Abstract
Background:
Gastric cancer is an aggressive malignancy with poor prognosis. Although obesity is a risk factor, an association between overweight and better survival has been reported. We explored the genomic implications of such association.
Methods:
Data from 940 patients were analyzed using Cox regression models and ROC curves to assess body mass index (BMI) and prognostic nutritional index (PNI) as predictors of survival. The exome sequencing of a random subset was analyzed to determine copy number variation (CNV) and single nucleotide variation (SNV), using Kruskal-Wallis and chi-square tests to evaluate their clinical implications.
Results:
Overall survival was lower in patients with BMI ≤ 24.9 and PNI ≤ 29 (p<0.001). BMI and survival were directly correlated (HR: 0.972, 95% CI: 0.953, 0.992; p-value<0.007). A higher PNI correlated with improved survival (HR: 0.586, 95% CI: 0.429, 0.801; p-value <0.001). We found a PNI cut-off point of 41.00 for overall survival. Genomic analysis showed an association between lower BMI, less CNV events (p-value = 0.040) and loss of tumor suppressor genes (p-value = 0.021).
Conclusions:
BMI and PNI are independent factors for overall survival in gastric cancer, probably linked to variations in genomic intratumoral alterations.
Keywords: Gastric cancer, Obesity, Body mass/BMI, Epidemiology
1. Introduction
Less than a century ago, gastric cancer (GC) was the most common cancer in the United States and perhaps around the world. Currently, GC ranks fifth in incidence and is the third leading cause of cancer-related mortality (1, 2). Different risks factors have been associated with the incidence of gastric adenocarcinoma (GA) -which is the most common type of gastric cancer generally divided into intestinal and diffuse subtype- such as dietary habits like high intake of salt, smoked or preserved food. It is estimated that, globally, 11% of stomach cancers are due to smoking. In Europe, 17% of the cases of GA were associated with smoking (3). Pathogens like the Epstein-Barr virus (EBV) and Helicobacter pylori [H. pylori] have also been associated with the development of GA (4). GC is assumed to originate from a sequential accumulation of molecular and genetic alterations at the gastric mucosa level (5), but the mechanism of carcinogenesis is complex and poorly understood (6, 7). Obesity is a risk factor for many types of cancers, with almost 20% of the overall cancer incidence related to this condition and many proposed molecular mechanisms linking them (8). From the molecular perspective obesity produces a chronic inflammatory status in patients with raised levels of cell proliferative factors like insulin, insulin growth factor (IGF), leptin, and reactive oxygen species (ROS), which are related to an increased risk for DNA damage and carcinogenesis (9).
A new GC classification was recently proposed according to molecular characteristics, and four subtypes were determined. This classification is a valuable complement to histopathology (10), and includes: I) EBV-infected tumors associated with PIK3CA mutation, PD-L1/2 overexpression, EBV-CIMP, CDKN2A silencing, and immune cell signaling; II) microsatellite instability (MSI) in tumors displaying characteristic hypermutations, gastric-CIMP, and MLH1 downregulation; III) the genomically stable (GS) type associated with diffuse tumors, CDH1 and RHOA mutations, or CLDN18-ARHGAP fusions; IV) chromosomal instability (CIN) in tumors associated with marked aneuploidy and focal amplification of receptor tyrosine kinases and TP53 mutations.
The association between body mass index (BMI) and gastric cancer risk has been explored in many studies. In a meta-analysis of 24 prospective studies, Chen et al. (2013) demonstrated that a high BMI is associated with gastric cardia cancer (GCC) but not with gastric non-cardia cancer (GNCC) (11); however, the exact biological mechanisms are not well defined yet. An intriguing relationship between overweight patients and better prognosis has been found in different cancers. For GC a large cohort study of 7760 South Korean patients revealed that a preoperative BMI ranging from 23 to 30 kg/m2, had better disease-specific and overall survivals, than normal-weight patients (12).
Several studies have shown that nutrition and immune status are closely associated with tumor progression and prognosis (13, 14). Moreover, the prognostic nutritional index (PNI), calculated by combining serum albumin levels and total circulating lymphocyte count, could reflect the nutritional and immune status of cancer patients. Introduced by Onodera et al. (1984), PNI showed a direct correlation between its value and the risk of postoperative complications in patients with gastrointestinal cancers (15). Furthermore, studies have shown that pretreatment PNI correlated with the prognosis of patients with a variety of human malignancies, such as lung cancer, esophageal squamous cancer, and gastric cancer (16–19).
The medical hypothesis known as “obesity paradox” suggests a potential protective effect on mortality in overweight and mild obese patients, but the underlying mechanisms are still elusive (20–22). Here we evaluated the significance of the most commonly used nutritional indexes, BMI and PNI, in predicting the overall survival of patients with gastric cancer by exploring the genomic mechanisms underlying the relationship between nutritional status and survival, using exome sequencing of a random subset of tumor samples, and correlating the results with such nutritional indexes.
2. Material and methods
2.1. Patients and data collection
All patients with diagnosis of gastric cancer treated at the National Cancer Institute (Mexico, NCI-Mx) between January 2005 and December 2018 were considered in this study. Retrospective information was obtained from the electronic medical records of each patient, such as physical examination, blood cytology, clinical biochemistry (including blood serum albumin [BSA] at diagnosis) and other indicators relevant to BMI, PNI, and survival. Social workers obtained information about socioeconomic status (SES) and education for each patient during the first interview, during the admission process to the Hospital (NCI-Mx). Then, this information is recorded in the electronic medical record (EMC). Therefore, the information about SES and education used in our analyses was collected from the ECM by trained personnel. SES classification is generated based on the patient’s income, home characteristics, and family environment, and is classified as follows: level 1 (lowest stratum) means that only 5% of the actual cost of medical care is paid, level 2 pays 10%, level 3 pays 25%, level 4 pays 53%, and level 5 pays 75%. This assessment is a standard for national public hospitals in Mexico. Surgery was classified as radical or partial gastrectomy. Some patients received chemotherapy according to the TNM staging. Patients without BSA levels or total lymphocytes counts or whose primary tumor was not of gastric origin were excluded from the study.
BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2) and classified into four categories, according to the World Health Organization (WHO) criteria: underweight (< 18.5 kg/m2), normal weight (18.5 – 24.9 kg/m2), overweight (> 25–29.9 kg/m2), and obese (> 30 kg/m2) (23). PNI was calculated as follows: (BSA in g/dL x 10) + (0.005 x total lymphocyte count in cells/mL), as previously reported (15) and grouped into quartiles Q1: < 29 (low); Q2: 29 – 34 (medium); Q3: > 34 (high).
A small cohort of 34 patients was randomly selected from the total of patients included in the study to explore genomic somatic mutations in gastric tumors throughout exome sequencing, which were then associated with BMI and PNI. Paraffin blocks of paired tumors and healthy gastric tissues obtained by gastrectomy were used to extract genomic DNA.
2.2. DNA extraction, library preparation, and exome sequencing
DNA of paired tumor:normal formalin fixed paraffin embedded tissues was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). Libraries were prepared using the Illumina Nextera Rapid Capture V1.2, and exome sequencing was performed using Illumina HiSeq 2500 following the manufacturer’s instructions. Bioinformatics software GATK, MuTec2, and CNVkit were used to identify pathogenic single nucleotide variations (SNV) and copy number variations (CNV) in cancer driver genes, whose clinical meaning was evaluated by comparing the results with ClinVar and COSMIC databases.
2.3. Statistical analysis
Survival curves were calculated using the Kaplan-Meier method. We used multivariable Cox regression models to investigate the associations between BMI and PNI in gastric cancer and overall survival. We estimated hazard ratios (HRs) and explored the influence of confounders by using the Akaike information criterion (AIC). Therefore, potential survival predictors were quantified and compared. For this purpose, we used ROC curves to quantify the powers or abilities of predictors of survival. A ROC curve is a graphical plot that illustrates the performance of a binary classifier system as its discrimination threshold is varied. The association between the patient’s mutational profile, BMI, and PNI was evaluated using Kruskal-Wallis and chi-square tests; p-values <0.05 were considered statistically significant. All analyses were done using R software (R Project for Statistical Computing).
3. Results
A total of 940 GC patients treated from 2005 to 2018 were included in this study. They had a mean age of 55.42 years (standard deviation [SD]: 13.92 years) at diagnosis. Most patients were male (57.56%) and had a very low socioeconomic status (49.89%). Most patients were also diagnosed in advanced stages (III and IV, 84.78%). The fundus and body of the stomach (33.82%) were the most affected areas, and adenocarcinoma was the most frequent histologic type (87.02%). Most subsets were positive for signet-ring cell formation (54.25%). Regarding the nutritional status, most patients were normal weight, based on the WHO classification (52.02% with BMI between 18.5 and 24.9 kg/m2) and had a mean PNI of 33.14 (SD: 0.83) at diagnosis; many of them had values higher than 34 (47.76%) (Table 1).
Table 1.
Sociodemographic, clinical, and histopathologic characteristics of patients with gastric cancer treated at the National Cancer Institute-Mexico from 2005 to 2018 (N=940).
| Variable | Mean | SD | |
|---|---|---|---|
| Age (years) | 55.42 | 13.92 | |
| Variable | n | % | |
| Sex | |||
| Female | 399 | 42.44 | |
| Socioeconomic status | |||
| 1 | 469 | 49.89 | |
| 2 | 361 | 38.4 | |
| 3 | 95 | 10.1 | |
| 4 | 8 | 0.85 | |
| 5 | 7 | 0.74 | |
| Education | |||
| Middle school or lower | 730 | 77.65 | |
| High school | 131 | 13.93 | |
| College or higher | 79 | 8.4 | |
| BMI (kg/m2) | |||
| Underweight (< 18.5 kg/m2) | 91 | 9.68 | |
| Normal weight (18.5 – 24.9 kg/m2) | 489 | 52.02 | |
| Overweight (≥ 25 kg/m2) | 360 | 38.29 | |
| PNI | |||
| < 29 | 254 | 27.02 | |
| 29 – 34 | 237 | 25.21 | |
| > 34 | 449 | 47.76 | |
| Clinical stage | |||
| IA | 11 | 1.17 | |
| IB | 25 | 2.65 | |
| IIA | 28 | 2.97 | |
| IIB | 39 | 4.14 | |
| IIIA | 53 | 5.63 | |
| IIIB | 19 | 2.02 | |
| IIIC | 156 | 16.59 | |
| IV | 502 | 53.4 | |
| Location | |||
| Fundus or body | 318 | 33.82 | |
| Antrum and pylorus | 255 | 27.12 | |
| Cardia | 177 | 18.82 | |
| Two or more locations | 190 | 20.21 | |
| Histology | |||
| Adenocarcinoma | 818 | 87.02 | |
| No adenocarcinoma | 122 | 12.97 | |
| Histologic subtypes (Lauren classification) | |||
| Diffuse | 431 | 45.85 | |
| Intestinal | 298 | 31.70 | |
| Mixed | 46 | 4.89 | |
| Histologic grade | |||
| Low | 190 | 20.21 | |
| High | 616 | 65.53 | |
| Signet-ring cell* | |||
| Positive | 510 | 54.25 | |
SD: Standard deviation. Socioeconomic status 1: very low, 5: very high. BMI: Body mass index. PNI: Prognostic nutritional index. Histologic grade: low (well differentiated and moderately differentiated), high (poorly differentiated).
For gastric adenocarcinoma only.
3.1. Distribution of clinical characteristics according to BMI and PNI
We did not find significant differences in age, height, sex, and socioeconomic status or education level regarding BMI. We also did not find differences related to BMI in tumor location and histology. In adenocarcinomas, histologic grade did not show a differential distribution regarding BMI. We found statistical differences in weight, stage, and histologic subtypes regarding BMI. This differential distribution was due to the absence of early stages in the BMI < 18.5 category, in comparison with 5.26% for the 18.5 to 24.9 group, and 4.12% for the ≥ 25 group. We also found a higher frequency of non-adenocarcinoma tumors in the highest BMI categories (12.88% for the 18.5 to 24.9 group and 13.61% for the ≥ 25 group) in comparison with the lowest BMI category (10.98%).
Regarding the PNI, we did not observe statistically significant differences in terms of sex distribution or histologic grade of adenocarcinomas. Remarkably, most of the sociodemographic and clinical factors showed statistically significant differences between the PNI categories. Patients in the highest PNI category (> 34) were 3.75 and 3.58 years younger than the lowest PNI categories.
We did not find a statistical relationship in the distribution of the PNI (low, intermediate, and high) in terms of sex, histologic type and grade, and the presence of signet-ring cells. Age (p < 0.001), weight (p < 0.001), and height (p = 0.036) were different according to the nutritional index. The highest percentage with the lowest PNI was found in the group with lower socioeconomic status, whereas a lower percentage was observed in those with higher socioeconomic levels in the groups with normal and elevated PNI (p = 0.043). We also found a higher percentage of low PNI in patients who did not attend high school (p < 0.001). Similarly, we observed the same pattern of decreased values of PNI in patients with advanced clinical stages (p = 0.001). According to the anatomical location, we found a higher prevalence of all ranges of PNI when the tumor was in the fundus or body of the stomach. We also found that the non-adenocarcinoma histologic subtypes were related to a lower frequency in PNI. The frequencies of the subgroups and the statistical significance of the distribution of clinical characteristics according to BMI and PNI are shown in detail in Table 2.
Table 2.
Socioeconomic and clinicopathologic characteristics of gastric cancer patients treated at the National Cancer Institute - Mexico, from 2005 to 2018 according to BMI and PNI (N=940).
| BMI < 18.5 | BMI 18.5 – 24.9 | BMI ≥ 25 | p-value | Low PNI | Medium PNI | High PNI | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (years) | 54.00 | 17.16 | 56.18 | 14.19 | 54.73 | 12.57 | 0.184 | 57.25 | 13.86 | 57.08 | 14.18 | 53.50 | 13.60 | <0.001 |
| Weight (kg) | 43.52 | 6.04 | 56.30 | 8.33 | 72.94 | 12.76 | 0.001 | 58.03 | 12.29 | 59.99 | 12.56 | 64.13 | 15.18 | <0.001 |
| Height (m) | 1.58 | 0.10 | 1.59 | 0.10 | 1.59 | 0.11 | 0.731 | 1.58 | 0.09 | 1.58 | 0.10 | 1.60 | 0.10 | 0.036 |
| n | % | n | % | n | % | p-value | n | % | n | % | n | % | p-value | |
| Sex | ||||||||||||||
| Female | 39 | 42.85 | 203 | 41.51 | 157 | 43.61 | 0.827 | 98 | 38.58 | 100 | 42.19 | 201 | 44.76 | 0.279 |
| Male | 52 | 57.14 | 286 | 58.48 | 203 | 56.38 | 156 | 61.41 | 137 | 57.80 | 248 | 55.23 | ||
| Socioeconomic status | ||||||||||||||
| 1 | 54 | 59.34 | 250 | 51.12 | 163 | 45.40 | 0.160 | 134 | 52.96 | 118 | 49.78 | 215 | 47.88 | 0.043 |
| 2 | 26 | 28.57 | 185 | 37.83 | 150 | 41.78 | 99 | 39.13 | 97 | 40.92 | 165 | 36.74 | ||
| 3 | 11 | 12.08 | 46 | 9.40 | 38 | 10.58 | 16 | 6.32 | 21 | 8.86 | 58 | 12.91 | ||
| 4 | 0 | 0.00 | 6 | 1.22 | 2 | 0.05 | 3 | 1.18 | 0 | 0.00 | 5 | 1.11 | ||
| 5 | 0 | 0.00 | 2 | 0.04 | 5 | 1.39 | 0 | 0.00 | 1 | 0.04 | 6 | 1.33 | ||
| Education | ||||||||||||||
| Middle school or lower | 76 | 83.51 | 385 | 78.73 | 269 | 74.72 | 0.378 | 213 | 83.85 | 201 | 84.81 | 316 | 70.37 | <0.001 |
| High school | 10 | 10.98 | 66 | 13.50 | 55 | 15.27 | 28 | 11.02 | 20 | 8.43 | 83 | 18.48 | ||
| College or higher | 5 | 5.50 | 38 | 7.77 | 36 | 10.00 | 13 | 5.11 | 16 | 6.75 | 50 | 11.13 | ||
| Clinical Stage | ||||||||||||||
| Early (IA-IB) | 0 | 0.00 | 23 | 5.26 | 13 | 4.12 | 0.010 | 2 | 0.09 | 6 | 2.83 | 28 | 6.84 | 0.001 |
| Advanced (IIA-IV) | 81 | 100 | 414 | 94.73 | 302 | 95.87 | 210 | 99.05 | 206 | 97.16 | 381 | 93.15 | ||
| Location | ||||||||||||||
| Antrum and pylorus | 24 | 26.37 | 136 | 27.81 | 95 | 26.38 | 0.790 | 65 | 25.59 | 67 | 28.27 | 123 | 27.39 | 0.036 |
| Cardia | 19 | 20.87 | 91 | 18.60 | 67 | 18.61 | 37 | 14.56 | 41 | 17.29 | 99 | 22.04 | ||
| Fundus or body | 27 | 29.67 | 159 | 32.51 | 132 | 36.66 | 84 | 33.07 | 85 | 35.86 | 149 | 33.18 | ||
| Two or more locations | 21 | 23.07 | 103 | 21.06 | 66 | 18.33 | 68 | 26.77 | 44 | 18.56 | 78 | 17.37 | ||
| Histology | ||||||||||||||
| Adenocarcinoma | 81 | 89.01 | 426 | 87.11 | 311 | 86.39 | 0.800 | 203 | 79.92 | 210 | 88.60 | 405 | 90.20 | <0.001 |
| No adenocarcinoma | 10 | 10.98 | 63 | 12.88 | 49 | 13.61 | 51 | 20.07 | 27 | 11.39 | 44 | 9.79 | ||
| Signet-ring cell* | ||||||||||||||
| Positive samples | 52 | 64.19 | 259 | 60.79 | 199 | 63.98 | 0.635 | 120 | 59.11 | 128 | 60.95 | 262 | 64.69 | 0.363 |
| Histologic subtypes | ||||||||||||||
| Intestinal | 22 | 29.33 | 165 | 40.84 | 111 | 37.50 | 0.034 | 73 | 39.45 | 85 | 42.92 | 140 | 35.71 | 0.395 |
| Diffuse | 43 | 57.33 | 218 | 53.96 | 170 | 57.43 | 100 | 54.05 | 100 | 50.50 | 231 | 58.92 | ||
| Mixed | 10 | 13.33 | 21 | 5.20 | 15 | 5.07 | 12 | 6.48 | 13 | 6.56 | 21 | 5.35 | ||
| Histologic grade (adenocarcinoma only) | ||||||||||||||
| Low | 15 | 20.00 | 111 | 26.11 | 64 | 20.91 | 0.196 | 44 | 22.11 | 50 | 24.15 | 96 | 24.00 | 0.854 |
| High | 60 | 80.00 | 314 | 73.88 | 242 | 79.08 | 155 | 77.88 | 157 | 75.84 | 304 | 76.00 | ||
Socioeconomic status: 1, very low; 5, very high. BMI: Body mass index, kg/m2. SD: Standard deviation. PNI: Prognostic nutritional index. Low PNI: < 29, Medium: 29 – 34, High: > 34. Histologic grade: low (well differentiated and moderately differentiated), high (poorly differentiated).
For gastric adenocarcinoma only.
3.2. Survival in gastric cancer according to the BMI and PNI
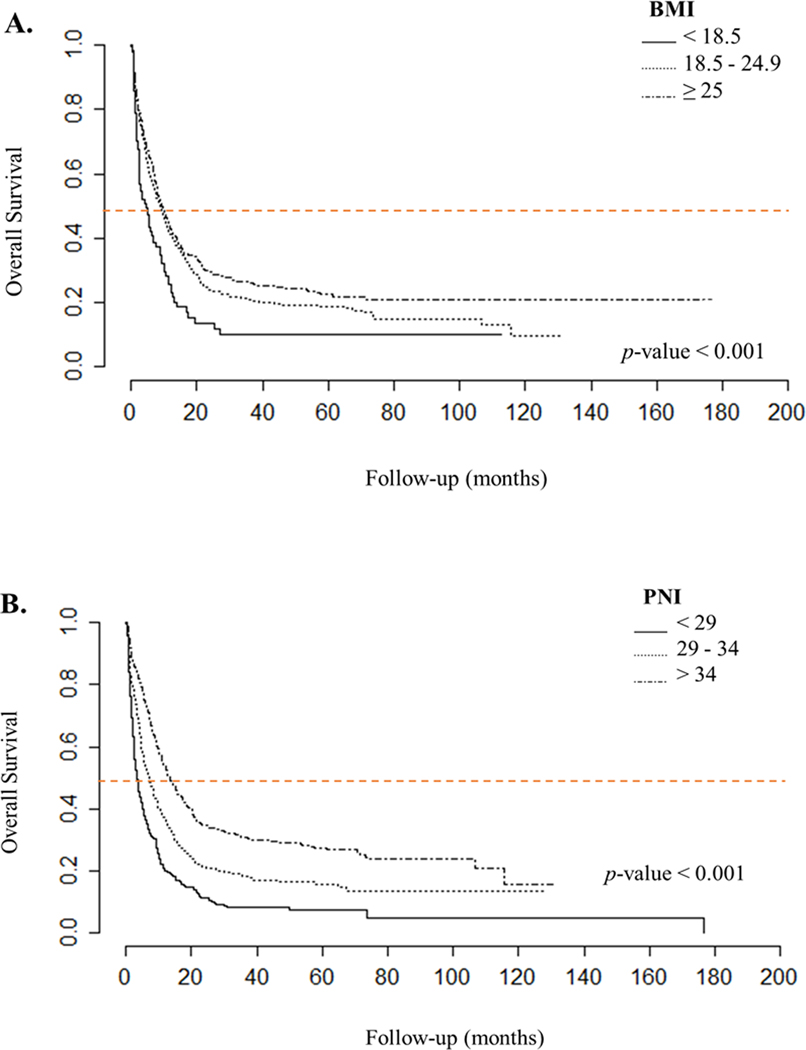
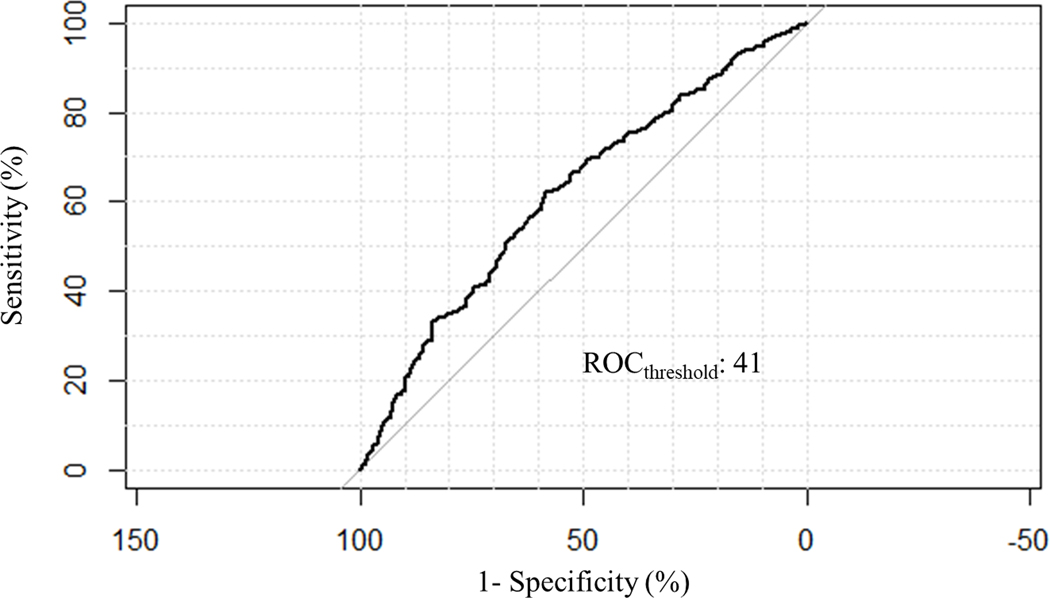
We observed that overweight patients (BMI ≥25 kg/m2) had better overall survival, followed by the group with normal BMI levels, whereas patients with low BMI levels had the worst prognosis (p < 0.001). The median overall survival was 9.4 months for the overweight group, 8.8 months for normal BMI group, and 4.7 months for the low BMI group. A similar pattern was observed in patients with high PNI, who had a median overall survival of 13.47 months, compared with patients with medium PNI (7 months) and low PNI (3.47 months) (p < 0.001, Figure 1). The association between overall survival and clinical stage, anatomical location, histologic type, subtype, and differentiation grade was significant (supplementary figure 1). The Cox regression analysis, adjusted for multiple variables, showed that BMI was a statistically significant independent predictor of survival in gastric cancer; however, the effect size was minimal (HR 0.972, IC95% 0.953–0.992, p = 0.007). Interestingly, when we built a Cox regression model including the PNI, we found that both PNI and BMI were independent predictors of overall survival (HR 0.586, IC95% 0.429 to 0.801, p < 0.001). These findings suggest that overweight and high PNI are protective factors for survival. The results are shown in detail in Table 3. In this same model, other predictors of overall survival were clinical stage, location, and age. Using ROC curves to evaluate the role of BMI and PNI in predicting survival, we observed that BMI was a poor predictor, and PNI had a threshold of 41.00875 (Figure 2). Finally, we found a weak positive correlation between BMI and PNI levels (r = 0.163, p < 0.001, Supplementary Figure 2).
Figure 1.
Kaplan-Meier plots for overall survival in patients with gastric cancer, treated at the National Cancer Institute - Mexico, 2005–2018 according to: A. Body mass index (BMI) (underweight [< 18.5 kg/m2], normal [18.5 kg/m2 − 24.9 kg/m2], overweight [≥ 25 kg/m2]) and B. Prognostic nutritional index (PNI) (low [< 29], medium [29 – 34], high [> 34]). Median survival is shown as an orange line on the graph. Statistical significance between the groups was obtained using the log-rank test.
Table 3.
Multivariable Cox regression model for the association between body mass index (BMI) and overall survival (top), and the association of prognostic nutritional index (PNI) and overall survival adjusted for BMI as a confounder (bottom) in patients with gastric cancer, treated at the National Cancer Institute - Mexico, from 2005 to 2018 (N=940).
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| BMI* | 0.972 | (0.953, 0.992) | 0.007 |
| Clinical stage** | 4.473 | (3.229, 6.196) | <0.001 |
| Histologic grade** | 1.174 | (0.921, 1.496) | 0.195 |
| Location** | 1.113 | (1.030, 1.204) | 0.006 |
| Histologic subtype** | 1.055 | (0.914, 1.217) | 0.462 |
| Age* | 1.007 | (1.001, 1.015) | 0.028 |
| Signet-ring cell** | 1.256 | (1.015, 1.553) | 0.035 |
| Variable | HR | 95% CI | p-value |
| PNI** | 0.586 | (0.429, 0.801) | <0.001 |
| BMI* | 0.975 | (0.956, 0.995) | 0.015 |
| Clinical stage** | 4.351 | (3.139, 6.031) | <0.001 |
| Histologic grade** | 1.170 | (0.918, 1.499) | 0.205 |
| Location** | 1.122 | (1.038, 1.212) | 0.004 |
| Histologic subtype** | 1.086 | (0.939, 1.256) | 0.265 |
| Age* | 1.007 | (1.001, 1.014) | 0.040 |
| Signet-ring cell** | 1.233 | (0.997, 1.524) | 0.053 |
HR: Hazard ratio, 95% CI: 95% Confidence interval. PNI: Prognostic nutritional index. BMI: Body mass index.
As continuous variable.
As dichotomous variable according to ROC threshold: PNI <41 or ≥41; Clinical stage: early (IA-IB) vs advanced (II-IV); Histologic grade: differentiated vs undifferentiated; Signet ring cell: positive or negative. Location: upper, middle vs lower; Histologic subtype: intestinal, diffuse vs others. Statistically significant values (p-value < 0.05) are shown in bold.
Figure 2.
Receiver operating characteristics (ROC) curve for overall survival according to prognostic nutritional index (PNI) in patients with gastric cancer treated at the National Cancer Institute - Mexico, between January 2005 and December 2018.
3.3. Mutational profile of gastric cancer samples
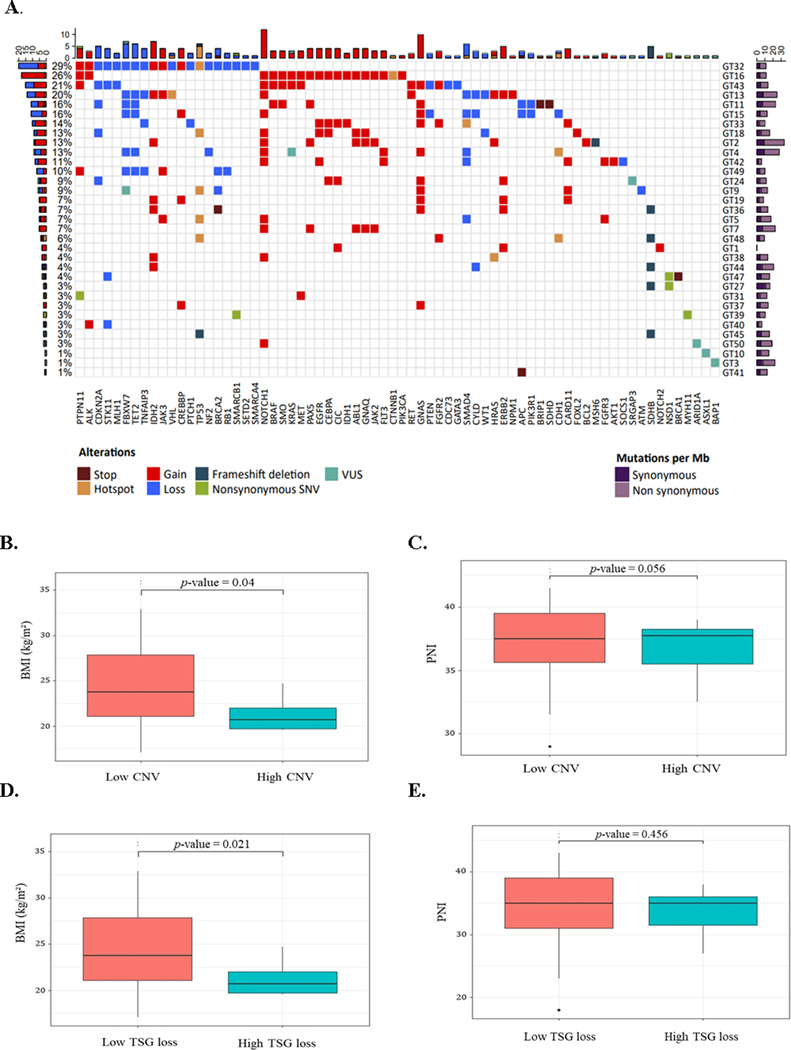
Formalin-fixed samples of tumors and paired healthy gastric tissues from 34 patients were randomly selected from the cohort of 940 patients and analyzed by whole-exome sequencing to explore the genomic landscape. A total of 938, 201 mutations were detected, with a mean of 26, 061 somatic mutations per patient. After exploring the impact of somatic mutations, we identified 106 cancer driver genes affected by CNV or SNV, of which 60 were classified as oncogenes and 46 as tumor suppressor genes. As shown in Figure 3, NOTCH1 was the most frequently mutated gene (35%), followed by GNAS (29%), FBXW7 (21%), and IDH2 (21%). A great number of C<T transitions associated to mutational signature 1 was found in all patients (Supplementary Figure 3). The liver cancer-related signature 23 of unknown origin was the second most common in this cohort, followed by mismatched related signature 6.
Figure 3.
Mutational profile in a subset of randomly selected patients (n=34) with gastric cancer according to body mass index (BMI) and prognostic nutritional index (PNI). A. Matrix of individual somatic alterations per patient (right, GTs) and frequency (left, %) of detection. B. - C. BMI (left) and PNI (right) according to the presence of copy number variation (CNV) events [low CNV < 6 events; high CNV ≥ 6 events]. D. - E. BMI (left) and PNI (right) according to the loss of tumor suppressor genes (TSG) [low TSG < 6 losses; high TSG ≥ 6 losses]. Dots indicate outliers. Statistical differences are shown in boxplots and were determined using Kruskal-Wallis tests. GT: patient code, VUS: variant of uncertain significance, SNV: single nucleotide variation, Mb: megabase.
3.4. PNI, BMI, and mutational profile
After exploring the mutational characteristics, PNI, and BMI of patients, we found associations regarding the number of events related to CNV or TSG loss. As shown in figure 3, a higher BMI was associated with a low number of CNV events (p = 0.040) and with a low number of TSG loss (p = 0.021). To assess the association between mutated genes and the patients, BMI and PNI, we selected those genes mutated in five or more patients. No associations were found between mutations in the GNAS, IDH2, TP53, SMAD4, ERBB2, PTPN11 genes, and CDKN2A and nutritional status. In contrast, mutations in NOTCH1, FBXW7, TET2, and SDHB were related to at least one of the indexes. A higher PNI was associated with unaltered NOTCH copy number (p = 0.041). A higher BMI was associated with unaltered FBXW7 copy number (p = 0.0077), as well as unaltered TET2 copy number (p = 0.0077). In the case of SDHB, we categorized the BMI values into underweight, normal weight, and overweight. We found that unmutated SDHB was associated with a BMI lower than 18.5 kg/m2 (p = 0.0167) (Supplementary Figure 4).
4. Discussion
As reported in previous studies, gastric adenocarcinoma mainly affects patients over 50 years with low socioeconomic status. In the Mexican population, a higher incidence of diffuse-type gastric adenocarcinoma has been found (24), whereas epidemiologic studies in other populations have shown that intestinal type gastric adenocarcinoma is more common (25).
Nutritional status has been studied as a predictor of survival in many diseases, especially in cancer. In our study, we observed that BMI is an independent predictor of survival, where a higher BMI was associated with a better prognosis. This finding agrees with other reports where BMI directly correlates with prognosis and TNM staging. It has been reported that low albumin levels (less than 3.9 g/dL) and low triglyceride concentrations (less than 1.6 mm/L) correlate with poor prognosis (26). Unlike breast cancer, where overweight and obesity are related to poorer survival rates than normal weight (27), we found that gastric cancer patients with a BMI > 25 kg/m2 had better survival rates than those with lower values.
On the other hand, many studies suggest that low PNI correlates with poor prognosis in patients with various cancers, including gastrointestinal cancers (17). Few studies correlate PNI with overall survival in gastric cancer. Although this index is not widely used, it could be more useful than BMI because it correlates the nutritional and immune status of patients. Therefore, we performed a systematic analysis to calculate and validate BMI and PNI as predictors of prognosis in gastric cancer patients according to overall survival, which allowed us to interpret in the best way the nutritional status of patients. We confirmed that BMI and PNI are independent factors of overall survival.
After evaluating the effects of BMI on overall survival, we found a protective relationship independent of other covariates. It is worth noticing that we found an association between higher BMI values and fewer CNV events and less TSG loss, which may indicate the mechanism of the protective effect; contrary to clear cell renal cell carcinoma, where overweight patients had a reduced risk of death, but no genomic alterations (single nucleotide variations [SNV], CNVs, and hypermethylation frequency) were associated with BMI (28).
We observed that the most frequently mutated gene was NOTCH1, in discrepancy with The Cancer Genome Atlas (TCGA) results, where NOTCH1 was mutated in about 7% of patients, and TP53 was the most frequently mutated gene. In fact, the five most frequently mutated genes we found in our study were mutated in less than 10% of the TCGA cohort (10). It is unknown if this difference in gene mutation frequency is due to ethnicity, other risk factors or due to a cohort effect; further studies are needed to evaluate the clinical impact of these findings. Regarding BMI and CNV and TSG events, it seems that an elevated BMI protects against genomic alterations. We observed the same association among the individual FBXW7, TET2, and SDHB genes, in which higher BMI values associated with the lack of mutations in these genes.
The FBXW7 gene codes for an E3 ubiquitin ligase that targets different oncogene protein products; hence FBXW7 is considered a tumor suppressor gene (29). The loss of this gene has been associated with poor prognosis in gastric cancer patients, as well as poor response to neoadjuvant chemotherapy (30). In the case of TET2, it was mutated in almost 3% of the TCGA cohort (10). These genes, FBXW7 and TET2, have been classified as tumor suppressor genes because of their role in DNA demethylation by methylcytosine oxidation. Their mutations have been mainly reported in myeloid leukemia. In gastric cancer, it has been shown that TET2 inhibits gastric cancer cell growth by upregulating the long non-coding RNA ANRIL and increasing the expression of P15, p16, and p14 proteins (31).
The SDHB gene codes for succinate dehydrogenase; its mutations are mainly associated with gastrointestinal stromal tumors. However, this gene was altered in almost 15% of our patients, compared with 1.4% of the patients in the TCGA cohort (10). Defects of the succinate dehydrogenase activity produce a succinate accumulation and create a pseudohypoxia-induced environment that activates HIF-proteins. The overactivation of the IGF-1 signaling pathway is also found in tumors with SDHB mutations (32).
One limitation of this study is the reduced number of patients included in the subset for the genomic analysis which restricted the statistical power of the genomic associations with clinical variables. However, we managed to find associations between nutritional indexes and the genomic profile of the patients, which need to be validated in further studies to define their molecular role and clinical utility. Another limitation was that not all patients had complete clinical records, for example, histologic subtype and grade could not be determined. These data could have provided more detailed information in terms of clinicopathologic characteristics if these tumors.
Interestingly, our results agree with the obesity paradox previously stated, which suggests that increased nutritional reserves provided by excess fat stores and higher lean body mass in obese patients (33) may provide an added advantage during periods of chronic illness (34), such as cancer. A rapid weight loss before diagnosis often indicates more aggressive cancers or advanced stages. Furthermore, in early-stage patients, lower weights may associate with a subclinical tumor activity. Weight changes can be observed six months before diagnosis, and noticeable subclinical impacts on lipid metabolism can start as early as two years before a diagnosis is made (35). Additionally, the association between BMI and CNV events or TSG loss could also be due to a decrease in the mutational threshold; hence less driver gene mutations are required to develop gastric carcinoma in obese patients, as it has been proposed for colon cancer (36). Thus, it is paramount to assess the BMI and PNI of newly diagnosed cancer patients and continue investigating the molecular mechanisms underlying the obesity paradox.
Nevertheless, we note that alternatively it may not necessarily be the case that overweight or obese individuals have better survival as compared with those who have a normal (healthy) body weight through diet and exercise. In this scenario, it is possible that intrinsic biological tumor characteristics may be associated with a BMI decrease in the preclinical stage before diagnosis. In these patients, cancer progression may cause the inability to maintain body weight due to conditions such as cachexia or poor appetite, resulting in worse prognosis. Understanding the independent roles of muscle and adiposity, the molecular architecture of the gastric tumors, and the interaction with other risk factors still need to be considered to better understand the role of BMI in GC.
Supplementary Material
ACKNOWLEDGMENTS
We thank Clementina Castro, MS in Basic Biomedical Research, for the critical review of this manuscript, as well as Marco Andonegui, PhD and Jaime Barrera for the contributions in the style review and samples collection and processing.
FUNDING
This study was supported by the National Institutes of Health [Grant R21ES027087, Prada] and by CONACYT (Consejo Nacional de Ciencia y Tecnología – México) – FOSISS (Fondo Sectorial de Investigación en Salud y Seguridad Social SS/IMSS/ISSSTE-CONACYT) [FOSISS 289503 Prada; FOSISS A3-S-49533 Prada; A3-S-48281 Garcia-Cuellar].
Footnotes
COMPETING INTERESTS
Authors declare that they have no conflicts of interest.
5. References
- 1.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.INTERNATIONAL WCRF. Diet, nutrition, physical activity and stomach cancer 2016. [Available from: https://www.wcrf.org/sites/default/files/Stomach-Cancer-2016-Report.pdf.
- 5.Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular pathology of gastric cancer: research and practice. Pathol Res Pract. 2011;207(10):608–12. [DOI] [PubMed] [Google Scholar]
- 6.Nobili S, Bruno L, Landini I, Napoli C, Bechi P, Tonelli F, et al. Genomic and genetic alterations influence the progression of gastric cancer. World J Gastroenterol. 2011;17(3):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun M, Nie FQ, Wang ZX, De W. Involvement of lncRNA dysregulation in gastric cancer. Histol Histopathol. 2016;31(1):33–9. [DOI] [PubMed] [Google Scholar]
- 8.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J, Zhao M, Li J, Lou G, Yuan Y, Bu S, et al. Obesity-associated digestive cancers: A review of mechanisms and interventions. Tumour Biol. 2017;39(3):1010428317695020. [DOI] [PubMed] [Google Scholar]
- 10.Network CGAR. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Liu L, Wang X, Wang J, Yan Z, Cheng J, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1395–408. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Park B, Joo J, Kook MC, Kim YI, Lee JY, et al. Body mass index and mortality in patients with gastric cancer: a large cohort study. Gastric Cancer. 2018;21(6):913–24. [DOI] [PubMed] [Google Scholar]
- 13.Chang WJ, Du Y, Zhao X, Ma LY, Cao GW. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol. 2014;20(16):4586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trestini I, Carbognin L, Bonaiuto C, Tortora G, Bria E. The obesity paradox in cancer: clinical insights and perspectives. Eat Weight Disord. 2018;23(2):185–93. [DOI] [PubMed] [Google Scholar]
- 15.Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi 1984;85(9):1001–5. [PubMed] [Google Scholar]
- 16.Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, et al. Preoperative Prognostic Nutritional Index Predicts Long-Term Surgical Outcomes in Patients with Esophageal Squamous Cell Carcinoma. World J Surg. 2018;42(7):2199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong S, Zhou T, Fang W, Xue C, Hu Z, Qin T, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389–97. [DOI] [PubMed] [Google Scholar]
- 18.Nakatani M, Migita K, Matsumoto S, Wakatsuki K, Ito M, Nakade H, et al. Prognostic Significance of the Prognostic Nutritional Index in Patients with Recurrent Esophageal Squamous Cell Carcinoma. Nutr Cancer. 2018;70(3):467–73. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol. 2016;42(8):1176–82. [DOI] [PubMed] [Google Scholar]
- 20.Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke. 2015;10(1):99–104. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165(1):55–61. [DOI] [PubMed] [Google Scholar]
- 22.Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370(3):233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. StatPearls. Treasure Island (FL) 2019. [PubMed] [Google Scholar]
- 24.Martínez-Galindo MG, Zamarripa-Dorsey F, Carmona-Castañeda A, Angeles-Labra A, Peñavera-Hernández R, Ugarte-Briones C, et al. Histopathologic characteristics of gastric adenocarcinoma in Mexican patients: a 10-year experience at the Hospital Juárez of Mexico. Rev Gastroenterol Mex. 2015;80(1):21–6. [DOI] [PubMed] [Google Scholar]
- 25.Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128(7):765–70. [DOI] [PubMed] [Google Scholar]
- 26.Liu BZ, Tao L, Chen YZ, Li XZ, Dong YL, Ma YJ, et al. Preoperative Body Mass Index, Blood Albumin and Triglycerides Predict Survival for Patients with Gastric Cancer. PLoS One. 2016;11(6):e0157401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L, Zhu Y, Qian Q, Tang L. Body mass index and prognosis of breast cancer: An analysis by menstruation status when breast cancer diagnosis. Medicine (Baltimore). 2018;97(26):e11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakimi AA, Furberg H, Zabor EC, Jacobsen A, Schultz N, Ciriello G, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105(24):1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao J, Ge MH, Ling ZQ. Fbxw7 Tumor Suppressor: A Vital Regulator Contributes to Human Tumorigenesis. Medicine (Baltimore). 2016;95(7):e2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li MR, Zhu CC, Ling TL, Zhang YQ, Xu J, Zhao EH, et al. FBXW7 expression is associated with prognosis and chemotherapeutic outcome in Chinese patients with gastric adenocarcinoma. BMC Gastroenterol. 2017;17(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng W, Wang J, Zhang J, Cai J, Bai Z, Zhang Z. TET2 regulates LncRNA-ANRIL expression and inhibits the growth of human gastric cancer cells. IUBMB Life. 2016;68(5):355–64. [DOI] [PubMed] [Google Scholar]
- 32.Miettinen M, Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs) - a review. Int J Biochem Cell Biol. 2014;53:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broughman JR, Williams GR, Deal AM, Yu H, Nyrop KA, Alston SM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. J Geriatr Oncol. 2015;6(6):442–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gioulbasanis I, Baracos VE, Giannousi Z, Xyrafas A, Martin L, Georgoulias V, et al. Baseline nutritional evaluation in metastatic lung cancer patients: Mini Nutritional Assessment versus weight loss history. Ann Oncol. 2011;22(4):835–41. [DOI] [PubMed] [Google Scholar]
- 35.Kritchevsky SB, Wilcosky TC, Morris DL, Truong KN, Tyroler HA. Changes in plasma lipid and lipoprotein cholesterol and weight prior to the diagnosis of cancer. Cancer Res. 1991;51(12):3198–203. [PubMed] [Google Scholar]
- 36.Bordonaro M, Lazarova D. Hypothesis: Obesity Is Associated with a Lower Mutation Threshold in Colon Cancer. J Cancer. 2015;6(9):825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.