Abstract
Background:
The prevalence and impact of inappropriate empiric antibiotic therapy for bloodstream infections (BSI) is unclear. We aimed to determine the population-level burden, predictors, and mortality risk of in vitro susceptibility-discordant empiric antibiotic therapy (DEAT) among BSI patients.
Methods:
Inpatients with BSI treated with systemic antibiotics on the day blood cultures were drawn or the following day were identified. DEAT occurred if the isolate was not susceptible in vitro to antibiotic(s) administered on blood culture sampling day. DEAT prevalence by hospital type was calculated using regression tree analysis and predictors were identified using Generalized Estimating Equations. Adjusted odds ratio (aOR) of in-hospital mortality was determined using logistic regression.
Findings:
At 131 hospitals between 2005–2014, 26,036 assessable BSI patients received empiric therapy on the day of or day after first blood culture collection. Seventeen percent (4,428) received no antibiotics on blood culture day. Of the remaining 21,608 patients, 4,165(19–3%) received DEAT [reliability-adjusted range by hospital type: 16·6(95% CI, 15·0–18·5)% to 21·1(95% CI, 20·1–22·1)%]. Antibiotic-resistant phenotypes strongly predicted DEAT [a0R=9·09(95% CI, 7·68–10·76);p<0·001]. Most DEAT events (73%) and associated deaths (76·8%) occurred among patients with S. aureus and Enterobacteriaceae BSIs. DEAT was independently associated with higher mortality (aOR=1·5[95% CI, 1·4–1·7];p<0·001), a relationship that was unaffected by the presence or absence of resistance or sepsis or septic shock.
Interpretation:
Approximately one in five BSI patients in U.S. hospitals received DEAT, which was closely associated with antibiotic-resistant pathogens. DEAT decreased survival even among those presenting without sepsis. Earlier identification of bloodstream pathogens and resistance is likely to improve population-level outcomes.
Funding:
NIH, U.S. CDC, AHRQ
Keywords: inappropriate, discordant, empiric, antibiotic, antimicrobial, bacteremia, bloodstream, infection, in vitro susceptibility
Introduction:
Prompt and effective antibiotic therapy is associated with improved outcomes in patients with serious infections such as bloodstream infection and sepsis.1,2 However, the rise of antibiotic resistance, and the inherent lag between sampling of cultures and reporting of in vitro susceptibility make empiric antibiotic selection challenging.3 Prescribing broad-spectrum regimens to all patients may increase de novo resistance, C. difficile infection, antibiotic-related toxicities, and costs.4,5 Understanding the burden and prognostic impact of inappropriate empiric antibiotic therapy in serious infections could inform the development of better antibiotic prescribing practices.
The population-level prevalence and impact of in-hospital inappropriate empiric therapy for serious infections is not clearly known; a meta-analysis1 reported a wide 14–79% range for the prevalence in serious infections. This and another meta-analysis of studies in sepsis2 found an association with lower survival, albeit with considerable heterogeneity attributed to variability in populations, risk-adjustment strategies and definitions for “inappropriate”.6 A more recent Danish population-based retrospective cohort study7 of 6,834 patients with BSI found an association between inappropriate empiric therapy and long-term but not short-term survival, but did not control for baseline acute illness severity. Even studies evaluating the same bloodstream pathogen have yielded mixed results8,9 on mortality risk.
The aim of this study was to assess the prevalence, predictors and mortality risk of in vitro susceptibility-discordant empiric antibiotic therapy (DEAT) in BSIs using electronic health record (EHR) data from a large cohort of U.S. hospitals. We hypothesized that robust estimation of population-level burden and impact might benefit from (a) focusing on BSIs, (b) using an objective definition of inappropriate empiric therapy that incorporates both timing (limited to the day of culture sampling) and in vitro concordance and (c) analyzing a large, well-distributed granular dataset enabling nuanced assessments of bacterial taxa, antibiotic-resistance phenotypes and illness severity.
Methods:
A retrospective cohort study was performed using patients with monomicrobial BSI at 131 U.S. hospitals between 2005 and 2014 (eFigure 1) that contribute data to the Cerner Healthfacts Database. The NIH Office of Human Subjects Research waived the need for institutional review board evaluation. Findings were reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines for reporting observational studies.10
Data Source and Study Population:
Cerner Healthfacts is a large de-identified EHR data repository from a well-distributed11 cohort of US hospitals; we limited the current analysis to the subset of hospitals that report microbiology data to Healthfacts.12 We included all patients with monomicrobial bacteremia due to common bloodstream pathogens (eTable 1) who received ≥1 systemic antibiotic on the day blood cultures were drawn or the following day and where susceptibility data were available. The analysis was limited to the first positive (index) blood culture per encounter.
Definitions
Empiric antibiotic therapy was considered discordant (DEAT) if the bloodstream isolate did not display in vitro susceptibility to any systemic antibiotic administered on the day of blood culture sampling. The assessment of DEAT was limited to patients in whom antibiotics were initiated specifically on the day of blood culture sampling in order to focus on those with suspected serious/bloodstream infection in whom providers considered it important to promptly initiate empiric therapy. Antibiotics initiated one day after blood cultures were drawn collectively represented a potential mix of delayed empiric, judiciously held and early targeted therapy. Hence these cases were excluded from the primary analysis of DEAT and analyzed separately. Activity of initial empiric antibiotics against the bloodstream pathogen’s broader gram-stain-based group (positive vs. negative) was also assessed. When not reported for the antibiotic(s) received, in vitro susceptibility or resistance was imputed from those interpretations that were reported within the same antibiotic category. For example, cefepime, a 4th generation cephalosporin was presumed to be active against an isolate in vitro if the isolate was reported as susceptible to the third-generation cephalosporin ceftriaxone. Furthermore, when susceptibility was not reported by laboratories due to intrinsic resistance (e.g., ceftriaxone and P. aeruginosa), the antibiotic was automatically adjudicated as discordant in vitro. These imputation rules enable maximal use of real-world microbiology data in an EHR repository. Imputation rules were predetermined by consensus among a microbiologist (JD) and four infectious diseases physicians (CR, SSK, RLD, and MK), subjected to a priori quality control checks, and are described elsewhere.13 Sepsis was defined according to Sepsis–3 criteria14 as BSI along with organ dysfunction measured as an increase in Sequential Organ Failure Assessment (SOFA) score (calculated as previously reported)15 by ≥2 from baseline. Septic shock was defined as BSI along with use of ≥1 vasopressor within 1 day of blood culture sampling (see supplement for additional methods and eTable 2 for additional definitions).
Analysis:
Baseline characteristics by in vitro-concordance of empiric therapy were reported. The overall and taxon-specific prevalence of DEAT and pre-selected in vitro phenotypes of antibiotic-resistance (eTable 1) were calculated. Patient-level predictors of DEAT were assessed using multivariable logistic regression and a generalized estimating equation with an exchangeable working correlation matrix, clustering on hospital ID using the geepack package in R.16 Given that hospital-level factors do not occur in isolation, recursive partitioning was performed using regression tree analysis to determine hospital-type associations with DEAT prevalence. The mean prevalence estimates were reliability-adjusted (i.e., shrinking prevalence of low-count hospitals closer to the overall rate) using a random effects model.17
The aOR of mortality (in-hospital death or discharge to hospice) associated with DEAT in BSI was calculated using logistic regression, controlling for patient, bloodstream isolate and center-level covariates. Candidate variables were selected based on a priori consensus among authors. The presence of multi-collinearity among covariates and effect modification using pre-specified interaction terms was assessed. The mortality model was also performed on subsets of individual taxa or by presence or absence of sepsis or septic shock and antibiotic-resistant phenotypes respectively.
Sensitivity analyses were performed to assess the aOR of mortality associated with (a) DEAT without applying pre-specified rules to impute susceptibility results, (b) DEAT re-defined as no in vitro active antibiotic received on the day of or day after culture sampling (as opposed to only culture day) and (c) delayed therapy (i.e., receiving no antibiotics on culture day) assessed separately in overall BSI and across sepsis strata. In order to isolate the mortality odds associated with delayed therapy per se, we also controlled for whether delayed therapy was in vitro susceptibility-discordant (vs. concordant). All statistical modeling was conducted using R 3·6·1 or SAS version 9·4.
Role of the funding source:
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. SSK and EER had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
We identified 26,036 patients with microbiologically-confirmed BSIs at 131 hospitals between January 2005 and December 2014 who received ≥1 systemic antibiotic on the day of or day after blood cultures were drawn (Figure 1). Of these, 4,428 (17%) did not receive antibiotics on the day blood cultures were drawn. Of 21,608 who received antibiotics on day that blood cultures were collected, 9,126 (42–2%) had sepsis and 3,154 (14–6%) had septic shock and 4,184 (19–4%) had antibiotic-resistant phenotypes. In addition, 792 (3.7%) received Gram-stain discordant empiric therapy (e.g. empiric vancomycin monotherapy in case of Gram-negative BSI) and 4,165 (19–3%) received DEAT based on retroactive assessment of final susceptibility results. Of the 21,608 patients, 21,050 (97.4%) were either already on or switched to concordant therapy by the day after susceptibility results were provided. Among 4,165 DEAT recipients, 1,958 (49%) had antibiotic-resistant bloodstream isolates. Vancomycin was the most frequently administered initial antibiotic among 37% of patients receiving concordant therapy whereas levofloxacin was most frequently administered initially among 23% of those receiving DEAT (eTable 4). Differences in other baseline characteristics are presented in Table 1 and eTables 2A–B). The crude proportion of DEAT ranged from 0% to 100% by hospital due to extreme values at centers with very low BSI and DEAT counts. The reliability-adjusted prevalence of DEAT ranged from 16·6 (95% CI, 15·0–18·5) at 8 teaching hospitals in the first quartile of baseline resistance in the Midwest, Northeast and West, to 21·1 (95% CI, 20·1–22·1)% at 28 teaching hospitals in the South and Midwest in the top 3 quartiles (Figure 2).
Figure 1: Case-selection flowchart.
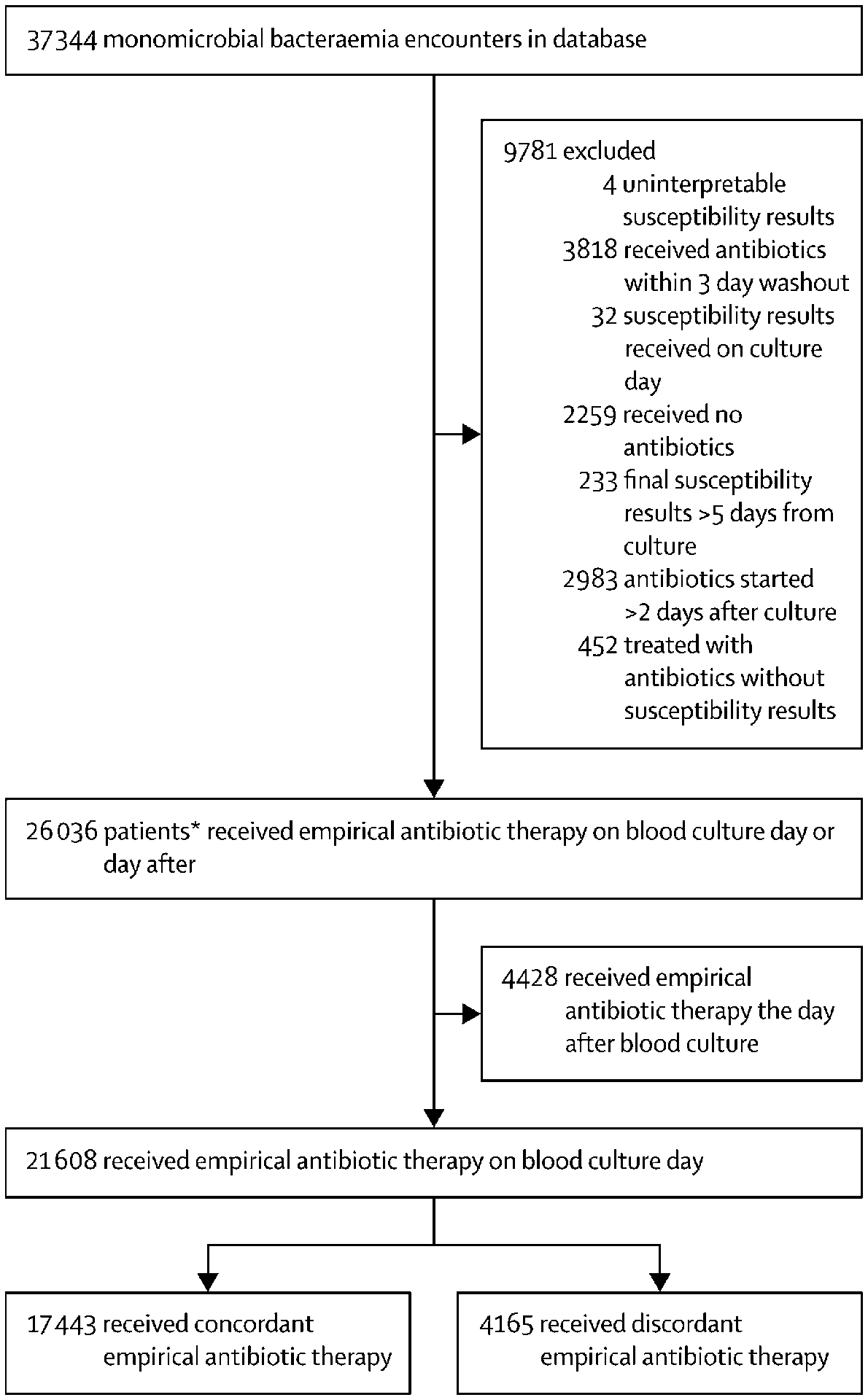
The figure describes the process of selecting patients with monomicrobial bloodstream infection due to common pathogens that were eligible for assessment of concordance of empiric antibiotic therapy. Empiric antibiotic therapy was considered discordant (DEAT) if the bloodstream isolate did not display in vitro susceptibility to any systemic antibiotic administered on the day of blood culture sampling.
Table 1:
Baseline Characteristics of Bacteremia Patients by Concordance of Empiric Antibiotic Therapy
| Concordant Empiric Antibiotic Therapy (n=17,443) | Discordant Empiric Antibiotic Therapy (n=4,165) | |
|---|---|---|
| PATIENT CHARACTERISTICS | ||
| Demographics | ||
| Year of Admission | ||
| 2005–2008 | 4824 (27·7) | 1041 (25·0) |
| 2009–2011 | 5943 (34·1) | 1384 (33·2) |
| 2012–2014 | 6676 (38·3) | 1740 (41·8) |
| Age - Median (IQR) | 66 (51–78) | 69 (56–80) |
| Male Gender | 8748 (50·2) | 2168 (52·1) |
| Race | ||
| White | 12550 (73·0) | 2948 (71·8) |
| Black | 3250 (18·9) | 860 (20·9) |
| Other | 1388 (8·1) | 299 (7·3) |
| Clinical | ||
| Congestive Heart Failure | 2958 (19·5) | 931 (24·8) |
| Diabetes Mellitus | 3118 (20·6) | 881 (23·5) |
| Renal Failure | 3604 (23·8) | 1041 (27·7) |
| Immunocompromised | 2017 (11·6) | 514 (12·3) |
| ICU | 3344 (19·2) | 715 (17·2) |
| Prior BSI encounter | 646 (3·7) | 239 (5·7) |
| Elixhauser Comorbidity Index1 - Median (IQR) | 6 (4–8) | 6 (4–10) |
| SOFA Score on Culture Day | ||
| 0 | 3331 (19·1) | 810 (19·4) |
| 1 | 3263 (18·7) | 753 (18·1) |
| 2 | 2796 (16·0) | 693 (16·6) |
| ≥3 | 8053 (46·2) | 1909 (45·8) |
| Presumed Source of BSI2 | ||
| Abdominal | 298 (1·7) | 49 (1·2) |
| Central Venous Catheter | 478 (2·7) | 138 (3·3) |
| Multi-site | 3698 (21·2) | 938 (22·5) |
| Other Known Site | 331 (1·9) | 51 (1·2) |
| Respiratory | 1454 (8·3) | 313 (7·5) |
| Skin & Soft Tissue | 803 (4·6) | 196 (4·7) |
| Unknown | 6844 (39·2) | 1519 (36·5) |
| Urinary | 3537 (20·3) | 961 (23·1) |
| Community-onset BSI3 | 16029 (91·9) | 3773 (90·6) |
| Antibiotic Resistant Phenotype | 2442 (14·0) | 1742 (41·8) |
| Status on Presentation | ||
| BSI without Sepsis | 7566 (43·4) | 1762 (42·3) |
| Sepsis without Shock4 | 7270 (41·7) | 1856 (44·6) |
| Septic Shock5 | 2607 (14·9) | 547 (13·1) |
| Empiric Therapy Type | ||
| Broad-spectrum | 7603 (43·6) | 1021 (24·5) |
| Multidrug | 9871 (56·6) | 1275 (30·6) |
| Gram stain-concordant | 0 | 792 (19·0) |
| Deceased6 | 1851 (10·6) | 615 (14·8) |
| CENTER CHARACTERISTICS | ||
| U.S. Region | ||
| Midwest | 2237 (12·8) | 524 (12·6) |
| Northeast | 6642 (38·1) | 1617 (38·8) |
| South | 6472 (37·1) | 1587 (38·1) |
| West | 2092 (12·0) | 437 (10·5) |
| ≥300 Beds | 8665 (49·7) | 2234 (53·6) |
| Teaching Facility | 12132 (69·6) | 3026 (72·7) |
| Urban Location | 14237 (81·6) | 3309 (79·4) |
| Quartile of Resistance | ||
| <15·8% | 4499 (25·8) | 919 (22·1) |
| 15·8–18·9% | 4181 (24·0) | 1005 (24·1) |
| 19·0–24·3% | 4435 (25·4) | 1137 (27·3) |
| ≥24·4% | 4328 (24·8) | 1104 (26·5) |
Based on ICD-9 codes and adapted from33
Based on ICD-9 codes and adapted from34
Defined as bacteremia identified on blood culture sampled within 2 days of hospital admission
Sepsis defined by Sepsis-III14 criteria of infection (bacteremia) and increase in SOFA score by ≥2 from baseline
Defined as bacteremic patients requiring ≥1 vasopressor within +/−1 day of blood culture sampling
includes discharges to hospice
Figure 2: Regression Tree Analysis: Association between Hospital Type and Reliability-adjusted Hospital Prevalence of Discordant Empiric Antibiotic Therapy in BSI.
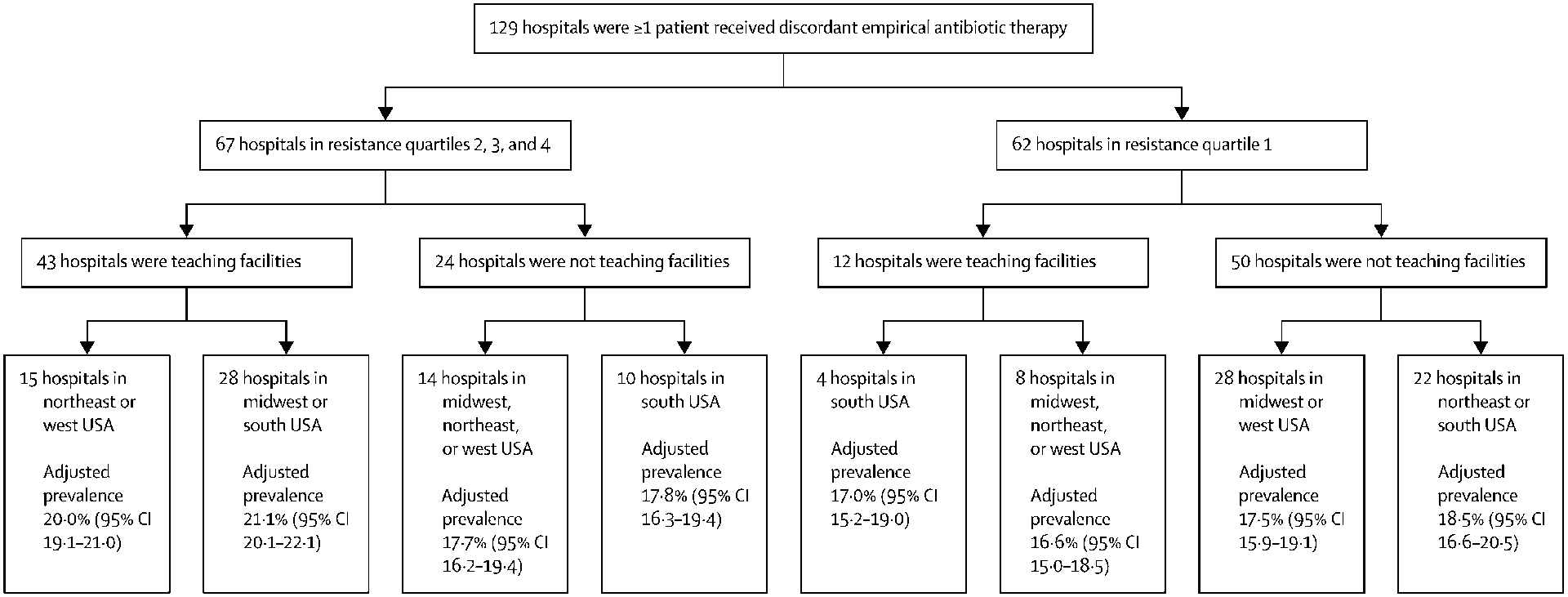
Given that hospital-level factors do not occur in isolation, recursive partitioning was performed by means of a regression tree analysis using combinations of the following characteristics: bed capacity, teaching status, geographic region, urban status and quartile of baseline resistance prevalence encountered at each hospital. Reliability adjustment of mean (95% CI) prevalence of DEAT in BSI for each of the 129 hospitals (with >1 BSI patient receiving DEAT) included was performed using a random effects model. Pruning was used to select the optimal nested subtree with the smallest misclassification cost. All calculations were performed in SAS version 9·4. The GLIMMIX procedure was used to fit the generalized linear mixed model and PROC HPSPLIT was used to conduct the regression tree analysis. The first split occurred at between baseline resistance quartiles 1 vs. 2,3,4, followed by teaching status and then region at which point the regression tree was pruned, yielding 7 distinct hospital types. The reliability-adjusted prevalence of DEAT in BSI ranged from 16·6% (95% CI,15·0–18·5) at 8 teaching hospitals in the first quartile of baseline resistance in the Midwest, Northeast and West, to 21·1% (95% CI, 20·1–22·1) at 28 teaching hospitals in the South and Midwest in the top 3 quartiles of baseline resistance.
Taxon-level variation in DEAT and antibiotic-resistant phenotypes
The proportion of patients with BSI receiving DEAT varied considerably by pathogen, ranging from 4–8% in β-hemolytic streptococci to 72–7% among E. faecium and 82–9% among S. maltophilia (Figure 3A). However, Enterobacteriaceae and S. aureus (predominantly methicillin-resistant S. aureus) were responsible for 1764 (44–5%) and 1256 (31–7%) of all BSIs treated with DEAT respectively. The proportion of bloodstream isolates with antibiotic-resistant phenotypes also varied considerably across taxa, ranging from 3–9% for E. faecalis to 77% for S. maltophilia (Figure 3B). The relative contribution of resistance to DEAT by taxon was determined by the ratio of count of patients receiving DEAT to patients with resistant organisms, which varied considerably from 1:3 for S. pneumoniae and 1:2 for S. aureus to 10:1 for E. faecalis (Figure 3C).
Figure 3 A-C: Discordant empiric antibiotic therapy and antibiotic-resistant phenotypes by taxon of bloodstream isolate.
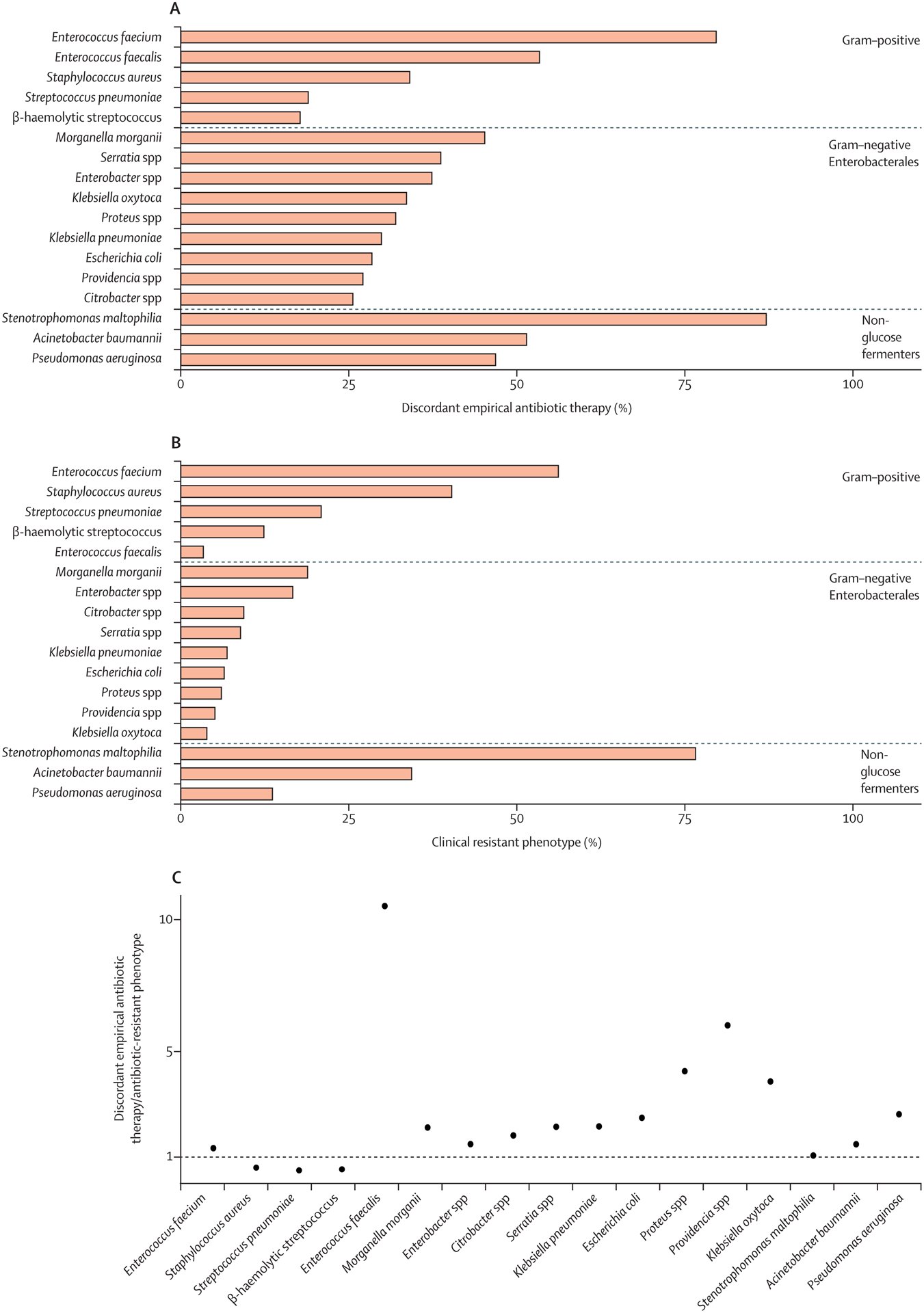
Horizontal bars in sub-figures A and B depict proportions of discordant empiric antibiotic therapy and antibiotic-resistant pathogens respectively. Counts to the right of the bar represents the denominator for each bar. Sub-figure C provides the ratio of count of discordant empiric antibiotic therapy vs. antibiotic-resistant phenotypes by taxon. Footnote: Antibiotic resistance phenotypes are defined for each pathogen as follows: Staphylococcus aureus – Methicillin (MRSA); Enterococcus spp. – Vancomycin (VRE); Enterobacteriaceae spp., non-glucose fermenters other than Stenotrophomonas maltophilia – Extended-spectrum cephalosporin (ESBL) or carbapenem (CR); Stenotrophomonas maltophilia – TMP/SMX, quinolone, or ceftazidime; β-hemolytic Streptococcus – Clindamycin; Pneumococcus spp. – Penicillin. Please refer to eTable 1 for additional details on resistance definitions.
Predictors of DEAT
Predictors of DEAT included the presence of antibiotic-resistant (vs. susceptible) phenotypes [aOR=9·09 (95% CI 7·68–10·76); p<0·001], BSIs due to non-glucose fermenting gram-negative organisms (vs. Enterobacteriaceae BSI) [aOR=3·08 (95% CI 2·02–4·71); p<0·001], and presence of Enterococci [aOR=4·73 (95% CI 3·91–5·71); p<0·001] (Table 2). Conversely, BSIs due to S. aureus [aOR=0·51 (95% CI 0·43–0·61); p<0·001] and Streptococci [aOR=0·18 (95% CI 0·1–0·32); p<0·001] were associated with lower odds of DEAT. Among predictors likely known on presentation, all non-urinary sources displayed greater odds of DEAT than urinary sources. Additionally, prior known hospitalization with BSI [aOR=1·24 (95% CI 1·03–1·49); p=0·024] was associated with greater odds of DEAT. Broad-spectrum or multidrug empiric therapy [a0R=0·32 (95% CI 0·27–0·37); p<0·001] and prior isolation of the pathogen from a non-blood site [a0R=0·58 (95% CI 0·42–0·82); p=0·002] during the same hospitalization were associated with lower odds of receiving DEAT. The odds of receiving DEAT rose with increasing age and Elixhauser co-morbidity index and decreased marginally with rising SOFA score, however, presence and severity of sepsis on presentation had no significant effect on odds of DEAT. Other than baseline resistance (specifically quartile 2 vs. 1) [aOR=1·37 (95% CI 1·16–1·61); p<0·001], other center-level factors were not independently associated with receiving DEAT.
Table 2:
Predictors of Discordant Empiric Antibiotic Therapy
| Estimate (95% CI) | p-value | |
|---|---|---|
| Intercept | 0·08 (0·05–0·13) | <0·001 |
| Factors Unknown on Presentation | ||
| Antibiotic Resistant Phenotype | 9·09 (7·68–10·76) | <0·001 |
| Enterococci spp. (Ref: Enterobacteriaceae spp.) | 4·73 (3·91–5·71) | <0·001 |
| A. baumannii (Ref: Enterobacteriaceae spp.) | 4·57 (2·53–8·26) | <0·001 |
| P. aeruginosa spp. (Ref: Enterobacteriaceae spp.) | 3·08 (2·31–4·1) | <0·001 |
| Other Non-glucose Fermenters (Ref: Enterobacteriaceae spp.) | 3·08 (2·02–4·71) | <0·001 |
| S. aureus spp. (Ref: Enterobacteriaceae spp.) | 0·51 (0·43–0·61) | <0·001 |
| Pneumococci spp. (Ref: Enterobacteriaceae spp.) | 0·2 (0·14–0·29) | <0·001 |
| Beta Hemolytic Streptococci (Ref: Enterobacteriaceae spp.) | 0·18 (0·1–0·32) | <0·001 |
| Factors Potentially Known on Presentation | ||
| Admission Year 2009–2011 (Ref: 2005–2008) | 1·32 (1·12–1·56) | 0·001 |
| Admission Year 2012–2014 (Ref: 2005–2008) | 1·24 (0·99–1·55) | 0·065 |
| Age | 1·0 (1·0–1·01) | <0·001 |
| Female Gender (Ref: Male) | 0·96 (0·89–1·03) | 0·235 |
| Black Race (Ref: White) | 1·17 (1·02–1·34) | 0·024 |
| Asian Race (Ref: White) | 1·14 (0·89–1·47) | 0·293 |
| Other Race (Ref: White) | 1·13 (0·9–1·43) | 0·302 |
| Hispanic Race (Ref: White) | 0·72 (0·53–0·98) | 0·034 |
| Immunocompromised | 0·97 (0·85–1·1) | 0·645 |
| Prior BSI Encounter | 1·24 (1·03–1·49) | 0·024 |
| Prior Administration of Antibiotic1 | 1·24 (0·92–1·66) | 0·159 |
| Prior Non-Bloodstream Isolation2 | 0·58 (0·42–0·82) | 0·002 |
| Broad Spectrum/Multi-drug Therapy | 0·32 (0·27–0·37) | <0·001 |
| Community-onset BSI3 | 0·98 (0·78–1·22) | 0·833 |
| Elixhauser Comorbidity Score4 | 1·02 (1·01–1·03) | <0·001 |
| Sepsis without Shock (Ref: BSI without Sepsis)5 | 1·08 (0·99–1·18) | 0·091 |
| Septic Shock (Ref: BSI without Sepsis)6 | 1·02 (0·87–1·19) | 0·844 |
| SOFA Score on Culture Day | 0·98 (0·96–1·0) | 0·048 |
| Presumed BSI Source7 - Abdominal (Ref: Urinary) | 2·66 (1·96–3·6) | <0·001 |
| Presumed BSI Source7 - Multi-site (Ref: Urinary) | 2·07 (1·55–2·75) | <0·001 |
| Presumed BSI Source7 - Respiratory (Ref: Urinary) | 1·75 (1·39–2·21) | <0·001 |
| Presumed BSI Source7 - Skin & Soft Tissue (Ref: Urinary) | 1·66 (1·27–2·16) | <0·001 |
| Presumed BSI Source7 - Other (Ref: Urinary) | 1·52 (1·24–1·85) | <0·001 |
| ≥300 Beds in Hospital | 0·98 (0·86–1·12) | 0·743 |
| Teaching Facility | 1·02 (0·87–1·2) | 0·776 |
| Urban Hospital | 1·0 (0·82–1·22) | 0·987 |
| Hospital Region: Northeast (Ref: South) | 1·03 (0·9–1·17) | 0·670 |
| Hospital Region: Midwest (Ref: South) | 0·87 (0·73–1·04) | 0·118 |
| Hospital Region: West (Ref: South) | 0·86 (0·69–1·09) | 0·214 |
| Resistance Quartile 2 - 15·8–18·9% (ref: Quartile 1 - <15·8%) | 1·37 (1·16–1·61) | <0·001 |
| Resistance Quartile 3 - 19·0–24·3% (ref: Quartile 1 - <15·8%) | 1·21 (1·01–1·46) | 0·044 |
| Resistance Quartile 4 - >24·4% (ref: Quartile 1 - <15·8%) | 0·99 (0·81–1·21) | 0·923 |
Administered between admission and 3 days prior to index blood culture sampling day
Prior isolation of same pathogen from non-bloodstream culture during same encounter
Defined as bacteremia identified on blood culture sampled within 2 days of hospital admission
Based on ICD-9 codes and adapted from 36
Sepsis defined by Sepsis-III24 criteria of infection (bacteremia) and increase in SOFA score by ≥2 from baseline
Defined as bacteremic patients requiring ≥1 vasopressor +/−1 day of blood culture sampling
Collapsed categories based on ICD-9 codes and adapted from53
Mortality Risk associated with DEAT
The crude mortality rate (including hospice discharges) among BSI patients was 12%, ranging from 10·6% for recipients of concordant empiric therapy vs. 13·2% for recipients of DEAT. The aOR of mortality associated with DEAT was 1·37 (1·24–1·51); p=<0·001) (Figure 4A, eTable 5). In taxon-specific models (Figure 4A, eTable 5), the aOR of mortality associated with DEAT remained significantly higher for patients with S. aureus [aOR=1·85 (95% CI 1·43–2·40); p<0·001], demonstrated a non-significant trend towards higher mortality odds among Enterobacteriaceae [aOR=1·23 (95% CI 1·00–1·52); p=0·054], and non-glucose fermenters [aOR=1·34 (95% CI 0·84–2·13); p=0·22], and had no effect on streptococcal [aOR=1·01 (0·51–0·99), p=0·98] or enterococcal [aOR=0·9 (0·55–1·48), p=0·69] BSIs respectively. DEAT was independently associated with a higher aOR of mortality regardless of whether patients presented with BSIs without sepsis [aOR=1·49(95% CI 1·01–1·82); p=0·008], sepsis without shock [aOR=1·54 (1·28–1·85); p<0·001)], or septic shock [aOR=1·30 (1·01–1·68); p=0·043] (Figure 4B, eTable 5), and whether bloodstream isolates displayed resistance [aOR=1–67 (95% CI 1·26–2·20), p<0·001] or not [aOR=1·36 (1·16–1·60), p<0·001] (Figure 4B, eTable 5). None of the pre-specified interaction terms improved model fit or significantly altered the DEAT-mortality relationship (data not shown) and were not included in final models.
Figure 4 A-D: Adjusted Odds Ratio of Mortality associated with Discordant and Delayed Empiric Antibiotic Therapy:
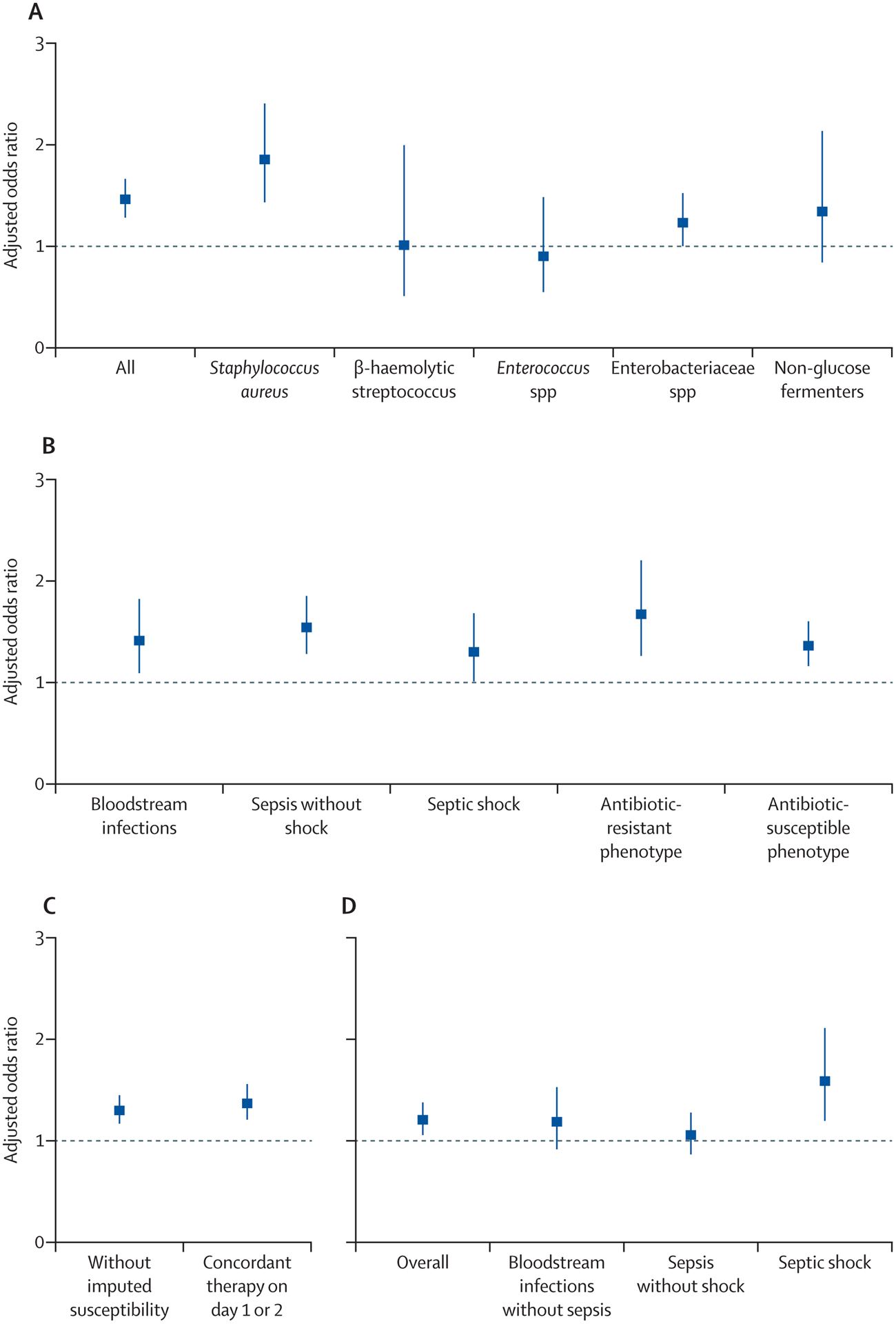
Primary, subset and sensitivity analyses. Estimates are represented by squares and 95% confidence intervals are represented by vertical bars. Sub-figure A displays results from overall and taxon-specific logistic regression models evaluating the odds ratio of mortality associated with discordant empiric antibiotic therapy adjusted for patient, pathogen and center-level characteristics. Sub-figure B examines the relationship between aOR for mortality associated with discordant empiric antibiotic therapy across sepsis strata and antibiotic resistant phenotypes. Sub-figure C examines sensitivity analyses without incorporating imputed susceptibilities and where concordant or discordant empiric antibiotic therapy were adjudicated based on empiric therapy in a window that included culture day or the next. Sub-figure D examines sensitivity analysis of the aOR of mortality associated with delayed therapy (i.e., where empiric therapy was initiated on the day after culture sampling day) while controlling for concordance of therapy.
Sensitivity analyses:
In sensitivity analyses (Figure 4 C–D; eTable 5), the association between DEAT and mortality was similar when analyzing the cohort for whom missing in vitro susceptibility results were not imputed [aOR=1·30 (95% CI 1·17, 1·45), p<0·001], as well as upon extending the period for adjudication of concordant vs. discordant therapy up to the day after blood culture sampling [aOR=1·37 (1·21–1·56), p<0·001]. Delayed empiric therapy (i.e., initiation on the day after culture sampling) while controlling for concordance was also associated with a higher odd of mortality [aOR=1·21 (1·06–1·38), p<0·001]. However, within sepsis strata, delayed therapy was only associated with significantly greater mortality risk in patients with BSI presenting with septic shock [aOR=1·59 (1·20 −2·11), p<0·001], but not in those with BSI without sepsis [aOR=1·19 (0·92–1·53), p=0·18] or sepsis without shock [aOR=1·06 (0·87–1·28), p=0·56] (See eTables 7–8 and eFigure 2–3 for quality-control analysis of missing antibiotic data).
Discussion
This work represents the largest study to date examining the burden and impact of in vitro susceptibility-discordant empiric antibiotic therapy (DEAT) in patients with BSI using routinely collected EHR data from U.S. hospitals. We attempted to address several pitfalls and challenges identified in previous studies1,6,7 by (1) investigating BSIs in lieu of other specimen sources that are more difficult to distinguish from colonization; (2) limiting “inappropriate” therapy to objectively-assessable in vitro susceptibility-discordant antibiotics administered on the day blood cultures were drawn; (3) maximizing real-world EHR data utilization by imputing susceptibility for antibiotic-pathogen combinations when not explicitly reported in microbiology data; and (4) performing comprehensive risk-adjustment that included controlling for underlying comorbidities, immunosuppression and baseline acute illness severity using a standardized score. Our study demonstrates that approximately one of every five patients with BSI received DEAT, which was associated with decreased survival amongst all patients that clinicians deem to require antibiotics on the same day blood cultures are drawn. DEAT was closely associated with antibiotic resistance among pathogens. DEAT was common across all study hospital types regardless of bed-capacity, teaching and urban/rural status or institutional and local resistance patterns, indicating that the problem is widespread and that efforts to optimize empiric prescribing would require large-scale implementation.
Our study throws light on the contribution of antibiotic-resistance to the probability of receiving DEAT, which could aid in prioritizing antibiotic stewardship strategies for early recognition and to improve prescribing practices. We found that a fifth of patients had BSIs due to resistant isolates, which were associated with 9-fold increased odds of receiving DEAT. Although our study identified resistance as the key determinant of DEAT occurrences, this was not invariably true; pathogens in nearly half the recipients of DEAT did not display pre-specified antibiotic-resistant phenotypes. Other factors besides resistance include initial therapy that could be missing coverage for a specific species, genus, or broader category of pathogens. We found that in the absence of resistant pathogens, DEAT was more often a consequence of incorrect initial empiric gram-positive or negative coverage (relative to bloodstream pathogen). Patients who received DEAT (vs. in vitro susceptibility concordant therapy) more often received narrow-spectrum or single-agent initial coverage. Furthermore, the rate of DEAT in E. faecalis BSIs was 10-fold higher than its rate of vancomycin resistance, suggesting that lack of initial coverage for routine enterococcus sp. (rather than VRE specifically) was the primary driver of DEAT for this pathogen. Several other unmeasured factors may have driven antibiotic choices, and in turn DEAT, such as patient allergies, signs and symptoms, institutional formularies, protocols, empiric therapy guidelines, as well as lack of a stewardship program and/or availability of appropriate diagnostic tools. Future studies where access to these additional data are available may further elucidate and consequently mitigate suboptimal prescribing behaviors and institutional factors that lead to inadequate coverage for BSI.
Our study provides a bird’s eye landscape of inpatient empiric therapy, BSI pathogens and resistance rates that has important implications for antibiotic stewardship and guidelines. We highlight the fact that patients with bloodstream isolates that are not generally covered by empiric guidelines for sepsis (e.g. Enterococcus, Acinetobacter, Pseudomonas and Stenotrophomonas sp.) are more likely to encounter DEAT. However, in order to justify further broadening the spectrum of recommended empiric therapy, it is also important to consider the overall likelihood of encountering these difficult-to-treat pathogens and the specific host populations. The highest proportions of DEAT were seen in BSI secondary to S. maltophilia and E. faecium, which likely reflects these organisms’ higher rates of intrinsic resistance.18,19 However, the population-level impact of DEAT from these two pathogens collectively represented only 3% of DEAT recipients. Furthermore, among 2,531 immune compromised patients with suspected and subsequently confirmed BSI in our study (in whom there may be greater concern for VRE than the general population), 161 (6.3%) had enterococcal BSI whereas only 28 (1.1%) had VRE. It may be difficult to justify empirically treating 91 patients with VRE-active agents to ensure 1 patient with VRE in the bloodstream is adequately treated. As such, these decisions are best made on a case-by-case basis by a comprehensive assessment of all available data elements including surveillance swabs, prior infection and antibiotic history, type and severity of illness on presentation and the risk of initial non-coverage.
S. aureus and Enterobacteriaceae, on the other hand, collectively represented three-quarters of the absolute burden of DEAT and associated deaths, suggesting that earlier knowledge specifically of these pathogens and consequent prompt administration of effective therapy could have a large impact on the overall population-level burden of mortality in patients with BSI. The beneficial impact of combining antibiotic stewardship efforts with data from rapid diagnostics has been well-described in the literature.20 This combined approach could benefit frontline providers who are often forced to exercise stressful guesswork when selecting empiric antibiotics in good faith for acutely ill and decompensating patients. Support for investigations on risk factors such as prior infection and colonization,21 followed by large-scale implementation of this knowledge to improve empiric prescribing is warranted, as is the ongoing development and deployment of reliable, affordable, point-of-care diagnostics22 particularly for earlier identification of high-burden pathogens.
Our primary and subset analyses report a relative excess of 20–60% in the odds of death associated with DEAT. However, BSIs per se are associated with a substantial mortality risk, even among those who received concordant empiric antibiotics. Using this cohort as the comparator group instead of non-bacteremic patients likely minimized the apparent detrimental impact of DEAT.23 Similarly, using culture day as baseline for severity-of-illness adjustment may have underestimated the impact of resistance on survival.24 Although we relied on in vitro susceptibility-concordance and discordance, these are not always synonymous with appropriateness25 or inappropriateness26 of therapy respectively. As such, in vitro concordant empiric therapy that is sub-efficacious may have also diluted the impact of DEAT on outcome. We did not adjust for switch to concordant therapy or the timing thereof given that most BSI patients assessed had eventually received concordant therapy by the day after in vitro susceptibilities had been reported. Furthermore, such adjustments risk introducing survival bias, as patients would need to remain alive until final susceptibility results were available and acted upon. We observed greater mortality risk associated with DEAT with or without resistant pathogens. Notably, some resistance traits are co-associated with hypervirulent bacterial strains that may produce worse outcomes independent of inappropriate therapy.27,28 Reducing inappropriate prescribing is likely to mitigate, but not eliminate the impact of antibiotic-resistant bacterial phenotypes.
Initial broad-spectrum therapy is advised in critically-ill patients with suspected sepsis and septic shock.3 However, the odds of receiving DEAT in BSI were not significantly influenced by presence of sepsis syndromes and only marginally lowered by increasing acute illness severity; broader antibiotic coverage may have been offset by greater prevalence of resistant pathogens among sicker patients.29 Importantly, the risk-benefit ratio of broad-spectrum empiric therapy for less-sick patients with BSIs without sepsis is less clear30 and warrants further study. The Centers for Medicare and Medicaid’s SEP-1 measure currently mandates all patients with suspected severe sepsis (infection and organ failure) or septic shock to receive empiric antibiotics within 3 hours of initial suspicion.31 Our sensitivity analysis on bacteremic patients that were not prescribed antibiotics until a day after blood culture sampling (including instances of missed vs. judiciously held empiric therapy) is potentially informative. Within this group, we found that delayed treatment of septic shock was associated with a statistically-significant increase in mortality risk, but this was not so for sepsis without shock or for BSI without sepsis. This signal is concordant with prior evidence on time-to-antibiotics in sepsis.32 However, this does not mean that patients presenting with suspected sepsis without shock should be denied prompt antibiotics. Rather, additional studies with finer temporal granularity and an objective and precise definition of “time-zero” for measuring time-to-antibiotics are needed to gauge the optimal immediacy of empiric antibiotics for patients with suspected sepsis who are not in shock.
Our study has limitations. Although our study represents a well-distributed cohort of U.S. hospitals and the largest ever studied for this question, the cohort may not be truly representative of all hospitals in the U.S. and our findings may not be generalizable to other global regions. We were unable to assess for the adequacy of source control given the absence of clinical documentation critical to make such determinations; we were unable to use minimum inhibitory concentration data; however, susceptibility interpretations guide most clinicians on their targeted antibiotic choices. Polymicrobial BSIs were excluded due to difficulty merging microbiology and susceptibility datasets for multi-organism samples, which may have impacted our estimates. Residual confounding may persist despite our efforts at risk-adjustment. While use of imputed missing susceptibilities based on consensus adjudication maximized use of available data, it may have added an element of subjectivity. However, we performed sensitivity analysis using the non-imputed dataset and found similar DEAT-outcome relationships. Despite our quality-control analyses, exclusion of encounters missing any antibiotic orders may have eliminated some cases where administration of antibiotics was truly missed. We were unable to generate hourly estimates of associations between time-to-antibiotics and outcomes due to insufficiently granular data.
CONCLUSION:
In summary, approximately one of every five patients with BSI in 131 U.S. hospitals received DEAT, most often due to antibiotic resistance. Discordant empiric therapy was associated with increased mortality amongst all patients whom clinicians elected to treat on the same day blood cultures were drawn, regardless of severity-of-illness or presence of resistant organisms. Our retrospective population-level analysis emphasizes that importance of earlier identification of bloodstream pathogens and their resistance profiles, particularly Enterobacteriaceae and S. aureus, as a means to potentially mitigate the mortality burden attributable to DEAT. We encourage investigators from other regions of the world where hospitals are powered by EHR systems to leverage their data to perform similar assessments of patterns and impact of empiric antibiotic prescribing, which may enhance local awareness and identify areas for improvement.
Preliminary data from this study were presented at the annual meeting of the Infectious Diseases Society of America (IDWeek) in San Francisco, CA (October, 2018) and will be presented at the 6th Decennial International Conference on Healthcare Associated Infections in Atlanta, GA (March, 2020).
Supplementary Material
Evidence before the study:
Selection of initial empiric antibiotic therapy for suspected serious infections remains challenging. We performed a preliminary review of evidence around the epidemiology of inappropriate (inadequate) empiric antibiotic therapy in serious infections such as bloodstream infection (BSI); a PubMed search identified studies over the last 30 years using the terms “(in)appropriate” OR “(in)adequate” AND “empiric” OR “empirical” AND “antibiotic” OR “antimicrobial”. The search, not restricted by infection site/type, language or patient age, revealed 141 articles. All relevant studies were observational. Two meta-analyses were identified representing populations with serious infection and sepsis respectively. The prevalence of inappropriate empiric antibiotics in serious infections varied considerably across studies. Although most studies in BSI and sepsis revealed a detrimental impact of inappropriate empiric therapy on survival, the signal of harm was not consistently observed across studies. Significant variability in study populations, risk-adjustment strategies and definitions for “inappropriate” across the identified studies or limited sample size precluded robust inferences.
Added value of this study:
Our retrospective cohort study of over 26,000 BSI patients at 131 U.S. hospitals represents the largest study to date examining the burden and impact of in vitro susceptibility-discordant empiric antibiotic therapy (DEAT) and demonstrates the feasibility of using routine electronic health record data (EHR) for this purpose. We found that a fifth of in-patients with BSI due to routine bloodstream pathogens initially received DEAT. Discordant empiric therapy was prevalent across hospital types. Although by proportions DEAT was most common among S. maltophilia and E. faecium, most cases of DEAT were in fact due to Enterobacteriaceae or S. aureus resistant to empiric agents. Antibiotic-resistant (vs. susceptible) bloodstream pathogens were associated with a 9-fold greater probability of receiving DEAT. Discordant empiric therapy was associated with lower survival in bacteremic patients regardless of the presence or absence of antibiotic-resistant phenotypes, sepsis or septic shock.
Implications of all the available evidence:
There is a need to bolster existing national efforts to control the development and spread of antibiotic resistance. However, our findings are not necessarily generalizable to other regions of the world. Wherever available, EHR data must be scrutinized to better understand reasons for failure of empiric coverage across diverse settings and patient populations, followed by system-wide implementation of interventions to optimize prescribing. Such optimization is likely to favorably impact survival of bacteremic patients regardless of whether they display sepsis or resistant pathogens. Earlier identification of bloodstream pathogens, especially Enterobacteriaceae and S. aureus and their resistance profiles may improve outcomes in BSI on a population level.
Acknowledgements:
The authors would like to thank Mr. David Fram of Commonwealth Informatics for assistance with curation of the dataset and Mrs. Kelly Byrne for assisting with formatting the manuscript.
Funding: This project has been funded in part with funds from the intramural research program of the National Institutes of Health Clinical Center, National Institutes of Allergy and Infectious Diseases, and National Cancer Institute, (under Contract No. HHSN261200800001E), as well as the US Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality.
Disclaimer: The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the National Institutes of Health, the US Centers for Disease Control and Prevention or the Agency for Healthcare Research and Quality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Powers III reports personal fees from Arrevus, Corbus, DaVolterra, Eicos, Eli Lilly, Gilead, MedImmune, Microbion, Otsuka, Roche, Romark, Shinogi, outside the submitted work. None of the other authors report any financial disclosures or conflicts of interest.
References:
- 1.Marquet K, Liesenborgs A, Bergs J, Vleugels A, Claes N. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care 2015; 19: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010; 54(11): 4851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017; 45(3): 486–552. [DOI] [PubMed] [Google Scholar]
- 4.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. JAntimicrob Chemother 2014; 69(4): 881–91. [DOI] [PubMed] [Google Scholar]
- 5.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014; 14: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGregor JC, Rich SE, Harris AD, et al. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis 2007; 45(3): 329–37. [DOI] [PubMed] [Google Scholar]
- 7.Gradel KO, Jensen US, Schonheyder HC, et al. Impact of appropriate empirical antibiotic treatment on recurrence and mortality in patients with bacteraemia: a population-based cohort study. BMC Infect Dis 2017; 17(1): 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SH, Park WB, Lee KD, et al. Outcome of inappropriate initial antimicrobial treatment in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 2004; 54(2): 489–97. [DOI] [PubMed] [Google Scholar]
- 9.Paul M, Kariv G, Goldberg E, et al. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 2010; 65(12): 2658–65. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370(9596): 1453–7. [DOI] [PubMed] [Google Scholar]
- 11.DeShazo JP, Hoffman MA. A comparison of a multistate inpatient EHR database to the HCUP Nationwide Inpatient Sample. BMC Health Serv Res 2015; 15: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadri SS, Lai YLE, Ricotta EE, et al. External Validation of Difficult-to-Treat Resistance Prevalence and Mortality Risk in Gram-Negative Bloodstream Infection Using Electronic Health Record Data From 140 US Hospitals. Open Forum Infect Dis 2019; 6(4): ofz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee C, Kadri SS, Dekker JP, Danner RL, Chen H, Fram D, Wang R, Klompas M Prevalence of Antibiotic Resistant Pathogens in Culture-Positive Sepsis and Outcomes Associated with Inappropriate and Unnecessarily Broad Empiric Antibiotics. 2020. [DOI] [PMC free article] [PubMed]
- 14.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315(8): 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee C, Zhang Z, Kadri SS, et al. Sepsis Surveillance Using Adult Sepsis Events Simplified eSOFA Criteria Versus Sepsis-3 Sequential Organ Failure Assessment Criteria. Crit Care Med 2019; 47(3): 307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Højsgaard S, Halekoh U., Yan J. The R Package geepack for Generalized Estimating Equations Journal of Statistical Software. 2006; 15(2): 1––11. [Google Scholar]
- 17.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res 2010; 45(6 Pt 1): 1614–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falagas ME, Valkimadi PE, Huang YT, Matthaiou DK, Hsueh PR. Therapeutic options for Stenotrophomonas maltophilia infections beyond co-trimoxazole: a systematic review. J Antimicrob Chemother 2008; 62(5): 889–94. [DOI] [PubMed] [Google Scholar]
- 19.CDC. Antibiotic Resistance & Patient Safety Portal. 2015.
- 20.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. The Effect of Molecular Rapid Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-analysis. Clin Infect Dis 2017; 64(1): 15–23. [DOI] [PubMed] [Google Scholar]
- 21.Isendahl J, Giske CG, Hammar U, et al. Temporal Dynamics and Risk Factors for Bloodstream Infection With Extended-spectrum beta-Lactamase-producing Bacteria in Previously-colonized Individuals: National Population-based Cohort Study. Clin Infect Dis 2019; 68(4): 641–9. [DOI] [PubMed] [Google Scholar]
- 22.van Belkum A, Rochas O. Laboratory-Based and Point-of-Care Testing for MSSA/MRSA Detection in the Age of Whole Genome Sequencing. Front Microbiol 2018; 9: 1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zilberberg MD, Shorr AF, Micek ST, Mody SH, Kollef MH. Antimicrobial therapy escalation and hospital mortality among patients with health-care-associated pneumonia: a single-center experience. Chest 2008; 134(5): 963–8. [DOI] [PubMed] [Google Scholar]
- 24.Perencevich EN. Excess shock and mortality in Staphylococcus aureus related to methicillin resistance. Clin Infect Dis 2000; 31(5): 1311–3. [DOI] [PubMed] [Google Scholar]
- 25.Harris PNA, Tambyah PA, Lye DC, et al. Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients With E coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018; 320(10): 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17(8): 1135–41. [DOI] [PubMed] [Google Scholar]
- 27.El-Solh AA, Hattemer A, Hauser AR, Alhajhusain A, Vora H. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med 2012; 40(4): 1157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Simmonds A, Uhlemann AC. Clinical Implications of Genomic Adaptation and Evolution of Carbapenem-Resistant Klebsiella pneumoniae. JInfect Dis 2017; 215(suppl_1): S18–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care 2014; 18(6): 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiens J, Snyder GM, Finlayson S, Mahoney MV, Celi LA. Potential Adverse Effects of Broad-Spectrum Antimicrobial Exposure in the Intensive Care Unit. Open Forum Infect Dis 2018; 5(2): ofx270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CMS. QualityNet. Inpatient Hospitals Specifications Manual.
- 32.Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. NEngl J Med 2017; 376(23): 2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43(11): 1130–9. [DOI] [PubMed] [Google Scholar]
- 34.Christensen KL, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB. Infectious disease hospitalizations in the United States. Clin Infect Dis 2009; 49(7): 1025–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


