Abstract
Background:
The Food and Drug Administration (FDA) is considering setting a nicotine standard for tobacco products to reduce their addictiveness. Such a standard should account for the apparent greater vulnerability to nicotine addiction in some subpopulations, such as adolescents with depression. The present study examined whether the reinforcement threshold and elasticity of demand (i.e., reinforcing efficacy) for nicotine in a genetic inbred rat model of depression (Flinders Sensitive Line [FSL]) differs from an outbred control strain.
Methods:
Acquisition of nicotine self-administration (NSA) across a wide range of nicotine doses was measured in both FSL and Sprague-Dawley (SD) control adolescent rats. At the highest dose, elasticity of demand was also measured. Nicotine pharmacokinetics was examined to determine whether it might modulate NSA, as it does smoking in humans.
Results:
FSL rats acquired self-administration quicker and showed more inelastic demand (greater reinforcing efficacy) than SDs at the highest unit dose. However, there was no strain difference in the reinforcement threshold of nicotine. FSL rats exhibited faster nicotine clearance, larger volume of distribution, and lower plasma and brain nicotine concentrations. However, these differences were not consistently related to strain differences in NSA measures.
Conclusion:
These findings are consistent with studies showing greater dependence and reinforcing efficacy of cigarettes in smokers with depression and those with relatively fast nicotine metabolism. However, these findings also suggest that a nicotine standard to reduce initiation of tobacco use should be similarly effective in both the general adolescent population and those with depression.
Keywords: Nicotine, Rat, Depression Model, Abuse Liability
1. Introduction
The 2009 Family Smoking Prevention and Tobacco Control Act charges the Food and Drug Administration (FDA) to regulate tobacco products in order to protect public health by reducing the prevalence of tobacco use and the toxicity of tobacco products (Hatsukami et al., 2013). Toward this end, the FDA Center for Tobacco Products (CTP) may set a nicotine standard for tobacco products that reduces the nicotine level below the threshold that produces reinforcing effects (i.e., the reinforcement threshold) as means to reduce tobacco use in established smokers and prevent adolescents who experiment with tobacco products from becoming addicted (Sofuoglu and LeSage; FDA, 2018).
A critical step toward setting a nicotine standard is understanding the extent of population variability in the nicotine reinforcement threshold. The CTP must consider data from vulnerable subpopulations whose nicotine reinforcement threshold might be lower than that of the general population, such as adolescents and those with psychiatric comorbidities. Adolescence is generally considered a period of greater vulnerability to substance abuse disorders, including tobacco addiction (Adriani and Laviola, 2004). It is well established that the majority of smokers begin smoking and can quickly become nicotine dependent during adolescence (DiFranza et al., 2002; O’Loughlin et al., 2003). Moreover, a variety of subpopulations of adolescents could exhibit nicotine reinforcement thresholds that differ from the general adolescent population. For example, depression in adolescents predicts subsequent onset of daily smoking and progression to dependence (Breslau and Peterson, 1996; Karp et al., 2006). Individuals with depression are three to four times more likely to develop nicotine dependence during adolescence (e.g., Kendler et al., 1993; Patton et al., 1998) and comprise 20–30% of adult smokers (e.g., Fond et al., 2015; Hebert et al., 2011; Shahab et al., 2014). Compared to non-depressed smokers, currently depressed smokers were twice as likely to choose smoking over other rewarding activities and reported needing a larger amount of an alternative reinforcer to quit smoking (Spring et al., 2003). Depression-prone smokers were also twice as likely as non-prone smokers to work for cigarette puffs versus money under a progressive-ratio schedule (Audrain-McGovern, et al., 2014). Although, these findings suggest that depression increases the reinforcing efficacy of smoking and risk of tobacco addiction, it is not yet clear whether the mechanism mediating this relationship is an increased sensitivity of depressed individuals to the reinforcing potency of nicotine per se (i.e. lower reinforcement threshold). To our knowledge, no dose-response studies have directly examined this issue using smoking or nicotine self-administration (NSA) methods in humans or nonhumans.
Given that it is ethically impossible to experimentally examine how depression moderates the nicotine reinforcement threshold for initiation of smoking in humans, animal models are needed to examine this issue (Donny et al., 2012). There are myriad animal models of depression from which to choose. Although none of these models can fully recapitulate the disorder, the Flinders Sensitive Line (FSL) is a genetic rat model of depression with considerable face, construct, and predictive validity (Overstreet, 2012; Overstreet and Wegener, 2012). FSL rats are hypersensitive to cholinergic agonists and have elevated levels of muscarinic and nicotinic acetylcholine receptors (Overstreet and Djuric, 2001, Tizabi et al., 1999; Tizabi et al., 2000). Given the hyperactivity of cholinergic systems in some depressed individuals (Janowsky et al., 1994; Mineur et al., 2011), the FSL rat is considered a model of this particular subtype of depression. Although people with this subtype of depression may be especially vulnerable to tobacco addiction compared to other subtypes, this has not been studied. FSL rats show many key behaviors resembling depression in humans, including reduced general activity, decreased social interaction, appetite suppression, greater stress-induced anhedonia in the sucrose preference test, and increased REM sleep (Overstreet and Wegener, 2012). All drugs with antidepressant effects in humans also show antidepressant effects in FSL rats (Overstreet and Wegener, 2012). In addition, FSL rats exhibit greater sensitivity to the antidepressant effects of nicotine and locomotor suppressant effects of nicotine withdrawal, as well as higher basal levels of α4β2 nicotinic receptors (a key mediator of nicotine reinforcement) and greater nicotine-induced α4β2 receptor upregulation (Tizabi et al., 1999; Tizabi et al., 2000).
There are several methods to determine the relative reinforcing efficacy of nicotine in rats. Most often researchers use low fixed-ratio (FR) schedules to compare rates of acquisition and/or the amount of responding maintained by intravenous self-administration across a range of doses (Ator and Griffiths, 2003; Banks and Negus, 2012). In rats, adolescents have been shown to acquire NSA quicker and maintain greater nicotine intake than adults (Adriani et al., 2002, 2003; Chen et al., 2007; Kota et al., 2007; Levin et al., 2007, 2003; Natividad et al., 2013; O’Dell et al., 2007), supporting the notion that adolescence is a period of greater vulnerability to nicotine addiction. Behavioral economics (Hursh, 1984) provides an alternative framework to measure the abuse liability of nicotine. In a behavioral economic analysis, drug intake is measured across a range of FR values (e.g., FR 1, 3, 6, 9, 15, etc.) to produce a demand curve. This demand curve allows several abuse liability factors to be collectively assessed, including demand intensity (i.e., the amount of consumption with relatively free access [e.g., an FR 1]) and demand elasticity (i.e., the rate of decrease in drug consumption in response to increasing unit price [FR/unit dose]), among others (Hursh et al., 2013). Of these measures, demand elasticity provides the primary metric of the abuse liability of a drug by quantifying how sensitive drug consumption is to increases in unit price (Hursh et al., 2013; Hursh and Roma, 2016). Demand is considered inelastic (i.e., reinforcing efficacy is greater) if consumption of a drug decreases slowly in proportion to increases in unit price, resulting in smaller α values (Hursh and Silberberg, 2008). To our knowledge, elasticity of demand for nicotine in adolescent animal models of depression has not been reported.
The present study examined acquisition of NSA across a range of nicotine doses (0, 1, 2, 4, 7, 15 & 30 ug/kg) in adolescent FSL and SD rats to determine if the reinforcement threshold for nicotine differs between the strains. Elasticity of demand for nicotine was also compared between strains at the highest unit dose, which is a common training dose for NSA. The primary hypothesis was that FSL rats would have a lower reinforcement threshold and less elastic demand for nicotine compared to SD rats. Another goal was to examine two factors that may moderate differences in these measures between or within strains. First, nicotine pharmacokinetic parameters (e.g., clearance) were measured to determine their association with metrics of abuse liability (i.e., α and NSA acquisition). Human studies show that slower nicotine metabolism and clearance predicts higher CPD and dependence in adolescents (Karp et al., 2006; Rubinstein et al., 2013). In addition, our previous studies show that nicotine clearance is a significant predictor of the nicotine reinforcement threshold and elasticity of demand in adult rats (Grebenstein et al., 2015). Second, males and females were included to allow analysis of sex differences in each strain. Sex differences in smoking, NSA, and comorbid disorders have been reported in humans (Fattore et al., 2008; Perkins, 2009), and some studies show that female adolescents in particular develop early milestones of addiction (e.g., cravings, tolerance, monthly smoking) faster than males, although incidence rates of later milestones (e.g., daily smoking, ICD-10 dependence) may be similar to males (DiFranza et al., 2007; DiFranza et al., 2002; Gervais et al., 2006). Previous studies by our lab and others using adolescent rats have also shown that females exhibit faster acquisition of NSA at lower doses, higher baseline intake, and/or higher breaking points under PR schedules than males (Chen et al., 2007; Grebenstein et al., 2013; Levin et al, 2003; Levin et al., 2003; Lynch, 2009; Sanchez et al., 2013). Regardless of whether sex differences exist, using both sexes is important to model the heterogeneity of participants in human studies that include both men and women, which may be vital to characterizing the full extent of variability in nicotine reinforcement thresholds.
2. Method
2.1. Animals
Male and female adolescent FSL (Duke University) and Sprague-Dawley (SD; Envigo, Madison, WI) rat pups were shipped with their dam to arrive on post-natal day (PND) 14 and were housed with free access to chow and water in a temperature- (22° C) and humidity-controlled colony room. Pups were weaned on PND 21 and individually housed with free access to chow and water until catheter implantation on PND 33. Following catheter implantation, rats were individually housed in an operant chamber and provided free access to water and restricted access to food, beginning at 13 g/day and escalated weekly to 16g and then 18g/day, where the food allotment remained for rest of the protocol. Based on pilot data, this regimen provides an amount of food per gram of body weight comparable to the level of food restriction often used in adult rats. Protocols were approved by the Hennepin Healthcare Research Institute’s Institutional Animal Care and Use Committee and were in accordance with NIH guidelines set forth in the Guide for the Care and Use of Laboratory Animals (National Reserach Council, 2011).
2.2. Apparatus
2.2.1. Self-administration Operant Chambers.
Nicotine self-administration chambers (Med-Associates, St. Albans, VT) were composed of aluminum and clear polycarbonate walls and a stainless-steel grid floor. The chamber had two response levers on the front panel, each with a white stimulus light located directly above, and the back panel contained a house light mounted centrally at the top with a waterspout below. Chambers were contained in sound-attenuating boxes equipped with ventilation fans that provided masking noise. Infusion pumps (Model RHSY, Fluid Metering, Syosset, NY) were connected to tygon tubing that attached to a swivel (Instech Inc., Plymouth Meeting, PA) affixed to a counter-balanced arm centered over the opening in the ceiling of the experimental chamber. Tubing from the swivel ran through a spring leash that attached to a vascular access harness (VAHD115AB, Instech) worn by the rat. A computer (OS: Windows 7®) running MED-PC IV® (Med Associates) orchestrated experimental sessions and recorded data.
2.2.2. Forced Swim Water Tanks.
A clear acrylic cylinder (33 cm diameter × 45 cm height; Med Associates) was used for forced-swim testing. Tests were video recorded and analyzed using ANY-maze (v. 4.99; Stoelting Co., Wood Dale, IL) software.
2.3. Drugs
(−) Nicotine (Sigma Chemical Co., St. Louis, MO) was dissolved in saline to formulate all nicotine doses (1 – 30 ug/kg; doses expressed as the base). The PH of each solution was adjusted to 7.4 with NaOH and then heparin was added (30 units/ml) to aid catheter patency. The concentration of nicotine in each dilution was verified using gas chromatography with nitrogen phosphorous detection using our routine assay (LeSage et al., 2003). Solutions varied by no more that ± 5% (average < 1%) from the target concentration.
2.4. Procedure
2.4.1. Forced Swim Test.
SD (N = 52) and FSL (N = 59) rats underwent forced swim testing on PND 32 to confirm a phenotype difference between strains. Prior to testing, rats were transported in their home-cage to the dimly-lit testing room and allowed 1 hour to acclimate before testing. The forced swim cylinder was filled with 25 °C water and the rat was placed in the testing cylinder to swim for 15 minutes. The water was emptied and the cylinder was cleaned following each test. All forced swim recordings were video-recorded and scored by Any-Maze software, which quantified the total time spent immobile (i.e., >50% body surface area overlap across a rolling 2 second time window) during the force swim test (Lee et al. 2017).
2.4.2. Surgery.
On PND 33, rats were implanted with a chronic indwelling catheter into the right jugular vein according to our standard procedures (LeSage et al., 2003, 2002) under i.m. ketamine (FSL: 20–30 mg/kg; SD: 75–90 mg/kg) and dexmedetomidine (FSL: 0.05 mg/kg; SD: 0.25 mg/kg) anesthesia. Different anesthetic doses were required for each strain because of the greater sensitivity of FSL rats. The catheters exited the body between the scapulae and attached to the vascular-access harness. Immediately following surgery, rats were administered atipamezole (5 mg/kg; s.c.) and extended-release meloxicam (2 mg/kg; s.c.). Rats recovered for four days, during which time they were given daily catheter flushes of heparinized saline (30 units/ml; i.v.) and ceftriaxone (5.25 mg). Infusions of methohexital (50 mg/ml, i.v.) were provided at critical experimental time points (i.e., following FR 1, FR 2, and the end of demand assessment) to confirm catheter patency. If a catheter became occluded or lost patency the rat was removed from study.
2.4.3. Acquisition of self-administration and demand elasticity.
Following surgery, rats were housed in the operant chambers to recover for four days. After recovery, rats were given access to nicotine during daily 23-h sessions (12:12 light dark cycle; lights off at 1000 hours). The start of each session was signaled by the illumination of the house light in the chamber and initiation of a fixed-ratio (FR 1) schedule of nicotine delivery. Under this schedule, a response on the active lever illuminated the stimulus light above the lever and delivered a nicotine infusion (100 μl/kg @ 50 μl/s). Each nicotine delivery was followed by a 7-second post-infusion timeout, wherein responses were recorded but had no programmed consequence. After the timeout, the stimulus light was darkened and nicotine was again available under the FR 1 schedule. A response on the inactive lever was recorded, but had no programmed consequence. Seven groups had access to one of six concentrations of nicotine: 0 [saline] (SD = 13, FSL = 14), 1 (SD = 15, FSL = 13), 2 (SD = 16, FSL = 13), 4 (SD = 12, FSL = 12), 7 (SD = 14, FSL = 11), 15 (SD = 13, FSL = 13), or 30 (SD = 26; FSL = 23) μg/kg/infusion. The higher sample size for the 30 μg/kg group was necessary to ensure that at least eight rats of each sex for each strain acquired NSA and completed demand assessment to allow analysis of sex differences. Ground food was placed on the active lever for the first session only to promote contact with the reinforcement contingency. Following 7 additional sessions, the FR requirement was increased to FR 2 for 7 more sessions. Thus, all rats were given a total of 15 sessions to acquire NSA. Nicotine demand elasticity was then assessed only in those rats that met acquisition criteria at the end of the FR 2 phase (last three sessions, see criteria in Section 2.5.3) in the 30 μg/kg group. During demand testing, the FR requirement was increased daily (per the progression: 3, 6, 9, 15, 30, 60, 120, 240, 480, etc.) until 0 infusions were earned, (see LeSage et al., 2016). Elasticity of demand at the 30 μg/kg unit dose was the primary data of interest because, at this dose, increases in unit price yield both relatively inelastic and elastic phases of consumption, providing a more exponential function for better curve fits and more accurate parameter estimates than occurs at lower unit doses where consumption is more variable and linearly related to unit price.
2.4.4. Assessment of nicotine pharmacokinetics.
The low blood volume in adolescent rats prevented collection of an adequate number and volume of blood samples at the end of the NSA protocol to estimate clearance of an i.v. bolus nicotine dose in each rat. Therefore, steady-state nicotine clearance during continuous nicotine infusion was measured in each rat at the completion of the NSA protocol (approximately PND 65). Nicotine clearance can be estimated equally well with bolus or continuous infusion. Drug clearance is an intrinsic property of each animal for a given drug and is not influenced by whether drug is administered by bolus or continuous infusion. Therefore, clearance during continuous infusion is indicative of clearance following i.v. bolus dosing during NSA. Rats (SD M = 48, F = 47; FSL M = 66, F = 46) were implanted with a subcutaneous osmotic mini pump (Alzet, Cupertino, CA 95014; 2ML2) under isoflurane anesthesia. Pumps provided a continuous infusion of nicotine at a rate of 3 mg/kg/day. Tail-vein blood samples (1 ml each) were taken daily for three days beginning at least 24 hours after pump implantation (data for 1 male SD and 2 female FSL rats from the 30 μg/kg dose group were not collected due to surgical complications). Immediately following the final blood sample, rats were sacrificed to collect brain.
To increase the rigor of the comparison of nicotine pharmacokinetics between strains, the pharmacokinetics of a single i.v. bolus nicotine dose were also examined in separate cohorts of rats that were not used in the NSA protocol. This allowed characterizing the time course of serum nicotine concentrations following a bolus dose as occurs during NSA and confirmation of the strain difference observed during continuous nicotine infusion. For this analysis, rats (SD M = 15, F = 15; FSL M = 14, F = 9) were anesthetized and implanted with a jugular and femoral catheter as described above at PND 65. Immediately following catheter implantation, an intravenous infusion of 0.1 mg/kg of nicotine was administered over 10-s via the jugular catheter. Blood samples (0.5 ml/each) were then taken via the femoral catheter, and each sample was replaced with 1 ml of saline. Due to the limited number of samples (i.e., 3) that could be taken in adolescent rats, two equal groups of each strain were formed to capture a 4-hr time-course with one group having samples at 15, 60 and 120 min and the other having samples at 15, 120 and 240 min (blood nicotine levels did not significantly differ at the 120 min timepoint for either strain). Nicotine levels were measured by gas chromatography with nitrogen-phosphorous detection. Brain nicotine levels were corrected for blood content.
2.5. Data analysis
2.5.1. Forced Swim Test Analysis.
Strain and sex differences in time immobile were quantified for the FST using a two-way (Strain X Sex) analysis of variance (ANOVA). In order to correct for the positive distribution skew observed in SDs, a square-root transform was applied to the data prior to analysis. Post-hoc comparisons were conducted upon significant (p < 0.05) main effects using Bonferroni-corrected t-tests (p < 0.025) for multiple comparisons.
2.5.2. Nicotine reinforcement threshold.
Due to violation of homogeneity of variance, strain differences in the nicotine infusion dose-response curve could not be analyzed by ANOVA. Therefore, the mean number of infusions at each unit nicotine dose was compared to saline via independent-samples t-tests with a Welch’s correction, using a significance level of p<0.0083 for six comparisons in each strain. The nicotine reinforcement threshold was defined as the lowest unit dose that maintained infusion rates significantly higher than saline.
2.5.3. Daily patterns of NSA.
Strain differences in daily patterns of NSA were examined using two methods. The first was to assess daily strain differences in active and inactive lever responding during acquisition using a separate two-way (Strain × Session) ANOVA for each FR phase at each unit dose with a Bonferroni correction applied to the main effect of strain (p > 0.025) at each FR. The second method was to assess the daily percentage of rats that met acquisition criteria across each session of the acquisition phase. Acquisition criteria were 1) an average ratio of 2:1 active to inactive lever responding across the most recent three consecutive sessions starting at session three and 2) the average nicotine infusions earned over the same set of sessions had to be above the 95% confidence interval of the mean of saline controls across the same three sessions. Under this analysis, a higher percentage of rats meeting criteria over time indicates faster acquisition for rats as a group at each unit dose (see Figure 4). To analyze differences in the proportion of rats that met these acquisition criteria under FR 1 and FR 2 at each dose, the sum of the proportions across sessions within each FR were compared between strains using a Chi-square test with a Bonferroni correction for the multiple comparisons across FR value (p > 0.025). Because no sex differences in these measures were observed, data were pooled across sex for these analyses.
2.5.3. Exponential demand quantification.
To quantify demand elasticity across a range of nicotine unit prices at the 30 μg/kg unit dose, exponential demand curves were fit to nicotine consumption in mg/kg at each FR value for both individual subjects and group means using the Hursh and Silberberg (2008) demand equation:
| (1) |
In this equation, Q is the quantity of a commodity consumed (mg/kg of Nic), C is unit-price cost of the commodity (FR/mg/kg Nic), and Q0 and α are free parameters resulting from the best-fit function and refer to maximal consumption at zero price (i.e., demand intensity) and rate of change in consumption across price (i.e., demand elasticity), respectively. The scaling parameter, k, is a constant that is fit globally across groups to normalize consumption. Such normalization allows for comparisons of free parameter estimates (i.e., α and Q0) of individual subjects between the different demand functions. Specifically, differences in demand elasticity were quantified using α, which is inversely related to reinforcing efficacy or essential value and characterizes how rapidly consumption decreases in response to increases in price. Commodities that have larger α values have more elastic demand (i.e., rapid decrease in consumption) and less reinforcing efficacy, whereas those with smaller α values have more inelastic demand (i.e., slower decrease in consumption) and greater reinforcing efficacy. An Excel template (Kaplan and Reed, 2014) was used to calculate Pmax (price at which consumption becomes relatively elastic) and Omax (maximum level of responding) values for each subject using the group fit k (2.16) and the individually fit Q0 and α values. The mean value of individually fit parameter values were employed as the primary analysis to assess strain differences using independent-samples t-tests with a Welch’s correction. Log transforms were used to normalize the distribution of α and Pmax values prior to statistical analysis. Data were pooled across sex because no sex difference was observed in these measures. To provide a complete demand function, 0 consumption at the highest unit price was replaced with 0.01 since 0 is undefined on a log scale and the log of 0.01 (i.e., log 0.01 = −2) is the next lowest log-unit value below the log of 1 infusion (i.e., log 0.03 = −1.52). Additionally, to make group fits of demand functions more representative of individual subjects, 0 infusions (i.e., 0.01) were interpolated for each subject from the point where 0 infusions were earned to the highest unit price achieved by an individual rat. These interpolated data were only used to illustrate group-level demand curves and were not used to determine the individual-subject curve fits or to conduct statistical analyses.
2.5.4. Pharmacokinetic data analysis.
Steady-state clearance during continuous nicotine infusion was calculated as pump delivery rate divided by the mean of the three serum concentrations. Mean clearance values were compared between strains via independent-sample t-test with Welch’s correction. Blood serum concentrations across the bolus-dose time-course were fit with a noncompartmental model using the PK Solver Excel template (Zhang et al., 2010) to derive the maximum plasma concentration (C0), Volume of Distribution (VD), Clearance (CL) and half-life (t50). Strain and sex differences in these parameters were assessed using a two-way ANOVA (p < 0.05). Post-hoc comparisons were conducted upon significant (p < 0.05) main effects using a Sidak correction for multiple comparisons. The derived pharmacokinetic parameters from the two groups sampled at different time points were combined for statistical analysis since, within each strain, there were no significant differences between the different sets of sample time points. Linear regression was used to determine whether pharmacokinetic parameters predicted self-administration measures.
3. Results
3.1. Forced swim test
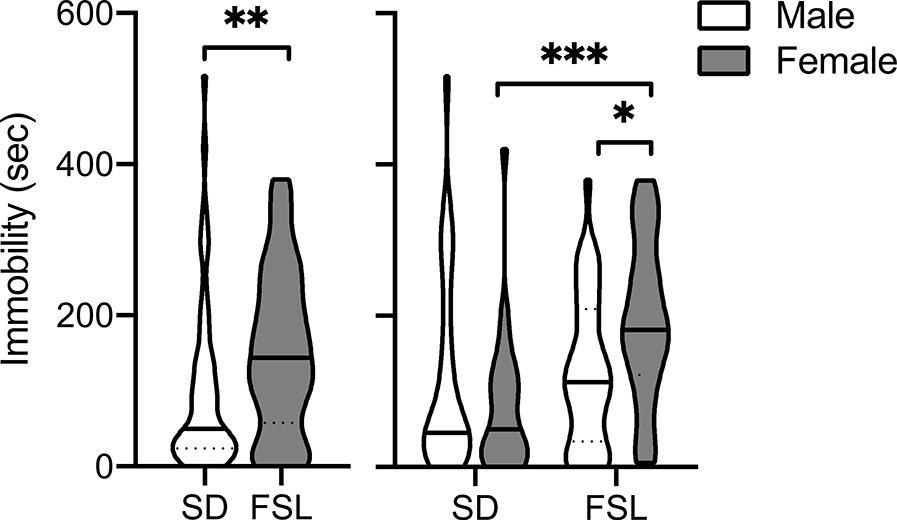
Figure 1 presents the total seconds spent immobile during the 15-min FST in SD (N = 53; male = 26, female = 27) and FSL (N = 62; male = 36, female = 26) rats. There was a significant main effect of Strain (F1, 111 = 8.22, p < 0.01) and a significant Sex X Strain interaction (F1, 111 = 6.73, p < 0.05), but no main effect of sex. Post-hoc tests revealed that female FSLs spent significantly more time immobile compared to female SDs and male FSLs (t111,0 = 3.74, p < 0.001; t111,0 = 2.43, p < 0.05; respectively).
Figure 1.
Violin plots showing the group median (solid line) and interquartile range (dashed lines) of time spent immobile during the 15 min forced swim test in male and female SD and FSL rats (N=52 and 59 for each strain, respectively). The shape of the plot represents the frequency distribution, with the width indicating the frequency of observations at the respective y value. The left panel shows all of the data for each strain. The right panel shows data for each sex in each strain. Significantly different, *p < 0.05, **p < 0.01, ***p < 0.001.
3.2. Reinforcement thresholds and patterns of acquisition
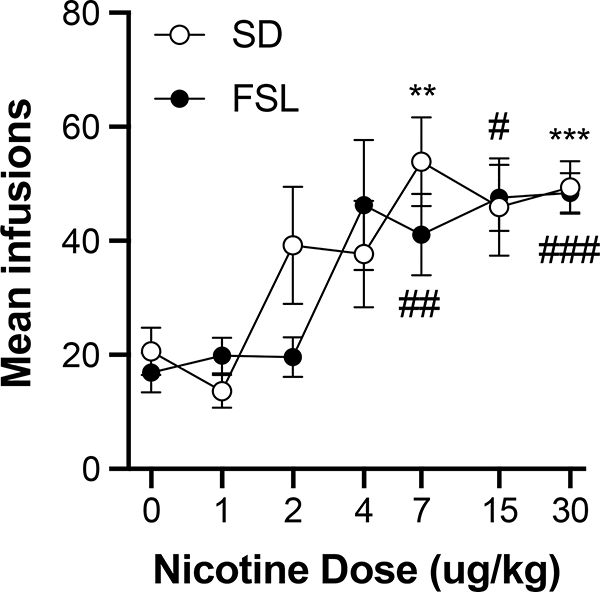
Figure 2 shows the mean number of infusions at each unit dose across the last three sessions at FR 2 in each strain. The dose response curves were similar between strains, with both showing infusion rates increasing with unit dose to maximal rates between 7 and 30 μg/kg. The 7, 15, and 30 μg/kg doses maintained infusions rates significantly above saline in FSL rats (7ug/kg: t19.7 = 3.752, p < 0.01; 15ug/kg: t17.0 = 2.654, p < 0.01; 30ug/kg: t34.5 = 4.619, p < 0.01), while the 7 and 30 μg/kg doses maintained infusion rates significantly above saline in SD rats (7ug/kg: t16.1 = 3.038, p < 0.01; 30ug/kg: t33.1 = 6.432, p < 0.01; the infusion rate at 15 μg/kg approached significance with p = 0.017). Therefore, the nicotine reinforcement threshold was 7 μg/kg for both strains.
Figure 2.
Group mean (± S.E.M.) infusions earned for SD and FSL rats across the final three sessions of FR 2 plotted as a function of dose; see Methods section for sample sizes per strain at each dose. Significantly different from saline in SD rats, **p < 0.01, ***p < 0.001, respectively. Significantly different from saline in FSL rats, ##p < 0.01 ###p < 0.001, respectively.
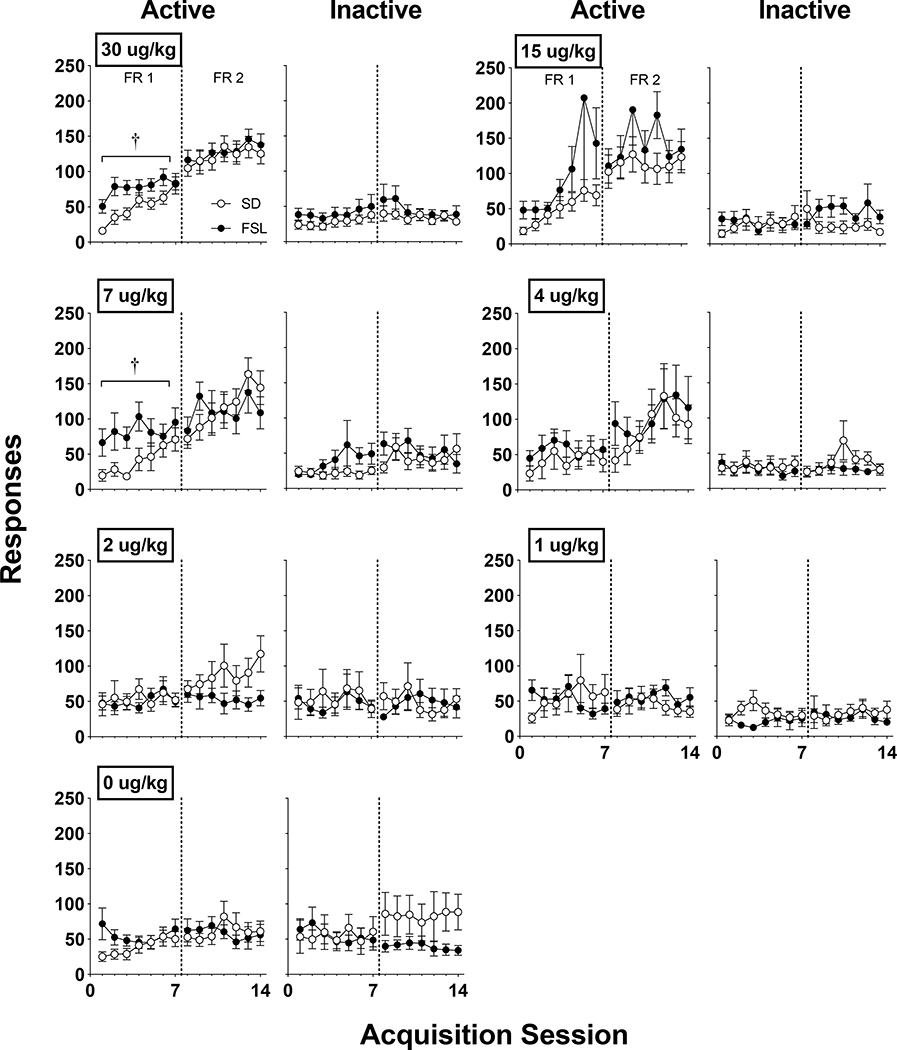
Figure 3 shows active and inactive responding during acquisition sessions in SD and FSL rats across unit nicotine doses. Active lever responding was significantly higher in FSL rats compared to SD rats during the initial FR 1 acquisition phase at the 7 ug/kg (F1, 23 = 5.792, p < 0.05) and 30 ug/kg (F1, 48 = 5.465, p < 0.05), but not at the 15 μg/kg dose. At doses below 7 ug/kg, active lever responses were similar between strains. By the end of the FR 2 phase, no significant group differences were apparent.
Figure 3.
Mean (± S.E.M.) group active and inactive responses for unit doses of nicotine in SD and FSL rats across acquisition sessions with active lever response requirements of FR 1 and FR 2 (left and right of the dotted line, respective); see the Methods section for sample sizes per strain at each dose. †Significant (p < 0.025) strain difference.
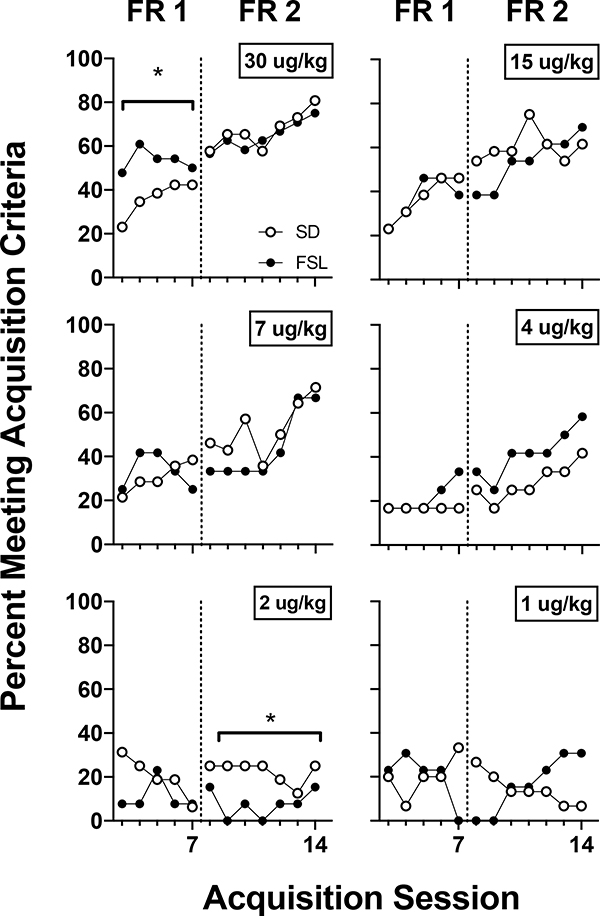
Figure 4 shows the percentage of SD and FSL rats that met acquisition criteria at each unit dose across acquisition sessions. At the 30 ug/kg dose, a greater proportion of FSL rats met acquisition criteria at FR 1 compared to SD rats (χ21, 248 = 7.445, p < 0.01; odds ratio is 5.802). At the 2 ug/kg dose under FR 2, a greater proportion of SDs met acquisition criteria than FSLs. However, this dose was below the reinforcement threshold for both strains and the percentage of rats meeting acquisition criteria did not show the same increasing trend that was observed with unit doses above threshold. The only unit doses that produced a positive slope in the proportion of rats meeting criteria across acquisition sessions was at 4 ug/kg and above. No sex differences were observed within each strain with regard to active responding, infusions earned or the percentage of rats meeting acquisition criteria at FR 1 or FR 2.
Figure 4.
The percentage of SD and FSL rats meeting acquisition criteria (see details in the Methods) during acquisition sessions across unit doses of nicotine; see the Methods section for sample sizes per strain at each dose. *Significant chi-square difference in the odds-ratio of acquiring nicotine self-administration.
3.3. Demand elasticity
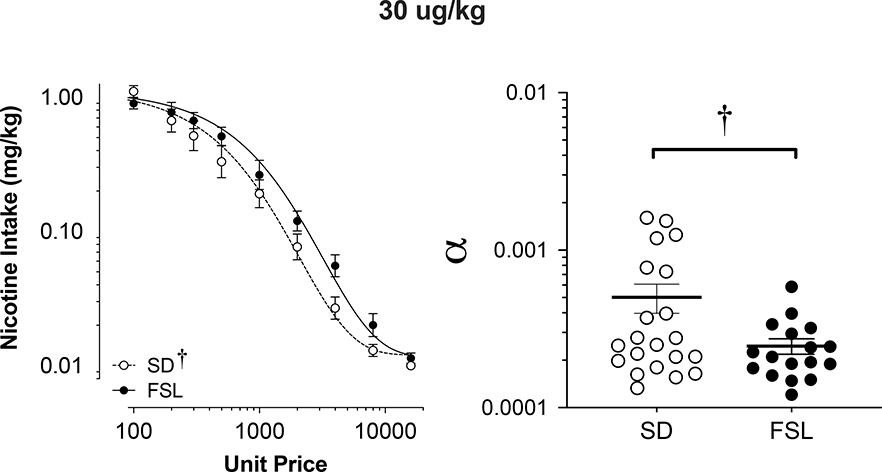
Figure 5 shows the mean consumption of nicotine at the 30 ug/kg dose (Left panel) and the best-fit individual demand elasticity α values in SD and FSL rats (Right panel). Table 1 shows individual and mean exponential demand parameter values. In general, FSL rats showed more inelastic demand at the 30 ug/kg dose of nicotine than SD rats, which was confirmed by significantly lower α values (t30.31 = 2.15, p < 0.05) and higher Pmax values (t35.57 =2.10, p < 0.05) in FSL vs. SD rats (Table 1). There was a non-significant trend toward FSL rats showing higher Omax values (p < 0.1), but no strain difference in the value of Q0 (demand intensity, see Table 1). No significant sex difference was observed in these parameters in either strain.
Figure 5.
Group mean (± S.E.M.) nicotine intake across unit price in SD and FSL rats during the demand assessment (left panel) at the 30 ug/kg unit nicotine dose and the resulting α values from the individually fit demand functions (right). †Significant (p < 0.05) difference in log alpha values (i.e. demand elasticity) between strains.
Table 1.
Exponential Demand Curve Parameters at the 30ug/kg dose of nicotine (k = 2.160).
| SD |
FSL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | |||||||||||
| ID # | α | Q0 | Pmax | Omax | r2 | ID # | α | Q0 | Pmax | Omax | r2 |
| 1 | 0.000396 | 1.49 | 442.7 | 210.5 | 0.97 | 22 | 0.000189 | 1.37 | 1007.8 | 440.1 | 0.98 |
| 2 | 0.000211 | 1.58 | 782.3 | 394.2 | 0.96 | 23 | 0.000294 | 0.80 | 1115.9 | 283.1 | 0.95 |
| 3 | 0.000773 | 1.47 | 230.2 | 107.7 | 0.97 | 24 | 0.000195 | 1.33 | 1013.6 | 428.1 | 0.98 |
| 4 | 0.000373 | 0.98 | 714.1 | 223.5 | 0.92 | 25 | 0.000338 | 1.79 | 432.3 | 246.5 | 0.94 |
| 5 | 0.000180 | 1.82 | 796.9 | 461.8 | 0.98 | 26 | 0.000321 | 1.63 | 499.3 | 259.7 | 0.99 |
| 6 | 0.000163 | 2.16 | 745.3 | 512.4 | 0.97 | 27 | 0.000160 | 0.26 | 6406.2 | 520.1 | 0.37 |
| 7 | 0.001608 | 2.39 | 67.9 | 51.8 | 0.96 | 28 | 0.000210 | 1.17 | 1060.3 | 395.7 | 0.95 |
| 8 | 0.000278 | 1.13 | 833.3 | 299.1 | 0.99 | 29 | 0.000585 | 2.73 | 163.9 | 142.4 | 0.86 |
| 9 | 0.001535 | 0.15 | 1107.9 | 54.2 | 0.49 | ||||||
| 10 | 0.000133 | 1.11 | 1773.5 | 624.6 | 0.98 | ||||||
| Mean | 0.000565 | 1.43 | 749.4 | 294.0 | 0.92 | Mean | 0.000287 | 1.39 | 1462.4 | 339.5 | 0.88 |
| SEM | 0.000178 | 0.20 | 149.7 | 63.1 | 0.05 | SEM | 0.000049 | 0.26 | 717.2 | 44.48 | 0.07 |
| Female | |||||||||||
| 11 | 0.001193 | 1.46 | 150.2 | 69.79 | 0.98 | 30 | 0.000190 | 1.841 | 745.6 | 437.5 | 0.91 |
| 12 | 0.001254 | 2.98 | 69.9 | 66.40 | 0.84 | 31 | 0.000244 | 0.626 | 1707.1 | 340.8 | 0.72 |
| 13 | 0.000251 | 1.54 | 678.6 | 331.99 | 0.95 | 32 | 0.000148 | 1.010 | 1746.4 | 562.2 | 0.87 |
| 14 | 0.000198 | 2.69 | 489.9 | 419.88 | 0.91 | 33 | 0.000396 | 1.625 | 406.1 | 210.3 | 0.89 |
| 15 | 0.000277 | 1.28 | 736.2 | 300.59 | 1.00 | 34 | 0.000150 | 1.750 | 993.2 | 553.9 | 0.96 |
| 16 | 0.000731 | 1.21 | 294.4 | 113.93 | 0.94 | 35 | 0.000121 | 1.207 | 1791.6 | 689.3 | 0.98 |
| 17 | 0.000220 | 0.81 | 1474.9 | 378.46 | 0.95 | 36 | 0.000179 | 1.472 | 994.2 | 466.5 | 0.89 |
| 18 | 0.000210 | 2.08 | 599.5 | 396.67 | 0.98 | 37 | 0.000242 | 1.985 | 544.9 | 344.8 | 0.96 |
| 19 | 0.000156 | 1.80 | 930.5 | 534.76 | 0.98 | 38 | 0.000225 | 1.129 | 1028.8 | 370.2 | 0.84 |
| 20 | 0.000164 | 1.82 | 872.7 | 507.08 | 0.98 | ||||||
| 21 | 0.000248 | 1.91 | 551.6 | 335.60 | 0.99 | ||||||
| Mean | 0.000446 | 1.78 | 622.6 | 314.11 | 0.95 | Mean | 0.000211 | 1.41 | 1106.4 | 441.7 | 0.89 |
| SEM | 0.000125 | 0.19 | 119.1 | 49.56 | 0.02 | SEM | 0.000027 | 0.15 | 175.1 | 48.2 | 0.03 |
|
Overall | |||||||||||
| Mean | 0.000502* | 1.61 | 683.0* | 304.5 | 0.94 | Mean | 0.000246* | 1.40 | 1274.0* | 393.6 | 0.88 |
| SEM | 0.000105 | 0.14 | 96.5 | 40.4 | 0.02 | SEM | 0.000025 | 0.14 | 304.4 | 36.2 | 0.05 |
- Significant difference (p < 0.05) between parameters, after log transformation.
3.4. Nicotine pharmacokinetics.
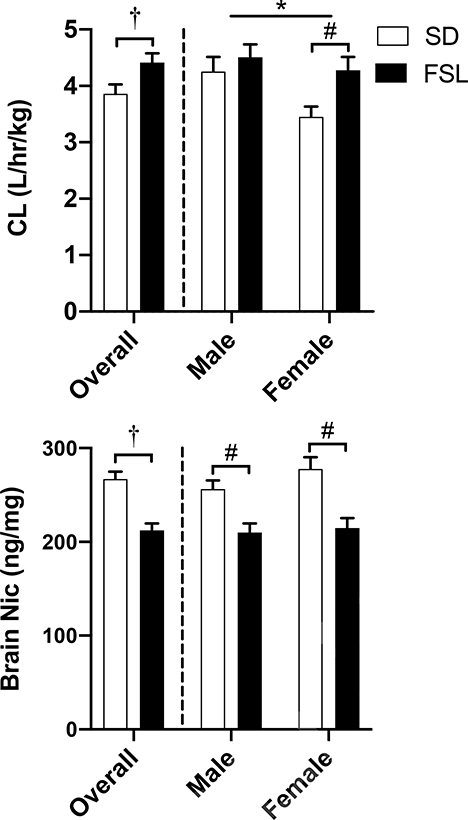
Figure 6 plots steady state clearance (upper panel) and brain concentration (lower panel) of nicotine in male and female FSL and SD rats when delivered via osmotic pump. There was a significant main effect of Strain (F1,210 = 5.394, p < 0.05) and Sex (F1,210 = 4.985, p < 0.05), indicating that FSL rats had higher rates of nicotine clearance compared to SDs. Sidak post-hoc tests revealed that in SDs males had higher clearance rates than females (t210 = 2.410, p < 0.05). In addition, post hoc tests found FSL females had significantly faster clearance than SD females (t210 = 2.397, p < 0.05). Sidak post-hoc tests revealed that there was a significant main effect of Strain (F1,202 = 19.72, p < 0.001) on brain nicotine concentrations, with FSLs having significantly lower brain nicotine levels compared to SDs in both males (t200 = 3.233, p < 0.05) and females (t200 = 4.026, p < 0.05).
Figure 6.
Mean (±SEM) nicotine clearance (upper panel) and brain nicotine concentrations (lower panel) in male and female SD and FSL rats (N=95 and 112 for each strain, respectively) implanted with osmotic pumps (3 mg/kg/day nicotine; sc) following the NSA phase of the study. †Significant (p < 0.05) strain difference. *Significant sex difference (p < 0.05). #Significant difference between sexes.
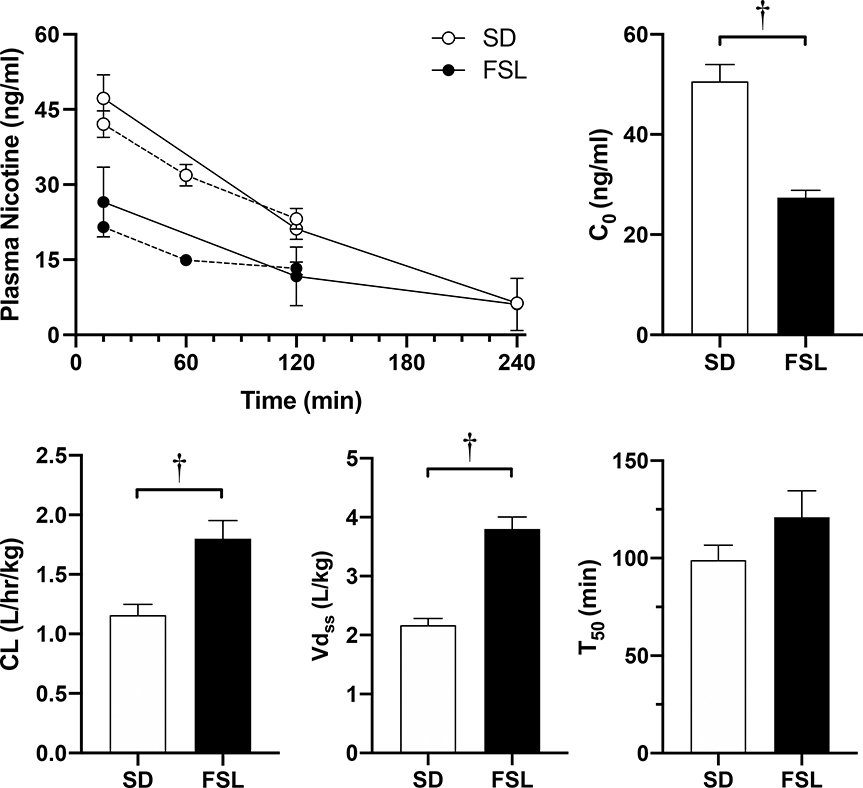
Figure 7 presents parameters from the bolus nicotine PK time-course in SD and FSL rats. Area under the plasma nicotine concentration curve was lower in FSL compared to SD rats (top-left panel; t50 = 5.08, p < 0.001). FSLs exhibited significantly higher clearance (bottom-left panel; F1,49 = 14.67, p < 0.001), volume of distribution (bottom-center panel; F1,49 = 59.78, p < 0.001), and lower estimated initial plasma concentration (C0, top-right panel; F1,49 = 29.76, p < 0.001) compared to SDs. The resulting half-life, however, was not significantly different between strains (bottom-right panel). Sex differences in these parameters were also found. There was a main effect of sex on clearance (F1,49 = 8.25, p < 0.01) and volume of distribution (F1,49 = 8.753, p < 0.01), with males being higher compared to females for both measures (data not shown). However, among FSL rats, there was no sex-difference in clearance.
Figure 7.
Mean plasma nicotine concentrations in SD and FSL rats (N=30 and 25, respectively) across minutes post-iv injection of 0.1 gm/kg nicotine (top-left) during the 2- and 4-hr pharmacokinetic assessments (dashed and solid lines, respectively), and the resulting initial plasma nicotine concentration (C0; top-right), clearance rate (CL; bottom-left), Volume of Distribution (Vd; bottom-center) and half-life (t50; bottom-right). †Significant (p < 0.05) difference in log alpha values (i.e. demand elasticity) between strains. (Note: Males showed significantly higher rates of CL and Vd compared to females, see results for details).
3.5. Relationship between nicotine CL and NSA measures.
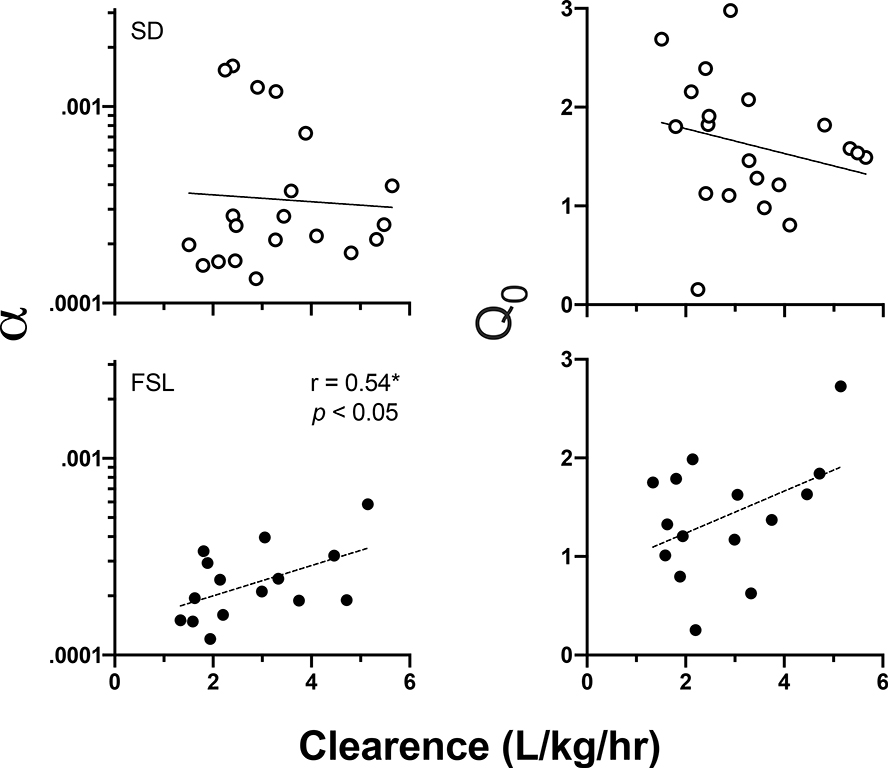
Figure 8 plots individual best-fit exponential demand α values as a function of nicotine clearance in SD (top-left panel) and FSL (bottom-left panel) rats. In SDs, there was no relationship between clearance and demand elasticity. In FSLs, however, larger alpha values (more elastic demand) were positively associated with higher clearance rates (r15 = 0.55, p < 0.05). There was no significant relationship in either strain between CL and Q0 (right column), or other measure of NSA.
Figure 8.
Individual α (demand elasticity; left column) and Q0 (demand intensity; right column) values at the 30 ug/kg unit nicotine dose as a function of nicotine clearance rates in SD (top panels) and FSL (bottom panels) rats. Note that smaller α values indicate more inelastic demand and greater reinforcing efficacy. *Significant positive correlation between greater clearance and more elastic demand in FSL rats.
4. Discussion
The primary findings of the present study were that the reinforcing efficacy of nicotine was greater in adolescent FSL rats as indicated by faster acquisition of NSA and more inelastic demand (i.e., greater reinforcing efficacy) at a typical reinforcing dose of nicotine (30 μg/kg/inf). However, the reinforcing potency of nicotine did not differ between strains, as indicated by similar nicotine unit dose-response curves and reinforcement thresholds between strains. The percentage of rats meeting acquisition criteria also did not differ between strains at doses below 30 μg/kg/inf. Strain differences in nicotine pharmacokinetics were observed, with FSL rats showing faster clearance, greater volume of distribution, and lower brain nicotine levels. However, these pharmacokinetic differences were not always consistent with strain differences in NSA measures, indicating that nicotine pharmacokinetics did not fully account for strain differences in nicotine’s reinforcing effects. To our knowledge, the present study is the first to examine the reinforcing efficacy and pharmacokinetics of nicotine in this genetic animal model of depression. The present study advances preclinical research on tobacco addiction comorbidities and has important clinical and policy implications.
The finding of greater demand for nicotine in FSL rats is consistent with human studies showing a positive association between smoking and depression, as indicated by heavier smoking, greater nicotine dependence, greater reinforcing efficacy of smoking, and more difficulty quitting in humans with depression and other psychiatric comorbidities (Aubin et al., 2012; Audrain-McGovern et al., 2014; Fluharty et al., 2016; Mathew et al., 2016; Tidey, 2016). They are also consistent with clinical studies showing adolescent smokers with depression exhibit greater nicotine dependence and faster progression to daily smoking (Breslau & Peterson, 1996; Karp et al., 2006). These human studies have primarily used cross-sectional or longitudinal observational methods to study the relationship between depression and smoking. As such, the lack of experimental control has made it impossible to determine whether the relationship is causal (Fluharty et al., 2016). Because animal models allow a level of experimental control that can’t be attained in human studies, the present study contributes to this literature by providing experimental data consistent with the notion that depression and tobacco addiction may be causally related.
The present findings have important policy implications for setting a nicotine standard for tobacco products to reduce tobacco use initiation in adolescents and continued dependence in adults. Although all animal models of tobacco addiction and depression have limitations, they are nonetheless vital to regulatory science because they allow studying issues (e.g., initiation in adolescents) that are difficult or impossible to address in humans for ethical, safety, or logistical reasons (Donney et al., 2012; LeSage, et al., 2018). As such, data from animal models are routinely used to conduct the risk assessments needed to set policy in other areas of public health (USHHS-FDA, 2005; White et al., 2009) and are being requested to inform tobacco control policy as well (see https://www.fda.gov/about-fda/fda-organization/center-tobacco-products). Regarding initiation of NSA, the reinforcement threshold for nicotine in the present study (4–7 μg/kg/infusion) was similar between strains, and somewhat higher than that reported in other strains (3–4μg/kg/infusion, e.g., Grebenstein et al., 2013, 2015; Shoaib et al., 1997; Sorge and Clarke, 2009; Smith et al, 2013). This finding suggests that setting a nicotine standard below the threshold for nicotine reinforcement in the general adolescent population may be sufficient to avoid initiation of tobacco use and development of tobacco addiction in adolescents with depression. However, our finding of less elastic demand in older FSL rats at the highest nicotine unit dose suggests that reducing already established tobacco dependence in young and older adults with depression may require a lower nicotine standard for tobacco products compared to the general adolescent population. Human studies are critical to examine these issues further to determine the validity of the present findings, and because any nicotine standard or similar tobacco regulatory policy cannot rely on animal data alone. (Donny et al., 2012; Sofuoglu & LeSage, 2012).
The reinforcing efficacy of nicotine and tobacco among individuals with depression may depend, in part, on the nature of the neural mechanisms mediating depression in a given smoker. Given that some depressed individuals exhibit hyperactivity of cholinergic systems (Janowsky et al., 1994; Mineur et al., 2011), the FSL rat could be considered a model of this particular subtype of depression. As such, the present findings may only generalize to smokers with this subtype of depression. Future human studies are needed to test the hypothesis that depressed individuals with heightened cholinergic activity may be more nicotine dependent and/or have greater difficulty quitting than those with normal cholinergic activity.
Few studies have examined nicotine self-administration in nonhuman models of depression. The present study is the first to demonstrate IV nicotine self-administration in FSL rats and the first to report greater reinforcing efficacy of nicotine in any nonhuman model of depression. These findings contrast with other studies in this area. One study showed no difference in oral nicotine intake between FSL and a control strain, and intake was lower in groups with access to nicotine than groups with access to tap water, indicating nicotine was not serving as a reinforcer in either strain (Djuric et al., 1999). Another study reported higher oral SA of nicotine in an olfactory bulbectomy (OBX) model of depression compared to sham controls (Vieyra-Reyes et al., 2008). Although the higher nicotine intake suggests that nicotine may have been more reinforcing in OBX rats, potential differences in peripheral sensory effects of nicotine between strains confound this interpretation. For example, the bitter taste of nicotine may have been lower in OBX rats, such that higher nicotine consumption was due to reduced punishing effects rather than increased reinforcing effects. Moreover, there was no evidence of nicotine preference in either OBX or sham controls under any condition, indicating that nicotine was not serving as a reinforcer. In a different genetic animal model of depression, the Wistar-Kyoto (WKY) rat strain (Malkesman et al., 2006; Tizabi et al., 2010), studies have shown that NSA is significantly lower in WKY rats compared to outbred Wistar controls under an FR schedule of NSA (de la Garza, 2005). However, only one nicotine unit dose was studied and reinforcing efficacy per se was not measured. Although certain procedural factors and experimental design issues could account for the differences between studies (e.g. route of administration, nicotine dose, failure to demonstrate nicotine reinforcement in control strains), the unique aspects of each of the depression models may also play a role. For example, the WKY model of depression is considered distinct from the FSL model because, unlike FSL rats, WKY rats exhibit an anxiety-like phenotype and do not respond to serotonergic antidepressants (Lopez-Rubalcava & Lucki, 2000). The differences in NSA between FSL rats in the present study and WKY rats in other studies may be due to differences in the neuropharmacological mechanisms mediating nicotine’s behavioral effects between these strains. Because all animal models of depression have limitations (Krishnan & Nestler, 2011), these findings highlight the need to utilize multiple depression models to gain a comprehensive understanding of the mechanisms mediating different aspects of depression and their relationship to tobacco addiction.
Despite the clear strain difference in nicotine pharmacokinetics, the lack of strain differences in some NSA measures (e.g. baseline infusion rates, nicotine dose-response curves), indicate that pharmacokinetic mechanisms alone cannot fully account for the strain differences in nicotine’s reinforcing effects. The strain differences in NSA are more likely mediated by an interaction of certain pharmacodynamic and pharmacokinetic mechanisms. FSL rats differ from outbred strains in regard to several neuropharmacological variables critical to nicotine’s reinforcing effects, including a higher density of α4β2 nAChRs in reinforcement-related brain regions (e.g. midbrain, striatum), higher α4, α7, and β2 nAChR subunit mRNA expression in striatum, greater nicotine-induced upregulation of α4β2 nAChRs, and greater nicotine-evoked dopamine (DA) release in striatum (Auta et al., 2000; Tizabi et al., 2000, 2009). The elevated nAChR expression and greater DA signaling in FSL rats may increase the magnitude of nicotine’s reinforcing effects, leading to faster acquisition and greater demand at the 0.03 mg/kg nicotine unit dose. However, the lack of strain differences in the nicotine unit dose-response curves and reinforcement threshold do not support this interpretation. If FSL rats were more sensitive to nicotine, one would expect their dose-response curve to be shifted to the left relative to SD rats. The strain differences in nicotine pharmacokinetics may have moderated any strain difference in nicotine’s neuropharmacological effects. FSL rats showed a higher apparent volume of distribution, which resulted in lower serum nicotine concentrations following an acute dose of nicotine. FSL rats also showed faster nicotine clearance during both acute and continuous nicotine dosing. Thus, although lower brain concentrations may have been achieved from NSA in FSL rats, those concentrations may have been sufficient to reinforce and maintain NSA rates similar to control rats due to the greater sensitivity of FSL rats to nicotine. Although the effect of depression per se on tobacco addiction in humans may not be mediated by nicotine pharmacokinetic mechanisms, studies are needed to directly examine this issue.
Human studies suggest that the effects of nicotine metabolism and clearance on tobacco addiction in humans manifests differently during adolescence than it does in adults. Adolescent smokers with slower clearance tend to have greater nicotine dependence and difficulty quitting smoking (e.g., Karp et al., 2006; Rubinstein et al., 2013), whereas the opposite relationship is observed in adults (Pianezza et al., 1998; Schoedel et al., 2004). In the earlier stages of smoking in adolescents, when nicotine intake is relatively low and intermittent, slower metabolism may be associated with greater tobacco addiction and difficulty quitting because it would result in prolonged presence of higher brain nicotine concentrations, which might result in a higher magnitude (or longer duration) of reinforcement. Our present and previous (Grebenstein et al., 2015) findings somewhat mirror these relationships in an animal NSA model. In the present study, inbred adolescent FSL rats with slower clearance showed more inelastic demand (i.e., greater abuse liability), whereas our previous work showed that outbred adult Holtzman rats (Grebenstein et al., 2015) with higher clearance showed more inelastic demand. However, there was no relationship between nicotine clearance and elasticity of demand in adolescent outbred SD rats. This strain difference in adolescent rats suggests that nicotine clearance may be a stronger moderator of tobacco addiction in adolescent smokers with depression, particularly in those with increased cholinergic function.
The lack of sex differences in NSA measures in the present study is consistent with our prior study showing a lack of sex differences in the nicotine reinforcement threshold and elasticity of demand for nicotine in adult rats during a unit dose reduction protocol. This suggests that nicotine standards to reduce the addictiveness of tobacco products would be equally effective in males and females regardless of age or stage of tobacco use (initiation or maintenance). However, our findings are inconsistent with some other studies showing sex differences in NSA in adolescent rats (Chen et al., 2007; Flores et al., 2017; Lynch, 2009 ), which may be due to different periods in adolescence when NSA began, different lever press training methods, different session durations, or different strains used between studies (Flores et al., 2017).
The sex difference observed during the initial FST suggests that the severity of depression is greater in female FSLs. This finding is consistent with other studies showing a more pronounced depressive phenotype, including greater immobility in the FST, in female FSLs than males (Dalla et al., 2010; Sanchez et al., 2018). However, there was no evidence of sex differences in the rate of acquisition, baseline NSA, reinforcement threshold, or elasticity of demand. There was also no significant relationship between FST scores and NSA measures among FSL rats. These findings indicate that individual differences in the severity of depression within the FSL strain were not associated with individual differences in the reinforcing efficacy of nicotine. However, the FST provides only one of many measures of depression-like behavior, and it’s validity is limited (Krishnan & Nestler, 2011). In order to obtain a more complete understanding of whether severity of depression influences nicotine’s reinforcing effects among FSL rats, further research should examine whether other measures of depression-like behavior (e.g. stress-induced anhedonia, reduced locomotor activity, increased REM sleep, etc.) are related to individual differences in NSA.
The present and prior findings raise some concern about the validity of NSA in FSL rats as a model of comorbid smoking and depression in humans. The similarity in baseline NSA between strains in the present study is not consistent with the higher CPD often observed in adolescent and adult smokers with depression (Fluharty e tla., 2016; Kendler et al., 1993; Patton et al., 1998). Moreover, prior studies show that FSL and control rats exhibit comparable basal DA and DA metabolite levels in NAC (Matthews et al., 1996) and levels of anhedonia, as indicated by similar baseline intracranial self-stimulation (ICSS) thresholds and sucrose preference (Pucilowski et al., 1993). These findings are inconsistent with the greater anhedonia and decreased motivation in most individuals with depression (Berton & Nestler, 2006). However, FSL rats show greater anhedonia and other depression-like behaviors when under stress (Overstreet and Wegener, 2012). The requirement of stress for the anhedonia phenotype to manifest in FSL rats suggests that this strain is better viewed as a model of the genetic predisposition to depression rather than of depression per se (see a review by Overstreet 2012; Willner & Mitchell, 2002). As such, use of the FSL model alone is a limitation of the present study because it fails to address the complex etiology of depression, which involves both genetic and environmental factors. Future studies should examine the effects of stress on nicotine reinforcement in FSL rats to better model the gene-environment interactions in the comorbidity of depression and smoking. For example, environmental stressors that are known to magnify nicotine’s reinforcing effects in outbred strains (Buczek et al., 1999; Yu et al., 2014; Yu & Sharp, 2015; Zislis et al.,, 2007; Zou et al., 2014) may do so to a greater degree in the FSL strain.
The present study is an important initial step in preclinical research on the comorbidity of tobacco addiction and depression, and the findings are consistent with human studies suggesting that the relationship between cigarette smoking and depression may be causal (Munafò and Araya, 2010; Fluharty et al., 2016). Our findings that FSL and control rats had similar thresholds for nicotine reinforcement suggests that setting a nicotine standard for combustible tobacco products based on the general population should be sufficient to limit development of tobacco dependence in adolescents with depression. However, to the extent that the present results can be generalized to humans, our finding of less elastic demand in FSL rats at the highest nicotine unit dose suggests that reducing maintenance of tobacco use and addiction in adolescent smokers with depression may require a lower nicotine standard for tobacco products compared to the general adolescent population. However, it will be important to examine this issue more directly by assessing demand in FSL rats during nicotine dose reduction, rather than during response cost escalation as in the present study. Moreover, assessing demand at other unit nicotine doses will be important to determine the generality of the strain difference in the present study, as elasticity of demand may depend on the maintenance dose of nicotine (Kohut and Bergman, 2016).
Highlights.
The reinforcement threshold and elasticity of demand (i.e., reinforcing efficacy) for nicotine was assessed in a genetic inbred rat model of depression (Flinders Sensitive Line [FSL])
FSL rats acquired self-administration quicker and showed more inelastic demand (greater reinforcing efficacy) than controls at the highest unit dose, but no strain differences in acquisition were observed at lower doses.
FSL rats exhibited faster nicotine clearance, larger volume of distribution, and lower plasma and brain nicotine concentrations, but were not consistently related to strain differences in NSA measures.
Results are consistent with the literature showing greater dependence and reinforcing efficacy of cigarettes in smokers with depression, but the lack of strain difference at lower doses suggests that a nicotine dose reduction will be effective in both the general adolescent population and those with depression.
Acknowledgements
We would like to thank Jonathan Resch, Marlee Curtis, Ben Dougan, and Theresa Harmon for their excellent technical assistance. Hennepin Healthcare Research Institute was formerly known as Minneapolis Medical Research Foundation (MMRF).
Role of Funding Source
This study was supported by NIDA grant R01-DA042525 (LeSage MG, PI) and a Career Development Award from Hennepin Healthcare Research Institute (MGL). These funding institutions had no role in study design, data collection and analysis, or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, and Laviola G, 2004. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol 15, 341–352 [DOI] [PubMed] [Google Scholar]
- Adriani W, Macrí S, Pacifici R, Laviola G, 2002. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 27, 212–224. doi: 10.1016/S0893-133X(02)00295-6 [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV, 2003. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci 23, 4712–4716. doi:23/11/4712 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin H-J, Rollema H, Svensson TH, Winterer G. 2012. Smoking, quitting, and psychiatric disease: a review. Neuroscience and biobehavioral reviews. 36, 271–284. doi: 10.1016/j.neubiorev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Auta J, Lecca D, Nelson M, Guidotti A, Overstreet D, Costa E, Javaid J, 2000. Expression and function of striatal nAChRs differ in the flinders sensitive (FSL) and resistant (FRL) rat lines. Neuropharmacology. 39, 2624–2631. 10.1016/s0028-3908(00)00082-4 [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR, 2003. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 70, S55–S72. doi: 10.1016/S0376-8716(03)00099-1 [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Wileyto EP, Ashare R, Cuevas J, Strasser AA, 2014. Reward and Affective Regulation in Depression-Prone Smokers. Bio Psych 76, 689–697. doi: 10.1016/j.biopsych.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS, 2012. Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration. Adv. Pharmacol. Sci 2012, 1–17. doi: 10.1155/2012/281768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Nestler E (2006). New approaches to antidepressant drug discovery: beyond monoamines Nat. Rev. Neuro 7, 137–151. 10.1038/nrn1846 [DOI] [PubMed] [Google Scholar]
- Buczek Y, Lê A, Wang A, Stewart J, Shaham Y, 1999. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology 144, 183–188. 10.1007/s002130050992 [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, 1996. Smoking cessation in young adults: Age at initiation of cigarette smoking and other suspected influences. Am. J. Public Health. 86, 214–220. doi: 10.2105/AJPH.86.2.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM, 2007. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharm 32, 700–709. doi: 10.1038/sj.npp.1301135 [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis P, Kokras N, Papadopoulou-Daifoti Z (2010). Sex Differences in Response to Stress and Expression of Depressive-Like Behaviours in the Rat. Curr. topics behav. neuro 8, 97–118. 10.1007/7854_2010_94 [DOI] [PubMed] [Google Scholar]
- De la Garza R (2005). Wistar Kyoto rats exhibit reduced sucrose pellet reinforcement behavior and intravenous nicotine self-administration. Pharmacol. Biochem. Beh 82, 330–337. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Pbert L, O’Loughlin J, McNeill AD, Ockene JK, Friedman K, Hazelton J, Wood C, Dussault G, and Wellman RJ 2007. Susceptibility to nicotine dependence: the Development and Assessment of Nicotine Dependence in Youth 2 study. Peds 120:e974–83. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C, 2002. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob. Control 11, 228–35. doi: 10.1136/tc.11.3.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurić V, Dunn E, Overstreet D, Dragomir A, Steiner M, 1999. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol. Behav 67(4), 533–537. 10.1016/s0031-9384(99)00091-8 [DOI] [PubMed] [Google Scholar]
- Donny EC, Taylor TG, LeSage MG, Levin M, Buffalari DM, Joel D, Sved AF, 2012. Impact of tobacco regulation on animal research: New perspectives and opportunities. Nicotine Tob. Res 14, 1319–1338. doi: 10.1093/ntr/nts162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Altea S, and Fratta W, 2008. Sex differences in drug addiction: a review of animal and human studies. Women’s health (London, England) 4:51–65 [DOI] [PubMed] [Google Scholar]
- Flores R, Uribe K, Swalve N, O’Dell L, 2017. Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiol. Behav 203, 42–50. doi: 10.1016/j.physbeh.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluharty M, Taylor A, Grabski M, Munafò M, 2016. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nic. tobacco res 19, 3–13. 10.1093/ntr/ntw140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Guillaume S, Jaussent I, Beziat S, Macgregor A, Bernard P, Courtet P, Bailly D, Quantin X, 2015. Prevalence and Smoking Behavior Characteristics of Nonselected Smokers With Childhood and/or Adult Self-Reported ADHD Symptoms in a Smoking-Cessation Program: A Cross-Sectional Study. J. Atten. Disord. 19, 293–300. doi: 10.1177/1087054713497396 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration, 2018. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Federal Register 83(52),11818–11843. [Google Scholar]
- Gervais A, O’Loughlin J, Meshefedjian G, Bancej C, and Tremblay M, 2006. Milestones in the natural course of onset of cigarette use among adolescents. Canadian Med. Assoc. J 175, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG, 2013. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: Implications for nicotine regulation policy. Pharmacol. Biochem. Behav 114–115, 70–81. doi: 10.1016/j.pbb.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein PE, Burroughs D, Roiko SA, Pentel PR, LeSage MG, 2015. Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy. Drug Alcohol Depend. 151, 181–193. doi: 10.1016/j.drugalcdep.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller M, 2013. Nicotine reduction: Strategic research plan. Nicotine Tob. Res 15, 1003–1013. doi: 10.1093/ntr/nts214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert KK, Cummins SE, Hernández S, Tedeschi GJ, Zhu S-H, 2011. Current Major Depression Among Smokers Using a State Quitline. Am. J. Prev. Med 40, 47–53. doi: 10.1016/j.amepre.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, 1984. Behavioral economics. J. Exp. Anal. Behav 42, 435–452. doi: 10.1901/jeab.1984.42-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Madden GJ, Spiga R, DeLeon IG, Francisco MT, 2013. The translational utility of behavioral economics: The experimental analysis of consumption and choice., in: APA Handbook of Behavior Analysis, Vol. 2: Translating Principles into Practice. American Psychological Association, Washington, pp. 191–224. doi: 10.1037/13938-008 [DOI] [Google Scholar]
- Hursh SR, Roma PG, 2016. Behavioral Economics and the Analysis of Consumption and Choice. Manag. Decis. Econ 37, 224–238. doi: 10.1002/mde.2724 [DOI] [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychol. Rev 115, 186–198. doi: 10.1037/0033-295X.115.1.186 [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Overstreet DH, and Nurnberger JI, 1994. Is cholinergic sensitivity a genetic marker for the affective disorders? Am. J. Med. Genetics. 54, 335–344. [DOI] [PubMed] [Google Scholar]
- Karp I, O’Loughlin J, Hanley J, Tyndale RF, Paradis G, 2006. Risk factors for tobacco dependence in adolescent smokers. Tob. Control 15, 199–204. doi: 10.1136/tc.2005.014118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC, 1993. Smoking and Major Depression. Arch. Gen. Psychiatry 50, 36. doi: 10.1001/archpsyc.1993.01820130038007 [DOI] [PubMed] [Google Scholar]
- Kohut SJ, Bergman J, 2016. Reinforcing effectiveness of nicotine in nonhuman primates: effects of nicotine dose and history of nicotine self-administration. Psychopharmacology, 233, 2451–2458. doi: 10.1007/s00213-016-4293-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI, 2007. Nicotine Dependence and Reward Differ between Adolescent and Adult Male Mice. J. Pharmacol. Exp. Ther 322, 399–407. doi: 10.1124/jpet.107.121616 [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler E (2011). Animal models of depression: molecular perspectives. Current topics in behavioral neurosciences 7(), 121–147. doi: 10.1007/7854_2010_108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Sern KR, Class MA, Bocz MD, Szumlinski KK, 2017. Anxiolytic Effects of Buspirone and MTEP in the Porsolt Forced Swim Test. Chronic Stress 1, 247054701771298 10.1177/2470547017712985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR, 2003. Effects of continuous nicotine infusion on nicotine self-administration in rats: Relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl). 170, 278–286. doi: 10.1007/s00213-003-1539-2 [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR, 2002. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol. Biochem. Behav 72, 279–289. doi: 10.1016/S0091-3057(01)00775-4 [DOI] [PubMed] [Google Scholar]
- LeSage MG, Smethells JR, Harris AC, 2018. Status and future directions of preclinical behavioral pharmacology in tobacco regulatory science. Behavior Analysis: Research and Practice 18(3), 252–274. doi: 10.1037/bar0000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Staley M, Muelken P, Smethells JR, Stepanov I, Vogel RI, Pentel PR, Harris AC, 2016. Abuse liability assessment of an e-cigarette refill liquid using intracranial self-stimulation and self-administration models in rats. Drug Alcohol Depend. 168, 76–88. doi: 10.1016/j.drugalcdep.2016.08.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA, 2007. Adolescent vs. adult-onset nicotine self-administration in male rats: Duration of effect and differential nicotinic receptor correlates. Neurotoxicol. Teratol 29, 458–465. doi: 10.1016/j.ntt.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Scott Swartzwelder H, 2003. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl). 169, 141–149. doi: 10.1007/s00213-003-1486-y [DOI] [PubMed] [Google Scholar]
- López-Rubalcava C, Lucki I, 2000. Strain Differences in the Behavioral Effects of Antidepressant Drugs in the Rat Forced Swimming Test Neuropsychopharmacology 22, 191–199. 10.1016/s0893-133x(99)00100-1 [DOI] [PubMed] [Google Scholar]
- Lynch WJ 2009. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol. Biochem. Behav 94, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Braw Y, Maayan R, Weizman A, Overstreet DH, Shabat-Simon M, Kesner Y, Touati-Werner D, Yadid G, and Weller A 2006. Two different putative genetic animal models of childhood depression. Bio. Psychiatry 59, 17–23. [DOI] [PubMed] [Google Scholar]
- Mathew AR, Hogarth L, Leventhal AM, Cook JW, Hitsman B., 2016. Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model. Addiction 112, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Baldo BA, Markou A, Lown O, Overstreet DH, Koob GF, 1996. Rewarding electrical brain stimulation: Similar thresholds for flinders sensitive line hypercholinergic and flinders resistant line hypocholinergic rats. Physiol. Behav 59, 1155–1162. doi: 10.1016/0031-9384(95)02212-0 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Einstein EB, Seymour PA, Coe JW, OʼNeill BT, Rollema H, and Picciotto MR, 2011. α4β2 nicotinic acetylcholine receptor partial agonists with low intrinsic efficacy have antidepressant-like properties. Behav. Pharmacol 22, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Araya R, 2010. Cigarette smoking and depression: a question of causation British Journal of Psychiatry 196(6), 425–426. doi: 10.1192/bjp.bp.109.074880 [DOI] [PubMed] [Google Scholar]
- National Reserach Council, 2011. Guide for the Care and Use of Laboratory Animals, Eigth. ed, Guide for the Care and Use of Laboratory Animals. The National Academic Press. doi: 10.1163/1573-3912_islam_DUM_3825 [DOI] [Google Scholar]
- Natividad LA, Torres OV, Friedman TC, O’Dell LE, 2013. Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behav. Brain Res 257, 275–285. doi: 10.1016/j.bbr.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA, 2007. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol. Teratol 29, 17–22. doi: 10.1016/j.ntt.2006.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, Clarke P, Hanley J, and Paradis G, 2003. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am. J. Preventive Med 25, 219–225 [DOI] [PubMed] [Google Scholar]
- Overstreet DH, 1993. The flinders sensitive line rats: A genetic animal model of depression. Neurosci. Biobehav. Rev 17, 51–68. doi: 10.1016/S0149-7634(05)80230-1 [DOI] [PubMed] [Google Scholar]
- Overstreet DH 2012. Modeling depression in animal models. Methods Mol. Biol 829:125–144 [DOI] [PubMed] [Google Scholar]
- Overstreet DH, and Djuric V 2001. A genetic rat model of cholinergic hypersensitivity: implications for chemical intolerance, chronic fatigue, and asthma. Ann. N.Y. Acad. Sci 933, 92–102. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Wegener G, 2012. The Flinders Sensitive Line Rat Model of Depression−-25 Years and Still Producing. Pharmacol. Rev 65, 143–155. doi: 10.1124/pr.111.005397 [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G, 1998. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am. J. Public Health 88, 1518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA 2009. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Nebr. Symp. Motiv 55, 143–169 [DOI] [PubMed] [Google Scholar]
- Pianezza MI, Sellers EM, Tyndale RF, 1998. Nicotine metabolism defect reduces smoking. Nature 393, 750. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS, 1993. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiology behav 54, 1215–20. 10.1016/0031-9384(93)90351-f [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL, 2013. Nicotine metabolism and addiction among adolescent smokers. Addiction 108, 406–412. doi: 10.1111/j.1360-0443.2012.04026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Khoury A, Hassan M, Wegener G, Mathé A, 2018. Sex-dependent behavior, neuropeptide profile and antidepressant response in rat model of depression Behavioural brain res 351, 93–103. 10.1016/j.bbr.2018.05.029 [DOI] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, and Lynch WJ, 2013. Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology 231, 1753–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF, 2004. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics 14, 615–626. doi: 10.1097/00008571-200409000-00006 [DOI] [PubMed] [Google Scholar]
- Shahab L, Andrew S, West R, 2014. Changes in prevalence of depression and anxiety following smoking cessation: results from an international cohort study. Psychol. Med 44, 127–141. doi: 10.1017/S095457941400090X [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR, 1997. Nicotine self-administration in rats: Strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl). 129, 35–43. doi: 10.1007/s002130050159 [DOI] [PubMed] [Google Scholar]
- Smith Tracy T, Levin Melissa E, Schassburger Rachel L, Buffalari Deanne M, Sved Alan F, Donny Eric C, 2013. Gradual and Immediate Nicotine Reduction Result in Similar Low-Dose Nicotine Self-administration. Nicotine & Tobacco Research 15 (11), 1918–1925. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, LeSage M, 2012. The reinforcement threshold for nicotine as a target for tobacco control. Drug and Alcohol Dependence 125, 1–7. 10.1016/j.drugalcdep.2012.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Clarke PBS, 2009. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J. Pharmacol. Exp. Ther 330, 633–640. doi: 10.1124/jpet.109.154641 [DOI] [PubMed] [Google Scholar]
- Spring B, Pingitore R, McChargue DE, 2003Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. Am J Psychiatry. 160, 316–322. [DOI] [PubMed] [Google Scholar]
- Tidey JW, 2016. A behavioral economic perspective on smoking persistence in serious mental illness. Prev Med 92, 31–35. doi: 10.1016/j.ypmed.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Hauser SR, Tyler KY, Getachew B, Madani R, Sharma Y, and Manaye KF, 2010. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog Neuropsychopharmacol Biol. Psychiatry 34:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis V. a, Clark E, Janowsky DS, Kling MA, 1999. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl). 142, 193–199. doi: 10.1007/s002130050879 [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Rezvani AH, Russell LT, Tyler KY, and Overstreet DH 2000. Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol Biochem Behav 66, 73–77. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration (2005). Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Center for Drug Evaluation Research, Rockville, MD. [Google Scholar]
- Vieyra-Reyes P, Mineur Y, Picciotto M, Túnez I, Vidaltamayo R, Drucker-Colín R, 2008. Antidepressant-like effects of nicotine and transcranial magnetic stimulation in the olfactory bulbectomy rat model of depression Brain research bulletin 77, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Cote I, Zeise L, Fox M, Dominici F, Burke T, White P, Hattis D, Samet J (2009). State-of-the-science workshop report: issues and approaches in low-dose-response extrapolation for environmental health risk assessment Environmental health perspectives 117(2), 283–287. doi: 10.1289/ehp.11502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Sharp B, 2015. Basolateral amygdala and ventral hippocampus in stress-induced amplification of nicotine self-administration during reacquisition in rat Psychopharmacology 232, 2741–2749. 10.1007/s00213-015-3911-4 [DOI] [PubMed] [Google Scholar]
- Yu G, Chen H, Sharp B, 2014. Amplified reacquisition of nicotine self-administration in rats by repeated stress during abstinence Psychopharmacology 231, 3189–3195. 10.1007/s00213-014-3501-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huo M, Zhou J, Xie S, 2010. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed 99, 306–314. doi: 10.1016/j.cmpb.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai T, Prado M, Shah H, Bruijnzeel A, 2007. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats Neuropharmacology 53, 958–966. 10.1016/j.neuropharm.2007.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Funk D, Shram M, Lê A, 2014. Effects of stressors on the reinforcing efficacy of nicotine in adolescent and adult rats Psychopharmacology 231, 1601–1614. 10.1007/s00213-013-3314-3 [DOI] [PubMed] [Google Scholar]