Abstract
Human laboratory studies play an important role in alcohol use disorder (AUD) medication development. Medications that are found to be safe and effective during human laboratory screening will then move to more expensive clinical trials in patient populations. Given the gatekeeping role of human laboratory studies in the medication development pipeline, it is critical that these studies accurately forecast how pharmacotherapies will perform under true-to-life clinical conditions. On the other hand, the design of these studies also must adhere to ethical guidelines: certain aspects of clinical reality cannot be incorporated into screening studies because doing so might place the participant at risk for harm or breach other ethical guidelines. Conventions exist that guide the resolution of these conflicting ideals. This article considers the practice of recruiting nontreatment-seeking heavy drinkers to participate in laboratory screening studies. By convention, volunteers are excluded from laboratory screening studies that involve alcohol administration if they are deemed “treatment seeking,” meaning that they recently stopped drinking or are motivated to do so. Although this common practice may reduce risk to participants, findings may not accurately predict medication effects on treatment seekers. Indeed, there is empirical evidence that treatment seekers differ from non-treatment seekers in their responses to medications (Ray et al., 2017; Mason et al., 2006). Here, we argue for the importance of recruiting treatment seekers for this research due to their qualitative difference from nontreatment seekers. We argue that these individuals should be the default population in human laboratory medication screening studies. We conclude by discussing 2 case examples of medication experiments led by our research groups that involved administering medications to treatment seekers.
Keywords: Alcohol administration, pharmacotherapy development, human laboratory, alcohol use disorder, research ethics
Human alcohol administration paradigms play a critical role in alcohol use disorder (AUD) treatment development research. Alcohol administration studies often provide “first-in-human” proof of concept that a novel medication or behavioral intervention is safe when co-administered with alcohol and can reduce alcohol intake (McKee et al., 2009, Wiers et al., 2010). In these studies, participants are typically treated with an intervention and complete experimental procedures that induce craving or allow participants to consume alcohol or examine responses to a standard alcohol challenge. Because behavior is directly observed in a closely controlled environment, laboratory paradigms produce precise estimates of a pharmacotherapy’s potential effect on drinking behavior. In practice, these human laboratory screening studies yield a go/no-go decision on novel therapeutic candidates: medications that reduce alcohol seeking, use and/or responses in the human laboratory will likely go on to be tested in resource-intensive phase 2 and 3 clinical trials, while medications that do not reduce alcohol use or responses will not (Litten et al., 2016). It is therefore critically important that these studies provide an accurate prediction of a medication’s clinical potential. This is particularly significant given the high cost of medication development and the very low rates of success in the trajectory of medication development processes (Litten et al., 2016).
Although it is not explicitly prohibited by the National Institutes of Health, the culture of alcohol research in the United States is such that investigators are discouraged from recruiting treatment-motivated people with AUD to participate in research involving alcohol administration. People who are likely to seek treatment for their alcohol use, described as treatment seekers1, are typically excluded from participating in studies in which alcohol exposure is expected. Rather, participants in these studies are almost uniformly nontreatment-seeking (e.g., Ray et al., 2017a, Covault et al., 2014, Childs et al., 2012, Verplaetse et al., 2016, Leggio et al., 2013, Vatsalya et al., 2015), as evidenced by a recent review of human laboratory studies in the AUD pharmacotherapy development area (Yardley and Ray, 2017). In many cases, only participants who have engaged in recent patterns of heavy drinking are recruited; those who meet criteria for AUD and/or express a desire to seek treatment or reduce their alcohol use are screened out (McKee et al., 2009, Covault et al., 2014).
Here, we argue for a shift in the currently accepted norms of human laboratory research. Our position is motivated by our belief that medication screening studies are most valid when they are designed to mimic how a medication might be used in the clinic. As such, it is important that participants in these studies are drawn from the same population as individuals who will eventually be treated with the medication. We are not the first group to make this argument. Just over a decade ago, a group of leading alcohol researchers made similar arguments in a position piece published in Alcoholism: Clinical & Experimental Research (ACER: Enoch et al., 2009). Though these researchers made a strong case that administering alcohol to treatment seekers in these studies could have major benefits to society, they abstained from making concrete recommendations noting a lack of adequate data to quantify risk to such participants. Our intention is to provide an update regarding their original arguments supported with new data, and to reconsider this issue in light of changes in the field that have occurred over the past decade.
What is the cost of systematically excluding motivated treatment seekers from participating in AUD medication screening studies? Excluding these volunteers may produce a biased sample that is not representative of patients that will be treated with the medication in subsequent clinical trials. Candidate medications may work differently in treatment seekers compared to non-treatment seekers. These differences matter: there are many documented patient characteristics and baseline variables that moderate responses to alcohol use pharmacotherapies (Garbutt et al., 2014; Ray et al., 2017b). A promising medication that reduces or eliminates drinking among non-treatment seeking heavy drinkers may have no effect on drinking patterns in treatment-motivated patients with severe AUD. Indeed, this type of discrepancy between laboratory screening studies and clinical trials may explain why some medications that show promise in human laboratory studies (e.g., mecamylamine; Blomqvist et al., 2002, Chi and de Wit, 2003) and go on to fail during phase 2 and 3 clinical trials (Petrakis et al., 2018). Perhaps more concerning, there is no way of knowing whether potential pharmacotherapies that are screened out by negative human laboratory results might have been highly effective in the clinic. It is therefore critical to maximize consilience between laboratory studies and clinical trials.
Our viewpoint here is consistent with a recent perspective paper on the broader state of translational research in AUD (Ray et al., 2020). Among other recommendations for improving consilience between laboratory and clinical research, this group of researchers advocated for recruiting treatment seekers at earlier stages of the medication development pipeline. Like these authors, we see including treatment seekers in laboratory alcohol administration as a key step in service of this broader goal.
It is nonetheless the current standard of practice to recruit non-treatment seekers to participate in these studies, primarily due to concern that alcohol administration procedures may expose treatment seekers to undue risk of harm (i.e., relapse). Is this concern warranted? As with any ethical problem, it is important to consider the risk/benefit ratio of the decision, as this is a basis of research ethics (Emanuel et al., 2000). In the following sections, we will argue that alcohol administration in pharmacotherapy screening exposes treatment seekers to a justifiable level of risk, and that the potential benefits to these participants and society at large warrant this risk. We conclude by making practical suggestions based on our own experience conducting pharmacotherapy screening studies involving alcohol administration, focusing on strategies that we used to minimize risk of harm to participating treatment seekers.
Ethical Guidelines
The potential risk of a protocol must be weighed against the benefit to the individual and society. Research ethics are guided by a set of principles that establish a framework for how these risk/benefits should be considered. These principles often conflict with one another, and these conflicts need to be navigated in the service of maximizing the favorability of the risk/benefit ratio. Moeller and Stoops (2015) provide a thorough examination of these principles as they relate to experimental administration of drugs of abuse; we will concisely summarize here and describe how these principles apply to alcohol administration in research settings in practice.
The first principle, respect for persons, requires that participants provide informed consent for participation in the research. Some bioethicists have argued that addiction compromises autonomy such that addicted individuals cannot truly consent to experimental drug administration (Cohen, 2002), because being offered alcohol may pose undue influence over decision-making in people with AUD (although readily available alcohol is a daily reality for most people with AUD, irrespective of their choice to participate in alcohol research). While it is true that addiction influences decision making in affect individuals, there is ample evidence that people with addictions can modify their drug use behavior consistent with higher-order goals, suggesting autonomy over their use (see Foddy and Savulescu, 2006). If this premise is accepted, then individuals with AUD have the capacity to decide whether to participate in alcohol administration research and therefore ought to be allowed to make that choice. Further, if addicted individuals were deemed incapable of providing informed consent, then all alcohol administration research involving participants with AUD, including non-treatment seekers, would be prohibited on ethical grounds.
The second major ethical principle, beneficence, states that researchers protect participants from exposure to unnecessary harm. Experimental alcohol exposure may harm treatment seekers by delaying abstinence or precipitating relapse among those who are already abstinent. This concern is predicated on the idea that a single alcohol exposure can prompt a full relapse to heavy drinking via the abstinence violation effect (Marlatt and Donovan, 2005) or alcohol priming (Ludwig et al., 1974). Relatively few studies have followed-up with participants in alcohol administration research to measure subsequent changes in alcohol use. Data addressing this issue in drinkers with AUD are relatively sparse. Available data suggests that laboratory alcohol administration does not increase, and may even decrease, subsequent naturalistic drinking (Drobes and Anton, 2000, Pratt and Davidson, 2005), although these studies were conducted with non-treatment seekers who were not abstinent from alcohol when participating in the research and brief interventions were conducted after the alcohol administration phases of the experiments. More recently, Sommer et al (2015) tracked naturalistic alcohol intake in two groups of young adult social drinkers 6 weeks before and after they participated in intravenous alcohol self-administration studies. In both samples, changes in drinking amount during the poststudy were negligible, suggesting that participating in the research did not increase alcohol consumption rates. Bacio et al (2014) examined alcohol use over a 1-month period following participation in an alcohol challenge experiment in a group of alcohol dependent non-treatment seekers. This protocol also included a motivational interviewing session following the challenge dose. Participants in this study reported a reduction in alcohol use from their pre- to post-challenge assessments, highlighting the utility of a brief intervention for offsetting any risk posed by alcohol exposure in the laboratory. This pattern of results mirrors findings from other drug classes (Kalapatapu et al., 2012, Elman et al., 2001, Vadhan et al., 2006).
Recent data from treatment seekers are sparser, perhaps due to the introduction of the current National Institute on Alcohol Abuse and Alcoholism (NIAAA) alcohol administration guidelines in 2004 (NIAAA, 2004). Kranzler et al (1990) administered an ethanol challenge to a group of alcoholics and measured their subsequent compliance with disulfiram treatment. These researchers found that the ethanol exposure group did not differ significantly in the number of days taking medication, a primary treatment outcome measure for disulfiram, compared to another group of inpatients who did not receive ethanol. Modell et al. (1993) similarly reported that receiving alcohol in a research context did not worsen outcomes in individuals with severe alcohol problems, although this study did not report outcomes quantitatively. Other studies conducted during this time period reach similar conclusions (Faillace et al., 1972, Løsberg et al., 1988) and are discussed in greater depth in previous reviews (Dolinsky and Babor, 1997, Enoch et al., 2009). However, research in another drug class support the notion that drug exposure during treatment does not necessarily translate to poorer outcomes. Roux et al (2012) tracked opioid use outcomes in groups of inpatients being treated for opioid use disorder in a clinical research setting. These participants underwent drug/money choice procedures or received challenge doses of opioid agonist medications. The researchers tracked their heroin use following release from the program, and encouragingly, self-reported heroin use significantly dropped at follow-up compared to their own pre-treatment baseline.
Available data continue to suggest that brief alcohol exposure in a research context does not promote negative outcomes; however, more research is still needed to confirm the safety of such procedures, particularly in treatment seekers. It is unclear how much a single alcohol exposure places a treatment seeker at risk for continued or increased drinking, although, as we discuss later, it is becoming more accepted that reduction in drinking levels may not be incompatible with a positive treatment outcome (Falk et al., 2019). An important research goal will be to evaluate rates of continued or increased drinking among treatment seekers following laboratory alcohol exposure so that this potential source of harm can be more carefully managed. However, as we discuss in the following section, we believe that this risk can be sufficiently managed by thoughtful research design that incorporates safeguards to protect participants from excessive risk.
As a brief aside, it is worth considering shifting viewpoints on the importance of achieving complete abstinence from alcohol as a treatment goal. It is becoming increasingly accepted that successful treatment outcome may be defined as something other than complete abstinence. A driving force in this reconsideration is the NIAAA Active Clinical Trials Initiative Workgroup (ACTIVE). Reanalysis of large-scale clinical trials and epidemiological samples find that people with AUD who reduce their drinking without achieving total abstinence show fewer alcohol use consequences, improved mental health, and reduced likelihood of meeting alcohol use disorder criteria (Witkiewitz et al., 2017, Knox et al., 2019, Hasin et al., 2017). A similar reanalysis found support for using drinking risk level reductions, as defined by World Health Organization (WHO) guidelines, to detect medication effects in clinical trials (Falk et al., 2019). This work has supported a long-existing view in the field that reduction of harmful alcohol use is a valid treatment goal (Sobell and Sobell, 1995). If less gravity is ascribed to continued drinking (albeit at non-harmful levels), then the risk of providing alcohol to a treatment seeker in an experimental context is also reduced.
The final principle is justice. This principle requires that the risks and benefits of the research must be equitably distributed among those who stand to benefit from the research. Categorical exclusion of a group of alcohol users, such as treatment seekers, from research participation should be avoided to satisfy the justice principle. Further, treatment seekers as a group are most likely to benefit from the knowledge gained through pharmacotherapy screening research, because these individuals are more likely to receive the medications during their regular treatment. Recruiting treatment seekers is therefore largely consistent with the justice principle.
The NIAAA provides guidelines on human alcohol administration based on expert interpretation of these ethical principles (NIAAA, 2004). The guidelines state that, “preferably, alcohol administration experiments should be conducted in individuals who are not seeking treatment.” That is, if an alcohol administration experiment will be equally valid if participants are or are not seeking treatment, then the guidelines favor using non-treatment seekers due the lower level of risk. This guideline is often interpreted as prohibiting alcohol administration in those seeking treatment; however, the document goes on to state, “In some circumstances alcohol exposure or alcohol cue exposure research may be appropriate in individuals who are seeking or receiving abstinence-oriented treatment. A strong scientific justification for why the question under study cannot be answered reasonably or validly without the subject’s participation and a strongly favorable risk/benefit assessment are both necessary.” In other words, experimental alcohol administration to treatment seekers is permissible per NIAAA guidelines when it is 1) scientifically necessary and 2) the risk/benefit ratio is favorable.
Treatment Seekers’ Participation Will Improve Prediction Accuracy of Human Laboratory Studies
What evidence supports our claim that recruiting treatment seekers to receive alcohol in medication screening studies will improve prediction accuracy? Although these experiments are necessarily approximations of naturalistic drinking, the guiding principle in designing these studies should be to make as them as applicable to the real world as possible (Yardley and Ray, 2017). Fewer modeled elements in these studies should translate to better predictive validity, maximizing consilience between the outcomes of the laboratory screening study and subsequent randomized clinical trials. Yet, the target population in these studies (i.e., treatment seekers with AUD) are often “modeled” as non-treatment seeking heavy drinkers who may meet mild AUD criteria, if meeting at all.
There is ample evidence that treatment seekers with AUD show distinct clinical and demographic characteristics compared to their non-treatment seeking counterparts. Those who seek treatment are more likely to be male, older, more educated, have more comorbid mental health diagnoses compared to the general population with AUD (Dawson, 1996, Green et al., 2002). Other similar studies report differences between treatment seekers/non-seekers on a range of personality and neurocognitive traits, such as impulsivity and general intellectual functioning (Rohn et al., 2017), and alcohol consumption amounts and trajectories (Fein and Landman, 2005). Non-treatment seekers also have different motivations for participation than do those who participate in clinical trials because they wish to change. They often are motivated by financial incentive and may be less concerned with treatment effectiveness, which may harm data quality (Yardley and Ray, 2017, Resnik and McCann, 2015).
Evidence also suggests that these differences between treatment seekers and non-treatment seekers influence medication responses. One study examined treatment motivation to achieve abstinence as a treatment moderator in a clinical trial of acamprosate for AUD (Mason et al., 2006), finding that participants who were motivated to achieve abstinence showed a larger medication effect compared to those who were not. In a direct test of this issue, Ray and colleagues (2017b) compared non-treatment seekers who participated in AUD medication laboratory screening studies to a sample of clinical trial participants from the COMBINE Study. This study also found that these two groups differed on many clinical and demographic variables. Specifically, treatment-seeking participants in the COMBINE Study were older, had more years of education, higher AUD severity, longer AUD duration, and consumed more alcohol compared to the non-treatment seeking human laboratory sample. Importantly, almost all these traits predicted treatment outcomes in the COMBINE Study, suggesting that these differences observed here can influence medication efficacy.
These results offer two conclusions that support the scientific advantage of designing medication screening studies to include treatment seekers: 1) there are clear differences between treatment seekers and non-treatment seekers, and 2) these differences likely affect treatment responses (but see Venegas and Ray [2020] for evidence that treatment and non-treatment seekers may not differ in their responses to alcohol cues in a frequently used medication screening paradigm). It follows that sampling participants in human laboratory studies and subsequent clinical trials from similar populations will produce more consistent results between these two stages of research.
Clinical researchers should consider a medication’s mechanism of action when evaluating the scientific importance of recruiting treatment seekers. Medications that reduce drinking by antagonizing the pleasurable reinforcing effects of alcohol, such as naltrexone (King et al., 1997), will presumably affect alcohol reactivity similarly in both treatment seekers/non-seekers (though this hypothesis has not been tested directly). On the other hand, some medications may target drinking via effects on processes that would not be readily apparent in non-treatment seekers. A medication that reduces drinking by enhancing cognitive control may require that participants are motivated to reduce their drinking to realize any benefit. Even if the medication is highly effective at improving cognitive control, it is unlikely that this medication effect would translate to reduced drinking among non-treatment seekers because they are not actually motivated to drink less. Other medications (e.g., acamprosate; Mason and Heyser, 2010) appear to work by targeting maladaptive neuroadaptations resulting from long-term patterns of severe alcohol abuse characteristic of people with more severe AUD, who are also likely to be engaged in treatment compared to their less severe counterparts (Enoch et al., 2009). These neuroadaptations are being increasingly recognized as central pathophysiological mechanisms maintaining AUD, and as such, important medication targets (Koob and Mason, 2016). The clinical potential of these medications may not be observable in a sample of non-treatment seeking heavy drinkers. In such cases, recruiting treatment seekers would be necessary to fully demonstrate the clinical potential of a medication.
Minimizing Risk: Two Case Examples
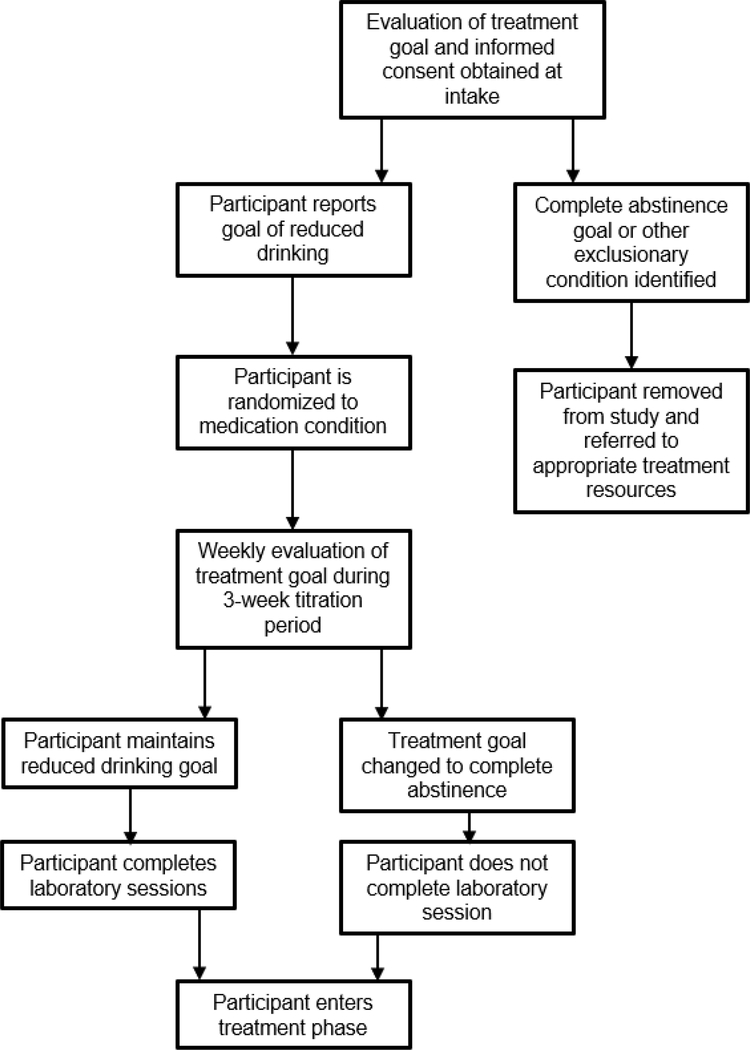
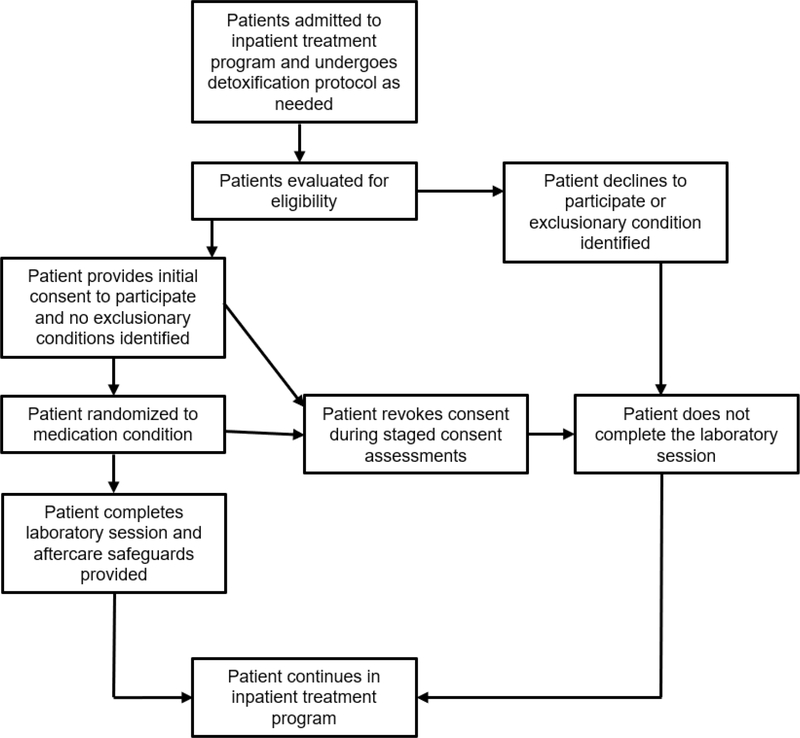
All research involving alcohol administration in humans exposes participants to risk. A shift towards including treatment seekers in alcohol administration studies will require developing a new set of safeguards and experimental procedures to manage the level of risk that these volunteers incur. We conclude this commentary by reviewing 2 case examples of experiments in which we safely administered alcohol to groups of treatment seekers with varying AUD severity. We will describe the methodology of each study and highlight design elements that were used to mitigate risk to participants. Whenever possible, our decisions are guided by the NIAAA guidelines on Administering Alcohol in Human Studies. This list is not meant to be exhaustive or authoritative; rather, we hope to further the conversation by offering strategies that we used in our own work involving alcohol administration in treatment seeking inpatients and outpatients. Flowcharts outlining safeguards that were used in participant recruitment and retention in both case example studies are presented in Figures 1 and 2.
Figure 1.
Participant progress flowchart illustrating protective measures used to mitigate risk to outpatient volunteers in mixed method laboratory study/outpatient clinical trial of extended release guanfacine for alcohol use disorder (NCT: 0376409)
Figure 2.
Participant progress flowchart illustrating protective measures used to mitigate risk to inpatient volunteers in a laboratory study of neural/subjective responses to acute alcohol administration in naltrexone-treated individuals with alcohol use disorder (Spagnolo et al., 2014).
A mixed-method trial of guanfacine for reducing alcohol use in outpatient treatment seekers.
This phase II clinical trial is an ongoing medication study was designed to evaluate the efficacy of extended release guanfacine for treating AUD in men and women. This study, which received IRB approval and is currently in the data collection phase, involves administering alcohol to treatment seekers in the context of a stress-induced drinking paradigm (PI: McKee, ClinicalTrials.gov identifier: NCT03764098). We are using a novel methodology in this trial in which participants who express interest in reducing or stopping their alcohol use complete laboratory alcohol administration sessions and then immediately transition to a 6-week outpatient treatment program. A licensed clinical psychologist (WR) completes a preliminary motivational interview with participants to identify goals and build motivation to reduce alcohol use. The remainder of the treatment phase is modeled after the medication management treatment used in the COMBINE trial (Anton et al., 2006). Medication treatment continues and participants attend weekly medication management with the clinical psychologist.
A primary strategy that we used to manage risk is to be selective in which treatment seekers we recruit. The NIAAA guidelines provide recommendations on this issue. Specifically, the guidelines state that “medical examination and screening to assure the absence of any medical or mental conditions for which alcohol exposure at the dose contemplated would be contraindicated” is necessary. As routine laboratory policy, we screen out participants (treatment seeking or otherwise) with medical characteristics that may contraindicate alcohol administration. Participants are screened for these conditions through medical examination with a study physician as well as extensive history taking during the intake appointment. Likewise, standardized psychiatric screening measures, such as the Structured Clinical Interview for DSM-5 (SCID-5; First et al., 2015), are used to assess for psychopathology that may impact risk of alcohol administration. Of primary concern is any history of severe, life-threatening alcohol withdrawal symptoms (e.g., seizures), as this significantly elevates the risk of alcohol administration to the volunteer. Other potential rule-out conditions are evaluated by the study physician and principal investigator.
We closely assess and monitor participants’ levels of alcohol use before and during their enrollment in the trial. As stated in the NIAAA guidelines, volunteers “who achieved a sustained period of abstinence should not be included as subjects in research involving alcohol administration.” Because this study involves a lower level of treatment involvement (relative to our inpatient example below), we only recruit volunteers who express interest in seeking treatment but have not yet made significant reductions in their drinking or achieved abstinence. This rule-out criterion also excludes volunteers who are already enrolled in AUD treatment programs. We also monitor participants for significant reductions in drinking during the 3-week medication titration period before the alcohol administration sessions. Participants who achieve abstinence or significant reduction during this time bypass the alcohol administration sessions and move directly to the medication maintenance treatment period. We also consider participants’ stated treatment goals at the outset of their participation, and we monitor their goals continuously throughout the trial. Volunteers who report that abstinence is a treatment goal are screened out. We chose to screen out individuals with abstinence goals for an abundance of caution, but we believe a strong case could be made based on our earlier arguments that abstinence-motivated volunteers could possibly be included. During the outpatient clinical trial, a clinical psychologist and other research staff continually assess participants for changes in their treatment goal. Participants who change their goals towards complete abstinence bypass the alcohol administration session and proceed to the outpatient treatment program, consistent with other research groups (Hammarberg et al., 2009). This is particularly important as participants desire to achieve complete abstinence can change rapidly between sessions.
We designed the study to facilitate connecting participants with AUD treatment following the alcohol administration episodes. This issue is addressed specifically in the NIAAA guidelines, which state that “… a serious and concerted effort should be made to link alcohol dependent research subjects who are not in treatment with treatment services. This linkage should be active in bringing together the subject with alcoholism treatment personnel, and not passive as in providing names of treatment programs and phone numbers to the research subject.” We view this step as critically important for reducing risk, so all participants complete the 6-week outpatient treatment program is that built into the protocol. At the end of this treatment period, participants who express interest in continuing treatment receive an active referral to local treatment resources. An additional benefit of this treatment period is that it provides preliminary data on medication effects on real world alcohol use.
Finally, the alcohol administration protocol of the experiment was developed with risk mitigation in mind. All alcohol in this study is self-administered. Participants who decide to abstain from drinking altogether are free to do so. This allows participants to retain control over their choice to drink. The maximum dose of alcohol available to participants is low relative to their typical doses, according to their self-reported drinking over the 90 days preceding intake. The laboratory sessions are conducted on a hospital research unit with medical oversight from physicians and nurses, and participants are kept overnight in the hospital. Conducting these laboratory sessions on an inpatient basis allows us to provide medical and behavioral support in case of adverse responses to alcohol administration. Because the participant has continued weekly contact with a psychologist with expertise in addiction treatment, any concerns regarding the alcohol administration procedures can be addressed and adaptive skill use can be encouraged.
In other protocols that do not involve integrated treatment components, participants could alternatively meet with a psychologist or addiction specialist after the laboratory session in lieu of a formal treatment program. The goal of this meeting could be to 1) process the alcohol exposure as a “teachable moment” in which the treater works with the participant to identify and utilize positive coping skills (Hutchison et al., 2006), and 2) conduct brief motivational enhancement treatment alongside an active referral to an appropriate treatment resource (i.e., “warm hand-off”).
An examination of naltrexone’s effects on neural responses to intravenous alcohol in treatment seeking inpatients.
The inpatient based research protocol was a laboratory examination of the effects of naltrexone on subjective and neural responses to alcohol in treatment-seeking individuals with alcohol dependence (Spagnolo et al., 2014). Participants in this experiment were recruited during inpatient treatment for alcohol dependence at the NIH Clinical Center. The laboratory session was conducted following titration on naltrexone and involved intravenous alcohol administration (approximately 3–4 standard drinks equivalent) and functional magnetic resonance imaging (fMRI) brain scans. After completing the protocol, participants remained inpatient in the alcohol dependence treatment program for at least 3 weeks, and standard retention protocols were used in case patients expressed interest in leaving the treatment program early. Compared to the outpatient experiment, participants in this study were experiencing a higher severity of AUD. However, the experiment was conducted on a completely inpatient basis, so risks to the participant (e.g., untreated medical complications, relapse) was mitigated by continuous medical oversight and close availability of treatment resources.
Volunteers in this study underwent extensive medical and psychological screening beginning with admission to the program. Like the outpatient protocol, participants who showed medical complications or psychiatric conditions for which alcohol administration is contraindicated did not participate. Participants underwent continued psychiatric and medical monitoring, and any changes that may have impacted their fitness to participate in the research were addressed accordingly. This continued evaluation was important, as participants in this study often underwent medically assisted detoxification. Volunteers were not eligible to participate until they showed no registerable BAC and did not require medications to treat active withdrawal symptoms. Any observed or reported history of severe withdrawal symptoms (i.e., seizures) were exclusionary. In recognition of the cognitive and motivational changes that can occur over the course of inpatient treatment, this study utilized an ongoing staged informed consent process, beginning with a signed consent form with numerous follow-up evaluations to ensure that changes in willingness to participate were detected.
Although referral to outpatient treatment was not an explicit part of this protocol, participants were expected to complete the inpatient treatment program. As such, they remained inpatient for at least 3 weeks following their participation. Although the experiment was not a treatment study per se, participants were given the option to continue to receive naltrexone in a treatment capacity after the session finished. Safeguards were used to prevent participants from leaving treatment as a result of alcohol administration. Behavioral health support (e.g., cognitive behavioral skills coaching) was available to participants following the alcohol infusions. Behavioral safeguards to dissuade participants from leaving the program (e.g., asking family/friends not to provide transportation to the patient immediately after the study) were used to minimize risk of participants leaving the treatment program as a result of their participation in the study.
Conclusions
Alcohol administration based human laboratory studies play an important role in screening medications for treating AUD. Despite hesitance to allow treatment seekers with AUD to participate in these research studies, there are clear methodological advantages to doing so. With some adjustments to standard protocols to mitigate risk, the risks to treatment seekers participating in this type of research can be effectively managed, providing better consilience with the ethical principles guiding human research. By including treatment seekers in medication screening research, these studies will produce more accurate estimates of candidate medications’ potential and, when combined with other recently proposed methodological improvements (Ray et al., 2020), allow better consilience with subsequent randomized clinical trials.
Acknowledgments
Support: This work was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants AA026890 (WR), AA025670 (TLV), and with ORWH U54AA027989 (SAM) and P01AA027473 (SAM). VAR is supported by the NIAAA Division of Intramural Clinical and Biological Research (Z1A000466). The opinions expressed in this article are the authors’ own and do not necessarily reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
For the purposes of determining eligibility for enrollment in alcohol administration studies, treatment seekers are operationally defined as people who report high motivation to stop or reduce their alcohol use in the near future. Seeking treatment services is one behavioral indicator of this state, but volunteers who report current intentions to reduce or stop drinking without the benefit of formal treatment would also be considered “treatment seeking.”
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017. [DOI] [PubMed] [Google Scholar]
- Bacio GA, Lunny KF, Webb JN, Ray LA (2014) Alcohol use following an alcohol challenge and a brief intervention among alcohol-dependent individuals. Am J Addict 23:96–101. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR (2002) Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcoho Clin Exp Res 26:326–331. [PubMed] [Google Scholar]
- Chi H, de Wit H (2003) Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res 27:780–786. [DOI] [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC, de Wit H (2012) Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res 36:906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PJ (2002) Untreated addiction imposes an ethical bar to recruiting addicts for non-therapeutic studies of addictive drugs. J Law Med Ethics 30:73–81. [DOI] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R, Arias AJ, Oncken C, Kranzler HR (2014) Dutasteride reduces alcohol’s sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology (Berl) 231:3609–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA (1996) Gender differences in the probability of alcohol treatment. J Subst Abuse 8:211–225. [DOI] [PubMed] [Google Scholar]
- Dolinsky ZS, Babor TF (1997) Ethical, scientific and clinical issues in ethanol administration research involving alcoholics as human subjects. Addiction 92:1087–1097. [PubMed] [Google Scholar]
- Drobes DJ, Anton RF (2000) Drinking in alcoholics following an alcohol challenge research protocol. J Stud Alcohol 61:220–224. [DOI] [PubMed] [Google Scholar]
- Elman I, Krause S, Karlsgodt K, Schoenfeld DA, Gollub RL, Breiter HC, Gastfriend DR (2001) Clinical outcomes following cocaine infusion in nontreatment-seeking individuals with cocaine dependence. Biol Psychiatry 49:553–555. [DOI] [PubMed] [Google Scholar]
- Emanuel EJ, Wendler D, Grady C (2000) What makes clinical research ethical? JAMA 283:2701–2711. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Johnson K, George DT, Schumann G, Moss HB, Kranzler HR, Goldman D, National Advisory Council on Alcohol A, Alcoholism (2009) Ethical considerations for administering alcohol or alcohol cues to treatment-seeking alcoholics in a research setting: can the benefits to society outweigh the risks to the individual? A commentary in the context of the National Advisory Council on Alcohol Abuse and Alcoholism -- Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation (2005). Alcohol Clin Exp Res 33:1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faillace LA, Flamer RN, Imber SD, Ward RF (1972) Giving alcohol to alcoholics: An evaluation. Q J Stud Alcohol 33: 85–90. [PubMed] [Google Scholar]
- Falk DE, O’Malley SS, Witkiewitz K, Anton RF, Litten RZ, Slater M, Kranzler HR, Mann KF, Hasin DS, Johnson B, Meulien D, Ryan M, Fertig J, Alcohol Clinical Trials Initiative Workgroup (2019) Evaluation of drinking risk levels as outcomes in alcohol pharmacotherapy trials: A secondary analysis of 3 randomized clinical trials. JAMA Psychiatry. 76: 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B (2005) Treated and treatment-naive alcoholics come from different populations. Alcohol 35:19–26. [DOI] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, Spitzer R (2015) Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Foddy B, Savulescu J (2006) Addiction and autonomy: can addicted people consent to the prescription of their drug of addiction? Bioethics 20:1–15. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Greenblatt AM, West SL, Morgan LC, Kampov-Polevoy A, Jordan HS, Bobashev GV (2014) Clinical and biological moderators of response to naltrexone in alcohol dependence: a systematic review of the evidence. Addiction 109:1274–1284. [DOI] [PubMed] [Google Scholar]
- Green CA, Polen MR, Dickinson DM, Lynch FL, Bennett MD (2002) Gender differences in predictors of initiation, retention, and completion in an HMO-based substance abuse treatment program. J Subst Abuse Treat 23:285–295. [DOI] [PubMed] [Google Scholar]
- Hammarberg A, Jayaram-Lindstrom N, Beck O, Franck J, Reid MS (2009) The effects of acamprosate on alcohol-cue reactivity and alcohol priming in dependent patients: a randomized controlled trial. Psychopharmacology (Berl) 205:53–62. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, Mann K, O’Malley SS, Scodes J, Robinson RL, Anton R, ACTIVE Workgroup (2017) Changes in non-abstinent WHO drinking risk levels and alcohol dependence: a 3 year follow-up study in the US general population. Lancet Psychiatry 4:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, Swift R (2006) The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology 31: 1310–1317. [DOI] [PubMed] [Google Scholar]
- Kalapatapu RK, Bedi G, Haney M, Evans SM, Rubin E, Foltin RW (2012) Substance use after participation in laboratory studies involving smoked cocaine self-administration. Drug Alcohol Depend 120:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP (1997) Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 129:15–22. [DOI] [PubMed] [Google Scholar]
- Knox J, Scodes J, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, Mann K, O’Malley SS, Anton R, Hasin DS, Alcohol Clinical Trials W (2019) Reduction in non-abstinent WHO drinking risk levels and depression/anxiety disorders: 3-year follow-up results in the US general population. Drug Alcohol Depend 197:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Mason BJ (2016). Existing and future drugs for the treatment of the dark side of addiction. Annu Rev Pharmacol Toxicol 56: 299–322. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Dolinsky Z, Kaplan RF (1990) Giving ethanol to alcoholics in a research setting: its effect on compliance with disulfiram treatment. Br J Addict 85:119–123. [DOI] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR, Addolorato G, Swift RM, Kenna GA (2013) A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Biochem Behav 103:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig JB (2016) Discovery, development, and adoption of medications to treat alcohol use disorder: goals for the phases of medications development. Alcohol Clin Exp Res 40:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løsberg T, Laberg JC, Andresen S, Larsen G (1988) Do drinking experiments have a negative effect on alcoholics’ recovery? Br J Addiction 83:1429–1437. [DOI] [PubMed] [Google Scholar]
- Ludwig AM, Wikler A, Stark LH (1974) The first drink: psychobiological aspects of craving. Arch Gen Psychiatry 30:539–547. [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism (2004). Administering alcohol in human studies. Available at: http://niaaa.nih.gov/Resources/ResearchResoures/job22.htm Accessed November 10, 2018. [Google Scholar]
- Marlatt GA, Donovan DM (2005). Relapse prevention: Maintenance strategies in the treatment of addictive behaviors (2nd ed.). New York: Guilford Press. [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P (2006) Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res 40:383–393. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Heyser CJ (2010). Acamprosate: a prototypic neuromodulator in the treatment of alcohol dependence. CNS Neurol Disord Drug Targets 9: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiat 66:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell JG, Glaser FB, Mountz JM (1993) The ethics and safety of alcohol administration in the experimental setting to individuals who have chronic, severe alcohol problems. Alcohol Alcohol 28:189–197. [PubMed] [Google Scholar]
- Moeller SJ, Stoops WW (2015) Cocaine choice procedures in animals, humans, and treatment-seekers: can we bridge the divide? Pharmacol Biochem Behav 138:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Gueorguieva R, O’Malley SS, Arias A, Sevarino KA, Jane JS, O’Brien E, Krystal JH (2018) Mecamylamine treatment for alcohol dependence: a randomized controlled trial. Addiction 113:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WM, Davidson D (2005) Does participation in an alcohol administration study increase risk for excessive drinking? Alcohol 37:135–141. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, Roche DJ, Heinzerling K, Miotto K (2017a) Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: a randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology 42:1776–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE (2017b) Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse 43:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Grodin EN, Leggio L, Bechtholt AJ, Becker H, Feldstein Ewing SW, Jentsch JD, King AC, Mason BJ, O’Malley SS, MacKillop J, Heilig M, Koob GF (2020). The future of translational research on alcohol use disorder. Addict Biol, e12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik DB, McCann DJ (2015) Deception by research participants. N Engl J Med 373:1192–1193. [DOI] [PubMed] [Google Scholar]
- Rohn MC, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L (2017) Differences between treatment-seeking and nontreatment-seeking alcohol-dependent research participants: an exploratory analysis. Alcohol Clin Exp Res 41:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Tindall C, Fugon L, Murray J, Vosburg SK, Saccone P, Sullivan MA, Manubay JM, Cooper ZD, Jones JD (2012) Impact of in-patient research participation on subsequent heroin use patterns: implications for ethics and public health. Addiction 107:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC (1995) Controlled drinking after 25 years: how important was the great debate? Addiction 90:1149–1177. [PubMed] [Google Scholar]
- Sommer C, Seipt C, Spreer M, Blumke T, Markovic A, Junger E, Plawecki MH, Zimmermann US (2015) Laboratory alcohol self-administration experiments do not increase subsequent real-life drinking in young adult social drinkers. Alcohol Clin Exp Res 39:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo PA, Ramchandani VA, Schwandt ML, Zhang L, Blaine SK, Usala JM, Diamond KA, Phillips MJ, George DT, Momenan R, Heilig M (2014) Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcohol-dependent patients. Alcohol Clin Exp Res 38:3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, Roe B, Colley J, Haney M, Foltin RW (2006) Substance use and psychosocial outcomes following participation in residential laboratory studies of marijuana, methamphetamine and zolpidem. Am J Drug Alcohol Abuse 32:589–597. [DOI] [PubMed] [Google Scholar]
- Vatsalya V, Gowin JL, Schwandt ML, Momenan R, Coe MA, Cooke ME, Hommer DW, Bartlett S, Heilig M, Ramchandani VA (2015) Effects of varenicline on neural correlates of alcohol salience in heavy drinkers. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas A, & Ray LA (2020). Comparing alcohol cue-reactivity in treatment-seekers versus non-treatment-seekers with alcohol use disorder. Am J Drug Alcohol Abuse 46:131–138. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA (2016) Effect of lowering the dose of varenicline on alcohol self-administration in drinkers with alcohol use disorders. J Addict Med 10:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F (2010) Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction 105:279–287. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Hallgren KA, Kranzler HR, Mann KF, Hasin DS, Falk DE, Litten RZ, O’Malley SS, Anton RF (2017) Clinical validation of reduced alcohol consumption after treatment for alcohol dependence using the World Health Organization risk drinking levels. Alcohol Clin Exp Res 41:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Ray LA (2017) Medications development for the treatment of alcohol use disorder: insights into the predictive value of animal and human laboratory models. Addict Biol 22:581–615. [DOI] [PMC free article] [PubMed] [Google Scholar]