Abstract
Purpose
Oral cancer causes over 120,000 deaths annually and affects the quality of life for survivors. Over 90% of oral cancers are derived from oral squamous cell carcinoma cells (OSCCs) which are generally resistant to standard cytotoxic chemotherapy agents. OSCC cells often exhibit increased TGFβ and PDPN receptor activity compared to nontransformed oral epithelial cells. Maackia amurensis seed lectin (MASL) can target the PDPN receptor and has been identified as a novel agent that can be used to treat oral cancer. However, mechanisms by which MASL inhibits OSCC progression are not yet clearly defined.
Methods
Here, we performed cell migration and cytotoxicity assays to assess the effects of MASL on OSCC motility and viability at physiologically relevant concentrations. We then performed comprehensive transcriptome analysis combined with transcription factor reporter assays to investigate the how MASL affects OSCC gene expression at these concentration. Key data were then confirmed by western blotting to evaluate the effects of MASL on gene expression and kinase signaling activity at the protein level.
Results
MASL significantly affected the expression of about 27% of approximately 15 thousand genes found to be expressed by HSC-2 cells used to model OSCC cells in this study. These genes affected by MASL include members of the TGFβ-SMAD, JAK-STAT, and Wnt-βCTN signaling pathways. In particular, MASL decreased expression of PDPN, SOX2, and SMAD5 at the RNA and protein levels. MASL also inhibited SMAD and MAPK activity and exhibited potential for combination therapy with doxorubicin and 5-fluorouracil.
Conclusions
Taken together, results from this study indicate that MASL decreases activity of JAK-STAT, TGFβ-SMAD, and Wnt-βCTN signaling pathways to inhibit OSCC growth and motility. These data suggest that further studies should be undertaken to determine how MASL may also be used alone and in combination with other agents to treat oral cancer.
Keywords: oral cancer, oral squamous cell carcinoma, chemotherapy, Maackia amurensis, lectin, podoplanin, TGFβ, SMAD, JAK-STAT
Introduction
Over 400,000 new oral cancer cases are diagnosed each year, causing over 120,000 deaths worldwide (Siegel et al. 2020). Moreover, current treatments rely on surgery and radiation procedures that cause pain, permanent disfiguration, and sequelae that decrease the quality of life for survivors. Chemotherapy agents, including taxanes, anthracyclines, alkylating agents, and antimetabolites, have not significantly increased survival rates or quality of life for oral cancer patients. New treatments are clearly needed to improve outcomes for this patient population (D’Cruz et al. 2018; Hertrampf et al. 2020; Retzbach et al. 2018).
Lectins have been identified as unique agents that can be administered orally to inhibit tumor cell growth, motility, and cancer progression (Hasan et al. 2007; Pryme et al. 2006; Pusztai et al. 2008; Yau et al. 2015). For example, Maackia amurensis seed lectin (MASL) has been used as a medicinal plant in parts of Asia for several centuries (Beloborodova et al. 2004; Fedoreev et al. 2010; Ian’kova et al. 2002; Saratikov et al. 2003; Shim et al. 2014; Wang et al. 2000) and is being examined in human clinical trials aimed at oral cancer (NCT04188665 2020).
Over 90% of oral cancers are oral squamous cell carcinomas (OSCC) (D’Cruz et al. 2018; Retzbach et al. 2018). OSCC cells often exhibit increased expression of tumor promoters including TGFβ and PDPN (Li et al. 2018; Retzbach et al. 2018). PDPN is a transmembrane receptor with an extracellular domain that can serve as a functionally relevant OSCC biomarker and chemotherapeutic target (Krishnan et al. 2019; Krishnan et al. 2018; Retzbach et al. 2018). Antibodies against PDPN can inhibit OSCC cell growth, motility, and tumor progression (Kaneko et al. 2017; Ochoa-Alvarez et al. 2015).
The extracellular domain of PDPN is O-glycosylated with sialic acid α2,3 linked to galactose (Quintanilla et al. 2019; Renart et al. 2015). MASL has a high affinity for these moieties (Imberty et al. 2000; Van Damme et al. 1997), and targets PDPN, and possibly other sialic acid modified receptors, to inhibit OSCC cell viability and motility (Krishnan et al. 2018; Ochoa-Alvarez et al. 2015; Retzbach et al. 2018). However, mechanisms by which MASL affects OSCC cell behavior have not been elucidated. In this study, we utilized comprehensive nonbiased transcriptome analysis combined with transcription factor reporter assays to investigate the effects of MASL on OSCC gene expression.
Methods
Cell culture and motility assays
HSC-2 cells (Riken RCB1945) were maintained in DMEM (Hyclone SH30021) supplemented with 25 mM HEPES (Hyclone SH30237) and FBS (Seradigm 1400–500) at 37°C in 5% CO2 and 100% humidity as described (Ochoa-Alvarez et al. 2015). Cells were plated at 80% confluence on 6 well tissue culture cluster plates (Falcon 353224), cultured overnight, treated with 0 nM, 770 nM, or 1925 nM MASL (Sentrimed) for 12 hours, washed thrice with PBS, transferred to microcentrifuge tubes with rubber policemen, pelleted at 14,000xg, aspirated, and frozen at −80°C. Sister plates were also scratched 6 hours after MASL addition, and incubated with MASL for an additional 18 hours to measure cell migration as the distance traveled from the edge into the center of the wounds as described (Krishnan et al. 2013; Krishnan et al. 2015; Ochoa-Alvarez et al. 2015).
RNA isolation and sequence analysis
Total RNA was extracted from frozen cell pellets using Qiagen RNeasy Plus Universal mini kit according to manufacturer’s instructions (Qiagen, Hilden, Germany). RNA library preparations and sequencing reactions were conducted at GENEWIZ, LLC. (South Plainfield, NJ, USA). RNA samples were quantified using Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA) and RNA integrity was checked using Agilent TapeStation 4200 (Agilent Technologies, Palo Alto, CA, USA).
RNA sequencing libraries were prepared using the NEBNext Ultra RNA Library Prep Kit for Illumina following manufacturer’s instructions (NEB, Ipswich, MA, USA). Briefly, mRNAs were first enriched with Oligo(dT) beads. Enriched mRNAs were fragmented for 15 minutes at 94 °C. First strand and second strand cDNAs were subsequently synthesized. cDNA fragments were end repaired and adenylated at 3’ends, and universal adapters were ligated to cDNA fragments, followed by index addition and library enrichment by limited-cycle PCR. The sequencing libraries were validated on the Agilent TapeStation (Agilent Technologies, Palo Alto, CA, USA), and quantified by using Qubit 2.0 Fluorometer (Invitrogen, Carlsbad, CA) as well as by quantitative PCR (KAPA Biosystems, Wilmington, MA, USA).
Sequencing libraries were clustered on 1 lane of a flowcell. After clustering, the flowcell was loaded on the Illumina HiSeq instrument (4000 or equivalent) according to manufacturer’s instructions. The samples were sequenced using a 2×150bp Paired End (PE) configuration. Image analysis and base calling were conducted by the HiSeq Control Software (HCS). Raw sequence data (.bcl files) generated from Illumina HiSeq was converted into fastq files and de-multiplexed using Illumina’s bcl2fastq 2.17 software. One mismatch was allowed for index sequence identification.
Raw data were converted to transcripts per million (TPM) values. RNA transcripts were filtered based on an initial TPM of greater than 1 in any sample group, and required to be either all above or all below untreated control samples. Differential gene expression was set at a threshold of 35% difference between each MASL treated sample, including both 770 nM and and 1925 nM treatments, and both untreated control samples with p<0.06 by t-test. The goa_human GO list (https://genome.sph.umich.edu/wiki/Gene_Ontology) was used to cluster differentially expressed genes based on biological process. Data from this study can be accessed with BioSample accession SAMN14979424 (https://www.ncbi.nlm.nih.gov/biosample/14979424).
Transcriptional reporter assays
Luciferase reporter constructs were transfected into Hela cells as previously described (Zaki et al. 2013). Pathways, targets, and inducers included Stat3 (TGCTTCCCGAATTCCCGAATTCCCGAATTCCCGAATTCCCGAATTCCCGAACGT) induced by IL6 (50ng/ml, R&D Systems 206-IL-010) (Zaki et al. 2013), SMAD (AGTATGTCTAGACTGAAGTATGTCTAGACTGAAGTATGTCTAGACTGA) induced by TGFβ (5 ng/ml, R&D Systems 240-B-002) (Zaki et al. 2013), Wnt (500ng/ml, Peprotech 315–20) (AGATCAAAGGGGGTAAGAKCAAAGGGGGTAAAATCAAAGGGGGCCCCCTTTGATCTTACCCCCTTTGATCTTACCCCCTTTGATCT) induced by Wnt3a (Zaki et al. 2013), AP-1 (TGAGTCATGAGTCATGAGTCATGAGTCA) induced by phorbol-12-myristate-13-acetate (PMA) (77 ng/ml, Sigma P8139) (Subbaramaiah et al. 2001; Zaki et al. 2013), NFkB (GCTACAAGGGACTTTCCGCTGGGGACTTTCCAGG) induced by PMA (Chang et al. 1994; Zaki et al. 2013), E2F (TTTCGCGCTTTCGCGC) induced by PMA (Subbaramaiah et al. 2001; Zaki et al. 2013), Myc (CACGTGCACGTGCACGTGCACGTG) induced by PMA (Laherty et al. 1997; Zaki et al. 2013), Ets (GACCGGAAGTAGTTCCGGTCGACCGGAAGTAGTTCCGGTC) induced by PMA (Subbaramaiah et al. 2001; Zaki et al. 2013), Notch (CAAACAAAAAATTCTTTTTCGTGAAGAACTCCAAAAAT) induced by PMA (Zaki et al. 2013), Hedgehog (GAAGACCACCCACAATGAAGACCACCCACAATGAAGACCACCCACAATGAAGACCACCCACAATGAAGACCACCCACAATGAAGACCACCCACAAT) induced by PMA (Zaki et al. 2013), FOXO (CAAAACAACAAAACAACAAAACAACAAAACAA) (Zaki et al. 2013), miR-21 (GTACATCAACATCAGTCTGATAAGCTACCCGGGTCAACATCAGTCTGATAAGCTAG) (Huang et al. 2009), K-Ras (AGGGCGGTGTGGGAAGAGGGAAGAGGGGGAGG) (Morgan et al. 2016), AhR (CTCCAGGCTCTTCTCACGCAACTCCGGGGCAC) (Saatcioglu et al. 1990), and pTK control vector (Zaki et al. 2013). Twenty four hours after transfection, cells were incubated with pathway inducers for 30 minutes, treated with MASL for 4–6 hours, lysed with One-Glo luciferase assay reagents (Promega), and luminescence was measured with a GloMax Multi+ detection system equipped with Instinct Software (Promega). Values were normalized to untreated HeLa control cells.
Drug cytotoxicity assays
MASL (Sentrimed), paclitaxel (VWR IC19353205), cisplatin (VWR 89150–632), doxorubicin (VWR IC0215910105), etoposide (VWR 102516–332), or 5-fluorouracil (VWR TCF0151–5G), methotrexate (VWR 97065–094) were added to 80% confluent HSC-2 cell cultures alone or in combination to yield final concentrations as indicated in text and corresponding figure legends. Cells were then incubated for 24 hours. Alamar Blue (BioRad BUF012A) was then added to wells at a 10% final concentration and incubated 4 hours to assay with excitation and emission at 570 nm and 600 nm, respectively. Effects of MASL on cell numbers were also evaluated by phase contrast microscopy on a Ziess Axiovert microscope as described (Ochoa-Alvarez et al. 2015; Ochoa-Alvarez et al. 2012).
Western blotting
Cells were rinsed with PBS, transferred to microcentrifuge tubes with rubber policemen, pelleted at 14,000xg, aspirated, and frozen at −80°C. Western blotting was performed as described previously (Alexander et al. 2004; Ochoa-Alvarez et al. 2015). Cells were lysed in a lysis buffer (2% SDS, 10% glycerol, 10 mM EDTA, 50 nM DTT, 50 mM NaF, 0.2 mM Na3VO4, and 1 mM PMSF in 62.5 mM Tris pH 6.8), sonicated, and clarified by centrifugation. Protein (15 μg/lane) was resolved by SDS-PAGE, transferred to Immobilon-P membranes (Millipore IH1079562), and incubated with antisera specific for PDPN (NZ-1 a gift from Yukinari Kato), Sox2 (Cell Signaling Technology 3579), pSTAT1 (Cell Signaling Technology 7649T), STAT1 (Cell Signaling Technology 14994T), phospho-Smad1/5 (Cell Signaling Technology 9516T), Smad5 (Cell Signaling Technology 12534T), MAPK (Cell Signaling Technologies 9102), phosphor-MAPK (Cell Signaling Technologies 9106), and β-actin (Sigma A1978). Primary antibodies were recognized by appropriate secondary anti-IgG antibodies conjugated to horseradish peroxidase including mouse (Invitrogen 31430), rat (EMD Millipore AP136P), and rabbit (Cell Signaling Technology 7074P2) and detected using enhanced chemiluminescence (Thermo Scientific 32106). Signal was quantitated with ImageJ software. Gels were stained with Coomassie and membranes were stained with India ink to verify equal loading and transfer after blotting.
Results
MASL inhibits OSCC cell motility
HSC-2 cells were used to model human OSCC cells in this study. These cells were derived from the mouth floor of a 69 year old male, are relatively drug resistant, HPV negative, and exhibit significant invasive tumorigenic potential (Harada et al. 2014; Krishnan et al. 2019; Ochoa-Alvarez et al. 2015; Retzbach et al. 2018; Yamanouchi et al. 2018). They also exhibit robust PDPN expression and are responsive to PDPN antibodies and MASL (Krishnan et al. 2019; Ochoa-Alvarez et al. 2015; Retzbach et al. 2018).
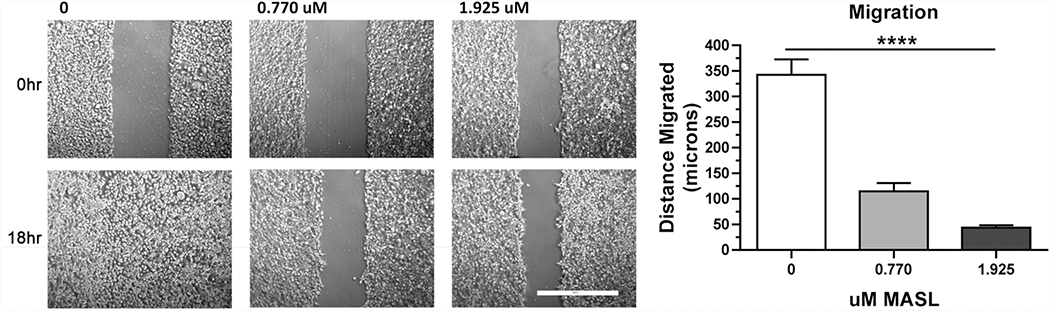
MASL inhibited HSC-2 cell motility in a dose responsive manner as previously reported (Ochoa-Alvarez et al. 2015). Effects of MASL on migration were assayed by wound healing assays as shown in Figure 1a. Quantitation of these data revealed that HSC-2 cells treated with 0, 770, or 1925 nM MASL migrated an average of 343±30, 115±16, and 44±5 microns (mean±SEM, n=3), respectively, as shown in Figure 1b. Therefore, 770 and 1925 nM MASL effectively inhibited HSC-2 cell motility by an average of 66% and 86%, respectively, in these assays.
Figure 1: MASL inhibits OSCC cell migration.
Confluent HSC-2 cells were treated with 0, 770, or 1925 nM MASL for six hours, scratched, and then incubated with MASL for an additional 18 hours. Cells were visualized directly after wounding (0 hr) and 18 hours after wounding (18 hr) as indicated (bar=500 microns). Cell migration was quantified as the distance migrated into the wound, measured at five locations per wound, and shown as mean+SEM (n=3). Quadruple asterisks indicate p<0.0001 by ANOVA.
MASL affects OSCC cell gene expression
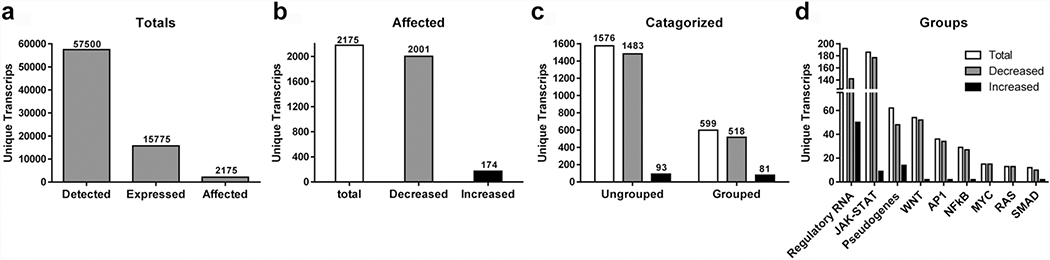
We employed RNA-Seq to acquire a nonbiased global assessment of the effects of MASL on the HSC-2 OSCC cell transcriptome. Cells were treated with MASL for 12 hours to allow for transcriptional effects, but limit MASL cytotoxic effects triggered by cell cycle checkpoints. A total of 57500 unique RNA transcripts were identified in HSC-2 cells by this method as shown in Figure 2a. These data compare well with 54261 total unique transcripts detected by RNA-Seq analysis of human OSCC tissue samples recently reported by Tang et al (Tang et al. 2020). Out of the total number of unique RNA transcripts, 15775 (27.4%) were deemed to be expressed at biologically relevant levels of at least one transcript per million (TPM) in either untreated or MASL treated cells as shown in Figure 2a.
Figure 2: MASL affects OSCC cell transcriptome composition.
RNA-Seq analysis was performed on HSC- 2 cells treated with 0, 770, or 1925 nM MASL for 12 hours. (a) 57500 gene transcripts were detected, with 15775 expressed at >1 transcript per million (TPM), and 2175 with differential expression compared to untreated control samples. (b) MASL decreased the expression of 2001 genes, and increased the expression of 174 genes compared to untreated controls (>35% difference with p<0.06 by t-test, n=2). (c) Differentially expressed transcripts were categorized by GO terms with 599 grouped (518 decreased, 81 increased) and 1576 without GO associations (1483 decreased, 93 increased). (d) MASL affected mRNA levels of 142 regulatory RNAs including miRNA and lncRNA (50 increased, 142 decreased); 186 genes involved in JAK-STAT signaling (8 increased, 177 decreased), 62 pseudogenes (14 increased, 48 decreased), 54 genes in WNT signaling (2 increased, 52 decreased), 36 genes in AP1 signaling (2 increased, 34 decreased), 29 genes in NFkB signaling (2 increased, 27 decreased), 15 genes decreased by Myc signaling, 13 genes decreased by Ras signaling, and 12 genes in TGFβ-SMAD signaling (2 increased, 10 decreased).
Of the 15775 “expressed” transcripts, 2175 displayed expression levels with at least a 35% difference between untreated controls compared to either group of MASL treated cells. MASL decreased the expression of 2001 of these genes, and induced the expression of 174. Therefore, MASL affected the production 13.8% of genes expressed by HSC-2 OSCC cells, with 92% of these differentially expressed genes (DEGs) suppressed by MASL and 8% induced by MASL as shown in Figure 2b. Gene ontology (GO) listings were used to categorize 599 of these DEGs, with expression of 518 (87%) decreased by MASL and 81 (13%) increased by MASL. The remaining 1576 genes were not grouped by gene ontology, with expression of 1483 (94%) decreased by MASL and 93 (6%) increased by MASL as shown in Figure 2c.
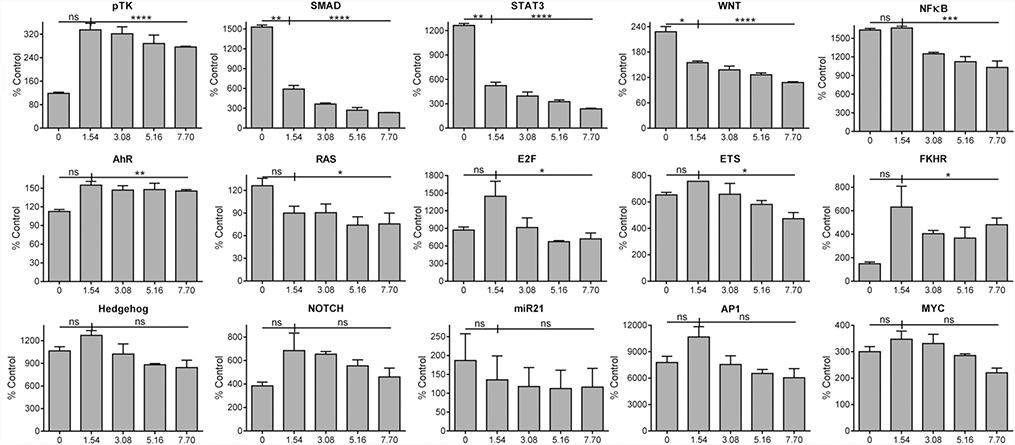
MASL affects the activity of specific transcriptional pathways
HeLa cells transfected with Luciferase reporter constructs were used to assess the effects of MASL on a variety of transcriptional signaling pathways including Stat3, SMAD, Wnt, AP-1, NFkB, E2F, Myc, Ets, Notch, Hedgehog (Hg), FKHR (FOXO), miR-21, K-Ras, and AhR. Inhibition of signaling events was seen in many of these pathways in response to MASL treatment. In particular, MASL significantly inhibited TGFβ-SMAD, JAK-STAT, and Wnt-βCTN signaling in a dose responsive manner across all concentrations ranging from 1.5 to 7.7 μM as shown in Figure 3.
Figure 3: MASL affects specific transcriptional activation pathways.
HeLa cells transfected with Luciferase reporter constructs to detect SMAD, STAT3, Wnt, NFκB, AhR, Ras, E2F, Ets, FKHR (FOXO1), Hedgehog, Notch, miR21, AP1, Myc, or the empty pTK parental vector were incubated with 0, 1.54, 3.08, 5.16, or 7.70 μM MASL for 4–6 hours as indicated. Luminescence values were normalized to untreated nontransfected control cells and are shown as percent control (mean+SEM, n=2). Single, double, triple, quadruple asterisks, and ns indicate p<0.05, 0.01, 0.001, 0.0001, and >0.05, respectively, by one-tailed t-test (0 vs 1.54 μM) or ANOVA.
Pathways analyzed by reporter constructs shown in Figure 3 were used with GO classifications to group differentially expressed genes identified by RNA-Seq. As shown in Figure 2d, regulatory RNA and JAK-STAT accounted for 32% and 31% of all categorized genes affected respectively. This indicates that MASL affected regulatory factors and the JAK-STAT pathway most prominently. MASL-treated OSCC cells downregulated 86% of the categorized genes overall. MASL treated cells showed a >74% downregulation of genes in all individual categories.
JAK-STAT related pathways had the highest number of genes downregulated at 177, followed by regulatory RNA genes at 142, Wnt-βCTN at 52, pseudogenes at 48, AP1 at 34, NFkB at 27, MYC at 15, RAS at 13 and SMAD pathways at 10. 1483 genes were downregulated that were not categorized in this investigation. Regulatory RNA had the highest number of upregulated genes at 50, followed by pseudogenes at 14, JAK-STAT at 9. The Wnt-βCTN, TGFβ-SMAD, NFκB and AP1 pathways each contained two genes upregulated induced by MASL. 93 genes were upregulated that were not categorized in this investigation as shown in Figure 2d.
MASL regulates expression of specific genes
GO categories classified 599 of the 2175 DEGs as shown in Figure 2d. About 42% of these products are noncoding RNAs and include regulatory RNAs such as lnRNA, miRNAs, and pseudogenes. MASL increase the expression of about 75% or these products, while about 25% were increased. The remaining 345 transcripts encode proteins grouped into members of the JAK-STAT, Wnt-βCTN, AP1, NFκB, Myc, Ras, and SMAD pathways as shown in Figure 2d.
MASL affected the expression of 192 regulatory RNAs. MASL increased the expression of 50, and decreased the expression of 142 unique transcripts. These include miRNAs and lnRNAs that are likely to regulate fundamental aspects of OSCC cell behavior. These effects are likely to be pleiotropic and complex in combination with effects of MASL on gene coding mRNAs.
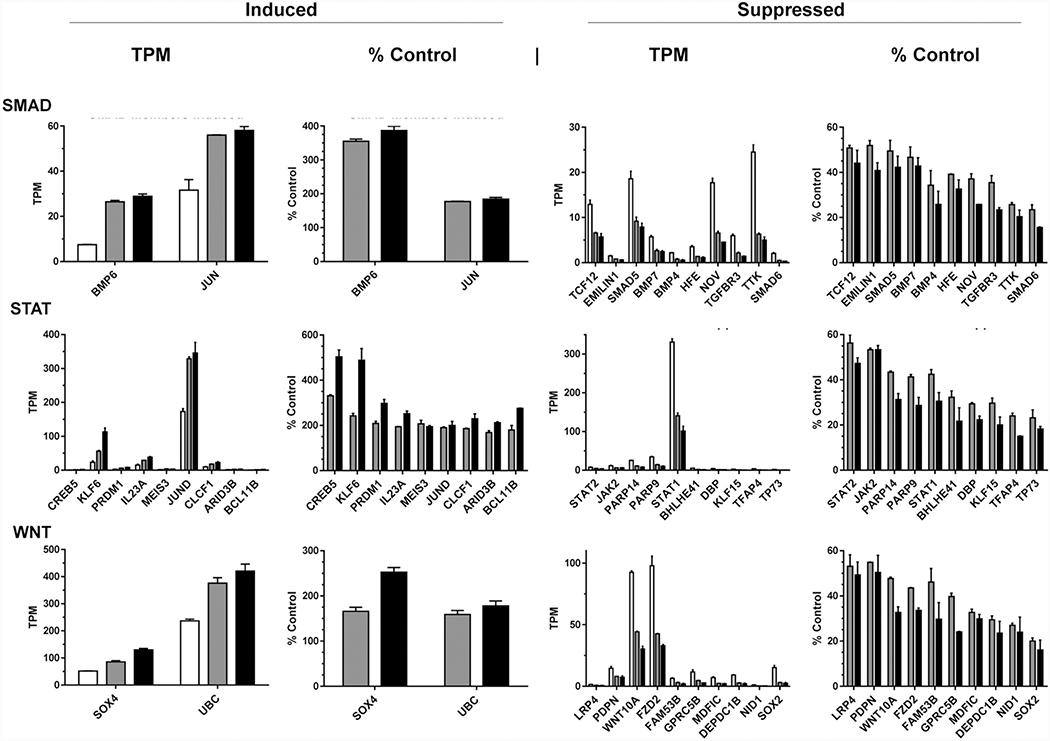
The largest classified group of protein coding DEGs (31%) were classified as members of the JAK-STAT pathway. MASL decreased the expression of 177 (95%) of these genes, and increase the expression of 9 as shown in Figures 2d. For example, expression of Creb5, Klf6, and Jund increased from an average of 0.43, 23, and 173 TMP in untreated cells to 1.4, 56, and 329 TPM in cells treated with 770 nM MASL, and 2.1, 113, and 346 TPM in cells treated with 1925 nM MASL, respectively, as shown in Figure 4. However, MASL decreased the expression of genes encoding JAK-STAT regulators including Jak2, Stat2, and Stat1 from an average of 7.5, 11, and 331 TMP in untreated cells to 4.3, 6.0, and 140 TPM in cells treated with 770 nM MASL, and 3.6, 6.0, and 101 TPM in cells treated with 1925 nM MASL, respectively, as shown in Figure 4. MASL also decreased the expression of genes encoding Parp14 and Parp9 from an average of 25 and 34 TMP in untreated cells to 11 and 14 TPM in cells treated with 770 nM MASL, and 7.6 and 9.8 TPM in cells treated with 1925 nM MASL, respectively, as shown in Figure 4.
Figure 4: MASL affects expression of specific genes involved in SMAD, STAT, and Wnt signaling in OSCC cells.
HSC-2 cells were treated for 12 hours with 0, 770, or 1925 nM MASL and examined by RNA-Seq. Expression of gene transcripts were quantitated and shown as TPM and the percent of untreated control cells (mean+SEM, n=2) as indicated.
MASL affected the expression of 54 genes classified as members of the Wnt signaling pathway. MASL inhibited the expression of 52 (96%) of these genes, and increased the expression of 2 as shown in Figure 2d. Expression of Sox4 (SRY-box transcription factor 4) and UBC (polyubiquitin C) increased from an average of 52 and 237 TMP in untreated cells to 85 and 376 TPM in cells treated with 770 nM MASL, and 130 and 421 TPM in cells treated with 1925 nM MASL, respectively, as shown in Figure 4. However, in contrast to Sox2, MASL decreased Sox4 mRNA expression from an average of 15 TPM in untreated cells to 3.0 and 2.4 TPM in cells treated with 770 and 1925 nM MASL, respectively. MASL also decreased expression of canonical Wnt signaling mediators including Wnt10a, Fzd2, and Lrp4 with average mRNA levels at 93, 98, 1.4 TPM in untreated cells to 44, 43, and 0.8 TPM in cells treated with 770 nM MASL, and 30, 33, and 0.7 TPM in cells treated with 1925 nM MASL, respectively, as shown in Figure 4. In addition to Wnt receptors, MASL also decreased Pdpn mRNA expression from an average of 15 TPM in untreated cells to 8.0 and 7.4 TPM in cells treated with 770 and 1925 nM MASL, respectively, as shown in Figure 4.
MASL affected expression of 12 genes classified as members of the TGFβ-SMAD pathways. MASL decreased the expression of 10 of these genes, and increase the expression of 2 as shown in Figures 2d. The expression of Bmp6 and Jun mRNAs increased from an average of 7.5 and 32 TMP in untreated cells to 27 and 56 TPM in cells treated with 770 nM MASL, and 28 and 58 TMP in cells treated with 1925 nM MASL, respectively, as shown in Figure 4. However, MASL also decreased the expression of Bmp7, Bmp4, Smad5, Smad6, and Tgfbr3 from an average of 5.7, 2.2, 19, 2.1, and 6.0 TMP in untreated cells to 2.7, 0.75, 9.2, 0.49, and 2.1 TPM in cells treated with 770 nM MASL, and 2.5, 0.57, 7.9, 0.32, and 1.4 TPM in cells treated with 1925 nM MASL, respectively, as shown in Figure 4.
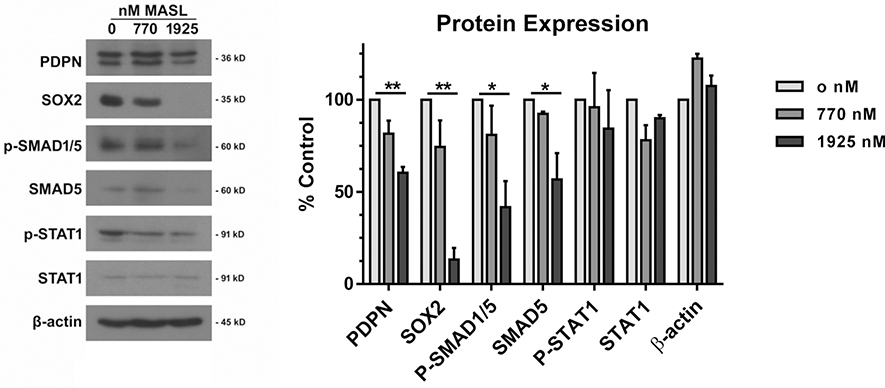
MASL regulates PDPN, SOX2, and SMAD protein expression.
Effects of MASL on gene expression were evaluated at the protein level by Western blot analysis. Treatment with 770 nM MASL decreased PDPN and SOX2 expression to 82% and 74% of levels expressed in untreated control cells, while 1925 nM MASL decreased their expression to 60% and 13% of controls, respectively, as shown in Figure 5. MASL also decreased levels of active and total SMAD expression consistent with decreased TGFβ signaling evident from reporter construct activity shown in Figure 3. Treatment with 770 nM MASL decreased phosphorylated SMAD1/5 and total SMAD5 expression to 81% and 92% of levels expressed by untreated control cells, while 1925 nM MASL decreased their expression to 41% and 57% of controls, respectively (see Figure 5). In contrast, MASL did not affect the expression of phosphorylated or total STAT1. Expression or β-actin was also not affected by MASL treatment as shown in Figure 5.
Figure 5: MASL inhibits expression of PDPN, SOX2, and SMAD proteins.
(a) HSC-2 cells were treated for 12 hours with 0, 770, or 1925 nM MASL and examined by Western blotting with apparent molecular weights shown as indicated. (b) Protein expression was quantitated by image densitometry and shown as percent of untreated control cells (mean+SEM, n=3). Single and double asterisks indicate p<0.05 and 0.01 by ANOVA.
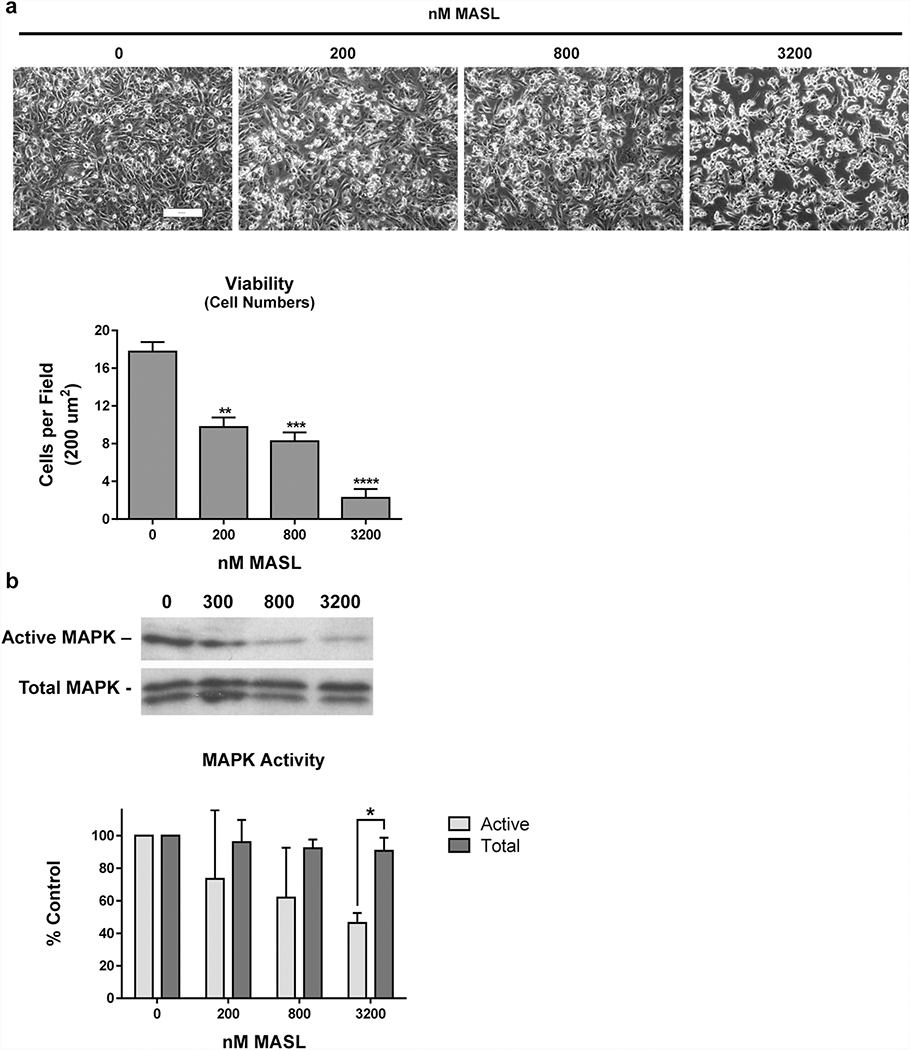
MASL inhibits MAPK activation and OSCC cell proliferation.
Previous studies have found that MASL can inhibit OSCC cell viability in addition to motility (Ochoa-Alvarez et al. 2015). In addition to inhibiting HSC-2 cell migration (shown in Figure 1), MASL inhibited HSC-2 cell viability in a dose responsive manner. MASL inhibited HSC-2 cell proliferation by an average of 24%, 47%, 61%, and 75% at 300, 600, 1200, and 2400 nM, respectively, as shown in Figure 6a.
Figure 6: MASL inhibits OSCC cell growth and MAPK activity.
(a) HSC-2 cells were exposed to 0, 200, 800, and 3200 nM MASL for 28 hours and visualized by phase contrast microscopy (bar=200 microns). Cell viability was measured as the number of cells per 200 μm2 field. (b) Levels of total and active MAPK were evaluated by Western blotting, quantitated by image densitometry, and converted to percent of untreated control cells. Data are shown as mean+SEM (n=4). Single, double, triple, and quadruple asterisks indicate p<0.05, 0.01, 0.001, and 0.0001 compared to nontreated controls, respectively.
MAPK activity is associated with PDPN expression and implicated in OSCC progression (Peng et al. 2018; Shao et al. 2020; Sikorska et al. 2019). Accordingly, the effects of MASL on MAPK activity correlated with cytotoxicity. MASL decreased MAPK activity along with inhibiting HSC-2 cell proliferation. MASL inhibited MAPK activity by an average of 26%, 48%, and 54%, while decreasing HSC-2 cell proliferation by 45%, 53%, and 87% at 200, 600, and 3200 nM, respectively, compared to untreated control cells as shown in Figure 6b.
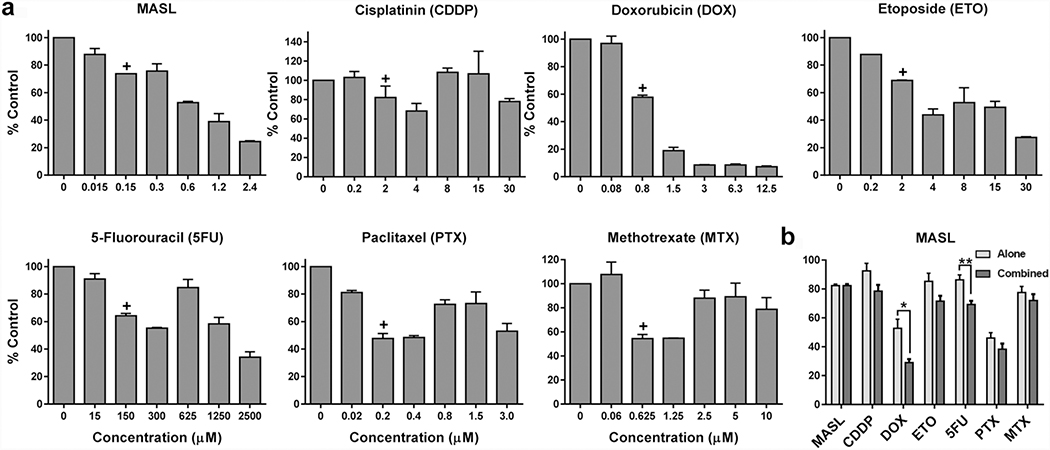
MASL inhibits OSCC cell growth in combination with other agents
Since combination therapies can often enhance squamous cell carcinoma treatment (Peng et al. 2019), MASL cytotoxicity was examined in combination with an array of standard traditional chemotherapy agents. These compounds included the alkylating agent cisplatin, anthracycline doxorubicin, topoisomerase inhibitor etoposide, nucleotide analog 5-fluorouracil, tubulin disruptor paclitaxel, and antimetabolite methotrexate. Concentrations of these agents that exhibited a cytotoxicity range between 10%–50% were combined with 150 nM MASL, which inhibited HSC-2 cell viability by an average of 18% as shown in Figure 7. While an additive effect was seen with MASL and these compounds, the most powerful synergy was seen with doxorubicin, which inhibited HSC-2 cell viability by an average of 53% alone compared to 71% in combination with 150 nM MASL.
Figure 7: MASL works with other agents to inhibit OSCC cell viability.
(a) HSC-2 cells were exposed to MASL, cisplatin (CDDP), doxorubicin (DOX), etoposide (ETO), 5-fluorouracil (5FU), paclitaxel (PTX), methotrexate (MTX) for 24 hours as indicated. (b) Cells were treated with concentrations of each compound indicated by plus sign in panel a in combination with 150 nM MASL or NZ1 antibody with and without complement as indicated. Cell viability was examined by alamar Blue and shown as percent control (mean+SEM, n=4). Single, double, triple, quadruple asterisks indicate p<0.05, 0.01, 0.001, and 0.0001 by t-test, respectively.
Discussion
OSCC kills and maims over 120 thousand people each year. New treatments are clearly needed for the patient population (Retzbach et al. 2018). MASL has been identified as a potential agent that can target OSCC cells to inhibit oral cancer development (Krishnan et al. 2019; Ochoa-Alvarez et al. 2015; Retzbach et al. 2018). This investigation was performed to elucidate epigenetic effects of MASL on OSCC cells. HSC-2 cells exhibit robust PDPN expression with invasive tumorigenic potential, are responsive to MASL (Krishnan et al. 2019; Ochoa-Alvarez et al. 2015; Retzbach et al. 2018), and were used to model human OSCC cells in this study. However, generalities of these findings are likely since MASL also inhibits motility and growth of OSCC cells derived from other patients, murine melanoma cells, and Src transformed fibroblasts (Ochoa-Alvarez et al. 2015; Ochoa-Alvarez et al. 2012; Retzbach et al. 2018). In addition, MASL also inhibits PDPN associated cytokine induced inflammation in reconstituted epidermis (Honma et al. 2017) and arthritic inflammation of human chondrocyte and mouse models (Carpintero-Fernandez et al. 2020). While specific pathways may vary across cell models, effects on gene expression are likely to be shared based on the fundamental consequence of reduced cell growth, motility, and inflammatory response in these varied systems.
MASL inhibited HSC-2 cell motility and growth in a dose responsive manner as previously reported (Ochoa-Alvarez et al. 2015). MASL also inhibited MAPK activity along with decreasing HSC-2 cell viability. In addition, results from this study indicate that MASL cytotoxicity can be enhanced in combination with other chemotherapeutic agents exemplified by doxorubicin and 5-fluorouricil. These data are consistent with reports indicating that combination therapies targeting MAPK offer potential to treat hypopharyngeal squamous cell carcinoma (Peng et al. 2019).
We employed RNA-Seq to obtain a nonbiased and comprehensive analysis of how MASL affects gene expression in HSC-2 OSCC cells. Twardziok et al reported that Mistletoe lectin affects the mRNA levels of 1004 genes, representing about 6% of the 17300 gene human Ewing Sarcoma cell transcriptome (Twardziok et al. 2017). Regarding OSCC, Tang et al identified 24,582 genes expressed by oral cancers, with 16% of these genes expressed to a different level compared to noncancerous oral epithelium (Tang et al. 2020). Wang et al reported similar finding of 15.5% of the transcriptome being affected by oral carcinogenesis (Wang et al. 2019). Here, we found that MASL affects the expression of 2175 genes, representing about 14% of the 15775 gene HSC-2 cell transcriptome. MASL affected the production of transcripts include regulatory RNAs which are likely to exert effects that act in concert with gene encoding proteins. Results from transcriptional reporter assays indicate that genes classified as members of the JAK-STAT, TGFβ-SMAD, and Wnt-βCTN signaling pathways are significantly affected by MASL exposure.
MASL affected 186 genes classified as members of the JAK-STAT pathway. For example, MASL increased the mRNA expression of JAK-STAT member Klf6, which is potential tumor suppressor associated with inhibition of OSCC motility, invasion, and tumor progression (Hsu et al. 2017). In addition, MASL decreased the expression of genes encoding JAK-STAT inducers including Jak2, Stat2, and Stat1. MASL also decreased the expression of genes encoding Parp14 and Parp9. These data suggest that MASL can coordinately decrease mitogenic JAK-STAT signaling (O’Shea et al. 2015; Thomas et al. 2015) and decrease the ability of cells to repair cytotoxic DNA damage (D’Andrea 2018; Morales et al. 2014). STAT3 and STAT5 promote OSCC viability and proliferation and are considered desirable targets for chemotherapy (Thomas et al. 2015). JAK1/JAK2 kinase blockers can decrease STAT3 expression and reduce OSCC tumor expansion (Sen et al. 2015). In fact, the use of JAK1/2 kinase inhibitors (ruxolitinib) are being investigated in clinical trials in OSCC patients (NCT03153982 2020).
MASL affected the expression of 12 genes classified as members of the TGFβ-SMAD pathway. Most notably, MASL decreased the expression of Bmp4, Bmp7, Tgfbr3, Smad5, and Smad6. These data suggest that MASL effectively inhibits TGFβ-SMAD signaling events in OSCC cells. TGFβ signaling plays important roles in OSCC tumor progression (Pang et al. 2018). For example, TGFβ activation induces matrix metalloproteinase (MMP) and integrin production to increase OSCC cell motility and invasion (Takayama et al. 2009). This has led to enticing therapeutic efforts to inhibit OSCC TGFβ activity. This is exemplified by development of a bifunctional anti-PD-L1/TGFb trap protein (M7824) which is being evaluated in clinical trials on oral cancer patients (NCT04247282 2020). However, OSCC TGFβ signaling events are complex and involve substantial paracrine crosstalk. For example, Li et al have reported that OSCC cells express PDPN which correlates with smooth muscle actin (αSMA) in adjacent fibroblasts, and promotes their conversion in cancer associated fibroblasts (CAFs). These activated CAFs produce TGFβ which signals through SMADs to induce PDPN expression in adjacent OSCC cells. PDPN then works in concert with EGF, AKT, and ERK signaling pathways to induce MMP expression and promote OSCC cell expansion (Li et al. 2018).
MASL targets PDPN, which is an oncogenic receptor suppressed by contact normalization. Contact normalization is mediated by intercellular communication that implicates cadherins and Wnt signaling in the MASL mechanism of action (Krishnan and Goldberg 2015; Krishnan et al. 2019). Wnt activated β-catenin signaling acts in concert with PDPN to promote tumorigenesis including malignant glioma (Lu et al. 2016), mammary carcinoma (Bresson et al. 2018), and OSCC development (Liu et al. 2017; Liu et al. 2013), and Wnt blockers exemplified by WNT974/LGK974 are currently being evaluated in clinical trials aimed at oral cancer (NCT01351103 2020). The Wnt-βCTN pathway in clearly of prime interest with respect to MASL mechanism of action.
MASL effected the expression of genes characterized as members of the Wnt-βCTN signaling pathway. For example, MASL increased Sox4 mRNA expression. Sox4 activity has been linked to OSCC development, treatment resistance, and tumor aggression (Liu et al. 2016; Yoon et al. 2015). However, Sox4 also protects p53 from ubiquitination and subsequent degradation to inhibit tumor cell proliferation, trigger apoptosis, and inhibit tumor progression (Pan et al. 2009). Interestingly, MASL also increased UBC mRNA expression. UBC was expressed at a higher level (over 4 fold) than Sox4, and maintains ubiquitin levels needed to degrade proteins in response to stress (Alfano et al. 2016). In contrast to Sox4, MASL decreased Sox2 mRNA expression. Sox 2 activity has been implicated in cancer stem cell production leading to OSCC progression, metastasis, and unfavorable patient prognosis (Chou et al. 2015; Chung et al. 2018; Ren et al. 2016; Schaefer and Lengerke 2020).These data suggest that consequences of increased UBC and decreased Sox2 might override effects of Sox4 induction that may occur in response to stress resulting MASL exposure. In addition to Sox2, MASL also decreased expression of mRNA encoding the Wnt10a ligand along with its Fzd2 and Lrp4 receptors. Along with Wnt receptors, MASL decreased Pdpn mRNA expression. The PDPN receptor has been identified as a powerful tumor promotor that can be targeted by MASL to inhibit OSCC growth and motility (Krishnan et al. 2019; Ochoa-Alvarez et al. 2015; Retzbach et al. 2018). These data suggest that MASL decreases the expression of Wnt ligands and receptors in concert with Sox transcription factors and PDPN to inhibit cancer stem cell production and tumor promotion.
Effects of MASL on PDPN, SOX2, and SMAD proteins were confirmed by Western blot analysis. Treatment with 770 nM and 1925 nM MASL decreased PDPN expression by 18% and 40%, and decreased SOX2 expression by 26% and 87%, respectively. Treatment with 770 nM and 1925 nM MASL also decreased levels of active SMAD1/5 expression by 19% and 59%, respectively. This inhibition of SMAD activation by MASL is consistent with suppression of TGFβ signaling exhibited by reporter construct activity. However, unlike SMAD activity, MASL did not affect the expression active STAT protein reflected by transcription reporter constructs. These contrasting results might be due to the relative stability of STAT1, with a half-life of 24 hours or more (Andrejeva et al. 2002; Lee et al. 1997), which is much higher than the relatively short SMAD half-life of 8 hours or less (Zhang et al. 2001). These dynamics would translate to inhibition of SMAD protein levels, but not STAT protein levels, during a 12 hour MASL treatment.
Conclusion
MASL targets PDPN and perhaps other extracellular moieties that contain α2,3-sialic acid residues to inhibit OSCC cell growth and motility. Taken together, results from this study indicate that this mechanism of action involves inhibition of JAK-STAT, TGFβ-SMAD, and Wnt-βCTN signaling events. Effects of MASL treatment on key elements of these pathways identified here should be evaluated in samples obtained from clinical studies of oral cancer patients treated with MASL (NCT04188665 2020). It will be helpful to determine the relevance of each pathway that may be used to develop novel therapies that combine MASL with other agents including STAT, SMAD, or Wnt blockers to more effectively treat oral cancer, and perhaps other cancers that utilize these pathways.
Acknowledgments
We are grateful to Dr. Yukinari Kato (Tohoku University) for providing PDPN specific antibody. This study was supported in part by funding from the Osteopathic Heritage Foundation, New Jersey Health Foundation, Sentrimed, Camden Health Research Initiative, and NIH grant CA235347 to GSG, and a contract from the University of Mississippi Medical Center Cancer Institute to PB.
Footnotes
Conflict of interest
GSG has intellectual property and ownership in Sentrimed, Inc. which is developing agents that target PDPN to treat diseases, including cancer and arthritis. No other authors have conflicts to report.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Alexander DB et al. (2004) Normal cells control the growth of neighboring transformed cells independent of gap junctional communication and SRC activity Cancer Research 64:1347–1358 [DOI] [PubMed] [Google Scholar]
- Alfano C, Faggiano S, Pastore A (2016) The Ball and Chain of Polyubiquitin Structures Trends Biochem Sci 41:371–385 doi: 10.1016/j.tibs.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Andrejeva J, Young DF, Goodbourn S, Randall RE (2002) Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons Journal of virology 76:2159–2167 doi: 10.1128/jvi.76.5.2159-2167.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloborodova EI, Gaisaev RO, Saratikov AS (2004) [Effect of the phytohepatoprotective agent maxar on the hepatic morphofunctional status in patients with chronic hepatitis] Klinicheskaia meditsina 82:39–42 [PubMed] [Google Scholar]
- Bresson L, Faraldo MM, Di-Cicco A, Quintanilla M, Glukhova MA, Deugnier MA (2018) Podoplanin regulates mammary stem cell function and tumorigenesis by potentiating Wnt/beta-catenin signaling Development 145 doi: 10.1242/dev.160382 [DOI] [PubMed] [Google Scholar]
- Carpintero-Fernandez P, Varela-Eirin M, Lacetera A, Gago-Fuentes R, Fonseca E, Martin-Santamaria S, Mayan MD (2020) New therapeutic strategies for osteoarthritis by targeting sialic acid receptors biomolecules 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Zhang J, Lombardi L, Neri A, Dalla-Favera R (1994) Mechanism of expression and role in transcriptional control of the proto-oncogene NFKB-2/LYT-10 Oncogene 9:923–933 [PubMed] [Google Scholar]
- Chou MY, Hu FW, Yu CH, Yu CC (2015) Sox2 expression involvement in the oncogenicity and radiochemoresistance of oral cancer stem cells Oral oncology 51:31–39 doi: 10.1016/j.oraloncology.2014.10.002 [DOI] [PubMed] [Google Scholar]
- Chung JH, Jung HR, Jung AR, Lee YC, Kong M, Lee JS, Eun YG (2018) SOX2 activation predicts prognosis in patients with head and neck squamous cell carcinoma Scientific reports 8:1677 doi: 10.1038/s41598-018-20086-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea AD (2018) Mechanisms of PARP inhibitor sensitivity and resistance DNA Repair (Amst) 71:172–176 doi: 10.1016/j.dnarep.2018.08.021 [DOI] [PubMed] [Google Scholar]
- D’Cruz AK, Vaish R, Dhar H (2018) Oral cancers: Current status Oral oncology 87:64–69 doi: 10.1016/j.oraloncology.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Fedoreev SA et al. (2010) Maksar: A preparation based on amur maackia Pharmaceutical Chemistry Journal 38:605–610 [Google Scholar]
- Harada K, Ferdous T, Ueyama Y (2014) Establishment of 5-fluorouracil-resistant oral squamous cell carcinoma cell lines with epithelial to mesenchymal transition changes International journal of oncology 44:1302–1308 doi: 10.3892/ijo.2014.2270 [DOI] [PubMed] [Google Scholar]
- Hasan SS, Ashraf GM, Banu N (2007) Galectins - potential targets for cancer therapy Cancer Lett 253:25–33 [DOI] [PubMed] [Google Scholar]
- Hertrampf K, Pritzkuleit R, Baumann E, Wiltfang J, Wenz HJ, Waldmann A (2020) Oral cancer awareness campaign in Northern Germany: first positive trends in incidence and tumour stages Journal of cancer research and clinical oncology 146:2489–2496 doi: 10.1007/s00432-020-03305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M, Shibuya T, Fujii M, Iinuma S, Saito N, Kishibe M, Ishida-Yamamoto A (2017) Maackia amurensis seed lectin can suppress IL-22induced hyperproliferative reconstituted epidermis Journal of Dermatalogical Science 86:E46 [Google Scholar]
- Hsu LS et al. (2017) KLF6 inhibited oral cancer migration and invasion via downregulation of mesenchymal markers and inhibition of MMP-9 activities Int J Med Sci 14:530–535 doi: 10.7150/ijms.19024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TH et al. (2009) Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion The Journal of biological chemistry 284:18515–18524 doi: 10.1074/jbc.M109.006676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ian’kova VI, Ivanova IL, Fedoreev SA, Kulesh NI (2002) [Antioxidant activity of the hepatoprotector maxar in experimental diabetes] Eksperimental’naia i klinicheskaia farmakologiia 65:33–36 [PubMed] [Google Scholar]
- Imberty A, Gautier C, Lescar J, Perez S, Wyns L, Loris R (2000) An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides JBiolChem 275:17541–17548 [DOI] [PubMed] [Google Scholar]
- Kaneko MK et al. (2017) ChLpMab-23: Cancer-Specific Human-Mouse Chimeric Anti-Podoplanin Antibody Exhibits Antitumor Activity via Antibody-Dependent Cellular Cytotoxicity Monoclonal antibodies in immunodiagnosis and immunotherapy doi: 10.1089/mab.2017.0014 [DOI] [PubMed] [Google Scholar]
- Krishnan H, Goldberg GS (2015) Contact normalization or escape from the matrix In: Kandous M (ed) Intercellular communication and cancer. Springer-Verlag, Heidelberg, pp 297–342 [Google Scholar]
- Krishnan H, Miller WT, Blanco FJ, Goldberg GS (2019) Src and podoplanin forge a path to destruction Drug Discov Today 24:241–249 doi: 10.1016/j.drudis.2018.07.009 [DOI] [PubMed] [Google Scholar]
- Krishnan H et al. (2013) Serines in the intracellular tail of podoplanin (PDPN) regulate cell motility The Journal of biological chemistry 288:12215–12221 doi: 10.1074/jbc.C112.446823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H et al. (2018) Podoplanin: An emerging cancer biomarker and therapeutic target Cancer science 109:1292–1299 doi: 10.1111/cas.13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H, Retzbach EP, Ramirez MI, Liu T, Li H, Miller WT, Goldberg GS (2015) PKA and CDK5 can phosphorylate specific serines on the intracellular domain of podoplanin (PDPN) to inhibit cell motility Exp Cell Res 335:115–122 doi: 10.1016/j.yexcr.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN (1997) Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression Cell 89:349–356 doi: 10.1016/s0092-8674(00)80215-9 [DOI] [PubMed] [Google Scholar]
- Lee CK, Bluyssen HA, Levy DE (1997) Regulation of interferon-alpha responsiveness by the duration of Janus kinase activity The Journal of biological chemistry 272:21872–21877 doi: 10.1074/jbc.272.35.21872 [DOI] [PubMed] [Google Scholar]
- Li YY, Zhou CX, Gao Y (2018) Interaction between oral squamous cell carcinoma cells and fibroblasts through TGF-beta1 mediated by podoplanin Exp Cell Res 369:43–53 doi: 10.1016/j.yexcr.2018.04.029 [DOI] [PubMed] [Google Scholar]
- Liu B, Chen W, Cao G, Dong Z, Xu J, Luo T, Zhang S (2017) MicroRNA-27b inhibits cell proliferation in oral squamous cell carcinoma by targeting FZD7 and Wnt signaling pathway Archives of oral biology 83:92–96 doi: 10.1016/j.archoralbio.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Liu J et al. (2013) Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974 Proceedings of the National Academy of Sciences of the United States of America 110:20224–20229 doi: 10.1073/pnas.1314239110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cui L, Huang J, Ji EH, Chen W, Messadi D, Hu S (2016) SOX4 Promotes Progression in OLP-Associated Squamous Cell Carcinoma J Cancer 7:1534–1540 doi: 10.7150/jca.15689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P et al. (2016) Malignant gliomas induce and exploit astrocytic mesenchymal-like transition by activating canonical Wnt/beta-catenin signaling Medical oncology 33:66 doi: 10.1007/s12032-016-0778-0 [DOI] [PubMed] [Google Scholar]
- Morales J et al. (2014) Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases Crit Rev Eukaryot Gene Expr 24:15–28 doi: 10.1615/critreveukaryotgeneexpr.2013006875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RK, Batra H, Gaerig VC, Hockings J, Brooks TA (2016) Identification and characterization of a new G-quadruplex forming region within the kRAS promoter as a transcriptional regulator Biochim Biophys Acta 1859:235–245 doi: 10.1016/j.bbagrm.2015.11.004 [DOI] [PubMed] [Google Scholar]
- NCT01351103 (2020) A Study of LGK974 in Patients With Malignancies Dependent on Wnt Ligands Clinicaltrialsgov
- NCT03153982 (2020) Ruxolitinib in Operable Head and Neck Cancer Clinicaltrialsgov
- NCT04188665 (2020) Using MASL to Combat Oral Cancer Clinicaltrialsgov
- NCT04247282 (2020) Anti-PD-L1/TGF-beta Trap (M7824) Alone and in Combination With TriAd Vaccine and N-803 for Resectable Head and Neck Squamous Cell Carcinoma Not Associated With Human Papillomavirus Infection Clinicaltrialsgov
- O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A (2015) The JAK-STAT pathway: impact on human disease and therapeutic intervention Annu Rev Med 66:311–328 doi: 10.1146/annurev-med-051113-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Alvarez JA et al. (2015) Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms Oncotarget 6:9045–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Alvarez JA et al. (2012) Plant lectin can target receptors containing sialic Acid, exemplified by podoplanin, to inhibit transformed cell growth and migration PLoSONE 7:e41845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X et al. (2009) Induction of SOX4 by DNA damage is critical for p53 stabilization and function Proceedings of the National Academy of Sciences of the United States of America 106:3788–3793 doi: 10.1073/pnas.0810147106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Tang YL, Liang XH (2018) Transforming growth factor-beta signaling in head and neck squamous cell carcinoma: Insights into cellular responses Oncology letters 16:4799–4806 doi: 10.3892/ol.2018.9319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Deng Z, Pan H, Gu L, Liu O, Tang Z (2018) Mitogen-activated protein kinase signaling pathway in oral cancer Oncology letters 15:1379–1388 doi: 10.3892/ol.2017.7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X et al. (2019) Co-targeting PI3K/Akt and MAPK/ERK pathways leads to an enhanced antitumor effect on human hypopharyngeal squamous cell carcinoma Journal of cancer research and clinical oncology 145:2921–2936 doi: 10.1007/s00432-019-03047-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryme IF, Bardocz S, Pusztai A, Ewen SW (2006) Suppression of growth of tumour cell lines in vitro and tumours in vivo by mistletoe lectins HistolHistopathol 21:285–299 [DOI] [PubMed] [Google Scholar]
- Pusztai A, Bardocz S, Ewen SW (2008) Uses of plant lectins in bioscience and biomedicine Front Biosci 13:1130–1140 [DOI] [PubMed] [Google Scholar]
- Quintanilla M, Montero-Montero L, Renart J, Martin-Villar E (2019) Podoplanin in Inflammation and Cancer Int J Mol Sci 20 doi: 10.3390/ijms20030707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZH, Zhang CP, Ji T (2016) Expression of SOX2 in oral squamous cell carcinoma and the association with lymph node metastasis Oncology letters 11:1973–1979 doi: 10.3892/ol.2016.4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart J, Carrasco-Ramirez P, Fernandez-Munoz B, Martin-Villar E, Montero L, Yurrita MM, Quintanilla M (2015) New insights into the role of podoplanin in epithelial-mesenchymal transition Int Rev Cell Mol Biol 317:185–239 doi: 10.1016/bs.ircmb.2015.01.009 [DOI] [PubMed] [Google Scholar]
- Retzbach EP et al. (2018) Podoplanin emerges as a functionally relevant oral cancer biomarker and therapeutic target Oral oncology 78:126–136 doi: 10.1016/j.oraloncology.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Saatcioglu F, Perry DJ, Pasco DS, Fagan JB (1990) Multiple DNA-binding factors interact with overlapping specificities at the aryl hydrocarbon response element of the cytochrome P450IA1 gene Mol Cell Biol 10:6408–6416 doi: 10.1128/mcb.10.12.6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saratikov AS, Livshits NS, Burchenkova FI, Kadychagova NG, Akhmedzhanov RR, Bashirova LV (2003) [Preclinical study of maxar safety] Eksperimental’naia i klinicheskaia farmakologiia 66:53–55 [PubMed] [Google Scholar]
- Schaefer T, Lengerke C (2020) SOX2 protein biochemistry in stemness, reprogramming, and cancer: the PI3K/AKT/SOX2 axis and beyond Oncogene 39:278–292 doi: 10.1038/s41388-019-0997-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M et al. (2015) JAK kinase inhibition abrogates STAT3 activation and head and neck squamous cell carcinoma tumor growth Neoplasia 17:256–264 doi: 10.1016/j.neo.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Cui D, Ma C, Chen P, Zhou B, Tao R, Wang J (2020) Transcriptome profiling of tolerogenic dendritic cells conditioned with dual mTOR kinase inhibitor, AZD8055 Int Immunopharmacol 81:106241 doi: 10.1016/j.intimp.2020.106241 [DOI] [PubMed] [Google Scholar]
- Shim M, Bae JY, Lee YJ, Ahn MJ (2014) Tectoridin from Maackia amurensis modulates both estrogen and thyroid receptors Phytomedicine : international journal of phytotherapy and phytopharmacology 21:602–606 doi: 10.1016/j.phymed.2013.10.022 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020 CA Cancer J Clin 70:7–30 doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Sikorska J, Gawel D, Domek H, Rudzinska M, Czarnocka B (2019) Podoplanin (PDPN) affects the invasiveness of thyroid carcinoma cells by inducing ezrin, radixin and moesin (E/R/M) phosphorylation in association with matrix metalloproteinases BMC cancer 19:85 doi: 10.1186/s12885-018-5239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Bulic P, Lin Y, Dannenberg AJ, Pasco DS (2001) Development and use of a gene promoter-based screen to identify novel inhibitors of cyclooxygenase-2 transcription J Biomol Screen 6:101–110 doi: 10.1177/108705710100600206 [DOI] [PubMed] [Google Scholar]
- Takayama S, Hatori M, Kurihara Y, Kinugasa Y, Shirota T, Shintani S (2009) Inhibition of TGF-beta1 suppresses motility and invasiveness of oral squamous cell carcinoma cell lines via modulation of integrins and down-regulation of matrix-metalloproteinases Oncology reports 21:205–210 [PubMed] [Google Scholar]
- Tang M et al. (2020) Transcriptome analysis of tongue cancer based on highthroughput sequencing Oncology reports 43:2004–2016 doi: 10.3892/or.2020.7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, Snowden JA, Zeidler MP, Danson SJ (2015) The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours British journal of cancer 113:365–371 doi: 10.1038/bjc.2015.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardziok M et al. (2017) Transcriptomic and proteomic insight into the effects of a defined European mistletoe extract in Ewing sarcoma cells reveals cellular stress responses BMC complementary and alternative medicine 17:237 doi: 10.1186/s12906-017-1715-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme EJ, Van Leuven F, Peumans WJ (1997) Isolation, characterization and molecular cloning of the bark lectins from Maackia amurensis GlycoconjJ 14:449–456 [DOI] [PubMed] [Google Scholar]
- Wang R et al. (2019) Integrative analysis of gene expression profiles reveals distinct molecular characteristics in oral tongue squamous cell carcinoma Oncology letters 17:2377–2387 doi: 10.3892/ol.2018.9866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Li JS, Jiang ZR, Kubo H, Higashiyama K, Ohmiya S (2000) Lupin alkaloids from Chinese Maackia amurensis Chem Pharm Bull (Tokyo) 48:641–645 [DOI] [PubMed] [Google Scholar]
- Yamanouchi R, Harada K, Ferdous T, Ueyama Y (2018) Low carbonyl reductase 1 expression is associated with poor prognosis in patients with oral squamous cell carcinoma Mol Clin Oncol 8:400–406 doi: 10.3892/mco.2018.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau T, Dan X, Ng CC, Ng TB (2015) Lectins with potential for anti-cancer therapy Molecules 20:3791–3810 doi: 10.3390/molecules20033791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon TM et al. (2015) SOX4 expression is associated with treatment failure and chemoradioresistance in oral squamous cell carcinoma BMC cancer 15:888 doi: 10.1186/s12885-015-1875-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MA et al. (2013) Cytotoxicity and modulation of cancer-related signaling by (Z)- and (E)-3,4,3’,5’-tetramethoxystilbene isolated from Eugenia rigida Journal of natural products 76:679–684 doi: 10.1021/np300893n [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chang C, Gehling DJ, Hemmati-Brivanlou A, Derynck R (2001) Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase Proceedings of the National Academy of Sciences of the United States of America 98:974–979 doi: 10.1073/pnas.98.3.974 [DOI] [PMC free article] [PubMed] [Google Scholar]