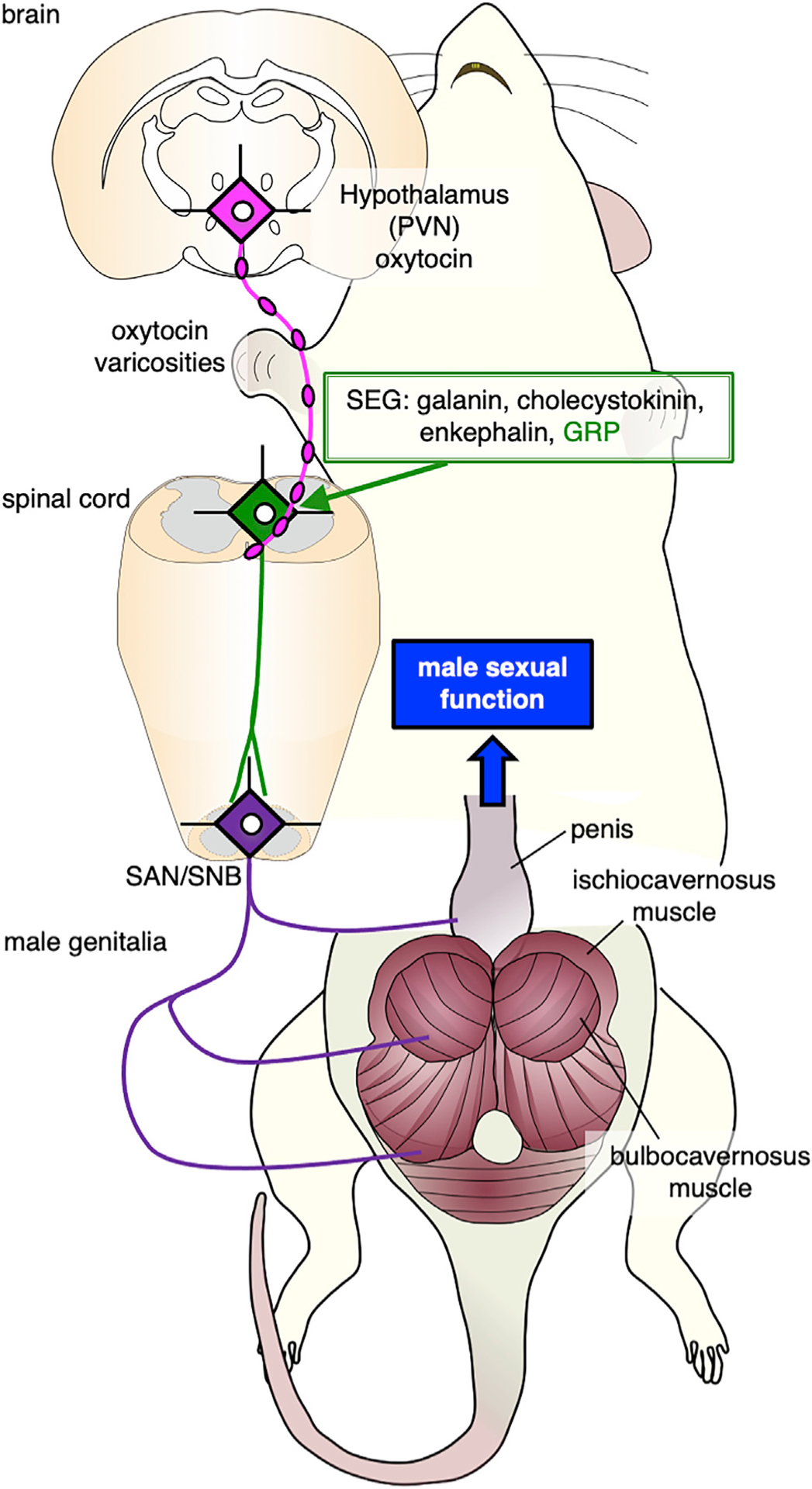
A group of oxytocin neurons located in the posterior part of the paraventricular nucleus (PVN) of the hypothalamus project to the lower spinal cord and control penile erection and ejaculation in male rats. The spinal ejaculation generator (SEG) neurons, which contain galanin, cholecystokinin, enkephalin, and gastrin-releasing peptide (GRP), project axons to both the autonomic (sacral autonomic nucleus [SAN]) and somatic (spinal nucleus of the bulbocavernosus [SNB]) centers of the lower lumbar spinal cord that mediate penile reflexes and trigger ejaculation.
Male sexual function in rodents is mediated by several spinal centers, which are referred to as the spinal pattern generator,27,28 the spinal pacemaker,29 the central pattern generator,30 and the spinal ejaculation generator (SEG)31,32 (Figure 1). These centers are believed to be influenced by supraspinal sites in the brainstem and hypothalamus.33,34 It has also been reported that galanin-containing neurons in the lumbar spinal cord (L3 to L4 level) play a pivotal role in the generation of ejaculatory behavior and may be a part of the “SEG”31,32,35, the neurons of which also contains cholecystokinin,36,37 enkephalin,38 and gastrin-releasing peptide (GRP)39 (Figure 1). GRP neurons are an important component of the SEG, and their axons project to the sacral autonomic nucleus (SAN) and to the somatic spinal nucleus in the lower lumbar and the upper sacral spinal cord (L5 to L6 and S1 level), which innervates bulbocavernosus and ischiocavernosus, striated muscles attached to the base of the penis.40 These nuclei control erection and ejaculation in an androgen-dependent manner.41 We have now examined the functional interaction between the spinal GRP system39,40 and the hypothalamo-spinal oxytocin system in rats. This has revealed a novel non-synaptic mode of oxytocin release driving this interaction via “en passant” release from axonal varicosities in the spinal cord to modulate male sexual activity.
RESULTS
SEG/GRP Neurons Controlling Ejaculation Express Oxytocin Receptors (OXTRs) and Are Activated via Oxytocin Signaling in the Lumbar Spinal Cord
We first examined the expression of phosphorylated extracellular signal-related kinases 1 and 2 (pERK), a marker for neuronal activation, after male sexual behavior, including ejaculation. The proportion of SEG/GRP neurons expressing pERK was significantly increased (>80%) 5 min after ejaculation (Figure 2), suggesting that spinal GRP neuron activation is important for ejaculation. In contrast, pERK expression was unchanged by mounts and intromissions alone (Figure 2). Double in situ hybridization in male rats revealed that ~70% Grp-positive SEG neurons express Oxtr mRNA (Figure 3A). If oxytocin is involved in penile function, then pharmacological stimulation of OXTRs at the lumbar spinal cord level should activate GRP neurons, and this would subsequently result in ejaculation. To test this hypothesis, we injected oxytocin intrathecally and found that, after 15 min, pERK expression in the spinal GRP neurons was increased (Figure 3B). We next used oxytocin-saporin (SAP), which consists of oxytocin conjugated to the toxin SAP that induces neuronal death when internalized via the OXTRs.31,42 Oxytocin-SAP (4 or 40 ng) was infused slowly into the L3 and L4 spinal cord at the location of the lumbar SEG/GRP neurons in sexually mature male rats. Two weeks after a single injection of oxytocin-SAP, over 50% of GRP+ neurons (40 ng) and ~30% (4 ng) of GRP+ neurons were lost (Figure 3C). In the lower spinal cords of oxytocin-SAP-treated rats, the intensity of GRP+ fibers in the SAN and the dorsal gray commissure (DGC) surrounding the central canal was also reduced (Figure 3D). Both the SAN and the DGC are involved in the autonomic regulation of genitalia.43 SAP conjugated to a random peptide sequence (blank-SAP), which served as the control, had no cytotoxic effects on GRP+ neurons (Figures 3C and 3D). Furthermore, infusion of blank-SAP did not cause any change in GRP+ fibers in the spinal dorsal horn, where the fibers are derived from a population of dorsal root ganglion GRP neurons44 (Figure 3D), which presumably process somatosensory stimuli, such as itch.42,45 Taken together, these results indicate that functional OXTRs are expressed in SEG/GRP neurons in the rat lumbar cord.
Figure 2. The Activity for the Spinal GRP Neurons Is Important for Ejaculation.
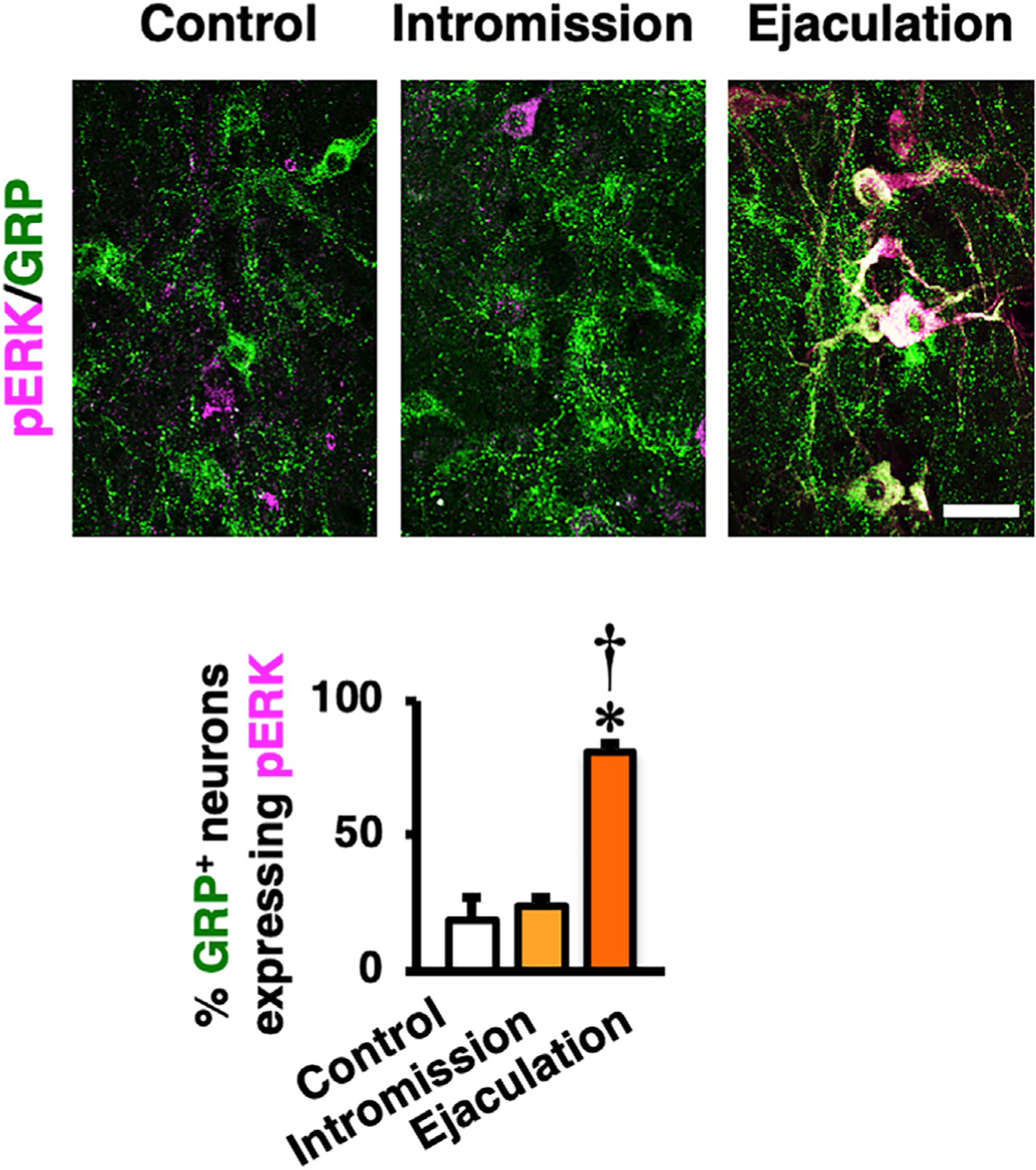
Expression of phosphorylated extracellular signal-related kinases 1 and 2 (pERK) (magenta) in rat spinal GRP neurons (green) after male sexual behavior. Left panel: control is shown. Middle panel: mounts and intromission only are shown. Right panel: after ejaculation is shown. Ejaculation significantly increased pERK expression in GRP+ neurons compared to control and intromission groups (data are presented as mean ± SEM; n = 4 in each group; one-way ANOVA; F2,9 = 36.3; *p < 0.05 versus control, †p < 0.05 versus intromission). Scale bar: 50 μm.
Figure 3. Oxytocin Receptor (Oxtr) Expression and Responsiveness to Oxytocin in the Spinal GRP Neurons.
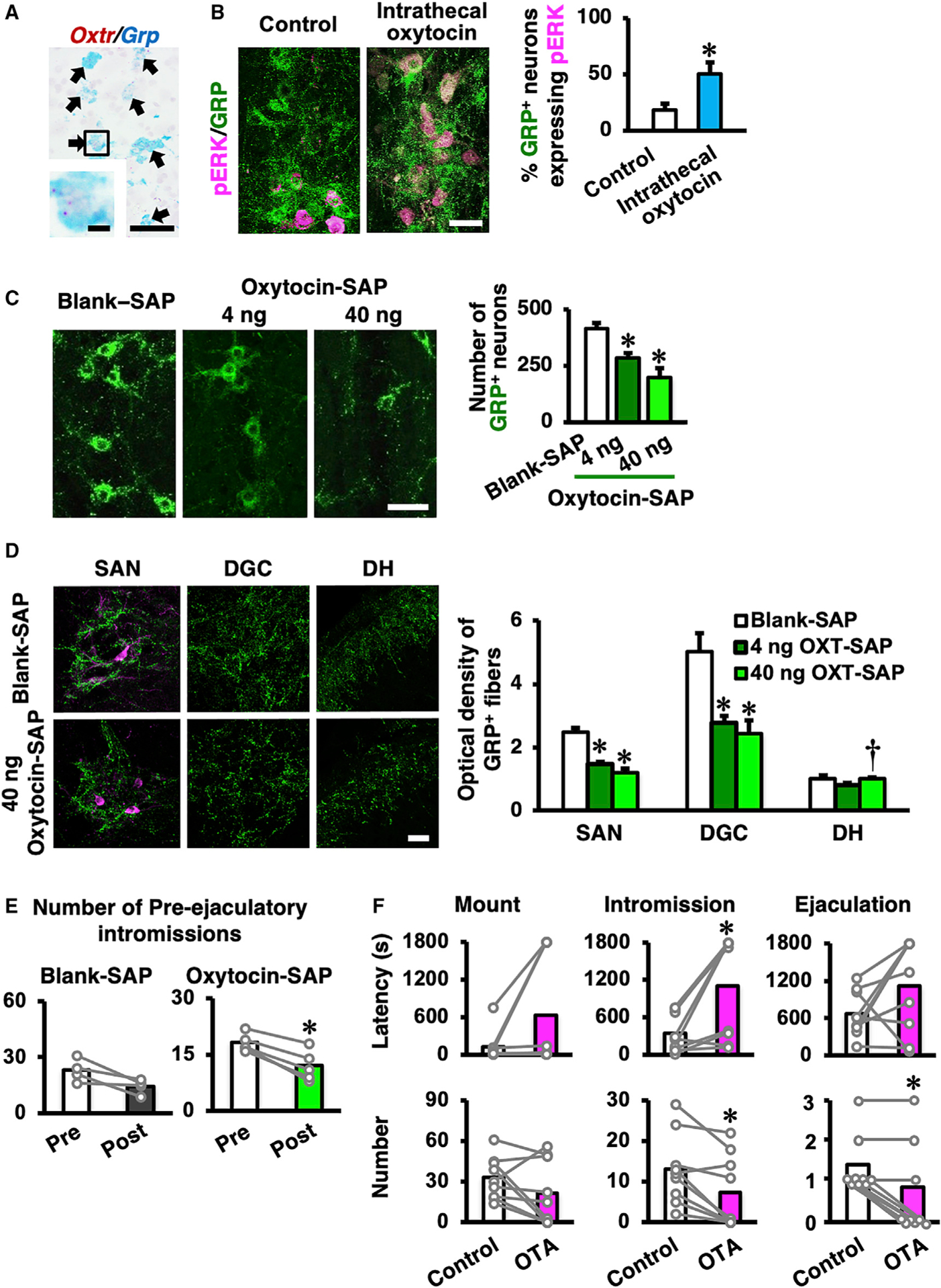
(A) Double in situ hybridization in male rats reveals that almost every Grp-positive neuron also expresses Oxtr mRNA. We could not find any Grp-positive but Oxtr-negative neurons in this study (n = 4, wild-type male rats). Scale bars: 50 μm (low magnification) and 10 μm (high magnification).
(B) Expression of pERK (magenta) in the spinal GRP neurons (green) after intrathecal oxytocin administration. Left panel: control group is shown (n = 4). Right panel: intrathecal oxytocin administration is shown (n = 5). Intrathecal oxytocin administration significantly increases pERK expression in GRP+ neurons compared to control (data are presented as mean ± SEM; Student’s unpaired t test; t6 = −2.74; *p < 0.05). Scale bar: 50 μm.
(C) The targeted toxin oxytocin-saporin (SAP), which consists of the toxin SAP conjugated to oxytocin was used. Oxytocin-SAP treatments (both 4 ng and 40 ng) significantly decrease the number of spinal GRP neurons (green) compared to the random peptide control, blank-SAP treatment. The left panel indicates blank-SAP-treated rats (n = 8). Middle panel: low-dose (4 ng) oxytocin-SAP-treated rats are shown (n = 7). Right panel: high-dose (40 ng) oxytocin-SAP-treated rats are shown (n = 6). Data are presented as mean ± SEM; one-way ANOVA; F2,18 = 15.2; *p < 0.05 versus blank-SAP. Scale bar: 50 μm. SEM, standard error of the mean.
(D) Optical density of GRP+ fibers in the lumbosacral spinal cord (L5–S1 level). Oxytocin-SAP-treated rats (4 ng, n = 7; 40 ng, n = 6) had fewer GRP+ fibers than did blank-SAP-treated rats (n = 8) in the sacral autonomic nucleus (SAN) and the dorsal gray commissure (DGC), but not in the dorsal horn (DH) (data as mean ± SEM one-way ANOVA; SAN, F(2, 18) = 20.5; DGC, F(2, 18) = 7.66; DH, F(2, 18) = 4.13; *p < 0.05 versus blank-SAP; †p < 0.05 versus 4 ng oxytocin-SAP). Scale bar: 50 μm.
(E) Effects of intraspinal administration of oxytocin-SAP (40 ng; n = 9) on male sexual behavior. Blank-SAP (n = 3) was used as control. The number of pre-ejaculatory intromissions of oxytocin-SAP (40 ng)-injected rats were decreased post-injection compared to pre-injection. No significant differences were observed in the other parameters of sexual behavior (data are presented as mean [highlighted] and individual dot [gray]; Student’s paired t test; pre-ejaculatory intromission number: blank-SAP t2 = 1.07; oxytocin-SAP t8 = 5.11; *p < 0.05).
(F) Effects of intrathecal administration of OXTR antagonist (OTA) on male sexual behavior. OTA intrathecal administration prolongs the latency to the first intromission and decreases the number of intromissions and ejaculations (data are presented as mean [highlighted] and individual dot [gray]; n = 9; Student’s paired t test; mount number: t8 = 1.63; intromission number: t8 = 3.43; ejaculation number: t8 = 3.16; *p < 0.05).
Sexual behavior tests in the oxytocin-SAP-injected male rats showed no significant differences compared with pre-injection for 5 of the 6 behavioral parameters that we measured; only the number of pre-ejaculatory intromissions was significantly reduced (Figure 3E). Similarly, intrathecal injection of oxytocin did not significantly influence sexual performance (Figure S1; Table S1). In contrast, the intrathecal injection of an OXTR antagonist (d(CH2)51, Tyr(Me)2, Thr4, Orn8, Tyr9-NH2)-vasotocin (OTA) (15 min before mating with a female) prolonged the latency to the first intromission, decreased the number of intromissions, and, most strikingly, decreased the number of ejaculations (indeed, 5 of the 9 males failed to ejaculate), clearly demonstrating a role for endogenous OXTR signaling (Figure 3F; Table S1). Thus, local inhibition of oxytocin at the spinal cord level not only attenuates ejaculation but also affects pre-ejaculatory behavior during normal sexual activity. SEG/GRP neurons therefore mediate the effect of oxytocin on male sexual activity, which is expressed as copulatory behavior (i.e., successful intromission and ejaculation; Figure 3F; Table S1).
The Oxytocin-Sensitive SEG/GRP System in the Lumbar Spinal Cord Controlling Male Reproductive Function Is Sexually Dimorphic and Male Biased
We generated EYFP (enhanced yellow fluorescent protein)-expressing bacterial artificial chromosome (BAC) transgenic rats in which the Oxtr promoter drives expression of the human diphtheria toxin receptor (Dtxr)-2A-channelrhodopsin-2-(ChR2)-EYFP fusion gene, referred to hereafter as Oxtr-ChR2-EYFP Tg rats. Because oxytocin neurons in the PVN express Oxtr, this Oxtr-ChR2-EYFP Tg rat would allow us to simultaneously opto-genetically stimulate oxytocin neurons in the brain (ChR2) and visualize GRP+ neurons in the spinal cord. Double in situ hybridization demonstrated colocalization of Oxtr and Dtxr mRNAs (Dtxr is inserted into the transgene) mRNAs in both the PVN (Figure 4A) and the lumbar spinal cord (L3 to L4 level; Figure 4B) in Oxtr-ChR2-EYFP Tg rats. In contrast, negative controls by RNAscope without specific probes for the Oxtr or Dxtr mRNA showed no specific signal (Figure S2). This reproduced the endogenous Oxtr pattern in the rat brain46 revealed as EYFP fluorescence (Figure S3A). Using Oxtr-ChR2-EYFP transgenic rats, in lamina X of the lumbar spinal cord (L3 to L4 level), we counted 3,766 GRP+ neurons (2,352 in males [n = 6] and 1,414 in females [n = 4]). Fluorescence microscopy of the same tissue revealed that 3,406 GRP+ neurons (2,035 in males and 1,371 in females) expressed EYFP. Quantitation of the EYFP+ neurons revealed 3,681 EYFP+ neurons (2,250 in males and 1,431 in females) in lamina X of the lumbar spinal cord (L3 to L4 level). The double-positive ratio for EYFP and GRP was therefore 94.6% ± 1.5% in males and 96.9% ± 0.5% in females (Figures S3B and S3C); the double-positive ratio for both sexes combined was 95.8% ± 0.9%. Oxytocin neurons and their axons were immunoidentified with the anti-oxytocin-neurophysin (NPI) antibody (PS60) that has been well characterized and frequently used as a marker for oxytocin neurons.47,48 We next demonstrated that the axonal distribution (Figures 4C and S4) around GRP neurons in the lumbar spinal cord (L3 to L4 level) exhibits a pronounced male-dominant sex difference in rats by using double immunofluorescence for NPI and GRP. Quantification of oxytocin fibers confirmed the male-biased distribution of oxytocin-positive fibers (Figure 4D).
Figure 4. Characterization of Oxtr-CHR2-EYFP Transgenic Rats.
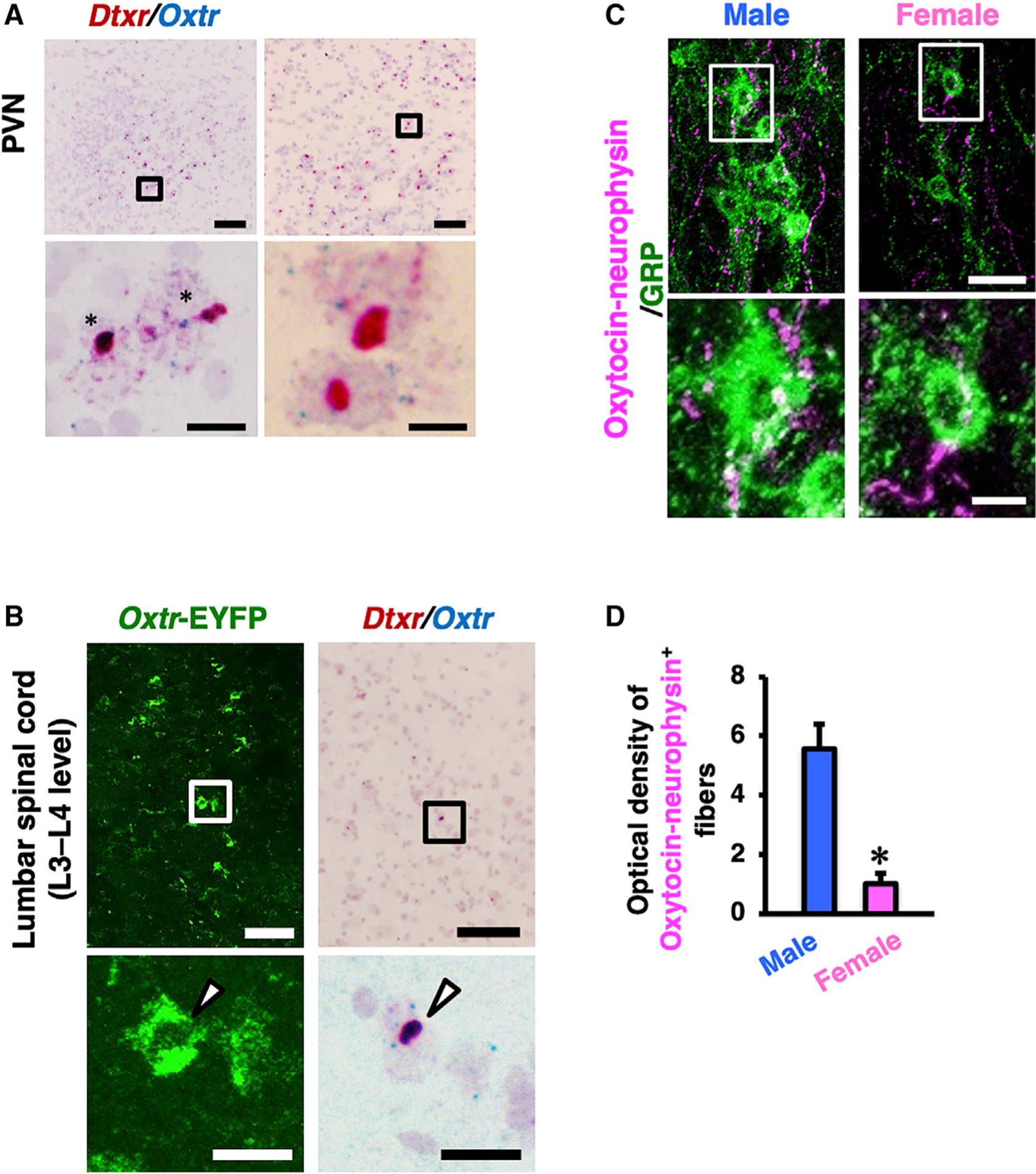
(A and B) Double in situ hybridization of human diphtheria toxin receptor (Dxtr) mRNA (red) and Oxtr mRNA (blue) in the PVN (A) and the lumbar spinal cord (L3 to L4 level; B). The lower panels are higher magnification images of the outlined areas in the upper panels. In the images of the PVN, almost all Oxtr+ neurons also express Dtxr (asterisks). In the images of the lumbar cord, arrow-heads indicate EYFP/Dxtr mRNA/Oxtr mRNA triple-positive neuronal somata. Scale bars: 100 μm (low magnification in A and B), 10 μm (high magnification in A), and 20 μm (high magnification in B).
(C) Double immunofluorescence for GRP (green) and oxytocin-neurophysin (magenta) in the lumbar cord of male and female rats demonstrated that oxytocin-containing axons surrounding GRP+ neurons exhibit a male-dominant sex difference.
(D) Quantitative analysis of oxytocin-neurophysin-immunoreactive axons in the lumbar spinal cord showed this sex difference (in the distribution—it is not really “distribution”). Outlined areas are enlarged in the lower panels. Data are presented as mean ± SEM. Student’s unpaired t test; oxytocin, t6 = 4.96; *p < 0.05; male rats (n = 4), female rats (n = 4). Scale bars: 50 μm in upper images and 20 μm in lower images.
See also Figures S2–S4.
We subsequently examined the activation of the spinal oxytocin-responsive (SEG/GRP) neurons after local hypothalamic stimulation of PVN oxytocin neurons in Oxtr-ChR2-EYFP transgenic males. After 10 min optogenetic stimulation, most oxytocinergic neurons in the PVN expressing ChR2-EYFP also expressed pERK (Figure S5). The proportion of Oxtr-EYFP+ neurons in the lumbar spinal cord (lamina X) expressing pERK was also significantly increased (by 80%) after the optogenetic stimulation (Figure 5A). Finally, we examined spinal cord oxytocin-responsive neuronal activity by in vivo extracellular recording. The frequency of firing was increased after oxytocin superfusion of the lumbar cord (Figures S6A and S6B), so we next performed in vivo extracellular recording after the optogenetic stimulation of the PVN in Oxtr-ChR2-EYFP transgenic rats. The frequency of oxytocin-responsive neuronal firing was significantly increased by the optogenetic stimulation of the PVN (Figure 5B), demonstrating that in vivo activation of PVN oxytocin neurons directly facilitates oxytocin-responsive (SEG/GRP) neuronal activity via axonal oxytocin release within the spinal cord. The increase in firing was detected from 10 to 60 min after the optogenetic stimulation and after a lag of 5.5 ± 0.9 min (Figure S6C).
Figure 5. Optogenetic Stimulation of the Oxytocin Neuron in the PVN and In Vivo Electrophysiology in the Lumbar Spinal Cord.
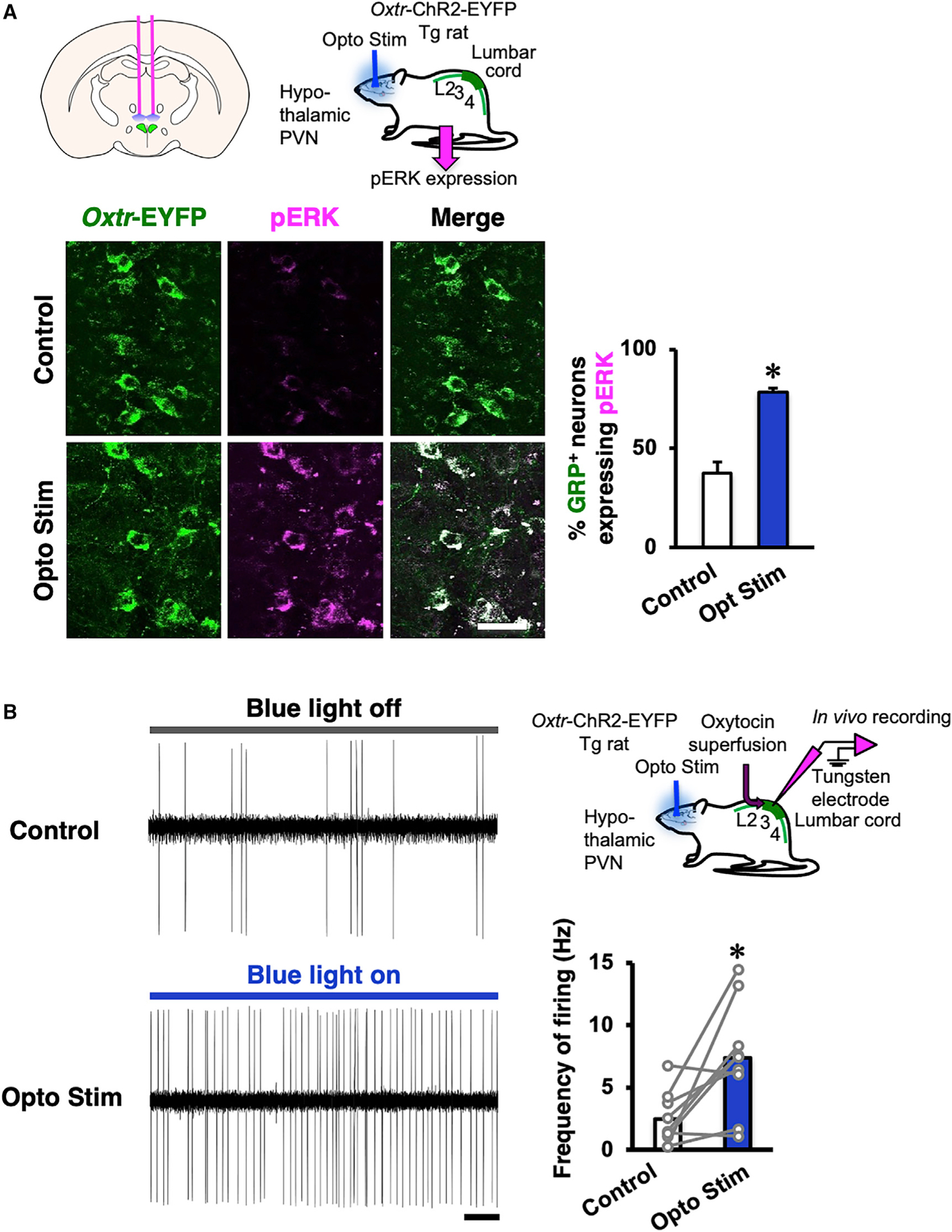
(A) The proportion of lumbar cord oxytocin-responsive neurons identified by their expression of the oxytocin receptor (Oxtr-EYFP) that also expressed pERK was significantly increased after optogenetic stimulation (opto stim) of the paraventricular nucleus (PVN) compared with control (n = 4 in each group). Scale bar: 50 μm.
Data are presented as mean ± SEM.
(B) In vivo electrophysiology revealed that the frequency of oxytocin-responsive neuronal firing was significantly increased by the optogenetic stimulation (blue light on) of PVN when compared with control (blue light off; n = 10 neurons from 5 animals; data are presented as mean [highlighted] and individual dot [gray]; Student’s paired t test; t8 = −3.18; *p < 0.05). Scale bar: 2 s. Tg, transgenic.
See also Figures S5 and S6.
Non-synaptic Axonal Release of Oxytocin in the Spinal Cord Regulates Male Reproductive Function
Electron microscopy finally revealed no classical synaptic contacts between oxytocin axons and spinal GRP neurons. Our double immunohistochemistry for GRP neurons and oxytocin fibers in the lumbar spinal cord supports this conclusion (Figure S4); oxytocin fibers containing oxytocin/NPI-immunoreactive dense-cored vesicles were never seen to form morphologically distinct synaptic connections.49,50 This suggests that oxytocinergic axons in the lumbar spinal cord release oxytocin in the vicinity of SEG/GRP neurons via a non-synaptic mechanism:7,12,51 a localized volume transmission. We therefore determined whether oxytocin is, indeed, secreted by exocytosis from the oxytocin-containing axonal varicosities in the lumbar spinal cord. Acute slices of the lumbar cord were prepared and pre-incubated in artificial cerebrospinal fluid for 15 min, followed by exposure to a high-potassium (56 mM) stimulation to induce exocytosis ex vivo. Slices were then fixed and processed for immunoelectron microscopy. Oxytocin-neurophysin immunoreactivity was detected in large numbers of dense-cored vesicles, many of which are located close to or in contact with the plasmalemma of axonal varicosities, in which no synaptic microvesicles or synapse-like membrane thickenings were visible (Figures 6 and S7). In some cases, the vesicles could be seen to discharge their contents by exocytosis into the surrounding extracellular space (Figure 6). This indicates that the release of oxytocin from axonal varicosities occurs by exocytosis at non-synaptic sites, as has been clearly demonstrated to occur in the hypothalamus.9
Figure 6. Localized Volume Transmission by Exocytosis of Oxytocin in the Lumbar Spinal Cord Controls the Spinal Ejaculation Center.
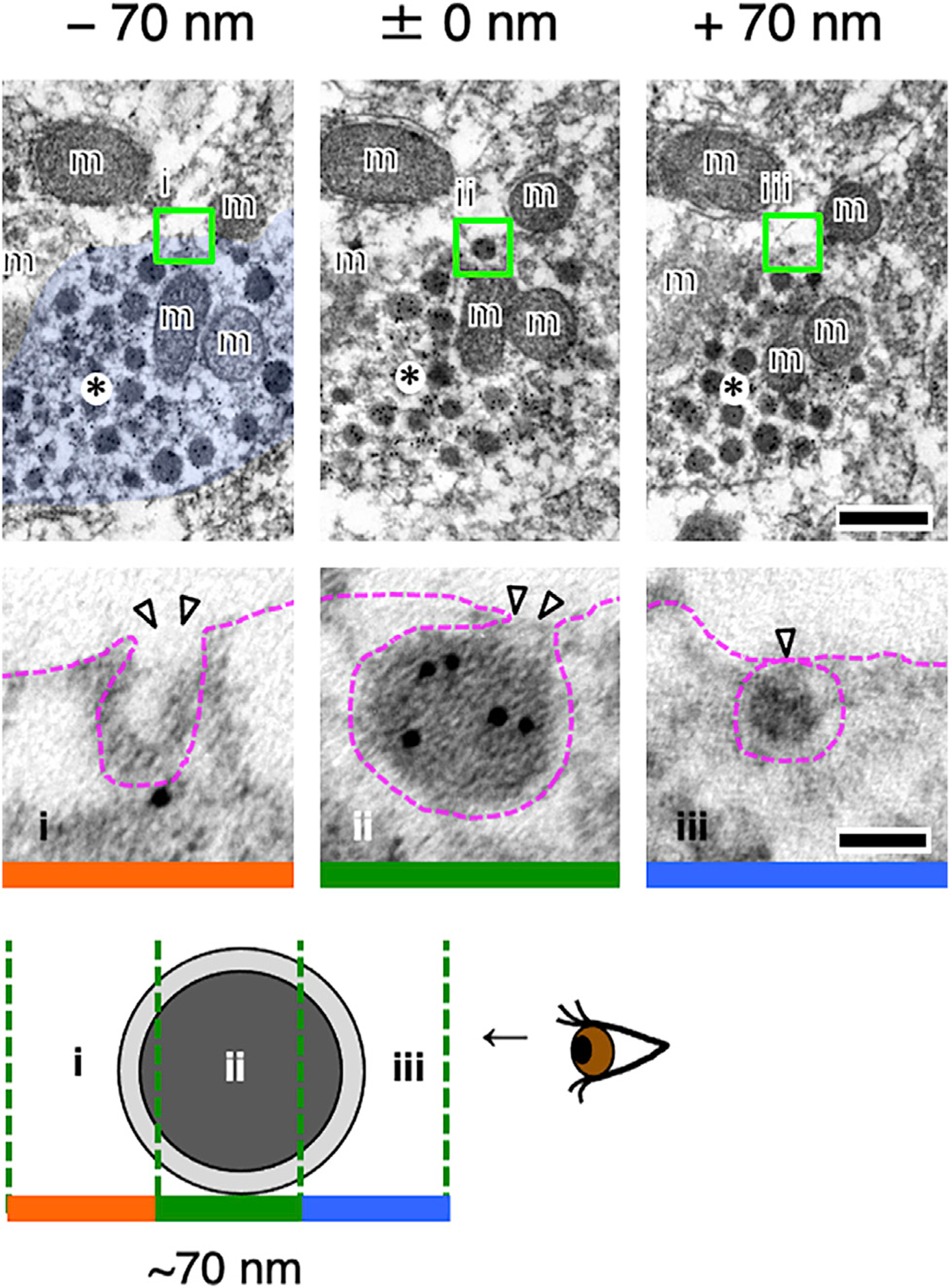
Three serial ultrastructural sections (~70 nm in thickness: i–iii) are displayed. Oxytocin-neurophysin is labeled with 10 nm gold particles. An oxytocin-neurophysin+ neurosecretory vesicle in the varicosity (asterisks) appears to be undergoing exocytosis at a non-synaptic site. Each blocked area is enlarged below, respectively. Arrowheads indicate exocytosis (i and ii) or docking (iii). m, mitochondrion. Scale bars: 200 nm in upper images and 50 nm in lower images. See also Figure S7.
DISCUSSION
Male sexual function in rodents is controlled by a brain-spinal cord network comprising the PVN, the SEG in the lumbar cord,16,20,21 and the SAN and SNB in the sacral cord. Axons from oxytocin neurons in the PVN pass down to the region of the lumbar spinal cord, which acts as the SEG. We have demonstrated that these SEG/GRP neurons express OXTRs and that interfering with oxytocin action in the lumbar spinal cord has clear effects on male sexual activity. We also show that, in the lumbar cord, oxytocin is secreted by exocytosis from axonal varicosities (not synaptic boutons) to act in a paracrine manner4,7,11,12—a localized volume (humoral) transmission— on SEG/GRP neurons expressing OXTRs (Figure 7).
Figure 7. Schematic Drawing of the Way in which Activation of PVN Oxytocin Neurons Causes Release of Oxytocin in the Lumbar Spinal Cord to Influence the Spinal Ejaculation Generator.
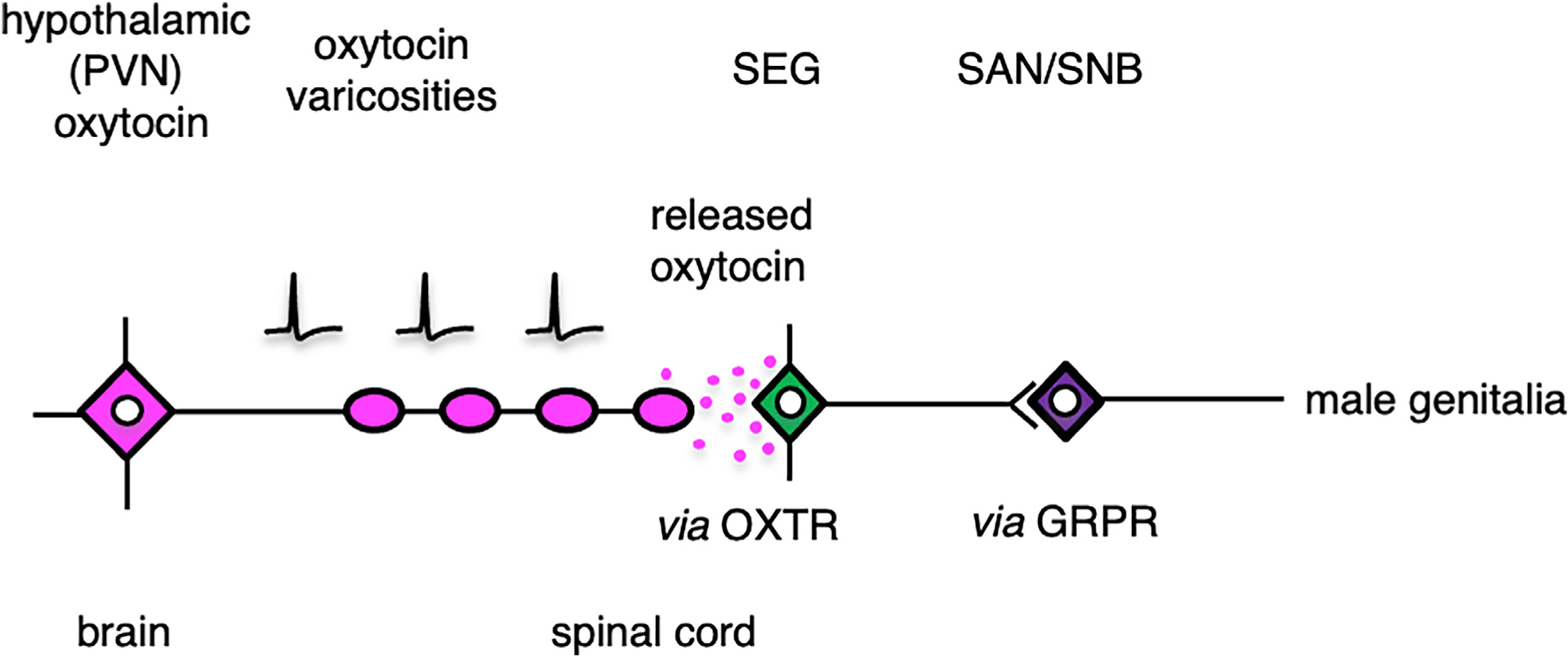
Our data demonstrate that the oxytocin in the lumbar cord, which facilitates sexual activity in male rats, is released from varicosities of axons originating from the PVN rather than from classical synaptic boutons originating from such axons and, consequently, that it reaches OXTR on GRP neurons by a localized volume distribution via the extracellular space. GRPR, gastrin-releasing peptide receptor; OXTR, oxytocin receptor; SAN, spinal autonomic nucleus; SEG, spinal ejaculation generator.
Truitt and Coolen,31 investigating the afferent limb of the sexual response circuit, have previously demonstrated that selective lesioning of the galanin-expressing spinothalamic neurons in the SEG completely eliminated the ability of male rats to ejaculate but that other aspects of male sexual behavior remained unaffected.
In this report, we have examined interneuron components of the sex response circuit: the SEG/GRP neurons; the action on them of PVN axon-derived oxytocin; and the resultant effect on the latency and number of intromissions and ejaculations. We have provided morphological evidence that the SEG/GRP neurons (identified by GRP immunoreactivity) express OXTRs in the lumbar spinal cord of Oxtr-ChR2-EYFP transgenic rats (Figures 4B, S3B, and S3C) and that locally applied oxytocin can stimulate the activation of the SEG/GRP neurons. Intrathecal administration of oxytocin in the lumbar spinal cord increased the expression of a neuronal activation marker (pERK) in the SEG/GRP neurons, and oxytocin superfusion increased the frequency of oxytocin-responsive (spinal GRP) neuronal firing in the lumbar spinal cord (Figure 3B). We have also provided functional evidence that the release of oxytocin from these axonal varicosities plays an important role in penile functions during copulatory behavior (mounting, intromission, and ejaculation) because local administration of an OXTR antagonist in the lumbar spinal cord not only attenuates the number of ejaculations but also affects pre-ejaculatory behavior (increased latency to intromission and reduced number of intromissions) during normal sexual activity (Figure 4). All of these findings suggest that oxytocin acts via the SEG/GRP neurons to stimulate sexual activity via the SAN and SNB. If release of oxytocin in the lumbar spinal cord does act in this way, then we might have expected a decrease in the number of ejaculations in the oxytocin-SAP-injected rats, but the decline in the number of ejaculations was not significant and the only behavior parameter that was altered was a small decrease in the number of pre-ejaculatory intromissions. However, the application of 40-ng oxytocin-SAP reduced the number of GRP neurons by only ~50%, and therefore, the remaining GRP neurons appear to have been sufficient to allow ejaculations, given that GRP is unlikely to be the only stimulus to the SAN/SNB. Also, although the intrathecal administration of the oxytocin antagonist markedly and significantly increased the latency to intromission and reduced the number of intromissions and ejaculations, the intrathecal infusion of oxytocin, despite stimulating the firing and increasing pERK expression of GRP neurons, did not significantly reduce the latency or increase the number of intromissions or ejaculations. We suggest that this was because, in the presence of a receptive female, endogenous release of oxytocin was already having a substantial effect on the GRP neurons and the effect of GRP neurons on the SAN and SNB was already maximal. Taken as a whole, our results strongly suggest that the oxytocin-responsive GRP neurons located in the lumbar spinal cord (lamina X; just dorsal to the central canal) are one important component of the SEG.
The projection of PVN neurons to the cord is well documented, and the only documented projection from the PVN to the lumbar spinal cord is that from the oxytocin neurons in the posterior part of the PVN.16,20 We have now demonstrated that optogenetic stimulation of Oxtr- and ChR2-expressing neurons in the PVN leads to activation of SEG neurons. Given that (1) there is abundant evidence that oxytocin neurons are themselves sensitive to oxytocin (e.g., express Oxtr)52 and (2) that the PVN contains a similar number of vasopressin neurons that do not respond to oxytocin53 and parvocellular neurons that project largely to the median eminence and not to the spinal cord,54 these data together indicate that the majority of OXTR-expressing (and therefore ChR2+ and EYFP+) neurons in the PVN that project to the spinal cord are oxytocin neurons. Furthermore, in the lumbar cord, almost all the oxytocin-immunoreactive axons are located in the immediate region of lamina X, where the GRP neurons are located, and 93% of the GRP neurons express EYFP and therefore also express OXTRs. Thus, although we cannot exclude the possibility that other non-oxytocin neurons in the PVN projecting to the spinal cord were activated during our optogenetic stimulation, there appears to be no positive evidence for such neurons but substantial evidence for an effect on SEG neurons of the posterior/parvocellular PVN oxytocin neurons that project to the lumbar cord.16,20,21
It is possible, even likely, that the dense-cored, oxytocin-containing vesicles in the axonal varicosities contain other peptides.55,56 Also, the detection of glutamate in the “synaptic” microvesicles in the perivascular endings of magnocellular neurosecretory neurons in the neurohypophysis57 raises the possibility that the varicose oxytocin axons in the lumbar cord also utilize a fast transmitter. However, this seems unlikely because (1) there are no synaptic microvesicles in the oxytocin-containing axonal varicosities in the lumbar cord, (2) no synaptic contacts (thickenings) were seen in any of the many hundreds of oxytocin-vesicle-containing axonal profiles in close proximity to GRP neurons, and (3) no oxytocin immunoreactivity was seen in any of the classic synaptic boutons in this region of the cord. Therefore, the oxytocin-containing axonal varicosities in the lumbar cord are morphologically and functionally similar to the preterminal “swellings” of magnocellular axons in the neurohypophysis rather than synaptic boutons.58 Fisher and Bourque,59 in a review that deals mostly with magnocellular neurons, note that depolarization can cause exocytotic release from both the many axonal swellings and endings of the neurons. However, in that paper, they refer to all release in the posterior pituitary as synaptic and contrast this with somatodendritic release in the hypothalamus. To our knowledge, there has been no attempt to identify any different controls on the release from the magnocellular “endings” (which also contain synaptic vesicles that have been shown to contain glutamate)57 and the preterminal swellings (varicosities).60 The release from dendrites is not tetrodotoxin sensitive,9,61 but our study of the spinal cord deals with axonal varicosities and not dendrites. Although we cannot completely rule out the possibility of some synaptic involvement, the balance of evidence clearly points to a non-synaptic release of oxytocin from axon varicosities in the lumbar cord. Further investigation of the effect of blocking synaptic release during optogenetic stimulation of the PVN might provide evidence for the non-synaptic release of oxytocin from axons in the cord.
Non-synaptic release from peptidergic axons is not unexpected because such exocytotic release has been clearly demonstrated for the preterminal axonal swellings in the neural lobe and for the dendrites in the hypothalamus.4,6–13 Non-synaptic release from lumbar cord axonal varicosities would be expected to occur at roughly the same time as that from the dendrites of the neurons in the PVN and their axonal varicosities in the hypothalamus. The electrical activity of the oxytocin neurons may well be important to synchronize brain and spinal cord functions in sexual activity.
Whereas release of transmitters at classical synapses has always received much attention because they are important components of fast neural networks, recent studies have shown that, in the hypothalamus, dendritic release of oxytocin and vasopressin acting as a paracrine signal in the brain4,8,10,11 can influence social behaviors.6,8,10,11,13,52,62 Many peptides, including oxytocin and vasopressin, appear to be released from multiple neuronal loci, including dendrites, axons, somata, as well as axon terminals.4,62 Understanding the differences between the evolutionarily ancient diffusion-based “humoral” transmission peptidergic systems and the newer, more rapid synaptic systems using conventional neurotransmitters could shed light on the evolutionary origin of the “synapse system” as a characteristic developed from the peptidergic nerve net of primitive creatures like Hydra.63 The two systems now coexist and are complementary in advanced Animalia.
Because the half-lives of oxytocin and vasopressin in the brain are long: ~20 min compared with just 2 min in the blood,52,62,64 diffusing peptides are not restricted spatially by rapid degradation or reuptake. A cloud of oxytocin is formed that can reach OXTRs, which are widespread in the central nervous system.65 Intracerebroventricular administration of oxytocin induces a dose-dependent increase in the number of penile erections and yawning episodes in male rats.17 Oxytocin concentrations in rat cerebrospinal fluid double 5 min after ejaculation66 and increase to three times the basal level 20 min after ejaculation.66 Given that circulating oxytocin also increases at the time of ejaculation in men, but not during sexual arousal,67 our observations could lead to new pharmacological approaches for the treatment of sexual dysfunction, such as erection and ejaculation difficulties. Intranasal administration of oxytocin has been reported to be effective in certain sexual and psychiatric disorders,14,24,25 but the mechanism of any action remains controversial.9,64
Peptide-containing axonal varicosities are a feature of many peptidergic neurons.68 Oxytocin and vasopressin systems act both as hormones in the systemic circulation and as neuromodulators in the brain extracellular fluid and cerebrospinal fluid.1,4 It has recently been shown that gonadotropin-releasing hormone (GnRH) neurons elaborate long-distance projections, which receive synapses and control GnRH secretion from the median eminence.69 Whether these unique projections, which exhibit properties of both a dendrite and an axon and are thus termed “dendrons”69, can release GnRH at any point (like magnocellular oxytocin and vasopressin neurons) is yet to be demonstrated, but GnRH certainly has behavioral effects when administered in the hypothalamus.70–74 It will therefore be important to determine how the peripheral and central actions of all these peptides are controlled and coordinated. We propose that axonal varicosities (swellings) of peptidergic neurons are sites for the storage of peptide-containing neurosecretory vesicles, which can be secreted by exocytosis. We conclude that the available evidence demonstrates a mechanism by which the sparse oxytocin fibers found in the central nervous system can influence regional OXTR signaling in populations of neurons to modulate social and sexual behaviors13 and that this mode of non-synaptic signaling is important for male sexual behavior controlled in part by GRP neurons in the lumbar spinal cord.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Request for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Hirotaka Sakamoto, Ph.D. (hsakamo@okayama-u.ac.jp).
Materials Availability
Research materials generated in this study are available from the corresponding author upon request.
Data and Code Availability
The published article includes all datasets analyzed during this study.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Rats
For experiments probing the expression site of the oxytocin receptor, adult transgenic Wistar rats bred in the animal facilities of Okayama University were examined. The transgenic rats were identified by standard PCR analysis of extracted ear DNA using primers detecting the Venus gene. In all other studies, adult wild-type Sprague-Dawley (SD) rats (Shimizu Laboratory Supplies Co., Ltd., Kyoto, Japan or Charles River Japan, Yokohama, Japan) were used in this study. All rats were maintained on a 12-h light/12-h dark cycle and were provided unlimited access to water and rodent chow. The Committee for Animal Research, Okayama University, Japan authorized the experimental procedures.
Oxtr-ChR2-EYFP transgenic rats
The oxytocin receptor (Oxtr) promoter-human heparin-binding epidermal growth factor-like growth factor (human diphtheria toxin receptor; Dxtr)-channelrhodopsin (ChR2)-enhanced yellow fluorescent protein (EYFP) BAC transgene was purified for microinjection using a slight modification of the procedure described previously.75 Oxtr-ChR2-EYFP transgenic rats were generated by pronuclear injection of Wistar rat embryos (Institute of Immunology Co., Ltd., Tokyo, Japan). EYFP expression was observed in the forebrain in 4% formaldehyde-fixed brain sections obtained from EYFP-positive rats.
METHOD DETAILS
In situ hybridization
Rats were deeply anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg kg−1 body weight) and perfused transcardially with physiological saline. The upper (L3–L4 level) spinal cords and brains were quickly removed and then quickly frozen using dry ice and stored at −80°C until use. Tissues were made at a slice thickness of 14 mm. RNA in situ hybridization was performed using RNAscope® 2.5 HD Duplex Reagent Kit (Advanced Cell Diagnostics, Hayward, CA) according to the manufacturer’s instructions for fresh frozen tissues. Chromogenic detection was performed using diaminobenzidine (DAB) followed by counterstaining with hematoxylin (131–09665, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). Using probes were listed below. Probes: Rn-Grp (497781), Rn-Oxtr (483671), Rn-Oxtr-C2 (483671-C2) and Hs-heparin-binding epidermal growth factor-like growth factor-C2 (Dxtr: 524821-C2) (Advanced Cell Diagnostics). RNAscope experiments were performed without specific probes for the Oxtr and/or Dxtr as negative controls (Figure S1). Stained sections were analyzed using an Olympus microscope.
Immunohistochemistry and immunofluorescence
Rats were deeply anesthetized with intraperitoneal injections of sodium pentobarbital (50 mg kg−1 body weight), and perfused via the left ventricle with 100 mL of physiological saline followed by 200 mL of 4% formaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Spinal cords were immediately removed and post-fixed in the same fixative for 3 h at room temperature. After immersion in 25% sucrose in 0.1 M PB for 48 h at 4°C for cryoprotection, lumbosacral cords were quickly frozen using powered dry ice and cut into 30-μm thick horizontal (the lumbar cord: L3–L4 level) and cross (brain and the lumbosacral cord: L5–S1 level)-sections on a cryostat (CM3050 S, Leica, Nussloch, Germany). Endogenous peroxidase activity was eliminated by incubation in 1% H2O2 in absolute methanol for 30 min followed by three 5-min rinses with phosphate-buffered saline (PBS, pH 7.4), This H2O2 treatment was omitted for immunofluorescence. After blocking nonspecific binding with 1% normal goat serum and 1% BSA in PBS containing 0.3% Triton X-100 for 30 min at room temperature, the sections were incubated with the primary rabbit antiserum against GRP (1:2,000 dilution) (11081; AssayPro, St. Charles, MO, RRID: AB_2571636) as described previously.39,44 The specificity of the anti-GRP serum in the spinal cord was demonstrated previously.39,44 Immunoreactive products were detected with a streptavidin-biotin kit (Nichirei, Tokyo, Japan), followed by diaminobenzidine (Dojindo, Kumamoto, Japan) development according to our previous method.39,44 To determine the density of GRP+ fibers in the lumbosacral spinal cord (L5–S1 level), at least ten sections per animal were analyzed using ImageJ software (ImageJ 1.44p, RRID_ SCR_003070) with a set threshold level. The GRP+ fiber pixel density was quantified as the average pixel density in three regions; the sacral autonomic nucleus (SAN), dorsal gray commissure (DGC) and dorsal horn (DH), of each animal, and was calculated as the ratio to the density in the DH in controls in each analysis.
To determine the distribution of EYFP signals in OXTR-ChR2-EYFP transgenic rat brain, we used the immunohistochemistry for detection of green fluorescent protein (GFP) antigens to intensify the YFP signal (for localizing Oxtr+ neurons). The sections were incubated with 1% H2O2 in absolute methanol solution for 30 min followed by three 5-min rinses with PBS. After blocking nonspecific binding, as described above, the sections were incubated with the primary chicken antiserum against GFP (1:2,000 dilution) (600-901-215; Rockland, Gilbertsville, PA, RRID: AB_1537402). After rinsing, the sections were incubated with biotinylated anti-chicken IgG raised in goats (1:1,000 dilution) (BA-9010; Vector laboratories, Inc., Burlingame, CA). Immunoreactive products were detected, followed by diaminobenzidine development, as described above.
To determine the distribution of oxytocin-containing axons, double immunofluorescence staining for GRP (1:1,000 dilution) and oxytocin-neurophysin (PS60; mouse monoclonal antibody, RRID: CVCL_G254) (1:1,000 dilution), a marker protein for oxytocin neurons, was performed. The PS60 antibody has previously been shown to be specific for oxytocin neurons at the ultrastructure level.47,48,76 Alexa Fluor 488-linked anti-rabbit IgG raised in goats (Molecular Probes, Eugene, OR) and Alexa Fluor 546-linked anti-mouse IgG raised in goats (Molecular Probes) were used for detection at a 1:1,000 dilution.
To determine the projection sites of GRP+ axons, double-immunofluorescence staining for GRP (1:1,000 dilution) and neuronal nitric oxide synthase (nNOS) (1:5,000 dilution) (A-11; mouse monoclonal antibody, Santa Cruz Biotechnology, Santa Cruz, CA, RRID: AB_626757), a marker protein for neurons in the SAN, was performed. Alexa Fluor 546-linked anti-mouse IgG (Molecular Probes) and Alexa Fluor 488-linked anti-rabbit IgG (Molecular Probes) were used for detection at a 1:1,000 dilution.
To examine the activation of GRP+ neurons, double immunofluorescence staining for GRP and phosphorylated extracellular signal-related kinases 1 and 2 (pERK), a marker protein for neuronal activation, was performed. After blocking nonspecific binding, as described above, the sections were incubated with the anti-pERK (1:1,000 dilution) (#9101; rabbit polyclonal antibody, Cell Signaling Technology, Danvers, MA, RRID: AB_331646)77 overnight at 4°C. After rinsing with PBS, sections were incubated for 1 h at room temperature with Dylight 549-linked Fab fragment goat anti-rabbit IgG (1:100 dilution) (Jackson Laboratory, Bar Harbor, ME). After rinsing, the sections were incubated with 1% H2O2 in absolute methanol for 20 min at room temperature. After blocking nonspecific binding, the sections were immersed overnight at 4 C in a 1:200 dilution of GRP antiserum. The second-primary immunoreaction was visualized as green by 1-h incubation with Alexa Fluor 488-linked anti-rabbit IgG (1:2,000 dilution) (Molecular Probes).
Immunostained or EYFP-positive sections were imaged with an all-in-one fluorescence microscope and cellSens Software (FSX100, Olympus, Tokyo, Japan, RRID: SCR_016238) or a confocal laser scanning microscope (FluoView 1000, Olympus, RRID: SCR_014215).
Surgery
Local intraspinal administration of oxytocin-saporin (SAP)
Male rats were anesthetized by inhaling isoflurane gas (concentration, 1.5% in air; flow rate, 1 L min−1). Spinal cords were fixed in a stereotaxic apparatus (STS-B; Narishige, Tokyo, Japan), and laminectomy was performed to expose the spinal cord. Oxytocin-saporin (SAP; IT-46, Advanced Targeting Systems, San Diego, CA) (4 and 40 ng μl−1) was microinjected slowly as a 1 μl injection into the midline at 1 to 1.5 mm below the dorsal surface of the spinal cord from L3 to L4. Blank-SAP (Advanced Targeting Systems) was used as control. Two concentrations of Blank-SAP were used in this paper; 4 ng μl−1 (n = 5) and 40 ng μl−1 (n = 3), which are equal amounts of those of oxytocin-SAP. Since no statistical difference in 2 concentrations of Blank-SAP was observed, we combined these data as the “Blank-SAP” control (n = 8). Following surgery, the wound was covered with gelfoam, and the muscle tissue and incision were closed with sutures and wound clips. Animals were killed 2 weeks after surgery, and spinal cords were sectioned.
Intrathecal administration of oxytocin
For immunohistochemistry of pERK in the spinal GRP neurons, male rats were anesthetized by inhaling isoflurane gas and the spinal cord was exposed as above, and 0.1 mM oxytocin (24274; AnaSpec Inc., Fremont, CA) in PBS (5 nmol 50 μl−1 PBS) was intrathecally administered in the upper lumbar spinal cord (L3–L4 level). Fifteen min after surgery, rats were quickly perfusion-fixed for immunohistochemical analyses. In addition, for sexual behavior tests after intrathecal administration of oxytocin, male rats were anesthetized by inhaling isoflurane gas as above. For installation of an intrathecal catheter, a small incision was made in the atlanto-occipital membrane and the catheter (polyethylene tube, PE-10; Nazme Seisakusho Co., Ltd., Tokyo, Japan) was inserted into the subarachnoid space, terminating in the L3–L4 level. Oxytocin (0.1 mM) diluted in artificial cerebrospinal fluid (aCSF)78 (n = 10; 1 μg 20 μl−1 aCSF) was locally administered via the installed catheter. Twenty min (including a period for 5 min-adaptation in the test cage) after the injection, sexual behavior test was performed as below. The sexually active rats that ejaculated after intrathecal administration of aCSF alone were used as controls.
Intrathecal administration of OXTR antagonist (OTA)
An intrathecal catheter was installed as above. aCSF was administered locally via the installed catheter. Twenty min (including a period for 5-min-adaptation in the test cage) after the injection, the sexual behavior test was performed as below. Sexually active rats that ejaculated after intrathecal administration of aCSF alone were used as controls. One week after the aCSF injection, the OXTR antagonist (OTA), [d(CH2)51, Tyr(Me)2, Thr4, Orn8, Tyr-NH29]-vasotocin (H-9405; Bachem, Torrance, CA) diluted in aCSF78 (n = 10; 1 μg 20 μl−1 aCSF) was locally administered and the sexual behavior test was again performed. When tested again 1–2 weeks after the OTA administration, ejaculations were observed in all rats, confirming that the earlier administration of OTA had not permanently disrupted the SEG.
Morphological analysis
We counted GRP+ neurons in the lumbosacral spinal cord. Immunofluorescence analysis of GRP expression in neurons of the anterior lumbar spinal cord (L3–L4 level) was performed as described above using horizontal sections (approximately eighteen-twenty-two 30-μm-thick sections per animal).79–81 Briefly, we counted the number of GRP+ cell bodies at × 200 magnification in all sections and analyzed a 600-μm2 area localized to the midline at the center. We acquired 5–15 micrographs per section, the number of which depended on the distribution of the GRP+ neurons. These digital micrographs were selected and processed using Adobe PhotoShop (Adobe Systems, San Jose, CA) and printed at 300 dots per inch on photographic paper. GRP neurons were identified by their following characteristics: densely immunostained, anatomical localization (mainly dorsal, dorsolateral, or both to the central canal in lamina X of lumbar segments III–IV), relatively large cell bodies (diameters approximately 20–30 μm), and clear round nuclei (diameters approximately 10–15 μm). To avoid overestimating cell number, only GRP+ neurons that contained a round, transected nucleus were counted. Because the mean diameter of the nuclei in the GRP neurons is much smaller than the 30-μm thick sections, this analysis reduced the overestimation of the number of neurons. All micrographs were coded and evaluated without the knowledge of the experimental group designation, and the code was not broken until the analysis was complete.
The number of Oxtr-EYFP BAC transgenic rats was 6 for males and 4 for females. We counted 3,766 GRP+ neurons (2,352 GRP+ neurons in males and 1,414 GRP+ neurons in females) in the lamina X of the lumbar spinal cord (L3–L4 level). We revealed 3,406 GRP+ neurons (2,035 GRP+ neurons in males and 1,371 GRP+ neurons in females) expressed EYFP. In addition, we counted 3,681 EYFP+ neurons (2,250 EYFP+ neurons in males and 1,431 EYFP+ neurons in females) in the lamina X of the lumbar spinal cord (L3–L4 level).
Sexual behavior test
For sexual behavior tests, stimulus females were ovariectomized and estradiol benzoate (5 μg 0.1 ml−1 sesame oil) was subcutaneously injected 2-d prior to testing. Progesterone (500 μg 0.1 ml−1 sesame oil) was subcutaneously injected 4–6 h prior to testing to induce sexual receptivity. Sexual behavior tests were performed for 30 min and the latency of the first mount, intromission and ejaculation and the number of mounts, intromissions and ejaculations were counted. Additional information is provided in Table S1.
Ex vivo analysis for exocytosis
To study exocytosis of oxytocin, male lumbar spinal cord slices were stimulated ex vivo by 56 mM potassium according to an established method.78,82 In brief, male rats (n = 6) were killed by decapitation under a deep pentobarbital anesthesia; lumbar cords (L3–L4) were quickly removed onto ice and horizontal slices (500 μm in thickness) containing DGC area were prepared by using a Vibrating Microtome (7000smz-2, Campden Instruments Ltd., Loughborough, UK). The slices were then incubated with aCSF pre-gassed with 95% O2/5% CO2 for 15 min at 37°C, then incubated with aCSF containing a high level (56 mM) of potassium and 0.2% tannic acid for 15 min at 37°C. After the high-potassium stimulation ex vivo, the slices were immersed in 4% formaldehyde and 1% glutaraldehyde in 0.1 M PB (pH 7.4) for 3 h at room temperature, washed with 0.1 M PB, then post-fixed with 1% osmium tetroxide in 0.1 M PB for 2 h at room temperature. The slices were washed with 0.1 M PB, dehydrated, and embedded in epoxy resin (Quetol-812; Nisshin EM, Tokyo, Japan) as described previously.83 Ultrathin sections were prepared as described above. The sections were then incubated with a 1:5 dilution of PS60 antibody for 1 h at room temperature, washed with TBS, then incubated with a 1:50 dilution of a goat antibody against mouse IgG conjugated to 10 nm gold particles (EM.GMHL10; BBI Solutions) for 1 h at room temperature.
Optogenetics
For pERK-immunohistochemical analyses in the lumbar spinal cord (L3–L4 level) after optogenetic activation of the PVN oxytocin neurons, a wireless stimulation system (Teleopt, Bio Research Center, Tokyo, Japan) was used. Oxtr-ChR2-EYFP transgenic male rats were implanted with the bilateral LED cannula unit (NA 0.50) targeting the posterior part of PVN (2 mm posterior to bregma, ± 0.6 mm bilateral to midline, 7.3 mm ventral to skull surface). Optogenetic activation was performed for 10 min with blue light (470 nm) stimulation (at least 10 mW, 15 ms light pulses, 20 Hz; Stimulation group; n = 4) or no stimulation (Control group; n = 4) condition. The delivery of light pulses was controlled by a schedule stimulator (COME-2-SPG2, Lucir Inc., Tsukuba, Japan). Fifteen min after blue light stimulation, rats were quickly perfusion-fixed for pERK-immunohistochemical analyses as described above.
For in vivo electrophysiological analysis of lamina X neurons in the lumbar spinal cord (L3–L4 level) during either superfusion of oxytocin to the lumbar cord (n = 9 neurons from 5 animals) or blue light stimulation (n = 9 neurons from 5 animals) to the PVN oxytocin neurons to induce axonal oxytocin secretion in the lumbar spinal cord (volume transmission), Oxtr-ChR2-EYFP transgenic male rats were implanted with an optical fiber (NA 0.50; THORLABS, Newton, NJ) targeting the posterior part of the left PVN (2 mm posterior to bregma, 1 mm left lateral to midline, 8 mm ventral to skull surface). The optical fiber was connected to an optical swivel (RJPSF-SMA, THORLABS), and then connected to a laser light source (470 nm; M470F3, THORLABS) that was controlled by either a schedule stimulator (Lucir Inc.) or without stimulation (Control). In vivo extracellular recordings during superfusion of oxytocin (1 μM, 2 min; AnaSpec Inc.) or blue light stimulation (at least 10 mW, 15 ms light pulses, 20 Hz) were performed as described below. We measured the firing rate from 10 min to 60 min after optogenetic stimulation of the PVN, because the latency of the increase in firing rate was 5.5 ± 0.9 min (Figure S7).
In vivo extracellular recording
The method used for in vivo extracellular recording from lamina X (GRP) neurons was similar to the established methods of recording from the superficial spinal dorsal horn neurons described previously.84 Briefly, the Oxtr-ChR2-EYFP transgenic male rats were anesthetized with urethane (1.2–1.5 g kg−1, i.p.), and implanted with an optical fiber into the PVN region as described above. Urethane produces a long-lasting steady level of anesthesia, which normally does not require administration of additional doses. A thoracolumbar laminectomy was performed exposing the dura from Th11 to L4 and the animal was then placed in a stereotaxic apparatus. After removing the dura and cutting the arachnoid membrane to make a window large enough to insert a tungsten microelectrode, the surface of spinal cord was irrigated with 95% O2/5% CO2-equilibrated Krebs solution (10–15 mL min−1) containing the following (in mM): 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 11 glucose, and 25 NaHCO3, through glass pipettes at 37 ± 1°C.
We first identified electrophysiologically oxytocin-responsive (GRP) neurons in the spinal cord after the oxytocin superfusion. The identification was based on the fact that the GRP neurons respond to oxytocin. This was followed by optogenetic stimulation of the PVN stimulation to induce activation of PVN oxytocin neurons. Extracellular single-unit recordings were obtained from oxytocin-responsive neurons (lamina X) at a depth of 890–1330 mm from the surface of the spinal cord. These cells were located within the lamina X gray matter just dorsolateral to the central canal as assessed by slices obtained from the same spinal cord level of adult male rats (2–3 month-old). Oxytocin-responsive neuronal firing was acquired with an amplifier (EX1; Dagan corporation, Minneapolis, MN). The data were digitized with an analog-to-digital converter (Digidata 1400A, Molecular Devices, Union City, CA), stored on a personal computer with a data acquisition program (Clampex version 10.2; Molecular Devices), and analyzed with a special software package (Clampfit version 10.2; Molecular Devices, RRID: SCR_011323).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed using KaleidaGraph (4.5.1; Synergy Software, Reading, PA, RRID_ SCR_014980). The plasma oxytocin or vasopressin concentrations, the oxytocin neurophysin+ fiber density, the number of GRP+ neurons in the lumbar spinal cord, the optical density of GRP+ fibers in the lumbosacral spinal cord and the differences of pERK expression ratio between oxytocin-injected males and controls are presented as the mean ± standard error of the mean (SEM) for each animal. Statistical analyses of the plasma oxytocin or vasopressin concentrations, the optical density of oxytocin+ fibers, the differences between males administered intrathecal oxytocin and the controls were assessed using Student’s unpaired t test. Statistical analyses of the number of mounts, intromissions, and ejaculations after oxytocin intrathecal administrations or OTA intrathecal administrations, and spike activity after oxytocin superfusion or optogenetic stimulation were assessed using the student’s paired t test. Statistical analyses of the latencies of mounts, intromissions, ejaculations, after oxytocin intrathecal administrations or OTA intrathecal administrations were assessed using the Wilcoxon signed-rank test. Statistical analyses of the number of GRP neurons and optical density of GRP+ fibers after oxytocin-SAP injections and the differences of pERK expression ratio after sexual behavior were performed using one-way analysis of variance (ANOVA). When significant main effects were found using ANOVA, the post hoc Tukey’s test was performed. All the various analyses in this study were conducted “blind.”
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Neuromedin C (GRP) | AssayPro | 11081-05015, RRID: AB_2571636 |
| Anti-GFP | RockLand | 600-901-215, RRID: AB_1537402 |
| PS60 | ATCC | CRL-1800, RRID: CVCL_G254 |
| Anti-NOS1 (A-11) | Santa Cruz Biotechnology | sc-5302, RRID: AB_626757 |
| Anti-p44/42 MAP kinase (phosphorylated Erk1/2) | Cell Signaling Technology | #9101, RRID: AB_331646 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Oxytocin-saporin | Advanced Targeting Systems | IT-46 |
| Oxytocin | AnaSpec Inc. | 24274 |
| [d(CH2)51, Tyr(Me)2, Thr4, Orn8, Tyr-NH29]-vasotocin | Bachem | H-9405 |
| Experimental Models: Organisms/Strains | ||
| Oxytocin receptor (Oxtr)-channelrhodopsin-2-(ChR2)-enhanced yellow fluorescent protein (EYFP) transgenic rat | This Paper | N/A |
| Oligonucleotides | ||
| Primer: EYFP (5$)-F: CACCATCTTCTTCAAGGACGAC EYFP (5$)-R: ATGATATAGACGTTGTGGCTGTTGT |
This paper | N/A |
| Software and Algorithms | ||
| ImageJ Software | NIH | RRID: SCR_003070 |
| FluoView FV10-ASW | Olympus | RRID: SCR_014215 |
| cellSens Software | Olympus | RRID: SCR_016238 |
| Clampfit | Molecular Devices | RRID: SCR_011323 |
| KaleidaGraph | Synergy Software | RRID: SCR_014980 |
| Other | ||
| Olympus all-in-one Fluorescent microscope FSX100 | Olympus | N/A |
| Olympus FluoView 1000 | Olympus | N/A |
| Teleopt | Bio Research Center | N/A |
| COME-2-SPG2 | Lucir Inc. | N/A |
| Digidata 1400A | Molecular Devices | N/A |
Highlights.
Oxytocin receptors are expressed in spinal ejaculation generator (SEG) neurons
Oxytocin directly activates SEG neurons and influences male sexual function in rats
Release of oxytocin in the lumbar spinal cord is not limited to conventional synapses
Released oxytocin acts by diffusion—a localized volume transmission—in the cord
ACKNOWLEDGMENTS
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) KAKENHI (to H.S.: 24680039, 15K15202, 15H05724, 15KK0257, and 16H06280 [ABiS]; to T.O.: 20K15837) and from the Japan Agency for Medical Research and Development (AMED) (to H.S.: 961149). T.O., K.S., and K.T. are supported by research fellowships of JSPS for young scientists. The contribution by L.J.Y. was supported by P50MH100023 to L.J.Y. and P51OD11132 to Yerkes National Primate Research Center (YNPRC). We thank Akito Otubo for help with the preparation of graphical illustrations.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.cub.2020.09.089.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Donaldson ZR, and Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904. [DOI] [PubMed] [Google Scholar]
- 2.Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, Onaka T, Mogi K, and Kikusui T (2015). Social evolution. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 348, 333–336. [DOI] [PubMed] [Google Scholar]
- 3.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, and Young LJ (2016). Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson ZV, and Young LJ (2017). Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev 76 (Pt A), 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell JA, and Leng G (1998). Sex, parturition and motherhood without oxytocin? J. Endocrinol 157, 343–359. [DOI] [PubMed] [Google Scholar]
- 6.Young LJ, and Wang Z (2004). The neurobiology of pair bonding. Nat. Neurosci 7, 1048–1054. [DOI] [PubMed] [Google Scholar]
- 7.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, Díaz-Cabiale Z, and Agnati LF (2012). On the role of volume transmission and receptor-receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res. 1476, 119–131. [DOI] [PubMed] [Google Scholar]
- 8.Takayanagi Y, Yoshida M, Takashima A, Takanami K, Yoshida S, Nishimori K, Nishijima I, Sakamoto H, Yamagata T, and Onaka T (2017). Activation of supraoptic oxytocin neurons by secretin facilitates social recognition. Biol. Psychiatry 81, 243–251. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig M, and Leng G (2006). Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci 7, 126–136. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, and Leng G (2002). Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature 418, 85–89. [DOI] [PubMed] [Google Scholar]
- 11.Chini B, Verhage M, and Grinevich V (2017). The action radius of oxytocin release in the mammalian CNS: from single vesicles to behavior. Trends Pharmacol. Sci 38, 982–991. [DOI] [PubMed] [Google Scholar]
- 12.Oláh S, Füle M, Komlósi G, Varga C, Báldi R, Barzó P, and Tamás G (2009). Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walum H, and Young LJ (2018). The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci 19, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young LJ, and Barrett CE (2015). Neuroscience. Can oxytocin treat autism? Science 347, 825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeMayo MM, Young LJ, Hickie IB, Song YJC, and Guastella AJ (2019). Circuits for social learning: a unified model and application to autism spectrum disorder. Neurosci. Biobehav. Rev 107, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackerman AE, Lange GM, and Clemens LG (1997). Effects of paraventricular lesions on sex behavior and seminal emission in male rats. Physiol. Behav 63, 49–53. [DOI] [PubMed] [Google Scholar]
- 17.Argiolas A, and Melis MR (2005). Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog. Neurobiol 76, 1–21. [DOI] [PubMed] [Google Scholar]
- 18.Clément P, Peeters M, Bernabé J, Denys P, Alexandre L, and Giuliano F (2008). Brain oxytocin receptors mediate ejaculation elicited by 7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT) in anaesthetized rats. Br. J. Pharmacol 154, 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson LW, and McKellar S (1979). The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J. Comp. Neurol 188, 87–106. [DOI] [PubMed] [Google Scholar]
- 20.Wagner CK, and Clemens LG (1991). Projections of the paraventricular nucleus of the hypothalamus to the sexually dimorphic lumbosacral region of the spinal cord. Brain Res. 539, 254–262. [DOI] [PubMed] [Google Scholar]
- 21.Wagner CK, and Clemens LG (1993). Neurophysin-containing pathway from the paraventricular nucleus of the hypothalamus to a sexually dimorphic motor nucleus in lumbar spinal cord. J. Comp. Neurol 336, 106–116. [DOI] [PubMed] [Google Scholar]
- 22.Witt DM, and Insel TR (1994). Increased Fos expression in oxytocin neurons following masculine sexual behavior. J. Neuroendocrinol 6, 13–18. [DOI] [PubMed] [Google Scholar]
- 23.Melis MR, Argiolas A, and Gessa GL (1986). Oxytocin-induced penile erection and yawning: site of action in the brain. Brain Res. 398, 259–265. [DOI] [PubMed] [Google Scholar]
- 24.Burri A, Heinrichs M, Schedlowski M, and Kruger TH (2008). The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology 33, 591–600. [DOI] [PubMed] [Google Scholar]
- 25.IsHak WW, Berman DS, and Peters A (2008). Male anorgasmia treated with oxytocin. J. Sex. Med 5, 1022–1024. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald K, and Feifel D (2012). Dramatic improvement in sexual function induced by intranasal oxytocin. J. Sex. Med 9, 1407–1410. [DOI] [PubMed] [Google Scholar]
- 27.Carro-Juárez M, and Rodríguez-Manzo G (2008). The spinal pattern generator for ejaculation. Brain Res. Brain Res. Rev 58, 106–120. [DOI] [PubMed] [Google Scholar]
- 28.McKenna KE, Chung SK, and McVary KT (1991). A model for the study of sexual function in anesthetized male and female rats. Am. J. Physiol 261, R1276–R1285. [DOI] [PubMed] [Google Scholar]
- 29.Sachs BD, and Garinello LD (1979). Spinal pacemaker controlling sexual reflexes in male rats. Brain Res. 171, 152–156. [DOI] [PubMed] [Google Scholar]
- 30.Carro-Juárez M, Cruz SL, and Rodríguez-Manzo G (2003). Evidence for the involvement of a spinal pattern generator in the control of the genital motor pattern of ejaculation. Brain Res. 975, 222–228. [DOI] [PubMed] [Google Scholar]
- 31.Truitt WA, and Coolen LM (2002). Identification of a potential ejaculation generator in the spinal cord. Science 297, 1566–1569. [DOI] [PubMed] [Google Scholar]
- 32.Truitt WA, Shipley MT, Veening JG, and Coolen LM (2003). Activation of a subset of lumbar spinothalamic neurons after copulatory behavior in male but not female rats. J. Neurosci 23, 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coolen LM (2005). Neural control of ejaculation. J. Comp. Neurol 493, 39–45. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto H (2012). Brain-spinal cord neural circuits controlling male sexual function and behavior. Neurosci. Res 72, 103–116. [DOI] [PubMed] [Google Scholar]
- 35.Newton BW (1992). A sexually dimorphic population of galanin-like neurons in the rat lumbar spinal cord: functional implications. Neurosci. Lett 137, 119–122. [DOI] [PubMed] [Google Scholar]
- 36.Ju G, Melander T, Ceccatelli S, Hökfelt T, and Frey P (1987). Immunohistochemical evidence for a spinothalamic pathway co-containing cholecystokinin- and galanin-like immunoreactivities in the rat. Neuroscience 20, 439–456. [DOI] [PubMed] [Google Scholar]
- 37.Phan DC, and Newton BW (1999). Cholecystokinin-8-like immunoreactivity is sexually dimorphic in a midline population of rat lumbar neurons. Neurosci. Lett 276, 165–168. [DOI] [PubMed] [Google Scholar]
- 38.Nicholas AP, Zhang X, and Hökfelt T (1999). An immunohistochemical investigation of the opioid cell column in lamina X of the male rat lumbosacral spinal cord. Neurosci. Lett 270, 9–12. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto H, Matsuda K, Zuloaga DG, Hongu H, Wada E, Wada K, Jordan CL, Breedlove SM, and Kawata M (2008). Sexually dimorphic gastrin releasing peptide system in the spinal cord controls male reproductive functions. Nat. Neurosci 11, 634–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakamoto H (2014). Sexually dimorphic nuclei in the spinal cord control male sexual functions. Front. Neurosci 8, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakamoto H, Takanami K, Zuloaga DG, Matsuda K, Jordan CL, Breedlove SM, and Kawata M (2009). Androgen regulates the sexually dimorphic gastrin-releasing peptide system in the lumbar spinal cord that mediates male sexual function. Endocrinology 150, 3672–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, and Chen ZF (2009). Cellular basis of itch sensation. Science 325, 1531–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan C, Nadelhaft I, and de Groat WC (1981). The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J. Comp. Neurol 201, 415–440. [DOI] [PubMed] [Google Scholar]
- 44.Takanami K, Sakamoto H, Matsuda KI, Satoh K, Tanida T, Yamada S, Inoue K, Oti T, Sakamoto T, and Kawata M (2014). Distribution of gastrin-releasing peptide in the rat trigeminal and spinal somatosensory systems. J. Comp. Neurol 522, 1858–1873. [DOI] [PubMed] [Google Scholar]
- 45.Sun YG, and Chen ZF (2007). A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, and Tohyama M (1993). Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology 133, 1239–1246. [DOI] [PubMed] [Google Scholar]
- 47.Castel M, Morris JF, Whitnall MH, and Sivan N (1986). Improved visualization of the immunoreactive hypothalamo-neurohypophysial system by use of immuno-gold techniques. Cell Tissue Res. 243, 193–204. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Ward AR, and Morris JF (1995). Oestradiol acutely stimulates exocytosis of oxytocin and vasopressin from dendrites and somata of hypothalamic magnocellular neurons. Neuroscience 68, 1179–1188. [DOI] [PubMed] [Google Scholar]
- 49.Tang Y, Rampin O, Giuliano F, and Ugolini G (1999). Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J. Comp. Neurol 414, 167–192. [PubMed] [Google Scholar]
- 50.Dobberfuhl AD, Oti T, Sakamoto H, and Marson L (2014). Identification of CNS neurons innervating the levator ani and ventral bulbospongiosus muscles in male rats. J. Sex. Med 11, 664–677. [DOI] [PubMed] [Google Scholar]
- 51.Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, and Agnati LF (1998). The emergence of the volume transmission concept. Brain Res. Brain Res. Rev 26, 136–147. [DOI] [PubMed] [Google Scholar]
- 52.Higashida H (2016). Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J. Physiol. Sci 66, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freund-Mercier MJ, Stoeckel ME, and Klein MJ (1994). Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J. Physiol 480, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swanson LW, and Kuypers HG (1980). The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol 194, 555–570. [DOI] [PubMed] [Google Scholar]
- 55.Meister B, Villar MJ, Ceccatelli S, and Hökfelt T (1990). Localization of chemical messengers in magnocellular neurons of the hypothalamic supraoptic and paraventricular nuclei: an immunohistochemical study using experimental manipulations. Neuroscience 37, 603–633. [DOI] [PubMed] [Google Scholar]
- 56.Wegrzyn JL, Bark SJ, Funkelstein L, Mosier C, Yap A, Kazemi-Esfarjani P, La Spada AR, Sigurdson C, O’Connor DT, and Hook V (2010). Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J. Proteome Res 9, 5002–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meeker RB, Swanson DJ, Greenwood RS, and Hayward JN (1991). Ultrastructural distribution of glutamate immunoreactivity within neurosecretory endings and pituicytes of the rat neurohypophysis. Brain Res. 564, 181–193. [DOI] [PubMed] [Google Scholar]
- 58.Mens WB, Witter A, and van Wimersma Greidanus TB (1983). Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 262, 143–149. [DOI] [PubMed] [Google Scholar]
- 59.Fisher TE, and Bourque CW (2001). The function of Ca(2+) channel subtypes in exocytotic secretion: new perspectives from synaptic and non-synaptic release. Prog. Biophys. Mol. Biol 77, 269–303. [DOI] [PubMed] [Google Scholar]
- 60.Morris JF (1976). Distribution of neurosecretory granules among the anatomical compartments of the neurosecretory processes of the pituitary gland: a quantitative ultrastructural approach to hormone storage in the neural lobe. J. Endocrinol 68, 225–234. [DOI] [PubMed] [Google Scholar]
- 61.Ludwig M, Callahan MF, and Morris M (1995). Effects of tetrodotoxin on osmotically stimulated central and peripheral vasopressin and oxytocin release. Neuroendocrinology 62, 619–627. [DOI] [PubMed] [Google Scholar]
- 62.Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, et al. (2007). CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45. [DOI] [PubMed] [Google Scholar]
- 63.Westfall JA, Yamataka S, and Enos PD (1971). Ultrastructural evidence of polarized synapses in the nerve net of Hydra. J. Cell Biol 51, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leng G, and Ludwig M (2016). Intranasal oxytocin: myths and delusions. Biol. Psychiatry 79, 243–250. [DOI] [PubMed] [Google Scholar]
- 65.Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, and Froemke RC (2016). A distributed network for social cognition enriched for oxytocin receptors. J. Neurosci 36, 2517–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes AM, Everitt BJ, Lightman SL, and Todd K (1987). Oxytocin in the central nervous system and sexual behaviour in male rats. Brain Res. 414, 133–137. [DOI] [PubMed] [Google Scholar]
- 67.Murphy MR, Seckl JR, Burton S, Checkley SA, and Lightman SL (1987). Changes in oxytocin and vasopressin secretion during sexual activity in men. J. Clin. Endocrinol. Metab 65, 738–741. [DOI] [PubMed] [Google Scholar]
- 68.Morris JF (2020). Neuroscretory vesicles: structure, distribution, release and breakdown In Neurosecretion: Scretory Mechanisms, Masterclass in Neuroendocrinology, Volume 8, Lemons JR, and Dayanithi G, eds. (Springer Nature Switzerland AG; ), pp. 81–102. [Google Scholar]
- 69.Herde MK, Iremonger KJ, Constantin S, and Herbison AE (2013). GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J. Neurosci 33, 12689–12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caraty A, Delaleu B, Chesneau D, and Fabre-Nys C (2002). Sequential role of e2 and GnRH for the expression of estrous behavior in ewes. Endocrinology 143, 139–145. [DOI] [PubMed] [Google Scholar]
- 71.Hull EM, Wood RI, and McKenna KE (2006). Neurobiology of male sexual behavior In Knobil and Neill’s Physiology of Reproduction, Third Edition, Neill JD, ed. (Elsevier), pp. 1729–1824. [Google Scholar]
- 72.Sirinathsinghji DJ (1983). GnRH in the spinal subarachnoid space potentiates lordosis behavior in the female rat. Physiol. Behav 31, 717–723. [DOI] [PubMed] [Google Scholar]
- 73.Sirinathsinghji DJ (1987). Inhibitory influence of corticotropin releasing factor on components of sexual behaviour in the male rat. Brain Res. 407, 185–190. [DOI] [PubMed] [Google Scholar]
- 74.Westberry J, and Meredith M (2003). The influence of chemosensory input and gonadotropin releasing hormone on mating behavior circuits in male hamsters. Brain Res. 974, 1–16. [DOI] [PubMed] [Google Scholar]
- 75.Abe K, Hazama M, Katoh H, Yamamura K, and Suzuki M (2004). Establishment of an efficient BAC transgenesis protocol and its application to functional characterization of the mouse Brachyury locus. Exp. Anim 53, 311–320. [DOI] [PubMed] [Google Scholar]
- 76.Satoh K, Oti T, Katoh A, Ueta Y, Morris JF, Sakamoto T, and Sakamoto H (2015). In vivo processing and release into the circulation of GFP fusion protein in arginine vasopressin enhanced GFP transgenic rats: response to osmotic stimulation. FEBS J. 282, 2488–2499. [DOI] [PubMed] [Google Scholar]
- 77.Staudt MD, de Oliveira CV, Lehman MN, McKenna KE, and Coolen LM (2010). Activation of MAP kinase in lumbar spinothalamic cells is required for ejaculation. J. Sex. Med 7, 2445–2457. [DOI] [PubMed] [Google Scholar]
- 78.Pow DV, and Morris JF (1989). Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience 32, 435–439. [DOI] [PubMed] [Google Scholar]
- 79.Katayama N, Oti T, Takanami K, Sakamoto T, and Sakamoto H (2016). Postnatal development of the gastrin-releasing peptide system in the lumbosacral spinal cord controlling male reproductive function in rats. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci 92, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oti T, Takanami K, Ito S, Ueda T, Matsuda KI, Kawata M, Soh J, Ukimura O, Sakamoto T, and Sakamoto H (2018). Effects of sex steroids on the spinal gastrin-releasing peptide system controlling male sexual function in rats. Endocrinology 159, 1886–1896. [DOI] [PubMed] [Google Scholar]
- 81.Oti T, Takanami K, Katayama N, Edey T, Satoh K, Sakamoto T, and Sakamoto H (2016). Perinatal testosterone exposure is critical for the development of the male-specific sexually dimorphic gastrin-releasing peptide system in the lumbosacral spinal cord that mediates erection and ejaculation. Biol. Sex Differ 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castel M, Morris J, and Belenky M (1996). Non-synaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. Neuroreport 7, 543–547. [DOI] [PubMed] [Google Scholar]
- 83.Sakamoto H, Arii T, and Kawata M (2010). High-voltage electron microscopy reveals direct synaptic inputs from a spinal gastrin-releasing peptide system to neurons of the spinal nucleus of bulbocavernosus. Endocrinology 151, 417–421. [DOI] [PubMed] [Google Scholar]
- 84.Andoh T, Uta D, Kato M, Toume K, Komatsu K, and Kuraishi Y (2017). Prophylactic administration of aucubin inhibits paclitaxel-induced mechanical allodynia via the inhibition of endoplasmic reticulum stress in peripheral Schwann cells. Biol. Pharm. Bull 40, 473–478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all datasets analyzed during this study.


