Abstract
Objective:
The United States Preventive Services Task Force recommends against breast self-examination. However, racial disparities exist in mammogram screening. We aimed to evaluate the presentation of women with newly diagnosed breast cancer in the underserved African-American and Hispanic community to provide insight regarding breast cancer screening in this population.
Methods:
This retrospective cohort study included women newly diagnosed with breast cancer from 1/1/2016 to 1/1/2018 in an inner city public community hospital. Data was collected via chart review. Patients were divided based on whether they presented with self-detected breast mass. Logistic regression was used for analysis.
Results:
59 women were newly diagnosed with breast cancer. 34 women (58%) were African-American, 20 (34%) were Hispanic, and 5 (8%) were other race. Of 59 women, 36 (61%) presented with self-detected breast mass, and only 21 (36%) reported prior mammography. For women who presented with breast mass, the odds of having prior mammography were 78% lower (OR=0.22, 95%CI 0.07–0.69, p=0.009), while the odds of having invasive ductal carcinoma were 4.33 times higher (OR=4.33, 95%CI 1.09–17.25, p=0.037), as compared to the odds for women not presenting with breast mass.
Conclusion:
Many of our newly diagnosed breast cancer patients were African-American or Hispanic women presenting with self-detected breast mass without prior screening mammography. Further studies should evaluate whether supplemental screening methods, such as breast self-examination or clinical examination, can help with early breast cancer detection in minority women with limited access to care, and such disparities should be considered by organizations when creating screening guidelines.
Keywords: breast cancer, screening, breast self-examination, African American, Hispanic
Introduction
Breast self-examination (BSE), clinical breast examination (CBE), and mammography all have been used alone or in combination for the screening of breast cancer. However, the most recent 2016 guidelines from the U.S. Preventive Services Task Force (USPSTF)1 and the American Cancer Society (ACS)2 no longer recommend BSE or CBE as a screening method for average-risk women of any age in the United States (US). On the other hand, the American College of Obstetrics and Gynecology (ACOG)3 and the National comprehensive cancer network (NCCN)4 recommends CBE every 1–3 years for women between 25 and 39 and annually for women 40 years and older, but BSE is not recommended for average-risk women due to a lack of evidence of benefit. BSE is a process whereby women examine their breasts regularly to detect any abnormal swelling or lumps in order to seek prompt medical attention. CBE is the examination of a woman’s breast by health care professionals who are trained to recognize abnormalities and warning signs in the breast5. Earlier studies have suggested that BSE and CBE may reduce breast-cancer mortality and improve survival6,7. In addition, patients presenting with palpable masses on SBE or CBE and normal mammograms within 1 year tend to have more aggressive tumors8. However, clinical trials of BSE training did not demonstrate lower mortality rates in the BSE group when compared to the controls9,10. In addition, false positive BSE or CBE may lead to unnecessary anxiety and worry about the cancer, as well as additional visits to the clinic, unwanted imaging and biopsies11,12.
However, the USPSTF and ACS recommendations assume that women have access to and are undergoing mammographic screening1,2, and subsequently should not be presumed to apply in settings where mammographic screening is lacking13,14. There is continued use of BSE and CBE to detect breast cancer in underserved parts of the world where screening mammography services are not available. Studies have demonstrated that even in developed countries like the United States, racial disparities exist in the use of screening mammography15. Black and Hispanic women are less likely to utilize screening mammography when compared to the white population15. It has been shown that access to breast cancer screening and medical care is a significant problem in the inner-city Hispanic community, related to recent immigration, undocumented immigration, and low income characteristics of its members16. Our goal is to evaluate the presentation of women newly diagnosed with breast cancer in a community hospital mainly serving an inner city African American and Hispanic population, in order to provide insight of breast cancer screening in low resource settings where minority women may have limited access to care.
Methods
We performed a retrospective cohort study. Adult female patients (≥18 years old) who were newly diagnosed with breast cancer at an inner-city community hospital between 1/1/2016 and 1/1/2018 were included. Patients with prior history of breast cancer were excluded from the analysis, in consideration of the possibility of higher breast cancer awareness and thus different screening patterns. Our community hospital is a public hospital primarily serving the poor and working class in an inner-city African American and Hispanic community, including uninsured city residents and new immigrants. The medical records of women newly diagnosed with breast cancer were reviewed. Age, race, presenting symptoms, imaging findings, pathologic findings, and risk factors were collected from patients’ medical records. The outcome of our study was defined as the percentage of women who presented with self-detected breast mass among these newly diagnosed with breast cancer. This study was approved by the institutional review board, and granted a waiver of consent. The study was compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Statistical Analysis
In the univariate analyses, χ2 tests were used for categorical variables and t-tests were used for continuous variables. To determine whether presentation of self-detected breast mass was independently associated with race, history of prior mammogram screening, and ER/PR/HER-2 status, we conducted the analysis using the logistic regression model. Potential confounding variables listed above were considered and those with p<0.2 in the univariate analyses were entered into the final multiple regression model. The adjusted odds ratios (ORs) and 95% confidence intervals (95% CI) were calculated from the logistic regression model to assess the magnitudes of the associations. Significance was based on α<0.05 and all hypothesis tests were 2-sided. The analyses were performed using Stata version 11.0 (Stata Corp, College Station, Texas).
Results
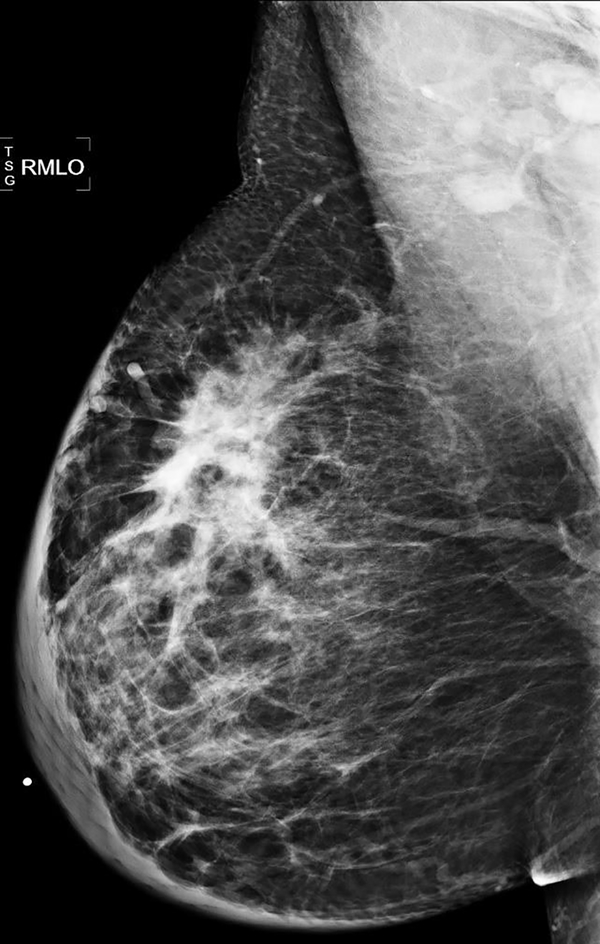
From 1/1/2016 to 1/1/2018, 6103 screening mammograms and 3361 diagnostic mammograms were performed on 6148 patients. 73 women had positive biopsy results. Among them, 59 women were newly diagnosed with breast cancer. Among those newly diagnosed with breast cancer, the average age (mean ± SD) at diagnosis was 59±15 years old, and the mean BMI was 29±6. 34 women (58%) were African American, 20 (34%) were Hispanic, and 5 (8%) self-identified as other races. 36 women (61%) presented with self-detected breast mass. Figure 1 shows the diagnostic mammogram of one of the women presented with self-detected breast mass. The mean size of breast cancer was 3.4±2.7 cm measured by ultrasound when masses were identified (n=41). Only 21 women (36%) reported prior screening mammograms in the U.S. based on pre-test questionnaires. 38 women (64%) had invasive ductal carcinoma, which is the most common diagnosis. 10 women (20%) were triple negative for ER/PR/HER-2.
Figure 1.
46-year-old woman presenting with a right self-detected breast mass. Right MLO mammography image demonstrates at 12:00, middle depth, a 4.0 cm irregular hyper-dense mass with associated skin thickening and trabecular coarsening. Prominent right axillary lymph nodes are also noted. Biopsy yielded invasive ductal carcinoma with nodal metastasis.
Table 1 shows the characteristics of the women newly diagnosed with breast cancer. Compared to women without self-detected breast mass, those who presented with self-detected breast mass were significantly less likely to report prior screening mammograms (22% vs. 57%, p=0.007), and significantly more likely to have larger tumor size when masses were identified on ultrasound (4.4±2.8 cm vs. 1.4±1.0 cm, p<0.001). Compared to women who did not present with self-detected breast mass, women who presented with self-detected breast mass were also more likely to be younger, have invasive ductal carcinoma as compared to ductal carcinoma in situ (DCIS), and triple negative cancer, however, the difference were not statistically significant (Table 1).
Table 1.
Patient characteristics by presentation of self-detected breast mass
| Total n (%) | Presentation of self-detected breast mass |
P value | ||
|---|---|---|---|---|
| Yes (n=36) | No (n=23) | |||
| Age | 59±15 | 57±17 | 61±9 | 0.244 |
| Race | 0.586 | |||
| Black | 34 (58%) | 21 (58%) | 13 (57%) | |
| Hispanic | 20 (34%) | 13 (36%) | 7 (30%) | |
| Other | 5 (8%) | 2 (6%) | 3 (13%) | |
| BMI | 29±6 | 29±6 | 29±6 | >0.999 |
| Size of cancer (cm) | 3.4±2.7 (n=41) | 4.4±2.8 (n=28) | 1.4±1.0 (n=13) | <0.001 |
| Self-reported Prior screening | 21 (36%) | 8 (22%) | 13 (57%) | 0.007 |
| Pathology | 0.088 | |||
| Invasive ductal carcinoma | 38 (64%) | 26 (72%) | 12 (52%) | |
| DCIS | 12 (20%) | 4 (11%) | 8 (35%) | |
| Other | 9 (16%) | 6 (17%) | 3 (13%) | |
| Triple negative (ER/PR/HER-2) | 10 (20%) | 7 (23%) | 3 (15%) | 0.470 |
In the univariate logistic regression as shown in Table 2, for women presented with self-detected breast mass, the odds of reporting prior screening mammography were 78% lower (OR=0.22, 95%CI 0.07, 0.69, p=0.009), while the odds of having invasive ductal carcinoma were 4.33 times higher (OR=4.33, 95%CI 1.09, 17.25, p=0.037), and the odds of having larger tumor size on ultrasound were 3.82 times higher (OR=3.82, 95%CI 1.49, 9.74, p=0.005), as compared to the odds for women who did not present with self-detected breast mass. Variables with p<0.2 in the univariate analyses were entered into the final multiple logistic regression model. The size of tumor was not included in the final model, because not all breast cancer were presented as identifiable masses on ultrasound. After adjusting for the factors in the logistic regression model, for women presented with self-detected breast mass, the odds of reporting prior screening mammography were 74% lower (OR=0.26, 95%CI 0.08, 0.89, p=0.031), as compared to the odds for women who did not present with self-detected breast mass (Table 2).
Table 2.
Unadjusted and adjusted associations between patient characteristics and presentation of self-detected breast mass
| Characteristics | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | |
| Age | 0.98 (0.94, 1.02) | 0.254 | NA | |
| Race | ||||
| Black | Reference | NA | ||
| Hispanic | 1.15 (0.36, 3.63) | 0.812 | ||
| Other | 0.41 (0.06, 2.81) | 0.366 | ||
| BMI | 1.00 (0.91, 1.10) | 0.997 | ||
| Size of cancer (cm) (n=41) | 3.82 (1.49, 9.74) | 0.005 | NA | |
| Self–reported prior screening | 0.22 (0.07, 0.69) | 0.009 | 0.26 (0.08, 0.89) | 0.031 |
| Pathology | ||||
| DCIS | Reference | Reference | ||
| Invasive ductal carcinoma | 4.33 (1.09, 17.25) | 0.037 | 3.05 (0.70, 13.22) | 0.136 |
| Other | 4.00 (0.64, 25.02) | 0.138 | 2.05 (0.28, 14.82) | 0.478 |
| Triple negative (ER/PR/HER-2) | 0.58 (0.13, 2.57) | 0.464 | NA | |
Discussion
Our public community hospital predominantly serves the inner city African American and Hispanic women who often have limited access to care. Among the women newly diagnosed with breast cancer, only 36% reported prior screening mammography. This is much lower when compared to the national average of 65.3% of women aged 40 and above who received a mammogram in the last 2 years17. Growing evidence suggests that limitations in the national survey databases lead to overestimations of mammogram use, particularly among low-income racial and ethnic minorities18. A prior study demonstrated that only half of the inner-city women were obtaining regular breast cancer screening19. The barriers to obtaining screening mammograms for medically underserved populations include cost, language and acculturation limitations, deficits in knowledge, lack of insurance and medical care18. Fear of cost due to no insurance has been reported as the most common reported barrier20. Low income minority and inner-city women continue to have relatively low breast cancer screening rates when compared to the general population21.
In our study, 61% of the women presented with self-detected breast mass. A prior study evaluating symptomatic women with breast cancer has shown that the most common symptom of presentation was breast lump22. IDC is more common in woman who presented with self-detected breast mass as compared to those who did not present with self-detected mass in our study. In addition, among the women with masses seen on ultrasound, the size of the primary tumor was larger in the woman who presented with self-detected breast mass as compared to those who did not. African-American women suffer higher breast cancer mortality23, which may be partially due to the disparities in screening mammography, tumor characteristics at diagnosis, biologic markers, and treatment24,25. It has been suggested that black women, when diagnosed at comparable disease stages as white women and treated appropriately, tend to experience similar breast cancer prognosis and survival26. Early detection of breast cancer might decrease the disease burden at the time of diagnosis, and potentially improve prognosis. Regular screening mammography detects breast cancer early and reduces breast cancer mortality27,28. Without screening mammography, the breast cancers may progress to later stages with palpable masses. Thus, it is important to establish outreach programs to the underserved communities to provide screening mammography for early cancer detection.
Given the high percentage of women presenting with self-detected breast mass in our study, further study is needed to evaluate whether BSE or CBE in medically underserved populations can help improve breast cancer awareness and overcome cultural barriers to early breast cancer detection. USPSTF does not recommend teaching women BSE29. They have also concluded that performing CBE do not offer any additional benefit beyond screening mammograms29. However, BSE is the recommended method in developing countries for breast cancer early detection because it is easy, convenient, private, safe and requires no specific equipment30,31. In addition, CBE is also considered useful to detect interval cancers detected in between screening mammography studies8,32. In our study, women who presented with self-detected breast mass also had higher rate of triple negative cancer. Thus, these women should be counseled about BSE and/or CBE, and encouraged to seek medical care early if they experience a change. Therefore, community outreach with BSE training and complimentary CBE programs may be important to improve early cancer detection in the underserved minority population.
There are several limitations in our study. First, our data was collected via retrospective chart review, and thus, the patients’ socioeconomic status, immigration status, insurance coverage and education level were not available in the electronic medical record (EMR). In addition, there was missing data for risk factors of breast cancer in the EMR, such as family history of breast cancer and reproductive history. Thus, we cannot evaluate the impact of these factors on breast cancer screening. Moreover, some of our patients decided to seek breast cancer treatment at different facilities after the initial diagnosis, and thus, we do not have data to assess the pathologic tumor size and lymph nodes status of these patients.
Conclusion
The majority of newly diagnosed breast cancer patients in our inner city hospital mainly serving the underserved minority community were African American or Hispanic women who presented with self-detected breast mass without prior screening mammography. These minority women who presented with self-detected breast mass were significantly more likely to have invasive ductal carcinoma and larger tumor size when masses were detected on ultrasound. Our study suggests that there needs to be outreach to the underserved communities with screening mammography for early cancer detection. In addition, future study is needed to evaluate whether supplemental screening methods, such as BSE and/or CBE, can help to improve breast cancer awareness and early breast cancer detection in minority women with limited access to care, and such disparities should be considered by organizations when creating screening guidelines.
Highlights.
Many African American and Hispanic women diagnosed with breast cancer presented with breast mass.
There needs to be outreach of screening mammography for early cancer detection.
Future study should assess if breast self-exam improves detection in minority with limited care.
Disparities in screening should be considered when creating breast cancer screening guidelines.
Acknowledgments
VLM was funded by the NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.U.S. Preventive Services Task Force. Final Recommendation Statement Breast Cancer: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening-:~:text=The%20USPSTF%20recommends%20biennial%20screening,aged%2050%20to%2074%20years.&text=The%20decision%20to%20start%20screening,should%20be%20an%20individual%20one Published 2016. Accessed June 8, 2020.
- 2.American Cancer Society. American Cancer Society Recommendations for the Early Detection of Breast Cancer. https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html Accessed June 8, 2020.
- 3.Committee on Practice B-G. Practice Bulletin Number 179: Breast Cancer Risk Assessment and Screening in Average-Risk Women. Obstet Gynecol. 2017;130(1):e1–e16. [DOI] [PubMed] [Google Scholar]
- 4.Bevers TB, Helvie M, Bonaccio E, et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2018;16(11). [DOI] [PubMed] [Google Scholar]
- 5.National Breast Cancer Foundation I. What’s The Difference Between A Breast Self-Exam And A Clinical Breast Exam? https://www.nationalbreastcancer.org/clinical-breast-exam Accessed June 8, 2020.
- 6.Greenwald P, Nasca PC, Lawrence CE, et al. Estimated effect of breast self-examination and routine physician examinations on breast-cancer mortality. N Engl J Med. 1978;299(6):271–273. [DOI] [PubMed] [Google Scholar]
- 7.Baines CJ. Breast self-examination. Cancer. 1992;69(7 Suppl):1942–1946. [DOI] [PubMed] [Google Scholar]
- 8.Haakinson DJ, Stucky CC, Dueck AC, et al. A significant number of women present with palpable breast cancer even with a normal mammogram within 1 year. Am J Surg. 2010;200(6):712–717; discussion 717–718. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst. 2002;94(19):1445–1457. [DOI] [PubMed] [Google Scholar]
- 10.Hackshaw AK, Paul EA. Breast self-examination and death from breast cancer: a meta-analysis. Br J Cancer. 2003;88(7):1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saslow D, Hannan J, Osuch J, et al. Clinical breast examination: practical recommendations for optimizing performance and reporting. CA Cancer J Clin. 2004;54(6):327–344. [DOI] [PubMed] [Google Scholar]
- 12.Barton MB, Harris R, Fletcher SW. The rational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: should it be done? How? JAMA. 1999;282(13):1270–1280. [DOI] [PubMed] [Google Scholar]
- 13.Anderson BO, Cazap E. Breast health global initiative (BHGI) outline for program development in Latin America. Salud Publica Mex. 2009;51 Suppl 2:s309–315. [DOI] [PubMed] [Google Scholar]
- 14.Anderson BO, Bevers TB, Carlson RW. Clinical Breast Examination and Breast Cancer Screening Guideline. JAMA. 2016;315(13):1403–1404. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed AT, Welch BT, Brinjikji W, et al. Racial Disparities in Screening Mammography in the United States: A Systematic Review and Metaanalysis. J Am Coll Radiol. 2016;14(2):157–165.e159. [DOI] [PubMed] [Google Scholar]
- 16.Fulton JP, Rakowski W, Jones AC. Determinants of breast cancer screening among inner-city Hispanic women in comparison with other inner-city women. Public Health Rep. 1995;110(4):476–482. [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Mammography use. https://www.cdc.gov/nchs/fastats/mammography.htm Accessed June 8, 2020.
- 18.Peek ME, Han JH. Disparities in screening mammography. Current status, interventions and implications. J Gen Intern Med. 2004;19(2):184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor VM, Thompson B, Montano DE, Mahloch J, Johnson K, Li S. Mammography use among women attending an inner-city clinic. J Cancer Educ. 1998;13(2):96–101. [DOI] [PubMed] [Google Scholar]
- 20.Fayanju OM, Kraenzle S, Drake BF, Oka M, Goodman MS. Perceived barriers to mammography among underserved women in a Breast Health Center Outreach Program. Am J Surg. 2014;208(3):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz SJ, Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage. Breast and cervical cancer screening in Ontario and the United States. JAMA. 1994;272(7):530–534. [PubMed] [Google Scholar]
- 22.Koo MM, von Wagner C, Abel GA, McPhail S, Rubin GP, Lyratzopoulos G. Typical and atypical presenting symptoms of breast cancer and their associations with diagnostic intervals: Evidence from a national audit of cancer diagnosis. Cancer Epidemiol. 2017;48:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institue. SEER Cancer Statistics Review (CSR) 1975–2016. https://seer.cancer.gov/archive/csr/1975_2016/ Accessed June 8, 2020.
- 24.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272(12):947–954. [DOI] [PubMed] [Google Scholar]
- 25.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112(1):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dignam JJ. Differences in breast cancer prognosis among African-American and Caucasian women. CA Cancer J Clin. 2000;50(1):50–64. [DOI] [PubMed] [Google Scholar]
- 27.American College of Radiology and Society of Breast Imaging. New ACR and SBI Breast Cancer Screening Guidelines Call for Significant Changes to Screening Process. 2018; https://www.acr.org/Media-Center/ACR-News-Releases/2018/New-ACR-and-SBI-Breast-Cancer-Screening-Guidelines-Call-for-Significant-Changes-to-Screening-Process Accessed June 12, 2020.
- 28.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA. 2015;314(15):1599–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244–255. [DOI] [PubMed] [Google Scholar]
- 30.Malak AT, Dicle A. Assessing the efficacy of a peer education model in teaching breast self-examination to university students. Asian Pac J Cancer Prev. 2007;8(4):481–484. [PubMed] [Google Scholar]
- 31.Philip J, Harris WG, Flaherty C, Joslin CA. Clinical measures to assess the practice and efficiency of breast self-examination. Cancer. 1986;58(4):973–977. [DOI] [PubMed] [Google Scholar]
- 32.Irwig L, Macaskill P, Houssami N. Evidence relevant to the investigation of breast symptoms: the triple test. Breast. 2002;11(3):215–220. [DOI] [PubMed] [Google Scholar]